Lichens are symbiotic between a fungal host and one or several algal and/or cyanobacterial partners. Whilst lichens are often perceived as pair-wise interactions between only one fungus and one photosynthetic symbiont, they frequently house a plethora of associated microorganisms, including specialised assemblages of bacteria and fungi. The associated organisms can occur both on lichen surfaces and deep within the photobiont layer and medulla of lichen thalli (Girlanda et al. Reference Girlanda, Isocrono, Bianco and Luppimosca1997; Grube et al. Reference Grube, Cardinale, de Castro, Müller and Berg2009, Reference Grube, Berg, Andrésson, Vilhelmsson, Dyer, Miao and Martin2014; Hodkinson & Lutzoni Reference Hodkinson and Lutzoni2009; Bates et al. Reference Bates, Cropsey, Caporaso, Knight and Fierer2011; Hodkinson et al. Reference Hodkinson, Gottel, Schadt and Lutzoni2012; U'Ren et al. Reference U'Ren, Lutzoni, Miadlikowska, Laetsch and Arnold2012; Aschenbrenner et al. Reference Aschenbrenner, Cardinale, Berg and Grube2014, Reference Aschenbrenner, Cernava, Berg and Grube2016; Sigurbjörnsdóttir et al. Reference Sigurbjörnsdóttir, Heiðmarsson, Jónsdóttir and Vilhelmsson2014).
True lichenicolous fungi are obligate associates of lichen-forming fungi and/or their photobionts (Rambold & Triebel Reference Rambold and Triebel1992; Lawrey & Diederich Reference Lawrey and Diederich2003). Approximately 1,750 species of obligate parasites, parasymbionts and/or saprophytes have so far been described, but recent estimates suggest that 5,000–7,500 species may exist (Lawrey & Diederich Reference Lawrey and Diederich2003, Reference Lawrey and Diederich2016; Werth et al. Reference Werth, Millanes, Wedin and Scheidegger2013). Some lichen parasites appear to have evolved from saprotrophic ancestors. In addition to enzymes that degrade the cell walls of their hosts, some lichenicolous fungi produce enzymes that degrade antifungal lichen compounds. Whilst such species may be rare, they have the potential to affect fungal community dynamics by enabling less specialised saprotrophs to colonise lichen thalli (Lawrey et al. Reference Lawrey, Torzilli and Chandhoke1999; Torzilli et al. Reference Torzilli, Mikelson and Lawrey1999; Lawrey & Diederich Reference Lawrey and Diederich2003; Werth et al. Reference Werth, Millanes, Wedin and Scheidegger2013).
Most lichen-forming fungi are ascomycetes, many of which produce unique secondary metabolites (Culberson Reference Culberson1969, Reference Culberson1970; Culberson et al. Reference Culberson, Culberson and Johnson1977; Huneck & Yoshimura Reference Huneck and Yoshimura1996; Lumbsch Reference Lumbsch, Kranner, Beckett and Varma2002). Whilst the possible physiological and/or ecological functions of most such lichen compounds are unknown, some protect lichen symbionts against UV-radiation (Rikkinen Reference Rikkinen1995; Solhaug et al. Reference Solhaug, Gauslaa, Nybakken and Bilger2003; Nguyen et al. Reference Nguyen, Chollet-Krugler, Gouault and Tomasi2013) and thallus-grazing animals (Lawrey Reference Lawrey1986; Nybakken et al. Reference Nybakken, Helmersen, Gauslaa and Selas2010; Asplund Reference Asplund2011; Asplund & Wardle Reference Asplund and Wardle2013). Some lichen compounds also protect lichen symbionts against viruses, bacteria and parasitic fungi (Lawrey Reference Lawrey1986; Halama & Van Haluwyn Reference Halama and Van Haluwyn2004; Ranković et al. Reference Ranković, Misić and Sukdolak2007; Fazio et al. Reference Fazio, Adler, Bertoni, Sepùlveda, Damonte and Maier2007).
Whilst the degree of host-specificity of most lichenicolous fungi remains poorly known, virulent parasites that indiscriminately kill their lichen hosts are generally rare (Hawksworth Reference Hawksworth1982a; Lawrey & Diederich Reference Lawrey and Diederich2003; Werth et al. Reference Werth, Millanes, Wedin and Scheidegger2013). A majority of host-specific lichenicolous fungi seem to be mildly parasitic or parasymbiotic, and the nature of their interactions may have been modified during coevolution with the hosts. Unfortunately, phylogenetic evidence of coevolution between lichen symbionts can be difficult to achieve, because photobiont switches and other community level effects may have effectively blurred signs of phylogenetic tracking between individual lineages (Rikkinen Reference Rikkinen2003a). The same applies to coevolution and/or evolutionary arms races between lichenicolous fungi and their hosts (Lawrey & Diederich Reference Lawrey and Diederich2003; Werth et al. Reference Werth, Millanes, Wedin and Scheidegger2013; Millanes et al. Reference Millanes, Truong, Westberg, Diederich and Wedin2014).
