The Hemiptera Linnaeus, Reference Linnaeus1758 is one of the most successful lineages of insects, with over 300 families recognised during its geological history and high variability of morphology (Shcherbakov & Popov Reference Shcherbakov, Popov, Rasnitsyn and Quicke2002; Szwedo et al. Reference Szwedo2004; Grimaldi & Engel Reference Grimaldi and Engel2005; Szwedo Reference Szwedo2018). The order is divided into six suborders: the extant Sternorrhyncha, Fulgoromorpha, Cicadomorpha, Coleorrhyncha and Heteroptera, and the extinct Paleorrhyncha. The last one currently comprises only one family, Archescytinidae, which needs re-study and reconsideration. The Hemiptera have been known since the Late Carboniferous (Nel et al. Reference Nel, Roques, Nel, Prokin, Bourgoin, Prokop, Szwedo, Azar, Desutter-Grandcolas, Wappler, Garrouste, Coty, Huang, Engel and Kirejtshuk2013) and they are likely to have been exclusively represented in the Palaeozoic by herbivorous taxa. Advanced plant sucking was the primary feeding adaptation in this lineage, which probably originated from ancestral Hypoperlida. The Permian Paleorrhyncha show some characters suggesting that both immatures and adults could have fed on gymnosperm ovules and/or immature seeds in cones, which is in accordance with the postulated plesiomorphic feeding on reproductive plant organs in general. Hemipterans evolved to shift from reproductive organs to photosynthetic tissues. Most of the Hemiptera since the Palaeozoic have been phloem feeders, whereas xylem feeding appeared in the Cicadomorpha in the Early Mesozoic. Mesophyll feeding probably also evolved during the Mesozoic. In the Hemiptera, predation is rare and occurs sporadically in modern Heteroptera; however, true bugs adopted zoophagy at the earliest stages of their evolution in the Triassic (Shcherbakov & Popov Reference Shcherbakov, Popov, Rasnitsyn and Quicke2002; Zherikhin Reference Zherikhin, Rasnitsyn and Quicke2002; Shcherbakov Reference Shcherbakov2008). Sap-feeding Hemiptera present one of the most extraordinary systems of close and mutual relationships containing obligate bacterial symbionts (Moran et al. Reference Moran, Tran and Gerardo2005; Szwedo Reference Szwedo2018).
The Palaeogene record of the Hemiptera is based on both compression fossils and amber inclusions throughout the world (e.g., EDNA 2018; PalaeoBioDB 2018 and references there).
Fossil assemblages from the Palaeocene are often dominated by the representatives of Fulgoroidea or Cercopoidea, while the other groups are not so abundant. Aphidomorpha are sometimes abundant, but Psyllodea are rather rare. Both Aleyrodomorpha and Coccomorpha are not very common in fossil assemblages. The most abundant water bugs are Nepomorpha (mainly Notonectidae and Corixidae), and the most abundant land bugs are Cimicomorpha (Miridae) and Pentatomomorpha (mainly Coreoidea and Pentatomoidea). Dominance of fulgoroids is usually related to warmer climatic conditions whereas a predominance of cercopoids represents a cooler climate. At the beginning of the Palaeogene a rapid evolution of the most specialised plant-sucking lineages of the Hemiptera continued, connected with the diversification of angiosperms and Cenophytic conifer lineages.
The early Cenozoic (Palaeogene) Bembridge Marls of the Isle of Wight is well known. Within it, the Insect Bed (Insect Limestone) occurs only in the northern half of the Isle of Wight. Most information dealing with the palaeontology, lithology and the age was summarised by Jarzembowski (Reference Jarzembowski1980) and more recently by Ross & Self (Reference Ross and Self2014). There has been disagreement about the age of the Insect Bed and on the position of the Eocene/Oligocene boundary. It has been considered to be Late Eocene (late Priabonian) or Early Oligocene (early Rupelian) in age (Jarzembowski Reference Jarzembowski1980; Collinson Reference Collinson, Prothero and Berggren1992; Hooker et al. Reference Hooker, Collinson, Van Bergen, Singer, De Leeuw and Jones1995, Reference Hooker, Collinson and Sille2004, Reference Hooker, Collinson, Grimes, Sille and Mattey2007, Reference Hooker, Grimes, Mattey, Collinson, Sheldon, Koeberl and Montanari2009; Ross & Self Reference Ross and Self2014). Gale et al. (Reference Gale, Huggett, Pälike, Laurie, Hailwood and Hardenbol2006, Reference Gale, Huggett and Laurie2007) investigated magnetostratigraphy, clay mineralogy, cyclostratigraphy and sequence stratigraphy postulating an Early Oligocene age for the Insect Bed. However, Hooker et al. (Reference Hooker, Collinson, Grimes, Sille and Mattey2007, Reference Hooker, Grimes, Mattey, Collinson, Sheldon, Koeberl and Montanari2009) disagreed. Hooker et al. (Reference Hooker, Grimes, Mattey, Collinson, Sheldon, Koeberl and Montanari2009) indicated that the Bembridge Marls were deposited over about 300,000 years, which would date the Insect Bed at about 34.2Ma (±/∼100,000 years) and indicate that the Insect Bed was deposited over about 10,000–15,000 years, i.e., Late Eocene (Ross & Self Reference Ross and Self2014), which is followed here. The Bembridge Marls fauna, with regards to its family composition, differs from other European Eocene faunas: Baltic (including Bitterfeld and Ukrainian) amber, Oise amber and Messel. The Bembridge Marls age can be directly compared with that of the Florissant Formation in the USA, which is radiometrically dated at 34.07 million years old (Meyer Reference Meyer2003).
The Hemiptera from the Bembridge Marls, Isle of Wight, were studied by Cockerell (Reference Cockerell1915, Reference Cockerell1921b, Reference Cockerellc, Reference Cockerell1922, Reference Cockerell1926, Reference Cockerell1927) and Klimaszewski & Popov (Reference Klimaszewski and Popov1993), and resulted in the description of several taxa of psyllids and aphids (Sternorrhyncha), planthoppers (Fulgoromorpha), leafhoppers (Cicadomorpha) and true bugs (Heteroptera).
The Palaeogene and Neogene record of Psyllodea is poor (Becker-Migdisova Reference Becker-Migdisova1985; Grimaldi & Engel Reference Grimaldi and Engel2005; Drohojowska Reference Drohojowska2011; Ouvrard et al. Reference Ouvrard, Burckhardt and Greenwalt2013). In addition to a few species from the Isle of Wight (Late Eocene), a few species are known from Eocene Baltic amber – Palaeopsylloides oligocaenicus (Enderlein, Reference Enderlein1915), Eogyropsylla eocenica Klimaszewski, Reference Klimaszewski1993b, E. jantaria Klimaszewski, Reference Klimaszewski1993b, Protoscena baltica Klimaszewski, Reference Klimaszewski1997b, Eogyropsylla magna Klimaszewski, Reference Klimaszewski1997c, E. parva Klimaszewski, Reference Klimaszewski1997c, Parascenia weitschati Klimaszewski, Reference Klimaszewski1997c (Enderlein Reference Enderlein1915; Klimaszewski Reference Klimaszewski1993b, Reference Klimaszewski1997b, Reference Klimaszewskic), Eogyropsylla sedzimiri Drohojowska, Reference Drohojowska2011 and E. paveloctogenarius Ouvrard et al., Reference Ouvrard, Burckhardt and Greenwalt2013 from the Middle Eocene Kishenehn Formation, Montana, USA, and a few from the terminal Eocene Florissant Formation in the USA (Scudder Reference Scudder1890). Representatives of the superfamily Psylloidea became more numerous in the fossil record from the Miocene, known from Dominican and Mexican ambers (Klimaszewski Reference Klimaszewski1993a, Reference Klimaszewski1996, Reference Klimaszewski1997a; Drohojowska et al. Reference Drohojowska, Wegierek and Solorzano-Kraemer2016).
Aleyrodomorpha are recorded in several Palaeogene ambers: ‘Aleurodes' aculeatus Menge, Reference Menge1856, Paernis gregorius Drohojowska & Szwedo, Reference Drohojowska and Szwedo2011b, Rovnodicus wojciechowskii Drohojowska & Szwedo, Reference Drohojowska, Perkovsky and Szwedo2015 in Drohojowska et al. Reference Drohojowska, Perkovsky and Szwedo2015 and Snotra christelae Szwedo & Drohojowska, Reference Szwedo and Drohojowska2016 from Baltic amber (Szwedo & Drohojowska Reference Szwedo and Drohojowska2016) and few additional specimens under survey; several taxa from the lowermost Eocene amber of Oise (Drohojowska & Szwedo Reference Drohojowska and Szwedo2013a) must be noted. Aleyrodomorpha as compression fossils from the Bembridge Marls were reported by Jarzembowski & Ross (Reference Jarzembowski and Ross1994). A few species were recorded from both compression fossils and amber from the Late Jurassic and Cretaceous (Schlee Reference Schlee1970; Shcherbakov Reference Shcherbakov2000a; Drohojowska & Szwedo Reference Drohojowska and Szwedo2011a, Reference Drohojowska, Szwedo, Azar, Engel, Jarzembowski, Krogmann, Nel and Santiago-Blay2013b, Reference Drohojowska and Szwedo2015), but this group remains poorly studied.
The scarcity of scale insect (Coccidomorpha) fossils is still a puzzle, but numbers of species representing both archeococcids and neococcids are known from fossil resins (Koteja Reference Koteja2000a, Reference Kotejab, Reference Koteja2001, Reference Koteja2008; Koteja & Azar Reference Koteja and Azar2008; Vea & Grimaldi Reference Vea and Grimaldi2012, Reference Vea and Grimaldi2015; Simon & Żyła Reference Simon and Żyła2015; Wang et al. Reference Wang, Xia, Wappler, Simon, Zhang, Jarzembowski and Szwedo2015). Compression fossils of scale insects are documented from the Lower Cretaceous of Transbaikalia (Koteja Reference Koteja1988, Reference Koteja1989) and England (Koteja Reference Koteja1999) – representing archeococcids (Matsucoccidae and Xylococcidae), Oligocene of North America (Scudder Reference Scudder1890), Miocene of Sicily (Pampaloni Reference Pampaloni1902, 1903; Koteja & Ben-Dov Reference Koteja and Ben-Dov2003), Eocene of Germany (Wappler & Ben-Dov Reference Wappler and Ben-Dov2008) and Miocene of Germany (Zeuner Reference Zeuner1938; Koteja Reference Koteja2000b) – representing neococcids (Diaspididae) – and the Miocene of Darjeeling in India (Bera et al. Reference Bera, Mitra, Banerjee and Szwedo2006).
Cenozoic fossil aphids (Aphidomorpha) have been studied in varying degrees. So far the largest number of species has been described from Baltic amber (about 100 species; Heie & Wegierek Reference Heie and Wegierek1998, Reference Heie and Wegierek2011). Late Eocene/Oligocene aphids (24 species) are known from several deposits around the world. Most of the compression fossils were described in the 18th and 19th Centuries: from Aix-en-Provence, France (Hope Reference Hope1847; Heer Reference Heer1856; Théobald Reference Théobald1937); Florissant, Colorado, USA (Scudder Reference Scudder1890; Cockerell Reference Cockerell1908b, Reference Cockerell1909, Reference Cockerell1913); Quesnel, British Columbia, Canada (Scudder Reference Scudder1890, Reference Scudder1894) and the Isle of Wight, UK (Cockerell Reference Cockerell1915, Reference Cockerell1921b). Some were later revised by Heie (Reference Heie1967, Reference Heie1970). Only specimens from East Siberia, Russia (Bolshaya Svetlovodnaya) and France (Céreste, Alpes de Haute Provence) were described much later (Heie Reference Heie1989; Heie & Lutz Reference Heie and Lutz2002). Where present, aphids are also numerous in Miocene insect deposits (20 species; Heie Reference Heie2005). Data on aphids from the more recent epochs (Pliocene and Pleistocene) are based on single species (Heie Reference Heie1968, Reference Heie1995).
The Fulgoromorpha is one of the most ancient lineages of the Hemiptera, and in the fossil record planthoppers have been known since the early Permian. The earliest Fulgoromorpha belong to the Permian superfamily Coleoscytoidea, the second taxon is the Permian–Triassic Surijokocixioidea; the Fulgoroidea have been known since the Jurassic. The Palaeogene record of Fulgoromorpha comprises both compression fossils and forms preserved in resins (ambers). These are present in the Palaeocene/Eocene Fur Formation of Denmark, Palaeocene/Eocene deposits of Menat in France, lowermost Eocene French amber, uppermost Palaeocene of Argentina, numerous specimens are known from Eocene Baltic amber, Eocene deposits of Germany, Eocene and Oligocene deposits of North America and China, and Miocene Dominican and Mexican ambers (Szwedo et al. Reference Szwedo2004; Petrulevičius Reference Petrulevičius2005; Szwedo Reference Szwedo2005a, Reference Szwedo2006a, Reference Szwedob, Reference Szwedo2007, Reference Szwedo2008, Reference Szwedo2011; Shcherbakov Reference Shcherbakov2006; Szwedo et al. Reference Szwedo, Bourgoin and Lefebvre2006; Szwedo & Wappler Reference Szwedo and Wappler2006; Stroiński & Szwedo Reference Stroiński and Szwedo2008, Reference Stroiński and Szwedo2011, Reference Stroiński and Szwedo2012; Emeljanov & Shcherbakov Reference Emeljanov and Shcherbakov2009; Lin et al. Reference Lin, Szwedo, Huang and Stroiński2010; Szwedo & Stroiński Reference Szwedo and Stroiński2010, Reference Szwedo and Stroiński2013, Reference Szwedo and Stroiński2017; Szwedo et al. Reference Szwedo, Stroiński and Lin2013, Reference Szwedo, Stroiński and Lin2015). Several fossil Fulgoroidea have been reported so far from the Late Eocene Bembridge Marls of the Isle of Wight. The first descriptions were by Cockerell (Reference Cockerell1921b), who described Poekilloptera melanospila Cockerell, Reference Cockerell1921b (transferred to Orthoptera by Nel et al. Reference Nel, Prokop and Ross2008; see Fulgoromorpha section). Later, more species were added under the names Hastites muiri Cockerell, Reference Cockerell1922, Hooleya indecisa Cockerell, Reference Cockerell1922 and Myndus wilmattae Cockerell, Reference Cockerell1926. These taxa are discussed below.
The Cicadomorpha is the second suborder formerly placed together with Fulgoromorpha as ‘Auchenorrhyncha', but not related directly to planthoppers (Bourgoin & Campbell Reference Bourgoin and Campbell2002; Szwedo Reference Szwedo2002; Szwedo et al. Reference Szwedo, Bourgoin and Lefebvre2004). The Palaeogene record of Cicadomorpha is also rich and known throughout the world (Metcalf & Wade Reference Metcalf and Wade1966; Lewis Reference Lewis1989; Szwedo Reference Szwedo2005b), but many taxa require re-examination and revision (Gębicki & Szwedo Reference Gębicki and Szwedo2006). Only a few species of Cicadidae and Tettigarctidae are known from the Palaeogene of France, Scotland and North America (Boulard & Nel Reference Boulard and Nel1990; Shcherbakov Reference Shcherbakov2009; Moulds Reference Moulds2018); most of the known fossil cicadas are from the Miocene of Eurasia. Cercopoidea are quite common in Palaeogene deposits of North America, Greenland and Europe as well as in amber but most must be re-studied and their taxonomic status revised and/or confirmed. Membracoidea, Cicadellidae in particular, are frequently reported, but only a few species from the Palaeogene have been formally described (Szwedo Reference Szwedo2002; Szwedo Reference Szwedo2005b; Gębicki & Szwedo Reference Gębicki and Szwedo2006; Szwedo & Gębicki Reference Szwedo2008; Szwedo et al. Reference Szwedo, Gębicki and Kowalewska2010; Dietrich & Gonçalves Reference Dietrich and Gonçalves2014). Over 220 specimens from the Insect Limestone of the Isle of Wight, deposited in the Natural History Museum (NHM) in London, Maidstone Museum and Sedgwick Museum, Cambridge, representing Fulgoromorpha and Cicadomorpha were investigated.
The first true bugs from the Bembridge Marls were described by Cockerell (Reference Cockerell1921c, Reference Cockerell1927). He referred them to the Tingidae (Celantia? seposita Cockerell, Reference Cockerell1921c), Lygaeidae (Lygaeites amabilis Cockerell, Reference Cockerell1921b) and Pentatomidae – Pentatomites acourti (Cockerell, Reference Cockerell1921c). The last one was previously considered to be a lygaeid (Cockerell Reference Cockerell1921c). A list of families of Hemiptera recorded from the Late Eocene Insect Limestone of the Isle of Wight is presented in Table 1.
Table 1 Families of the Hemiptera recorded from the Late Eocene Insect Limestone of the Isle of Wight.

1 Including specimens recognised as representing separate species, but not formally described.
The insects are preserved in concretions or tabular bands of very fine-grained micrite, known as Insect Limestone. The unit where these concretions/bands occur is known as the Insect Bed, which lies towards the base of the Bembridge Marls Member (Solent Group: Bouldnor Formation). The most extensive collection from the Insect Limestone are specimens preserved at the NHM. They belong to the collections of E.J. A'Court Smith (purchased 1877 and 1883), Reverend P. B. Brodie (purchased 1898) and R. W. Hooley (purchased 1924). They are labelled ‘Gurnard Bay' or ‘Gurnet Bay' (which is an old name for Gurnard Bay); however, A'Court Smith collected specimens all the way from West Cowes to Newtown River on the NW side of the Isle of Wight (Jarzembowski Reference Jarzembowski1980; Ross & Self Reference Ross and Self2014). Most of the specimens probably came from Thorness Bay (Jarzembowski Reference Jarzembowski1976). Brodie and Hooley acquired parts of Smith's collection, so parts and counterparts of individual insects have turned up in all three collections. The parts and counterparts often have different numbers because they were registered at different times. An additional collection was discovered at the Sedgwick Museum, Cambridge, by A. J. Ross. This collection has also yielded counterparts of specimens at the NHM, which indicates that this is another part of the Smith collection. A label with ‘1883' on it suggests that the Sedgwick Museum acquired this collection in 1883, the same year that the NHM purchased specimens from Smith.
The following collections have been examined and contained the Hemiptera in their care:
BMB – Booth Museum of Natural History, Brighton
CAMSM – Sedgwick Museum of Earth Sciences, University of Cambridge.
MIWG – Museum of Isle of Wight Geology.
NHMUK – Department of Earth Sciences, Natural History Museum, London.
USNM – Department of Paleobiology, National Museum of Natural History, Smithsonian Institution, Washington, DC, USA.
MNEMG – Maidstone Museum & Bentlif Art Gallery.
With the financial support of Project INTAS 03-51-4367, concerning the fauna and flora of the Isle of Wight, formerly described species were revised and additional material was examined resulting in new taxa that are described herein.
1. Systematic palaeontology
Order Hemiptera Linnaeus, Reference Linnaeus1758
Jumping plant-lice (Hemiptera: Sternorrhyncha: Psylloidea)
by Jowita Drohojowska
The Eocene witnessed the emergence of the first representatives of the superfamily Psylloidea, commonly known as jumping plant-lice. The psyllid fauna of this epoch is known largely from Baltic amber (Enderlein Reference Enderlein1915; Klimaszewski Reference Klimaszewski1993a, Reference Klimaszewski1997a, Reference Klimaszewskib; Drohojowska Reference Drohojowska2011; Ouvrard et al. Reference Ouvrard, Burckhardt and Greenwalt2013). Late Eocene fossils from the Bembridge Marls of the Isle of Wight were studied by Cockerell (Reference Cockerell1915, Reference Cockerell1921b) and Klimaszewski in Klimaszewski & Popov (Reference Klimaszewski and Popov1993). The record was not exceptionally rich, with all the species of this age classified in one family, the Aphalaridae, and even in one subfamily, the Aphalarinae. No new taxa are described here; however, there have been some taxonomic changes since Klimaszewski & Popov (Reference Klimaszewski and Popov1993) was published so the fauna is summarised below. All the specimen numbers in Klimaszewski & Popov (Reference Klimaszewski and Popov1993) are incorrect – they used field numbers, not registration numbers. Paratypes are from the same locality as the holotype unless stated otherwise.
In Early Miocene Dominican amber (Klimaszewski Reference Klimaszewski1996, Reference Klimaszewski1997c), species appear that belong to several other families. Apart from the Aphalaridae, there are also species of Psyllidae, Carsidaridae and Triozidae (Becker-Migdisova Reference Becker-Migdisova1964; Klimaszewski Reference Klimaszewski1993a). In the Miocene Mexican amber the first fossil psyllid from family Liviidae was described (Drohojowska et al. Reference Drohojowska, Wegierek and Solorzano-Kraemer2016). In the Paleogene the family Aphalaridae dominated, then in the Neogene the family Psyllidae outnumbered other forms. This has continued to the present day, with the domination of Triozidae (over 1000 described species) and Psyllidae (nearly 1200 species) (Ouvrard Reference Ouvrard2019).
Suborder Sternorrhyncha Amyot & Audinet-Serville, Reference Amyot and Audinet-Serville1843
Infraorder Psyllodea Flor, Reference Flor1861
Superfamily Psylloidea Latreille, Reference Latreille1807
Key to the genera of jumping plant-lice from the Bembridge Marls
1. Cells m1 and cu1 large, vein M1+2 on the forewing the same length or longer than vein M Carsidarina
– Cells m1 and cu1 small, Vein M1+2 on the forewing visibly shorter than vein M 2
2. Cell cu1 relatively high, approximately as long as cell m1 Lapidopsylla
– Cell cu1 long and flat, distinctly longer than cell m1 3
3. Vein Cu1a very long and almost straight, cell cu1 almost flat (much longer than high) Paleopsylloides
– Vein Cu1a arcuate, cell cu1 not flat (much longer than high) Proeurotica
Family Aphalaridae Löw, Reference Löw1879
Subfamily Aphalarinae Löw, Reference Löw1879
Tribe Paleopsylloidini Becker-Migdisova, Reference Becker-Migdisova1985
Genus Proeurotica Becker-Migdisova, Reference Becker-Migdisova1985
Type species. Psylla exhumata Cockerell, Reference Cockerell1915; by original designation.
Plesioaphalara Klimaszewski in Klimaszewski & Popov, Reference Klimaszewski and Popov1993
Type species. Plesioaphalara arcana Klimaszewski in Klimaszewski & Popov, Reference Klimaszewski and Popov1993; by original designation.
Diagnosis (after Becker-Migdisova Reference Becker-Migdisova1985 and Klimaszewski & Popov Reference Klimaszewski and Popov1993). Length/width coefficient of forewing 2.3:1. Stem R+M short, slightly longer (1.1–1.2 times) than M+CuA and CuA. Rs long, straight, slightly curved at apex anteriad; stem R half of M+CuA stem length, 1.8 times shorter than stem CuA. Branches of M short, distinctly shorter than stem M. Cell cu1 with length/width coefficient 3.1–4.5.
Description. Forewing elongated, with long, narrow cell cu1. Stem R+M+CuA short, subequal to stem R. Cell m shorter than stem M.
Remark. The genus Plesioaphalara was synonymised under Proeurotica by Ouvrard et al. (Reference Ouvrard, Burckhardt and Greenwalt2013). Proeurotica exhumata (type species) differs from all other species recently moved by Ouvrard et al. (Reference Ouvrard, Burckhardt and Greenwalt2013) to the genus Proeurotica by the lack of a pterostigma. However, better preserved material is necessary to confirm or reject this situation.
Proeurotica exhumata (Cockerell, Reference Cockerell1915)

Plate 1 (1) Proeurotica exhumata (Cockerell, Reference Cockerell1915), holotype, USNM No. 61427. (2) Proeurotica arcana (Klimaszewski in Klimaszewski & Popov, Reference Klimaszewski and Popov1993) holotype, BMB 018433, forewing. (3) Proeurotica paulula (Klimaszewski in Klimaszewski & Popov, Reference Klimaszewski and Popov1993) holotype, BMB 018440, forewing. (4) Proeurotica inanima (Klimaszewski in Klimaszewski & Popov, Reference Klimaszewski and Popov1993) holotype, BMB 018441, forewing. (5) Lapidopsylla thornessbaya Klimaszewski in Klimaszewski & Popov, Reference Klimaszewski and Popov1993 holotype, BMB 018443, forewing. (6) Lapidopsylla memoranda Klimaszewski in Klimaszewski & Popov, Reference Klimaszewski and Popov1993 holotype, BMB 018444, forewing. (7) Carsidarina hooleyi (Cockerell, Reference Cockerell1921) holotype, NHMUK In. 24358a, part, forewing. (8) Carsidarina jarzembowskii (Klimaszewski in Klimaszewski & Popov, Reference Klimaszewski and Popov1993) holotype, BMB 018424-5, part, forewing. Scale bar=1mm.

Figures 1–8 Forewing. (1) Proeurotica exhumata (Cockerell, Reference Cockerell1915), holotype, USNM No. 61427. (2) Proeurotica arcana (Klimaszewski in Klimaszewski & Popov, Reference Klimaszewski and Popov1993) holotype, BMB 018433. (3) Proeurotica paulula (Klimaszewski in Klimaszewski & Popov, Reference Klimaszewski and Popov1993) holotype, BMB 018440. (4) Proeurotica inanima Klimaszewski in Klimaszewski & Popov, Reference Klimaszewski and Popov1993 holotype, BMB 018441. (5) Lapidopsylla thornessbaya Klimaszewski in Klimaszewski & Popov, Reference Klimaszewski and Popov1993 holotype, BMB 018443. (6) Lapidopsylla memoranda Klimaszewski in Klimaszewski & Popov, Reference Klimaszewski and Popov1993 holotype, BMB 018444. (7) Carsidarina hooleyi (Cockerell, Reference Cockerell1921c) holotype, NHMUK In.24358a, part. (8) Carsidarina jarzembowskii (Klimaszewski in Klimaszewski & Popov, Reference Klimaszewski and Popov1993) holotype, BMB 018424, part. Scale bar=1mm.
1915 Psylla exhumata Cockerell, p. 487, pl. 63, fig. 6.
1985 Proeurotica eshumata [sic]: Becker-Migdisova, p. 82.
1985 Proeurotica exhumata: Becker-Migdisova, p. 83, fig. 63.
1993b Paleopsylloides exhumatus: Klimaszewski, p. 10.
2013 Proeurotica exhumata Cockerell, Reference Cockerell1915: Ouvrard et al., p. 24.
Holotype. USNM No. 61427, Lacoe Collection 7619, Insect Limestone, NW Isle of Wight.
Remarks. Klimaszewski (Reference Klimaszewski1993b) transferred Proeurotica exhumata (Cockerell Reference Cockerell1915) to the genus Paleopsylloides Becker-Migdisova, Reference Becker-Migdisova1985 on the basis of misinterpreted characters.
Proeurotica arcana (Klimaszewski in Klimaszewski & Popov, Reference Klimaszewski and Popov1993) comb. nov.
1993 Plesioaphalara arcana Klimaszewski in Klimaszewski & Popov, pp. 19–20; fig. 2b; pl. 2, figs 1–3.
2013 Plesioaphalara arcana Klimaszewski, Reference Klimaszewski and Popov1993: Ouvrard et al., p. 24.
Holotype. BMB 018433 (BLS 1423); Insect Limestone, Thorness Bay, collected by A. A. Mitchell.
Paratypes. BMB 018434 (BLS 603), 018435 (BLS 1112), 018436 (BL 1319), 018437 (BL 131), 018438 (BL 145), all collected by A. A. Mitchell; 018439 (IL 61), collected by M. J. Warren.
Proeurotica paulula (Klimaszewski in Klimaszewski & Popov, Reference Klimaszewski and Popov1993) comb. nov.
1993 Plesioaphalara paulula Klimaszewski in Klimaszewski & Popov, pp. 20–21; fig. 3a; pl. 2, fig. 4.
2013 Plesioaphalara paulula Klimaszewski, Reference Klimaszewski1993a: Ouvrard et al., p. 24.
Holotype. BMB 018440 (BLS 723–32); Insect Limestone, Thorness Bay, collected by A. A. Mitchell.
Proeurotica inanima (Klimaszewski in Klimaszewski & Popov, Reference Klimaszewski and Popov1993) comb. nov.
1993 Plesioaphalara inanima Klimaszewski in Klimaszewski & Popov, p. 21; fig. 3b; pl. 2, figs. 5, 6.
2013 Plesioaphalara inanima Klimaszewski, Reference Klimaszewski and Popov1993: Ouvrard et al., p. 24.
Holotype. BMB 018441 (BLS 381); Insect Limestone, Thorness Bay, collected by A. A. Mitchell.
Paratype. BMB 018442 (BLS 978), Mitchell Collection
Key to the species of Proeurotica Becker-Migdisova
1. Costal margin of forewing straight, vein Rs subparallel to anterior margin of forewing exhumata
– Costal margin of forewing arcuate, vein Rs not subparallel to anterior margin of forewing 2
2. Length of forewing up to 1mm, width 0.45mm, very short and high cell m1 paulula
– Length of forewing more than 1mm, width more 0.5mm, cell m1 long 3
3. Length of forewing more 2mm, vein Cu1 longer than vein M+Cu1 inanima
– Length of forewing up to 2mm, vein Cu1 always shorter than vein M+Cu1 arcana
Genus Lapidopsylla Klimaszewski in Klimaszewski & Popov, Reference Klimaszewski and Popov1993
Type species. Lapidopsylla thornessbaya Klimaszewski in Klimaszewski & Popov, Reference Klimaszewski and Popov1993; by original designation.
1993 Lapidopsylla Klimaszewski: Klimaszewski & Popov, p. 21.
Lapidopsylla thornessbaya Klimaszewski in Klimaszewski & Popov, Reference Klimaszewski and Popov1993
1993 Lapidopsylla thornessbaya Klimaszewski in Klimaszewski & Popov, Reference Klimaszewski and Popov1993, p. 22; fig. 3c; pl. 2, fig. 7.
2013 Lapidopsylla thornessbaya Klimaszewski, Reference Klimaszewski1993a, Reference Klimaszewski1993b: Ouvrard et al., p. 24.
Holotype. BMB 018443 (BLS 850-1); Insect Limestone, Thorness Bay, collected by A. A. Mitchell.
Lapidopsylla memoranda Klimaszewski in Klimaszewski & Popov, Reference Klimaszewski and Popov1993
1993 Lapidopsylla memoranda Klimaszewski in Klimaszewski & Popov, Reference Klimaszewski and Popov1993, pp. 22–23; fig. 3d; pl. 2, fig. 8.
2013 Lapidopsylla memoranda Klimaszewski, Reference Klimaszewski and Popov1993: Ouvrard et al., p. 24.
Holotype. BMB 018444 (BLG 203); Insect Limestone, Thorness Bay, collected by A. A. Mitchell.
Key to the species of Lapidopsylla Klimaszewski
1. Length of forewing more than 2mm, vein M long, about twice as long as vein M1+2, vein Rs visibly, arched towards forewing margin memoranda
– Length of forewing about 1.5mm, vein M short, 1 5 times as long as vein M1+2, vein Rs almost straight thornessbaya
Genus Carsidarina Becker-Migdisova, Reference Becker-Migdisova1985
Type species. Livilla hooleyi Cockerell, Reference Cockerell1921c; by original designation.
Remarks. Ouvrard et al. (Reference Ouvrard, Burckhardt and Greenwalt2013, p. 31) synonymised genus Palaeoaphalara Klimaszewski, Reference Klimaszewski1993b with Carsidarina Becker-Migdisova, Reference Becker-Migdisova1985, thus three species described by Klimaszewski (in Klimaszewski & Popov Reference Klimaszewski and Popov1993) should be moved to Carsidarina. The genus Carsidarina Becker-Migdisova, Reference Becker-Migdisova1985 was moved from Carsidaridae to the tribe Palaeopsylloidini Becker-Migdisova, Reference Becker-Migdisova1985 of Aphalaridae: Aphalarinae (Ouvrard et al. Reference Ouvrard, Burckhardt and Greenwalt2013).
Carsidarina hooleyi (Cockerell, Reference Cockerell1921)
1921 Livilla hooleyi Cockerell, Reference Cockerell1921c, p. 476, fig. 44.
1985 Carsidarina hooleyi: Becker-Migdisova, p. 86, fig. 66.
1992 Livilla: Carpenter, p. 253.
2013 Carsidarina hooleyi Cockerell, Reference Cockerell1921 [sic]: Ouvrard et al., p. 24.
Holotype. NHMUK In. 24358a, b (H. 430/H.445) (part and counterpart) Hooley Collection, Insect Limestone, NW Isle of Wight.
Paratype. NHMUK In. 24359 (H. 449). Hooley Collection.
Carsidarina jarzembowskii (Klimaszewski in Klimaszewski & Popov, Reference Klimaszewski and Popov1993) comb. nov.
1993 Palaeoaphalara jarzembowskii Klimaszewski in Klimaszewski & Popov, pp. 16–17; fig. 1a–c; pl. 1, figs 1–4.
1996 Palaeoaphalara jarzembowski [sic!]: Klimaszewski, p. 25.
2013 Palaeoaphalara jarzembowskii Klimaszewski, Reference Klimaszewski and Popov1993: Ouvrard et al., p. 24.
Holotype. BMB 018424-5 (IL 67 a, b) (part and counterpart); Insect Limestone, Thorness Bay, collected by A. J. Ross.
Carsidarina ampla (Klimaszewski in Klimaszewski & Popov, Reference Klimaszewski and Popov1993) comb. nov.

