The first half of the Carboniferous record of actinopterygians is marked by two remarkable – and likely interrelated – events: the increase in numerical dominance relative to other groups of fishes, and accompanying substantial anatomical innovation (Sallan Reference Sallan2014; Friedman 2015). Long appreciated by palaeontologists (e.g., Woodward Reference Woodward1895), these complementary patterns have recently been viewed through the lens of evolutionary recovery following the end-Devonian or Hangenberg extinction event (Sallan & Coates Reference Sallan and Coates2010; Friedman & Sallan Reference Friedman and Sallan2012). The early Carboniferous record provides the oldest unambiguous evidence for key functional innovations in feeding (e.g., durophagy) and locomotion (e.g., body elongation) that would become repeated motifs over the subsequent evolutionary history of ray-finned fishes (Bellwood Reference Bellwood2003; Claverie & Wainwright Reference Claverie and Wainwright2014).
Although the Carboniferous ray-finned fish record is rich, most available fossil material is heavily flattened, restricting investigations to the limited suite of characters apparent in the external dermal skeleton. However, some deposits from the United States and United Kingdom do yield three-dimensionally preserved actinopterygian specimens that permit more detailed examination of internal structures. Such material has been studied through traditional mechanical preparation and observation (Moodie Reference Moodie1915; Watson Reference Watson1928; Case Reference Case1937; Bradley Dyne Reference Bradley Dyne1939; Poplin & Véran Reference Poplin, Véran and Milner1996; Coates Reference Coates1999), physical tomography (Moodie Reference Moodie1920; Poplin Reference Poplin1974, Reference Poplin1984; Hamel & Poplin Reference Hamel and Poplin2008), and, more recently, computed tomography (Giles & Friedman Reference Giles and Friedman2014; Pradel et al. Reference Pradel, Maisey, Mapes and Kruta2016; Coates & Tietjen Reference Coates and Tietjen2019). Fossils collected from Wardie on the south shore of the Firth of Forth, Scotland, are of particular interest because of their age, preservation and the diversity of fauna represented. Of Viséan age, the Wardie assemblage includes the oldest substantial collection of three-dimensionally preserved Carboniferous actinopterygian remains. It lies stratigraphically above the Tournaisian-earliest Viséan Romer's Gap, an interval variously interpreted as characterised by poor sampling, biotic recovery from extinction or some combination of the two (Ward et al. Reference Ward, Labandeira, Laurin and Berner2006; Smithson et al. Reference Smithson, Wood, Marshall and Clack2012; Clack et al. Reference Clack, Bennett, Carpenter, Davies, Fraser, Kearsey, Marshall, Millward, Otoo, Reeves, Ross, Ruta, Smithson, Smithson and Walsh2016). Wardie yields roughly a dozen nominal actinopterygian species divided between seven anatomically divergent genera, and many of these are known from almost complete individuals preserved in concretions (Wood Reference Wood1975; Dineley & Metcalf Reference Dineley and Metcalf1999). These include taxa that differ from most geologically older ray-finned fishes in terms of body size and shape, as well as major modifications to jaw and dental structure. The most recent substantive examination of any of the Wardie ray-fins is over half a century old (Gardiner Reference Gardiner1963), and itself represents only an incremental advance on foundational early works (Traquair Reference Traquair1867, Reference Traquair1875, Reference Traquair1877–1914; Watson Reference Watson1928). The tenacious ironstone matrix that characterises and surrounds Wardie fossils impedes mechanical preparation, although some success has been achieved with chondrichthyan material (Dick Reference Dick1978, Reference Dick1981, Reference Dick1998). Microcomputed tomography (μCT) was first applied to material from Wardie as an alternative more than a decade ago (Anderson et al. Reference Anderson, Carroll and Rowe2003), and subsequent efforts targeting tetrapods (Pardo et al. Reference Pardo, Szostakiwskyj, Ahlberg and Anderson2017) and chondrichthyans (Coates & Tietjen Reference Coates and Tietjen2018) have revealed new anatomical details with important functional and phylogenetic implications.
Here we employ μCT to examine the jaws and dentition of the eurynotiform (sensu Sallan & Coates Reference Sallan and Coates2013) actinopterygian Eurynotus from Wardie. Although available details are incomplete, it is nevertheless clear that the feeding apparatus of Eurynotus is substantially modified relative to primitive actinopterygian conditions. This taxon is of particular interest in being among the earliest ray-finned fishes with functional modifications consistent with the processing of hard prey. Egerton (Reference Egerton1850, p. 3) first noted ‘blunt and rounded teeth' based on correspondence from, and casts provided by, Hugh Miller (Young Reference Young1866, p. 314, provides text of Miller's letters). Drawing on several specimens from various Scottish localities, Traquair (Reference Traquair1867, Reference Traquair1879) provided more detailed accounts of the palate, parasphenoid, and upper and lower jaws, accompanied by illustrations of the maxilla, ‘pterygoid' and some teeth. Watson (Reference Watson1928) subsequently described and figured a single specimen from Wardie showing bones of the palate and jaws in articulation, but the specimen was badly broken and his figure lacks detail as a consequence. More recently, Coates (Reference Coates1994, fig. 7) described and illustrated a flattened specimen of Eurynotus from East Kirkton with a disarticulated head showing some details of the jaws and toothplates. By clarifying the anatomy of the feeding apparatus of Eurynotus, we seek to address previous hypotheses of its relationships, provide interpretations of its palaeoecology and determine its implications for patterns of trophic turnover among fishes between the Devonian and Carboniferous.
1. Material and methods
1.1. Specimen and background
Two of us (M.F. and S.E.P.) identified a three-dimensionally preserved specimen (Museum of Comparative Zoology, Harvard University, Cambridge, MA, USA (MCZ) 10508) of Eurynotus from Wardie Beach, Edinburgh, Scotland (Fig. 1). Material from Wardie at MCZ represents a personal collection sold by Thomas Stock, and received in two instalments in November 1883 and March 1884; MCZ 10508 arrived as part of the first shipment. This individual comprises much of the skull except for the anterior tip of the snout. The nodule is broken behind the skull, but includes the pectoral girdle. The left side of the skull is exposed, while the opposite face of the specimen is completely buried within the enclosing siderite matrix and was therefore undamaged by splitting of the concretion. Locality information is written on the external surface of the concretion: ‘Eurynothus [sic], Trinity, below Railway Station'.

Figure 1 Eurynotus crenatus, MCZ 10508, Wardie Shales Member, Gullane Formation, Edinburgh, Scotland. Specimen in (A) right-lateral view; (B) left-lateral view. Images copyright President and Fellows of Harvard College.
Agassiz (Reference Agassiz1835) named two species of Eurynotus from the early Carboniferous of Scotland: the type specimen E. crenatus, based on several specimens from Burdiehouse (Hopetun Member of the West Lothian Oil-Shale Formation) housed (in part) at the National Museum of Scotland, Edinburgh, UK (NMS 1878.18.11, 1878.18.12, 1878.18.13, 1878.18.14, 1950.38.99, 1950.38.100; Agassiz Reference Agassiz1835: pl. 14a, b; Henrichsen Reference Henrichsen1970) and the referred E. fimbratus, based on a portion of trunk from Wardie at the Oxford University Museum, Oxford University Museum of Natural History, Oxford, UK (OUMNH E.03152; Agassiz Reference Agassiz1835: pl. 14c, figs 1–3). The genus is a characteristic member of the so-called ‘Oil-Shale fish fauna' (Coates Reference Coates1994) of Scotland, and its abundance has been noted by Traquair (Reference Traquair1879: 349–350). Traquair temporarily adopted the convention of identifying material of Eurynotus from Wardie as belonging to E. fimbratus (e.g., Traquair Reference Traquair1867), but here we follow Woodward (Reference Woodward1891) and Traquair (Reference Traquair1903) in considering material from Wardie as referable to the type species E. crenatus. Taxonomy of other probable examples of Eurynotus, including a specifically unassigned example from East Kirkton (Coates Reference Coates1994; NMS G 1993.6.30) and the Belgian ‘Platysomus' insignis (De Koninck Reference De Koninck1878; Royal Belgian Institute of Natural Sciences, Brussels, Belgium (RBINS) 10.445; pers. obs. M.F.), is in need of revision, but this is beyond the scope of the current report.
1.2. Geological context
The shales at Wardie have been known for their fossils since the first half of the 19th Century, when materials collected by Lord Greenock were described by Agassiz in his Recherches (Reference Agassiz1835). At present these deposits are recognised as the Wardie Shales Member of the Gullane Formation which, along with the underlying Arthur's Seat Volcanic Formation and overlying Westlothian Oil-Shale Formation, comprise the Strathclyde Group in West Lothian (Chisholm et al. Reference Chisholm, McAdam and Brand1989; Chisholm & Brand Reference Chisholm and Brand1994; Waters et al. Reference Waters, Browne, Jones, Somerville and Waters2011a, fig. 44). Goniatites from the MacGregor Marine Bands above the Wardie Shales Member are assigned to zone B2 of the English succession (Currie Reference Currie1954; Wilson Reference Wilson1989; Waters et al. Reference Waters, Somerville, Stephenson, Cleal, Long and Waters2011b), which is contained entirely within the Asbian substage of the Viséan stage of the Mississipian (Waters et al. Reference Waters, Somerville, Stephenson, Cleal, Long and Waters2011a). The Lochriea mononodosa Zone is the oldest conodont zone restricted entirely to the overlying Brigantian substage. The base of the nodosa Zone is no less than 333.95±0.39Ma, the estimated age of the base of the younger Lochriea ziegleri Zone (Davydov et al. Reference Davydov, Korn, Schmitz, Gradstein, Schmitz and Ogg2012); this defines a youngest age constraint for the Wardie Shales Member. 40Ar/39Ar dating of intrusive rocks of the Arthur's Seat Volcanic Formation that are overlain by the Gullane Formation yields an age estimate of 335.1±0.6Ma (Monoghan et al. Reference Monoghan, Browne and Barford2014), and provides an oldest age constraint. Thus the age of the Wardie Shales Member, and the specimen of Eurynotus described here, is restricted to a relatively narrow window of ∼333.5–335.5Ma.
