The genus Tempskya Corda is a group of extinct ferns composed of species that are characterised by a false trunk, which consists of numerous intermingled rhizomes and a large number of adventitious roots coming out from the rhizomes. This false trunk can reach up to 50cm in diameter and 6m high (Taylor et al. Reference Taylor, Taylor and Krings2009). Due to the unique morphology of this false trunk, Tempskya represents an extinct monotypic family Tempskyaceae Read & Brown ex L.C.A. Martínez, Reference Martínez and Olivo2015.
However, the systematic affinity of the family has remained unclear to date because of the lack of attached leaves and reproductive organs (Tidwell & Ash Reference Tidwell and Ash1994). Recently, Martínez & Olivo (Reference Martínez and Olivo2015) emended the Tempskyaceae family based on exceptionally well-preserved silicified false trunks, which included possible frond segments and attached sori with sporangia containing spores. The characters of these reproductive remains allowed the authors to attribute this family to the Cyatheales. Some other Tempskya remains have also been compared to the Anemiaceae, although no direct evidence supporting this affinity exists (Tidwell & Ash Reference Tidwell and Ash1994).
The genus Tempskya has been recorded exclusively in Cretaceous deposits from the Valanginian (Barale & Viera Reference Barale and Viera1989; Martínez & Olivo Reference Martínez and Olivo2015) to the Santonian (Nishida Reference Nishida1986). Records of Tempskya are found in both Lower and Upper Cretaceous deposits from the Laurasia domain, including North America, but the genus has also been reported from the Lower Cretaceous of Argentina and Australia within the Gondwana realm (see Section 2.4). The remains of this fern are generally represented in Eurasia by silicified fragments of false trunks that have been recorded in the Lower and ‘Middle' Cretaceous from the Valanginian–Hauterivian (Barale & Viera Reference Barale and Viera1989) and Hauterivian–Barremian (Puente-Arauzo et al. Reference Puente-Arauzo, Sender, Villanueva-Amadoz, Bienvenido-Diez and Torcida Fernández-Balor2014) in Spain, the ‘Wealden Beds' in England (Seward Reference Seward1894; Stopes Reference Stopes1915; Austen & Batten Reference Austen, Batten and Batten2011), Germany (Jung Reference Jung1983) and France (Corda Reference Corda1845; Carpentier Reference Carpentier1923), and from the Cenomanian in the Czech Republic (Corda Reference Corda1845; Velenovsky Reference Velenovsky1888). Nevertheless, this taxon has not previously been recorded from Albian deposits in western Eurasia.
Here, we describe the first Albian remains of Tempskya false trunks in western Eurasia from middle to upper Albian deposits in Spain. These consist of several false trunks of differing sizes, some of which also present possible evidence of palaeo-wildfire damage. These new findings fill the temporal gap of this genus in Eurasia, providing new evidence for inferring the palaeoenvironment and distribution patterns of this genus during the Cretaceous.
1. Material and methods
1.1. Geographical and geological setting
The fossil plants studied in this paper were collected at the ‘Carretera-1' fossil site near the village of Escucha in Teruel Province (Aragón, NE Spain; Fig. 1). This village is situated 70km N of Teruel city and 5km E of Utrillas village, which is a well-known reference locality for both Albian geology and palaeobotany in the region. The fossil locality is in the Cuencas Mineras district, and the stratigraphic level containing Tempskya (Fig. 2a) belongs to the Upper Member of the Escucha Formation (Aguilar et al. Reference Aguilar, Ramírez del Pozo and Oriol Riba1971). This formation is mainly a continental detrital unit that consists of alternate sandstones and clays intercalated with coal seams and sporadic marine limestones, which were deposited in a tidally influenced delta-estuary system (Pardo Reference Pardo1979; Pardo & Villena Reference Pardo and Villena1979; Querol Reference Querol1990; Pardo et al. Reference Pardo, Ardevol and Villena1991; Querol et al. Reference Querol, Salas, Pardo, Ardevol, McCabe and Parrish1992; Salas et al. Reference Salas, Guimerà, Mas, Martín-Closas, Meléndez, Alonso, Ziegler, Cavazza, Robertson and Crasquin-Soleau2001).