Plate 2 (1) Carsidarina ampla (Klimaszewski in Klimaszewski & Popov, Reference Klimaszewski and Popov1993) holotype, BMB 018431, forewing. (2) Carsidarina media (Klimaszewski in Klimaszewski & Popov, Reference Klimaszewski and Popov1993) holotype, BMB 018426, forewing. (3) Paleopsylloides? anglica (Cockerell, Reference Cockerell1915), holotype, USNM No. 61426, forewing (arrowed), with the holotype of the ant Emplastus hypolithus (Cockerell, Reference Cockerell1915). (4) Aleyrodidae indet., MNEMG IB, SEM photo, puparium. (5–6) Aleyrodidae (?) indet., NHMUK II.2986a, b, puparium (?).

Figures 9–13 (9–11) Forewing: (9) Carsidarina ampla (Klimaszewski in Klimaszewski & Popov, Reference Klimaszewski and Popov1993) holotype, BMB 018431, forewing; (10) Carsidarina media (Klimaszewski in Klimaszewski & Popov, Reference Klimaszewski and Popov1993) holotype, BMB 018426; (11) Paleopsylloides? anglica (Cockerell, Reference Cockerell1915), holotype, USNM No.61426. Scale bar=1mm. (12) Schizoneurites brevirostris Cockerell, Reference Cockerell1915, holotype, counterpart NHMUK I.9850: (A) general view; (B) right antenna. Scale bar=0.05mm. (13) Hormaphis? longistigma Wegierek sp. nov., holotype, NHMUK I.9595: (A) general view. Scale bar=0.05mm; (B) part of antennal segment. Scale bar=0.02mm.
1993 Palaeoaphalara ampla Klimaszewski in Klimaszewski & Popov, pp. 17–18; fig. 2a; pl. 1, fig. 7.
2013 Palaeoaphalara ampla Klimaszewski, Reference Klimaszewski and Popov1993: Ouvrard et al., p. 24.
Holotype. BMB 018431 (BL 64); Insect Limestone, Thorness Bay, collected by A. A. Mitchell.
Carsidarina media (Klimaszewski in Klimaszewski & Popov, Reference Klimaszewski and Popov1993) comb. nov.
1993 Palaeoaphalara media Klimaszewski in Klimaszewski & Popov, Reference Klimaszewski and Popov1993, p. 17; fig. 1d; pl. 1, figs. 5, 6.
2013 Palaeoaphalara media Klimaszewski, Reference Klimaszewski and Popov1993: Ouvrard et al., p. 24.
Holotype. BMB 018426-7 (IL 62a, b) (part and counterpart); Insect Limestone, Thorness Bay, collected by A. J. Ross.
Paratypes. BMB 018428-9 (IL 6), collected by T. B. E. Jarzembowski; BMB 018430 (BL 50), collected by A. A. Mitchell.
Key to the species of Carsidarina Becker-Migdisova
1. Vein Rs straight, vein M in distal portion concave hooleyi
– Vein Rs gently curved, vein M in distal portion not concave 2
2. Cell m1 is shorter than cell cu1 jarzembowskii
– Cell m1 longer or the same length as cell cu1 3
3. Length of forewing more than 3mm, width about 1.4mm, vein M+Cu1 1.47 times as long as vein Cu1 ampla
– Length of forewing no more than 2.7mm, width no more than 1.15mm, vein M+Cu1 1.8–2.0 times as long as vein Cu1 media
Subfamily Aphalarinae Löw, Reference Löw1879
Tribe Aphalarini Löw, Reference Löw1879
Genus Paleopsylloides Becker-Migdisova, Reference Becker-Migdisova1985
Type species. Strophingia oligocaenica Enderlein, Reference Enderlein1915; by original designation by Becker-Migdisova, Reference Becker-Migdisova1985, p. 82.
Paleopsylloides? anglica (Cockerell, Reference Cockerell1915)
1915 Necropsylla anglica Cockerell, p. 487, pl. 63, fig. 5.
1985 Camaratoscena? anglica: Becker-Migdisova, p. 81, pl. 61.
1993b Paleopsylloides? anglica?: Klimaszewski, p. 11.
1993b Necropsylla angelica [sic]: Klimaszewski, p. 21.
2013 Camaratoscena? anglica (Cockerell, Reference Cockerell1915): Ouvrard et al., p. 24.
Holotype. USNM No. 61426, Lacoe Collection 7671. Next to the holotype ant (Formicidae) wing of Emplastus hypolithus (Cockerell Reference Cockerell1915), USNM No. 61411. Insect Limestone, NW Isle of Wight.
Remarks. This species has been described on the basis of a fragment of a forewing. The preserved part comprises only veins M1 and Cu1, the distal part of veins M+Cu1 and cells M1 and Cu1. Klimaszewski (Klimaszewski & Popov Reference Klimaszewski and Popov1993) suggested that this species should be transferred to the monotypic genus Paleopsylloides, distinguished by Becker-Migdisova (Reference Becker-Migdisova1985). Despite the paucity of data offered by the forewing, this suggestion seems plausible. The use of a synonymous name – Necropsylla angelica [sic] – in Klimaszewski (Reference Klimaszewski1993b) seems to have been a mere oversight.
Whiteflies (Hemiptera: Sternorrhyncha: Aleyrodidae)
by Jacek Szwedo and Jowita Drohojowska
The oldest known whiteflies so far are of the Upper Jurassic of Kazakhstan (Shcherbakov Reference Shcherbakov2000a); some others are recorded from the Early Cretaceous of England, Early Cretaceous Lebanese amber, Mid-Cretaceous Burmese amber Early Eocene Oise amber and Middle Eocene Baltic amber (Schlee Reference Schlee1970; Shcherbakov Reference Shcherbakov2000a; Azar Reference Azar2007; Drohojowska & Szwedo Reference Drohojowska and Szwedo2011a, Reference Drohojowska and Szwedob, Reference Drohojowska and Szwedo2013a, Reference Drohojowska, Szwedo, Azar, Engel, Jarzembowski, Krogmann, Nel and Santiago-Blayb, Reference Drohojowska and Szwedo2015; Drohojowska et al. Reference Drohojowska, Perkovsky and Szwedo2015; Szwedo & Drohojowska Reference Szwedo and Drohojowska2016), Middle Eocene Geiseltal fossil Lagerstätte (Weigelt Reference Weigelt1940). Whiteflies are also recorded in Miocene Mexican and Dominican ambers (Poinar Reference Poinar1992; Wu Reference Wu1996), and Miocene Ethiopian amber (Schmidt et al. Reference Schmidt, Perrichot, Svojtka, Anderson, Belete, Bussert, Dörfelt, Jancke, Mohr, Mohrmann, Nascimbene, Nel, Nel, Ragazzi, Roghi, Saupe, Schmidt, Schneider, Selden and Vávra2010) and Pliocene of Germany (Rietschel Reference Rietschel1983).
Suborder Sternorrhyncha Amyot & Audinet-Serville, Reference Amyot and Audinet-Serville1843
Infraorder Aleyrodomorpha Chou, Reference Chou and Cockerell1963
Superfamily Aleyrodoidea Westwood, Reference Westwood1840
Family Aleyrodidae Westwood, Reference Westwood1840
Aleyrodidae gen. and sp. indet.
1993 ‘aleyrodoid': Jarzembowski & Ross, p. 218, fig. 2.
2000a ‘pupal case of Aleyrodoidea': Shcherbakov, p. 35.
Material. Specimen No. MNEMG IB, collected by A. A. Mitchell. Insect Limestone, NW Isle of Wight.
Description. Fossil of pupal case, dorsal view, 0.9mm long, 0.65mm wide. Margin smooth, thoracic tracheal pore not differentiaded from the margin. Submarginal area wide, with distinct submarginal lines. Cephalothoracic suture absent. Longitudinal moulting suture not reaching margin; transverse moulting suture not reaching margin of pupal case, slightly curved anteriad, but lateral portions not distinctly bent, but gently curved at wide angle. Abdomen with intersegmental sutures distinct, not extending into subdorsum. Abdominal rhachis absent. Lateral portions of abdominal segments with eminences (subdorsal pores?, wax pores?). Vasiform orifice triangular. Operculum rounded, lingula spatulate.
Remarks. A SEM (scanning electron microscope) photograph of this specimen was figured by Jarzembowski & Ross (Reference Jarzembowski and Ross1994, fig. 2). The subfamilial and tribal placement of the specimen needs further studies. A few additional specimens, with numbers, MNEMG 2018.6.719; MNEMG 2018.6.773; MNEMG 2018.6.1478; MNEMG 2018.6.2717; MNEMG 2018.6.3060 (field numbers 719, 773, 1478, 2717 and 3060 respectively, collected by Tony Mitchell) preliminarily identified as Aleyrodidae are stored in the Maidstone Museum. One specimen was found in the collection of the NHM, London, NHMUK II.2986a, b (Pl. 2: 5–6), but only provisionally ascribed to Aleyrodidae.
The Aleyrodomorpha is a group with great taxonomic difficulties. In the Aleyrodomorpha, the taxonomy of recent forms is based on the last pre-adult instar, the so called ‘puparium', but that of the fossil forms, on winged adults (Gill Reference Gill and Gerling1990; Shcherbakov Reference Shcherbakov2000a; Martin Reference Martin2003). The Aleyrodidae comprises a single family including around 1550 currently valid species and subspecies names (Martin & Mound Reference Martin and Mound2007; Ouvrard & Martin Reference Ouvrard2019). Relationships between Aleyrodidae and their host plants are still unclear, and this problem was addressed by Manzari & Quicke (Reference Manzari and Quicke2006) and Dubey & Ko (Reference Dubey and Ko2006). Most studies on whitefly biology deal with plants of economic importance (Lenteren & Noldus Reference van Lenteren, Noldus and Gerling1990) and the lack of reliable behaviour and ecology data hampers the understanding of evolutionary patterns in this group. Few whitefly species are known as monophagous, most being oligo- or polyphagous. According to Mound & Halsey (Reference Mound and Halsey1978) the majority of aleyrodids are recorded only from dicotyledonous angiosperms and a smaller, but significant, number feed on monocots, particularly grasses and palms. Few present-day whiteflies feed on non-angiosperm hosts, the record of a whitefly feeding on a gymnosperm, involving the highly polyphagous Trialeurodes vaporariorum is exceptional (Martin et al. Reference Martin, Mifsud and Rapisarda2000; Manzari & Quicke Reference Manzari and Quicke2006). A few species habitually feed on ferns and other pteridophytes such as Selaginella (Mound et al. Reference Mound, Martin and Polaszek1994); these are very much exceptions to the rule (Martin et al. Reference Martin, Mifsud and Rapisarda2000). Whiteflies appear to have evolved quite a long time ago, with the oldest known fossil remains from the Late Jurassic – the extinct Bernaeinae Shcherbakov Reference Shcherbakov2000a surviving to the Mid-Cretaceous. The oldest Udamoselinae are recorded from Lower Cretaceous Lebanese amber (Hauterivian–Aptian), the first Aleurodicinae were found in Burmese amber (Cenomanian). No confirmed fossil record of Aleyrodinae is available at the moment (Shcherbakov Reference Shcherbakov2000a). The present-day distribution of Aleyrodidae lineages shows that Aleurodicinae are distributed mainly in the Neotropical and Australasian regions, while Aleyrodinae are distributed worldwide (Mound & Halsey Reference Mound and Halsey1978; Martin & Mound Reference Martin and Mound2007; Evans Reference Evans2008). This distributional pattern and the availability of fossil data suggest a Palaeotropical origin of the whiteflies (Mound Reference Mound1984; Bink-Moenen & Mound Reference Bink-Moenen, Mound and Gerling1990; Manzari & Quicke Reference Manzari and Quicke2006). The question of ancestral host plants of the Aleyrodidae is still open. It seems that the group evolved in relation to some Jurassic gymnosperms (or pro-angiosperms?); however, their accelerated diversification probably took place in concordance with the diversification of angiosperms and biotic reorganisation of the biosphere in the Mid-Cretaceous (Rasnitsyn Reference Rasnitsyn and Ponomarenko1988; Drohojowska & Szwedo Reference Drohojowska and Szwedo2015; Szwedo & Drohojowska Reference Szwedo and Drohojowska2016). Manzari & Quicke (Reference Manzari and Quicke2006) stated that the diversification pattern of Aleyrodidae with their host plants is obscured by widespread host switching. However, it could be said that the evolution of aleyrodid host plant affiliations appears not to be random as some groups have species feeding on related plants.
Coccids (Hemiptera: Sternorrhyncha: Coccidomorpha)
by Jacek Szwedo and Ewa Simon
The scarcity of scale insects among Palaeogene fossils is still a puzzle, but the number of species representing both archeococcids (Orthezioidea) and neococcids (Coccoidea) are known from fossil resins (Koteja Reference Koteja2000a, Reference Kotejab, Reference Koteja2001, Reference Koteja2004, Reference Koteja2008; Koteja & Azar Reference Koteja and Azar2008; Vea & Grimaldi Reference Vea and Grimaldi2012, Reference Vea and Grimaldi2015; Simon & Żyła Reference Simon and Żyła2015; Wang et al. Reference Wang, Xia, Wappler, Simon, Zhang, Jarzembowski and Szwedo2015). The oldest neococcids are known from Lower Cretaceous Lebanese amber, and in the Palaeogene they represent a diverse group. It would be interesting to know on which host plants ancestral and fossil scale insects fed on in various periods of geological time, and with which types of vegetation and climatic conditions they were associated. Scale insects appeared as an abundant and diversified group in the Early Cretaceous, but their roots are unknown even if the supposed time of their origin is Triassic (Koteja Reference Koteja1985, Reference Koteja2001). It is believed that the most recent periods of scale insects diversification are related to the evolution of two other groups of organisms intimately associated with coccids: angiosperm plants and ants (Koteja Reference Koteja1985; Grimaldi & Engel Reference Grimaldi and Engel2005). To test the hypothesis about scale insect phylogeny and relationships, data on their biology, host–parasite relationships, origins of gall induction, biogeography, evolution of chromosome systems and molecular characteristics (Cook et al. Reference Cook, Gullan and Trueman2002; Gullan & Cook Reference Gullan and Cook2007; Hodgson & Hardy Reference Hodgson and Hardy2013), as well as morphology-based palaeontological and neontological research and correlation of radiation events are necessary (Koteja Reference Koteja2000a, Reference Koteja2008; Koteja & Azar Reference Koteja and Azar2008; Vea & Grimaldi Reference Vea and Grimaldi2012, Reference Vea and Grimaldi2015; Wang et al. Reference Wang, Xia, Wappler, Simon, Zhang, Jarzembowski and Szwedo2015).
Suborder Sternorrhyncha Amyot & Audinet-Serville, Reference Amyot and Audinet-Serville1843
Infraorder Coccidomorpha Heslop-Harrison, Reference Heslop-Harrison1952
Superfamily Coccoidea Fallén, 1814 indet.
(Pl. 3: 1)

Plate 3 (1) Coccoidea, MNEMG HL1b, male, forewing. (2) Schizoneurites brevirostris Cockerell, Reference Cockerell1915, holotype, USNM 61428 part, dorsal part of body, without legs. (3) Hormaphis? longistigma Wegierek sp. nov., holotype, NHMUK I.9595. (4) Eriosoma gratshevi Wegierek sp. nov., holotype, NHMUK I.8585. Scale bar=1mm.
Material. Specimen No. MNEMG HL1a, b, Jarzembowski Collection, Maidstone Museum. Insect Limestone, Hampstead Ledge.
Description. Imago, male, lanceolate forewing, 2.15mm long, 1mm wide. Subcostal ridge curved along forewing margin, slightly sigmoidal at base, not strongly curved in apical portion.
Compression fossil scale insects (Hemiptera: Sternorrhyncha: Coccomorpha) were documented from the Early Cretaceous of Transbaikalia (Koteja Reference Koteja1988, Reference Koteja1989) and England (Koteja Reference Koteja1999) – representing archeococcid males (Orthezioidea: Matsucoccidae and Xylococcidae). Only adult male scale insects have wings and we cannot glean anything about insect and plant interactions because the adult males do not feed. These records provide only morphological details. However, feeding stages of females and larvae preserved on fossil dicotyledonous leaves were documented from the Middle Eocene of Messel, Germany (Wappler & Ben-Dov Reference Wappler and Ben-Dov2008), Miocene deposits of Sicily (Pampaloni Reference Pampaloni1902; Koteja & Ben-Dov Reference Koteja and Ben-Dov2003), Germany (Zeuner Reference Zeuner1938; Koteja Reference Koteja2000b), New Zealand (Harris et al. Reference Harris, Bannister and Lee2007) – representing neococcids (Coccoidea: Diaspididae) and unidentified scale insects (Schmidt et al. Reference Schmidt, Kaulfuss, Bannister, Baranov, Beimforde, Bleile, Borkent, Busch, Conran, Engel, Harvey, Kennedy, Kerr, Kettunen, Philie Kiecksee, Lengeling, Lindqvist, Maraun, Mildenhall, Perrichot, Rikkinen, Sadowski, Seyfullah, Stebner, Szwedo, Ulbrich and Lee2018) – and India (Bera et al. Reference Bera, Mitra, Banerjee and Szwedo2006).
Aphids (Hemiptera: Sternorrhyncha: Aphidomorpha)
by Piotr Wegierek
The earliest published data on aphids from the Insect Limestone of the Isle of Wight concerned two species known from single specimens, which suggested that aphids were very rare in these strata and their taxonomic diversity was low. As a result of the present research, 25 fossils have been identified as Aphidoidea. The findings suggest a relatively high degree of taxonomic diversity (seven species representing one extinct and three extant families), comparable with Eocene/Oligocene deposits where aphids have been found (Scudder Reference Scudder1890; Heie Reference Heie1989). In the present paper aphid species formerly described from the Isle of Wight, i.e., Aphis gurnetensis Cockerell, Reference Cockerell1921a, Reference Cockerellb, Reference Cockerellc and Schizoneurites brevirostris Cockerell, Reference Cockerell1915 are revised, and other fossils are described for the first time.
Schizoneurites brevirostris Cockerell, Reference Cockerell1915 redescribed below, is the last known representative of the extinct family Elektraphididae. In contrast to the Baltic amber fauna, most of the described species are placed within recent genera. The condition of specimens prevents a more detailed comparative analysis.
Suborder Sternorrhyncha Amyot & Audinet-Serville, Reference Amyot and Audinet-Serville1843
Infraorder Aphidomorpha Becker-Migdisova & Aizenberg, 1962
Superfamily Aphidoidea Geoffroy, 1762
Family Elektraphididae Steffan, Reference Steffan1968
Genus Schizoneurites Cockerell, Reference Cockerell1915
Type species. Schizoneurites brevirostris Cockerell, Reference Cockerell1915, Insect Limestone, NW Isle of Wight, UK; by original designation.
Remarks. This genus was redescribed by Heie (Reference Heie1970). He proposed that the genera Antiquaphis Heie, Reference Heie1967 and Elektraphis Steffan, Reference Steffan1968 should be regarded as junior synonyms and belonged to the family Elektraphididae (Heie Reference Heie1976). Steffan & Schlüter (Reference Steffan and Schlüter1981) restored the formerly synonymised genera. However, the systematic position of the genus Schizoneurites was not specified. In the original description Cockerell (Reference Cockerell1915) emphasised its similarity to the genera Schizoneura Hartig, Reference Hartig1839 or Eriosoma Leach, Reference Leach1818 of the family Eriosomatidae. The five-segmented antennae with transverse grooves, veins CuA1 and CuA2 connected basally suggest that this genus should be placed in the family Elektraphididae.
Schizoneurites brevirostris Cockerell, Reference Cockerell1915
1915 Schizoneurites brevirostris Cockerell, p. 488.
1992 Schizoneurites brevirostris: Carpenter, p. 248.
1970 Schizoneurites brevirostris: Heie, p. 114.
1976 Schizoneurites brevirostris: Heie, p. 54.
1998 Schizoneurites brevirostris: Heie & Wegierek, p. 183.
2011 Schizoneurites brevirostris: Heie & Wegierek, p. 56.
Holotype part. USNM 61428, Lacoe collection. Imprint of dorsal part of body, right antenna and fragments of legs preserved. Left fore- and hindwings visible, cubital veins on right wing distinct.
Counterpart. NHMUK I. 9850, Brodie Collection Imprint of ventral part of body, without legs. Right antenna and left forewing preserved. The counterpart was not seen for the original description.
Diagnosis. Veins CuA1 and CuA2 thick, with a common stem. Vein M undivided. Antennae five-segmented, with transverse grooves. Primary rhinaria invisible. In contrast to other representatives of this genus, the basal portion of vein M visible.
Redescription. Length of body 1.2mm. Antennae five-segmented, segment III as long as IV (0.05mm) and markedly shorter than longest segment V (about 0.06mm). Basal part of segment III narrow, apical part wide. Segment IV approximately cylindrical, segment V tapering apically. All segments of flagellum with distinct transverse grooves.
Compound eyes extend to the ventral part of head. Front coxae contiguous with clypeus. Forewings 1.3mm long. Pterostigma almost as long as the common stem of veins Sc+R+M, four times longer than wide. Cubital veins (CuA1 and CuA2) thicker than others, forming a short common stem (CuA1+2), whose length equals the width of the pterostigma. Bases of cubital veins close to the pterostigma, distanced from it by two widths of the pterostigma. Vein M distinct in the basal part, branching off from the basal part of the pterostigma, basal part of Rs invisible.
Family Hormaphididae Mordvilko, Reference Mordvilko1908
Genus Hormaphis Osten-Sacken, Reference Osten-Sacken1861
Type species. Hormaphis hamamelidis Osten-Sacken, Reference Osten-Sacken1861, recent species, by original designation.
Hormaphis? longistigma Wegierek sp. nov.
Etymology. From ‘longistigma', Latin – ‘elongated pterostigma'.
Holotype. NHMUK I.9595, Brodie Collection; Insect Limestone, NW Isle of Wight. Part of head, thorax, abdomen, forewings and part of antenna.
Diagnosis. Veins CuA1 and CuA2 connected basally to form a common stem. Vein M with a single fork. Pterostigma long. Antennae with annular rhinaria.
Description. Body about 1.0mm long and 0.4mm wide across abdomen. Antennae five-segmented, about 0.2mm long. Length of antennal segments in millimetres: II 0.04, III 0.06, IV 0.03-0.05, V 0.05–0.06. Last segment with annular rhinaria. Mesothoracic lobe not well developed. Forewings 1.3mm long, 0.35–0.5mm wide. Cubital veins CuA1 and CuA2 connected basally to form a common stem equal in length to vein CuA2 or half the length of CuA1. Vein M in the basal part invisible, in the apical part forked. Pterostigma thin and long, ten times longer than wide. Vein Rs s-shaped, branching off in the basal part of the pterostigma. Segment boundaries of abdomen well defined.
Remarks. Modern aphids rarely possess a common stem of CuA1 and CuA 2. Such wing venation with annular secondary rhinaria is typical for Hormaphididae. Similar branching of CuA veins is to be found in the recent European species Hormaphis betulae Mordvilko. The newly described species, contrary to the recent one, has a forked vein M and elongated pterostigma.
Family Eriosomatidae Kirkaldy, Reference Kirkaldy1905
Genus Eriosoma Leach, Reference Leach1818
Type species. Eriosoma lanigera Hausmann, Reference Hausmann1802 recent species.
Eriosoma gratshevi Wegierek sp. nov.
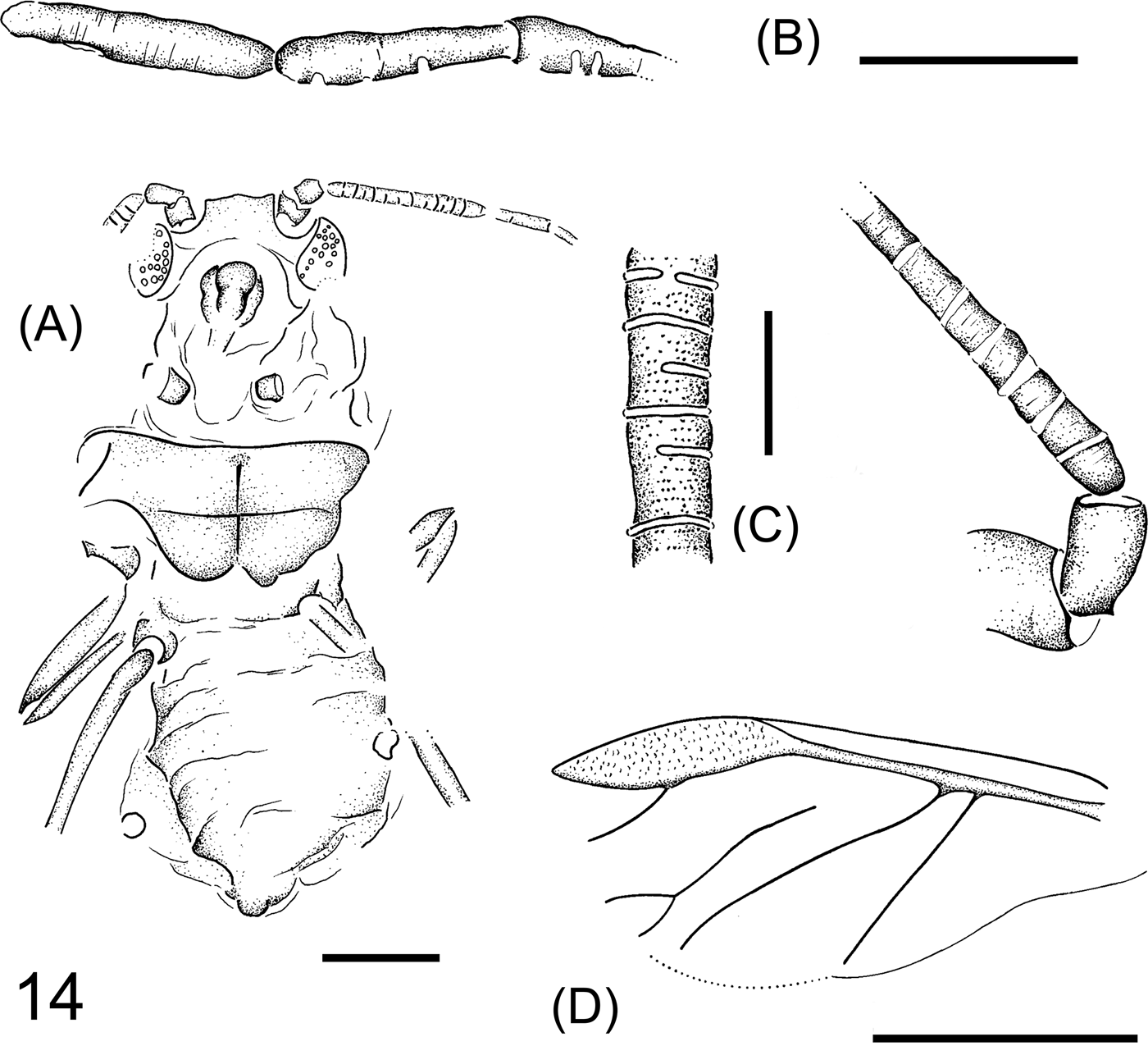
Figure 14 Eriosoma gratshevi Wegierek sp. nov. (A) General view, paratype NHMUK I.8712. Scale bar=0.02mm. (B) Left antenna, paratype NHMUK I.8712. Scale bar=0.05mm. (C) Part of antennal segment, holotype NHMUK I.8585. Scale bar=0.1mm. (D) Left forewing, holotype NHMUK I.8585. Scale bar=0.5mm.
Etymology. In honour of Vadim G. Gratshev, the late Russian entomologist and good friend of the author.
Holotype. NHMUK I.8585, Brodie Collection, Insect Limestone, NW Isle of Wight. Ventral part of head, right antenna and mesosternum. Right forewing well visible.
Paratype. NHMUK I.8712, Brodie Collection. Ventral part of body, basal portions of legs and fragments of antennae.
Diagnosis. As in recent representatives of the genus Eriosoma, antennal segment III very long, with annular rhinaria. Segments IV and V with single rhinaria, last segment only with primary rhinarium. Vein M forked. Veins CuA1 and CuA2 branch off independently. Siphunculi porous.
Description. Length of body 1.3mm. Compound eyes large, with distinct triommatidium, extending to the ventral side of head. Antennae six-segmented, 0.7mm long. Length of antennal segments in mm: I 0.05, II 0.05–0.06, III 0.29, V 0.11, VIa 0.10, VIb 0.03. Antennal segment III with many annular rhinaria, segments IV and V with single semiannular rhinaria, the last segment only with primary rhinarium. Front coxae 0.04–0.05mm long. Middle femora 0.28mm long. Forewings about 1.2mm long, 0.5mm wide. Veins CuA1 and CuA2 branch off from the common stem independently, CuA1 arcuate, CuA2 straight. Vein M close to the base of CuA1 but not reaching the common stem of Sc+R+M because its basal part is not developed; in the apical part forked. Pterostigma lenticular, four times longer that wide. Rs branching off in the middle of pterostigma. Segment boundaries of abdomen clearly marked; siphunculi porous, 0.04mm in diameter.
Genus Colopha Monell, Reference Monell1877
Type species. Byrsocrypta ulmicola Fitch, Reference Fitch1859, recent species; by original designation.
Colopha? incognita Wegierek sp. nov.
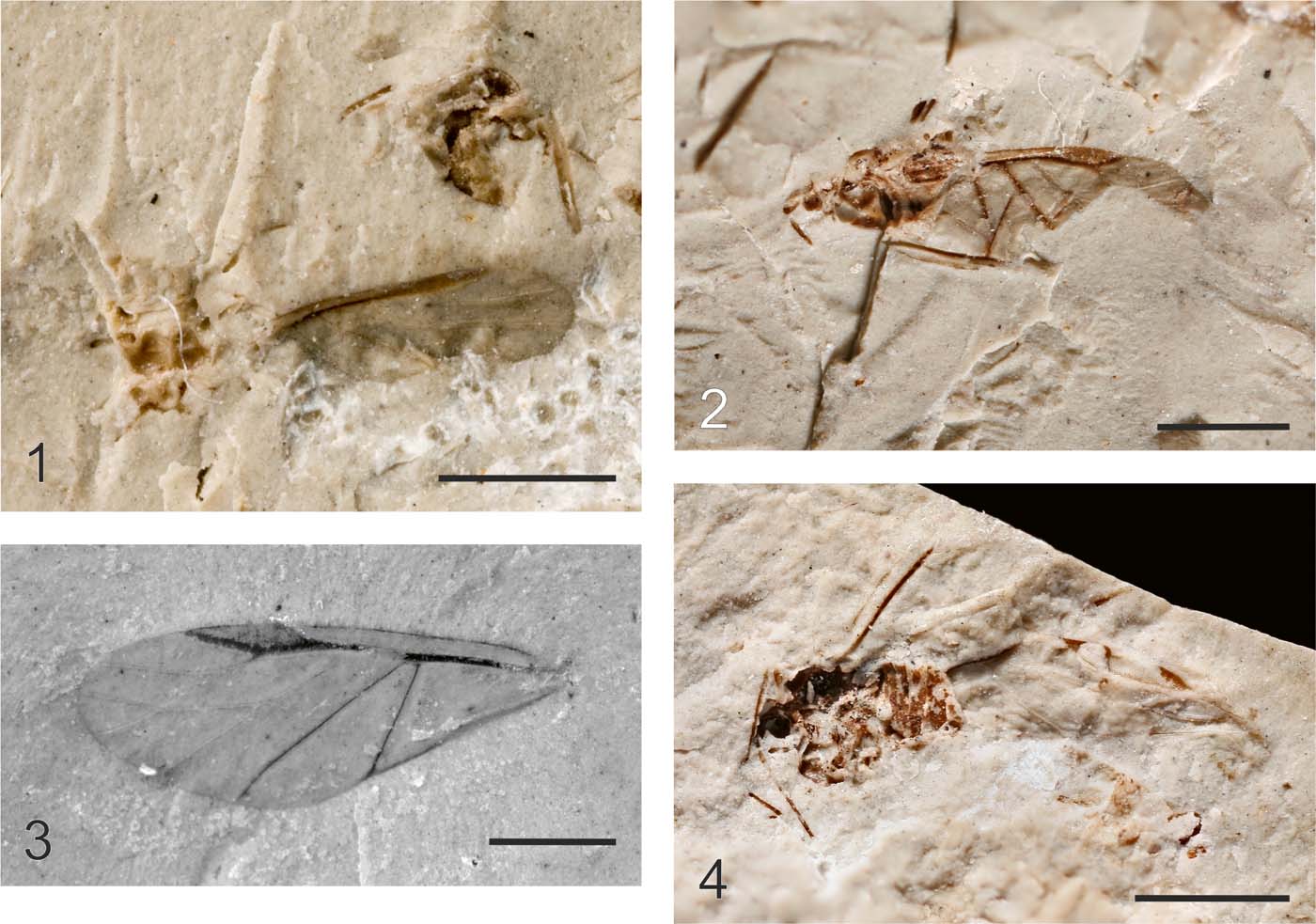
Plate 4 (1) Colopha? incognita Wegierek sp. nov., holotype, NHMUK I.9203. (2) Panfossilis anglicus Wegierek gen. et sp. nov., holotype, NHMUK I.9033. (3) Phyllaphis gurnetensis (Cockerell, Reference Cockerell1921b), holotype, NHMUK In.24357. (4) Betulaphis kozlovi Wegierek sp. nov., holotype, NHMUK I.9411.