The Wardie Shale Member consists of roughly 325m of shales, calcareous mudstones and sandstones. The depositional environment is interpreted as deltaic with occasional marine influence (Greensmith Reference Greensmith1962). Fossil fishes are best known from outcrops exposed along the Wardie shore between Granton and Newhaven. These include abundant plants indicating proximity to the shore, brachiopods, bivalves, ostracods, chondrichthyans, actinopterygians, sarcopterygian fishes and the tetrapod Lethiscus (Traquair Reference Traquair1903; Wood Reference Wood1975; Dineley & Metcalf Reference Dineley and Metcalf1999). Vertebrates are preserved within siderite nodules found within a series of distinctive fish beds. These show differences in faunal composition and relative abundance, as well as the structure of fossil-bearing nodules (Wood Reference Wood1975). Sumner (Reference Sumner1991) investigated the taphonomy of Wardie nodules, which are rarely barren and most frequently contain coprolites. Wood's (Reference Wood1975) fish beds 2–6 yield Eurynotus crenatus. In no case is the species the most common actinopterygian, and instead falls within the middle of the abundance distribution (Wood Reference Wood1975).
1.3. Computed tomography and segmentation
MCZ 10508 was scanned at the Center for Nanoscale Systems, Harvard University using a HMXST225 X-Tek Micro-CT System. X-rays (220kV, 110μA) were filtered with 2mm of copper, with a resulting scan resolution of 33.965μm. Tomograms were saved as a TIFF image stack and loaded into the segmentation software Mimics v.19.0 (http://biomedical.materialise.com/mimics). Surface files exported from Mimics were rendered in Blender (blender.org) for figures.
2. Description
We restrict our description to components of the skull intimately associated with feeding: the palate, jaws and hyoid arch (Figs 2, 3). Other components visible in tomograms, but not presented here, include fragmentary ceratobranchials and portions of the dermal shoulder girdle.
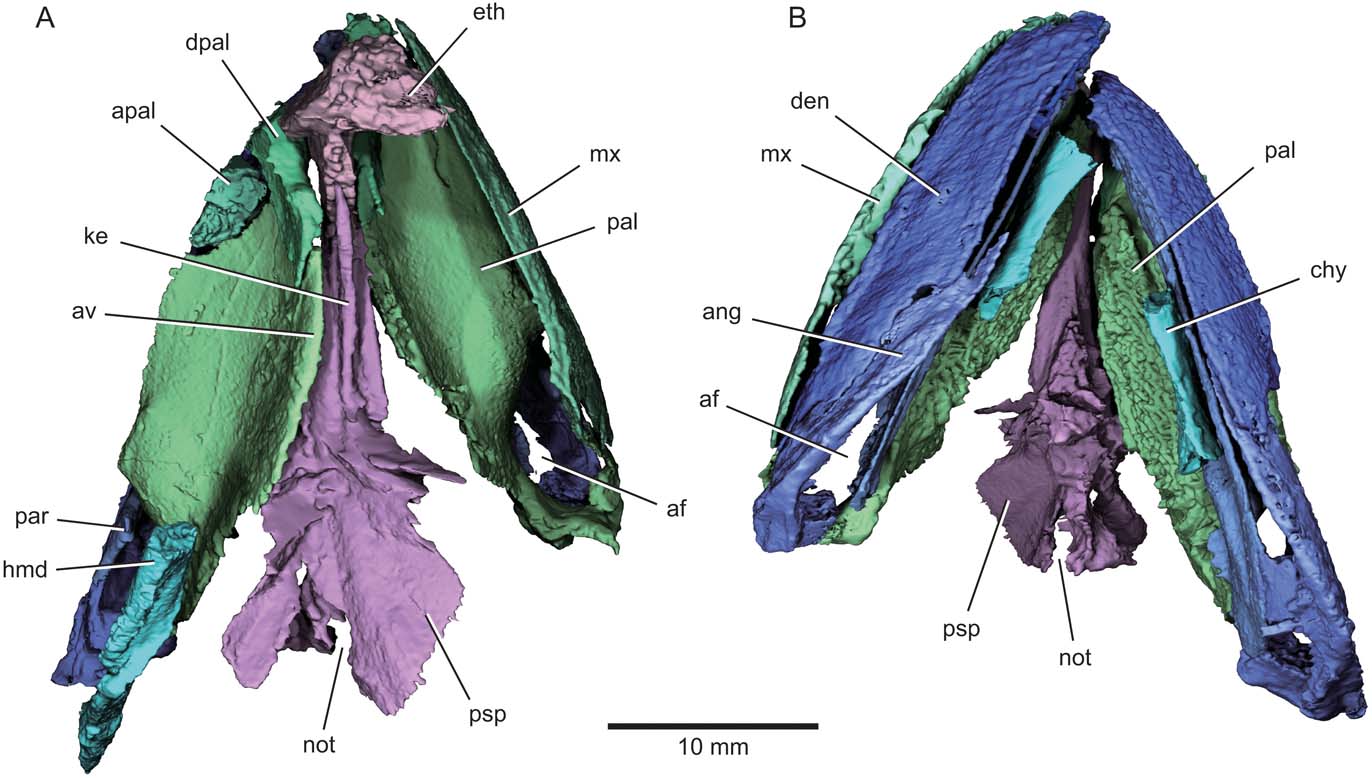
Figure 2 Eurynotus crenatus, MCZ 10508, Wardie Shales Member, Gullane Formation, Edinburgh, Scotland. μCT model of jaws, palate, and hyoid arch in (A) dorsal view; (B) ventral view. Abbreviations: af = adductor fossa; ang = angular; apal = autopalatine; av = accessory vomer; chy = ceratohyal; den = dentary; dpal = dermoplalatine; eth = ethmoid; hmd = hyomandibula; ke = dorsal keel of parasphenoid; mx = maxilla; not = aortic notch; pal = palate; par = prearticular; psp = parasphenoid. Colour coding of the skeleton (adopted in subsequent figures): blue = lower jaw complex; green = upper jaw complex; purple = braincase and parasphenoid; turquoise = hyoid arch.

Figure 3 Eurynotus crenatus, MCZ 10508, Wardie Shales Member, Gullane Formation, Edinburgh, Scotland. μCT model of jaws, palate, and hyoid arch in (A) left-lateral view; (B) right-lateral view. Abbreviations: ang = angular; apal = autopalatine; asc = ascending process of the parasphenoid; av = accessory vomer; cot = articular cotyle; den = dentary; dpal = dermopalatine; eth = ethmoid; fac = articular facet for autopalatine; hmd = hyomandibula; ?ment = possible mentomeckelian ossification; mx = maxilla; pal = palate; par = prearticular; psp = parasphenoid; qu = quadrate; ?sang = surangular.
2.1. Upper jaw
The maxilla (Figs 3, 4) is triangular, with external ornament comprising thin ridges. Two prominent features are found on the inner surface. First, a mesially directed flange (fl, Fig. 4B) extends parallel to, but offset from, the oral margin. Second, a low ridge (ri, Fig. 4B) that traces the oral margin of the bone appears to bear small teeth (dent, Fig. 4b) not visible from the external surface of the maxilla. Both features are restricted to the anterior two-thirds of the maxilla, and together define a broad trough that embraces the ventrolateral margin of the palate anterior to the level of the adductor chamber. The premaxilla is not preserved.

Figure 4 Eurynotus crenatus, MCZ 10508, Wardie Shales Member, Gullane Formation, Edinburgh, Scotland. μCT model of right maxilla. (A) Lateral view. (B) Mesial view. Abbreviations: dent = denticles; fl = flange on inner surface of maxilla; mx = maxilla; ri = ridge.
2.2. Palate
Individually ossified palatal bones include an autopalatine and two anterior dermopalatines (Figs 5, 6). An accessory vomer is continuous with the dorsal margin of the palatal toothplate on one side of the specimen, with a clear suture dividing the two structures. All remaining bones – including the quadrate, entopterygoid, ectopterygoid and any additional dermal ossifications – are either co-ossified or too tightly sutured to be distinguished from one another. No independent vomers are apparent, although the tip of the snout is missing. Posteriorly, each half of the palate is widely separated from its antimere. However, only a narrow gap separates them anteriorly, and this appears to broadly reflect life position of the jaws (Fig. 2).