Some groups of lichens seem to harbour more host-specific lichenicolous fungi than others. For example, a relatively high diversity of lichenicolous species has been described from species of Peltigeraceae as compared to those of Parmeliaceae, despite the much larger number of species in the latter family. These differences may be partly explained by differences in thallus morphology and types of lichen compounds produced (Hawksworth Reference Hawksworth1982b), but also by differences in speciation rates between families, etc. (Kraichak et al. Reference Kraichak, Divakar, Crespo and Lumbsch2015).
Lichen fossils are rare in comparison to plant and animal fossils. The oldest fossils of fungal–algal symbioses are from the Lower Devonian Rhynie chert from Scotland (Taylor et al. Reference Taylor, Hass and Kerp1997; Karatygin et al. Reference Karatygin, Snigirevskaya and Vikulin2009) and some of them share many structural features with extant lichens (Honegger et al. Reference Honegger, Edwards and Axe2013). Younger lichen fossils have been found from different Paleogene amber deposits, and many of them can be assigned to modern lichen families and genera (e.g., Rikkinen & Poinar Reference Rikkinen and Poinar2002, Reference Rikkinen and Poinar2008; Hartl et al. Reference Hartl, Schmidt, Heinrichs, Seyfullah, Schäfer, Gröhn, Rikkinen and Kaasalainen2015; Kaasalainen et al. Reference Kaasalainen, Heinrichs, Krings, Myllys, Grabenhorst, Rikkinen and Schmidt2015, Reference Kaasalainen, Schmidt and Rikkinen2017).
Here, we describe diverse fossils of lichen-associated filamentous fungi from Bitterfeld and Baltic amber, some of which were briefly reported by Kettunen et al. (Reference Kettunen, Schmidt, Diederich, Grabenhorst and Rikkinen2016). Several distinct morphologies of filamentous fungi growing on crustose and foliose lichens are exquisitely preserved, suggesting that a high diversity of dematiaceous hyphomycetes has occurred on epiphytic lichens at least since the Paleogene.
1. Material and methods
Fossils of lichen-associated fungi are enclosed in a total of ten pieces of Bitterfeld and Baltic amber (Table 1).
Table 1 Origin and repository of the amber pieces containing lichen-associated filamentous fungi. Collections: GZG=Geoscientific Collections of the Georg August University, Göttingen, Germany; Grabenhorst = Heinrich Grabenhorst Amber Collection, Wienhausen, Germany.

Bitterfeld amber originates from the “Bernsteinschluff” Horizon in the upper part of the Cottbus Formation of the Goitzsche mine, near the city of Bitterfeld, Germany. The upper Oligocene amber-bearing sediment has an absolute age of 25.3–23.8 million years (Knuth et al. Reference Knuth, Koch, Rappsilber and Volland2002; Blumenstengel Reference Blumenstengel2004). A previous notion that Bitterfeld amber represents re-deposited Eocene Baltic amber is based on the fact that there is a significant proportion of identical arthropod morphologies in amber from both localities (Weitschat Reference Weitschat1997). Redeposition of Baltic amber is unlikely, based on the reconstruction of the sedimentary environment of this huge amber deposit (Standke Reference Standke, Rascher, Wimmer, Krumbiegel and Schmiedel2008). A local reworking of pre-Chattian amber, however, has not been dispelled so far (see Dunlop Reference Dunlop and Penney2010 for discussion). In any case, Bitterfeld amber is Paleogene in age and its minimum age is c.24 million years.
The majority of Baltic amber derives from the amber-bearing marine ‘Blue Earth’ layers that are predominantly exposed on the Samland Peninsula of the Kaliningrad district (Russia), but Baltic amber is also often found washed ashore along the coast of the Baltic Sea and in neighboring areas. The commercially mined amber-bearing strata is Priabonian in age (34–38 Ma, using the International Chronostratigraphic Chart v2017/02 www.stratigraphy.org), though there is a lower horizon of Lutetian age (41–48 Ma) (Standke Reference Standke, Rascher, Wimmer, Krumbiegel and Schmiedel2008).
For investigation, the amber pieces were further ground and polished manually, using a series of wet silicon carbide papers (grit from FEPA P 600–4000 (25.8 μm to 5 μm particle size), Struers, Germany) to produce smooth opposite surfaces for investigation. A fraction of a millimetre of amber surface was gradually removed from each amber piece, while frequently checking the preparation under a stereoscope to ensure that the inclusion was not further damaged (see Schmidt et al. Reference Schmidt, Jancke, Lindquist, Ragazzi, Roghi, Nascimbene, Schmidt, Wappler and Grimaldi2012 for protocols).