Figure 15 Colopha? incognita Wegierek sp. nov. (A) Paratype, NHMUK In.24837, forewing and hindwing. (B) Holotype, NHMUK I.9203, right forewing. Scale bar=0.5mm.
Etymology. From ‘incognitus', Latin – ‘unrecognisable.'
Holotype. NHMUK I.9203 (Fig. 15B), Brodie Collection, Insect Limestone, NW Isle of Wight. Ventral side of body without abdomen, right forewing preserved.
Paratypes. NHMUK I.8662, Brodie Collection – forewings and hindwing; In.17178, Smith Collection – forewings; In.17198, Smith Collection – head with parts of antenna and fragments of dorsal part of thorax; In.24837 (Fig. 15A), Hooley Collection – thorax and side of head, forewings and hindwing.
Diagnosis. Vein Rs branches off approximately in the middle of pterostigma, the fork shifted towards its base. M with a single fork, hindwings with a single vein.
Description. Forewing 1.7–2.0mm long, 0.7–0.8mm wide. Pterostigma 4.5–5.5 times longer than wide, in the apical part pointed, in the basal part broadening rapidly. Vein Rs branches off approximately in the middle of pterostigma, the fork shifted towards its basal part. Vein M separates from the common stem Sc+R+M in the midpoint between the base of pterostigma and the base of vein CuA1, with a single fork. The common stem of M as long as M3+4. Veins CuA1 and CuA2 leave the common stem independently, almost parallel, CuA1 slightly arcuate. Hindwings 1.1mm long with a single vein.
Remarks. The preserved forewings resemble those in several genera of the subfamily Eriosomatinae (sensu Heie Reference Heie1980). However, the set of characters – with vein M forked, the unique shape of the basal part of the pterostigma and hindwings with a single vein – seems closest to the genus Colopha.
Family Drepanosiphidae Herrich-Schäffer in Koch, Reference Koch1857
Genus Panfossilis Wegierek gen. nov.
Etymology. The name of a recent genus Panaphis Kirkaldy, Reference Kirkaldy1904, to which it bears resemblance, and Latin fossilis, ‘fossil'.
Type species. Panfossilis anglicus sp. nov.; here designated.
Diagnosis. Circular rhinaria only on segment III, terminal process as long as the width of segment IVa at base. Pterostigma short, vein Rs short, bases of veins CuA1 and CuA2 wide apart.
Description. Antennae six-segmented; segment III with many circular secondary rhinaria along the whole segment. Other segments of flagellum without secondary rhinaria, with rows of small transverse depressions, which may be remnants of delicate spinules. Terminal process very short, blunt, as long as the width of segment VI at base. Pterostigma short and wide. Vein Rs short, arcuate, in the middle invisible. Veins CuA1 and CuA2 branch off independently, their bases wide apart.
Panfossilis anglicus Wegierek sp. nov.
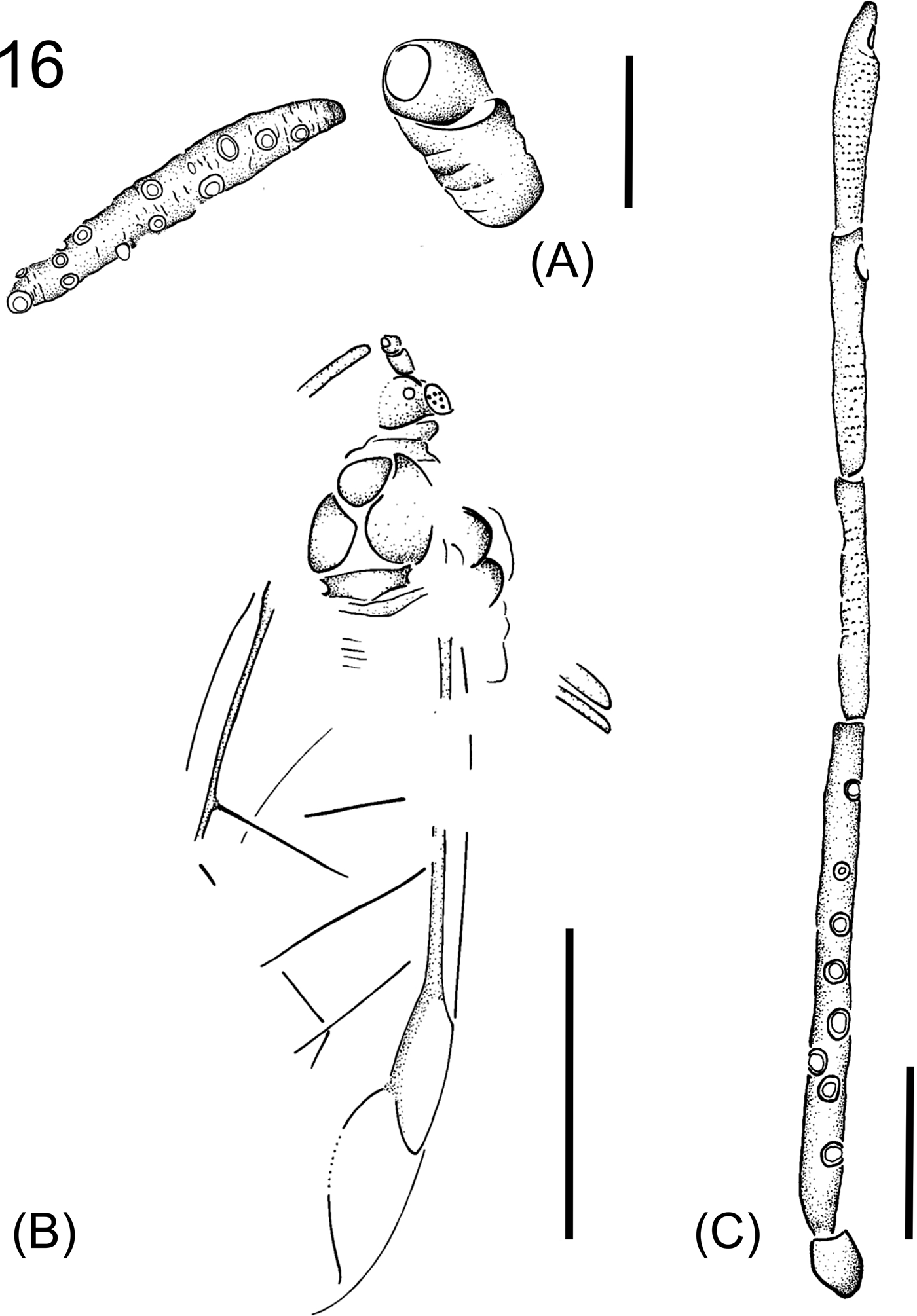
Figure 16 Panfossilis anglicus gen. and sp. nov. (A) Holotype, NHMUK I.9033, part of antenna. Scale bar=0.1mm. (B) General view. Scale bar=0.5mm. (C) Paratype, NHMUK I. 8661, left antenna. Scale bar=0.1mm.
Etymology. From ‘Anglia' – the Polish name for England, part of Great Britain.
Holotype. NHMUK I.9033, Brodie Collection, Insect Limestone, NW Isle of Wight. Head with part of antenna, thorax in dorsal view, right forewing and base of left forewing.
Paratype. NHMUK I.8661, Brodie Collection Ventral side of body, whole left antenna preserved.
Diagnosis. As for genus as it is the only included species.
Description. Length of body 1.4mm. Compound eyes situated at sides of head. Antennal segment III shorter than the total length of other segments of flagellum. Length of antennal segments in millimetres: I 0.05–0.08, II 0.07, III 0.33, IV 0.15, V 0.15, VIa 0.13, VIb 0.02. Diameter of secondary rhinaria approximately as long as half the width of antennal segment III. Middle femora 0.32mm long, hind coxae 0.09mm long, hind femora 0.38mm long. Pterostigma three times longer than wide. Base of vein Rs shifted beyond the midpoint of the pterostigma towards the apex.
Bases of veins CuA1 and CuA2 wide apart. The distance between the bases of veins M and CuA1 equals the distance between the bases of cubital veins.
Genus Phyllaphis Koch, Reference Koch1856
Type species. Chermes fagi Linnaeus, Reference Linnaeus1761, recent species; by original monotypy.
Phyllaphis gurnetensis (Cockerell, Reference Cockerell1921b) comb. nov.
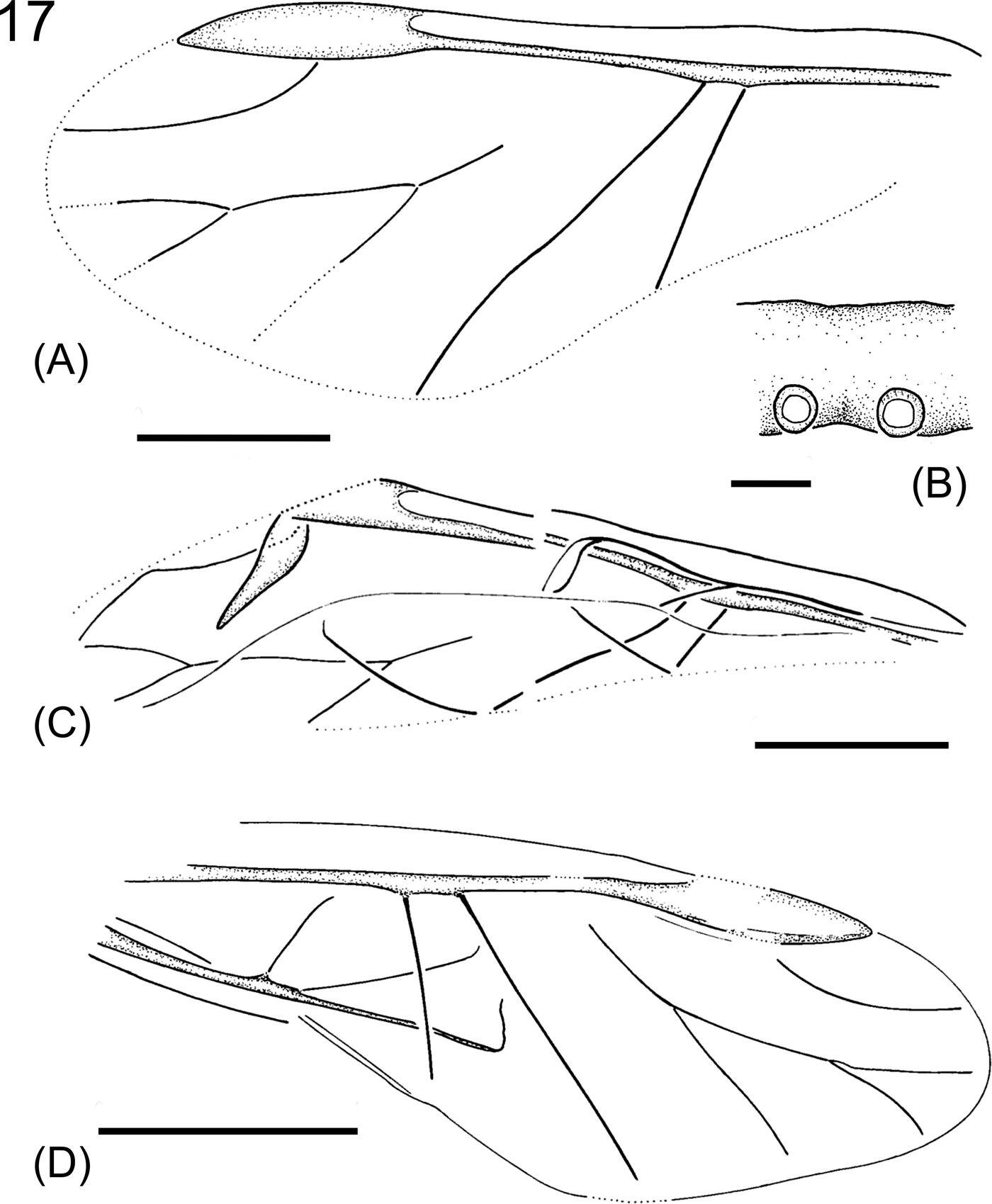
Figure 17 Phyllaphis gurnetensis (Cockerell, Reference Cockerell1921b). (A) Holotype NHMUK In. A 24357, forewing. Scale bar=0.5mm. (B) Part of antennal segment, NHMUK I.8512. Scale bar=0.02mm. (C) Forewing and hindwing, NHMUK I.8512. Scale bar=0.5mm. (D) NHMUK I.9098, forewing and hindwing. Scale bar=1mm.
1921 Aphis gurnetensis Cockerell, Reference Cockerell1921b, p. 476, fig. 43.
1962 Aphis gurnetensis: Becker-Migdisova & Aizenberg, p. 198, fig. 577.
1967 Aphis gurnetensis: Heie, p. 13.
1991 Aphis gurnetensis: Becker-Migdisova & Aizenberg, p. 273, fig. 577.
1998 Aphis gurnetensis: Heie & Wegierek, p. 164.
Holotype. NHMUK In.24357 (H 1124) (Fig. 17A), Hooley Collection, Insect Limestone, NW Isle of Wight. Forewing.
Additional material. NHMUK I.8512 (Fig. 17B, C), Brodie Collection – distorted body, poorly preserved antenna, creased forewings and hindwings; I.9098 (Fig. 17D), Brodie Collection – part of thorax and abdomen in dorsal view, right forewing and hindwing.
Diagnosis. Antennal segment III with circular secondary rhinaria arranged in a single row. Pterostigma lenticular, vein Rs arcuate, vein M with two forks, M1 approximately as long as M1+2. Cubital veins branch off independently, not far away from each other. Hindwings with two veins, their bases close together.
Description. Antennal segment III with circular secondary rhinaria located along the lower margin, their diameter as long as 1/3 of the segment width. Forewings 2.5–3.8mm long and 1.0–1.5mm wide. Pterostigma lenticular. Vein Rs arcuate, branching off in the middle of pterostigma. Vein M with two forks, the common stem of M approximately as long as M3+4. M1 as long as or only slightly shorter that M1+2. Veins CuA1 and CuA2 branch off independently from the common stem, distance between their bases as long as or slightly shorter than the width of pterostigma. CuA2 straight, CuA1 slightly arcuate. Hindwings about 2.1mm long, with two transverse veins located in the middle part of the wing, distance between their bases shorter than the width of pterostigma on forewing.
Remark. Becker-Migdisova & Aizenberg (1962, 1991) erroneously listed this species as originating from ‘Oligocene, North America'. The species has been placed within the genus Phyllaphis on the basis of fore and hindwing structure and venation as well as on a highly characteristic shape of secondary rhinaria and their arrangement on antennal segment III.
Genus Betulaphis Glendenning, Reference Glendenning1926
Type species. Betulaphis occidentalis Glendenning, Reference Glendenning1926, recent species; by original designation.
Betulaphis kozlovi Wegierek sp. nov.

Figure 18 Betulaphis kozlovi Wegierek sp. nov. (A) Forewing and hindwing, paratype, NHMUK In.24499. Scale bar=1mm. (B) Part of antennal segment, holotype, NHMUK I.9411. Scale bar=0.1mm. (C) Forewing, holotype, NHMUK I.9411. Scale bar=0.5mm.
Etymology. In honour of the late Mikhail A. Kozlov, renowned Russian entomologist.
Holotype. NHMUK I.9411 (Fig. 18B, C), Brodie Collection, Insect Limestone, NW Isle of Wight. Head with part of antenna, dorsal side of thorax, fragment of front femur and tibia. Basal part of right forewing and creased left wing.
Paratype. NHMUK In.24499 (Fig. 18A). Forewing and hindwing.
Diagnosis. Antennal segment III with few semiannular rhinaria arranged in a single row. Pterostigma lenticular, vein Rs arcuate. Vein M with two forks, the common stem of M short, veins M1 and M2 very short. Veins CuA1 and CuA2 leave the common stem independently. Hindwings with two transverse veins.
Description. Compound eyes situated at sides of the head. Legs and antennae with rows of small transverse depressions, which may be remnants of delicate spinules. Antennal segment III with semiannular rhinaria arranged ventrally in a single row at a distance of at least the width of the segment from each other. Forewings 3.4mm long and 1.2mm wide. Pterostigma thin, lance-shaped, pointed, four to five times longer than wide. Vein Rs arcuate, branching off in the middle of the pterostigma, with the base shifted towards its basal portion. Base of vein M in the middle of the distance between the bases of CuA1 and Rs. Vein M with two forks. The common stem of M1+2+3+4 short, half the length of M3+4. Veins M1 (shorter than half the length of M1+2) and M2 (shorter than 1/3 the length of M1+2) very short. Veins CuA1 and CuA2 branch off from the common stem independently, their bases at the distance of the width of pterostigma from each other. Hindwings with two transverse veins.
Aphidoidea incertae sedis
Material. NHMUK I.9304, I.9596, I.9700, I.9943, I.10210, Brodie Collection; In.17198, In.17210 (2, 3) (with paratype of Aeolothrips jarzembowskii Shmakov, Reference Shmakov2014), In.17214, A'Court Smith Collection; In.24625, Hooley Collection; II.2766a, b, II.2861, II.2862, II.3028 [det. J. Szwedo]. Insect Limestone, NW Isle of Wight.
Discussion. Most of the described aphid fauna was probably associated with arborescent angiosperm plants or angiosperm shrubs, mainly of the families Fagaceae, Betulaceae, Ulmaceae, Juglandaceae and Lauraceae. Representatives of the family Eriosomatidae might have migrated onto secondary hosts of Asteraceae (Compositae), Cyperaceae or Poaceae (Graminae). Today a group of species of the family Hormaphididae is also associated with Poaceae (especially Bambuseae) or Palmaceae. It is possible that Elektraphididae, like recent Adelgidae, were associated with Pinaceae.
Planthoppers, froghoppers, singing cicadas, leafhoppers (Hemiptera: Fulgoromorpha & Cicadomorpha)
by Jacek Szwedo
The Fulgoromorpha comprises one of the most ancient lineages of the Hemiptera, and in the fossil record planthoppers have been known since the Early Permian (Szwedo et al. Reference Szwedo2004; Szwedo Reference Szwedo2018). The earliest Fulgoromorpha are placed in the Permian superfamily Coleoscytoidea Martynov, Reference Martynov1935; the second group is the Permian–Triassic Surijokocixioidea Shcherbakov, Reference Shcherbakov2000b and the Fulgoroidea have been known since the Jurassic.
Several fossil Fulgoroidea have been reported so far from the latest Eocene Bembridge Marls of the Isle of Wight. The first descriptions were by Cockerell (Reference Cockerell1921b), who described Poekilloptera melanospila Cockerell, Reference Cockerell1921b (transferred to Orthoptera, see Nel et al. Reference Nel, Prokop and Ross2008). Later, more species were added under the names Hastites muiri Cockerell, Reference Cockerell1922, Hooleya indecisa Cockerell, Reference Cockerell1922 and Myndus wilmattae Cockerell, Reference Cockerell1926. These taxa are discussed below.
Cicadomorpha is the second suborder formerly placed together with Fulgoromorpha as ‘Auchenorrhyncha', but not related directly to planthoppers (Bourgoin & Campbell Reference Bourgoin and Campbell2002; Szwedo Reference Szwedo2002, Reference Szwedo2018; Szwedo et al. Reference Szwedo, Bourgoin and Lefebvre2004). Cicadomorpha comprises ancient lineages, some of them extinct (Dysmorphoptiloidea Handlirsch, Reference Handlirsch1906 (in 1906–8), Hylicelloidea Evans, Reference Evans1956, Palaeontinoidea Handlirsch, Reference Handlirsch1906, Pereborioidea Zalessky, Reference Zalessky1930, Prosboloidea Handlirsch, Reference Handlirsch1906 and Prosbolopseoidea Becker-Migdisova, Reference Becker-Migdisova1946). The placement of the paraphyletic Scytinopteroidea Handlirsch, Reference Handlirsch1906 – forms ancestral to Coleorrhyncha Myers & China, Reference Myers1929 and Heteroptera (Shcherbakov & Popov Reference Shcherbakov, Popov, Rasnitsyn and Quicke2002) – remains unresolved, but close to Cicadomorpha (Shcherbakov & Popov Reference Shcherbakov, Popov, Rasnitsyn and Quicke2002; Szwedo et al. Reference Szwedo, Bourgoin and Lefebvre2004; Szwedo Reference Szwedo2018). Only representatives of the superfamilies Cercopoidea, Cicadoidea and Cicadelloidea (together with the extinct Hylicelloidea Evans, Reference Evans1956, extant Myerslopioidea Evans, Reference Evans1957 and Membracoidea Rafinesque, Reference Rafinesque1815 forming the clade Clypeata Qadri, Reference Qadri1967) are reported here. Only a single representative of Cicadomorpha from the Bembridge Marls has been reported so far, named Aphrophora woodwardi Cockerell, Reference Cockerell1922.
The venation interpretations of Fulgoromorpha follow Szwedo & Żyła (Reference Szwedo and Żyła2009) and Bourgoin et al. (Reference Bourgoin, Wang, Asche, Hoch, Soulier-Perkins, Stroiński, Yap and Szwedo2015), and for Cicadomorpha follow interpretations of Emeljanov (Reference Emeljanov1987), Wang et al. (Reference Wang, Zhang and Szwedo2009) and Nel et al. (Reference Nel, Roques, Nel, Prokin, Bourgoin, Prokop, Szwedo, Azar, Desutter-Grandcolas, Wappler, Garrouste, Coty, Huang, Engel and Kirejtshuk2013).
Suborder Fulgoromorpha Evans, Reference Evans1946
Superfamily Fulgoroidea Latreille, Reference Latreille1807
Family Cixiidae Spinola, Reference Spinola1839
Subfamily Bothriocerinae Muir, Reference Muir1923
Genus Klugga Szwedo gen. nov.
Etymology. Name is derived from Proto-Celtic word ‘klugga' meaning ‘stone'. Gender: feminine.
Type species. Klugga gnawa sp. nov., here designated.
Diagnosis. Tegmen with venation similar to Bothriobaltia Szwedo, Reference Szwedo2002, but differs in having a larger stigma (stigma elongate and narrow in Bothriobaltia); wider basal cell (basal cell narrow and elongate in Bothriobaltia); shorter common stem ScP+R (common stem longer, reaching level of claval veins junction in Bothriobaltia); branching of vein ScP+RA1 slightly basad of forking of vein CuA (forking of ScP+RA1 slightly apicad of CuA forking in Bothriobaltia).
Description. Tegmen with costal margin slightly curved at base, striations on costal and apical margins distinct. Stigma distinct, about twice as long as wide, with corrugated texture. Basal cell about twice as long as wide. Stems of veins ScP+R, MP and CuA leaving basal cell independently; stem ScP+R leaving basal cell slightly basad of stem M; branch ScP+RA branched slightly basad of forking of stem CuA, terminal ScP+RA1 distinctly curved anteriad, widened in apical portion; vein RP forked at same level as forking of anterior branch of vein MP, with three terminals; stem M forked at level of nodal line, anterior branch forked again at level of RP branching, and again at level of posterior branch of vein MP forking, vein MP with five terminals; stem CuA forked slightly posteriad of ScP+RA branching, posteriad of claval veins junction. Nodal line distinct, veinlet rp-mp oblique, veinlet mp-cua more or less oblique. Clavus short with apex reaching nearly half of tegmen length; claval veins Pcu and A1 fused at about half of clavus length.
Klugga gnawa Szwedo sp. nov.

Plate 5 (1) Klugga gnawa Szwedo gen. et sp. nov., holotype, NHMUK In.24513, tegmen. (2–3) Klugga regoa Szwedo gen. et sp. nov., holotype, NHMUK In.24512: (2) tegmen; (3) SEM photo of stigma area. (4–7) Liwakka gelloa Szwedo gen. et sp. nov.: (4) holotype, NHMUK In.26034, tegmen; (5) SEM photo of the paratype NHMUK In.25457, tegmen; (6) SEM photo of stigmal area; (7) SEM photo of stigmal area sensory pores. Scale bars=1mm for 1, 2 and 4.
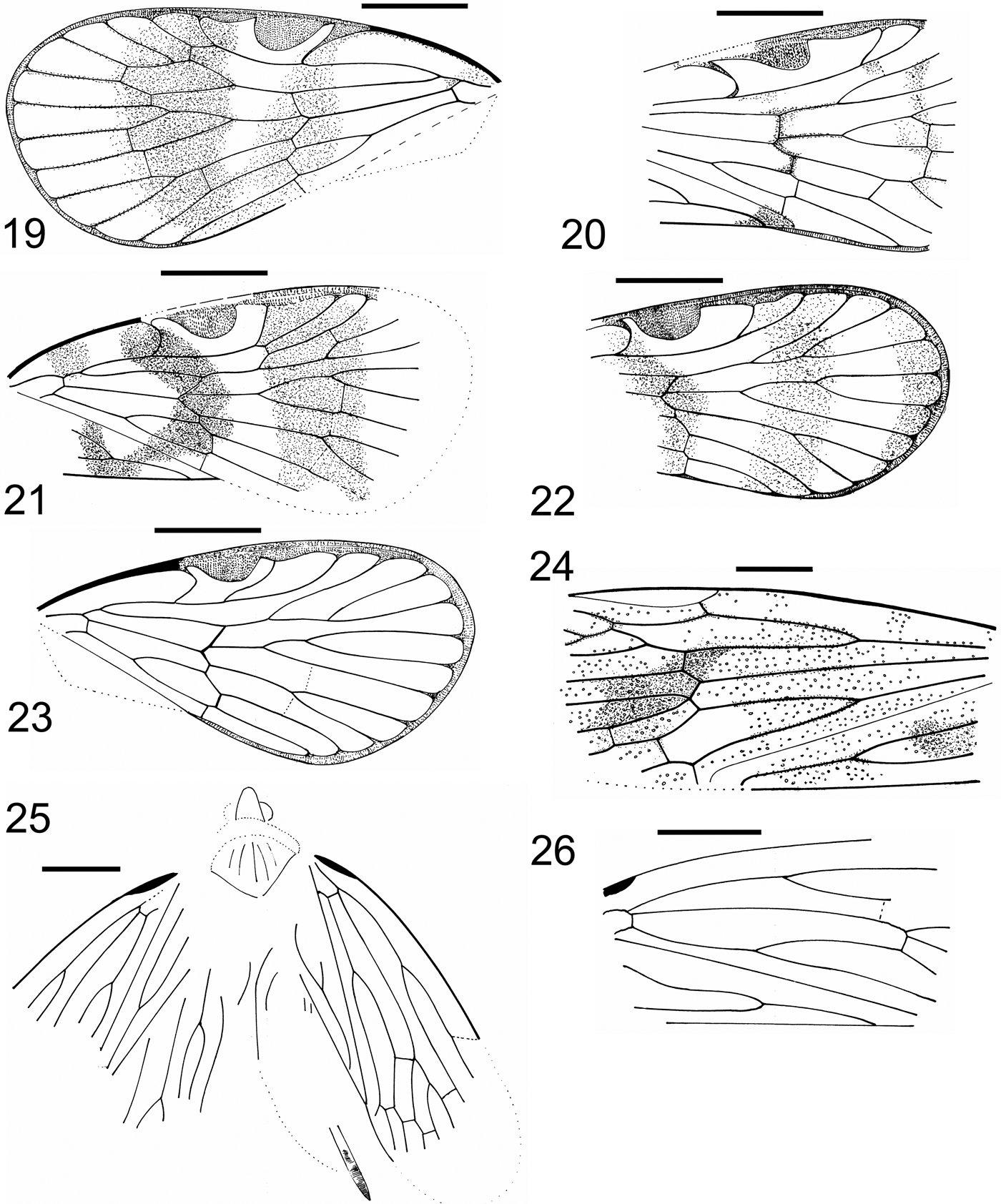
Figures 19–26 (19–24) Tegmen: (19) Klugga gnawa, Szwedo gen. et sp. nov., holotype, NHMUK In.24513; (20) Klugga regoa Szwedo gen. et sp. nov., holotype NHMUK In.24512; (21) Liwakka gelloa Szwedo, gen et sp. nov., holotype NHMUK In.26034; (22) Liwakka gelloa Szwedo, gen et sp. nov., paratype NHMUK In.25457; (23) Delwa morikwa Szwedo gen. et sp. nov., holotype NHMUK I.8657; (24) Kommanosyne wrikkua Szwedo gen. et sp. nov., holotype NHMUK In.24521. (25) Kernastirdius nephlajeus Szwedo gen. et sp. nov., holotype NHMUK I.9023, anterior part of body, parts of tegmina and hindwing. (26) Kernastirdius? sp., NHMUK In.25480, tegmen. Scale bar=1mm.
Etymology. Specific epithet is derived from Proto-Celtic word ‘gnawo' meaning ‘clear'.
Holotype. NHMUK In.24513; Hooley Collection, Insect Limestone, NW Isle of Wight. Tegmen with missing clavus and coloration partly preserved.
Diagnosis. Nodal veinlet m-cu not distinctly oblique; apical line of veinlets indistinct; cell C5 about as long as stigma; median portion of tegmen with darker, transverse, wide band.
Description. Length of tegmen 4.05mm, width at widest point 2.05mm. Basal and apical portion not coloured, median 1/3 of tegmen with darker transverse, wide band, with lighter portion mediad of stigma to anterior branch of vein M. Veins slightly darker.
Klugga regoa Szwedo sp. nov.
Etymology. Specific epithet is derived from Proto-Celtic word ‘rego' meaning ‘band'.
Holotype. NHMUK In.24512; Hooley Collection, Insect Limestone, NW Isle of Wight. Impression of median portion of tegmen.
Diagnosis. Nodal veinlet m-cu distinctly oblique; apical line of veinlets distinct; apical veinlet m-cu oblique; cell C5 slightly longer than stigma; tegmen with slightly darker, narrow band at level of nodal line, passing to apex of clavus, darker band at level of apical veinlets.
Description. Length of preserved portion of tegmen 2.75mm, width at widest point 1.9mm. Tegmen with veins slightly darkened, two slightly darker transverse bands, first at level of nodal line and second at level of apical line of veinlets.
Genus Liwakka Szwedo gen. nov.
Etymology. Name is derived from Proto-Celtic word ‘liwakk' meaning ‘stone'. Gender: feminine.
Type species. Liwakka gelloa sp. nov.; here designated.
Diagnosis. Venation similar to Klugga gen. nov., but differs in branching of vein ScP+RA slightly posteriad of vein CuA forking (branching of vein ScP+RA slightly anteriad of CuA forking in Klugga); claval veins Pcu and A1 fused at level of CuA forking (claval veins fused basad of CuA forking in Klugga); differs from Bothriobaltia Szwedo, Reference Szwedo2002 by shorter stem ScP+R (stem ScP+R longer in Bothriobaltia); bigger stigma (stigma narrow and elongate in Bothriobaltia).
Description. Costal margin slightly curved at base, costal and apical margin with distinct striations. Stigma about twice as long as wide, with corrugated texture. Basal cell twice as long as wide. Stems of veins ScP+R, MP and CuA leaving basal cell independently; stem ScP+R leaving basal cell slightly basad of stem MP; branch ScP+RA1 branched slightly apicad of forking of stem CuA, distinctly curved anteriad; vein RP forked at same level as forking of anterior branch of vein MP, with three terminals; stem MP forked at level of nodal line, anterior branch forked again at level of RP branching, and again at level of posterior branch of vein MP forking, vein MP with five terminals; stem CuA forked slightly basad of ScP+RA1 branching, at level of claval veins junction. Nodal line distinct, veinlet rp-mp oblique, veinlet mp-cua short, straight, parallel to veinlet icu. Clavus short with apex reaching half of tegmen length; claval veins Pcu and A1 fused apicad of half of clavus length.
Liwakka gelloa Szwedo sp. nov.
Etymology. Specific epithet is derived from the Proto-Celtic word ‘gello' meaning ‘yellow, brown'; it refers to the coloration of the specimen.
Holotype. NHMUK In.26034 (Pl. 5: 4; Fig. 21); Hooley Collection, Insect Limestone, NW Isle of Wight. Tegmen, with part of clavus missing.
Paratype. NHMUK In.24547 (Plate 5: 5–7; Fig. 22); Hooley Collection. Tegmen with basal and claval portion not preserved.
Diagnosis. Nodal line very distinct, veinlets mp-cua and both veinlets icu straight, subparallel. Wide, transverse, brown band at level of nodal line, second wide, transverse, brown band in subapical portion of tegmen; clavus pigmented; veins darkened, brown.
Description. Length of tegmen 3.75mm, width at widest point 1.9mm. Wide, transverse, brown band at nodal line arcuate apicad, wide, transverse, brown band in subapical portion not arcuate; clavus brown.
Genus Delwa Szwedo gen. nov.
Etymology. Name from the Proto-Celtic word ‘delwa' meaning ‘form'. Gender: feminine.
Type species. Delwa morikwa sp. nov.; here designated.
Diagnosis. Similar to Liwakka gen. nov., but differs by more basad forking of anterior branch of MP; longer common stem of veins ScP+R, longer than arculus (in Liwakka and Klugga common stem ScP+R about as long as arculus).
Description. Costal margin merely curved, costal and apical margins with distinct striations. Stigma distinct, about twice as wide as long. Basal cell about 2.5 times as long as wide. Stems of veins ScP+R, MP and CuA leaving basal call independently; stem ScP+R leaving basal cell slightly basad of stem MP; common stem of vein ScP+R about three times as long as arculus; branch ScP+RA1 branched slightly apicad of forking of stem CuA, distinctly curved anteriad; vein RP forked about at same level as forking of anterior branch of vein MP, with three terminals; stem MP forked at level of nodal line, anterior branch forked again at level of first RP branching, and again apicad of posterior branch of vein MP forking, vein MP with five terminals; stem CuA forked slightly basad of ScP+RA1 branching. Nodal line distinct, veinlet rp-mp oblique, veinlet mp-cua straight, parallel to veinlet icu. Clavus short, with apex reaching half of tegmen length.
Delwa morikwa Szwedo sp. nov.