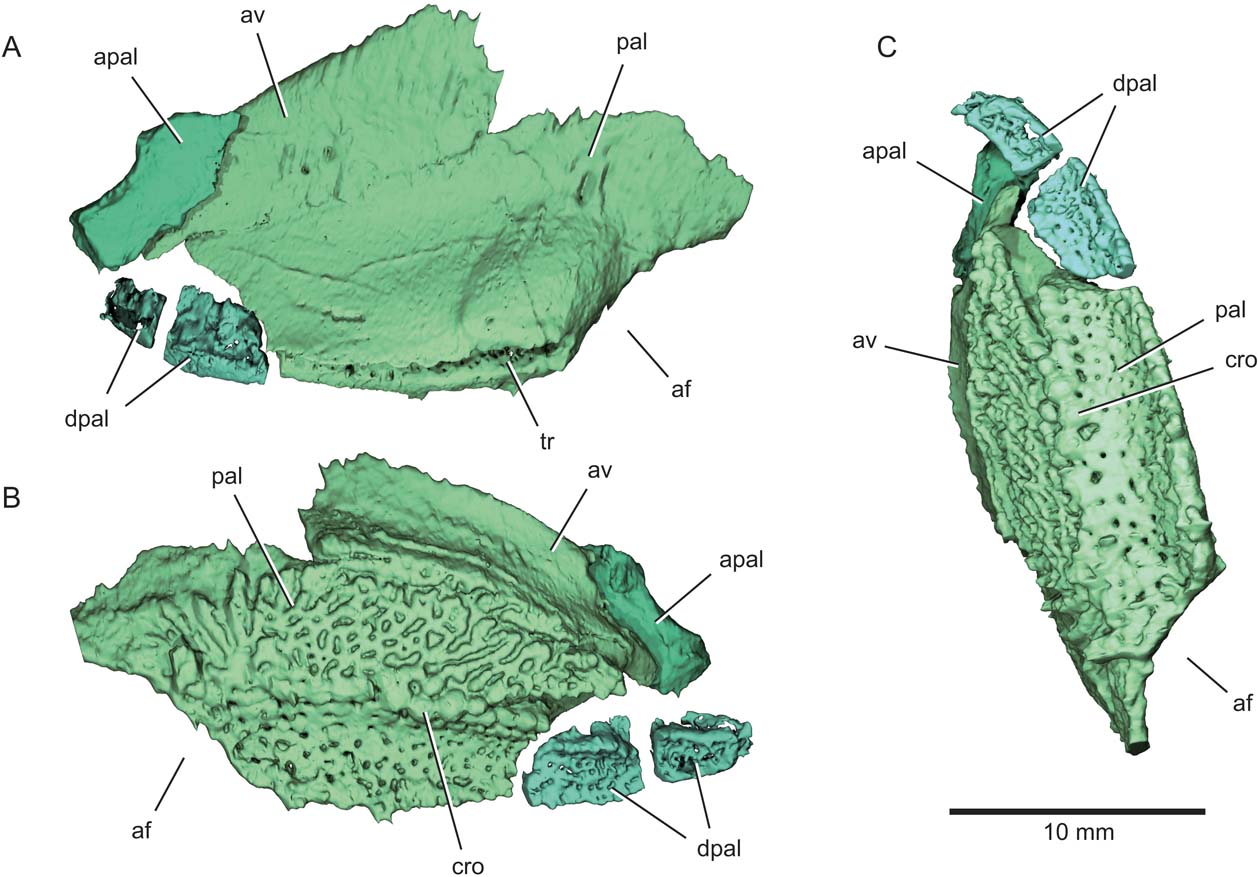
Figure 5 Eurynotus crenatus, MCZ 10508, Wardie Shales Member, Gullane Formation, Edinburgh, Scotland. μCT model of left palatoquadrate complex. (A) Dorsolateral view. (B) Ventromesial view. (C) Ventral view. Abbreviations: af = adductor fossa; apal = autopalatine; av = accessory vomer; cro = crown; dpal = dermopalatine; pal = palate; tr = trough marking articulation with maxilla.
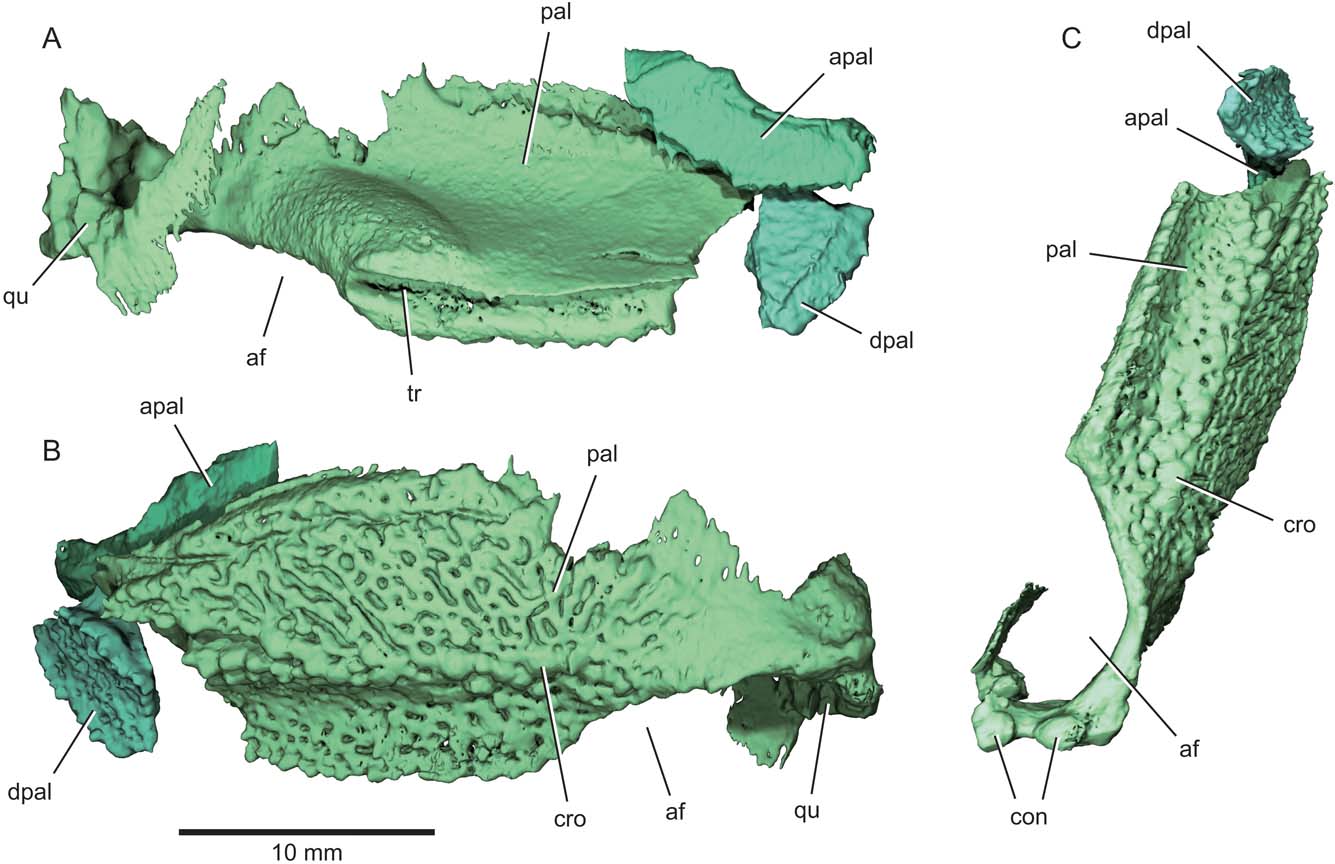
Figure 6 Eurynotus crenatus, MCZ 10508, Wardie Shales Member, Gullane Formation, Edinburgh, Scotland. μCT model of right palatoquadrate complex. (A) Lateral view. (B) Mesial view. (C) Ventral view. Abbreviations: af = adductor fossa; apal = autopalatine; con = quadrate condyle; cro = crown; dpal = dermopalatine; pal = palate; qu = quadrate; tr = trough marking articulation with maxilla.
The gently curved autopalatine is free from the dermal palate (apal, Figs 2, 3, 5, 6). Its posteroventral margin appears unfinished, and much of its dorsal margin consists of a long, slightly concave surface that articulates with the ethmoid region of the braincase. The two free dermopalatines are the most anterior preserved portions of the dermal palate (dpal, Figs 2, 3, 5, 6). The first dermopalatine is convex medially, and articulates posteriorly with the trapezoidal second dermopalatine. Coalesced teeth cover the buccal surfaces of both dermopalatines, with gaps between them resulting in an irregular pattern of large perforations in an otherwise smooth dental surface. Bones and teeth show homogeneous greyscale values in tomograms of the specimen, and we can detect no obvious internal structure of any palatal bones beyond their cross section. However, it appears that all the structures are formed from a single generation of teeth because there is no obvious trace of superimposed dentition. Low dental ridges mark the lateral and mesial edges of the bones, and are particularly well developed on the second dermopalatine. The ventral surface of the dermopalatine series is interrupted by a concave trough bounded by these marginal ridges. Both the trough and ridges align with similar features on more posterior portions of the palate, and mark the region of occlusion with the convex toothplate of the lower jaw.
Posterior to the dermopalatines, the buccal surface of the palatal toothplate (pal, Figs 2, 3, 5, 6) consists of two regions: a horizontal surface continuous with that of the dermopalatines, and a vertical lamina corresponding to the expected position of the entopterygoid (Figs 2, 5, 6). We are unable to detect any divisions within this plate, which occupies the region of several distinct ossifications in many early actinopterygians (Watson Reference Watson1928; Gardiner Reference Gardiner1984). The horizontal portion of the upper toothplate occludes directly with the mandibular toothplate. It forms a shallow, anteroposteriorly oriented gutter that conforms to the convex dorsal surface of the lower dentition. As on the dermopalatines, teeth are coalesced, with no indication in tomograms of underlying tooth generations.