The amber inclusions were studied under a Carl Zeiss AxioScope A1 compound microscope, equipped with a Canon 5D digital camera. In most instances, incident and transmitted light were used simultaneously. The light-microscopical images (Figs 1, 3–6) are digitally stacked photomicrographic composites of up to 70 individual focal planes, obtained using the software package Helicon Focus 5.0 for a better illustration of the three-dimensional inclusions.

Figure 1 Lichen substrates preserved in Bitterfeld and Baltic amber: (A) specimen GZG.BST.27298 (Bitterfeld amber) contains a crustose lichen colonised by Sporidesmium-like fungi. Three apothecia of the host lichen are indicated (Ap); (B) specimen Grabenhorst-Ri-51 (Baltic amber) contains a partly immersed crustose lichen colonised by a minute toruloid fungus. Laminal soralia (So), with globose heaps of soredia (granular symbiotic propagules consisting of algal cells enveloped with fungal hyphae), are seen on the upper surface of the thallus. Scale bars=1 mm (A); 100 µm (B).
To confirm that the filamentous fungi were growing on lichen thalli, substrate fragments from some amber specimens were exposed using a scalpel to remove the overlaying amber, and transferred to a carbon-covered SEM-mount using a wet hair from a superfine brush, sputtered with goldplatinum/palladium (2×120 seconds at 20 mA, 10 nm coat thickness) using an Automatic Sputter Coater (Canemco Inc.) and examined under a field emission scanning-electron microscope (Carl Zeiss LEO 1530 Gemini).
After investigation, the pieces were fully embedded in a high-grade epoxy (Buehler Epoxicure) under vacuum (see Nascimbene & Silverstein Reference Nascimbene, Silverstein and Grimaldi2000 for protocols) to ensure long-term preservation of the fossils.
Institutional repositories. GZG, Geoscientific Collections of the Georg August University, Göttingen, Germany; Grabenhorst, Heinrich Grabenhorst Amber Collection, Wienhausen, Germany.
2. Results
All the filamentous fungi grew on epiphytic lichens attached to tree bark and were preserved together with their substrate. As many crustose lichens grow tightly attached to or even partly immersed in their substrate, it can be extremely difficult to recognise their fossils as such, especially if deeply imbedded in refracting amber. In some cases, distinctive reproductive structures such as apothecia (Fig. 1A) or soredia (Fig. 1B) were present, but in most cases only degraded fragments of leprose or areolate thalli were present. The SEM analysis of some thallus fragments confirmed that their internal structure was lichen-like; i.e., that both fungal hyphae and photobiont cells were present within a stratified thallus (Fig. 2). On the basis of very few preserved features, none of the crustose lichens can be identified with any precision, but they probably represent species of the predominately lichen-forming order Lecanorales with trebouxioid green algal photobionts.
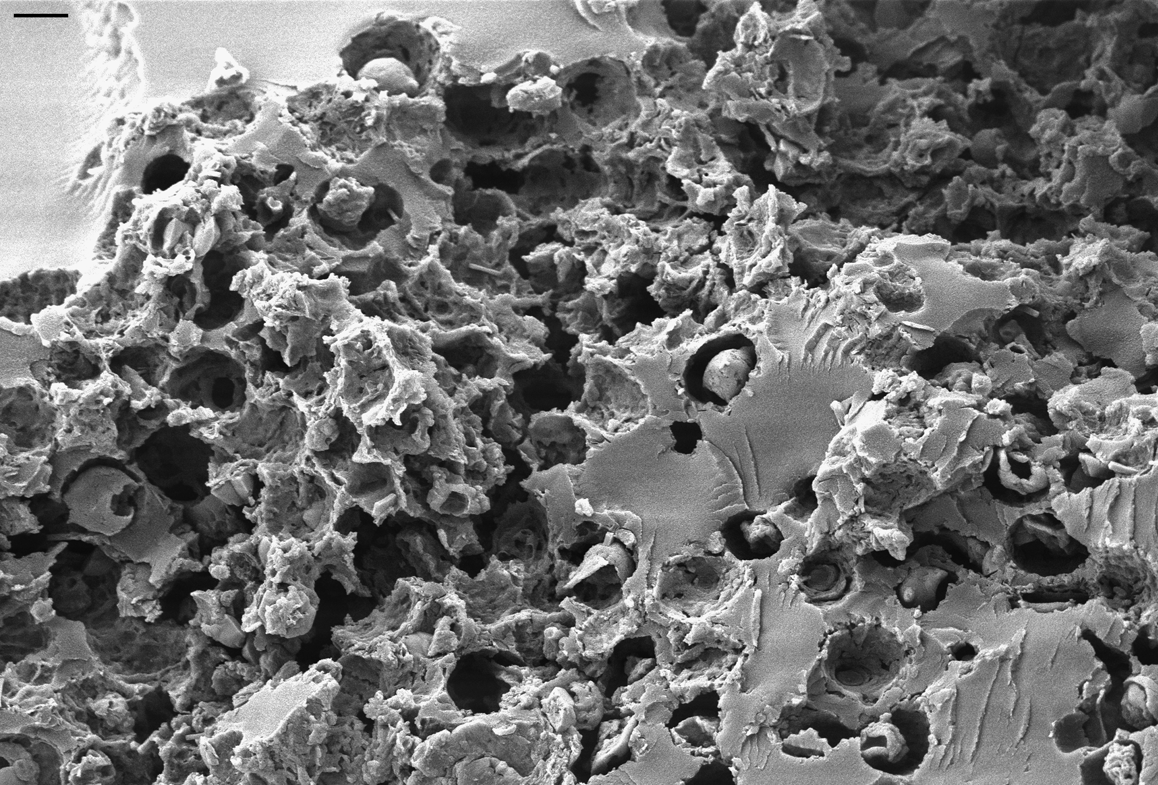
Figure 2 Scanning electron microscopical image of photobiont layer in crustose lichen in Bitterfeld amber (specimen GZG.BST.27299). Several shrivelled photobiont cells and details of the mycobiont–photobiont interface have been preserved. Scale bar=2 μm.