Figure 1 (A) Geographical location of the plant fossil site. (B) Geological map of the fossil site near Escucha village.

Figure 2 (A) Synthetic stratigraphic section of part of the Escucha and Utrillas Formations, indicating depositional environments and sub-environments (modified from Villanueva-Amadoz Reference Villanueva-Amadoz2009). The stratigraphic level bearing Tempskya is indicated by an arrow. (B) Fossil site image with the layer containing Tempskya indicated by an asterisk. See a hammer for scale (28cm long), to the left above the asterisk.
The Escucha Formation in this area is limited at the base by the carbonated marine deposits of the Villarroya de los Pinares Formation (Aptian in age) and is overlain by sandy fluvial deposits of the Utrillas Formation (middle–late Albian). The most recent palynological studies in the area, where the remains of Tempskya were found, indicate a middle to late Albian age for the Upper Member of the Escucha Formation (Villanueva-Amadoz Reference Villanueva-Amadoz2009). The lithology of the stratigraphic interval in the Escucha locality bearing the studied fossils exhibits white cross-bedded sandstones intercalated with shales and grey-coloured clays (Fig. 2b) deposited in a fluvio-tidal environment (Pardo et al. Reference Pardo, Ardevol and Villena1991; Querol et al. Reference Querol, Salas, Pardo, Ardevol, McCabe and Parrish1992).
1.2. Studied material
Fragments of permineralised false trunks (24 pieces) possibly represent 14 individual plants. The largest specimen measures 1.20m long (Fig. 3a). All false trunks are preserved as silicified remains, although they have undergone both recrystallisation and weathering, causing some alteration of internal structures (Fig. 4a). In addition, some samples exhibit compressional deformation. Three false trunks contain some charcoalified remains preserved within the surrounding silicified tissues (Figs 4b, c, 6, 7).
Fossils were first cleaned with fresh water and then five fragments of fossil trunks were cut transversally in order to obtain polished and thin sections. Photographs of the false trunks and the polished sections were taken using a Nikon D90 camera with an AF-S Micro Nikkor 60mm macro lens. Thin sections of less deformed false trunks were prepared at the Hard Materials Preparation Service at the University of Zaragoza (Spain). Images of these thin sections were obtained using a Leica DM 2000 LED stereomicroscope with an attached Leica ICC50 W digital camera at the University of Vigo (Spain). Small pieces of charcoalified roots were extracted from the interior of the false trunks using small needles, and were then mounted on a stub and covered with gold. These charcoalified remains were photographed using a FESEM Carl Zeiss MERLIN electron microscope at the Microscopy Imaging Service at the University of Zaragoza.
The specimens are housed at the Museo de Ciencias Naturales de la Universidad de Zaragoza (Natural Sciences Museum of the University of Zaragoza) in Zaragoza city (Spain) under the designations MPZ2009/345–MPZ2009/347 and MPZ2017/531–MPZ2017/541.
2. Results and discussion
2.1. Taxonomic description
Division Pteridophyta
Family Tempskyaceae Read &
Brown ex L.C.A. Martínez, Reference Martínez and Olivo2015
Genus Tempskya Corda emend. Kidston & Gwynne-Vaughan, Reference Kidston and Gwynne-Vaughan1911 Tempskya sp.
(Figs 3–7)

Figure 3 (A) Most complete specimen of Tempskya sp. (MPZ2017/538), measuring 1.2m long. (B) Apical part of false trunk (specimen MPZ2017/541) presenting abundant surface moulds of rhizomes. (C) Basal part of Tempskya (specimen MPZ2017/539) almost completely covered by adventitious roots. Old rhizomes are covered and obscured by root mantle. (D) Central part of a false trunk (specimen MPZ2017/533) showing well-marked external moulds of dichotomising rhizomes ascending the false trunk. (E) Detail of central area of (D) enlarged, showing the contour of rhizomes. (F) A linear sequence of equally spaced circular marks corresponding to leaf scars (specimen MPZ2017/540). Scale bars=20cm (A); 5cm (B–D); 2cm (E); 1cm (F).