Raised dental ridges define either side of the longitudinal trough of the upper toothplate, and bear separate crowns (cro, Figs 5, 6) as noted by Traquair (Reference Traquair1867, Reference Traquair1879). Anteriorly, each ridge appears to comprise two or more radiating rows of teeth. Dentition on the vertically oriented region of the toothplate shows a contrasting arrangement to that of the trough-like occlusal area. Individual teeth are coalesced, but they form an anastomosing series of ridges separated by irregular gaps rather than a smooth sheet punctated by subcircular pits. Ridges define a radiating pattern in the area of the toothplate immediately anterior to the adductor chamber. A plate-like accessory vomer is sutured to the dorsal margin of the toothplate (av, Figs 2, 3, 5). Apart from an apparent dental ridge extending along its ventral margin, the buccal surface of the accessory vomer is devoid of any obvious teeth.
The external surface of the toothplate-bearing bone is smooth, and bears a broad swelling immediately anterior to the excavation for the jaw adductor muscles. A well-defined trough extends along the thick lateral face of the toothplate (tr, Figs 5, 6). This trough clasps the complementary flange on the inner surface of the maxilla. Neither side of the skull appears to preserve the posterior of the palate in its entirety. The right quadrate is present (qu, Figs 3, 6), and is connected to the remainder of the palate by a bowed sheet of bone that forms the mesial wall of the adductor chamber. Two convex facets (con, Fig. 6), the mesial offset anteriorly relative to the lateral, mark the jaw joint on the ventral surface of the quadrate, matching paired depressions (cot, Fig. 7) on the articular of the mandible.
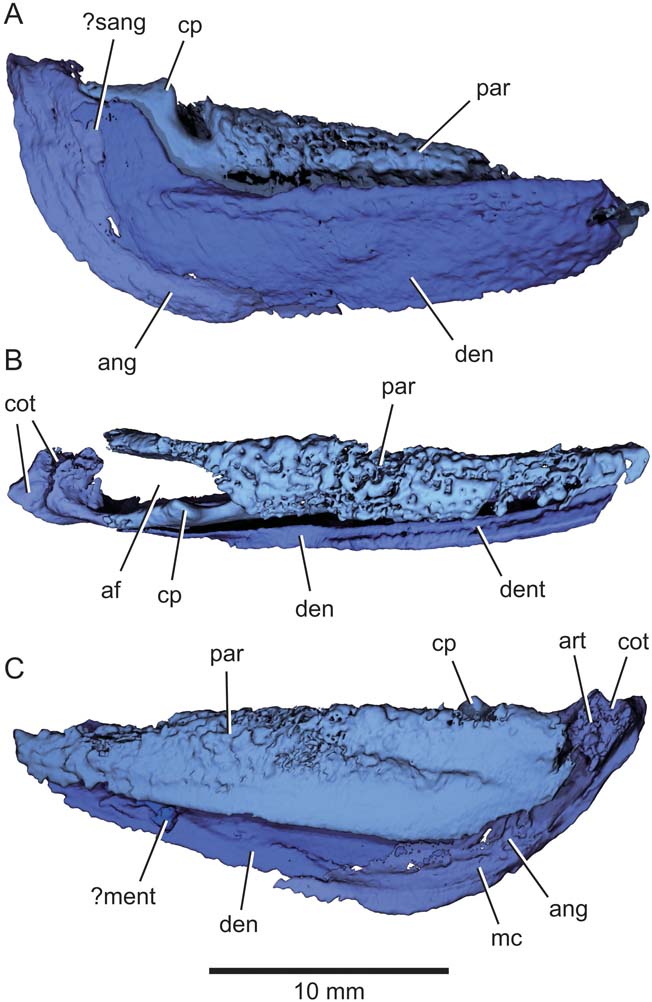
Figure 7 Eurynotus crenatus, MCZ 10508, Wardie Shales Member, Gullane Formation, Edinburgh, Scotland. μCT model of right mandible. (A) Lateral view. (B) Dorsal view. (C) Mesial view. Abbreviations: af = adductor fossa; ang = angular; art = articular; cot = articular cotyle; cp = coronoid process; den = dentary; dent = denticles; mc = mandibular canal; ?ment = possible mentomeckelian ossification; par = prearticular = ?sang = possible surangular.
2.3. Mandible
The mandible (Figs 2, 3, 7) is stout, with a maximum depth equivalent to one-third of overall jaw length. External dermal bones of the lower jaw comprise a dentary, angular and possible surangular. The dentary (den, Figs 2, 3, 7) is ‘L'-shaped, with a long horizontal arm forming the principal ramus of the jaw, and a short dorsally directed process at its posterior end. Anterior to this dorsal process, the oral margin of the dentary is thickened. This appears as a low band laterally and a short shelf mesially. Externally, there is no obvious dentition visible along the dorsal edge of the jaw, but roughness on the inner surface appears to represent a band of denticles (dent, Fig. 7). A low ridge extending along the inner surface of the dentary close to its ventral margin marks the course of the mandibular sensory canal (mc, Fig. 7). The posterior margin of the dentary overlaps the crescentic angular (ang, Figs 2, 3, 7), the mesial surface of which also bears a raised ridge corresponding to the mandibular canal. A possible surangular (?sang, Figs 3, 7) occupies the space between the angular and the dorsal process of the dentary. It is lenticular, extends beyond the adjacent anterior margin of the angular, and terminates anteriorly in a pointed tip. This region of the possible surangular is concealed by the dentary in lateral view.
Divisions between mesial dermal bones of the lower jaw are not apparent. Although it is not clear whether multiple ossifications were present, we refer to the entire complex as the prearticular (par, Figs 2, 3, 7). The most conspicuous feature is a well-developed dental surface, which comprises a series of closely packed to partially coalesced bluntly rounded teeth forming a toothplate that extends far above the dorsal margin of the dentary in lateral view. The fusion of adjacent teeth results in a pattern of large pits distributed across the occlusal surface of the tooth plate. Ventral and mesial to this toothplate, the prearticular consists of a smooth, vertical lamina of bone. There is a broad gap between the lower margin of the prearticular and that of the dentary and infradentaries. This lamina defines the mesial wall of the adductor fossa, and joins the articular at its posterior end. The lateral wall is defined by an additional posterior projection of the prearticular that sutures with the surangular. This posterior process bears a ‘U'-shaped notch in lateral view that separates the toothplate from a low, rounded coronoid process (cp, Fig. 7). An excavation on the posterior margin of the prearticular represents the contact with the infradentary bones.
The articular defines the posterior wall of the adductor fossa, and bears two large, dorsally directed cotyles, one lateral and one mesial (cot, Fig. 7). The level of the articular cotyles is offset dorsally relative to the occlusal surface of the toothplates (Figs 3, 7A). There is limited ossification of the mesial surface of Meckel's cartilage ventral to the prearticular on one jaw. Poorly preserved structures, restricted to the anterior quarter of each mandible and almost completely concealed by the prearticular and dentary, might represent mentomeckelian ossifications (ment, Figs 3, 7).
2.4. Parasphenoid and braincase
The parasphenoid (Fig. 8) consists of a narrow anterior corpus and a wider posterior stalk. The posterior stalk is deeply notched (not, Figs 2, 8), with dorsolaterally extensive wings that would have embraced much of the otic sidewall of the neurocranium in life. A slender ascending process (asc, Fig. 8), preserved only on one side, lies at the junction between these two regions. Anterior to the ascending process, the ventral surface of the parasphenoid bears a greatly thickened teardrop-shaped platform with its pointed tip directed anteriorly. Irregular pits mark the buccal surface of this plinth, which appears to be a hypertrophied denticle field (dent, Fig. 8). The ventral surface of the parasphenoid is gently convex in axial section anterior to this dentition, with no conspicuous features. Passage of the buccohypophyseal canal through the parasphenoid is not apparent, and there is no indication of a foramen on either the dorsal or ventral surface. A longitudinal trough extends along the dorsal surface of the parasphenoid corpus. A high keel emerges dorsally from the midline of this trough. In lateral view, the dorsal margin of the keel rises from its origin anterior to the ascending process, reaching its greatest height at the intersection between the parasphenoid and the ethmoid (ke, Fig. 8). Curiously, there are no indications of a basipterygoid process or alternative means of articulation between the posterior portion of the palate and basicranium.
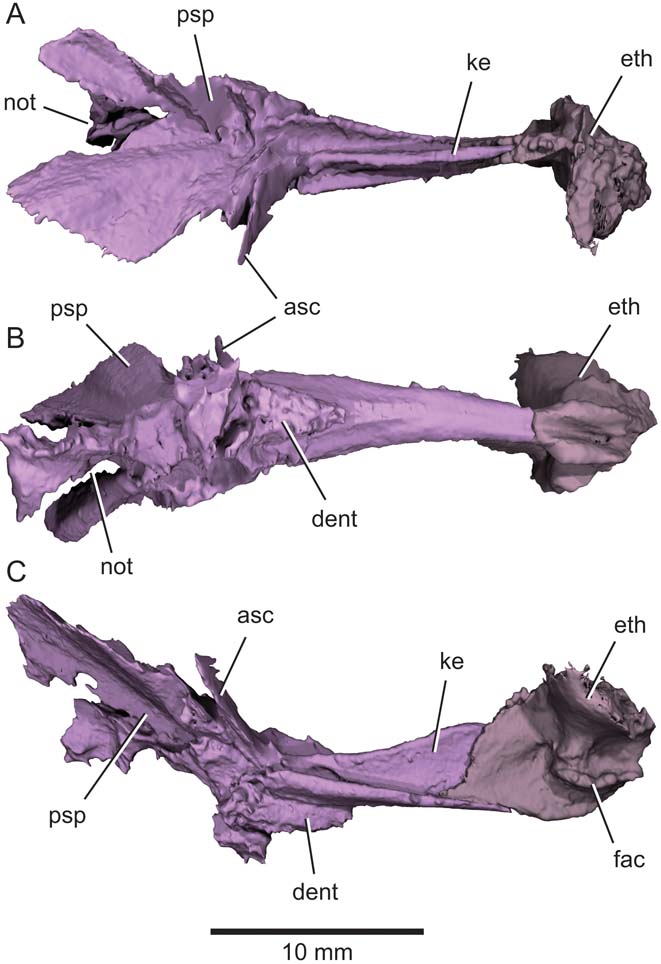
Figure 8 Eurynotus crenatus, MCZ 10508, Wardie Shales Member, Gullane Formation, Edinburgh, Scotland. μCT model of parasphenoid and ethmoid ossification. (A) Dorsal view. (B) Ventral view. (C) Lateral view. Abbreviations: asc = ascending process of the parasphenoid; dent = denticles; eth = ethmoid ossification; fac = articular facet for autopalatine; ke = keel; not = aortic notch; psp = parasphenoid.