2.1. Sporidesmium-like fungi
Bitterfeld amber (specimens GZG.BST.27298 and GZG.BST.27294) contains three slightly distinguished morphologies of fungi resembling the extant genus Sporidesmium Link, 1809 (Fig. 3). One form, growing on the thallus surface of an Ochrolechia-like crustose lichen, formed dark brown to black, punctiform colonies, forming clusters of upright conidiophores. Conidiophores are macronematous, unbranched, mid to dark brown, 10–50 µm long and 6–9 µm wide (Fig. 3B). Conidiogenous cells appear monoblastic, doliiform or lageniform, lighter brown, and sometimes integrated. Conidia are multicellular, 55–120 µm long and 6–15 µm wide phragmoconidia, with cells tapering gradually towards the apex. Conidia are produced solitarily, acrogenous, straight or curved, obclavate, with cells being moniliform or doliiform, brown to dark brown. Conidial cells are usually broader than long, 6–15 µm wide (usually 10–12 µm) and 4–10 µm long (usually 7–8 µm). Apical cells are elongate, light brown or hyaline. The conidia have at least 7–11 septa and their detachment is schizolytic (Fig. 3C).

Figure 3 Sporidesmium-like fungi from Bitterfeld amber: (A) colony possessing prominent apical extensions and globular to pyriform structures (GZG.BST.27294); (B) clusters of upright conidiophores with conidia on an elevated lichen thallus ridge (GZG.BST.27298); (C) detached conidium (GZG.BST.27298); (D, E) possible immature stages with two to three thin and lighter brown appendages on wide trunk-like structures (GZG.BST.27294). Other microfossils associated with the Sporidesmium-like fungus include small toruloid cell chains (upper arrowhead) and hyphae of sooty moulds (lower arrowhead). Scale bars=50 µm (A, B, D, E); 20 mm (C).
A second form of Sporidesmium-like fungi differs in having prominent apical extensions and in producing globular to pyriform structures presumed to be conidial initials (Fig. 3A). Colonies are dark brown to black, formed solitarily or in clusters of upright conidiophores. Mycelium is immersed or partially superficial, hyphae 1–3 µm wide. Conidiophores are macronematous, unbranched, mid to dark brown, length and width often difficult to assess, but up to at least 30 µm long and 6 µm wide. Conidiogenous cells appear monoblastic, doliiform or lageniform and brown. Conidia are multicellular phragmoconidia, 75–160 µm long and 9–15 µm wide (at the widest part), cells tapering gradually towards the apex. Conidia are produced solitarily; they are acrogenous, straight or curved and obclavate. Conidial cells at the widest point are moniliform or doliiform, brown to dark brown. The apical cells are lighter brown or hyaline, forming elongated apexes 30–100 µm long and 3–6 µm wide. Some of the apical parts have formed globular, subglobular to pyriform septate structures 12–18 µm long and 9–12 µm wide (one non-septate initial stage 9 µm long and 6 µm wide), possibly representing the initial stages of new conidia. Detachment of the conidia appears to be schizolytic. No germinating conidia have been seen. The fungus grows on a degraded lichen thallus.
The third form of Sporidesmium-like fungi (Fig. 3D, E) may either represent immature stages, another species or indicate a wide morphological variety of a single fossil species. These fungi produced dark brown trunk-like structures with two to three thin and lighter brown appendages branching out from the apex. The structures are 30–70 µm long, the widest parts being 12–15 µm and the thinnest parts 3–6 µm wide. There are also some small flat and globular initial stages 9–15 µm in diameter. Attached to these structures there are dark fungal hyphae 1–3 µm wide (Fig. 3D).
