The Silurian Period was marked by a series of climatic disturbances that had a significant impact on the evolution of marine life. In the late Wenlock, one of these disturbances, the Mulde Event, is associated with sea level fluctuations (Calner et al. Reference Calner, Kozłowska, Masiak and Schmitz2006) and two probable glaciations at the beginning and middle of the event (Jeppsson & Calner Reference Jeppsson and Calner2003). These severe climatic shifts coincided with a collapse in the abundance and diversity of marine fauna (Lenz et al. Reference Lenz, Noble, Masiak, Poulson and Kozłowska2006; Calner Reference Calner and Elewa2008; Steeman et al. Reference Steeman, Vandenbroucke, Williams, Verniers, Perrier, Siveter, Wilkinson, Zalasiewicz and Emsbo2016; Venckutė-Aleksienė et al. Reference Venckutė-Aleksienė, Radzevičius and Spiridonov2016; Spiridonov Reference Spiridonov2017). Among the most striking of the biotic disturbances of the Mulde interval was the early Homerian lundgreni graptolite extinction event (Koren’ & Urbanek Reference Koren’ and Urbanek1994; Urbanek et al. Reference Urbanek, Radzevičius, Kozłowska and Teller2012), a severe, although short-lived, biodiversity disturbance detected in the fossil record of Baltic ostracods (Rinkevičiutė et al. Reference Rinkevičiutė, Stankevič, Radzevičius, Meidla, Garbaras and Spiridonov2021) and graptolites (Urbanek et al. Reference Urbanek, Radzevičius, Kozłowska and Teller2012). This disturbance saw the family Monograptidae reduced to a single species, Pristiograptus dubius parvus Ulst (Koren’ & Urbanek Reference Koren’ and Urbanek1994; Urbanek et al. Reference Urbanek, Radzevičius, Kozłowska and Teller2012). Pristiograptus dubius parvus was followed by an anagenic successor in Pristiograptus dubius frequens (Jaekel), and during and following the Mulde interval that stem lineage gave rise to a broad swath of morphologically disparate taxa (Urbanek Reference Urbanek1997; Rickards & Wright Reference Rickards and Wright2003; Urbanek et al. Reference Urbanek, Radzevičius, Kozłowska and Teller2012; Whittingham et al. Reference Whittingham, Radzevičius and Spiridonov2020). The diverse monograptids from that time are especially remarkable as many species both from the low paleolatitudes of the Baltic Basin (Urbanek et al. Reference Urbanek, Radzevičius, Kozłowska and Teller2012), Canadian High Arctic (Lenz & Kozłowska-Dawidziuk Reference Lenz, Kozłowska-Dawidziuk, Gutierrez-Marco and Rabano1998), and Australia (Rickards et al. Reference Rickards, Packham, Wright and Williamson1995; Rickards & Wright Reference Rickards and Wright1999), and from the mid-paleolatitude Bohemia (Štorch et al. Reference Štorch, Manda and Loydell2014) possess the same novel trait, known as ‘sicular annuli’.
Annuli are thickened bands on the sicula (Fig. 1) or, very rarely, on the first theca (Fig. 1a) of some monograptids. These were observed to be disassociated with the growth rings (fusellae) of the sicula (Kozłowski Reference Kozłowski1948; Walker Reference Walker1953), refuting claims that the annuli were representative of slowed periods of growth in the sicula (Kraft Reference Kraft1926). Walker (Reference Walker1953) also showed that sicular annuli were formed during the development of the sicula, instead of being constructed after its completion. Later observations (Urbanek Reference Urbanek1958) revealed the consistent presence of an initial annulus, at the boundary of the prosicula and metasicula (Figs 1c, d), and showed that the initial annulus consisted of material deposited on both the inside and outside surfaces of the sicula, contrasting with the metasicular annuli, which exist only on the inside of the sicula. Urbanek also noted broad variation in the number of sicular annuli per specimen in a given species, including some specimens with none. This variability has been since expanded upon through two key findings: (1) that sicular annuli at the boundary of the prosicula and metasicula are first consistently identifiable across multiple species after the lundgreni Biozone of the lower Homerian (Lenz & Kozłowska-Dawidziuk Reference Lenz, Kozłowska-Dawidziuk, Gutierrez-Marco and Rabano1998), and (2) that annuli vary in number between the nassa graptolite Biozone of middle Homerian and the leintwardinensis graptolite Biozone of the earliest Ludfordian (Urbanek Reference Urbanek1997; Urbanek et al. Reference Urbanek, Radzevičius, Kozłowska and Teller2012).
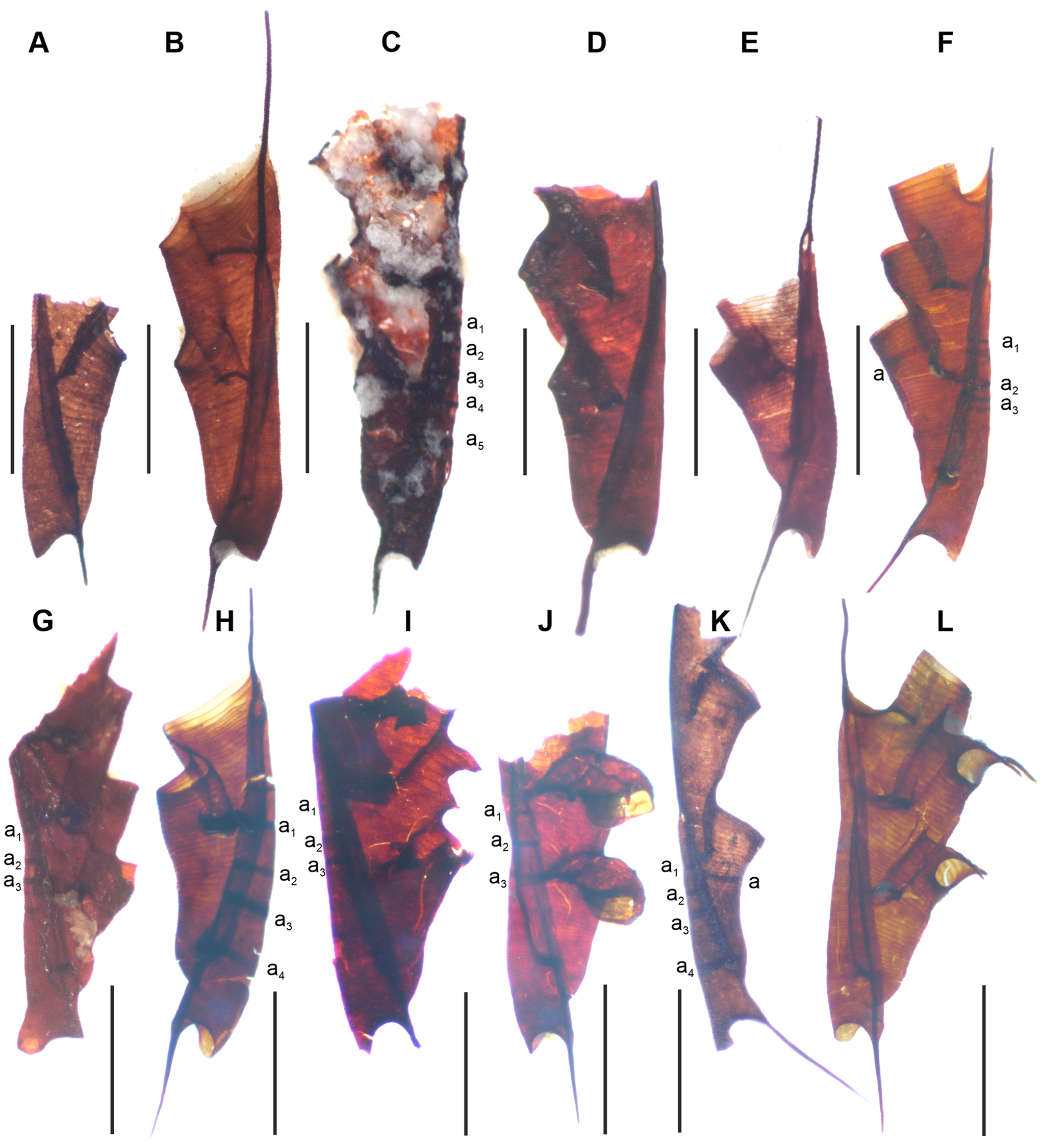