The ethmoid (eth, Figs 2, 8) is the only region of the braincase that is clearly preserved. A vertical sheet of bone that embraces the dorsal keel of the parasphenoid forms the posterior half of the ethmoid. The anterior half of the ethmoid is massive, and has a ‘T'-shaped profile in axial section. A large, curved facet (fac, Fig. 8) for the autopalatine dominates the ventral half of the lateral face of the ethmoid in this anterior region.
2.5. Hyoid arch
Preserved components of the hyoid arch are limited to the hyomandibula and ceratohyal (Fig. 9). The anteroventral margin of the hyomandibula is gently curved. By contrast, the posterodorsal margin of the hyomandibula appears more angular, with distinct dorsal and ventral arms that join the apex of a modest opercular crest (opc, Fig. 9). The lateral surface of the hyomandibula is smooth, with no obvious features, but the inner face of the dorsal limb bears a low ridge that extends from the opercular process to near the ventral edge of the proximal facet of the bone. The hyomandibula is somewhat flattened mediolaterally, with an elliptical cross section. No foramina pierce the bone, although a deep groove likely housed the hyomandibular trunk of the facial nerve (VIIfhm) and there is no fusion with the dermohyal.
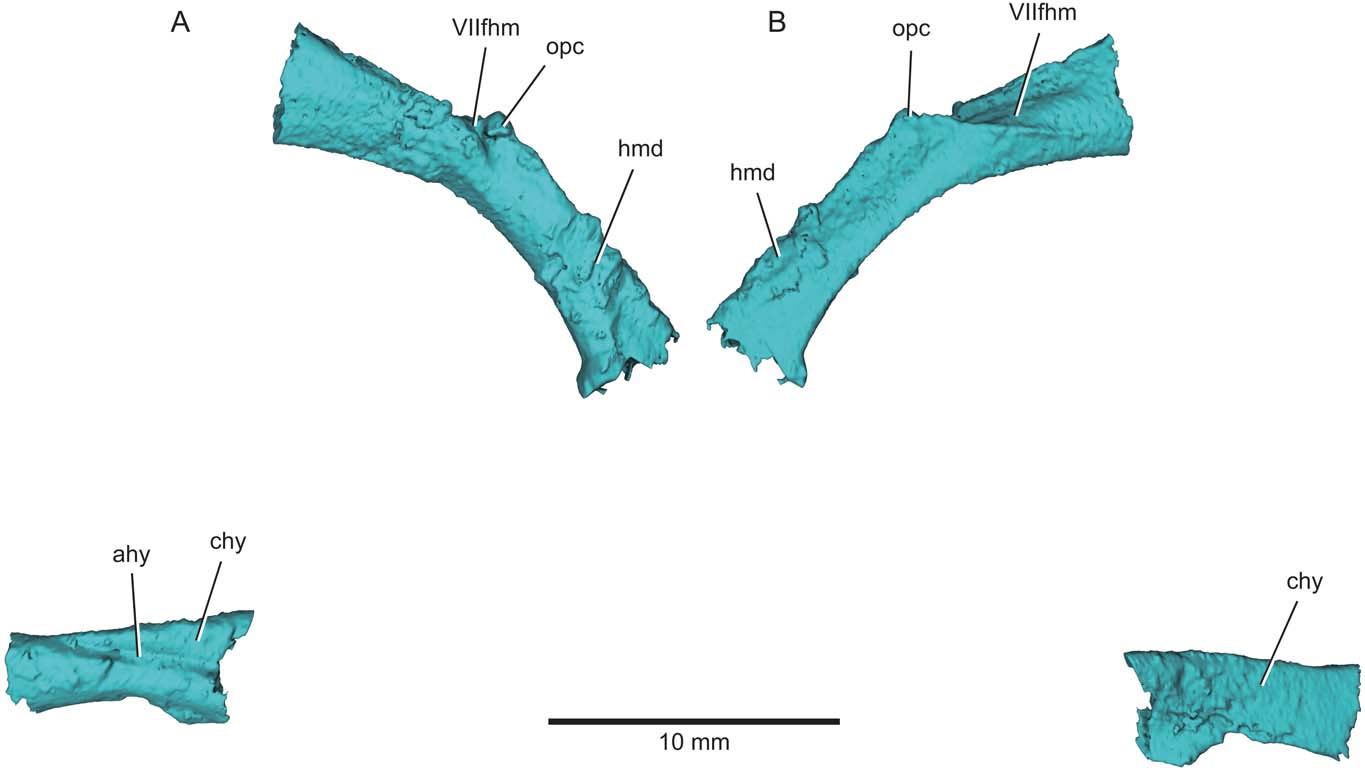
Figure 9 Eurynotus crenatus, MCZ 10508, Wardie Shales Member, Gullane Formation, Edinburgh, Scotland. μCT model of left hyoid arch. (A) Lateral view. (B) Mesial view. Abbreviations: ahy = groove for afferent hyoid artery; chy = ceratohyal; hmd = hyomandibula; opc = opercular process; VIIfhm = groove for hyomandibular branch of the facial nerve.
The ceratohyal has a convex external surface and a concave inner face. A deep groove extends longitudinally along the external surface of the bone and blends into its dorsal rim (ahy, Fig. 9). A straight dorsal margin and convex ventral margin give the ceratohyal a boot-like shape in lateral view.
3. Discussion
3.1. Comparison with past accounts
The handful of accounts of the jaw anatomy in Eurynotus draw on either disarticulated (Traquair Reference Traquair1867, Reference Traquair1879; Coates Reference Coates1994) or badly broken (Watson Reference Watson1928) material, and only the maxilla and palate have been illustrated beyond schematic reconstructions (Traquair Reference Traquair1867, pl. 45, fig. 13; Traquair Reference Traquair1879, pl. 3, figs 10–15; Watson Reference Watson1928, fig. 12).
Although our μCT models add considerable information on the upper jaw and palate, there is little in the accounts of Traquair (Reference Traquair1867, Reference Traquair1879) that requires substantial revision. Our specimen shows a greater coalescence of individual teeth on the horizontal component of the palatal toothplate than his disarticulated example of an undetermined species of Eurynotus from the younger Queensferry beds. Similarly, where our specimen preserves a smooth mesial surface adjacent to the oral margin of the maxilla, in material from Queensferry, Loanhead and Burdiehouse, Traquair (Reference Traquair1879) illustrates a distinct strip of large denticles.
Watson (Reference Watson1928) illustrated a more complete palate from a specimen of E. crenatus from Wardie that indicates divisions between individual ossifications. Although the position of these sutures is plausible based on conditions in other early actinopterygians, we cannot detect these divisions in our fossil. The tongue-and-groove connection between the palate and maxilla is accurately described by Watson (Reference Watson1928, p. 63), with the articular flange on the inner surface of the maxilla clearly figured – but not discussed – by Traquair (Reference Traquair1879).
Our specimen substantially improves understanding of the lower jaw, which is only superficially described in previous accounts. Traquair (Reference Traquair1867, Reference Traquair1879) reconstructed the mandible of Eurynotus, whereas Watson (Reference Watson1928) and Coates (Reference Coates1994) provided specimen illustrations. Traquair's figure shows a series of individual cusps rather than a coalesced plate, while both Watson's text and illustration are ambiguous.
The parasphenoid and braincase of Eurynotus have only previously been described by Watson (Reference Watson1928). In terms of overall shape of the parasphenoid, our models confirm his illustration, and we can confirm the presence of an ascending process. Watson's broken specimen shows only the base of the keel on the upper face of the parasphenoid, and he did not appreciate the dorsal extent of this structure. We have not detected clearly defined pits or capsules for the olfactory bulbs in our scans, but otherwise Watson's account of the ethmoid appears accurate given the limitations of his material.