Plate 6 (1–2) Delwa morikwa Szwedo gen. et sp. nov., holotype, NHMUK I.8657: (1) tegmen; (2) SEM photo of stigmal area sensory pores. (3) Kommanosyne wrikkua Szwedo gen. et sp. nov., holotype, NHMUK In.24521, tegmen. (4) Kernastiridius nephlajeus Szwedo sp. nov., holotype, NHMUK I.9023, anterior part of body, parts of tegmina and wings. (5) Margaxius angosus Szwedo sp. nov., holotype, NHMUK In.24620, tegmen with clavus missing. (6) Dweivera reikea Szwedo gen. et sp. nov., holotype, NHMUK I.10375, tegmen, counterpart. (7) Samaliverus bikkanus Szwedo gen. et sp. nov., holotype, NHMUK I.9199(2), tegmen. (8) Langsmaniko marous Szwedo gen. et sp. nov., holotype, NHMUK In. 25286(1), tegmen. (9) Komsitija tuberculata Szwedo gen. et sp. nov., holotype, NHMUK Pl II 2999, tegmen.
Etymology. Specific epithet is derived from Proto-Celtic word ‘morikwa' meaning ‘sea-shore'.
Holotype. NHMUK I.8657; Brodie Collection, Insect Limestone, NW Isle of Wight. Tegmen, with clavus missing.
Diagnosis. Tegmen twice as long as wide at widest point; cell C5 two times as long as stigma; nodal line distinct; stem MP thickened, branch MP3+4 at nodal line thickened; apex of clavus reaching half of tegmen length.
Description. Length of tegmen 3.8mm, width at widest point 1.9mm. Other features as for the genus as it is the only included species.
Remark. Bothriocerinae is a group in need of revisionary studies. Recent species are unevenly distributed in the genera Bothrioceretta Caldwell, Reference Caldwell1950 with four species and Bothriocera Burmeister, Reference Burmeister1835 with 45 species present from south of the USA to Brazil and Bolivia, and the only known so far extinct genus Bothriobaltia Szwedo, 2001 with single species from Baltic amber (Bourgoin Reference Bourgoin2017).
Subfamily Cixiinae Spinola, Reference Spinola1839
Tribe Mnemosynini Emeljanov, Reference Emeljanov1992
Genus Kommanosyne Szwedo gen. nov.
Etymology. Genus name is derived from Proto-Celtic word ‘kommano' meaning ‘memory', combined with generic name of cixiid planthopper Mnemosyne Stål, Reference Stål1866. Gender: feminine.
Type species. Kommanosyne wrikkua sp. nov.; here designated.
Diagnosis. Tegmen venation similar to other genera of Mnemosynini, e.g., Stalisyne Szwedo et al., Reference Szwedo, Bourgoin and Lefebvre2006, Mnaomaia Szwedo et al., Reference Szwedo, Bourgoin and Lefebvre2006 and Autrimpus Szwedo, Reference Szwedo2004. Differs from them by forking of common stem ScP+R at same level as stem CuA forking (stem ScP+R forked distinctly more basad in Stalisyne, Mnaomaia and Autrimpus); stigma very long, about eight times as long as wide (stigma shorter in Stalisyne, Mnaomaia and Autrimpus); cell C5 widened in apical portion (cell C5 not widened in apical portion in Stalisyne, Mnaomaia and Autrimpus).
Description. Tegmen with common stems of veins ScP+R and CuA forked at same level, at level of claval veins Pcu and A1 junction. Stigma narrow and elongate, about eight times as long as wide. Cell C1 elongate, longer than cell C5, tapered in apical portion. Vein RA with single terminal; vein RP forked at level of half of length of stigma, anterior branch of RP forked again near apical end of stigma; anterior branch of vein MP, i.e., MP1+2, not forked before apical end of stigma, posterior branch forked immediately after first MP forking at level of nodal line, i.e., veins MP3 and MP4 separated since the nodal line; stem CuA forked at level of claval veins junction; anterior margin shifted mediad, to the level of nodal line, then posteriad, posterior branch parallel to CuP; clavus long, distinctly exceeding half of tegmen length; claval veins Pcu and A1 fused at level of stems ScP+R and CuA forkings. Veinlet ir between branches RA and RP very short; nodal veinlet rp-mp straight, perpendicular to branch MP1+2; nodal veinlet mp-cua oblique, about as long as common stem MP3+4; veinlet icu closing cell C5 merely oblique, at level of half of stigma length. Tegmen partly covered with setiferous tubercles.
Kommanosyne wrikkua Szwedo sp. nov.
Etymology. Specific epithet is derived from Proto-Celtic word ‘wrikku' meaning ‘bristle' and is referring to the presence of the setiferous tubercles on the tegmen.
Holotype. NHMUK In.24521; Hooley Collection, Insect Limestone, NW Isle of Wight. Part of tegmen, with basal and apical portions not preserved.
Diagnosis. Basal portion of cell C1, radial cell, medial cell, base of cell C5, cubital cell, claval cells with numerous setiferous tubercles; few setiferous tubercles in cell C2, C3a, C3b and C4; veins darkened, indistinct colour pattern: darker markings in basal portion of clavus, at level of nodal line, on cells C3a and C3b.
Description. Length of preserved part of tegmen 6.1mm (estimated length of tegmen about 9mm), width at widest point 2.74mm. Other features as for the genus as it is the only included species.
Tribe Pentastirini Emeljanov, Reference Emeljanov and Yemel'yanov (Emeljanov)1971
Genus Kernastiridius Szwedo gen. nov.
Etymology. Generic name is combination of the Proto-Celtic word ‘kerna' meaning ‘head', and Pentastiridius – generic name of pentastirine planthopper. Gender: masculine.
Type species. Kernastiridius nephlajeus sp. nov.; here designated.
Diagnosis. Tegmen venation similar to Pentastiridius Kirschbaum, Reference Kirschbaum1868, but differs in more basal forking of stems ScP+R and CuA, at level of claval veins Pcu and A1 junction (stems ScP+R and CuA forked apicad of claval veins junction in Pentastiridius); stem MP forked immediately apicad of nodal veinlet mp-cua (short stalk present before forking in Pentastiridius); first veinlet rp-mp apicad of nodal line, at level of claval apex (veinlet rp-mp basad of claval apex, basad of nodal veinlet mp-cua in Pentastiridius).
Description. Vertex slightly wider at base than long in mid line, anterior margin arcuately acute; head with compound eyes narrower than pronotum. Rostrum very long, exceeding length of body. Mesonotum about as long in mid line as wide, with five distinct carinae. Tegmen about 2.9 times as long as wide; costal margin more curved at base, then mildly curved, with slightly widened basicostal area; basal cell about three times as long as wide. Stems of veins ScP+R, MP and CuA leaving basal cell independently, stem ScP+R slightly anteriad of stem MP, but close to it; stem ScP+R forked slightly posteriad of CuA forking, posteriad of claval veins junction; stem MP forked basad of claval apex, merely apicad of nodal veinlet m-cu, anterior branch, i.e., branch MP1+2 forked at level of claval apex; posterior branch, i.e., branch MP3+4 forked apicad of claval apex; cell C5 more than twice as long as cell C4. Clavus long, with apex reaching 2/3 of length of tegmen, claval veins Pcu and A1 fused at level of stem CuA forking, at half of clavus length.
Kernastiridius nephlajeus Szwedo sp. nov.
Etymology. Specific epithet derived from the Proto-Celtic words ‘ne' meaning ‘not' and ‘phlaje' meaning ‘fold'.
Material. Holotype NHMUK I.9023; Brodie Collection, Insect Limestone, NW Isle of Wight. Compression of anterior part of body, parts of tegmina and wings.
Diagnosis. Tegmen with cell C5 widest at level of nodal veinlet mp-cua, then narrowing apicad; cell C4 about half of length of cell C5, subequal in length with cell C3b.
Description. Total length 5.9mm, length of pronotum in mid-line ca.1mm, length of preserved part of right tegmen 4.1mm, estimated total length of tegmen ca.5mm, width at widest point 1.7mm. Other features as for the genus as it is the only included species.
Remark. Another specimen (Fig. 26) NHMUK In.25441/In.25480 (part and counterpart), Hooley Collection could belong to the genus Kernastiridius Szwedo. It is the median portion of a tegmen.
Pentastirini gen. et sp. indet.
(Fig. 27)
Figures 27–35 (27) Cixiidae: Penatastirini, NHMUK I.10344, parts of the body, portion of tegmen and (probably) part of hindwing. (28) Margaxius angosus Szwedo gen. et sp. nov., holotype, NHMUK In.24620, tegmen. (29) Dweivera reikea Szwedo gen. et sp. nov., holotype, NHMUK I.9193, part. (30) Dweivera reikea Szwedo gen. et sp. nov., holotype NHMUK I.10375, counterpart. (31) Samaliverus bikkanus Szwedo gen. et sp. nov., holotype, NHMUK I.9199(2), tegmen. (32) Langsmaniko marous Szwedo gen. et sp. nov., holotype, NHMUK In. 25286(1), tegmen. (33) Komsitija tuberculata Szwedo gen. et sp. nov., holotype, NHMUK Pl II 2999, tegmen. (34) Komnixta jarzembowskii Szwedo sp. nov., holotype, MNEMG IL 87a, tegmen, part. (35) Worodbera nimakka Szwedo sp. nov., holotype, NHMUK In.43470, tegmen. Scale bar=1mm.
Material. NHMUK I.10344; Brodie Collection, Insect Limestone, NW Isle of Wight. Mesonotum, parts of the body, portion of tegmen and (probably) part of hindwing.
Description. Length of mesonotum 1.2mm. Length of tegmen 5.7mm. Mesonotum with five distinct longitudinal carinae. Tegmen with base slightly curved at base, then only mildly curved, stigma elongate, about 3.6 times as long as broad. Stem ScP+R forked anteriad of stem CuA forking, branch RA with two terminals. Stem MP not forked before nodal veinlet rp-mp.
Remark. The specimen is not complete enough for the formal description of a new taxon. It differs in size and venation from Kernastiridius Szwedo, but could be placed in tribe Pentastirini.
Tribe Cixiini Spinola, Reference Spinola1839
Genus Margaxius Szwedo gen. nov.
Etymology. Genus name is combination of the Proto-Celtic word ‘marga' meaning ‘marl' and Cixius – generic name of cixiine planthopper. Gender: masculine.
Type species. Margaxius angosus sp. nov.; here designated.
Diagnosis. Tegmen venation resembles Cixius Latreille, Reference Latreille1804, but differs in barely curved basal portion of costal margin (more curved in Cixius); early branching of stems ScP+R and CuA, at about basal ¼ of tegmen length (stems ScP+R and CuA forked at about 1/3 of tegmen length in Cixius); presence of veinlet rp-mp distinctly basad of nodal line (lack of basal veinlet rp-mp in Cixius).
Description. Tegmen narrow, elongate, three times as long as wide. Costal margin mildly curved since base, apical margin elongately rounded. Basal cell about twice as long as wide. Stems ScP+R, MP and CuA leaving basal cell independently, stem ScP+R slightly anteriad, but very close to stem MP. Stem ScP+R forked slightly apicad of CuA forking, at about basal ¼ of tegmen length; branch ScP+RA forked slightly apicad of nodal veinlet rp-mp; RA with two terminals, RP forked slightly basad of veinlet rp-mp of apical line, with three terminals; stem MP forked merely basad of veinlets rp-mp and mp-cua of nodal line, anterior branch, i.e., branch MP1+2 forked slightly apicad of nodal line, then forked apicad of veinlets of apical line, upper branch, i.e., branch MP3+4 forked slightly basad of imp veinlet of apical line. Stem CuA forked slightly apicac of stem ScP+R forking, slightly basad of basal rp-mp veinlet. Clavus long, with apex reaching 2/3 of tegmen length. Apical line of veinlets rp-mp, imp, mp-cua and icu stepwise.
Remark. This genus is tentatively placed in the tribe Cixiini.
Margaxius angosus Szwedo sp. nov.
Etymology. Specific epithet derived from the Proto-Celtic word ‘angos' meaning ‘narrowness', referring to shape of the tegmen.
Holotype. NHMUK In.24620; Hooley Collection Insect Limestone, NW Isle of Wight. Tegmen with clavus missing.
Diagnosis. Apical line of veinlets stepwise; cell C1 longer than cell C5; cells C3a and C3b similar in length; apical cells elongate subequal in length to subapical ones; nodal veinlets r-m and m-cu and apical veinlets darkened.
Description. Length of tegmen 8.8mm, width at widest point 2.9mm. Other features as for the genus as it is the only included species.
Remark. Probably also specimen NHMUK In.17369, Smith Collection could be referred to the genus Margaxius.
Genus Dweivera Szwedo gen. nov.
Etymology. Name derived from Proto-Celtic word ‘dwei' meaning ‘two' (feminine form) and generic name of cixiid planthopper – Kuvera. Gender: feminine.
Type species. Dweivera reikea sp. nov.; here designated.
Diagnosis. Tegmen venation similar to Kuvera Distant, Reference Distant1906, but differs in short, but distinct common stem of veins ScP+R and MP leaving basal cell (stems ScP+R and MP leaving basal cell separately or with very short stem in Kuvera); nodal veinlet mp-cua slightly basad of stem MP forking (veinlet mp-cua distad of stem MP forking in Kuvera); cell C3a elongate (cell C3a short in Kuvera).
Description. Tegmen with costal margin distinctly curved at base, then mildly convex, apical margin elongately rounded. Basal cell about twice as long as wide. Stems ScP+R and MP leaving basal cell with a short common stem. Stem ScP+R forked slightly apicad of CuA forking, basad of half of tegmen length; branch ScP+RA forked apicad of nodal veinlets rp-mp and mp-cua; RA with single terminal, RP forked slightly apicad of apical line of veinlets, with three terminals. Stem MP forked distinctly apicad of nodal veinlet rp-mp and merely apicad of nodal veinlet mp-cua; branch MP1+2 forked basad of branch MP3+4 forking; branch MP3+4 forked apicad of apical line of veinlets. Stem CuA forked at about basal 1/3 of tegmen length, slightly basad of stem ScP+R forking. Veins with small tubercles. Clavus long, with apex reaching 2/3 of tegmen length.
Remark. This genus is tentatively placed in the tribe Cixiini.
Dweivera reikea Szwedo sp. nov.
Etymology. Specific epithet is derived from Proto-Celtic word ‘reike' meaning ‘tear'.
Holotype. NHMUK I.9193/I.10375; Brodie Collection (part and counterpart), Insect Limestone, NW Isle of Wight. Tegmen with claval and apical portion not preserved (part); body and tegmen with claval and apical portion of tegmina not preserved (counterpart).
Diagnosis. Costal cell about as wide as cell C1; cell C4 subquadrate, cell C5 more than twice as long as cell C4; veinlet icu basad of veinlet mp-cua, close to veinlet icu connecting posterior branch of CuA with tegmen posterior margin; apex of clavus reaching 2/3 of tegmen length.
Description. Length of tegmen 5.5mm, width at widest point 1.9mm. Other features as for the genus as it is the only included species.
Genus Samaliverus Szwedo gen. nov.
Etymology. Generic name derived from the Proto-Celtic word ‘samali' meaning ‘similar', combined with masculine form of the generic name of cixiid planthopper Dweivera. Gender: masculine.
Type species. Samaliverus bikkanus sp. nov.; here designated.
Diagnosis. Tegmen shape and venation similar to Dweivera gen. nov., but differs in smaller size; stem ScP+R forked basad of stem CuA forking (stem ScP+R forked slightly apicad of stem CuA forking in Dweivera); nodal veinlet rp-mp slightly apicad of stem MP forking (nodal veinlet rp-mp distinctly basad of stem MP forking in Dweivera); nodal veinlet mp-cua merely apicad of stem MP forking (nodal veinlet mp-cua basad of stem MP forking in Dweivera).
Description. Costal margin barely curved, apical margin elongately rounded. Stigma about three times as long as wide. Basal cell narrow. Stem ScP+R and MP leaving basal cell with common stem; stem ScP+R+MP forked distinctly basad of claval veins junction. Stem ScP+R forked slightly apicad of claval veins junction, vein ScP+RA1 forked at level of fusion of claval veins Pcu+A1 with posterior margin, vein RA with two terminals, vein RP forked distinctly apicad of nodal line, with three terminals. Vein MP with branch MP1+2 forked basad of apical line, branch MP3+4 slightly apicad of apical line; vein MP with five terminals. Stem CuA forked apicad of stem ScP+R forking and apicad of claval veins junction. Clavus with apex at 2/3 of tegmen length, claval veins Pcu and A1 fused at half of clavus length.
Remark. This genus is only tentatively placed in the tribe Cixiini.
Samaliverus bikkanus Szwedo sp. nov.
Etymology. Specific epithet derived from the Proto-Celtic word ‘bikkano' meaning ‘small'.
Holotype. NHMUK I.9199(2), with holotype of the braconid wasp Taphaeus cervicalis (Cockerell Reference Cockerell1921a); Brodie Collection, Insect Limestone, NW Isle of Wight. Tegmen with claval portion partly missing.
Diagnosis. Cell C1 very long, distinctly longer than cell C5; cell C3 shorter than cell C2, not divided into cells C3a and C3b basad of apical line of veinlets.
Description. Length of tegmen 4.06mm, width at widest point 1.32mm. Other features as for the genus as it is the only included species.
Genus Langsmaniko Szwedo gen, nov.
Etymology. Genus name from the Proto-Celtic word ‘langsmaniko' meaning ‘jumping'. Gender: neuter.
Type species. Langsmaniko marous sp. nov.; here designated.
Diagnosis. Tegmen with venation similar to Samliverus Szwedo, but differs in short common stalk ScP+R (distinctly longer in Samaliverus); branch RA forked slightly apicad of nodal veinlet ir; branch MP1+2 forked slightly apicad of nodal veinlet r-m (branch MP1+2 forked distinctly apicad of nodal veinlet rp-mp in Samaliversus).
Description. Tegmen with costal margin distinctly curved in basal portion, then nearly straight. Basal cell narrow, about 3.5 times as long as broad. Stalk ScP+R+MP, leaving basal cell very short. Stem ScP+R forked distinctly basad of stem CuA forking. Branch RA forked slightly basad of nodal veinlet ir. Stem MP not forked basad of nodal line veinlets; branch MP1+2 forked slightly apicad of nodal veinlet rp-mp, forked again distinctly basad of apical veinlets, then anteriad branch forked again apicad of apical veinlets. Stem CuA forked apicad of stem ScP+R forking. Nodal veinlets ir, rp-mp and mp-cua close each other, stepwise; apical line of veinlets regular, stepwise.
Langsmaniko marous Szwedo sp. nov.
Etymology. Specific epithet derived from the Proto-Celtic word ‘maro' meaning ‘remain'.
Holotype. NHMUK In.25286(1) (with Diptera: Sciaridae wing); Hooley Collection, Insect Limestone, NW Isle of Wight.
Diagnosis. Cell C1 shorter than cell C5. Cell C3a merely shorter than cell C3b; cell C3b of same length as cell C4.
Description. Estimated length of tegmen 7.4mm. Indistinct narrow darker band apicad of Sc+R forking, longitudinal veins darker, veinlets of apical line distinctly darker.
Genus Komsitija Szwedo gen. nov.
Etymology. Genus name from the Proto-Celtic word ‘komsitija' meaning ‘equal length'. Gender feminine.
Type species. Komsitija tuberculata sp. nov.; here designated.
Diagnosis. Tegmen venation similar to Macrocixius Matsumura, Reference Matsumura1914, but it differs in distinctly smaller length (about half of tegmen length of Macrocixius); forkings of stems ScP+R and CuA at same level (forking ScP+R slightly anteriad in Macrocixius); vein RA with two terminals (vein RA with single terminals in Macrocixius); nodal veinlet mp-cua very short, at level of MP3+4 branch (nodal veinlet mp-cua long, apicad of branch MP3+4 in Macrocixius); cells C3a and C3b subequal in length (cell C3b longer than cell C3a in Macrocixius).
Description. Costal margin curved at base, then gently curved, thickened. Stigma narrow, about 4.4 times as long as wide. Basal cell elongate, about 4 times as long as wide. Stems ScP+R and MP leaving basal cell at same point. Stem ScP+R forked at basal 1/3 of tegmen length, at same level as stem CuA forking; vein ScRA1 forked basad of stem MP forking, branch RA forked at level of posterior margin of stigma, with two terminals; branch RP forked apicad of nodal line, at about half of length of stigma, then both branches forked again, i.e., RP with four terminals. Stem MP forked at level of claval apex, anterior branch, i.e., branch MP1+2 forked again distinctly basad of apical line of veinlets, posterior branch, i.e., branch MP3+4, forked apicad of apical line, vein MP with five terminals. Stem CuA forked at same level as stem ScP+R, at basal 1/3 of tegmen length, posterior branch forked at level of apical line, i.e., vein CuA with three terminals. Veinlet ir between RA and RP very short, nodal veinlet mp-cua very short; apical veinlets ir, rp-mp, im, icu forming distinct apical line.
Remark. This genus is tentatively placed in the tribe Cixiini.
Komsitija tuberculata Szwedo sp. nov.
Etymology. Specific epithet refers to the presence of distinct setiferous tubercles along the veins.
Holotype. NHMUK Pl II 2999; donated D. Azar, Insect Limestone, Thorness Bay. Tegmen with clavus missing.
Diagnosis. Costal cell wider than cell C1; cell C1 tapered in apical portion, cell C2 very long, delimited posteriorly by apical veinlet rp-mp; cells C1 and C5 subequal in length, cell C4 short, distinctly shorter than adjoining cell C3b.
Description. Length of tegmen 5.1mm, width at widest point 1.77mm. Other features as for the genus as it is the only included species.
Genus Komnixta Szwedo gen. nov.
Etymology. Genus name from the Proto-Celtic word ‘komnixta' meaning ‘first cousin (female)'. Gender: feminine.
Type species. Komnixta jarzembowskii sp. nov.; here designated.
Diagnosis. Tegmen venation similar to Dweivera, but differs in cell C5 longer than cell C1 (cell C5 shorter than C1 in Dweivera); cell C3b twice as long as cell C3a (cell C3b about 1.2 times as long as cell C3a in Dweivera); stem ScP+R forked apicad of stem CuA forking (stem Sc+R forked basad of stem CuA forking in Dweivera).
Description. Stem ScP+R forked apicad of CuA forking; branch ScP+RA forked slightly apicad of nodal veinlets r-m and m-cu; Stigma more than twice as long as wide; stem RA with single terminal, RP forked at level of ir veinlet, then forked apicad of line of apical veinlets; with three terminals. Stem MP forked distinctly apicad of nodal veinlet r-m and merely apicad of nodal veinlet mp-cua, at level of ScP+RA1; branch MP1+2 forked basad of branch MP3+4 forking, then its anterior branch forked at level of apical veinlets; branch MP3+4 not forked. Stem CuA forked basad of stem ScP+R forking. Cell C5 longer than cell C1, widest at level of nodal vein mp-cua. Veins with small tubercles.
Remark. This genus is tentatively placed in the tribe Cixiini.
Komnixta jarzembowskii Szwedo sp. nov.