Figure 1 (A) Pristiograptus parvus Ulst; no. VU-S.V-131_356b, Vilkaviškis – 131 borehole, depth 1085.8 m, parvus Biozone, Gėluva Regional Stage, Homerian, Wenlock; (B, C) Pristiograptus dubius frequens Jaekel, Šiupyliai – 69 borehole: (B) without sicula annuli no. VU-P.S-69_292, depth 998.4 m, nassa Biozone, Gėluva Regional Stage, Homerian Wenlock; (C) with sicula annuli, VU-S.S-69_011b, depth 952.4 m, progenitor Biozone, Dubysa Regional Stage, Gorstian, Ludlow; (D) Pristiograptus virbalensis Paškevičius, no. VU-S.V-131_012, Vilkaviškis – 131 borehole, depth 1076.3 m, praedeubeli Biozone, Gėluva Regional Stage, Homerian, Wenlock; (E, F) – Colonograptus praedeubeli (Jaeger), Vilkaviškis – 131 borehole, praedeubeli Biozone, Gėluva Regional Stage, Homerian, Wenlock: (E) without sicula annuli, no. VU-S.V-131_015, depth 1076.3 m; (F) no. VU.MW.V-131_022, depth 1077.3 m; (G) Colonograptus deubeli (Jaeger), no. VU-S.V-131_016, Vilkaviškis – 131 borehole, depth 1067.3 m, deubeli Biozone, Gėluva Regional Stage, Homerian, Wenlock; (H) Pseudomonoclimacis dalejensis (Bouček), no. VU.MW.S-69_018, Šiupylai – 69 borehole, depth 964.3 m, nilssoni Biozone, Dubysa Regional Stage, Gorstian, Ludlow; (I) Colonograptus gerhardi (Kühne), no. VU.MW.B-2_019, Baubliai – 2 borehole, depth 1641.1 m, nilssoni Biozone, Dubysa Regional Stage, Gorstian, Ludlow; (J) Uncinatograptus uncinatus (Tullberg), no. VU.MW.S-69_025, Šiupylai – 69 borehole, depth 964.3 m, nilssoni Biozone, Dubysa Regional Stage, Gorstian, Ludlow; (K) Bohemograptus bohemicus bohemicus (Barrande), no. VU.S.B-2_016, Baubliai – 2 borehole, depth 1628.8 m, scanicus Biozone, Dubysa Regional Stage, Gorstian, Ludlow; (L) Saetograptus chimaera (Barrande), no. VU.MW.S-96_012, Šiupylai – 69 borehole, depth 951.2 m, progenitor Biozone, Dubysa Regional Stage, Gorstian, Ludlow. Abbreviations: a = dense fusellae marking interrupted growth; an = annuli of sicula. Scale bars = 1 mm.
The fluctuation of annuli between the middle Homerian and Ludfordian was recognised as occurring in phylogenetically independent lineages by Urbanek (Reference Urbanek1997), with the veracity of that phylogenetic independence later being supported via stratocladistics (Whittingham et al. Reference Whittingham, Radzevičius and Spiridonov2020). However, the ecological and evolutionary significance of sicular annuli remains unclear. It is that fluctuation in the number of annuli per specimen during the nassa–leintwardinensis Biozone interval that may provide insight into the life habit of middle–late Silurian graptolites and, if the fluctuations are independent of species, the changing climate of the Mulde interval. The purpose of this study is to establish whether the fluctuations in sicular annuli through the late Homerian and early Gorstian are independent of taxonomy, as predicted by Urbanek (Reference Urbanek1958), and if so, what factors may instead be responsible for that fluctuation.
1. Material and methods
Material consists of 367 graptolites (see Supplementary material available at https://doi.org/10.1017/) from the Viduklė-61 [56.049°N, 23.090°E] (upper nassa–scanicus biozones), Šiupyliai-69 [55.395°N, 22.910°E] (praedeubeli–scanicus biozones), and Vilkaviškis-131 [54.764°N, 22.849°E] (praedeubeli–deubeli biozones) boreholes from the Lithuanian region of the Baltic Basin (Fig. 2), an area located near the equator during the late Wenlock and early Ludlow (Torsvik & Cocks Reference Torsvik, Cocks, Harper and Servais2013). Graptolite rhabdosomes were prepared from rock using hydrochloric acid and hydrofluoric acid solutions. In the absence of an available infrared camera, specimens were stripped of their outermost cortical layer by being placed in a combined solution of 15 mL of 65 % nitric acid, 15 mL distilled water, and 5 g of potassium chlorate for 5–7 days, rendering the specimens red-brown and translucent to make the sicular annuli more clearly visible. This procedure serves only to enhance visibility and does not damage specimens or affect annulus counts (Green Reference Green and Green2001). Specimens were prepared and counted for sicular annuli if they possessed both an intact sicula and first theca. Species diagnoses were made for Pristiograptus dubius frequens Jaekel, Pristiograptus virbalensis Paškevičius, Colonograptus praedeubeli (Jaeger), and Colonograptus deubeli (Jaeger) based on descriptions by Urbanek et al. (Reference Urbanek, Radzevičius, Kozłowska and Teller2012), for Pseudomonoclimacis dalejensis (Bouček) based on descriptions of Pseudomonoclimacis haupti (Kühne) (e.g., Urbanek Reference Urbanek1958), which was synonymised with Ps. dalejensis by Štorch et al. (Reference Štorch, Manda and Loydell2014), for Uncinatograptus uncinatus (Tullberg) based on descriptions by Urbanek (Reference Urbanek1958), and for Saetograptus chimaera (Barrande) based on descriptions by Walker (Reference Walker1953). Additionally, the genera Bohemograptus, Lobograptus, and Neodiversograptus were diagnosed according to descriptions by Urbanek (Reference Urbanek1963, Reference Urbanek1970) and included in total counts of annuli but not identifiable to species in enough samples to warrant inclusion in individual species metrics (see Supplementary material). Mean counts of sicular annuli were plotted using PAST v.3.09 (Hammer et al. Reference Hammer, Harper and Ryan2015). Additional plots were only made for individual species if they were present and identifiable to species in more than one sample to maintain consistency in taxonomic scale. Counts of annuli for each individual species were compared via Tukey's pairwise comparisons in PAST v.3.09 with a significance threshold of 95 % in any core sample where two or more species were represented by at least two specimens to determine any major divergences in mean counts of annuli between species in the same environment. Per-species counts of annuli were also compared to total counts via linear regression to determine if total annulus counts were predictive of counts in any given species.