Figure 4 Polished transverse sections of false trunks of Tempskya sp. (A) Many elliptical to round rhizomes are dispersed randomly within a root mass. Difference in colour is due to weathering caused by water percolation from cracks in the middle of the sample (specimen MPZ2017/535). (B) Specimen MPZ2017/534, showing hollows corresponding to dissolution of the interior part of rhizomes. Red dashed lines indicate positions of charcoalified remains, which comprise three concentric curves parallel to the false-stem external surface. (C) Upper-right area of (B) enlarged, showing black charcoalified roots (arrows). Scale bars=1cm (A, C); 2cm (B).

Figure 5 Transverse thin sections of false trunks of Tempskya sp.. (A) Nearly oval-shaped rhizomes with departing petiole bases, showing dorsoventrally oriented rhizome (specimen MPZ2017/532). (B) Upper-left part of (A) enlarged, showing a rhizome departing a C-shaped leaf trace. Notice a leaf trace in preparation on the upper-left side of the rhizome. (C) Specimen MPZ2017/532, with part of a rhizome xylem cylinder and surrounding tissues cut slightly oblique, showing deformed xylem cells between other recrystallised tissues. (D) Lower part of (A) enlarged, showing a dichotomising rhizome having formed two solenosteles, each developing leaf traces. (E) Close-up of a petiolar leaf trace with a distorted C-shape and only xylem cells preserved. (F) Specimen MPZ2017/535, showing a single root preserving the cortex, protoxylem and the metaxylem in the centre. (G) A group of three roots in transversal section from the same specimen as in (F). (H) Root tracheids showing scalariform thickenings in an oblique longitudinal section. Abbreviations: c = cortex; lt = leaf trace; mx = metaxylem; ptx = protoxylem; x = xylem. Scale bars=1cm (A); 1mm (B), 200μm (C); 3mm (D); 400μm (E, F); 500μm (G); 100μm (H).

Figure 6 Thin sections of charcoalified roots from specimen MPZ2017/534 in Figure 4b. (A, B) Two charcoalified roots partially fused or cracked by probable fire influence. (C) Detail of (A), showing distinct type of preservation between charcoalified tissues (in black) and silicified tissues (yellow to ochre). (D) Detail of (B). Some charcoalified cells are broken, resulting in large holes due to recrystallisation. Scale bars=500μm (A, B); 200μm (C, D).

Figure 7 SEM images of charcoalified roots from specimen MPZ2017/534 in Figure 4b. (A) general view of a root fragment with oriented arrangement of grouping tracheids. (B) Detail of (A), showing tracheids. (C) Broken cells with fused lamellae (arrows). (D) detail of some of the broken cells (tracheids), showing fused lamellae due to the influence of fire (arrows). Scale bars = 200 μm (A); 50 μm (B); 25 μm (C); 5 μm (D).
The false trunks measure up to 1.20m long in the largest specimen (Fig. 3a), with an average diameter of 10cm, and consist of thick root mantles (adventitious roots) and embedded rhizomes (Figs 3, 4). Most specimens are somewhat compressed laterally, resulting in an oval outline in cross-sectional view of the false trunk (Fig. 4a, b).
The rhizomes are clearly visible on the surface of both medial and apical parts of the false trunks (Fig. 3b, d), but are often protruded or obscured by root mantles in the basal part (Fig. 3c). The rhizomes are distributed randomly in the false trunks (Fig. 3b–e) and proceed distally from the base to the apex. Interval dichotomisation of rhizomes is ca.5cm on average, with the next order of rhizomes also dividing dichotomously at approximate intervals of 1.5–2cm (Fig. 3d, e). In cross section, rhizomes are oval-shaped and arranged randomly in the false trunks (Fig. 4). The leaf traces depart from the dorsal side of the rhizomes, either singly or as part of aggregations of several traces, with shorter internodes in most rhizomes (Figs 5a, b, d). One specimen (MPZ2017/540) shows leaf petiole scars arranged in a definite interval of nearly 0.7cm on the ventral side of the rhizome (Fig. 3f). Histological details are best preserved in specimen MPZ2017/532 (Fig. 5a), although the preservation is still insufficient to observe tissue details.