Plate 7 (1–3) Komnixta jarzembowskii Szwedo gen. et sp. nov., holotype: (1) MNEMG IL 87a, part; (2) MNEMNG IL 87b, counterpart; (3) SEM image of MNEMG IL 87a, part. (4) Worodbera nimakka Szwedo gen. et sp. nov., holotype, NHMUK In.43470, tegmen. (5) Hooleya indecisa Cockerell, Reference Cockerell1922, holotype, NHMUK In.24364, tegmen. (6) Sognotela emeljanovi Szwedo gen. et sp. nov., holotype, NHMUK In.25335, tegmen. (7) Catulliastites muiri (Cockerell, Reference Cockerell1922) gen. nov., holotype, NHMUK In.24365, tegmen. (8) Reteotissus hooleyi Szwedo gen. et sp. nov., holotype, NHMUK In.24806, tegmen.
Etymology. Specific epithet is given in honour to Dr Edmund A. Jarzembowski, eminent palaeoentomologist.
Holotype. Part and counterpart. Labelled MNEMG IL 87a (part); MNEMG IL 87b (counterpart), Insect Limestone, NW Isle of Wight. Apical part of tegmen, basal portion and clavus missing.
Diagnosis. Costal cell slightly wider than cell C1; stigma ca.2.2 times as long as wide; cell C1 longer but narrower than cell C2; cell C4 of similar length as adjoining cell C3b; cell C3b more than twice as long as cell C3a; apical veinlets icu, mp-cua, im stepwise.
Description. Estimated length of tegmen ca.6–7mm; width ca.2mm; darker band at level of apical veinlets. Other features as for genus.
Tribe Pintalini Metcalf, 1938
Genus Worodbera Szwedo gen. nov.
Type species. Worodbera nimakka sp. nov.; here designated.
Etymology. Genus name is derived from the Proto-Celtic word ‘*wor-od-ber-o-' meaning ‘additional work'. Gender: feminine.
Diagnosis. Venation of apical part of tegmen similar to some species of Pintalia Stål, Reference Stål1862. Appendix wide up to claval apex (as in Pintalia), RA with two terminals, RP with four terminals, stem MP forked slightly apicad of transverse veinlets rp-mp and mp-cua, branch MP1+2 forked basad of branch MP3+4 fork, at level of forst RP fork; cell C5 nearly twice as long as cell C3; Apical line of veinlets regular.
Description. Tegmen with apical margin rounded, with widened appendix, stigma area (?) (thickened); stem ScP+R forked basad of stem MP fork, branch RA with two terminals reaching margin, branch RP with four terminals reaching margin, first fork, basad of ir veinlet, slightly apicad of RA fork, and slightly basad of terminal MP1 fork, second fork slightly apicad of rp-mp veinlet, third fork before reaching margin; stem MP forked slightly apicad of nodal veinlets rp-mp and mp-cua, branch MP1+2 forked slightly apicad of branch RP first fork, then terminal MP1 forked more apicad before reaching the margin, branch MP3+4 forked slightly basad of branch MP1+2 fork; stem MP reaching apical margin with five terminals; stem CuA forked distinctly basad of stem M, reaching margin with two terminals. Cell C3 about 0.6 times as long as cell C2a.
Worodbera nimakka Szwedo sp. nov.
Etymology. Specific epithet is derived from the Proto-Celtic words ‘makko-' meaning ‘surety' with a negative particle ‘ni'.
Holotype. NHMUK In.43470; Hooley Collection, Insect Limestone, NW Isle of Wight. Tegmen, costal portion weakly preserved.
Diagnosis. Cell C5 about 2.3 times as long as cell C3; cell C2a longer than cell C4a; cell C3a about 0.6 as long as cell C3; cell C3 narrowing toward apex; costal margin strengthened at level of nodal line, nodal line veinlet rp-mp oblique, slightly anteriad of straight nodal line mp-cua; apical line of veinlets regular.
Description. Preserved portion of tegmen 2.16mm long. Costal margin strengthened at level of nodal line, appendix widened. Apical line of veinlets regular, composed of ir, rp-mp, im and icu veinlets.
Family Achilidae Stål, Reference Stål1866
Subfamily Achilinae Stål, Reference Stål1866
Tribe Achillini Emeljanov, Reference Emeljanov and Yemel'yanov1991
Genus Hooleya Cockerell, Reference Cockerell1922
Type species. Hooleya indecisa Cockerell, Reference Cockerell1922, p. 160, by monotypy.
Diagnosis. Differs from Achilla Haglund, Reference Haglund1899 and Maurisca Emeljanov, Reference Emeljanov2005 by stem ScP+R leaving basal cell separately from stem of vein MP (short common stem ScP+R+MP in Achilla and Maurisca); branches of RA reaching costal margin obliquely, inclined apicad (branches of RA perpendicular or slightly inclined basad in Achilla and Maurisca); forkings of vein RP close each other (forkings of RP more widely spaced in Achilla and Maurisca), first veinlet r-m apicad of third forking of vein MP (apicad of first branching of MP in Achilla and Maurisca).
Description. Costal margin distinctly curved at base, then nearly straight; basicostal area distinct, comparatively wide at base and narrow in remainder portion, up to stigmal area. Costal margin at level of stigmal area slightly widened, striated. Basal cell relatively narrow, about three times as long as wide. Costal cell wide, slightly wider than cell C2, about twice as wide as cell C1. Stem ScP+R short, leaving basal cell separately from stem MP; vein RP separates from stem ScP+R at level of CuA forking; branch ScP+RA1 strongly oblique, branches RA less oblique, five preserved terminals visible (probably seven or eight terminals); RP with three visible terminals, narrowly separated each other; vein MP forked at level of ScP+RA1 forking, with four visible branches preserved (probably five terminals); vein CuA forked at level of stem ScP+R forking. Apex of clavus probably exceeding half of tegmen length. First veinlet rp-mp apicad of second MP forking, second veinlet rp-mp placed close to first, near third forking of RP; veinlet mp-cua perpendicular to lower branch of CuA, at level of ScP+RA1 forking.
Hooleya indecisa Cockerell, Reference Cockerell1922
Figures 36–44 (36) Hooleya indecisa Cockerell, Reference Cockerell1921b, holotype, NHMUK In.24364, tegmen. (37) Hooleya indecisa Cockerell, Reference Cockerell1921b, reconstruction of tegmen venation after Emeljanov (Reference Emeljanov and Emeljanov1994). (38–43) Tegmen: (38) Sognotela emeljanovi Szwedo gen. et sp. nov., holotype, NHMUK In.25335; (39) Catulliastites muiri (Cockerell, Reference Cockerell1922) gen. nov., comb. nov., holotype, NHMUK In.24365; (40) Reteotissus hooleyi Szwedo gen. et sp. nov. holotype, NHMUK In.24806; (41) Phatanako wilmattae (Cockerell Reference Cockerell1926) gen. nov., comb. nov., holotype, NHMUK In.26637; (42) Keriophettus atibenus Szwedo gen. et sp. nov., holotype, NHMUK In.24387; (43) Senogaetulia kwalea Szwedo gen. et sp. nov., holotype, IWCMS 2018.49. (44) Dakrutulia mikhailkozlovi Szwedo gen. et sp. nov., holotype, NHMUK In.24602, tegmen, part. Scale bar=1mm.
v*1922 Hooleya indecisa Cockerell, p. 160, fig. 2.
1992 Hooleya indecisa: Carpenter, p. 256.
1994 Hooleya indecisa: Emeljanov, p. 77: fig. 1a, pl. 7, fig. 1.
2004 Hooleya indecisa: Szwedo et al., p. 42.
2006 Hooleya indecisa: Szwedo, 2006a, p. 167.
Holotype. NHMUK In.24364; Hooley Collection, Insect Limestone, NW Isle of Wight. Basal portion of tegmen, with part of claval portion preserved, apical portion of membrane missing.
Diagnosis. Tegmen with basicostal area elongate, widest at base. Branch ScP+RA1 oblique, first terminal of vein RA subparallel to branch ScP+RA1. Cell C1 half as wide as costal cell; cell C5 narrower than cell C2, about as wide as cell C1. First veinlet rp-mp oblique. First and second forkings of lower branch of vein MP at similar distance, about as width of cell C2.
Description. Length of preserved fragment of tegmen 8.8mm, width 4.0mm. Other features as for the genus as it is the only included species.
Remark. Originally placed in Derbidae, but transferred to Achilidae: Achillini by Emeljanov (Reference Emeljanov and Emeljanov1994). Emeljanov (Reference Emeljanov and Emeljanov1994) presented also a reconstruction of tegmen venation (Fig. 35). Carpenter (Reference Carpenter1992) listed it in ‘Family uncertain' section.
Another, but very incomplete specimen, NHMUK In. 24822; Hooley Collection, could belong to the genus Hooleya Cockerell.
Family Tropiduchidae Stål, Reference Stål1866
Subfamily Tropiduchinae Stål, Reference Stål1866
Tribe Tambiniini Kirkaldy, Reference Kirkaldy1907
Genus Sognotela Szwedo gen. nov.
Etymology. Combination of Proto-Celtic words ‘sogno' meaning ‘net' and extinct dictyopharid generic name Netutela, to which it is superficially similar. Gender: feminine.
Type species. Sognotela emeljanovi sp. nov.; here designated.
Diagnosis. Similar to some species of the genus Tambinia Stål, Reference Stål1859, but tegmen more elongate, as in Garumna Melichar, Reference Melichar1914. It differs by shorter prenodal portion of cell C5 (more than twice as long as wide in Tambinia), less oblique nodal line, rounded postnodal line, postnodal cells about as long as apical cells (postnodal cells shorter than apical cells in Garumna); stem MP3+4 forked merely apicad of nodal line level (stem MP3+4 not forked or forked apicad of nodal line level in Tambinia).
Description. Tegmen elongate, narrow, costal margin slightly thickened, apical margin elongately rounded, clavus reaching 0.6 of tegmen length. Stem ScP+R long, forked slightly basad of apex of clavus, apicad of CuA forking; single terminal ScP+RA1 and RA2, RP with two terminals. Stem MP long, forked at level of nodal line, stem MP1+2 forked at apical line, stem MP3+4 forked merely apicad of nodal line, distinctly apicad of ScP+R and CuA forkings, lower branch forked at level of apical line of veinlets, with two terminals, upper branch forked merely apicad of nodal line, with two terminals. Stem CuA forked basad of ScP+R forking, basad of apex of clavus. Nodal line distinct, apical line of veinlets distinct, arcuate.
Sognotela emeljanovi Szwedo sp. nov.
Etymology. Species is named in honour of Professor Alexander F. Emeljanov, an eminent specialist on planthoppers and leafhoppers.
Holotype. NHMUK In.25335; Hooley Collection, Insect Limestone, NW Isle of Wight. Tegmen, with claval portion missing.
Diagnosis. Cell C1 short, subtriangular, cell C3a shorter than cell C3, with posterior margin arcuate. Single terminal of RA, two terminals of RP, four terminals of MP, veinlet icu apicad of nodal line present.
Description. Length of tegmen 3.9mm, width at widest point 1.22mm. Other features as for the genus as it is the only included species.
Tribe Catullini Melichar, Reference Melichar1914
Genus Catulliastites Szwedo nom. nov.
Etymology. Genus name is derived from the Catullia – generic name of tropiduchid planthopper combined with Hastites original, but homonymic name given to this fossil by Cockerell. Gender: masculine.
Type species. Hastites muiri Cockerell, Reference Cockerell1922, p. 161; by monotypy.
Hastites Cockerell, Reference Cockerell1922 (Insecta: Hemiptera: Cixiidae) nec Hastites Mayer-Eymar, Reference Mayer-Eymar1883 (Mollusca: Cephalopoda: Belemnitida: Hastitidae)
1883 Hastites Mayer-Eymar, pp. 640, 642 (Mollusca); type species: Belemnites clavatus Schlotheim, Reference Schlotheim1820, p. 49.
1922 Hastites Cockerell, p. 161 (Insecta); type species: Hastites muiri Cockerell, Reference Cockerell1922, p. 161.
1992 Hastites Cockerell; Carpenter, p. 256 (Insecta).
2004 Hastites Cockerell; Szwedo et al., p. 93 (Insecta).
Diagnosis. Venation pattern similar to other genera of Catullini, but differs in more apicad line of transverse veinlets forming nodal line. It differs from Catullia Stål, Reference Stål1870 by higher number of veinlets in basicostal area (only a few in Catullia); more apical forking of stem Sc+R, slightly apicad of apex of clavus (forking of stem ScP+R slightly basad of apex of clavus in Catullia).
Description. Tegmen with costal margin mildly curved. Basicostal area narrow, with few veinlets. Basal cell elongate, about three times as long as wide. Stem ScP+R leaving basal cell separately from stem MP; first forking of ScP+R slightly apical of apex of clavus; two terminals: ScP+RA1 and RA2; branch RP with single terminal. Stem MP very long, first forking more apicad than ScP+R forking, with four terminals. Stem CuA forked slightly basad of apex of clavus, with four terminals. Clavus long, with apex reaching 0.6 of tegmen length, claval veins Pcu and A1 fused distinctly apicad of half of clavus length. Single veinlets between Pc+CP and ScP+R; RP and MP, and MP and CuA branches, apical row of veinlets rp-mp, im and mp-cua present.
Remark. According to Muir's opinion cited in Cockerell (Reference Cockerell1922), this genus could be placed in Dictyopharidae, relating it to the genera Hasta Melichar, Reference Melichar1914 and Thanatodictya Kirkaldy, Reference Kirkaldy1906. It is not mentioned in Metcalf & Wade's (Reference Metcalf and Wade1966) catalogue. Carpenter (Reference Carpenter1992) listed it under ‘Homoptera, Family uncertain'. The venation characters exclude it from Dictyopharidae and place it in the Tropiduchidae tribe Catullini.
Catulliastites muiri (Cockerell, Reference Cockerell1922) comb. nov.
v*1922 Hastites muiri Cockerell, Reference Cockerell1922, p. 161, fig. 3.
1992 Hastites muiri; Carpenter, p. 256.
2004 Hastites muiri; Szwedo et al., p. 93.
Description. Length of tegmen 5.6mm, width at widest point 1.6mm. Other features as for the genus as it is the only included species.
Holotype. NHMUK In.24365; Hooley Collection, Insect Limestone, NW Isle of Wight. Tegmen, with some portion of basal portion not preserved.
Diagnosis. Tegmen elongate, length/width ratio about 3.46:1. Costal area present, long and narrow, exceeding apex of clavus, with few transverse veinlets. Nodal line of veinlets shifted apicad of apex of clavus, subapical line of a few veinlets. Cell C2 about as long as cell C3. Single terminals of ScRA1, RA2 and RP, vein M with four terminals, vein CuA with four terminals.
Tribe Trypetimorphini Melichar, Reference Melichar1914
Genus Reteotissus Szwedo gen. nov.
Etymology. Combination of the Celtic word ‘reteo' meaning ‘run' and tropiduchid generic name Ommatissus. Gender: masculine.
Type species. Reteotissus hooleyi sp. nov.; here designated.
Diagnosis. Differs from Ommatissus Fieber, Reference Fieber1875 by more simple venation, with veins not forked apicad of nodal line; stems ScP+R, MP and CuA leaving basal cell separately (common stem of M and CuA in Ommatisus). Common terminal of posterior branch of MP and anterior branch of CuA apical of nodal line reaching apical margin (terminals of MP and CuA reaching apical margin separately in Ommatisus).
Description. Costal margin gently curved, thickened, apical margin elongately rounded, veins thick, apex of clavus reaching 0.6 of tegmen length; basal cell elongate, narrow. Stems of veins ScP+R, MP and CuA leaving basal cell independently, but close each other. Stem ScP+R not forked before nodal line, single terminal ScP+RA, two terminals of RP; stem MP not forked before nodal line, posterior branch as common terminal with anterior branch of CuA; vein CuA forked at level of ScP+RA branching.
Reteotissus hooleyi Szwedo sp. nov.
Etymology. Specific epithet is given after collector R. W. Hooley.
Holotype. NHMUK In.24806; Hooley Collection, Insect Limestone, NW Isle of Wight. Tegmen with clavus missing.
Diagnosis. Venation reduced, costal margin thickened; five cells apicad of nodal line.
Description. Length of tegmen 4.2mm, width of tegmen at widest point 1.3mm. Other features as for the genus as it is the only included species.
Tribe Jantaritambini Szwedo, 2000
Genus Phatanako Szwedo gen. nov.
Etymology. The name is derived from Proto-Celtic word ‘phatanako' meaning ‘winged'. Gender: masculine.
Type species. Myndus wilmattae Cockerell, Reference Cockerell1926; here designated.
Diagnosis. Venation similar to Isporisa Walker, Reference Walker1857 (Isporisini) and Jantaritambia Szwedo, 2000 (Jantaritambini). From Isporisa it differs in tegmen widest at level of claval apex (tegmen widest near middle in Isporisa); apex of clavus exceeding ¾ of tegmen length (clavus not extending beyond middle of clavus in Isporisa); apical margin shallowly rounded (deeply rounded in Isporisa); nodal line neither even nor oblique (nodal line apical cells distinctly shorter than half of subapical cells; apical cells longer than half of subapical cells in Isporisa). From Jantaritambia it differs by apex of clavus distinctly exceeding ¾ of tegmen length (clavus reaching ¾ of tegmen length in Jantaritambia); basal cell about twice as wide as long (three times as long as wide in Jantaritambia); apical cells less than half of length of subapical cells (apical cells more than half of length of subapical cells in Jantaritambia).
Description. Tegmen unpigmented, not coriaceous, widest slightly anteriad of apex of clavus, without basicostal area and transverse veinlets branching from Pc+CP. Nodal line nether oblique nor even, apical line not distinctly stepwise. Basal cell about twice as long as wide. Vein R forked once before nodal line, RA with single terminal, RP with three terminals; vein MP not united basally with vein CuA, not forked before nodal line, with five terminals; vein CuA forked once before nodal line, with two terminals. First veinlet icu nearly perpendicular to lower branch of CuA, lying at nodal line.
Phatanako wilmattae (Cockerell, Reference Cockerell1926) comb. nov.

Plate 8 (1) Phatanako wilmattae (Cockerell, Reference Cockerell1926) gen. nov., comb. nov., holotype, NHMUK In.26637, tegmen. (2) Keirophettus atibenus Szwedo gen. et sp. nov., holotype, NHMUK In.24387, tegmen. (3) Senogaetulia kwalea Szwedo gen. et sp. nov., holotype, IWCMS 2018.49, tegmen. (4) Dakrutulia mikhailkozlovi Szwedo gen. et sp. nov., holotype, NHMUK In.24602, tegmen. (5) Krundia korba Szwedo gen. et sp. nov., holotype, NHMUK Pl II 2740a, tegmen, part. (6) Breukoscelis vadimgratshevi Szwedo gen. et sp. nov., holotype, NHMUK In.26043, tegmen. (7) Breukoscelis phrikkosus Szwedo gen. et sp. nov., holotype, NHMUK In.26035, tegmen. (8) Uphodato garwoterus Szwedo gen. et sp. nov., holotype, NHMUK In.24502, tegmen.
v*1926 Myndus wilmattae Cockerell, p. 322, Fig. 12.
2004 Myndus wilmattae; Szwedo et al., p. 97.
Holotype. NHMUK In.26637, Insect Limestone, NW Isle of Wight. Tegmen, with claval portion incomplete, basal portion not distinct.
Diagnosis. Tegmen with cell C1 slightly longer than cell C3, cell C3 slightly longer than cell C4, cell C5 the longest, nearly twice as long as cell C4. Tegmen with ten apical cells, distinctly shorter than subapical ones. Apical portion of tegmen widely rounded.
Description. Length of tegmen 5.2mm, width at widest point 2.05mm. Costal margin curved at base. Claval portion incomplete, other features as for the genus as it is the only included species.
Remark. Shcherbakov (Reference Shcherbakov2006) placed Jantaritambia loculata (Germar & Berendt Reference Germar, Berendt and Berendt1856) and ‘Cixius' succineus Germar & Berendt, Reference Germar, Berendt and Berendt1856 in the tribe Jantariambini, both from Eocene Baltic amber.
Genus Keirophettus Szwedo gen. nov.
Etymology. Generic name is derived from Proto-Celtic words: ‘keiro' meaning dark brown and ‘phett' wing. Gender: masculine.
Type species. Keirophettus atibenus sp. nov.
Diagnosis. Differs from Phatanako by very oblique stem portion of ScP+RA prolonged by ScP+RA1, reaching costal margin (ScP+RA1 less oblique in Phatanako, more arcuate in Jantaritambia); branch RA reaching margin with four terminals (single terminal in Phatanako, two terminals in Jantaritambia); branch MP3+4 single (as in Jantaritambia, forked in Phatanako); cell C1 narrow, without veinlet (cell C1 about as wide as cell C2, with nodal line veinlet ir in Phatanako and Jantaritambia).
Description. Costal margin strengthened, appendix with transverse wrinkles visible. Stem ScP+R forked slightly apicad of stem CuA fork, distinctly basad of stem MP forking; branch ScP+RA basad of ScP+RA1 terminal about as long as terminal ScP+RA1, branch RA after ScP+RA1 leaving slightly arcuate, reaching margin with four terminals; branch RP forked in apical portion of membrane, reaching margin with three terminals; stem MP forked at nodal line level, slightly anteriad of nodal veinlets rp-mp and mp-cua; branch MP1+2 forked again on membrane, basad of RP forking; branch MP3+4 single; stem CuA forked basad of stem ScP+R forking. Nodal line not fully developed, composed of nodal veinlet rp-mp, perpendicular to branch RP, basal portions of MP1+2 and MP3+4 branches and oblique veinlet mp-cua. Membrane with subapical veinlet ir and rp-mp, and apical veinlet ir (other veinlets not preserved). Stigmal area with indistinct sigmoid veinlet. Cell C1 narrow, about 7 times as long as wide, closed apically by subapical veinlet ir; Cell C2a about 2.5 times as long as wide; cell C5 long, lanceolate.
Keirophettus atibenus Szwedo sp. nov.
Etymology. Specific epithet derived from Proto-Celtic ‘atibena' meaning cut.
Holotype. NHMUK In.24387 (H102), Hooley Collection, Insect Limestone, NW Isle of Wight. Part of tegmen, with basal and apical portions deteriorated; traces of coloration preserved.
Diagnosis. Cell C1 about 2.2 times as long as cell C2a; terminal ScP+RA1 forked near connection to costal margin; forking of branch RP more apical than forking of branch MP1+2; branch RP2 forked again apically; cells formed between terminals of RA, narrow, longer than wide.
Description. Length of preserved portion of tegmen 3.1mm, width 1.33mm. Basal and apical portion not preserved, clavus missing. Apical half darkened, with several patchy lighter spots; basal portion light, with a few indistinct darker patches. Other features as for the genus as it is the only included species.
Subfamily Elicinae Melichar, Reference Melichar1915
Tribe Elicini Melichar, Reference Melichar1915
Genus Senogaetulia Szwedo gen. nov.
Etymology. Combination of Proto-Celtic ‘seno' meaning ‘old' and generic name Gaetulia Stål, Reference Stål1864. Gender: feminine.
Type species. Senogaetulia kwalea sp. nov.; here designated.
Diagnosis. Tegmen with venation pattern similar to Indogaetulia Schmidt, Reference Schmidt1919. It differs by tegmen with basicostal area wider than costal cell (basicostal area about as wide as costal cell in Indogaetulia), cell C5 long, not separated by veinlet (cell C5 short, delimited by transverse veinlet in Indogaetulia); cell C3 about 3 times as long as wide (twice as long as wide in Indogaetulia); first forkings of stem MP and CuA at same level (stem CuA forked slightly anteriad of stem MP forking in Indogaetulia).
Description. Tegmen with costal margin distinctly curved at base, anterior angle angulate, apical margin arcuate, posterior angle angulate. Clavus long, apex of clavus reaching 2/3 of tegmen length. Basicostal area wider than costal cell, with few veinlets. Basal cell wide, stems ScP+R, MP and CuA leaving basal cell independently; stem ScP+R forked.
Senogaetulia kwalea Szwedo sp. nov.
Etymology. Specific epithet from Proto-Celtic word ‘kwale' meaning ‘dig'.
Holotype. IWCMS 2018.49, Insect Limestone, NW Isle of Wight. Tegmen, with apical portion partly not preserved.
Diagnosis. Cell C3 about as long as cell C4; posteroapical margin of tegmen angulately rounded; four darker spots near the apical margin of tegmen.
Description. Length of tegmen 7.5mm, width at widest point 4.2mm. Other features as for the genus, as it is the only included species.
Genus Dakrutulia Szwedo gen. nov.
Etymology. Generic name is combination of the Proto-Celtic word ‘dakru' meaning ‘tear' with nogodinid planthopper genus name Gaetulia Stål, Reference Stål1864. Gender: feminine.
Type species. Dakrutulia mikhailkozlovi sp. nov., here designated.
Diagnosis. Tegmen with pattern of venation similar to Indogaetulia Schmidt, Reference Schmidt1919. It differs by short and more rounded shape of tegmen (tegmen elongate), about twice as long as wide in Indogaetulia; cell C1 longer than cell C3 (cells C1 and C3 subequal in length in Indogaetulia); cell C3 with three cell adjoining cells apicad (two adjoining cells apicad in Indogaetulia); cell C5 elongate, without preapical icu veinlet (preapical veinlet icu present in Indogaetulia).
Description. Tegmen short, rounded; apical margin roundly arcuate. Stem ScP+R forked slightly basad of stem MP forking. Branch ScP+RA with a few terminals, branch RP with four terminals. Stem MP with branch MP1+2 with five terminals, branch MP3+4 with three terminals, i.e., MP with eight terminals; stem CuA forked at level of stem MP forking, then at level of branch MP3+4 forking, with three terminals. Subapical line of veinlets strongly stepwise; apical line distinct, regular. Cells apically adjoining cells C1, C2 and C3, shorter of them.
Remark. The fossil tegmen consists of strongly torn remnants, but the pattern of venation could be reconstructed. It matches the venation pattern found in the representatives of the tribe Gaetulini.
Dakrutulia mikhailkozlovi Szwedo sp. nov.
Etymology. Specific epithet is given in honour of the late Mikhail A. Kozlov, specialist on Hymenoptera: Proctotrupoidea.
Holotype. part NHMUK In.24602, Hooley Collection; counterpart CAMSM X.50140.33 (TN 83), Smith Collection, Insect Limestone, NW Isle of Wight. Tegmen, torn, with clavus, basal and costal portions missing.
Diagnosis. Both cells adjoining cell C2 apicad distinctly shorter of it; basal veinlet r-m basad of veinlet m-cu; apical cells slightly shorter than subapical cells.
Description. Preserved length of tegmen 5.1mm, width 4.2mm. Other features as for the genus as it is the only included species.
Family Issidae Spinola, Reference Spinola1839
Subfamily Issinae Spinola, Reference Spinola1839
Tribe Issini Spinola, Reference Spinola1839
Genus Krundia Szwedo gen. nov.
Etymology. Genus name derived from the Proto-Celtic word ‘krundi' meaning ‘round'. Gender: feminine.
Type species. Krundia korba sp. nov.; here designated.
Diagnosis. In shape of tegmen and pattern of venation resembling some genera formerly placed in the tribe Thioniini Melichar, Reference Melichar1906 (Gnezdilov Reference Gnezdilov2013). In respect to strong convexity of tegmen it resembles representatives of Issidae: Hemisphaeriini Melichar, Reference Melichar1906. It could be characterised by the following combination of characters: tegmen curved at costal margin, widely rounded at apical margin; common stalk ScP+R relatively long, stem ScP+R forked slightly apicad of stem MP forking; stem CuA single, nodal line of veinlets basad of apex of clavus, apical line of veinlets regular, distinct; apical cells shorter than subapical cells.
Description. Tegmen about 1.8 times as long as wide. Costal margin slightly curved, apical margin widely rounded. Basal cell about twice as long as wide; very short stem ScP+R+MP leaving basal cell; stem ScP+R shorter than stem MP. Stem ScP+R forked at ¼ of tegmen length, slightly basad of stem MP forking; vein ScP+RA1 forked at level of nodal line, branch RA forked apicad of nodal line; branch RP forked basad of apical line of veinlets. Stem MP forked slightly apicad of stem ScP+R forking, anterior branch forked apicad of apical line of veinlets; posterior branch forked slightly basad of nodal line, then mediad branch forked again basad of apical line. Stem CuA single. Apex of clavus exceeding half of tegmen length.
Remark. The genus is tentatively placed in the subfamily Issinae, tribe Issini. In shape of tegmen and pattern of venation it resembles some of Issidae: Issinae: Thioniini. In respect to strong convexity of tegmen it resembles representatives of Issidae: Hemisphaeriini. Strongly convex tegmina are also present in the genus Breukoscelis Szwedo gen. nov. from the same deposit (see below in the same section).
Krundia korba Szwedo sp. nov.
Figures 45–54 (45) Krundia korba Szwedo gen. et sp. nov., holotype In. Pl II 2740a, tegmen, part. (46–51) Tegmen: (46) Breukoscelis vadimgratshevi Szwedo gen. et sp. nov., holotype, NHMUK In.26043; (47) Breukoscelis phrikkosus Szwedo gen. et sp. nov., holotype, NHMUK In.26035; (48) Uphodato garwoterus Szwedo gen. et sp. nov., holotype, NHMUK In.24502; (49) Ambitaktoa stoumma Szwedo gen. et sp. nov., holotype, NHMUK In.17282; (50) Phariberea gurdonika Szwedo gen. et sp. nov., holotype, NHMUK I.17096; (51) Wixskimoa torxsea Szwedo gen. et sp. nov., holotype, MIWG 3609. (52) Wixskimoa torxsea Szwedo gen. et sp. nov., holotype, MIWG 3609, visible portion of hindwing. (53, 54) Tegmen: (53) Niadrima yulei Szwedo gen. et sp. nov., holotype, NHMUK II.3046; (54) Ankwomwarius brodiei Szwedo gen. et sp. nov., holotype, NHMUK I. 8803. Scale bar=1mm.
Etymology. Specific epithet derived from the Proto-Celtic word ‘korb' meaning ‘stain' and refers to colour pattern of the tegmen.
Holotype. NHMUK Pl II 2740a, b (part and counterpart); collected by C. Buckley, Insect Limestone, Thorness Bay. Tegmen with clavus missing.
Diagnosis. Costal cell about as wide as cell C1; nodal veinlet ir distinctly oblique, nodal veinlets rp-mp, and im arranged in row; apical veinlets arranged in row; anterocubital cell, between stems CuA and CuP, about half of width of cell C4 at widest point.
Description. Length of tegmen 3.2mm, width at widest point 1.76mm. Darker wide, transverse band apicad of half of tegmen length; darker spot at level of ScP+RA1 branch. Other features as for the genus as it is the only included species.
Remark. Another specimen, NHMUK Pl II 2714, collected by the ‘Polish Team' 23 May 2005, N. end Thorness Bay, is probably also related to Krundia.
Genus Breukoscelis Szwedo gen. nov.
Etymology. Combination of the Proto-Celtic word ‘breuko' of not recognised meaning and generic name of the planthopper – Caliscelis Laporte, Reference Laporte1833. Gender: masculine.
Type species. Breukoscelis vadimgratshevi sp. nov., here designated.
Diagnosis. General shape of tegmen and venation similar to various genera of Issidae. It can be recognised by the following combination of characters: tegmen elongate, strongly convex; basicostal area very distinct, thickened, elongate; short common stem ScP+R+MP; stem MP forked basad of stem ScP+R forking; stem RP forked at level of stem MP1+2 forking, with several terminals; stem MP1+2 with several terminals, stem MP3+4 with a few terminals; stem CuA single.
Description. Tegmen about 2.5 times as long as wide, base of costal margin strongly curved, with distinct and thickened, elongate basicostal area, median portion of costal margin merely curved, posterior margin widely arcuate. Common stem ScP+R and MP short; stem ScP+R forked apicad of stem MP forking; branch ScP+RA relatively short, reaching costal margin with terminals ScP+RA1 and RA2; branch RP forked at level of RA2 branching, anterior branch of RP with four terminals reaching costal margin and anterior angle; posterior branch of RP reaching apical margin with a few terminals. Stem MP forked shortly after separation of stem ScP+R, basad of stem ScP+R forking; branch MP1+2 forked at level of branch RP forking, then forked at level of apical line of veinlets. Stem CuA single. Veinlet mp-cua placed distinctly basad of subapical veinlets ir and im; subapical veinlets ir and im not forming distinct line; apical row of veinlets regular.
Remark. The genus described above presents a very peculiar set of characters. In some respect its venation could resemble patterns found in macropterous forms of Caliscelidae Amyot & Audinet-Serville, Reference Amyot and Audinet-Serville1843. However Issidae: Issinae and Acanaloniidae also present high variability in venation patterns, and some similarities can be found. Forms with strongly convex tegmina are present in all of these families. In Emeljanov's (Reference Emeljanov1999) opinion Issidae are distinguished by pterygomonomorphism with slightly diminished, peculiar, case-shaped or steeply tectiform tegmina. Caliscelidae are distinguished by the pterygonodimorphism with prevalence of strongly brachypterous forms. Acanaloniidae are characteristic of tegmina without costal area. Based only on the tegminal characters it is not possible to resolve the taxonomic placement of the genus. However, it is tentatively placed in the subfamily Issinae, but given its very particular pattern of venation it could represent separate taxon of tribal or subfamilial level.
Breukoscelis vadimgratshevi Szwedo sp. nov.
Etymology. Specific epithet is given in the honour of the late Vadim G. Gratshev, specialist on curculionid beetles.
Holotype. NHMUK In.26043; Hooley Collection, Insect Limestone, NW Isle of Wight. Tegmen with basal portion and clavus missing.
Diagnosis. Branch RP forked at level of forking of branch MP1+2, anteriad branch with four terminals, mediad branch forked at level of apical line, with two terminals; branch MP1+2 forked at level of branch RP forking, with seven terminals; branch MP3+4 with single terminal; short veinlet rp-mp apicad of subapical veinlets ir and im; cell C1 less than 1.5 times as long as adjoining subapical cell; cell C2 not extending to apical row of veinlets, delimited apically by short veinlet rp-mp.
Description. Length of tegmen 5.3mm, width at widest point 1.9mm.
Breukoscelis phrikkosus Szwedo sp. nov.
Etymology. Specific epithet is derived from the Proto-Celtic word ‘phrikko' meaning ‘furrow'.
Holotype. NHMUK In.26035; Hooley Collection, Insect Limestone, NW Isle of Wight. Tegmen with basal portion and clavus missing.
Diagnosis. Tegmen smaller than in Breukoscelis vadimgratshevi sp. nov.; branch RP with eight terminals (six terminals in B. vadimgrachevi), branch MP1 with three terminals, branch MP2 with two terminals, branch MP3+4 with two terminals respectively (five, two and one terminals respectively in B. vadimgrachevi); cell C1 about 1.5 times as long as adjoining subapical cell; cell C2 narrow and elongate, delimited apically by veinlet rp-mp of apical row.
Description. Length of tegmen 4.7mm, width at widest point ca.2mm; dark, triangular spot in median portion of tegmen.
Genus Uphodato Szwedo gen. nov.
Etymology. Genus name derived from the Proto-Celtic word ‘uphodato' meaning ‘sediment'. Gender: neuter.
Type species. Uphodato garwoterus sp. nov.; here designated.
Diagnosis. Tegmen with venation superficially resembling Breukoscelis Szwedo, but distinctly smaller. Tegmen tuberculate along veins. Common stalk ScP+R+MP elongate (similarly to Breukoscelis); stem ScP+R forked distinctly more basad than stem M forking (stem ScP+R forked apicad of stem MP forking in Breukoscelis); stem CuA forked apicad of nodal line (stem CuA not forked in Breukoscelis); subapical line of veinlets apicad of apex of clavus (subapical veinlets basad of apex of clavus in Breukoscelis).
Description. Tegmen with widened basicostal area, about 2.5 times as long as wide, with rounded apical portion; with apex reaching 2/3 of tegmen length. Basal cell elongate, about twice as long as broad. Common stalk ScP+R+MP elongate, longer than basal cell. Stem MP forked basad of ¼ of tegmen length; stem ScP+R forked well basad of stem MP first forking. Branch ScP+RA forked slightly basad of subapical line of veinlets; vein RA with two terminals; vein RP forked basad of apical line of veinlets, reaching margin with three terminals. Branch MP1+2 not forked basad of apical line of veinlets; branch MP3+4 forked at level of claval apex, basad of subapical veinlets rp-mp and im, reaching apical margin with three terminals. Stem CuA forked at level of apex of clavus. Clavus elongate, claval veins Pcu and A1 fused basad of half of tegmen length, at level of subapical veinlet m-cu. Apical veinlets ir, rp-mp, im and mp-cua forming nearly regular line.
Remark. Placement of this genus in Issidae: Issinae is only tentative. Similar forms with tubercular tegmina are known among recent Nogodinidae Melichar, Reference Melichar1898 – Andrewsiella Izzard, Reference Izzard1936 and Distiana Metcalf, Reference Metcalf1958. Venation pattern, placement of tubercles between veins and very long clavus reaching near the apex of tegmen differs them easily from Uphodato Szwedo. The two above-mentioned recent genera were placed in the family Acanaloniidae Amyot & Audinet-Serville, Reference Amyot and Audinet-Serville1843, which was recently redefined (Gnezdilov Reference Gnezdilov2012a) and the genera were moved to Nogodinidae. Also the generic content of the family Issidae was revised recently (Gnezdilov Reference Gnezdilov2013), with a new tribal classification proposed.
Uphodato garwoterus Szwedo sp. nov.
Etymology. Specific epithet is derived from the Proto-Celtic word ‘garwotero' meaning ‘roughness'.
Holotype. NHMUK In.24502; Hooley Collection, Insect Limestone, NW Isle of Wight. Tegmen with basicostal portion and part of clavus missing.
Diagnosis. Tegmen with subapical cells adjoining to cells C1 and C2 of same length; cell C4 shorter than cell C3; apical cells shorter than subapical ones; basal portion of tegmen darkened; wide, darker band basad of half of tegmen length; apical portion with indistinct dark pattern.
Description. Length of tegmen 4.1mm, width of tegmen 1.56mm. Other features as for the genus as it is the only included species.
Family Nogodinidae Melichar, Reference Melichar1898
Subfamily Ambitaktoinae Szwedo subfam. nov.
Type genus. Ambitaktoa gen. nov.; here designated.
Diagnosis. Differs from all other subfamilies of Nogodinidae by the following combination of characters: tegmen with stems ScP+RA and RP separated since base or with a short common stalk; stem MP forked very basad, about ¼ of tegmen length, then forked again; two rows of veinlets: subapical and apical, relatively regular; stem CuA single; clavus very long, reaching nearly to the posterior angle of tegmen.
Remark. The subfamily Ambitaktoinae subfam. nov. is tentatively placed within the Nogodinidae. The content of the family needs to be reconsidered. Emeljanov (Reference Emeljanov1999) suggested that Nogodinidae sensu stricto comprises forms with tegmen with costal area (‘precostal' in his nomenclature) present and with veinlets; hind tibiae with lateral spines, and postclypeus with lateral keels. He also redefined the Acanaloniidae Amyot & Audinet-Serville, Reference Amyot and Audinet-Serville1843 in his key to Issidae-like families distinguishing characters of these families are compared in couplets of the key opposing them to Nogodinidae. Shcherbakov (Reference Shcherbakov2006) discussed several extinct taxa and their placement in Nogodinidae sensu lato. The content and definitions of subfamilies and tribes placed in Nogodinidae were recently discussed by Gnezdilov (Reference Gnezdilov2017). Representatives of Ambitaktoini resemble some taxa placed in the Tonginae (group placed in Issidae, later Acanaloniidae, then Nogodinidae – Gnezdilov, 2007, recently redefined and partly supressed under Nogodinidae – Gnezdilov, 2017), but tegminal characters which are available are not enough for definite conclusion of placement of Ambitaktoinae.
The suprageneric classification and phylogeny of the family Nogodinidae is not understood. Fennah (Reference Fennah1978, Reference Fennah1984, Reference Fennah1987) divided it into two subfamilies: Nogodininae Melichar, Reference Melichar1898 with seven tribes (Nogodinini Melichar, Reference Melichar1898, Bladinini Kirkaldy, Reference Kirkaldy1907, Mithymnini Fennah, Reference Fennah1967, Pisachini Fennah, Reference Fennah1978, Varciini Fennah, Reference Fennah1978, Epacriini Fennah, Reference Fennah1978, Lipocalliini Fennah, Reference Fennah1984), and the monotypical Gastriniinae Fennah, Reference Fennah1987. Later, Gnezdilov (Reference Gnezdilov2007) supplemented Nogodininae with the tribe Tongini Kirkaldy, Reference Kirkaldy1907 (moved and downgraded from the family Issidae), but excluded the subtribe Gaetuliina Fennah, Reference Fennah1978 from the tribe Bladinini Kirkaldy, and transferred it to the family Tropiduchidae (Gnezdilov Reference Gnezdilov2007, Reference Gnezdilov2013). In addition, Gnezdilov (Reference Gnezdilov and Gnezdilov2012b) changed the status and position of Colpopterini Gnezdilov, Reference Gnezdilov2003 (formerly placed in Issidae) as subfamily Colpopterinae in Nogodinidae. Recent, molecular investigations (Sun et al. Reference Sun, Meng and Wang2015) supported separation of Tonginae from Issinae sensu stricto, but placement within Issidae is postulated by these authors. Gnezdilov (Reference Gnezdilov2017) divided Nogodinidae into four subfamilies with 11 recent tribes: Bladininae Kirkaldy, Reference Kirkaldy1907 with Bladinini, Colpopterinae Gnezdilov, Reference Gnezdilov2003 with Colpopterini Gnezdilov Reference Gnezdilov2003, Gastriniinae with Gastriniini Fennah, Reference Fennah1987, Nogodininae with tribes Lipocalliini, Nogodinini, Bilbiliini Gnezdilov, Reference Gnezdilov2017, Mithymini, Pisachini, Varciini, Tongini and Epacriini. Extinct Celinapterixini Petrulevičius, Reference Petrulevičius2005 are to be placed in Nogodininae (Bourgoin Reference Bourgoin2017).
Genus Ambitaktoa Szwedo gen. nov.
Etymology. Generic name is derived from the Proto-Celtic word ‘ambitakto' meaning ‘wander'. Gender: feminine.
Type species. Ambitaktoa stoumma sp. nov.; here designated.
Diagnosis. Characterised by the following combination of features: costal margin strongly curved at base, then gently curved, thickened; short common stem ScP+R leaving basal cell short, forked very basally; branch ScP+RA curved, subparallel to costal margin, branch RP nearly straight; stem MP forked at about ¼ of tegmen length, anterior branch forked again; costal cell narrower than cell C1.
Description. Tegmen with costal margin thickened, strongly curved at basal portion than gently curved; anterior apical angle angulately rounded, posterior angle rounded. Stems ScP+R, MP and CuA leaving basal cell independently. Common stem ScP+R very short; branch ScP+RA curved, subparallel to costal margin, without additional branches, branch RP forked at level of basal line of veinlets, with three terminals. Stem MP forked at ¼ of tegmen length, anterior branch forked again, mediad branch forked at level of basal veinlets, anteriad branch forked slightly apicad of basal line of veinlets; posterior branch forked at level of basal line of veinlets, i.e., vein MP with six terminals. Stem CuA not forked, reaching to posterior angle near apex of clavus. First veinlet ir basad of basal line of veinlets; basal line veinlets rp-mp, im and mp-cua almost in one line. Apical line of veinlets subparallel to apical margin.
Ambitaktoa stoumma Szwedo sp. nov.