2.2. Taeniolella-like fungi
Bitterfeld amber (specimens GZG.BST.27299 and Grabenhorst-Ri-49) contains fungal fossils closely resembling species of the extant genera Taeniolella Hughes, Reference Hughes1958 s. l. and Taeniolina Ellis, Reference Ellis1976. Colonies are effuse or pulvinate, brown to dark brown (Fig. 4A–D). Mycelium is mostly immersed. Conidiogenous cells appear monoblastic, integrated, terminal, determinate, cylindrical or doliiform. Conidia are multicellular, doliiform, brown to dark brown, in simple or sparingly branched 50–180 µm long chains. Conidial cells are 6–9 µm wide and 3–8 µm long, smooth or somewhat ornamented. Some detached conidia have been preserved (Fig. 4E–G). Mycelia of pale, narrow (approx. 1–2 µm wide) hyphae, some of them growing partially attached to the lichen thalli of specimen GZG.BST.27299, indicate that the fungi grew on decomposing lichens.

Figure 4 Taeniolella-like fungi from Bitterfeld amber: (A) overview of colony and detached conidia on crustose lichen (GZG.BST.27299); (B) three colonies and degraded remains of crustose lichen (Grabenhorst-Ri-49). (C) hyphae and conidia on the ridge of a lichen thallus (Grabenhorst-Le-91); (D) colony with numerous mature conidia on a lichen thallus ridge (GZG.BST.27299); (E–G) conidia and robust vegetative hyphae (GZG.BST.27299). Scale bars=50 µm.
Amber specimen Grabenhorst-Le-91 (Bitterfeld) contains a fungus (Fig. 4C) that closely resembles the Taeniolella-like fungus from specimens GZG.BST.27299 and Grabenhorst-Ri-49, but it has slightly smaller conidial cells that are 3–6 µm wide and 3–6 µm long. The colonies are also less dense.
2.3. Other lichen-associated fungi
In addition to the Sporidesmium-like and Taeniolella-like hyphomycetes described above, we found six other fungal morphologies associated to lichen thalli.
Sporidesmium-like fungi are sometimes accompanied by a more delicate hyphomycete (Fig. 5G-I). Conidiophores of this fungus are macronematous, dark brown and 75–100 µm high. Conidiogenous cells are polyblastic and brown. Conidia are multicellular, 3–6-septate, brown, elliptical or oblong, 3–5 µm wide and 9–12 µm long, individual cells 3–5 µm wide and 1.5–3 µm long (Fig. 5I). Conidial ontogeny is acroblastic; larger cells develop at the tips of a conidiophore and gradually become multiseptate.

Figure 5 Lichen-associated dematiaceous hyphomycetes from Bitterfeld amber: (A–F) hyphomycete with dark upright conidiophores and curved multiseptate conidia (Grabenhorst-Ri-30); (G–I) conidiophores and conidia in specimen GZG.BST.27298. Scale bars=20 µm.