Thin sections of the rhizomes show their structure from the centre outwards, consisting in this order of the pith, the vascular cylinder, the cortex and the epidermis. The different tissues can be recognised as concentric areas preserved differently both in colour and in structure (Fig. 5b, d). The cortex is composed of the outer layer, which is preserved as a pale-coloured zone that is probably parenchymatous, and the inner layer, which consists of a dark-coloured band and is possibly sclerenchymatous (Fig. 5b). The vascular cylinder is amphiphloic, with a central ring of scalariform tracheids (Fig. 5h). Possible phloem is observed as more recrystallised, light-coloured areas. The pith is poorly preserved, but as with the outer cortex, is probably parenchymatous. Some rhizomes preserve circular-shaped cells in the xylem and inner cortex, although these cells are usually crushed due to deformation during diagenesis (Fig. 5c).
The leaf traces are V-, C- or U-shaped depending on the orientation of the thin sections (Fig. 5b, d), although they usually present crescent morphologies due to deformations that also caused poor tissue preservation (Fig. 5e). The adaxial margins of the leaf traces are slightly incurved (Fig. 5d).
Roots are the most abundant and best-preserved elements in the false trunks, and form a compact mesh between the rhizomes and departing leaf bases (Fig. 5a, b, d). In cross section, the roots are circular to oval-shaped (Fig. 5f, g), 400–500μm in diameter, or, if deformed, 600–800μm in maximum dimensions, and consist of central diarch xylem, poorly preserved phloem and a cortex composed of two layers (Fig. 5f). Root metaxylem tracheids show scalariform thickening in longitudinal section (Fig. 5h).
2.2. Affinities
The external part of the false trunks is well preserved, showing some morphological features characteristic of the family Tempskyaceae Read & Brown ex L.C.A. Martínez – namely, three-dimensional networks of slender rhizomes embedded in a thick matrix of adventitious roots. The rhizomes are solenostelic, departing petioles have a single V-, C- or U-shaped vascular bundle and the diarch roots also confirm the family diagnosis.
Detailed histological structures of most specimens have been altered or even destroyed due to recrystallisation. However, partially preserved portions of false trunks provide some internal features. Due to the limited number of taxonomically valid characters, it is not possible to assign our fossils to a definite species or to designate them as a new taxon within the genus, and it is thus only possible to describe them as Tempskya sp. Despite these limitations, it is notable that the short internodes observed in most stem cross sections (Figs 4a, 5a) differ from the long internodes observed in specimen MPZ2017/540 (Fig. 3f), which suggests the presence of false trunks showing different morphologies that could represent two species. However, we do not have sufficient histological information to confirm this hypothetical taxonomic assertion.
2.3. Palaeoenvironmental implications
The presence of abundant remains of larger pieces of false trunks, some of which still preserve the apex (Fig. 3a, b), and the spindle-like original shape would seem to indicate short-distance transportation from the growing habitat. The sedimentological evidence of the fossil-containing deposits near the Escucha locality (Pardo et al. Reference Pardo, Ardevol and Villena1991; Querol et al. Reference Querol, Salas, Pardo, Ardevol, McCabe and Parrish1992) suggests that the Tempskya plants studied here would have grown near the tidally influenced fluvial channels that developed on the western coast of the Tethys Ocean on the Iberian Plate during the middle–late Albian.