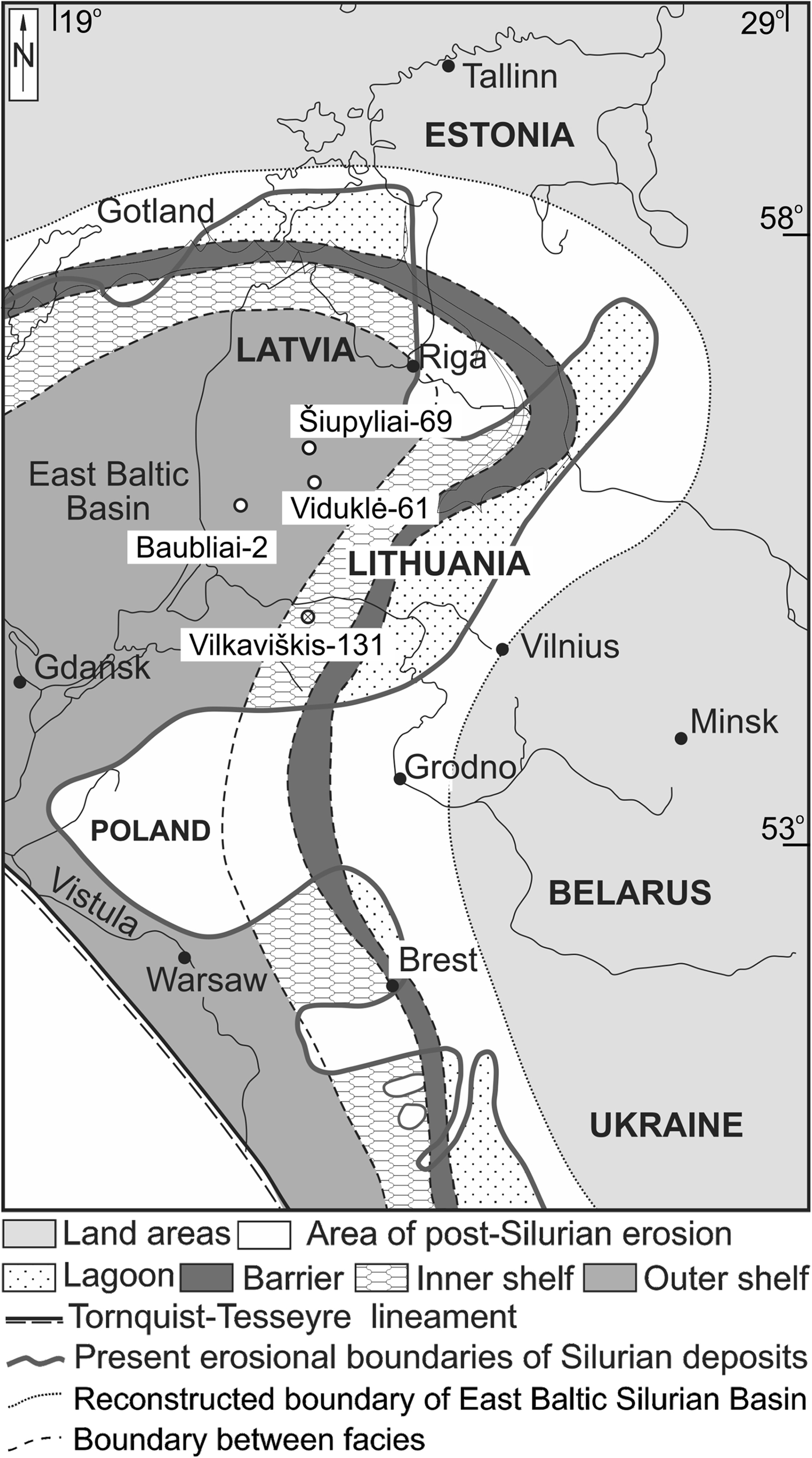
Figure 2 Facies map of the Baltic Silurian Basin during the nassa graptolite Biozone (after Einasto et al. Reference Einasto, Abushik, Kaljo, Koren, Modzalevskaya, Nestor, Klaamann, Kaljo and Klaamann1986) and location of boreholes.
Boreholes were correlated by applying a graphical correlation approach using the troughs and peaks of the double-peaked Mulde excursion akin to that used by Spiridonov (Reference Spiridonov2017), where the δ13C curve was used as an independent source of correlation between cores and was an independent test of the veracity of correlations derived from recurrence plots and conodont abundance data. There, and also in the current application, maxima and minima of mid- to late Homerian δ13C excursions were used in a simple graphic correlation procedure. Here, Viduklė-61 was used as a reference core, and all measurements of sicular annuli were projected to correlation lines and combined in a single data for use in all subsequent statistical analyses.
In order to test the environmental associations of sicular annuli, the average number of sicular annuli per sicula in a sample was compared with the combined model of fourth- and fifth-order sea level cycles, which are presumably driven by sea level fluctuations, and approximately correspond to the 400 Ka and 100–130 Ka Milankovitch cycles (Radzevičius et al. Reference Radzevičius, Spiridonov and Brazauskas2014, Reference Radzevičius, Tumakovaitė and Spiridonov2017). This model is created from the high resolution (~0.1 m) natural gamma ray log of the Viduklė-61 core (Radzevičius et al. Reference Radzevičius, Spiridonov and Brazauskas2014) and the extraction of statistically significant frequencies using phase-preserving Gaussian filtering (Radzevičius et al. Reference Radzevičius, Tumakovaitė and Spiridonov2017). The major pattern of sea level change is widely recognised to be of global significance and is associated with the positive late Homerian δ13C excursions and major biotic perturbations (Cramer et al. Reference Cramer, Brett, Melchin, Maennik, Kleffner, McLaughlin, Loydell, Munnecke, Jeppsson, Corradini and Brunton2011; Venckutė-Aleksienė et al. Reference Venckutė-Aleksienė, Radzevičius and Spiridonov2016; Spiridonov et al. Reference Spiridonov, Venckutė-Aleksienė and Radzevičius2017b).