Some Taeniolella-like fungi are associated with light brown filamentous fungi. Cells of thin hyphae and conidial chains are cylindrical, rounded or doliiform, 2–3 µm wide and 3–6 µm long (Fig. 6 H–I).
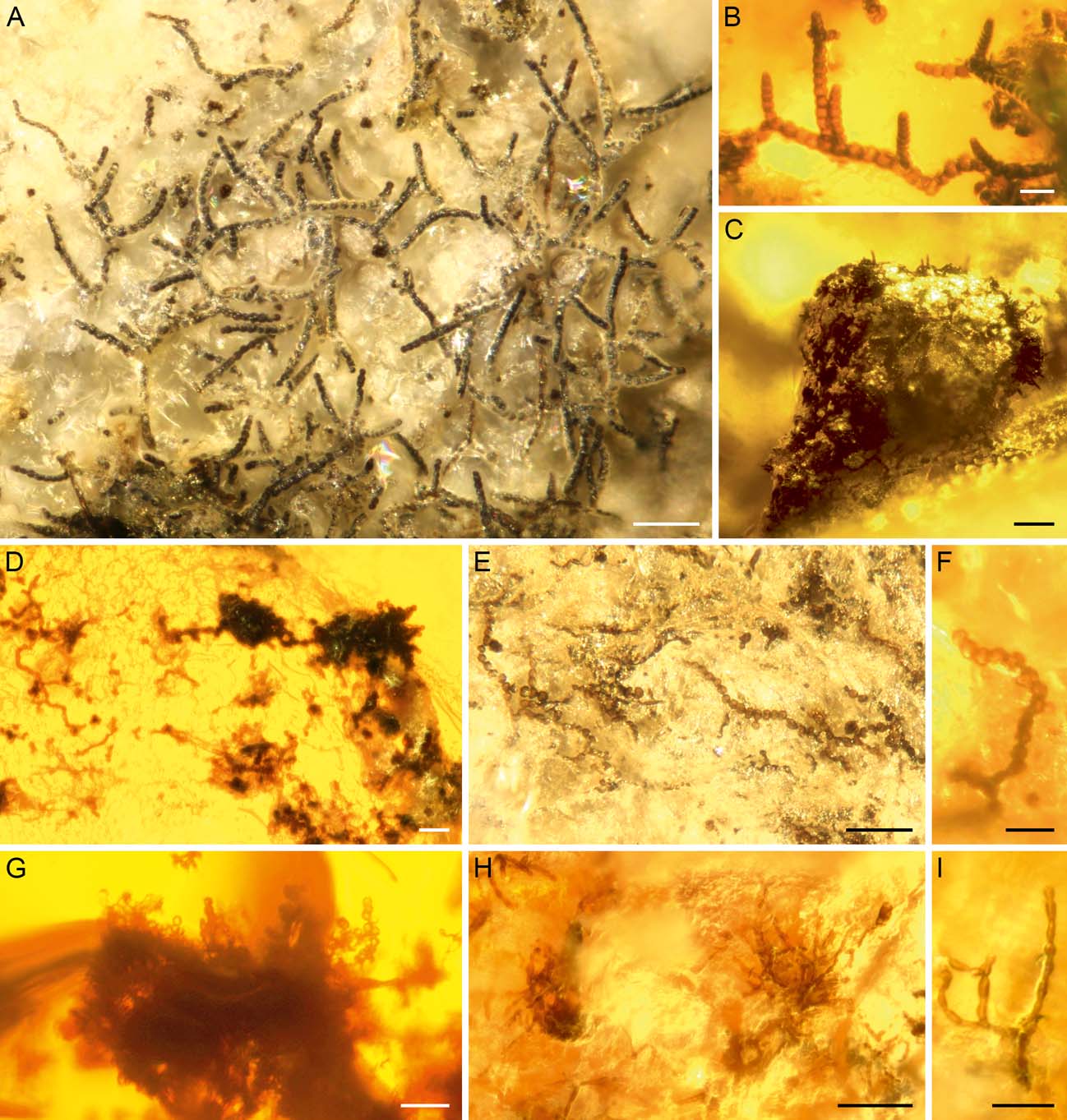
Figure 6 Diverse lichen-associated filamentous fungi from Bitterfeld and Baltic amber: (A) sooty mould in Baltic amber (Grabenhorst-Ri-35); (B) sooty mould in Bitterfeld amber (GZG.BST.27294); (C), (D), (F) toruloid microfungi in Bitterfeld amber: (C) GZG.BST.27293; (D) GZG.BST.27294; (F) GZG.BST.27298; (E) toruloid microfungus in Baltic amber (Grabenhorst-Ri-51); (G) microfungus in Bitterfeld amber (GZG.BST.27299); (H, I) hyphae and conidial chains in Bitterfeld amber (GZG.BST.27299). Scale bars=50 µm (A, C, E, H); 20 mm (B, D, F, G, I).
Bitterfeld (specimen Grabenhorst-Ri-30) and Baltic (specimen Grabenhorst-Ri-54) ambers contain a lichen-associated hyphomycete with dark stalked conidiophores and curved multiseptate conidia (Fig. 5A–F). Colonies are effuse, dark brown to black. Mycelium is mostly superficial, 3–6 µm wide, dark brown. Conidiophores are macronematous, growing solitarily or in groups, dark brown to black, multiseptate and unbranched, percurrent, 180–360 µm long and 6–9 µm wide. Conidiogenous cells are monoblastic, forming chains of ellipsoid to oblong cells at the apical region of the conidiophore, brown, 9–12 µm wide and 12–15 µm long. Conidia are multicellular phragmoconidia, 10–14 µm wide and up to 75 µm long. They are produced solitarily, acrogenous, curved when mature and up to 10-septate, brown to dark brown. The detachment of the conidia appears to be schizolytic.
Lichens from Bitterfeld and Baltic ambers are sometimes covered by dense mycelia of sooty moulds (Figs 3E, 6A, B). The tapering branching vegetative hyphae of these fungi are brown to dark brown and 6–12 µm wide.
Small-celled ’toruloid’ fungi are quite frequent on the fossil lichen thalli (Table 1, Figs 3E, 6C, E, F). These grew parallel to the lichen surface, sometimes producing protruding cell chains (Figs 3E, 6C–G). Cells are usually 3–6 µm in diameter, globular or slightly oblong, brown to dark brown, attached to 2–4 µm-wide hyphae. These fungi form flat, globular clusters consisting of round cells and hyphae that are partly embedded in the lichen thallus. The clusters can be around 50 µm in diameter, and possibly represent young initials of ascomata or microsclerotium-like resting phases. Sometimes cell chains protrude from these structures.