3.2. Systematic implications
Deep-bodied actinopterygians first appear in the fossil record during the early Carboniferous. The relationships of these taxa to one another, to more ‘generalised' contemporary species, and to deep-bodied taxa from younger deposits has long been confused (see summary in Sallan & Coates Reference Sallan and Coates2013). Traquair (Reference Traquair1879) rejected earlier associations of deep-bodied Palaeozoic taxa with Mesozoic groups like pycnodonts and dapediids, and restricted Platysomidae to those forms appearing in the early Carboniferous. Evidence for anatomical diversity among platysomids presented by Traquair and others led Moy-Thomas (Reference Moy-Thomas1939) to elevate the group to subordinal status and include within it two separate families: Platysomidae (e.g., Platysomus) and Amphicentridae (e.g., Amphicentrum). Notably, the hypothesised close relationship between these platysomoid lineages persists, recurring in cladistic surveys (Gardiner & Schaeffer Reference Gardiner and Schaeffer1989, although queried by Coates Reference Coates1993) and results of formal phylogenetic analysis (Wilson et al. Reference Wilson, Pardo and Anderson2018). However, Sallan & Coates (Reference Sallan and Coates2013) argued for multiple, distinct origins of deep-bodied Carboniferous actinopterygians, with parallel acquisition of this geometry in the two ‘platysomoid' divisions recognised by past workers (see also Zidek Reference Zidek and Zidek1992, who anticipated this arrangement by placing platysomids and amphicentrids in separate orders). Their thesis rests on the interpretation of the styracopterids Fouldenia (late Tournaisian) and Styracopterus (early Viséan) as a fusiform sister group of Amphicentridae (used here in a manner corresponding roughly to the concept of the group outlined by Moy-Thomas Reference Moy-Thomas1939) to the exclusion of Platysomidae. Sallan & Coates (Reference Sallan and Coates2013) recognise the clade comprising styracopterids and amphicentrids as Eurynotiformes, and present a list of derived features – including many of the jaws and dentition – supporting the monophyly of the group. This argumentation is verbal, but the proposed systematic arrangement has been corroborated by recent analyses (Giles et al. Reference Giles, Xu, Near and Friedman2017; Wilson et al. Reference Wilson, Pardo and Anderson2018), which we use as a broad framework within which to assess possible patterns of character evolution within amphicentrids (here taken to include Amphicentrum, Benedenius, Cheirodopsis, Eurynotus, Mesolepis, Proteurynotus and Wardichthys).
Most amphicentrids are known almost exclusively from external anatomy. Detailed knowledge of internal structure is limited to Eurynotus and Amphicentrum, with fewer, as yet unreported, details of the palatal toothplate preserved in Cheirodopsis (pers. obs. M.I.C.). Relative to primitive actinopterygian conditions (e.g., Mimipiscis; Gardiner Reference Gardiner1984), Eurynotus and Amphicentrum share conspicuous derived features, including a posterior stalk of the parasphenoid formed of broad, winglike processes; a slender anterior corpus of the parasphenoid; a prominent dorsal keel of the parasphenoid (NMS SPW 2273); a well-developed ethmoid ossification; and narrowly separated and anteroposteriorly elongate autopalatine facets (NMS SPW 2273). Unfortunately, none of these features can be assessed for other eurynotiforms, and their implications for interrelationships within the group remain ambiguous pending new data. However, dental specialisations of Eurynotus and Amphicentrum suggest broader patterns of relationships. All eurynotiforms in which such conditions can be assessed appear to bear rows of blunt dentition on the palate and inner dermal bones of the lower jaw. However, individual crowns appear to remain separate from one another in styracopterids (Sallan & Coates Reference Sallan and Coates2013, fig. 14) and the amphicentrids Benedenius (Boulenger Reference Boulenger1902) and Mesolepis (Traquair Reference Traquair1879, pl. 4, figs 6–8). Outgroup comparison indicates this is a primitive arrangement. By contrast, individual teeth are placed so closely in Eurynotus that their crowns are mostly fused in both the upper and lower toothplates. The resultant smooth surface appears formed from only a single generation of teeth, as we have not detected buried crowns. In this way, amphicentrid toothplate construction probably differs from the ‘phyllodont' (i.e., comprising multiple superimposed sets of replacement teeth) upper and lower toothplates present in Platysomus and bobasatraniids (Zidek 1992; Böttcher Reference Böttcher2014).
We suggest the condition in Amphicentrum is an exaggeration of the arrangement seen in Eurynotus, to the degree that gaps are no longer present at the occlusal surface of the toothplate, and individual cusps can only be recognised in dental ridges. The shared presence of extensive dental coalescence implies that Amphicentrum and Eurynotus form a group to the exclusion of at least some amphicentrids. Furthermore, we suggest that dental structure in Eurynotus provides a model for understanding the evolution of fully consolidated dental plates of Amphicentrum, with tomographic or histological study of dentition in the latter genus representing a critical test of this dental coalescence hypothesis.
One of the more clearly defined groups of early actinopterygians, eurynotiforms are geologically long-lived, ranging in age from the early Mississippian (late Tournaisian) to the late Pennsylvanian (Kasimovian), a span of nearly 50 million years. Generic diversity is highest early in the history of the group, with only Amphicentrum reported from the Pennsylvanian; whether this reflects either a genuine biological pattern or neglect of stratigraphically younger material is not certain. In any case, it is clear that eurynotiforms represented important components of a range of aquatic settings in the Carboniferous. Although Eurynotus is restricted to freshwater or brackish units (Coates Reference Coates1994: 325), other eurynotiforms are known from sites with a stronger marine influence. In the Mississippian, these include the Tournaisian of Foulden, Scotland (Fouldenia; Clarkson Reference Clarkson1985; Sallan & Coates Reference Sallan and Coates2013), the Viséan of Glencartholm, Scotland (Styracopterus, Proteurynotus, Cheirodopsis; Moy-Thomas & Bradley Dyne Reference Moy-Thomas and Bradley Dyne1938; Schram Reference Schram1983), and Denée, Belgium (Benedenius; Mottequin et al. Reference Mottequin, Pouty and Prestianni2015), and the Serpukhovian of Bearsden, Scotland (Amphicentrum; Wood Reference Wood1982; Coates Reference Coates1993). Amphicentrum is known from both marine and freshwater deposits in North America and Europe during the Pennsylvanian (Zidek 1992; Bardack Reference Bardack, Shabica and Hay1997). Eurynotiforms have only rarely been included within formal phylogenetic analyses, and their position within the actinopterygian tree is unclear. Gardiner & Schaeffer (Reference Gardiner and Schaeffer1989, fig. 12) placed their ‘Platysomus Group' (comprising platysomids and eurynotiforms as recognised here) within the actinopterygian crown, as part of a large clade of fossil forms representing the sister lineage of crown Actinopteri. By contrast, Giles et al. (Reference Giles, Xu, Near and Friedman2017) do not resolve eurynotiforms and platysomids as sister lineages (cf. Sallan & Coates Reference Sallan and Coates2013), placing the former just outside crown Actinopterygii and the latter within the actinopteran crown. Subsequently, Wilson et al. (Reference Wilson, Pardo and Anderson2018) recovered platysomids and eurynotiforms as successive stem members of the Chondrostei. Most recently, Latimer & Giles (Reference Latimer and Giles2018) resolve eurynotiforms as stem actinopterygians and platysomids as sister to a clade comprising cladistians and chondrosteans (although the implausibility of this latter clade is noted in the text). Additional study of well-preserved eurynotiform material, including the use of μCT, will be vital in providing further anatomical data that might be helpful in constraining the phylogenetic position of the clade.
3.3. Functional considerations
Beginning with Agassiz's (Reference Agassiz1835) initial description, authors have emphasised the distinctive jaw geometry and dentition of Eurynotus, with many drawing explicit functional inferences from these structures (Traquair Reference Traquair1879, p. 361; Watson 1928, p. 63; Coates Reference Coates1994, p. 325). Here we build on these accounts by examining the anatomy of Eurynotus in terms of both qualitative comparisons with other groups showing similar structural modifications and examination of explicit quantitative metrics.
Eurynotus and other eurynotiforms show modifications seen elsewhere among early lungfishes and holocephalans, most notable of which is an elaboration of the palatal dentition. All three groups show a decrease in the midline gap between the two halves of the palate, with lungfishes and holocephalans exhibiting midline contact between dental surfaces associated with the right and left sides of the palate. The geometry in lungfishes and holocephalans is achieved by fusion between the palatoquadrate and neurocranium, whereas the palate remains separate from the braincase in Eurynotus and other eurynotiforms in which the condition can be assessed (Bradley Dyne Reference Bradley Dyne1939; Sallan & Coates Reference Sallan and Coates2013). As in those other groups, eurynotiform toothplates appear to be derived from the union or coalescence of non-shedding teeth on the palate and lower jaw. While the mechanisms of toothplate growth in lungfishes (Ahlberg et al. Reference Ahlberg, Smith and Johanson2006) and holocephalans (Stahl Reference Stahl and Schultze1999) are reasonably well understood, additional study of eurynotiform dentitions – particularly Amphicentrum – is necessary. It seems likely that μCT study of well-preserved material could yield important information, with studies of fossil tetraodontiform dentitions providing proof-of-concept for this approach (Close et al. Reference Close, Johanson, Tyler, Harrington and Friedman2016; Bemis et al. Reference Bemis, Tyler, Bemis, Kumar, Rana and Smith2017). In addition to bearing surfaces that are flat or gently curved, the toothplates of Eurynotus, Amphicentrum and many early lungfishes bear low cusps, often crowned by acute tips, and are arranged in rows. In shape, these correspond broadly to tooth geometries that are most effective at fracturing hard prey under experimental conditions (Crofts & Summers Reference Crofts and Summers2014). Lungfishes, holocephalans and eurynotiforms also share a rearrangement of the suspensorium that permits more perpendicular – rather than oblique – insertion of adductor muscles on the mandible, more effectively transmitting force for jaw closing (see below).