The variability of average frequencies of annuli per sicula is strongly non-Gaussian. In ~18 % of samples annuli are completely absent. This creates a ‘clipped’ from below pattern – widespread in positive direction and sudden collapse of variability at zero. In order to mitigate this bias two approaches were employed: (1) ln(x = 1) transformation of original number of sicular annuli and later ordinary least square regression; (2) transformation of number of sicular annuli into the categorical binary variable – ‘0’ if annuli are absent, and ‘1’ if annuli are present. In the second approach the logistic regression generalised linear model with binomial error structure and logit link function was used. The regression analysis was performed in PAST v.3.09. The statistically significant positive relations would indicate positive association of the prevalence of annuli in high-stand (presumably warm and humid) conditions. Due to the lack of a cyclic model associated with the depths of the highest (youngest) five collected sicular annuli samples from the Ludlow (scanicus Biozone), those samples were not included in environmental comparisons, but were still used in analyses of within-sample similarity between species. While not commonly distinguished from one another on a global scale, the praedeubeli and deubeli graptolite biozones are herein identified as separate as they are useful and easily distinguishable within Uzbekistan (Koren’ & Suyarkova Reference Koren’ and Suyarkova1994) and the Baltic Basin (Radzevičius Reference Radzevičius2006; Koren’ & Suyarkova Reference Koren’ and Suyarkova2007).
2. Results
If present, annuli were always found at the boundary of the prosicula and metasicula, but showing no distinct pattern of appearance elsewhere. Species appearing in multiple samples were as follows: Pristiograptus dubius frequens Jaekel, Pristiograptus virbalensis Paškevičius, Colonograptus praedeubeli (Jaeger), Colonograptus deubeli (Jaeger), Pseudomonoclimacis dalejensis (Bouček), Uncinatograptus uncinatus (Tullberg), and Saetograptus chimaera (Barrande). However, both S. chimaera and P. dalejensis occur primarily in the five omitted samples from the scanicus Biozone, and so are mostly only informative for within-sample comparisons of counts of annuli between species. Additionally, the genera Bohemograptus, Lobograptus, and Neodiversograptus were found and included in average and total counts of annuli but not identifiable to species in enough samples to warrant inclusion in individual species metrics (see Supplementary material). In all cases where annuli were found, there was an annulus observed at the boundary of the prosicula and metasicula as described by Urbanek (Reference Urbanek1958).
The beginning of the sicular annuli dataset approximately corresponds to the upper nassa Biozone (middle of the Ho2 stage slice) of the Homerian, and ends with the lower scanicus Biozone (Go1 stage slice) of the early Gorstian (Cramer et al. Reference Cramer, Brett, Melchin, Maennik, Kleffner, McLaughlin, Loydell, Munnecke, Jeppsson, Corradini and Brunton2011).
The counts collected showed changes in overall mean sicular annuli in multiple distinct phases over the late Wenlock and early Ludlow (Fig. 3). Prior to the nassa Biozone, counts of annuli could not be collected from any of the studied Lithuanian boreholes. As such, precise estimates of average counts of annuli prior to the nassa Biozone cannot be determined. However, evidence from specimens of Testograptus testis from the lundgreni Biozone of the late Wenlock indicate that at least some pre-nassa monograptids possessed an annulus on the prosicula (Lenz & Melchin Reference Lenz and Melchin2008), while there are no known reports of sicular annuli from the lone monograptid species Pristiograptus dubius parvus from the proceeding parvus Biozone. The following interval (nassa