Plate 9 (1) Ambitaktoa stoumma Szwedo gen. et sp. nov., holotype, NHMUK In.17282, tegmen. (2) Phariberea gurdonika Szwedo gen. et sp. nov., holotype, NHMUK In.17096, tegmen. (3) Wixskimoa torxsea Szwedo gen. et sp. nov., holotype, MIWG 3609, tegmen, part of wing and part of body. (4) Niadrima yulei Szwedo gen. et sp. nov., holotype, NHMUK II.3046, tegmen. (5) Ankomwarius brodiei Szwedo gen. et sp. nov., holotype, NHMUK I.8803, tegmen. (6) Ankwlanno bluga Szwedo gen. et sp. nov., holotype, NHMUK In.26069(1)b, tegmen, counterpart. (7) Kintusamo boulardi Szwedo gen. et sp. nov., holotype, NHMUK In.25267, tegmen. (8) Cicadidae, NHMUK In.25244, hindwing.
Etymology. Specific epithet is derived from the Proto-Celtic word ‘stoumma' meaning ‘bend'.
Holotype. NHMUK In.17282, Smith Collection. Tegmen with clavus missing.
Diagnosis. Cell C3a less than two times than adjoining subapical cell; cell C3b more than two times as long as adjoining subapical cells; apical line veinlet ir very short; three veinlets uniting posterior branch of vein MP and stem CuA in apical portion of tegmen.
Description. Length of tegmen 3.59mm, width at widest point 1.5mm. Other features as for the genus as it is the only included species.
Genus Phariberea Szwedo gen. nov.
Etymology. Generic name derived from the Proto-Celtic word ‘pharibere' meaning ‘enjoy'. Gender feminine.
Type species. Phariberea gurdonika sp. nov.; here designated.
Diagnosis. Larger than Ambitaktoa gen. nov.; costal margin strongly thickened (not so strongly thickened in Ambitaktoa); branch RA forked, with a few terminals (branch RA not forked in Ambitakoa); branch RP forked basad of first veinlet ir (branch RP not forked basad of ir in Ambitakoa); posteriad branch of MP forked (anteriad branch of stem MP forked in Ambitaktoa); costal cell wider than cell C1 (costal cell narrower than cell C1 in Ambitaktoa).
Description. Tegmen with costal margin strongly thickened, distinctly curved at base then gently curved; apical margin angulately rounded. Costal cell widest near base of tegmen, then narrowing, with a few branches of ScP+RA stem, reaching margin. Basal cell about twice as long as broad. Stems ScP+RA, RP, MP and CuA leaving basal cell separately. Stems ScP+RA and RP separated at base; stem RP forked distad of half of tegmen length, with six terminals. Stalk MP short, forked at ¼ of tegmen length, then posterior branch forked again, i.e., three prenodal branches, branches of MP forked at level of preapical veinlets and apicad of preapical veinlets, probably eight terminals. Stem CuA forked slightly basad of nodal line. Clavus long, with apex exceeding 2/3 of tegmen length; claval veins Pcu and A1 fused at half of clavus length. Preapical line of veinlets rp-mp, im, and mp-cua distinct, regular.
Phariberea gurdonika Szwedo sp. nov.
Etymology. Specific epithet derived from the Proto-Celtic word ‘gurdoniko' meaning ‘rough'.
Holotype. NHMUK In.17096, Smith Collection, Insect Limestone, NW Isle of Wight. Tegmen, with some parts of membrane missing.
Diagnosis. Single prenodal veinlet rp-mp at level of nodal mp-cua veinlet; cell C3a elongate, cell C3b, about ¾ of cell C3a length; additional veinlets rp-mp, ir and mp-cua apicad of preapical line of veinlets.
Description. Length of tegmen 4.8mm, width at widest point ca.2mm. Other features as for the genus as it is the only included species.
Genus Wixskimoa Szwedo gen. nov.
Etymology. Generic name derived from the Proto-Celtic word ‘wixskimo' meaning ‘turbulent'. Gender: feminine.
Type species. Wixskimoa torxsea sp. nov.; here designated.
Diagnosis. Tegmen smaller in size, with venation pattern similar to Phariberea gen. nov.; costal margin of tegmen more thickened than in Phariberea; branch RP forked distinctly apicad of first veinlet ir (branch RP forked basad of first ir in Phariberea); stem ScP+RA forked anteriad of branch MP3+4 forking (ScP+RA forked posteriad of branch MP3+4 forking in Phariberea); posterior branch of stem MP and anterior branch of stem CuA fused at point (distinct veinlet mp-cua present in Phariberea). Hindwing with single terminals of veins ScP+RA, RP and MP, CuA with three terminals.
Description. Tegmen with costal margin strongly thickened, distinctly curved at base, then nearly straight, apical margin rounded. Costal cell widest at base of tegmen then narrowing, transected by ScP+RA stem branches. Basal cell about twice as long as broad. Stems ScP+RA+RP, MP and CuA leaving basal cell separately. ScP+RA+RP with a very short common stem, ScP+RA forked anteriad of branch MP3+4 forking, vein RA with three terminals reaching margin. Stem RP forked at level of preapical veinlet im, with three terminals. Stem MP forked at basal ¼ of tegmen length, then posterior branch forked again at level of first RA terminal; two branches of MP forked basad of apical line of veinlets, five terminals reaching margin. Stem CuA forked slightly basad of veinlet ir. Clavus long with apex exceeding 2/3 of tegmen length. Preapical veinlet ir slightly apicad of stem CuA branching; preapical veinlets rp-mp and im stepwise. Apical line of veinlets regular. Hindwing wide. Stem ScP+R with terminals ScRA and RP. Stem M single. Stem CuA forked distinctly basad of veinlets rp-mp and mp-cua, anterior branch forked again basad of veinlet mp-cua. Stem CuP single shifted from CuA. Intercubital fold distinct.
Wixskimoa torxsea Szwedo sp. nov.
Etymology. Specific epithet from Proto-Celtic work ‘torxse' meaning ‘break'.
Holotype. MIWG 3609 (part and counterpart), Insect Limestone, NW Isle of Wight. Tegmen, part of wing and part of body.
Diagnosis. Cell C3a twice as long as cell C3b; cell C3b about ¾ of length of adjoining subapical cell; subapical cells about 1.5 times as long as apical cells.
Description. Length of tegmen 4.35mm, width of tegmen at widest point 1.6mm. Other features as for the genus as it is the only included species.
Remark. It is the only specimen with a hindwing partly preserved. Visible portion of venation matches the hindwing venation of Nogodinidae as defined by Shcherbakov (Reference Shcherbakov and Shcherbakov1982).
Subfamily Colpopterinae Gnezdilov, Reference Gnezdilov2003
Tribe Niadrimini Szwedo trib. nov.
Type genus. Niadrima Szwedo gen. nov.; here designated.
Diagnosis. Differs from Colpopterini by tegmina not so elongated, without apical narrowing, membrane with row of apical veinlets (numerous, often irregular veinlets in Colpopterini); stem ScP+R forked basad, stem MP forked apicad of stem CuA forking (similarly to Colpopterini, sometimes stem CuA single in Colpopterini); ScP+RA with two (three) terminals (as in Colpopterini), branches of M not forking on membrane (branches of MP forking on membrane in Colpopterini); branch CuA1 single (CuA1 sometimes branching or amalgamated with branches of MP in Colpopterini).
Genus Niadrima Szwedo gen. nov.
Etymology. Generic name is derived from the Proto-Celtic name ‘*ad-rīmā' meaning number with negative particle ‘ni'. Gender: feminine.
Type species. Niadrima yulei sp. nov.; here designated.
Diagnosis. Tegmen with costal margin thickened, postcostal cell very wide, with prenodal veinlets; RP with two terminals reaching margin; stem MP forked apicad of stem CuA fork, branch MP1+2 forked basad of apical line of veinlets; branch MP3+4 single; branches CuA1 and CuA2 reaching margin as single terminals. Cell C3 about as long as cell C5. Radial cell with veinlets in apical portion.
Description. Tegmen with postcostal cell wide, intersected by prenodal veinlets. Stem ScP+R forked basad, branch ScP+RA reaching margin at anteroapical angle; branch RP forked at level of nodal area, with two terminals reaching margin at anteroapical angle. Stem MP forked slightly apicad of stem CuA forking, branch MP1+2 forked slightly apicad of nodal line, reaching margin with single terminals MP1 and MP2, branch MP3+4 not forked on membrane reaching margin as single terminal. Stem CuA forked in basal portion, terminals CuA1 and CuA2 single. Radial cell with a few transverse veinlets; nodal area with veinlets ir, and two veinlets rp-mp; apical line of veinlets composed of thick rp-mp, im, mp-cua and icua.
Niadrima yulei Szwedo sp. nov.
Etymology. Specific epithet is given in honour to the collector of the specimen, Mr Andy Yule.
Holotype. NHMUK II.3046; Yule Collection, Insect Limestone, NW Isle of Wight. Apical portion of tegmen, with clavus missing.
Diagnosis. Branch RP forked at level of nodal area; terminal ScRA1 forked; prenodal veinlets sigmoid; veinlets of radial cell oblique; apical veinlets im and mp-cua oblique. Cell C3a about 1/3 as long as cell C3. Apical cells longer than preapical cells C3a and C2c.
Description. Preserved portion of tegmen about 4.77mm long. Venation distinct, veins thick. Anteroapical angle widely rounded. Costal cell about three times as wide as radial cell; radial cell with transverse veinlets in apical portion, basad of nodal area. apical margin with traces of darker coloration, apical cells intersected with narrow, darker band, anteroapical angle and apical line of veinlets with traces of darker coloration, nodal area with darker spot, traces of coloration along longitudinal veinlets on corium. Other characters as for genus.
Family Lophopidae Stål, Reference Stål1866
Genus Ankomwarius Szwedo gen. nov.
Etymology. Genus name derived from the Proto-Celtic word ‘ankomwari' meaning ‘disarranged'. Gender: masculine.
Type species. Ankomwarius brodiei sp. nov.; here designated.
Diagnosis. Regarding tegmen venation similar to extant genera Serida Walker, Reference Walker1857 and Sarebasa Distant, Reference Distant1909, but differs in more branches of posteriad branch of vein CuA (mediad branch of CuA more branched in Serida and Sarebasa); stems MP1+2 and M3+4 branched apicad of half of tegmen length (stems MP1+2 and MP3+4 branched basad of half of tegmen length in Serida and Sarebasa); stem MP1+2 branched more anteriad of stem MP3+4 branching (stems MP1+2 and MP3+4 branched at same level in Sarebasa); stem RP forked posteriad of stem MP3+4 branching (RP forked at same level in Serida, RP forked anteriad of stem MP3+4 forking in Sarebasa); costal cell wider than basicostal field, with a few veinlets (costal cell without veinlets in Serida and Sarebasa, as wide as basicostal field in Serida, narrower than basicostal field in Sarebasa).
Description. Tegmen about 2.5 times as long as wide, with anterior margin gently curved. Basicostal field narrower than costal cell, with transverse veinlets. Stem ScP+R with a short common stalk. Stem ScP+RA forked slightly posteriad of stem RP forking. Stem RP forked basad of stem MP1+2 forking, then mediad branch of RP forked basad of anteriad branch of RP. Stem MP forked apicad of stem ScP+R forking, basad of stem CuA forking. Branch MP1+2 forked apicad of branch MP3+4 forking. Stem CuA forked distinctly basad of stem MP3+4 forking, then posteriad branch forked again, basad of stem MP3+4 forking.
Ankomwarius brodiei Szwedo sp. nov.
Etymology. Specific epithet is given in honour of eminent British palaeoentomologist and collector Rev. P. B. Brodie.
Holotype. NHMUK I.8803, Brodie Collection, Insect Limestone, NW Isle of Wight. Part of tegmen.
Diagnosis. Cell C1 longer than cell C3; veinlet ir oblique, placed basad of branch ScP+RA forking; veinlet im slightly apicad of ir.
Description. Length of tegmen ca.14mm width at widest point 5.5mm. Other features as for the genus as it is the only included species.
Remark. The state of preservation is too incomplete for definite placement of the genus. Observable characters place it in the Sarebasa+ group as defined by Soulier-Perkins (Reference Soulier-Perkins1998, Reference Soulier-Perkins2000). It comprises a group of 11 extant genera, four of which are widely distributed on Oriental and Australian regions, and two present in Afrotropical, Oriental and Australian regions. Some of these genera have members which show a great ability to disperse. Biogeographic hypothesis for the group infers their common ancestor originated in Southeast Asia (Soulier-Perkins Reference Soulier-Perkins, Ouvrard, Attié and Bourgoin2000). Baninus thuringiorum Szwedo & Wappler, 2006, representing another group of Lophopidae – the Bisma+ group – is recorded from the Middle Eocene of Messel Maar in Germany (Szwedo & Wappler 2006). The oldest fossil Lophopidae comes from the Palaeocene of Tibet (Szwedo et al. 2015). The species described above also proves the presence of Lophopidae in Europe during the Palaeogene. The tropic relationships of Lophopidae need further attention, as the full host plant range is not entirely known (Soulier-Perkins Reference Soulier-Perkins1998, Reference Soulier-Perkins2000; Soulier-Perkins et al. Reference Soulier-Perkins, Ouvrard, Attié and Bourgoin2007). The ancestor of the family Lophopidae was postulated as feeding on Arecaceae, with two later changes to Poaceae and Musaceae (Soulier-Perkins et al. Reference Soulier-Perkins, Ouvrard, Attié and Bourgoin2007). The Sarebasa+ group is the lineage which shifted to feed on Poaceae (Soulier-Perkins et al. Reference Soulier-Perkins, Ouvrard, Attié and Bourgoin2007).
It could be postulated that Ankomwarius Szwedo had been inhabiting rather open areas in which monocotyledon host plants, maybe Poaceae, have been present. Massive diversification and expansion in Poaceae took place in the Palaeogene (Jones et al. Reference Jones, Burke and Duvall2014; Magallón et al. Reference Magallón, Gómez-Acevedo, Sánchez-Reyes and Hernández-Hernández2015). The success of some extant Lophopidae, particularly in African savannahs, could be related to expansion of grassy habitats during the Miocene (Soulier-Perkins Reference Soulier-Perkins2000).
Family Ricaniidae Amyot & Audinet-Serville, Reference Amyot and Audinet-Serville1843
Genus Ankwlanno Szwedo gen. nov.
Etymology. Genus name derived from the Proto-Celtic word ‘ankwlanno' meaning ‘having no clan'. Gender: neuter.
Type species. Ankwlanno bluga sp. nov.; here designated.
Diagnosis. Tegmen with common stem ScP+R very short (longer common stalk present in Hammapteryx anglica Cockerell, Reference Cockerell1920); common stem of M long. From Hammapteryx anglica, described from the Middle Eocene Bagshot Beds, Bournemouth, by distinctly narrower costal cell (distinctly wider than costal cell in H. anglica); stem RP not forked distinctly basad of subapical line (stem RP forked distinctly basad of subapical line in H. anglica).
Description. Tegmen about 2.5 times as long as wide, costal margin distinctly curved at base, then gently curved, anterior apical angle widely rounded. Costal cell narrower than costal cell. Stem ScP+R leaving basal cell with a very short common stalk, stem ScP+RA forked slightly basad of preapical line of veinlets. Stem RP forked slightly basad of preapical line of veinlets. Stem MP forked at about basal 1/3 of tegmen length, stem MP1+2 forked at level of preapical veinlets, stem MP3+4 forked distinctly more basad. Preapical line of veinlets almost straight and regular; apical line of veinlets arcuately stepwise, regular. Anteapical cells longer than apical cells.
Remark. Placement of particular species ascribed to the genus Hammapteryx remains unclear. Szwedo et al. (2004) placed the genus in the family Ricaniidae. Petrulevičius (Reference Petrulevičius2005) transferred Hammapteryx paucistriata Henriksen, Reference Henriksen1922 to the newly erected genus Henriksenopteryx Petrulevičius, Reference Petrulevičius2005. Shcherbakov (Reference Shcherbakov2006) moved the whole genus Hammapteryx to Nogodinidae sensu lato, discussing some of its venational characters, and placing generic status of Hammapteryx in doubt, but with no further discussion of this opinion. Placement of these fossils was not discussed by Gnezdilov (Reference Gnezdilov2007, Reference Gnezdilov2017), and the features of Nogodinidae and Ricaniidae need much more attention as most of the venational characters used to distinguish these families need to be re-examined and re-interpreted.
Ankwlanno bluga Szwedo sp. Nov.
Figures 55–65 (55) Ankwlanno bluga Szwedo gen. et sp. nov., holotype, NHMUK In. 26069(1)a, tegmen, part. (56) Kintusamo boulardi Szwedo gen. et sp. nov., holotype, NHMUK In.25267, tegmen. (57) Cicadidae, NHMUK In.25244, hindwing. (58) Blenniphora woodwardi (Cockerell, Reference Cockerell1922), holotype, NHMUK In. 24363, tegmen, counterpart. (59–61) Tegmen: (59) Blenniphora skaka Szwedo gen. et sp. nov., holotype, CAMSM X.50140.90; (60) Blenniphora bikkanoa Szwedo gen. et sp. nov., holotype, NHMUK In.24503; (61) Luisphantyelus briwus Szwedo gen. et sp. nov., holotype, NHMUK In. 24533. (62) Natajephora lijanka Szwedo gen. et sp. nov., holotype NHMUK I.8874, tegmen, part. (63–65) Hindwing: (63) Aphrophoridae gen. et sp. indet. 1, NHMUK In.24496; (64) Aphrophoridae gen. et sp. indet. 2, NHMUK In.24501; (65) Aphrophoridae gen. et sp. indet. 3, NHMUK In.24560. Scale bar=1mm.
Etymology. Specific epithet from Proto-Celtic word ‘bluga' meaning ‘piece'.
Holotype. NHMUK In.26069(1) a, b (part and counterpart, with a psychodid fly figured by Krzeminski et al. Reference Krzemiński, Blagoderov, Azar, Lukashevich, Szadziewski, Wedmann, Nel, Collomb, Waller and Nicholson2019), Hooley Collection, Insect Limestone, NW Isle of Wight. Incomplete tegmen.
Diagnosis. Tegmen with costal field equally wide near to the apical portion, when tapered; vein RP with six terminals; anteapical cells about 1.5 times as long as adjoining apical cells.
Description. Length of tegmen 6.8mm, width at widest point 2.75mm. Other features as for the genus as it is the only included species.
Remark. Placement of the genus Ankwlanno Szwedo described above in Ricaniidae is only tentative. It possesses some venation features close to the Afrotropical genera Privesa Stål, Reference Stål1862a and Acroprivesa Schmidt, Reference Schmidt1912. It also resembles Henriksenopterix Petrulevičius, Reference Petrulevičius2005 (created for Hammapteryx paucistriata based on tegmen), which was placed in Nogodinidae sensu lato (Petrulevičius Reference Petrulevičius2005; Shcherbakov Reference Shcherbakov2006). This genus and Celinapterix bellisima Petrulevičius, Reference Petrulevičius2005 were placed in Nogodinidae sensu lato on account of bidentate second and long first metatarsomeres discovered in Celinapterix and also RA not reaching tegminal apex, tornus remote from claval apex and one or two RA-RP branches sigmoidal (Petrulevičius Reference Petrulevičius2005; Shcherbakov Reference Shcherbakov2006). A monobasic tribe Celinapterixini is described from the Late Paleocene of Argentina (Petrulevičius Reference Petrulevičius2005). The correct name of this tribe should be Celinapterygini, but according to Article 29.4 of the ICZN the spelling ‘Celinapterixini' is to be maintained. However, the characters mentioned above are also found in some Ricaniidae, e.g., in the primitive genus Cotrades Walker, Reference Walker1858 (Fennah Reference Fennah1949, 1968, fig. 3), that also lack a precostal lobe in the hindwings, characteristic of Ricaniidae (Shcherbakov Reference Shcherbakov and Shcherbakov1982, Reference Shcherbakov2006). In the genus Ankwlanno Szwedo vein RA seems not to reach apex, and branches of RA-RP are more or less sigmoidal, so its placement in Nogodinidae sensu lato or in Ricaniidae cannot be resolved. Placement of the species included in the genus Hammapteryx Scudder, Reference Scudder1890 requires further research. In particular, Hammapteryx anglica Cockerell from the Middle Eocene of the UK and some other Old World fossil species ascribed to this genus need revising. Some of them seem to represent Ricaniidae, while some others could be members of Nogodinidae sensu lato. Placement of some extinct taxa ascribed formerly to Ricaniidae, Nogodinidae and Flatidae was recently discussed by Shcherbakov (Reference Shcherbakov2006), who transferred some of them to Nogodinidae sensu lato.
Suborder Cicadomorpha Evans, Reference Evans1946
Superfamily Cicadoidea Latreille, Reference Latreille1802
Family Cicadidae Latreille, Reference Latreille1802
Genus Kintusamo Szwedo gen. nov.
Etymology. Genus name derived from the Proto-Celtic word ‘kintusamo' meaning ‘first'. Gender: neuter.
Type species. Kintusamo boulardi sp. nov.; here designated.
Diagnosis. Similar in size to Paracicadetta Boulard & Nel, Reference Boulard and Nel1990 but differs by unfused and separated stems MP and CuA at basal cell (stems MP and CuA meeting at basal cell in Paracicadetta); forking of CuA1–CuA2 forming an acute angle (forking of CuA1–CuA2 forming an obtuse angle in Paracicadetta); apical cell a8 elongately rhomboidal, about twice as long as broad (cell a8 trapezoidal, about three times as long as broad in Paracicadetta).
Description. Tegmen about three times as long as broad. Pterostigma present, weak. Stem ScP+R close to costal margin. Branch MP1+2 in basal part slightly concave. Veinlet r-mp in line prolonging basal portion of MP1+2. Apical cell a8 trapezoid, about three times as long as broad; apical cells a6 and a5 of similar length.
Kintusamo boulardi Szwedo sp. nov.
Etymology. Specific epithet is given in honour of eminent specialist on Cicadoidea – Michel Boulard.
Holotype. NHMUK In.25267, Insect Limestone, NW Isle of Wight. Tegmen with apical portion and clavus indistinct.
Diagnosis. Apical cell a8 elongately rhomboidal; subapical veinlets mp-cua and im placed slightly apicad of stem MP1+2 and MP3+4 forkings.
Description. Preserved length of tegmen 15mm, width at widest point 5.3mm.
Remark. Few fossil Cicadidae have been described from Cenozoic deposits (Cooper Reference Cooper1941; Metcalf & Wade Reference Metcalf and Wade1966; Wagner Reference Wagner1967; Fujiyama Reference Fujiyama1969, Reference Fujiyama1979, Reference Fujiyama1982; Kinugasa & Miyatake Reference Kinugasa and Miyatake1976; Kinugasa & Yorio Reference Kinugasa and Yorio1979; Boulard & Riou Reference Boulard and Riou1988, Reference Boulard and Riou1999; Boulard & Nel Reference Boulard and Nel1990; Carpenter Reference Carpenter1992; Riou Reference Riou1995; Prokop & Boulard Reference Prokop and Boulard2000; Moulds Reference Moulds2018). The higher classification of Cicadoidea was recently presented by Moulds (Reference Moulds2005), and alternative views are presented by Sanborn (Reference Sanborn2014). Unfortunately the venation of the tegmen is of limited use for taxonomic purposes. The specimen of Kintusamo Szwedo is only part of tegmen and it is very difficult to make a formal placement for it in one of the recently recognised subfamilies and tribes. According to Moulds (Reference Moulds2005, Reference Moulds2018) separation of the stems MP and CuA at basal cell excludes it from Cicadettini Buckton, Reference Buckton1890. Presence of pterostigma relates it to Cryptotympanini Handlirsch, Reference Handlirsch and Schröder1925, the tribe to which some Early Oligocene taxa from Europe and North America are placed, and with fossil East Asian Leptopsaltriini Moulton, Reference Moulton1923 (Moulds Reference Moulds2018), to which it is also similar in structure of basal part of MP1+2 branch and prolonging r-mp veinlet.
Gen. et sp. indet.
Material. NHMUK In.24511/In.25244 (part and counterpart) Hooley Collection, Insect Limestone, NW Isle of Wight. Hindwing with anal and apical portions missing.
Description. Preserved portion of hindwing length 8.33mm.
Remark. The venation of the hindwing is of limited use for taxonomic purposes. The specimen is only partly preserved and it is impossible to make a formal placement for it. As the appendix is relatively wide, it seems more likely to belong to the Cicadidae than the Tettigarctidae; however, Tettigarctidae were present in Europe during the Palaeogene (Zeuner Reference Zeuner1944).
Superfamily Cercopoidea Leach, Reference Leach and Brewster1815
Family Aphrophoridae Amyot & Audinet-Serville, Reference Amyot and Audinet-Serville1843
Genus Blenniphora Szwedo gen. nov.
Etymology. Genus name derived from the Proto-Celtic word ‘blenni' meaning ‘spittle'. Gender: masculine.
Type species. Aphrophora woodwardi Cockerell, Reference Cockerell1922; here designated.
Diagnosis. Tegmen shape and venation similar to Aphrophora Germar, Reference Germar1821, but differs in stem MP distinctly curved mediad apicad of half of tegmen length (stem MP nearly straight in Aphrophora); terminal branches of RP not forked before apex (terminal branches of RP usually forked just before apex in Aphrophora).
Description. Tegmen about three times as long as wide, costal margin distinctly curved, apical portion acutely rounded, clavus long, surface punctate. Stem Sc+R forked basad of half of tegmen length, but apicad of stem MP+CuA forking. Vein ScRA1 distinctly oblique, RA with three terminals. Vein RP forked apicad of r-m veinlet, with three terminals. Stem M+CuA forked distinctly basad of half of tegmen length; vein M distinctly curved mediad apical of half of tegmen length. Vein CuA sigmoidal.
Blenniphora woodwardi (Cockerell, Reference Cockerell1922) comb. nov.