A very interesting preservation feature exhibited by three specimens (MPZ2017/533, MPZ2017/534 and MPZ2017/537) is the presence of coalified tissues embedded in the false trunks. The polished section of specimen MPZ2017/534 shows some black-coloured charcoalified roots that seem to be arranged in a circular pattern forming three nearly concentric sequences of incomplete rings parallel to the external contour of the false trunk (Fig. 4b, c). Moreover, these circular sequences of black roots are confined to one side, accounting for nearly one third of the false trunk (Fig. 4b). These roots show a different type of preservation to the surrounding tissues, because they are charcoalified whereas all other elements in the false trunks are silicified. This can also be observed in thin sections as a distinct compositional difference between the charcoalified roots and other silicified roots (Fig. 6a, b), as well as the broken cell walls only present in the former (Fig. 6d). The charcoalification process in these roots is also identifiable under a scanning electron microscope (SEM), showing cell lamellae that are partially fused (Fig. 7c, d) due to the possible influence of high temperatures on the cell walls (Scott Reference Scott2000, Reference Scott2010).
The presence of charcoalified plant tissues, which are arranged in three parallel lines on the same side of transverse sections of specimen MPZ2017/534, suggests the possible influence of fire on some of the false trunks, from the same direction. Nevertheless, only a limited number of tissues were affected by fire, while the other surrounding roots remain unaltered (non-charcoalified) (Fig. 6a–c).
One possible explanation for the localised presence of charcoalified roots in some false trunks is that several direction-orientated wind–fire events may have occurred various times during tree fern growth (Fig. 8). Multiple occurrences of such fire events that could have damaged the local plant community have been previously inferred from burnt plant leaves and charcoalified wood remains from the Albian deposits of the Escucha Formation in this area (Villanueva-Amadoz et al. Reference Villanueva-Amadoz, Pons, Diez, Ferrer and Sender2010, Reference Villanueva-Amadoz, Sender, Alcalá, Pons, Royo-Torres and Diez2015; Sender Reference Sender2012; Sender et al. Reference Sender, Villanueva-Amadoz, Pons, Diez and Ferrer2015). In our case, it is possible that the fire only affected a specific part of the plant surface – those dead and/or dried roots, leaving major parts – most living and/or humid roots – intact. It could be also possible that most burnt roots were eroded or washed away before the fire-affected surface was covered again by new roots, leaving limited numbers of charcoalified roots in the false trunk.
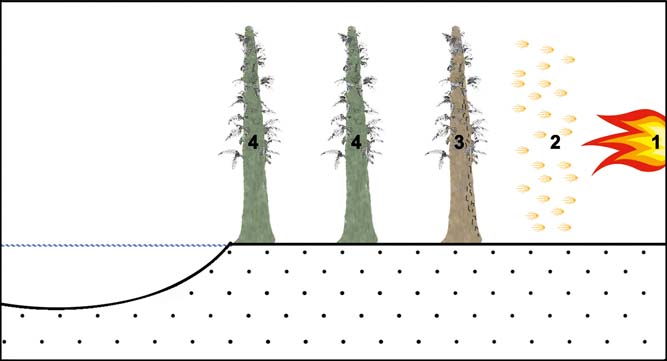
Figure 8 Schematic palaeoenvironmental reconstruction of the hypothetical fire event affecting Tempskya sp. plants, which grew near a fluvio-tidal channel close to the coast. (1) Recurrent wildfires occurred in relatively distant neighbouring forests. (2) Hot winds or weak fires coming from main wildfires. (3) The heat from fires partially burnt some Tempskya plants or parts of them. (4) More distant coeval Tempskya plants remain intact.
Wildfire effects have been documented both in extant plants (Gutsell & Johnson Reference Gutsell and Johnson1996; Scott et al. Reference Scott2000; Stambaugh et al. Reference Stambaugh, Smith and Dey2017 and references therein) and in fossil plants (Scott Reference Scott2000, Reference Scott2010; Scott et al. Reference Scott, Cripps, Collinson and Nichols2000; Brown et al. Reference Brown, Scott, Glasspool and Collinson2012 and references therein). However, in our case, we hypothesise that only the hot winds produced by these fires, and not direct flames, would have affected the false trunks, thus burning only some of the tissues; both the number and position of the charred roots contained within the false trunks are selective, and this could be due to the respective differences in the humidity of the roots within the false trunk. Partially burnt and charred plant tissues caused by wildfires have been documented previously in several extant (Scott Reference Scott2010) and fossil records, including gymnosperm and angiosperm remains (Jones et al. Reference Jones, Scott and Mattey1993; Scott Reference Scott2010; Degani-Schmidt et al. Reference Degani-Schmidt, Guerra-Sommer, Cazzulo-Klepzig, Iannuzzi, Mendonça, Mendonça, Jasper, Cazzulo-Klepzig and Iannuzz2015), and similar effects of fire have been reported in experiments on living lycopods and ferns (Walker & Boneta Reference Walker and Boneta1995; Vogel et al. Reference Vogel, Piatkowski, Dooley and Poli2011).