to late praedeubeli biozones) shows fluctuation of mean counts of annuli between 1 and 3, with some rare specimens lacking annuli. This is followed by an interval spanning the upper praedeubeli and lower deubeli biozones showing the disappearance of sicular annuli in conjunction with major excursions in fourth- and fifth-order filtered gamma ray logs, phytoplankton diversity, and δ18O and δ13C stable isotope curves. Counts of annuli then rebound in the latter half of the deubeli Biozone. The remainder of the Wenlock and early Ludlow are characterised by elevated counts of annuli and generally increasing within-sample variability in the same species which show few or no annuli in the praedeubeli–deubeli interval, with counts as high as seven or eight in some specimens in the upper nilssoni and scanicus biozones (Fig. 3).
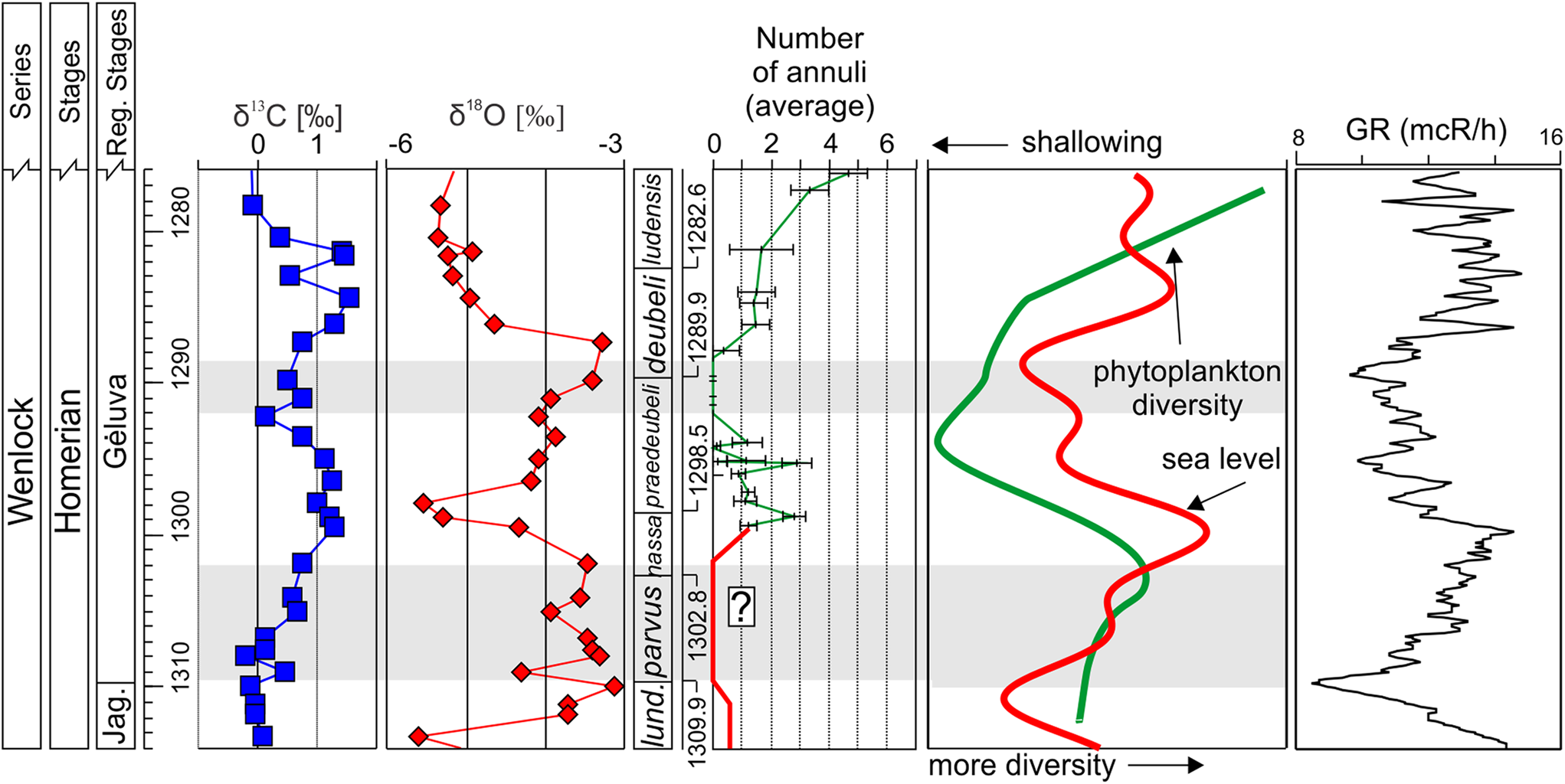
Figure 3 Homerian stable carbon and oxygen isotopic ratios in carbonates from Viduklė-61 (Martma et al. Reference Martma, Brazauskas, Kaljo, Kaminskas and Musteikis2005), the trend in average frequencies of sicular annuli in graptolites, and filtered fourth- and fifth-order cycles in natural gamma, interpreted as representing sea level fluctuations, the generalised phytoplankton diversity trend (Venckutė-Aleksienė et al. Reference Venckutė-Aleksienė, Radzevičius and Spiridonov2016; Radzevičius et al. Reference Radzevičius, Tumakovaitė and Spiridonov2017), and gamma ray (GR) data from Radzevičius et al. (Reference Radzevičius, Spiridonov and Brazauskas2014). The filtered fourth- and fifth-order sea level model, gamma ray, isotopic data, and phytoplankton diversity trends are derived from analyses of the Viduklė-61 core. The graptolite sicular annuli data were combined using a graphic correlation dataset projected on to the composite standard (Vidulkė-61 core). Intervals with extremely rare or no sicular rings are shown with grey bars. The section marked in red in and below the nassa Biozone was not included within the study range and is based on the lack of reporting of sicular annuli in Pristiograptus dubius parvus from other works, and the reporting of some singular annulus occurrences in Testograptus testis in the lundgreni Biozone (Lenz & Melchin Reference Lenz and Melchin2008), both unconfirmed in the geographical context of this study.
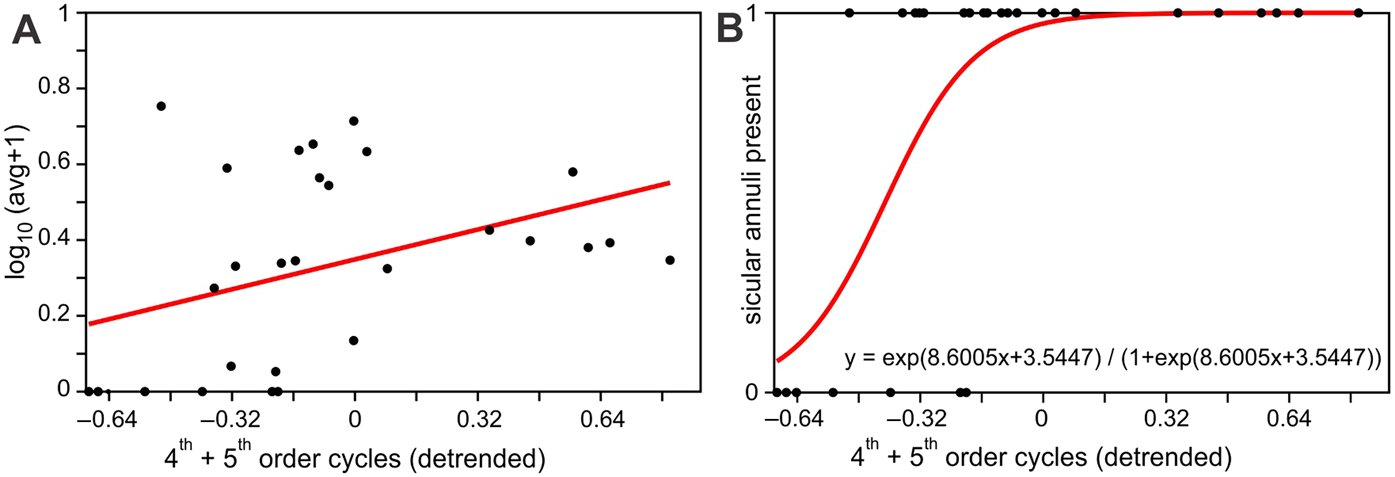
The least square regression of log transformed average sicular annuli numbers per sicula per sample show statistically significant (at P < 0.05) relation with the sea level cycles, represented by fourth- and fifth-order cycle gamma ray values (Fig. 4a). This conclusion is supported by the logistic regression of absence or presence of sicular annuli in graptolite samples with the sea level cycles (Fig. 4b). The positive connection between presence of annuli and the high-stand conditions here is even more apparent and statistically highly significant (P < 0.001). The intervals which are typified by graptolite sicula without annuli fall completely into the regressive parts of sea level cycles (where standardised fourth- and fifth-order cycle values are <0). Least square regression also showed a statistically significant (at P < 0.05) negative correlation between average counts of annuli and δ13C values despite the latter proxy being of rather moderate resolution.
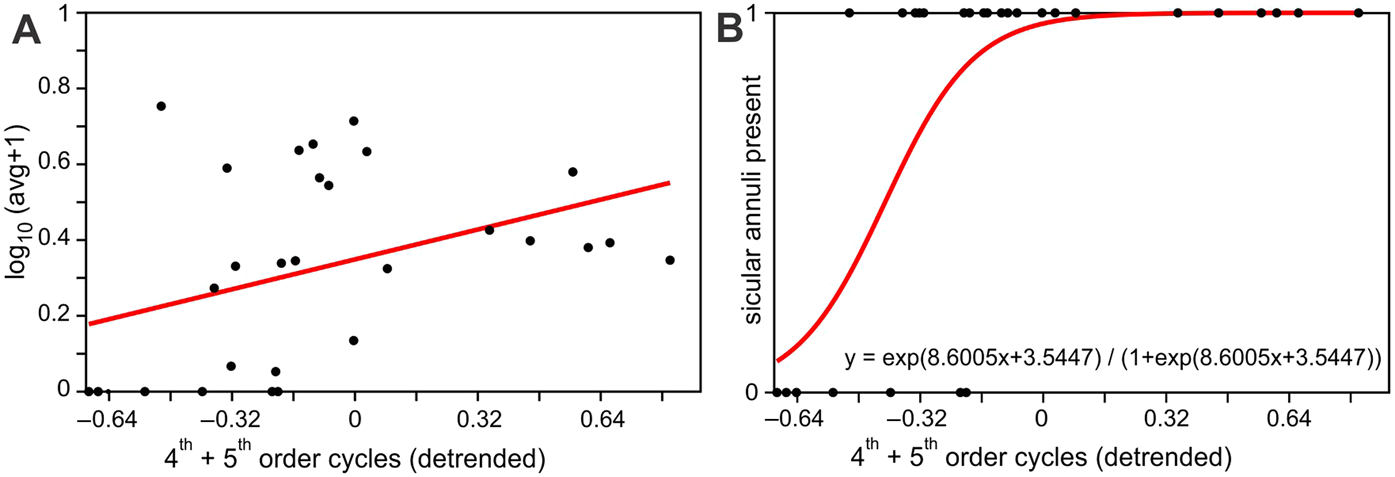
Figure 4 (A) Ordinary least squares regression of average log number of monograptid sicular annuli per specimen in a sample against the combined model of fourth- and fifth-order sea level cycles (a(slope) = 0.24; b(intercept) = 0.34; r = 0.39, P = 0.03, n = 28); (B) logistic regression of presences (‘1’) or absences (‘0’) of sicular annuli in graptolite specimens in samples against the combined model of fourth- and fifth-order sea level cycles (G = 14.49; P(slope = 0) = 0.0001; n = 28).