Plate 10 (1) Blenniphora woodwardi (Cockerell, Reference Cockerell1922), holotype, NHMUK In. 24363, tegmen, counterpart. (2) Blenniphora skaka Szwedo gen. et sp. nov., holotype, CAMSM X.50140.90, tegmen. (3) Blenniphora bikkanoa Szwedo gen. et sp. nov, holotype, NHMUK In.24503, tegmen. (4) Luisphantyelus briwus Szwedo gen. et sp. nov., holotype, NHMUK In. 24533, tegmen, part. (5) Natajephora lijanka Szwedo gen. et sp. nov., holotype, NHMUK I.8874, tegmen, part. (6) Berro enissuextaensis Szwedo gen. et sp. nov., holotype, NHMUK In.43467, tegmen. (7) Teniwitta andrewrossi Szwedo gen. et sp. nov., holotype, NHMUK In.20529, body and tegmen. (8) Cicadellidae nymph/exuvium, NHMUK I.9637.
v*1922 Aphrophora woodwardi Cockerell, p. 159, fig. 1.
1992 Aphrophora Cockerell, Reference Cockerell1922; Carpenter, p. 231.
Holotype. NHMUK In. 24363/In.64098 (part and counterpart), Hooley Collection, Insect Limestone, NW Isle of Wight. Tegmen with clavus and median part of costal portion missing.
Diagnosis. Tegmen about three times as long as wide, costal margin distinctly arcuate, apical portion acutely rounded. Narrow darker band at basal 1/3 of tegmen length. Veins thick, darker. Surface covered with minute punctuation. Darker band at level of stem ScP+R forking, darker spot at level of vein RA2 branching.
Description. Length of tegmen 6.5mm, width of tegmen 1.9mm.
Remark. Subfamilial and tribal assignment could not be given on the basis of preserved material.
Blenniphora skaka Szwedo sp. nov.
Etymology. Specific epithet from Proto-Celtic word ‘skak' meaning ‘jump'.
Holotype. CAMSM X.50140.90 (TN 145), Smith Collection, Insect Limestone, NW Isle of Wight. Tegmen with clavus missing.
Diagnosis. Larger than Blenniphora woodwardi (Cockerell). Stem RP curved mediad in apical portion, forked slightly apicad of veinlet ir; anteriad branch forked again just before margin.
Description. Length of tegmen 7.2mm, width 2.6mm. Tegmen about 2.8 times as long as broad. Stem Sc about as long as common stalk ScP+R. Veins darker. Dark band slightly basad of stem ScP+R forking, continuing on stem MP+CuA forking, with more dark spot near costal margin. Darker spot on the vein MP at level of vein ScP+RA1 branching. Two darker spots slightly basad of vein RA2 forking.
Blenniphora bikkanoa Szwedo sp. nov.
Etymology. Specific epithet from Proto-Celtic word ‘bikkano' meaning ‘small'.
Holotype. NHMUK In.24503, Hooley Collection, Insect Limestone, NW Isle of Wight. Tegmen with clavus missing.
Diagnosis. Smaller than Blenniphora woodwardi (Cockerell). Branch MP sinuate, branch RP in apical part curved mediad, forked at level of apical veinlet ir. Subapical cubital cell widest in apical portion.
Description. Length of tegmen 5.8mm, width of tegmen 1.9mm.
Remark. Subfamilial and tribal assignment could not be given on the basis of preserved material.
Genus Luisphantyelus Szwedo gen. nov.
Etymology. Genus name derived from the Proto-Celtic word ‘luisphant' meaning ‘toad, frog' combined with Ptyelus, genus name of cercopoid. Gender: masculine.
Type species. Luisphantyelus briwus sp. nov.; here designated.
Diagnosis. Tegmen similar to Blenniphora Szwedo, but differs in vein MP almost straight (stem MP distinctly curved in Blenniphora); stem ScP+R forked apicad of half of tegmen length (stem ScP+R forked basad of half of tegmen in Blenniphora); vein RP with single terminal (vein RP with three terminals in Blenniphora).
Description. Tegmen elongate, about three times as long as wide. Costal margin curved, apical margin rounded, clavus long. Stem Sc long. Common stem ScP+R forked distinctly apicad of half of tegmen length. Branch RA with three (?) terminals, branch RP single. Stem MP+CuA forked distinctly basad of half of tegmen length, branch MP nearly straight.
Luisphantyelus briwus Szwedo sp. nov.
Etymology. Specific epithet from Proto-Celtic word ‘briwo' meaning ‘fragment'.
Holotype. NHMUK In. 24533/In. 24540 (part and counterpart), Hooley Collection, Insect Limestone, NW Isle of Wight.
Diagnosis. Apex of tegmen rounded; apical veinlet m-cu basad of apical veinlet ir; subapical median cell about as long as half of tegmen.
Description. Length of tegmen 6.5mm, width of tegmen 2.3mm. Other features as for the genus as it is the only included species.
Genus Natajephora Szwedo gen. nov.
Etymology. Genus name derived from the Proto-Celtic word ‘nataje' meaning ‘jump, fly', combined with suffix ‘∼phora', common for cercopoids. Gender: feminine.
Type species. Natajephora lijanka sp. nov.; here designated.
Diagnosis. Similar to Luisphantyelus Szwedo, but tegmen slightly longer; stem ScP+R forked basad of half of tegmen length (stem ScP+R forked apicad of half of tegmen length in Luisaphantyelus); subapical veinlet mp-cua present (lack of such veinlet in Luisaphantyelus).
Description. Tegmen with costal margin distinctly curved at base, mildly curved in median portion, apical portion elongately rounded. Stem ScP long, common stalk ScP+R short, forked distinctly basad of half of tegmen length. Vein ScP+RA1 reaching margin at level of claval apex, vein RA with two terminals; vein RP single. Stem MP nearly straight, with single terminal. Preapical and apical veinlets rp-mp oblique.
Natajephora lijanka Szwedo sp. nov.
Etymology. Specific epithet from Proto-Celtic word ‘lijank' meaning ‘stone'.
Holotype. NHMUK I.8555/I.8874, Brodie Collection, Insect Limestone, NW Isle of Wight. Tegmen, clavus missing.
Diagnosis. Interradial cell slightly shorter than half of tegmen length
Description. Length of tegmen 7.3mm, width 2.2mm.
Stratigraphical range. Insect Limestone, Bembridge Marls, Bouldnor Formation, Priabonian, latest Eocene.
Occurrence. NW Isle of Wight, UK.
Gen. et sp. indet. 1
(Fig. 63)
Material. NHMUK In.24496, Hooley Collection, Insect Limestone, NW Isle of Wight. Part of hindwing, with apex missing.
Description. Preserved length 6.4mm, width 3.4mm. Hindwing with vein RP forked before apex, apicad of veinlet rp-mp. Vein CuA not forked before apex. Veinlet mp-cua short, placed slightly apicad of stem ScP+R forking. Anal field heavily wrinkled.
Remark. Specimens number In. 24515 and In. 24523 from the same locality and collector; may belong to the same taxon.
Gen. et sp. indet. 2
(Fig. 64)
Material. NHMUK In.24501, Hooley Collection, Insect Limestone, NW Isle of Wight. Part of hindwing, with base and anal portion missing.
Description. Similar to gen. et sp. indet. 1, but slightly smaller, preserved length 5.56mm. Hindwing with vein RP single, vein CuA forked distinctly apicad of mp-cua veinlet. Apical portion at ambient vein darker.
Remark. CAMSM X.50140.145 (TN 203), Smith Collection, may also belong to this taxon.
Gen. et sp. indet. 3
(Fig. 65)
Material. NHMUK In.24560, Smith Collection, Insect Limestone, NW Isle of Wight. Part of hindwing, with base and anal portion missing.
Description. Preserved length 6.5mm. Hindwing with vein RP single, vein MP single. Veinlet rp-mp close to the apex; veinlet m-cu at level of vein CuA forking.
Family Cercopidae Westwood, Reference Westwood1838
Genus Berro Szwedo gen. nov.
Etymology. Genus name derived from the Proto-Celtic word ‘berro' meaning ‘short'. Gender: neuter.
Type species. Berro enissuextaensis sp. Nov.; here designated.
Diagnosis. Tegmen with venation similar to extant genus Aufidus Stål, Reference Stål1863, but smaller. Tegmen with three cells formed by terminals of stem ScP+RA (five or six cells in Aufidus). Four big apical cells as in Aufidus.
Description. Tegmen about twice as long as broad, apical margin rounded, with distinct appendix. Branch ScP+RA1 forked slightly basad of branch CuA2, vein RA with three terminals. Vein RP with single terminal, vein MP with single terminal. Apical veinlet ir slightly oblique. Apical cells slightly longer than wide.
Berro enissuextaensis Szwedo sp. nov.

Figures 66–72 (66) Berro enissuextaensis Szwedo gen. et sp. nov., holotype NHMUK In.43467. (67) Teniwitta andrewrossi gen. et sp. nov., holotype NHMUK In.20529, parts of body and tegmina. (68) Cicadellidae: Typhlocybinae gen. et sp. indet., NHMUK In.17142(3). (69) Cicadellidae: Typhlocybinae gen. et sp. indet., NHMUK I.9720 , tegmen. (70) Cicadellidae: Typhlocybinae gen. et sp. indet., NHMUK I.9095. (71) Cicadellinae gen. et sp. indet. 1, NHMUK In.24507, tegmen. (72) Cicadellidae gen. et sp. indet. 2, NHMUK In.43466, part of hindwing. Scale bar=1mm.
Etymology. Specific epithet from ancient British name of Isle of Wight – Eniss Uexta.
Holotype. NHMUK In.43467, Hooley Collection, Insect Limestone, NW Isle of Wight. Tegmen, with base and clavus missing
Diagnosis. Apical cells a2 and a3 of similar length; apical cells a1 and a4 shorter than cells a2 and a3.
Description. Length of tegmen. 4.7mm, width of tegmen 2.3mm.
Remark. Tribal assignment could not be given on the basis of preserved material.
Superfamily Cicadelloidea Latreille, Reference Latreille1802
Family Cicadellidae Latreille, Reference Latreille1802
Subfamily Mileewinae Evans, Reference Evans1947
Tribe Mileewini Evans, Reference Evans1947
Genus Teniwitta Szwedo gen. nov.
Etymology. Genus name derived from the Proto-Celtic words ‘teni' meaning ‘delicate' and ‘witta' meaning ‘vein'. Gender: feminine.
Type species. Teniwitta andrewrossi sp. nov.; here designated.
Diagnosis. In general habitus similar to extant genus Ujna Distant, Reference Distant1908 and extinct genera Eomileewa Gebicki & Szwedo, Reference Gębicki and Szwedo2001 and Youngeewa Gebicki & Szwedo, Reference Gębicki and Szwedo2001, but distinctly smaller. Length of tegmen less than 2.5mm (over 3mm in Ujna, Eomileewa and Youngeewa). Tegmen uniformly narrow (more widened in postclaval portion in Ujna, Eomileewa and Youngeewa); head with apex more round than in Ujna, Eomileewa and Youngeewa.
Description. Vertex in mid line about as long as wide, compound eyes distinct. Pronotum in mid-line longer than vertex in mid-line. Tegmen uniformly wide, merely widened in post claval portion. Longitudinal veins delicate. Apex of clavus exceeding half of tegmen length.
Teniwitta andrewrossi Szwedo sp. nov.
Etymology. Species named in honour of Dr Andrew J. Ross, eminent palaeoentomologist and Principal Curator of Palaeobiology at the National Museum of Scotland, Edinburgh, who made the studies of Insect Limestone material possible.
Holotype. NHMUK In.20529, Smith Collection, Insect Limestone, NW Isle of Wight. Right tegmen, body and part of left tegmen.
Diagnosis. Head and pronotum darkly coloured. Tegmen 3.75 times as long as wide, apex of clavus exceeding half of tegmen length, appendix narrow. Tegmen with indistinct patchy coloration.
Description. Length of preserved part of body 1.35mm, head with compound eyes 0.47mm, vertex in mid line 0.2mm long. Length of tegmen 2.2mm.
Remark. The Mileewinae were redefined by Dietrich (Reference Dietrich2011). Previously Mileewinae included only the nominotypical tribe, and redefined include tribes Mileewini, Tinteromini Godoy & Webb, Reference Godoy and Webb1994 and Makilingiini Evans, Reference Evans1947 (both previously treated as separate subfamilies), as well as tribe Tungurahualini Dietrich, Reference Dietrich2011.
Subfamily Typhlocybinae Kirschbaum, Reference Kirschbaum1868
Gen. et sp. indet.
Material. NHMUK In.17142(3) (Fig. 68), (with a paratype of Mastotermes anglicus Rosen, Reference Rosen1913 and a cockroach tegmen (Blattodea: Ectobiidae) described by Ross (Reference Ross2019)), Smith Collection, Insect Limestone, NW Isle of Wight. Head, parts of thorax and abdomen, tegmen and hindwing partly preserved. NHMUK I.9720 (Fig. 69), remnants of head, thorax, abdomen and tegmina. NHMUK I.9095 (Fig. 70), remnants of body and tegmen.
Remark. These specimens belong to the subfamily Typhlocybinae, but the state of preservation does not allow the placement of these specimens in one of the recognised extinct or extant tribes (Gębicki & Szwedo Reference Gębicki and Szwedo2006). The extant Typhlocybinae are leafhoppers distributed worldwide, with a known diversity of about 470 genera and 5200 species (Dietrich Reference Dietrich2006). Fossil representatives of the group are poorly known and these scarce records needs to be reconsidered. The oldest known representatives of Typhlocybinae are placed in the extinct tribe Protodikraneurini Gębicki & Szwedo, Reference Gębicki and Szwedo2006, from Eocene Baltic amber. Several other fossil taxa described from Late Eocene Baltic amber, Middle Eocene deposits of Roan Mountain, Colorado, Late Eocene deposits of Florissant, Colorado, USA, clearly represent Typhlocybinae, but need to be re-examined and redescribed (Gębicki & Szwedo Reference Gębicki and Szwedo2006). The oldest representatives of the extant tribe Dikraneurini is a specimen mentioned and described by Dietrich & Vega (Reference Dietrich and Vega1995), from Miocene Dominican amber.
Subfamily Cicadellinae Latreille, Reference Latreille1802
Gen. et sp. indet. 1
(Fig. 71)
Material. NHMUK In.24507, Hooley Collection, Insect Limestone, NW Isle of Wight. Tegmen.
Description. Tegmen 3.8mm long, 1.1mm wide. Costal margin slightly arcuate, apex of clavus at about ¾ of tegmen length. Stem ScP+R+MP forked basad of basal ¼ of tegmen length. MP forked slightly basad of 2/3 of tegmen length.
Remark. Placement of this specimen in the subfamily Cicadellinae is only tentative. The interpretation of Cicadellinae follows Young (Reference Young1968, Reference Young1977, Reference Young1986) in including only the tribes Cicadellini and Proconiini (Dietrich Reference Dietrich2005).
Cicadellidae gen. et sp. indet. 2
(Fig. 72)
Material. NHMUK In.43466/II.2866 (part and counterpart), Hooley Collection, Insect Limestone, NW Isle of Wight. Part of hindwing, with base and anal portion missing.
Description. Preserved portion 3.5mm long. Hindwing with vein RP single, vein MP single. Vein CuA forked basad of veinlet rp-mp. Veins RP and MP close each other, veinlet rp-mp short. Veinlet mp-cua long, oblique. Wing smoked.
Cicadellidae nymph/exuvium
Material. Nymph/exuvium, NHMUK I.9637, Brodie Collection, Insect Limestone, NW Isle of Wight.
Discussion. Over 220 specimens deposited in the NHM in London, Maidstone Museum and Sedgwick Museum, Cambridge were investigated. Eight families of Fulgoromorpha (Fulgoroidea) and four families of Cicadomorpha (Cicadoidea, Cercopoidea and Cicadelloidea) were identified. Thirty-five new species of Fulgoromorpha and Cicadomorpha are described (27 and eight respectively) in addition to four species formerly described.
The Bembridge Marls fauna, preserved in the late Eocene Insect Bed of the Isle of Wight, with regard to family composition differs from the fauna of the Eocene Baltic amber. Unfortunately, Baltic amber planthoppers (Fulgoromorpha) and leafhoppers (Cicadomorpha) are still insufficiently studied, which prevents simple comparison. The coeval fauna of planthoppers and leafhoppers of Florissant, Colorado is also insufficiently known (Scudder Reference Scudder1890, Reference Scudder1892; Cockerell Reference Cockerell1906, Reference Cockerell1908a; Lewis & Heikes Reference Lewis and Heikes1991) and the taxa described require urgent revisionary studies. This fossil site presents representatives of Achilidae, Dictyopharidae, Fulgoridae, Nogodinidae sensu lato, Cicadidae, Aphrophoridae, Clastopteridae and Cicadellidae, similarly to the Bembridge Marls fauna. Unfortunately, the state-of-the art on planthoppers and leafhoppers from the Florissant beds precludes the detailed comparison with these insects preserved in the Bembridge Marls. Few species of planthoppers and leafhoppers have been reported form the earliest Miocene strata of Rott in Germany (Statz Reference Statz1950); Cicadellidae are dominant in this fossil assemblage, but the material requires revision. Taphonomic conditions during fossilisation of planthoppers and leafhoppers in the Insect Bed of the Isle of Wight and in Baltic amber were different. However, both faunas share some common elements at the family level. Representatives of the family Cixiidae are most numerous in both faunas. Bothriocerine cixiids, recently limited in distribution to the New World, are present in Baltic amber and in the Insect Bed. The oldest record of Bothriocerinae is found in the Palaeocene Fur Formation of Denmark (undescribed), while the New World record is from Miocene Dominican amber (Schlee Reference Schlee1990). One may assume that the taxon originated in Europe, and migrated to North America, then spread to Central and South America. It could be hypothesised that this migration took place during the Palaeocene–Eocene Thermal Maximum, about 55.5Mya, through the Thulean Bridge, a corridor connecting Europe, Greenland and North America (McKenna Reference McKenna, Bott, Saxov, Talwani and Thiede1983; Sanmartín et al. Reference Sanmartín, Enghoff and Ronquist2001; Smith et al. Reference Smith, Rose and Gingerich2006). Recent distribution and diversity of Bothriocerinae resulted rather from the reduction of the ancestral range and secondary diversification on the islands of the Caribbean (Szwedo 2002). The biology of Bothriocerinae is poorly known; host plants (Wilson et al. Reference Wilson, Mitter, Denno, Wilson, Denno and Perfect1994) comprise the families Pinaceae (gymnosperms), Fagaceae, Rubiaceae, Polygonaceae, Chrysobalanaceae, Solanaceae, Asteraceae (dicotyledone angiosperms), Arecaceae, Juncaceae, Cyperaceae, Commelinaceae, Poaceae (monocotyledone angiosperms). Representatives of the subfamily Cixiinae found among the Bembridge Marls fossils are Mnemosynini, Pentastirini and Cixiini. The record of Mnemosynini is known since the Palaeocene Fur Formation of Denmark (undescribed), uppermost Palaeocene of Menat in France and Eocene Baltic amber (Szwedo Reference Szwedo2004; Szwedo et al. Reference Szwedo, Bourgoin and Lefebvre2006), and Miocene Mexican amber (Fennah Reference Fennah1963). Recent representatives of Mnemosynini are distributed in subtropical and tropical zones of the world, with only two host plant records on Poaceae and Cactaceae (Myers Reference Myers and China1929; Stalle Reference Stalle1987; Wilson et al. 1994). Fossil representatives ascribable to Pentastirini are reported from mid-Cretaceous Burmese amber (Grimaldi et al. Reference Grimaldi, Engel and Nascimbene2002); other undescribed forms are known from the Palaeogene of Denmark and described taxa from the Eocene Baltic amber (Szwedo & Stroiński Reference Szwedo and Stroiński2002), Miocene Dominican amber (Szwedo Reference Szwedo2000) and the earliest Miocene strata of Germany (Statz Reference Statz1950). In respect to their host plants, Pentastirini are recorded from a wide spectrum of plant families: Agavaceae, Amaranthaceae, Arecaceae, Asteraceae, Batidaceae, Betulaceae, Blechnaceae (fern), Brassicaceae, Caricaceae, Chenopodiaceae, Convolvulaceae, Dicksoniaceae, Ebenaceae, Ericaceae, Euphorbiaceae, Fabaceae, Flacourtiaceae, Frankeniaceae, Gesneriaceae, Hydrangiaceae, Lamiaceae, Liliaceae, Malvaceae, Moraceae, Myrtaceae, Oleandraceae, Pinaceae, Poaceae, Rhizpohoraceae, Salicaceae, Solanaceae, Tamaricaceae, Urticaceae, Verbenaceae, Zygophyllaceae, but at the species level most Pentastirini are oligophagous. Representatives of the tribe Cixiini were previously known from Baltic amber and from Dominican amber (Szwedo et al. 2004). These planthoppers are related to plant families: Arecaceae, Chenopodiaceae, Cupressaceae, Ehteriaceae, Ericaceae, Fagaceae, Juglandacaea, Malvaceae, Oleaceae, Pinaceae, Plumbaginaceae, Poaceae, Rosaceae, Salicaceae, Sapindaceae and Tamaricaceae (Wilson et al. Reference Wilson, Mitter, Denno, Wilson, Denno and Perfect1994). The host plants of Pintalini are virtually unknown; the only record comes from Alismataceae (Wilson et al. Reference Wilson, Mitter, Denno, Wilson, Denno and Perfect1994). The family Achilidae is represented among the Bembridge palaeoentomofauna by Hooleya indecisa Cockerell of the tribe Achillini and two poorly preserved additional specimens. Most Achilidae are not phloem feeders like the majority of planthoppers but feed on the hyphae of fungi. Achillini are represented in the extant fauna by only two genera from Western and Central Africa, so the finding of another representative of the tribe in the Isle of Wight from Late Eocene deposits is very interesting. Achilidae are quite common in Eocene Baltic amber, but represented by taxa of other tribes including two extinct ones (Szwedo 2006a). The sole achilid described from the Late Eocene of Florissant requires revision. Inclusions in Miocene Dominican amber are representative of the most numerous recent tribe, Plectoderini Fennah, Reference Fennah1950. Among the Bembridge palaeoentomofauna the family Tropiduchidae is represented by the extant tribes Catullini and Trypetimorphini and by a very interesting find of the tribe Jantaritambini, previously known only from Baltic amber. Host plant range of recent Catullini comprises Rutaceae, Poaceae and Cyperaceae, and genera of the tribes occur in Afrotropical and Oriental Regions. The Trypetimorphini host plant range comprises Arecaceae, Poaceae and Cyathaceae and today the tribe is widely distributed in Afrotropical, Palaearctic, Oriental and Australian regions (Metcalf Reference Metcalf1954; Fennah Reference Fennah1982; Wilson et al. Reference Wilson, Mitter, Denno, Wilson, Denno and Perfect1994). Genera of Tropiduchidae: Gaetulini are recently distributed in tropical and subtropical zones all around the world (Fennah Reference Fennah1984). The fossils from the Bembridge Marls seem to be related to extant taxa from the Oriental region. The family Issidae is distributed worldwide with widely known host plant ranges (Wilson et al. Reference Wilson, Mitter, Denno, Wilson, Denno and Perfect1994): Arecaceae, Asteracae, Chenopodiaceae, Clusiaceae, Corylaceae, Cupressaceae, Cyathacae, Ericaceae, Ephedraceae, Euphorbiaceae, Fabaceae, Fagaceae, Hypericaceae, Moraceae, Pinaceae, Piperaceae, Poaceae, Polygonaceae, Rosaceae, Santalaceae, Scrophulariaceae, Solanaceae, Tamaricaceae, Verbenaceae and Vitac eae, with a number of polyphagous forms. The host plant spectrum of Nogodinidae is poorly known; it comprises Apocynaceae, Asteraceae, Myrtaceae and Steruliacae (Wilson et al. Reference Wilson, Mitter, Denno, Wilson, Denno and Perfect1994). The tribe Ambitaktoini are known only from the Insect Bed of the Isle of Wight. The fossil record of Nogodinidae is relatively rich (Szwedo et al. 2004; Shcherbakov Reference Shcherbakov2006). Host plant records of the family Lophopidae comprise Arecaceae, Bignoniaceae, Fabaceae, Poaceae, Myrtaceae and Rubiaceae (Wilson et al. Reference Wilson, Mitter, Denno, Wilson, Denno and Perfect1994; Soulier-Perkins et al. Reference Soulier-Perkins, Ouvrard, Attié and Bourgoin2007), but lophopids are believed to feed mainly on the monocot families Arecaceae, Musaceae and Poaceae (Soulier-Perkins Reference Soulier-Perkins1998, Reference Soulier-Perkins2000; Soulier-Perkins et al. Reference Soulier-Perkins, Ouvrard, Attié and Bourgoin2007). Recently, the family is distributed in the tropical zone of the Afrotropical, Oriental, Australian and Neotropical Regions. Known fossils occur in the Palaeocene of Menat in France, Palaeocene deposits of Tibet and the Middle Eocene Messel Maar in Germany (Szwedo & Wappler 2006; Stroiński & Szwedo Reference Stroiński and Szwedo2012; Szwedo et al. 2015). The host plant range of recent Ricaniidae comprises the families Araceae, Arecaceae, Canabidaceae, Fabaceae, Flacourtiaceae, Malvaceae, Moraceae, Myrtaceae, Oleaceae, Pandanaceae, Pittosporaceae, Poaceae, Rubiaceae, Santalaceae and Theaceae. The fossil record of the family needs reconsideration and revision as numerous taxa previously placed in the Ricaniidae were proposed to belong to Nogodinidae (Shcherbakov Reference Shcherbakov2006).
In contrast to phloem-feeding Fulgoromorpha, cicadomorphan Cicadidae (singing cicadas) are notable xylem-feeders. Singing cicadas are peculiar with endogeic nymphal life habits, with prolonged nymphal development lasting up to 17 years in some recent Nearctic species (Karban Reference Karban, Taylor and Karban1986). It is noteworthy that for cicadas (and other xylem feeders as well) woody and tall-growing species of host plants do not play a role. In particular, the nymphs are seldom recorded from woody plants, although after emergence relatively numerous species perform an obligate stratum shift from the soil or herbaceous layer up to tall herbs or up to the canopies (Nickel Reference Nickel2003). Another group of Cicadomorpha – Cercopoidea – feed almost exclusively on xylem sap and are believed to be oligophagous to widely polyphagous on a wide variety of host plants. Very generally speaking, Aphrophoridae are regarded as feeding on conifers and herbaceous dicotyledones, while Cercopidae tend to feed on herbaceous monocotyledones (Carvalho & Webb Reference Carvalho and Webb2005). Within the diversity of host plants, numerous cercopoids have a predilection for nitrogen-fixing plants. The Cercopidae prefer plants with associative nitrogen fixation through root zone bacteria (Thompson Reference Thompson2004), while Aphrophoridae prefer legumes, plants of the Fabaceae and related families (Carvalho & Webb Reference Carvalho and Webb2005).
Phloem feeding is widespread among the biggest cicadomorphan family, Cicadellidae, with some exceptions. The subfamily Typhlocybinae is the only group adapted to mesophyll feeding, which required dramatic morphological and physiological adaptations. Within Cicadellidae numerous phloem-feeding subtaxa are largely arboreal (e.g., Fagacae, Betulaceae, Tiliaceae, Aceraceae, Salicaceae) while others predominate on Poaceae, Cyperaceae and Juncaceae (Nickel Reference Nickel2003). Very little is known about host plants of Mileewinae, only a scant record on Asteraceae and Urticaceae exists (Chiang & Knight Reference Chiang and Knight1991) and they are found in moist habitats in the understory of shrubs and small trees in montane tropical and subtropical forest regions (Linnavuori Reference Linnavuori1979; Nielson & Godoy Reference Nielson and Godoy1995). The tribe is recently widespread in the Ethiopian, Oriental and Neotropical Regions, and comprises only four endemic genera with 90 species (Nielson & Knight Reference Nielson and Knight2000; Dietrich Reference Dietrich2006), plus three extinct genera are known from European deposits. Mesophyll feeding Typhlocybinae, exploiting advanced herbaceous angiosperms such as Lamiaceae, Asteraceae, Poaceae, Cyperaceae, which are likely to constitute a more recent evolutionary trend. However, more basal tribes – Empoascini and Dikraneurini – seem to present a more general, ancestral trait in comparison to ‘higher' Typhlocybinae (Nickel Reference Nickel2003). Typhlocybinae is a large, distinctive, cosmopolitan group, that is especially rich in the Oriental region (Nielson & Knight Reference Nielson and Knight2000), with a relatively rich fossil record (Gębicki & Szwedo Reference Gębicki and Szwedo2006).
It could be hypothesised that planthoppers and leafhoppers of the Bembridge Marls Insect Bed present a non-homogenous picture of taxa inhabiting more open habitats, shrubland and or woodland during the Late Eocene. Taxa supposed to live in more open habitats, related to herbaceous plants and mainly xylem feeders, constitute at least about 20%. It is difficult to make the same estimation for phloem feeders, but the ratio of planthoppers (and possibly also cicadellids) living in open habitats seems to be rather high. However, some forms believed to be restricted or closely related to woody plants also occur in the sample. The lack of the evidence precludes a conviction on the exact types of habitats just on the basis of taxic diversity of Fulgoromorpha and Cicadomorpha. Some distributional patterns observed on the basis of planthoppers and leafhoppers from the Insect Bed assemblage of the Isle of Wight could be related to changes of vegetation and habitat conditions in the Palaeogene and Neogene. Cooling climates in the Late Palaeogene–Early Neogene forced large assemblages of warm temperate to subtropical biotas to retreat from medium- to high-latitude circumboreal distribution southwards, to large refugial regions that preserved the warm wet climate that they needed. These refugia, termed Tertiary relict floras, are found in East Asia, SE North America, western North America and SW Eurasia (Milne Reference Milne2006). It seems reasonable to hypothesise that the plant feeding taxa migrated with their host plants and part of the recent diversity of planthoppers and leafhoppers in the tropics and subtropics originated due to these migrations. Notwithstanding the scarce data and still weak knowledge of fossil planthoppers and leafhoppers, unknown ancestral areas of the higher taxa and dispersal routes, it seems that migration, resulting in wide ranges of higher taxa, was an important process in the past. The biogeographic scenario proposed for Lophopidae (Soulier-Perkins Reference Soulier-Perkins2000) could be tested in respect to the Bembridge Marls fossils and other fossils of the family as well. The oldest fossil species of Lophopidae comes from Palaeocene deposits of Tibet and France (Stroiński & Szwedo Reference Stroiński and Szwedo2012; Szwedo et al. 2015). It could be assumed that Lophopidae separated in the Late Cretaceous. These planthoppers benefited from exploitation of the host plant's expansion and availability of new habitats due to the Mid-Cretaceous re-organisation of biosphere (Szwedo & Soulier-Perkins 2010; Stroiński & Szwedo Reference Stroiński and Szwedo2012). This separation took place probably somewhere in the ancestral area of Arecaceae, their presumed ancestral host plants. Lophopidae committed a rather rapid diversification and spreading coincident with them. The ecological shift of the Sarebasa+ clade to Poaceae was postulated to take place in Southeast Asia (Soulier-Perkins et al. Reference Soulier-Perkins, Ouvrard, Attié and Bourgoin2007). This shift could be related to Poaceae massive diversification and expansion in the Palaeogene (Jones et al. Reference Jones, Burke and Duvall2014; Magallón et al. Reference Magallón, Gómez-Acevedo, Sánchez-Reyes and Hernández-Hernández2015). The finding of Lophopidae from the Sarebasa+ group among Bembridge Marls fossils could support this opinion. The tempo of insect migrations could be estimated only by comparison with other better sampled groups with more fossils (and fossil sites) elaborated. Some assumptions could be present for example on the basis of rapid expansion of true primates during 100,000 years duration of the Palaeocene–Eocene Thermal Maximum (Smith et al. Reference Smith, Rose and Gingerich2006). In respect to this, the understanding of the recent distribution (and phylogeny) of planthoppers and leafhoppers cannot ignore fossil data. The entomofauna preserved in the Bembridge Marls Insect Bed could be regarded as representative of the Palaeogene hothouse world and together with other fossil assemblages of these times is a precious source of phylogenetic, biogeographic, palaeoclimatic and palaeoecological information.
True bugs (Heteroptera)
by Yuri A. Popov
During my visit to the NHM (London) I examined and studied the collection of true bugs from the Bembridge Marls (Insect Limestone) containing early findings (120 specimens), the finds made during fieldwork (under the guidance of Dr A. J. Ross) in May of 2005, and nine specimens from the collection of the Sedgwick Museum (Cambridge) belonging to Corixidae (one hemelytron), Gerridae (one hemelytron), Lygaeidae (two), Cydnidae (one) and Pentatomidae (two in the genus Podopinites Popov gen. nov. and one hemelytron probably belonging to the genus Teleoschistus Scudder, Reference Scudder1890). One specimen of Cydnidae was also found in the collection of Mr Andy Yule (the Dinosaur Isle Museum). Altogether about 150 fossils of these hemipteran insects have been examined.
It is apparent that there is a low taxonomic diversity of Heteroptera in the Insect Limestone, represented by fewer than a dozen families (compared to about 100 recent and extinct families known so far). The fauna is dominated by the Pentatomidae (64 specimens) and Lygaeidae (30 specimens), which are among the largest extant families with more than 10,000 recent species each. Pentatomidae are represented by no more than two or three genera, mostly represented by large hemelytra (10–15mm) and probably can be referred to as the genus Teleoschistus widespread in the middle Cenozoic (e.g., Florissant, USA; Mo Clay, Denmark; Oeningen, Germany). There is an analogous situation with Lygaeidae in that the fossils are represented almost exclusively by bodies. The third group of bugs is represented by the relatively small pentatomoid family Cydnidae (over ten specimens), comprising only about 90 genera and 680 living species (Lis Reference Lis, Aukema and Rieger2006). The rest of the heteropteran families have a few specimens: Belostomatidae (one), Corixidae (six), Gerridae (one), Anthocoridae? (one), Miridae (three), Alydidae (one), and Coreidae (two). Here the only representative of the first family may definitely be referred to the modern genus Lethocerus Latreille, Reference Latreille1802 (giant water bugs). Aquatic Corixidae are similar to the Miocene genus Diacorixa Popov, Reference Popov1971, numerous representatives of which are known from the southern Germany (Randeck Maar) and northern Kirghizia (Popov Reference Popov1989), but these represent another, new genus.
It is interesting to note that the first fossil findings of the pentatomid subfamily Podopinae are represented by the new (formal) genus Podopinites Popov gen. nov. consisting of P. acourti (Cockerell, Reference Cockerell1921c) and P. coloratus Popov sp. nov. Lace bugs (Tingidae) are of a special interest here. One of them belongs to the extant genus Parasinalda (Cantacaderinae, Phatnomini), whose recent species (14) are exclusively distributed in the tropics and subtropics (mainly in South Africa), and three species are known from Eocene Baltic amber (Golub & Popov Reference Golub and Popov2005a). The presence of the genus Parasinalda Heiss & Golub, Reference Heiss and Golub2013 in the Eocene of Europe tells us about correspondence of climatic conditions in this period to ecological requirements of the species of this genus. Only very wide biogeographical connections for a long time between Europe and Africa could provide such a great shift of the genus southward (Golub & Popov Reference Golub and Popov2005a) and these changes are probably related to the temperature reduction during the Neogene and Pleistocene (Nel et al. Reference Nel, Waller, de Plöeg and Nel2004). The present discovery of a Late Eocene European tingid belonging to the Phatnomini supports the opinion (Golub & Popov Reference Golub and Popov1999) about the significance and diversity of the Cantacaderinae among the lace bug fauna of the European Paleogene (Heiss Reference Heiss2002; Wappler Reference Wappler2003; Nel et al. Reference Nel, Waller, de Plöeg and Nel2004; Golub & Popov Reference Golub and Popov2005a, Reference Golub and Popovb). In the Eocene fauna of East Europe representatives of the subfamily Cantacaderinae, especially of the tribe Phatnomini, were widespread and highly diverse. In particular, only from Baltic amber do three genera of Phatnomini occur (Parasinalda, Intercader Golub & Popov, Reference Golub and Popov1998 and Tingicader Golub & Popov, Reference Golub and Popov1998) and two genera of Cantacaderini (Paleocader Froeschner, Reference Froeschner1996 and Weitschatiella Heiss, Reference Heiss2002). The morphological features of the species in Intercader and Tingicader genera combine features of two related taxonomic groups of a higher rank: Intercader – Phatnomini and Cantacaderini, Tingicader – Phatnomini and the subfamily Tinginae (Golub & Popov Reference Golub and Popov1998, Reference Golub and Popov2002; Golub Reference Golub2001). Thus, in the Eocene, judging from the diversity of some genera and the representations of intermediate forms between the Tingidae taxa on tribal and subfamily levels in the territory of Central Europe, there have probably been intensive form developing processes. In the Eocene or maybe even in the Paleocene the differences between the highest taxa of the family (Cantacaderini, Phatnomini and Tinginae) reached the modern level. Later on, such intensive processes of adaptive radiation in the subfamily Cantacaderinae (Cantacaderini and Phatnomini) moved southward. In the modern fauna both tribes Cantacaderinae (Cantacaderini and Phatnomini) are represented exclusively in the tropics and only partially in the subtropics. As compared to the Eocene, by the present time in Eurasia the northern limit of the range of Cantacaderini has moved to the south by approximately 15 latitudinal degrees, while Phatnomini by approximately 25 degrees (Golub & Popov Reference Golub and Popov2005b).
From the late Middle Eocene of Grube Messel (Germany) another cantacaderid, Exmesselensis disspinosus Wappler, Reference Wappler2003, was also found and placed in Phatnomini (Wappler Reference Wappler2003). One more very peculiar cantacaderine (?Phatnomini) lace bug Parazetekella eocenica Nel et al., Reference Nel, Waller, de Plöeg and Nel2004 was described from the lowermost Eocene amber of the Paris Basin (Nel et al. Reference Nel, Waller, de Plöeg and Nel2004). A new genus Viktorgolubia Popov gen. nov. is being established within the subfamily Tinginae for Celantia (?) seposita (Cockerell Reference Cockerell1921c) from the Bembridge Marls. A specimen identified as Tingidae incertae sedis by Nel (Reference Nel1992) from the uppermost Eocene of France also belongs to this genus.
Suborder Heteroptera, Latreille, Reference Latreille1810
Infraorder Nepomorpha Popov, Reference Popov1968
Superfamily Corixoidea Leach, Reference Leach and Brewster1815
Family Corixidae Leach, Reference Leach and Brewster1815
Subfamily Corixinae Leach, Reference Leach and Brewster1815
Genus Diacorixites Popov gen. nov.
Etymology. After the Miocene genus Diacorixa Popov, Reference Popov1971. Gender: masculine.
Type species. Diacorixites szwedoi sp. nov.; here designated.
Description. Hemelytra medium sized (5–6mm), smooth, finely punctate, sclerotised, not differentiated into proximal coriaceous and distal membranaceous part; transverse dark stripes, outer border of membrane dark; embolium (embolar groove) quite wide and rather flattened; white frosted (pruinous) area (prenodal and postnodal pruinoses) well developed; postnodal pruina quite short, less than two times as long as prenodal pruina; embolium rather flattened and wide; costal fracture (nodal furrow) almost vertical; border between corium and membrane hardly distinguished.
Remarks. This formal genus is the closest to the Miocene genus Diacorixa Popov (Popov Reference Popov1971, Reference Popov1989) – whose species are known from the Miocene sediments of North Kirghizia (D. miocaenica Popov, Reference Popov1971) and southern Germany (D. germanica Popov). The greatest similarities of its melytral features are as follows: rather flattened costal margin (especially embolium) and clavopruinous area of hemelytra, as well as the dark outer border of membrane (as in D. germanica). Yet the differences, more sclerotised hemelytra, different ration of pruinose areas (very short postnodal pruina), transversal position of costal fracture, and well-developed dark stripes, clearly distinguish it from the species of the genus Diacorixa.
Diacorixites szwedoi Popov sp. nov.