Similarities are not restricted to coeval durophages, and Eurynotus anticipates features arising independently in later groups of ray-finned fishes either interpreted or confirmed as hard-prey specialists. Broad dental surfaces on the lower jaw and palate are found in numerous groups, including the Mesozoic semionotiforms (López-Arbarello & Sferco Reference López-Arbarello and Sferco2011), dapediids (Thies & Herzog Reference Thies, Herzog, Arratia and Schultze1999; Smithwick Reference Smithwick2015), pycnodonts (Nursall Reference Nursall, Arratia and Schultze1999) and several modern lineages including gymnodont tetraodontiforms (Tyler Reference Tyler1980). The independent histories of these groups is apparent in specific arrangements of an otherwise similar feeding system, including contrasting composition of upper (vomer in pycnodonts; dermopalatines and possibly more internal bones of the palate in dapediids and some semionotiforms; premaxilla in gymnodonts) and lower (coronoids or prearticular in pycnodonts, dapediids, and some semionotiforms; dentary in gymnodonts) dental plates (Tyler Reference Tyler1980; Nursall Reference Nursall, Arratia and Schultze1999; Thies & Herzog Reference Thies, Herzog, Arratia and Schultze1999; López-Arbarello & Sferco Reference López-Arbarello and Sferco2011; Smithwick Reference Smithwick2015). In addition to the obvious convergent specialisation of palatal bites mediated by closely packed, molariform teeth or continuous dental surfaces, some of these groups share other specialisations with eurynotiforms including beak-like oral jaws or modified anterior teeth for prey manipulation (gymnodonts, pycnodonts) and deeply keeled parasphenoids that might confer additional rigidity to the skull in compression (dapediids, gymnodonts; Tyler Reference Tyler1980; Nursall Reference Nursall, Arratia and Schultze1999; Latimer & Giles Reference Latimer and Giles2018).
Qualitative comparisons of anatomy are suggestive of durophagy in Eurynotus and its relatives, but quantification of specific, biomechanically relevant attributes permit more explicit comparison. Overall geometry of the mandibles also carries functional consequences, and these can be quantified using simple metrics. Here we compare such measures for Eurynotus with those presented for other early jawed vertebrates by Anderson et al. (Reference Anderson, Friedman, Brazeau and Rayfield2011). We draw on their sample of Devonian actinopterygians (n=13) as being representative of primitive jaw mechanics for ray-finned fishes and toothplate-bearing Devonian lungfishes (n=20) as a roughly contemporaneous osteichthyan group with dental modifications similar to – but derived independently of – those in Eurynotus (Fig. 10).

Figure 10 Functionally relevant measures of the mandible of Eurynotus (grey-filled circle) in comparison with Devonian actinopterygians (open circles) and lungfishes (black-filled circles). The ratio of mandibular depth to length plotted against: (A) jaw-closing mechanical advantage (anterior); (B) jaw-closing mechanical advantage (posterior). Measurements for taxa apart from Eurynotus taken from Anderson et al. (Reference Anderson, Friedman, Brazeau and Rayfield2011). Silhouettes adapted from Traquair (Reference Traquair1879; Eurynotus), Ahlberg & Trewin (Reference Ahlberg and Trewin1995; Dipterus), Choo (Reference Choo2012; Mimipiscis).
Jaw-closing mechanical advantage (Barel Reference Barel1983) has been applied to a variety of extant and fossil fishes to investigate contrasts in feeding mode (Bellwood Reference Bellwood2003; Westneat Reference Westneat2004). This metric models the mandible as a simple third-order lever, and incorporates the distance between the jaw joint and a designated point along the dentition (outlever) as well as the site of muscle attachment (inlever) and the angle of that muscle attachment. Here we consider outlever measurements to both the most anterior and posterior points of the dentition. The ratio of the outlever to the inlever yields the unitless jaw-closing mechanical advantage (MA) of the mandible, with higher values indicating greater force transmission from jaw closing muscles to the dentition and, in turn, prey item. The simple approximation of MA provided by Anderson et al. (Reference Anderson, Friedman, Brazeau and Rayfield2011) treats the angle of adductor insertion as being perpendicular to the inlever because of uncertainties in many fossil taxa (cf. Bellwood Reference Bellwood2003). Anterior jaw-closing MA for Eurynotus (0.22) narrowly exceeds that reported for Devonian actinopterygians (maximum MA=0.19; mean MA=0.15), but falls well below the minimum (0.3) value recorded for toothplate-bearing Devonian lungfishes (Fig. 10A). With toothplates that extend posterior to the midpoint of the adductor fossa, Eurynotus has a posterior jaw-closing MA (0.83) substantially larger than that of Devonian actinopterygians (maximum MA=0.68; mean MA=0.50) but within the range of Devonian lungfishes (0.69–1.37). A major caveat accompanies these observations. The assumption of uniform angles of muscle insertion across taxa is likely to lead to an overestimate in MA is some cases. This overestimation is most probable for Devonian actinopterygians due to the oblique orientation of the suspensorium – and presumably adductor musculature (Schaeffer & Rosen Reference Schaeffer and Rosen1961) – in these taxa relative to the more vertical arrangement characteristic of Eurynotus and lungfishes that permits an approximately perpendicular orientation of jaw-closing muscles relative to the input lever.
In addition to a relatively high MA compared to Devonian actinopterygians, the mandible of Eurynotus also shows a ratio of maximum jaw depth to maximum jaw length that deviates from more general conditions. Given simplifying assumptions about aspects including jaw thickness and material properties, this value is taken as a proxy for flexural stiffness of the mandible when loads are applied vertically, as during biting (Anderson et al. Reference Anderson, Friedman, Brazeau and Rayfield2011 and references therein). The value of 0.3 for Eurynotus exceeds that of any Devonian actinopterygian (mean=0.2), and falls within the lower half of the range of toothplate-bearing Devonian lungfishes (mean=0.34). Thus, the shift in jaw depth in Eurynotus relative to earlier actinopterygians might represent an adaptation for accommodating the increased loads associated with the consumption of hard prey.
3.4. A review of durophagy in Devonian–Carboniferous fishes and possible impacts of the Hangenberg event
Apparent specialisations for durophagy appear early in the history of jawed vertebrates (Anderson et al. Reference Anderson, Friedman, Brazeau and Rayfield2011), and are most clearly manifest in broad dental plates and changes to mandibular geometry. In our discussion below, we principally consider those taxa with anatomical structures consistent with the consumption of hard-shelled prey rather than simply the processing of animals with a stiff (but possibly weakly mineralised) carapace or exoskeleton. As such, our definition of durophagy is more restrictive than the broadest sense of the term.
The Lochkovian Diabolepis provides the earliest unambiguous evidence for this feeding mode in the form of a palatal bite, upper and lower toothplates, and changes in jaw proportions relative to more generalised sarcopterygians (Chang Reference Chang1995). The incertae sedis osteichthyan Megamastax has recently been advanced as an even older specialist on hard prey, based on the presence of low, rounded prominences in the position of the coronoids (Choo et al. Reference Choo, Zhu, Zhao, Jia and Zhu2014), although the slender morphology of the mandible in this taxon conflicts with this interpretation. In any case, lungfishes, including Diabolepis, were the dominant group of durophagous osteichthyans during the Devonian, obtaining considerable taxonomic and morphological diversity, and inhabiting environments ranging from lakes to reefs (Campbell & Barwick Reference Campbell and Barwick1990; Ahlberg et al. Reference Ahlberg, Smith and Johanson2006; Lloyd et al. Reference Lloyd, Wang and Brusatte2012).
Several lineages of durophagous placoderms appeared during the Devonian. The most diverse of these, the ratfish-like ptyctodonts, never achieved levels of richness or disparity similar to their lungfish contemporaries. Lockhovian remains attributed to ptyctodonts consist of thoracic plates (Mark-Kurik Reference Mark-Kurik and Menner1977), with the distinctive dental plates of the group appearing later (Denison Reference Denison and Schultze1978, Reference Denison1985). Isolated dental plates represent the most common remains of ptyctodonts, which have greatly reduced cranial and thoracic armour relative to other placoderms. It is possible that reduction of the skeleton might, in part, be responsible for the low sampled diversity of ptyctodonts, which only number roughly a dozen genera during the entirety of the Devonian (Trinajstic & Long Reference Trinajstic and Long2009). Ptyctodonts are joined by two or possibly three genera of mylostomatids, durophagous arthrodires of uncertain relationships. Apart from rare Givetian material (Case Reference Case1931), myostomatids are restricted to the Late Devonian and achieved larger sizes than most ptyctodonts (Denison Reference Denison and Schultze1978). Their dentition comprises broad tritors on the supragnathals opposed to a similar surface on the infragnathals. Unlike ptyctodonts, some of which are known from lacustrine settings, mylostomatids appear to have been exclusively marine (Denison Reference Denison and Schultze1978; Dineley & Metcalf Reference Dineley and Metcalf1999). Mylostomids are the most diverse group of durophagous arthrodires, but Hlavin & Boreske (Reference Hlavin and Boreske1973, p. 9) argued for similar feeding ecologies in other arthrodire lineages, including the selenosteid Paramylostoma and the bungartiid Bungartius, both of which are known from the latest Devonian Cleveland Member of the Ohio Shale.