Finally, yet another morphologically simple conidiogenous fungus accompanies the Taeniolella-like colonies and other fungi in Bitterfeld amber specimen GZG.BST.27299, forming clusters of small, round conidia, 2–4 µm in diameter (Fig. 6G).
3. Discussion
The superb preservation of delicate structures, such as upright conidiophores, in the lichen-associated fungi indicates that they were already fully developed on the lichen surfaces when their substrates were engulfed by fresh resin and finally preserved in amber. Although epiphytic lichens were most likely quite common in Paleogene amber forests, particularly foliose and fruticose species were not likely candidates for preservation. Due to their three-dimensional structure, some parts of the thallus were almost invariably left outside the resin, allowing microbial decomposers to also degrade the submerged parts of the lichen. However, several lichen-forming ascomycetes have so far been described from European amber, and recent findings indicate that such fossils are more common than previously thought (Rikkinen & Poinar Reference Rikkinen and Poinar2002; Rikkinen Reference Rikkinen2003b; Schmidt et al. Reference Schmidt, Dörfelt, Grabenhorst, Tuovila, Rikkinen, Rascher, Rappsilber and Wimmer2013; Hartl et al. Reference Hartl, Schmidt, Heinrichs, Seyfullah, Schäfer, Gröhn, Rikkinen and Kaasalainen2015; Kaasalainen et al. Reference Kaasalainen, Heinrichs, Krings, Myllys, Grabenhorst, Rikkinen and Schmidt2015, Reference Kaasalainen, Schmidt and Rikkinen2017).
Saprotrophic filamentous fungi, including species of Cladosporium Link, Reference Link1816, Penicillium Link, Reference Link1809 and Aspergillus Micheli ex Haller, Reference Haller1768, can occasionally grow on extant lichens, especially on dead and decomposing thalli (Hawksworth Reference Hawksworth1979, 1982a; Petrini et al. Reference Petrini, Hake and Dreyfuss1990; Girlanda et al. Reference Girlanda, Isocrono, Bianco and Luppimosca1997). However, saprotrophic fungi are clearly more common and diverse on decomposing plant remains. The relative scarcity of saprotrophic fungi on lichens may reflect the common presence of biologically active, potentially mycotoxic lichen compounds in lichen thalli. It is possible that the fossilised fungi were able to tolerate the presence of such metabolites in their substrates.
All the lichen-associated fungi here described were probably saprophytes or, at most, weak parasites which continued to grow on dead and decomposing lichen thalli (Hawksworth Reference Hawksworth1982a). Their placement on the host thalli was clearly not random, which may indicate a level of substrate specialisation. For example, the conidial clusters of the Sporidesmium-like fungi consistently developed on thallus ridges and other high spots on the substrate (Figs 3A, B, E, 4A, C), which presumably represented favourable spots for the wind dispersal of conidia.
Whilst over 400 species have been described in Sporidesmium, the genus is clearly polyphyletic and consists of several unrelated lineages with convergent morphologies (Shenoy et al. Reference Shenoy, Jeewon, Wu, Bhat and Hyde2006). Only three species have been described from lichens: Sporidesmium bacidiicola Alstrup, Reference Alstrup1991, growing on Bacidia rubella (Hoffmann) Massalongo, Reference Massalongo1852 in Sweden (Alstrup Reference Alstrup1991); S. lichenicola Iturriaga, Hawksworth & Crane, Reference Iturriaga, Hawksworth and Crane2008, growing on a degraded Leptogium (Acharius) Gray, Reference Gray1821 specimen in Venezuela (Iturriaga et al. Reference Iturriaga, Hawksworth and Crane2008); and S. usneae Etayo, Reference Etayo2017, growing on Usnea in Peru (Etayo Reference Etayo2017). In their overall morphology, the fossils more resemble S. lichenicola, but have longer conidia and apical extensions (especially in Bitterfeld specimen B). Sporidesmium lichenicola is either a saprotroph or a parasite that persists on the host lichen after its death (Iturriega et al. Reference Iturriaga, Hawksworth and Crane2008). The fossilised Sporidesmium-like fungi may have had a very similar ecology.