For all but one core sample there were no significant between-species differences in mean counts of annuli, that lone exception being in the Šiupyliai-69 core at a depth of 951.2 m between samples of Saetograptus chimaera and Pristiograptus dubius frequens (P < 0.001). The discrepancy at this depth is large between the two species, with S. chimaera having a mean annulus count of 2.18 ± 0.78 and P. dubius frequens having a mean annulus count of 5.71 ± 0.66. However, that sample represents the only significant departure in sicular annuli means between S. chimaera and P. dubius frequens. Furthermore, comparison via linear regression (Fig. 5) showed significant correlation between total counts of annuli and counts from each of P. dubius frequens (r 2: 0.9749, P < 0.001), Colonograptus praedeubeli (r 2: 0.9305, P < 0.001), Colonograptus deubeli (r 2: 0.773, P: 0.05), and S. chimaera (r 2: 0.6542, P: 0.1). These results indicate that there is no correlation between counts of annuli and taxonomy.
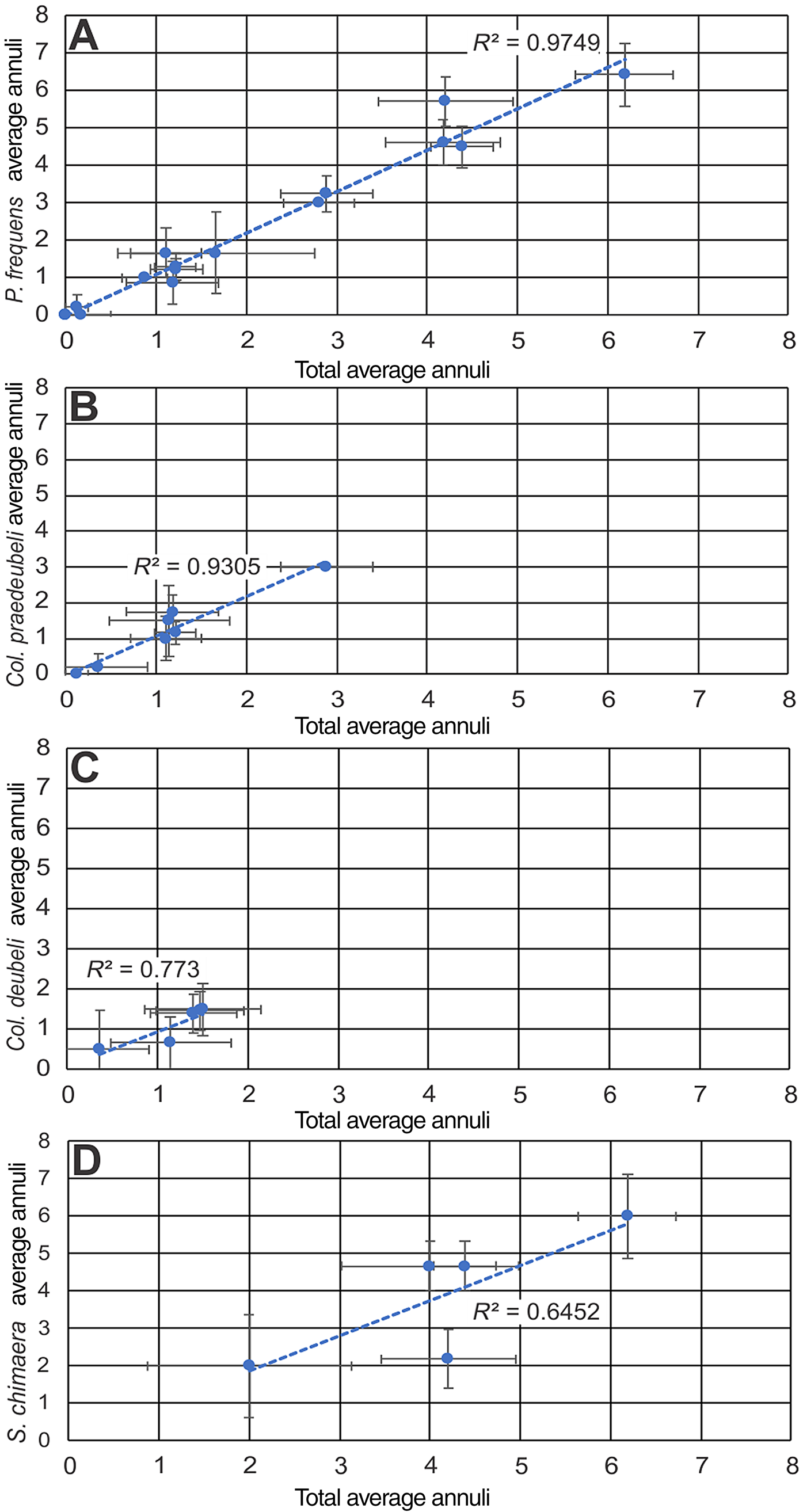
Figure 5 Ordinary least squares regression of mean counts of annuli per sample between an individual monograptid species and the remainder of species within the same samples. Tested species are (A) Pristiograptus dubius frequens (P < 0.001), (B) Colonograptus praedeubeli (P < 0.001), (C) Colonograptus deubeli (P < 0.05), and (D) Saetograptus chimaera (P < 0.1).
3. Discussion
From the curve of mean sicular annuli through the late Wenlock (Fig. 3) it is clear that there is significant fluctuation in sicular annuli between the praedeubeli and ludensis graptolite biozones. Given the concurrent fluctuations in counts of annuli between species and lack of significant difference between mean counts of annuli in different species (Fig. 5), it is apparent that the variation in counts of annuli is not taxonomically significant. These findings imply dependence of counts of annuli on extrinsic environmental factors, indicating that the variation in the trait is ecophenotypic. As there are no reported cases of prosicular annulus absence in the presence of metasicular annuli, it is highly unlikely that the inclusion of prosicular annuli would confound the results. If prosicular annuli were to be formed independently of environmental conditions promoting metasicular annulus growth, they should bias the results towards a false-negative signal in the correlation between annulus counts and environmental factors, particularly in the comparisons between environmental conditions and annulus presence and absence. This bias is overcome where measured, even in the presence–absence results (Fig. 5b), indicating that the environmental conditions promoting the development of metasicular annuli are also relevant to the development of the prosicular annulus.