Holocephalans are the last group of durophagous fishes to appear in the Devonian, where they are known exclusively from toothplates. Reliable examples appear restricted to the Late Devonian (especially Famennian; Stahl Reference Stahl and Schultze1999), but Darras et al. (Reference Darras, Derycke, Blieck and Vachard2008) report a questionable specimen of Givetian age. These Middle Devonian fossils substantially predate the oldest holocephalan body fossils that bear crushing dentition (Viséan; Finarelli & Coates Reference Finarelli and Coates2012), as well as the earliest skeletal remains attributed to the total group (Famennian; Coates & Sequeira Reference Coates and Sequeira2001; Coates et al. Reference Coates, Gess, Finarelli, Criswell and Tietjen2017). By the time the earliest complete skeletons of durophagous holocephalans appear in the fossil record in the early Carboniferous, the group had already undergone substantial anatomical change relative to generalised chondrichthyan conditions, including major modifications to the skull (e.g., autostyly) and evidence of substantial experimentation in postcranial anatomy that anticipates the considerable postcranial diversity of later Permo-Carboniferous holocephalans (Stahl Reference Stahl and Schultze1999; Finarelli & Coates Reference Finarelli and Coates2012).
The early Carboniferous is marked by proliferation of holocephalans and ray-finned fishes, including the first appearance of durophagous lineages in the latter, and follows the complete extinction of placoderms and the apparent environmental marginalisation of lungfishes. This pattern has long been appreciated (Woodward Reference Woodward1891; Romer Reference Romer1966; Signor & Brett Reference Signor and Brett1984), but it is only recently that this substantial taxonomic turnover within the durophagous fish guild has been viewed as a consequence of recovery from the end-Devonian extinction or Hangenberg event (Sallan & Coates Reference Sallan and Coates2010, Reference Sallan and Coates2013; Sallan et al. Reference Sallan, Kammer, Ausich and Cook2011; Friedman & Sallan Reference Friedman and Sallan2012; Sallan & Friedman Reference Sallan and Friedman2012; Richards et al. Reference Richards, Sherwin, Smithson, Bennion, Davies, Marshall and Clack2018). It is clear that the extinction did not result in a complete taxonomic shift, as members of the dominant Devonian and Carboniferous durophagous fish groups overlapped to varying degrees in the Late Devonian and early Carboniferous (Sallan et al. Reference Sallan, Kammer, Ausich and Cook2011). The only groups not present both before and after the Devonian–Carboniferous boundary are durophagous placoderms, which persist to the latest Famennian (Denison Reference Denison and Schultze1978), and durophagous actinopterygians, which first appear in the late Tournaisian, roughly 10 million years after the Devonian–Carboniferous boundary (Sallan & Coates Reference Sallan and Coates2013). Significantly, durophagy probably arose multiple times within actinopterygians during the early Carboniferous. This mirrors, to some degree, the proliferation of new durophagous groups in the aftermath of other extinction events: neopterygian fishes and marine reptiles in the Triassic (Tintori Reference Tintori1998; Rieppel Reference Rieppel2002; Lombardo & Tintori Reference Lombardo and Tintori2005; Latimer & Giles Reference Latimer and Giles2018), and the many groups of modern teleost durophages like sparids, wrasses and tetraodontiforms in the early Palaeogene (Bellwood Reference Bellwood2003).
Precise patterns of turnover among durophagous fishes during the Devonian–Carboniferous are obscured by taxonomic neglect. In particular, the diversity of tooth morphologies co-occurring in articulated holocephalans casts doubts on the value of systematic interpretations drawn from isolated dental material (Finarelli & Coates Reference Finarelli and Coates2012) and, in turn, palaeobiological conclusions drawn from counts of dental form taxa. Further complicating the picture, recent efforts to better sample vertebrate diversity during ‘Romer's Gap' have provided evidence – at least on a local scale – for high dental disparity and taxonomic richness among lungfishes during the Tournaisian (Smithson et al. Reference Smithson, Richards and Clack2016). This adds nuance to a longstanding narrative depicting the post-Devonian interval of dipnoan evolution as one of reduced anatomical innovation and increasing environmental restriction (Westoll Reference Westoll, Jepsen, Simpson and Mayr1949; Lloyd et al. Reference Lloyd, Wang and Brusatte2012). These Tournaisian lungfishes, and indeed post-Devonian taxa more generally, overwhelmingly bear dentitions with well-developed dental ridges suggesting precise occlusion between upper and lower toothplates. Similar patterns are also found in some earlier lungfishes, and mark a pronounced shift from the broad flattened or gently rounded dental surfaces typical of exclusively Devonian assemblages like ‘dipnorhynchids' and ‘chirodipterids' (Campbell & Barwick Reference Campbell and Barwick1990). This ridged geometry foreshadows the condition in modern lepidosirenid lungfishes, where precise occlusion between blade-like dental ridges marks a shift to shearing from the grinding found in Neoceratodus and, presumably, many fossil lungfishes (Bemis Reference Bemis1986). By contrast, the relatively flat dental surfaces of Eurynotus and some other eurynotiforms are geometrically similar to those of contemporary holocephalans (Stahl Reference Stahl and Schultze1999; Finarelli & Coates Reference Finarelli and Coates2012).
Such differences between these groups indicate contrasting feeding modes, a suggestion bolstered by a further outstanding distinction between toothplated actinopterygians and their holocephalan and lungfish contemporaries. Crucially, these early actinopterygians retain cranial kinesis: palatal toothplates are not fused to the basicranium; maxillae and premaxillae remain separate; the mandibular symphysis is never fused. It follows that these persistent articulations allowed movement between left and right sides of the jaws, and mediolateral movement between the grinding surfaces of upper and lower toothplates. Thus, in addition to a refined taxonomic framework, a more detailed appraisal of patterns of turnover and replacement among groups will require more sophisticated analyses of the attributes of ‘crushing' dentitions, along with consideration of ecologically relevant attributes like body size and environmental associations, which are readily available (Sallan & Coates Reference Sallan and Coates2010; Sallan & Galimberti Reference Sallan and Galimberti2015).
4. Conclusion
μCT of Eurynotus crenatus from Wardie reveals considerable new anatomical data on the feeding apparatus of one of the earliest durophagous actinopterygians. Eurynotus shares many distinctive specialisations with the stratigraphically younger Amphicentrum, the only other eurynotiform in which the internal structure of the skull is described in detail. These traits are specialised in comparison with outgroups, but their implications for eurynotiform intrarelationships will remain ambiguous until anatomy in other members of the group is better known. With the exception of Wardichthys and some isolated parts of Cheirodopsis, much of the relevant material is compressed (Traquair Reference Traquair1879; Moy-Thomas & Bradley Dyne Reference Moy-Thomas and Bradley Dyne1938; Sallan & Coates Reference Sallan and Coates2013), but it is possible that μCT of even flattened specimens might yield important details of robust and potentially informative structures like dental plates. Additional information might help resolve the relationships within the structurally diverse and stratigraphically long-ranging eurynotiforms, thereby providing a framework for documenting the assembly of the extreme morphologies of its later occurring – and presumably anatomically derived – members.
It is probable that such data will also prove useful in addressing broader questions of ray-finned fish evolution. Apart from the braincase (Rayner Reference Rayner1952; Poplin Reference Poplin1974; Schaeffer & Dalquest Reference Schaeffer and Dalquest1978; Coates Reference Coates, Norman, Milner and Milner1998, Reference Coates1999; Giles & Friedman Reference Giles and Friedman2014; reviewed in Friedman & Giles Reference Friedman, Giles, Clack, Fay and Popper2016), internal skeletal structure is largely unknown for Permo-Carboniferous actinopterygians (but see Watson 1925, 1928). Instead, knowledge of character-rich anatomical systems like palates and hyoid and branchial arches in early ray-finned fishes has historically derived from a few Late Devonian examples from Gogo (Gardiner Reference Gardiner1984) and structurally disparate – and likely to be phylogenetically derived – taxa from the Early Triassic of Greenland (Nielsen Reference Nielsen1942, Reference Nielsen1949). More details have emerged recently for Devonian forms (e.g., Choo Reference Choo2012; Giles et al. Reference Giles, Darras, Clément, Blieck and Friedman2015), but these are broadly consistent with classical accounts. This anatomical gap for Permo-Carboniferous actinopterygians is associated with considerable phylogenetic instability of those same taxa (Giles et al. Reference Giles, Xu, Near and Friedman2017). Ambiguous patterns of relationships have consequences extending beyond taxonomy and systematics, and represent an obstacle to dissecting the striking patterns of anatomical and taxonomic diversification apparent in the fossil record in a rigorous, comparative framework. Along with the results presented here for Eurynotus, emerging μCT results for three-dimensionally preserved Permo-Carboniferous fossils point to unanticipated and phylogenetically informative characters that might help to clarify this important episode in actinopterygian evolution (Pradel et al. Reference Pradel, Maisey, Mapes and Kruta2016; Coates & Tietjen, Reference Coates and Tietjen2019). We anticipate that μCT study of ray-finned fishes from key localities including Wardie will provide critical new insights on the relationships, ecology and evolution of ray-finned fishes during their Carboniferous rise to taxonomic dominance.
5. Acknowledgements
It is our pleasure to present this paper in honour of Prof. Jennifer Clack and her contributions to both the study of Devonian and Carboniferous vertebrates as well as our own careers. Carol Abraczinskas (University of Michigan Museum of Paleontology) improved the figures. We thank Marcello Ruta, Tim Smithson, and Susie Bloor for their editorial input, and two anonymous referees for their constructive comments on an earlier draft of this paper. This study was financed by the College of Literature, Art, and Science and the Department of Earth and Environmental Sciences, University of Michigan (to M.F.), Harvard University (to S.E.P.), NSF DEB 1541491 (to M.I.C.), and a L'Oréal-UNESCO International Rising Talents Fellowship and Royal Society Dorothy Hodgkin Research Fellowship (to S.G.).