Baker & Dugan (Reference Baker and Dugan2013) recorded the distribution, size and shape of fire scars on trunks of an extant conifer, and they found that the scar orientations were usually aligned towards the direction of the fire. In our case, only charred tissues but not fire scars were preserved; although the anatomy of tree ferns precludes a classical fire scar, we can also estimate the direction of the fire based on the distribution pattern of the charcoalified roots in the false trunk. Following the distribution of charcoalified remains, at least three fire events that could have influenced the plant community of the Tempskya from Spain have been identified (Fig. 4b, c). After each event, subsequent plant regrowth covered the charcoalified roots, embedding them within the false trunk. It is also noteworthy that this is the first report worldwide of the possible effects of wildfire on false trunks of Tempskya.
Another possibility concerning the explanation for the selective charcoalification of roots in the studied false trunks of Tempkya could be a result of low-intensity surface fire, causing charring on the leeward side of the false trunk. In this process, the char would detach from the false trunks due to both the brittle nature of charcoal and the less dense nature of the Tempskya tissue compared to true trunks. This may have been followed by growth of new roots over the injured face (Dr Tamara Fletcher, personal communication). However, we did not find any base of preserved Tempskya and, therefore, we are not able to check the possible charcoalified remains in the area, which would indicate the action of a surface fire.
2.4. Palaeophytogeographical dispersal of genus Tempskya during the Cretaceous
To date, the genus Tempskya has been found in both hemispheres and on all continents except Africa and Antarctica (Table 1). The fossils occur most abundantly in Albian–Cenomanian deposits from western regions of the US (Ash & Read Reference Ash and Read1976), but this abundance could be due to extensive palaeontological field campaigns and excavations carried out at the beginning of the 20th Century by a number of North American scientists. Nevertheless, Tempskya has also been recovered from many other locations worldwide in the last three decades.
Table 1 Data of Tempskya species, indicating their geographic distribution and age.

Some articles on Tempskya have tried to map its distribution patterns during the Cretaceous (Tidwell & Wright Reference Tidwell and Wright2003; Clifford & Dettmann Reference Clifford and Dettmann2005). Unfortunately, these attempts have failed to show chronological phytogeographical changes because they only used one palaeogeographical map for a given age. Here, we present five palaeogeographical maps from Scotese (Reference Scotese2013), on which we have plotted the locations of Tempskya at five age intervals, i.e., the Valanginian, Hauterivian–Barremian, Aptian–Albian, Cenomanian and Santonian (Fig. 9).

Figure 9 Palaeophytogeographical distribution of Tempskya in time, based on fossil sites (indicated by red dots) associated with the number referring to the species reported for each site. (A) Santonian. (B) Cenomanian. (C) Albian–Aptian. (D) Barremian–Hauterivian. (E) Valanginian. 1, 2. Tempskya dernbachii Tidwell & Wright emend. Martínez; 3. T. riojana Barale & Viera; 4. T. aff. riojana Puente, Arauzo et al.; 5. Tempskya sp. in Carpentier; 6. T. schimperi Corda; 7. Tempskya sp. in Jung; 8. T. erosa Stopes; Star Tempskya sp. (this paper); 9. T. whitei Berry; 10. T. superba Arnold; 11. T. reesidei Ash & Read; 12. T. zelleri Ash & Read; 13. T. minor Read & Brown; 14. T. readii Tidwell & Hebbert; 15. T. stichkae Tidwell & Hebbert; 16. T. wyomingensis Arnold; 17. T. grandis Read & Brown; 18. T. wesselii Arnold; 19. T. knowltoni Seward (1924); 20. T. jonesii Tidwell & Hebbert; 21. T. judithae Clifford & Dettmann; 22. T. pulchra Corda; 23. T. varians Velenovsky; 24. T. cretacea Hosius & Marck; 25. T. rossica Kidston & Gwynne-Vaughan; 26. T. iwatensis Nishida; 27. T. uemurae Nishida. Palaeogeographical maps modified from Scotese (Reference Scotese2013).