While the beginning of the parvus interval is accompanied by stable isotope and gamma ray excursions associated with the aftermath of the early part of the Mulde event both in the Viduklė-61 core and elsewhere in the Baltic Basin (Radzevičius et al. Reference Radzevičius, Spiridonov and Brazauskas2014, Reference Radzevičius, Tumakovaitė and Spiridonov2017, Reference Radzevičius, Raczynski, Užomeckas, Norkus and Spiridonov2019; Venckutė-Aleksienė et al. Reference Venckutė-Aleksienė, Radzevičius and Spiridonov2016), the diversity of sampled localities and species analysed for counts of annuli is too low in the parvus Biozone to allow for any inferences of environmental correlation.
With sicular annuli likely ecologically controlled in number, local environmental data represent the best opportunity to explain their fluctuation. The correlations between counts of annuli and fourth- and fifth-order gamma ray logs indicate a positively correlated relationship between annuli and sea level. The fourth- and fifth-order cycles are derived from the natural gamma ray curve, which reflects the amount of radioactive isotopes of potassium, uranium, and thorium. The uranium shows affinity with clayey rocks and especially organics, thus indirectly showing the amount of preserved organic matter (Fig. 4). The combined fourth- and fifth-order cycles show periodic trends at the regional scale by reducing local noise, allowing for correlation with the regional scale graptolite data composited from multiple sites in the Baltic Basin. The close relationship between natural gamma values and the preserved carbon was previously clearly found in integrated studies of the Silurian Baltic Basin (Gelūnaitė & Spiridonov Reference Gelūnaitė and Spiridonov2015; Cichon-Pupieni et al. Reference Cichon-Pupienis, Littke, Froidl and Lazauskienė2020; Spiridonov et al. Reference Spiridonov, Stankevič, Gečas, Brazauskas, Kaminskas, Musteikis, Kaveckas, Meidla, Bičkauskas, Ainsaar and Radzevičius2020). Moreover, no bentonites, which may affect gamma ray logs if present, have been recorded from nassa–scanicus biozones of the studied cores (Kiipli et al. Reference Kiipli, Radzevičius, Kallaste, Motuza, Jeppsson and Wickstrom2008). The sedimentary and sea level cycles are congruent with the statistically estimated phytoplankton diversity grand fluctuations (Venckutė-Aleksienė et al. Reference Venckutė-Aleksienė, Radzevičius and Spiridonov2016), which suggest causal connection of high-stands, higher overall biological productivity in warmer eutrophic conditions as revealed by natural gamma curves, and the higher diversity of phytoplankton. The phytoplankton diversity fluctuations can be considered indicative of changes in primary productivity, as there is a general positive correlation between phytoplankton diversity and productivity (Irigoien et al. Reference Irigoien, Huisman and Harris2004), particularly where primary productivity is not already very high (Vallina et al. Reference Vallina, Follows, Dutkiewicz, Montoya, Cermeno and Loreau2014), as is the case during the late Homerian. The low-stands in the Silurian Baltic Basin are associated with development of reef and carbonate factories during the late Homerian post-Mulde interval (Calner Reference Calner2005), mid-Ludfordian Lau Event (Spiridonov et al. Reference Spiridonov, Stankevič, Gečas, Šilinskas, Brazauskas, Meidla, Ainsaar, Musteikis and Radzevičius2017a), and the recently recognised early Pridoli Šilalė Event (Spiridonov et al. Reference Spiridonov, Stankevič, Gečas, Brazauskas, Kaminskas, Musteikis, Kaveckas, Meidla, Bičkauskas, Ainsaar and Radzevičius2020). For example, during the Šilalė Event, a significant ≈5 °C drop in sea water temperatures (Žigaitė et al. Reference Žigaitė, Joachimski, Lehnert and Brazauskas2010) was associated with the development of carbonate reefs (Kaminskas et al. Reference Kaminskas, Michelevičius and Blažauskas2015), and drop in gamma ray values, as well as amount of preserved organic carbon (proxy for productivity), and orders of magnitude decrease in abundance of nektobenthic carnivores–conodonts (Spiridonov et al. Reference Spiridonov, Stankevič, Gečas, Brazauskas, Kaminskas, Musteikis, Kaveckas, Meidla, Bičkauskas, Ainsaar and Radzevičius2020). All these patterns point to the prevalence of decreased productivity in the water column and, thus, oligotrophy during cooling events and concurrent sea level regressions. This is particularly relevant due to the presence of annuli in specimens of Colonograptus praedeubeli from the low-palaeolatitude Canadian High Arctic (Lenz & Kozłowska-Dawidziuk Reference Lenz, Kozłowska-Dawidziuk, Gutierrez-Marco and Rabano1998) and the apparent absence of annuli in contemporaneous high-palaeolatitude material from Bolivia (Maletz et al. Reference Maletz, Suarez Soruco and Egenhoff2003), indicating that the formation of annuli may have been unique to tropical monograptids. Moreover, the low-stand at the end of the lundgreni Zone and beginning of the parvus Zone was associated with the extreme dominance of very small plankton, which also confirms the dominance of oligotrophic and low-productivity conditions at low-stands/lower values of natural gamma curves in the Baltic Basin (Spiridonov et al. Reference Spiridonov, Venckutė-Aleksienė and Radzevičius2017b). Only one genus (Leiosphaeridia) was studied for variation in size by Spiridonov et al. (Reference Spiridonov, Venckutė-Aleksienė and Radzevičius2017b), due to it possessing a simple spherical cyst shape, which eases calculations. Although just a part of the overall assemblage, Leiosphaeridia absolutely dominated the microphytoplankton communities, constituting approximately 85 % of all preserved individual organic microfossils (19,091 out of 22,338 counted individuals; reported in Venckutė-Aleksienė et al. Reference Venckutė-Aleksienė, Radzevičius and Spiridonov2016). Therefore, the conclusions on the size dynamics of the genus should, at first approximation, correctly represent states of whole communities.
Nowhere is the association of inferred sea level and productivity with counts of annuli more pronounced than during the praedeubeli–deubeli interval wherein all monograptid species entirely lack annuli, an interval concurrent with major excursions in fourth- and fifth-order gamma ray and stable isotope records (Fig. 4), and with a cooling event induced from data from Gotland from the same interval (Jepsson & Calner Reference Jeppsson and Calner2003). While the interval of zero annuli does not tightly co-occur with the nadir of phytoplankton diversity, it does closely align with the nadir of the fourth- and fifth-order gamma ray curve and generally comparatively low phytoplankton diversity. These combined observations indicate that the interval of zero annuli coincided with very low primary productivity.
These patterns represent a correlation between palaeoclimate and graptolite ecophenotypy, wherein higher sea levels (and likely higher primary productivity) are associated with higher counts of annuli, and low-sea-level regimes are associated with few or no annuli.