Most extant species of Taeniolella s. l. are saprophytes that grow on decomposing plant material, bark or wood, but the genus also includes lichenicolous species. Hawksworth (Reference Hawksworth1979) described four Taeniolella species from lichens, all of them with 1–3 septate conidia; and the many other known lichenicolous species (Lawrey & Diederich Reference Lawrey and Diederich2016) also produce conidia with only one or a few septa. In a phylogenetic analysis, Ertz et al. (Reference Ertz, Heuchert, Braun, Freebury, Common and Diederich2016) recovered Taeniolella as strongly polyphyletic: the generic type Taeniolella exilis (Karsten) Hughes, Reference Hughes1958 belongs to Kirschsteiniotheliaceae (Dothideomycetes); other saprotrophic species belong to Sordariomycetes; whilst the lichenicolous taxa belong to Asterotexiales (Dothideomycetes). The Taeniolella-like fossils have multiseptate conidia and thus more resemble extant plant saprophytic species such as T. stilbospora (Corda) Hughes, Reference Hughes1958. Whereas secession of conidia in extant Taeniolella is schizolytic (Seifert et al. Reference Seifert, Morgan-Jones, Gams and Kendrick2011), some fossil conidia are rhexolytically ruptured (Figs 4E–G). It is possible, however, that the conidia broke accidentally during embedment in the tree resin.
In addition, some Taeniolina species, such as Taeniolina scripta (Karsten) Kirk, Reference Kirk1981 (formerly Taeniolella scripta (Karsten) Hughes, Reference Hughes1958), are quite similar to the fossils, and the latter species is known to occasionally grow on lichen thalli (Hawksworth Reference Hawksworth1979, Reference Hawksworth2003). The fossils in Bitterfeld amber specimens GZG.BST.27299 and Grabenhorst-Ri-49 are morphologically indistinguishable, but it is possible that the small fungus in Bitterfeld amber specimen Grabenhorst-Le-91 represents a second species. It is noteworthy that all three fossils were found on the surfaces of epiphytic lichens, indicating that these fungi must have been common on lichens in the Bitterfeld amber forest.
In their overall habit, the fungi shown in Fig. 5A–F somewhat resemble modern species of the genera Troposporopsis Whitton, McKenzie & Hyde, Reference Whitton, McKenzie and Hyde1999 and Penzigomyces Subramanian, Reference Subramanian1992. However, species of the former genus grow on plants and have helicoid conidia with distinct areas of light and dark pigmentation (Whitton et al. Reference Whitton, McKenzie and Hyde1999). Extant Penzigomyces species grow on bark, wood and dung and have not been reported from lichens. The genus was established by Subramanian (Reference Subramanian1992) to accommodate Sporidesmium-like fungi with lageniform, doliiform or nodose percurrent proliferations in the conidiophores. The fossil has such proliferations, but the distinctly curved conidia do not correspond with those of Penzigomyces.
Minute fungi, morphologically more or less identical to the small toruloid fungus in several amber specimens (Fig. 6D–F), are exceedingly common on extant lichens and often grow partly immersed into the vegetative thallus or apothecia of their hosts. However, only a few examples of such fungi have been studied in any detail and have usually been placed in the genus Intralichen Hawksworth & Cole, Reference Hawksworth and Cole2002. Thus, it is not possible to assign the minute toruloid fossil fungi to any modern group.
Based on characteristic gradually tapering vegetative hyphae, the sooty moulds preserved on the fossil lichens are assignable to the family Metacapnodiaceae (Capnodiales, Ascomycota). Several fossils of sooty moulds have been found from Paleogene amber, some growing on lichen thalli (Rikkinen et al. Reference Rikkinen, Dörfelt, Schmidt and Wunderlich2003; Schmidt et al. Reference Schmidt, Beimforde, Seyfullah, Wege, Dörfelt, Girard, Grabenhorst, Gube, Heinrichs, Nel, Nel, Perrichot, Reitner and Rikkinen2014). Many extant sooty moulds get their nutrition from insect excretions, especially from the honeydew produced by sap-sucking aphids and scale insects. Modern sooty moulds are also occasionally found on lichens (Braun et al. Reference Braun, Heuchert and Diederich2009). It is quite possible that the fossil lichens also grew on resin-producing trees that also supported sap-feeding insects, with honeydew dripping on the lichen thalli. Thus, we interpret the occurrence of sooty moulds on the fossil lichens as incidental, and not as evidence of an ecological association with lichens.
Bitterfeld and Baltic ambers preserved distinct morphologies of filamentous microfungi from epiphytic lichens, demonstrating that a range of presumably specialised microfungi lived on dead and decomposing lichen thalli. The host lichens most probably grew on resin-producing trees and became embedded in resin flows, together with their fungal associates. These new fossil findings add a previously unknown ecological component to the as yet poorly known mycota of the ancient European amber forests.
4. Acknowledgements
The authors would like to thank Volker Arnold (Heide) for generously donating an amber specimen for this study and Dorothea Hause-Reitner (Göttingen) for assistance with field emission microscopy. We are grateful to the reviewers Uwe Braun (Halle) and George O. Poinar (Corvallis) for their helpful comments on the manuscript. The research was supported by grants from the Otto A. Malm Foundation and Jenny and Antti Wihuri Foundation (grant to EK).