These mapped distributions show that the first presence of Tempskya in the Hauterivian was recorded in two very distant areas, namely Spain (Barale & Viera 1989) and Argentina (Martínez & Olivo 2015) (Fig. 9e). Another interesting point deduced from the maps is the constant presence of Tempskya from the Valanginian to Albian in the area occupied today by the Iberian Peninsula (Fig. 9c–e), and a possible distributional shift of the genus from the central Atlantic Ocean area to the Far East during the Cenomanian–Santonian (Fig. 9a, b). These data suggest that the area occupied by the Iberian Peninsula during the Valanginian–Albian may have been a dispersal pathway for this genus, at least to North America, as the distribution of land masses and seas changed, developing land bridges and barriers to dispersal. This hypothesis regarding the possible dispersal of plant taxa between North America and the islands that constituted the Iberian Plate during the Early Cretaceous was presented recently by Sender et al. (Reference Sender, Villanueva-Amadoz, Diez, Sánchez-Pellicer, Bercovici, Pons and Ferrer2012, Reference Sender, Doyle, Villanueva-Amadoz, Pons, Diez and Ferrer2016) based on similar distributional patterns of angiosperm taxa in both areas. Therefore, it is also possible that Tempskya could have dispersed along the same route, probably through Greenland and islands that once connected North America and the Iberian Plate during the Albian.
3. Conclusions
• The first record of the genus Tempskya from the Albian of western Eurasia is reported based on silicified false trunks found in the Upper Member of the Escucha Formation in NE Spain.
• These findings fill the biostratigraphic gap of Tempskya between the basal Early Cretaceous and the early Late Cretaceous in western Eurasia.
• The presence of large fragments representing several individuals and the taphonomic data from the fossil-bearing deposits indicate that the plants grew near tidally influenced fluvial channels.
• The remains of charred roots in some false trunks suggest environmental conditions involving recurrent occasional fires.
• Nearby wildfires from the same direction could have caused hot winds that promoted partial charring of some exposed surfaces of the false trunks at several distinct times during plant growth.
• These new records of the Tempskya tree fern from the Albian of Spain may support the hypothesis that the genus dispersed from western Eurasia to both North America and eastern Eurasia during the mid-Cretaceous.
4. Acknowledgements
The authors thank D. Juan José Martínez, who discovered the fossil site and for the donation of several of the studied fossils, Antonio Sender for donation of some other samples and Teófilo Gracia for his support during the fieldworks. The authors would like to acknowledge the use of Servicio General de Apoyo a la Investigación-SAI, Universidad de Zaragoza, especially to Manuel Tricás and Felipe Barbed from Hard Materials Preparation Service of the University of Zaragoza for polished and thin sections and to Ana Cristina Gallego from Microscopy Imaging Service of the University of Zaragoza for FESEM images. We also thank the ‘Dirección General de Patrimonio Cultural' of Aragón Region Government for permissions and grants to conduct fieldwork. We are also grateful for the comments of two referees (Dr Tamara Fletcher and Dr Leandro Martínez), which have improved the manuscript. The English text has been corrected by Dr Christopher Evans from the University of Barcelona (Spain). This work is a contribution to project CGL2015-69805-P of the ‘Ministerio de Ciencia e Innovación' and to project CGL2013-41295-P of the ‘Ministerio de Economía y Competitividad' of the Spanish Government, which supported LMS and JBD. The authors were also supported by project GRC2015/020 of the ‘Consellería de Cultura, Educación y Ordenación Universitaria' of the Galician Government, with additional support from Chuo University International Research Grant 2016 to HN.