Though control of the trait's expression is extrinsic, origination of sicular annuli and their plasticity appears more likely to be evolutionary than a product of environmental factors. Given the extreme paucity of taxa at the time of the origination of sicular annuli, and the apparent resultant radiation of all future monograptids from that stem (Whittingham et al. Reference Whittingham, Radzevičius and Spiridonov2020), it is fair to interpret that sicular annuli arose once in Pristiograptus dubius frequens, and that the plasticity of their number was homologous and plesiomorphic in later monograptids. The plasticity of sicular annuli and the conditions controlling their number appear to have been inherited in all measured descendant clades of P. dubius frequens, as shown by the presence and fluctuation of annuli in Pristiograptus, Colonograptus, Saetograptus, Uncinatograptus, Pseudomonoclimacis, Bohemograptus, Lobograptus, and Neodiversograptus (Appendix 1). This is a phenomenon that has been observed in a variety of animal groups (Scheiner & Lyman Reference Scheiner and Lyman1989; Cheetham et al. Reference Cheetham, Jackson and Hayek1995; Nussey et al. Reference Nussey, Postma, Gienapp and Visser2005; Pelletier et al. Reference Pelletier, Réale, Coltman and Fest-Bianchet2007). The evolution of plasticity in various morphological and behavioural traits has been documented as an apparent adaptive response to significant environmental disturbances in the modern biota across multiple clades (Grottoli et al. Reference Grottoli, Rodrigues and Palardy2006; Nicotra et al. Reference Nicotra, Atkin, Bonser, Davidson, Finnegan, Mathesius, Poot, Purugganan, Richards, Valladares and van Kleunen2010), though the same has not yet been observed in the fossil record of graptolites. While the precedent set by the modern data indicates that significant disturbances such as those seen in the late Wenlock could influence the adaptive evolution of plastic traits, the extreme evolutionary bottlenecking of monograptids in the aftermath of the lundgreni extinction event could also have easily resulted in the fixation of nonadaptive traits. It therefore remains unclear as to whether sicular annuli and their plasticity were a functional, adaptive response to environmental disturbance, or simply an accident borne of random sorting.
As graptolites are understood to have been primary consumers (Berry & Wilde Reference Berry and Wilde1990; Underwood Reference Underwood1993; Cooper et al. Reference Cooper, Rigby, Loydell and Bates2012), it is likely that the formation of annuli was the result of a cascading effect of sea level changes on primary productivity, influencing nutrient availability for monograptids.
If counts of annuli were, at least in part, controlled by primary productivity as indicated, phytoplankton blooms may have contributed to increased annulus construction. In a low-latitude, tropical marine setting like the late Silurian Baltic Basin (Torsvik & Cocks Reference Torsvik, Cocks, Harper and Servais2013), large phytoplankton blooms are standard by-products of upwelling caused by monsoons (Brock et al. Reference Brock, McClain, Luther and Hay1991; Tudhope et al. Reference Tudhope, Lea, Shimmield, Chilcott and Head1996; Tiwari & Rao Reference Tiwari and Rao2004) and tropical storms (Chen et al. Reference Chen, Liu, Chuang, Yang, Shiah, Tang and Chung2003; Roman et al. Reference Roman, Adolf, Bichy, Boicourt, Harding, Houde, Jung, Kimmel, Millwe and Zhang2005; Lugo-Fernández & Gravois Reference Lugo-Fernández and Gravois2010; Gittings et al. Reference Gittings, Raitsos, Krokos and Hoteit2018). Monsoon seasons are understood to increase in intensity and variability with temperature (Goswami et al. Reference Goswami, Venugopal, Sengupta, Madhussodanan and Xavier2006; Sharmila et al. Reference Sharmila, Joseph, Sahai, Abhilash and Chattopadhyay2015) and are significantly subdued under cooler, dryer conditions (Prell & Kutzbach Reference Prell and Kutzbach1987). Likewise, the frequency of intense tropical cyclones is positively tied to high sea-surface temperatures (Yeh et al. Reference Yeh, Kang, Kirtman, Kim, Kwon and Kim2010; Yan et al. Reference Yan, Wei, Korty, Kossin, Zhang and Wang2016), with cooler, dryer conditions being associated with reduced tropical cyclone intensity (Hobgood & Cerveny Reference Hobgood and Cerveny1988), aligning with the patterns observed in mean counts of annuli (Fig. 3). In mid- and high latitudes, storm events and storm-related upwelling are also projected to increase in frequency with increasing sea levels and sea-surface temperatures (Francis & Vavrus Reference Francis and Vavrus2012; Mathis et al. Reference Mathis, Pickart, Byrne, McNeil, Moore, Juranek, Liu, Ma, Easley, Elliot, Cross, Reisdorph, Bahr, Morison, Lichendorf and Feely2012; Pickart et al. Reference Pickart, Schulze, Moore, Charette, Arrigo, van Dijken and Danielson2013; Tebaldi et al. Reference Tebaldi, Strauss and Zervas2012). These processes may then serve as potential drivers of increased annulus counts. As estimates of graptolite sicula growth are on the scale of a few weeks to a few months (Rigby & Dilly Reference Rigby and Dilly1993), storm events are the more likely candidates to instigate annulus growth multiple times over the development of a sicula, as storm-related upwelling events may occur multiple times per month (Pickart et al. Reference Pickart, Schulze, Moore, Charette, Arrigo, van Dijken and Danielson2013). If this hypothesis is true, storm-related upwelling events would have either been directly responsible for the formation of sicular annuli, or would at least have significantly increased the likelihood of annulus formation. Some tempestites have been recorded from the upper Homerian and Gorstian of Estonia (Nestor et al. Reference Nestor, Einasto, Nestor, Märss and Viira2001), supporting the idea that storm action may have contributed to the formation of sicular annuli, and further recording of tempestites from periods of high annulus counts in Lithuania would help strengthen this hypothesis. In this way, mean counts of annuli may serve as a potential proxy for storm event frequency in a given locality.
4. Conclusion
Our results paint a comprehensive picture of the origination and variation in sicular annuli in upper Wenlock and lower Ludlow monograptids. The ecophenotypic nature of the plasticity in the trait is borne out by the lack of statistically significant differences between mean counts of annuli between all species from the same time intervals and by the correlation between total counts and species-specific counts. That plasticity appears to be borne out of the extreme evolutionary bottlenecking of the aftermath of the lundgreni extinction event. The construction of annuli is likely a product of the effects of sea level change cascading through changes in primary productivity. Upwelling events, possibly as a result of more intensified and/or more frequent tropical cyclones, would have further encouraged increases of primary productivity, driving increased annulus construction. Overall, our findings show the presence of an easily measured ecophenotypic character that may provide further insight into the changing climate of the mid- to late Silurian.
5. Supplementary material
Supplementary material is available online at https://doi.org/10.1017/S1755691021000402.
6. Acknowledgements
This study was supported by a grant from the Research Council of Lithuania (grant number S-MIP-19-15: ‘Ecosystem construction and collapse in the Silurian – survival of biodiversity in the extreme climate’).