Plate 11 (1–3) Diacorixites szwedoi Popov gen. et sp. nov.: (1) holotype, NHMUK II.3045, tegmen; (2) paratype, NHMUK I.8943, tegmen and body remains; (3) paratype, NHMUK I.10371, tegmen. (4) Parasinalda wappleri Popov sp. nov., holotype, NHMUK I.9644. (5) Viktorgolubia seposita (Cockerell, Reference Cockerell1921c) gen. nov., comb. nov., holotype, NHMUK In.24360, tegmen. (6) Viktorgolubia sp., NHMUK In.24361.
Etymology. Species is named after my friend and colleague Dr Jacek Szwedo (Department of Invertebrate Zoology and Parasitology, University of Gdańsk, Gdańsk, Poland), an eminent specialist on the fossil hemipterans.
Holotype. NHMUK II.3045, Yule Collection, Insect Limestone, NW Isle of Wight.
Paratypes. NHMUK I.8943, hemelytron part (membranous part is not preserved); I.10371 (labelled as ‘Coleoptera'), both Brodie Collection and In.24561 Hooley Collection
Diagnosis. As for genus. Holotype length 6.6mm, width 1.6mm.
Description. As for the genus.
Infraorder Cimicimorpha Leston et al., Reference Leston, Pendergrast and Southwood1954
Superfamily Tingoidea Laporte, Reference Laporte1833
Family Tingidae Laporte, Reference Laporte1833
Subfamily Cantacaderinae Stål, Reference Stål1873
Tribe Phatnomatini Drake & Davis, Reference Drake and Davis1960
Genus Parasinalda Heiss & Golub, Reference Heiss and Golub2013
Type species. Phatnoma baltica Drake, Reference Drake1950; by original designation.
Emended diagnosis (for fossil species). The genus is recognised by the presence of a dorsomedial tubercle or spine of head, quite narrow paranota (not recurved above itself) with only one or two rows of areolae along most of its length and usually also narrow costal area containing one or two rows of areolae which are larger than the areolae of subcostal area. Rostrum surpassing fore coxae or extending middle coxae reaching hind ones sometimes.
Remarks. Such characters as the narrow and somewhat oblique, very weakly bilobed paranota with only two rows of areolae and also narrow costal area of hemelytra containing usually one or two rows of areolae (like in the recent type species Sinalda elegans Distant, Reference Distant1904 and also S. sinuaticollis Linnavuori, Reference Linnavuori1977 or S. haplotaxis Froeschner, Reference Froeschner1968) strongly differentiate them from the fossil species from Eocene Baltic amber placed in the genus Sinalda by Froeschner (Reference Froeschner1996) and Golub & Popov (Reference Golub and Popov1998). After studies of additional material of S. baltica (Drake Reference Drake1950), Heiss & Golub (Reference Heiss and Golub2013) transferred Baltic amber species S. baltica, S. froeschneri Golub & Popov, Reference Golub and Popov1998 and Parasinalda groehni Heiss & Golub, Reference Heiss and Golub2013 to the genus Parasinalda. Nevertheless, for a resolution of taxonomical generic problem between Sinalda and Parasinalda all fossils and recent species referred to these genera should be restudied.
Parasinalda wappleri Popov sp. nov.

Figures 73–79 (73) Parasinalda wappleri Popov sp. nov., holotype, NHMUK I.9644. (74) Viktorgolubia seposita (Cockerell, Reference Cockerell1921c), holotype, NHMUK In.24360. (75) Gurnardinia herczeki Popov sp. nov., holotype NHMUK In.17232. (76) Gurnardobaya rossi Popov sp. nov, holotype, NHMUK I.9074. (77) Eocenocydnus lisi Popov gen. et sp. nov., holotype, MIWG DIX 205.36. (78) Podopinites coloratus Popov gen. et sp. nov., holotype, NHMUK In.24516. (79) Podopinites acourti (Cockerell Reference Cockerell1921c) gen. nov, comb. nov., holotype, NHMUK I.8658. Scale bar=1mm.
Etymology. After Torsten Wappler (Bonn), the German palaeoentomologist famed for his excellent monograph on the Eocene insects from Eckfelder Maar, SW Germany (Wappler Reference Wappler2003) and some publications on fossil Tingidae.
Holotype. NHMUK I.9644, Brodie Collection, Insect Limestone, NW Isle of Wight, old label ‘Coleoptera'. Female (?), macropterous form, counterpart (ventral), moderately preserved insect (head partly preserved, legs and most of right side of body missing).
Description. Body oblong oval, twice as long as wide, length from head (apparently longer than preserved) to apices of hemelytra 3.2mm, preserved width 1.3mm. Eyes moderate size, weakly protrude laterally; rostrum quite short, slightly extended beyond middle of mesothorax. Pronotum relatively short, narrowing apically, anterior pronotal margin distinctly concave, paranota no projecting anterior angles, narrow, with probably two cell rows. Hemelytra very long and extended much beyond abdominal apex; outline of hemelytra arc-like; costal area narrow, with one regular row of rectangular cells of middle size along its almost full (except in basal part) length; subcostal area very wide, with not less than ten transversal thick veins and with five or six rows of quite small, irregular pentagonal or quadrangular cells; hemelytral membrane rather wide, with about ten cell rows in the widest part.
Subfamily Tinginae Laporte, Reference Laporte1832
Genus Viktorgolubia Popov gen. nov.
2016 Viktorgolubia ‘Popov gen. nov. (in press)': Golub & Popov, Reference Golub and Popov2016, p. 70 – nomen nudum.
Etymology. The specific epithet is a patronymic honouring my old friend and colleague, Russian Heteropterist Viktor B. Golub, the World's eminent specialist on the family Tingidae.
Type species. Celantia (?) seposita Cockerell, Reference Cockerell1921c; here designated.
Diagnosis (hemelytron). Hemelytral areolae mostly moderately large and mainly pentagonal and rectangular areolae; hemelytra clearly subdivided by raised veins into costal, subcostal and discoidal areas, and membrane, without stenocostal area and distinctly elevated transverse veins; hypocostal (Sc) vein delimiting costal and subcostal areas; costal area very wide (especially along preapical sinus), with three rows of areola in its widest part; subcostal area very narrow, raised to a vertical position and therefore weakly visible from above, with one or two rows of areolae; discoidal area short, slightly shorter of half length of hemelytron (R+M and Cu fused at middle of hemelytron) and almost flat; membrane wide; clavus clearly separated from corium by suture; hypocostal and R+M+Cu reaching hemelytron apex.
Remarks. Judging from the absence of the stenocostal area and elevated prominent transversal veins of hemelytra Viktorgolubia Popov gen. nov. belongs to the subfamily Tinginae. The presence of a strongly reduced clavus, separated from the corium by a groove, does not contradict the placement of this genus in this subfamily, because the clavus is also separated from the corium by the suture or a fracture in representatives of some genera of the Tinginae. The type of areolae and the relation of the length between hemelytral areae (a short discoidal area) show that the new genus may be similar to the following four recent American genera: Corythucha Stål, Reference Stål1873 (North and South America), Gargaphia Stål, Reference Stål1862b (North and South America), Leptopharsa Stål, Reference Stål1873 (North and South America) and Pliobyrsa Drake & Hambleton, Reference Drake and Hambleton1946 (Central and South America). In this case Leptopharsa is also known from Dominican amber (Golub & Popov Reference Golub and Popov2003). The general form of the hemelytron Viktorgolubia is reminiscent of the cosmopolitan genus Tingis Fabricius, Reference Fabricius1803 differing from it by the narrower subcostal area and the shorter discoidal area.
Cockerell's (Reference Cockerell1921c) placement of the species seposita in the recent genus Celantia Distant, Reference Distant1903, whose recent species are known from India, Ceylon, Bismarck Archipelago and Australia, was incorrect. In the type species, C. vagans Distant, Reference Distant1903 the discoidal area of hemelytra is much longer and comprises over a half of the hemelytron length, while in species of the genus Viktorgolubia the subcostal area is much shorter than half the length of the whole hemelytron. Also in C. vagans the subcostal area is considerably broader than in the extinct species of the new genus and more flattened (not recurved above itself).
In addition, the new genus differs from Corythucha, Gargaphia, Leptopharsa and Pliobyrsa, primarily by its narrow subcostal area, located almost vertically, which from above it is almost not visible, that is why Cockerell (Reference Cockerell1921c), in his drawing of Celantia (?) seposita missed the subcostal area. In the representatives of the above four genera it is broader and much more flattened. In Corythucha, Gargaphia, Leptopharsa and Pliobrysa the discoidal area is more or less concave, while in the species of the genus Viktorgolubia it is practically flat.
Viktorgolubia seposita (Cockerell, Reference Cockerell1921c) comb. nov.
1921 Celantia (?) seposita Cockerell, Reference Cockerell1921c, p. 542, fig. 1.
1960 Celantia (?) seposita; Drake & Ruhoff, p. 11.
1965 Celantia (?) seposita; Drake & Ruhoff, p. 118.
1992 Celantia (?) seposita; Nel, p. 103.
1999 Celantia (?) seposita; Golub and Popov, p. 36.
2003 Celantia (?) seposita; Wappler, p. 26.
2016 Viktorgolubia seposita Cockerell, 1903 [sic]: Golub & Popov, p. 70.
2016 Viktorgolubia seposita: Popov, comb. nov. (in press): Golub & Popov, p. 70.
Holotype. NHMUK In.24360, Hooley Collection, Insect Limestone, NW Isle of Wight.
Description (left hemelytron, measurements in mm). Length 2.8, width 1.0; width of costal area 0.4; width of subcostal area 0.1; discoidal area: length 1.5, width 0.3; width of sutural area (membrana) 0.6; clavus: length 0.9, width 0.15. All hemelytral areolae large and almost the same size. Subcostal area with two rows of areolae along R+M vein and one row of areolae along R+M+Cu. Discoidal area with four rows of areolae in the widest part of it. Clavus distinctly narrowing to its apex, with three rows of areolae at base, two rows of areolae in other part and one areola at apex.
Remark. Another specimen, NHM In.24361 (Pl. 11, fig. 6) Hooley Collection, length 2.3mm, width 0.9mm, probably belongs to the same genus.
Family Miridae Hahn, Reference Hahn1833
Subfamily Phylinae Douglas & Scott, Reference Douglas and Scott1865
Genus Gurnardinia Popov gen. nov.
Etymology. Derived from the locality Gurnard Bay. Gender: feminine.
Type species. Gurnardinia herczeki sp. nov.; here designated.
Diagnosis. Medium sized, not more 5mm; generally oblong-oval body; dorsal surface smooth and bare. General coloration dark-brown. Antennae long and thin. Pronotum small, more than seven times shorter of body length; trapezoidal, clearly transverse, more than twice as wide as long. Mesoscutum narrowly exposed. Hemelytral membrane biareolate, minor cell weakly marked.
Remarks. The main characters typical for genera of the Phylinae, such as the absence of pronotal collar and calli, and also the impunctate body, are sufficient to place the new genus in this subfamily and possibly the tribe Phylini. The recent taxonomy of all recent mirid subfamilies is mainly based on the pretarsal structures and modifications of the male genitalia.
Gurnardinia herczeki Popov sp. nov.

Plate 12 (1–2) Gurnardinia herczeki Popov gen. et sp. nov.: (1) holotype, NHMUK In.17231, counterpart; (2) holotype, NHMUK In.17232, part. (3–4) Gurnardobaya rossi Popov gen. et sp. nov.: (3) holotype, NHMUK I.9074; (4) paratype, CAMSM X.50140.71.
Holotype. In.17231/In.17232 (part and counterpart), Smith Collection, Insect Limestone, NW Isle of Wight.
Etymology. Named in honour of my old best friend and colleague Prof. Dr hab. Aleksander Herczek (Silesian University, Katowice, Poland), a well-known specialist on the family Miridae.
Description. Macropterous. Body length ca.4mm. Pronotum convex; strongly transverse, 2.36 times as wide as long; lateral margins strongly narrowing toward (LSP=3.25), posterior margin straight. Outer margin of hemelytra parallel and almost straight; proportion of hemelytron, corium and cuneus length along outer side 4.8–3.4–1.4. maximal length of cuneus almost 2.5 times less than corium length; large membrane cell 1.7 times shorter than membrane length.
Measurements (in mm). Body length including hemelytra 4mm (3.8 without head), width 1.7; antennal joints II: III: IV=∼0.9: 0.9: 0.6; pronotum: length 0.55, width 0.4 (ant.) and 1.3 (post.); mesoscutum length 0.15; scutellum: length 0.5, width 0.7.
Infraorder Pentatomomorpha Leston et al., Reference Leston, Pendergrast and Southwood1954
Superfamily Lygaeoidea Schilling, Reference Schilling1829
Family Lygaeidae Schilling, Reference Schilling1829
Subfamily Lygaeinae? Schilling, Reference Schilling1829
Tribe Gurnardobayini Popov, trib. nov.
Type genus. Gurnardobaya Popov gen. nov.; here designated.
Diagnosis. Body slender; antennae long, eyes rather small and weakly projecting; hemelytra narrow, subcostal area with numerous transversal short veins, veins R, M and Cu almost parallel each other.
Remarks. The peculiar venation of this form strongly differing from all other lygaeids allows the erection of a new taxon at tribal level.
Genus Gurnardobaya Popov gen. nov.
Etymology. The generic name is derived from the place Gurnard Bay where Joseph A'Court Smith lived.
Type species. Gurnardobaya rossi sp. nov.; here designated.
Description. Medium sized, not more than 5mm. Body elongate, slender, granular, six times as long as wide. Head quite long, somewhat longer than wide and pronotum length; antennae long and quite thin. Pronotum roughly granulate, convex and relatively elongate, slightly longer than wide or equal size of length and width; pronotal collar well developed and the same thickness as antennal joints; lateral margins weakly narrowing toward and slightly emarginated, posterior one straight; its posterior angles rounded. Hemelytra strongly elongated, about four times as long as wide; three main veins R, M and Cu distinctly expressed along of all wing length, R and M are fused at distal part of wing; membrane with four free parallel veins. Scutellum medium size. Legs slender and femora of all legs are equal thickness.
Gurnardobaya rossi Popov sp. nov.
Etymology. Named after Dr Andrew J. Ross, palaeoentomologist at the National Museum of Scotland, Edinburgh.
Holotype. NHMUK I.9074a, b (part and counterpart) (Fig. 3); Brodie Collection, Insect Limestone, NW Isle of Wight. Male.
Paratype. CAMSM X.50140.71 (TN122) (Fig. 4), Smith Collection. Male.
Description. Macropterous. Body length ca. 4mm., elongate body almost six times as long as wide; general coloration pale-yellowish, antennae and legs dark-brown; dorsal surface bare. Antennal joints II–IV of equal thickness, second joint longest. Hemelytra slightly longer than abdomen.
Measurements (in mm). Body length 4.0mm, width 0.65; head: length 0.7, width 0.6; pronotum: length 0.65, width 0.6; hemelytron: length 2.7, width 0.7; abdomen: length 2.0, width 0.7.
Remarks. There is another specimen from the Sedgwick Museum, Cambridge, whose main characters (same size of body, slender and granulate surface of body, somewhat elongate head and pronotum, and especially the same peculiar venation of hemelytra) correspond well (Pl. 12: 4) with the type specimen (Pl. 12: 3). However, the apical parts of corium of these specimens are somewhat different them. Unfortunately, the incomplete preservation of both of them does not allow to solve their taxonomical position. Therefore this specimen is tentatively included into this species as a paratype.
Family Lygaeidae Schilling, Reference Schilling1829? incertae sedis
Genus Lygaeites Heer, Reference Heer1853
Lygaeites amabilis Cockerell, Reference Cockerell1921

Plate 13 (1) Lygaeites amabilis Cockerell, Reference Cockerell1921c, holotype, NHMUK In.24362, hemelytron. (2) Eocenocydnus lisi Popov gen. et sp. nov., holotype, MIWG DI.X.205.36. (3) Podopinites coloratus Popov gen. et sp. nov., holotype, NHMUK In.24516. (4) Podopinites acourti (Cockerell, Reference Cockerell1921c) comb. nov., holotype, NHMUK I.8658.
1921 Lygaeites amabilis Cockerell, Reference Cockerell1921c, p. 542; fig. 1., pl. 2, fig. 1.
1927 Lygaeites amabilis; Cockerell, p. 590
1964 Lygaeites amabilis; Slater, p. 1503.
Holotype. NHMUK In.24362, Hooley Collection, Insect Limestone, NW Isle of Wight. Hemelytron.
Description. (after Cockerell Reference Cockerell1921c): Tegmen length 2.9mm, width 1.2mm, beautifully marked as shown in the figure. The corium has white marks on a black ground; the membrane is light reddish brown, with four curved, broad, white lines.
Superfamily Pentatomoidea Leach, Reference Leach and Brewster1815
Family Cydnidae Billberg, Reference Billberg1820
Subfamily Sehirinae? Amyot & Audinet-Serville, Reference Amyot and Audinet-Serville1843
Genus Eocenocydnus Popov gen. nov.
Etymology. From the geological name and the genus Cydnus Fabricius, Reference Fabricius1803. Gender: masculine.
Type species. Eocenocydnus lisi sp. nov.; here designated.
Diagnosis. Large size, more than 5mm. Body oval, deeply punctate, less than 1.5 times as long as wide. Head markedly transverse, without spinules at anterior margin; clypeus quite narrow, slightly projecting beyond jugal plates; eyes of moderate size, almost transversely triangular, touching anterior pronotal margin, vertex rather broad. Pronotum convex, markedly transverse, only less than 1.5 times as wide as long, with anterior margin distinctly sinuate, posterior one weakly concave, lateral margins clearly convex; its anterior angles subacuate, posterior ones weakly rounded. Scutellum triangular, apparently a little wider than long. Abdomen very broad, distinctly wider than length.
Remarks. This genus is distinct from other Palaeogene cydnids and probably from all known Neogene ones by the strongly transverse vertex, pronotum and abdomen, and also coarsely punctured body. The incomplete preservation of body (antennae, legs, and hemelytra are missed) does not allow to put this Oligocene cydnid in a definite subfamily. But judging from the large size of this burrower bug, open anteriorly clypeus and absence of spinules along the anterior margin of the head, it may apparently be placed in the subfamily Sehirinae and one can compare it with the recent genus Sehirus Amyot & Audinet-Serville, Reference Amyot and Audinet-Serville1843 having similar features. However, the triangular eyes of this Eocene cydnid clearly distinguish it from the species of Sehirus.
Eocenocydnus lisi Popov sp. nov.
Etymology. After the well known heteropterologist Professor Dr hab. Jerzy A. Lis, outstanding specialist of the burrow bugs (Cydnidae).
Holotype. MIWG DI.X 205.36, female, part, Insect Limestone, NW Isle of Wight.
Description. The head almost twice as wide as long. Vertex more than five times wider than eye; pronotum nearly twice as wide as long and abdomen; very broad abdomen, some more 1.5 times as wide as long.
Measurements (in mm). Body length 7.5, width 5.5; head: length 1.1, width (across eyes) 1.6; eye width 0.25; vertex width 1.25; pronotum: length 2.25, width 2.0 (ant.) and 4.5 (post.); scutellum width 3.6; abdomen: length 3.5, width 5.5.
Family Pentatomidae Leach, Reference Leach and Brewster1815
Subfamily Podopinae Amyot & Audinet-Serville, Reference Amyot and Audinet-Serville1843
Remarks. Of great interest is the presence of the new (formal) genus Podopinites Popov gen. nov. based on P. acourti (Cockerell Reference Cockerell1921c) and the newly discovered P. coloratus Popov sp. nov. The humeral angles of pronotum, strongly convex and large scutellum covering the abdomen, which is shorter than the posterior margin of the pronotum definitely point to them being members of the pentatomid subfamily Podopinae – the first fossil representatives of this peculiar subfamily. This widely distributed subfamily has 65 genera and 255 species (Davidová-Vilímová Reference Davidová-Vilímová1993; Schuh and Slater Reference Schuh and Slater1995).
Genus Podopinites Popov gen. nov.
Etymology. From the generic name of a true bug – Podops Laporte, Reference Laporte1832 and ending ‘ites'. Gender: masculine.
Type species. Podopinites coloratus Popov sp. nov.; here designated.
Diagnosis. Body elongated, distinctly convex, about 10mm. Scutellum very large and reaching apex of abdomen, almost twice as long as wide; surface distinctly dark-coloured and deeply punctuate, with distinct longitudinal medial carina; base of scutellum almost the same size as posterior margin of pronotum, the latter straight along almost all of its length.
Composition. The genus contains the type species which is described below and one other form which was described as Pentatomites acourti Cockerell, Reference Cockerell1921c.
Remarks. Despite the incomplete preservation of the body (head, basal part of the pronotum and legs are missing) of these specimens, it is possible to put them in the Pentatomidae. The scutellum covering the whole abdomen is known only in two pentatomoid families: Scutelleridae Leach, Reference Leach and Brewster1815 and Podopinae (Pentatomidae). But the base of the scutellum, which is not broader than the posterior margin of the pronotum is the main feature for members of the pentatomid subfamily Podopinae and it allows these fossils to be placed here. However, the equal length of the posterior margin of the pronotum and the base of the scutellum and also strongly convex and significantly elongate, especially P. acourti (Cockerell), body are features that taxonomically might bring them close to the Scutelleridae.
Podopinites coloratus Popov sp. nov.
Etymology. Derived from ‘coloratus' (Latin), coloured.
Holotype. NHMUK In.24516, Hooley Collection, Insect Limestone, NW Isle of Wight.
Diagnosis. This new species clearly differs from P. acourti in the angular humeral angles of pronotum and the type of coloration (first of all by two longitudinal stripes of the scutellum); also the body is less convex in P. coloratus.
Description. Head and most of part of pronotum, and legs missing. Humeral angles of pronotum broadly rounded. Scutellum moderate convex, 1.89 as long as wide; strongly pigmented, with two clearly expressed longitudinal white stripes; apex moderately rounded.
Measurements. Preserved length 7.8mm, width 3.7mm.
Podopinites acourti (Cockerell, Reference Cockerell1921c) comb. nov.
1921 Lygaeites acourti Cockerell, Reference Cockerell1921c, p. 543, fig. 3.
1927 Pentatomites acourti: Cockerell, p. 590.
1964 Lygaeites acourti: Slater, p. 1503.
Holotype. NHMUK I.8658, Brodie Collection, Insect Limestone, NW Isle of Wight.
Redescription. Head and most part of pronotum, and legs missing. Humeral angles of pronotum angularly rounded. Scutellum strongly convex, 1.88 as long as wide; coloured, there is a pattern of quite large, mainly round and pale spots; apex widely rounded.
Measurements. Width of pronotum 4.6mm; length of scutellum 7.5mm, width 4mm.
Remark. Undescribed representatives of the Heteroptera are presented on Plate 14. Water true bugs are represented by Gerridae – sole tegmen, preliminarily identified as belonging to the genus Gerris Fabricius, Reference Fabricius1794 (Pl. 14: 1) and Belostomatidae Leach, Reference Leach and Brewster1815, probably belonging to the genus Lethocerus Mayr, Reference Mayr1853 (Pl. 14: 7) – I.9425, tegmen length 42mm, width 24mm. Land bugs are represented by the families Coreidae (Pl. 14: 2) – In.24505, tegmen length 6.4mm, width 2.0mm, Lygaeidae (Pl. 14: 3–4) – I.8856, tegmen length 4.0mm, width 1.2mm; In.24505 tegmen length 6.4mm, width 2.0mm, Cydnidae (Pl. 14: 5) – In.17441b, tegmen length 5.8mm, preserved width of abdomen 2.5mm and Pentatomidae, tentatively identified as belonging to the extinct genus Teleoschistus Scudder, Reference Scudder1890 (Pl. 14: 6) – II.2713a tegmen length 12mm, width 6mm.

Plate 14 (1) Tegmen of Gerridae: Gerris sp., CAMSM X.50140.44 (TN94) (Smith Collection). (2) Tegmen of Coreidae, NHMUK In.24505 (Hooley Collection). (3) Tegmen of Lygaeidae, NHMUK I.8856 (Brodie Collection). (4) Tegmen of Lygaeidae, NHMUK I.8857 (Brodie Collection). (5) Cydnidae, NHMUK In.17441b (Smith Collection). (6) Tegmen of Pentatomidae: ?Teleoschistus sp., NHMUK II.2713a (Collection Polish Team). Scale bar=1mm. (7) Belostomatidae; Lethocerus sp., NHMUK I.9425 (Brodie Coll). Scale bar=5mm.
Discussion. By composition, the Bembridge Marls Heteroptera fauna differs considerably from the family composition in Baltic amber. Partially this may be explained by taphonomic reasons. Unfortunately, Heteroptera of Baltic amber are still in the early stage of study and one can compare only individual groups. Primarily this refers to the main thermophilous Tingidae, which are represented by subfamily Tinginae, dominating in the modern fauna, and the genus Viktorgolubia is close to the genera spread predominantly in the tropics and subtropics of the Eastern Hemisphere. One of them (Leptopharasa) is rather common in Dominican amber (Golub and Popov Reference Golub and Popov2000, Reference Golub and Popov2003). Yet other specimen belonging to this genus (identified as Tingidae incertae sedis by Nel (Reference Nel1992) is known from the uppermost Eocene of France. It also became clear that the genus Parasinalda of the subfamily Cantacaderinae is present only in the Insect Limestone of the Bembridge Marls and Baltic amber. Hemelytra of the water family Corixidae closely resemble those of the Miocene genus Diacorixa known from southern Germany (Randecker Maar) and Kirghizia (Popov 1971, 1989). Representatives of two terrestrial bug families Pentatomidae and Lygaeidae are dominant among all other heteropterans. Most of them resemble pentatomids and lygaeids from the Late Eocene of Florissant (Colorado, USA). One can note that the heteropteran assemblage of the Bembridge Marls looks rather poor in taxonomic composition – its fauna is represented by less than a dozen families (compared to about 100 recent and extinct families known so far). Among them, dominating are Pentatomidae (64 specimens) and Lygaeidae (30 specimens) which belong to the largest extant families including more than 10,000 recent species each. There is poor taxonomic diversity in the Bembridge Marls fauna. In addition to Podopinites gen. nov. Pentatomidae are represented by large hemelytra (10–15mm) that most probably can be referred to the genus Teleoschistus, though Polioschistus Scudder, Reference Scudder1890, Poteschistus Scudder, Reference Scudder1890 and Pentatomites Scudder, Reference Scudder1890 from the Late Eocene of Florissant (Scudder Reference Scudder1890) are other possibilities. There is an analogous situation in Lygaeidae (Pl. 14: 3–4). The difference is that the fossils are represented almost exclusively by bodies. The third group of bugs is represented by the pentatomoid family Cydnidae (a dozen specimens; Pl. 14: 5) comprising only about 600 living species. The rest of the Heteroptera families usually have few specimens: Coreidae Leach, Reference Leach and Brewster1815 (two; Pl. 14, fig. 2), Alydidae (one), Miridae (three), Tingidae (two), ?Anthocoridae (one), Gerridae (one), Corixidae (six) and Belostomatidae (one). The representative of the Belostomatidae (giant water bugs) can be identified as belonging to the recent cosmopolitan genus Lethocerus (Pl. 14: 7), whose representatives prefer staying in water habitats. Sometimes they can exist in saline estuaries draining into a sea or even ocean. Therefore Lethocerus could have lived in the brackish lagoon and estuarine environment of Bembridge Marls, though the presence of only adult specimens and the absence of nymphs does not support this. Specimens belonging to the Corixidae are distributed worldwide, but predominantly found in temperate and subtropical areas. Various corixids (e.g., species of the subgenus Sigara (Vermicorixa)) survive well in salt water (e.g., salt lakes). It should be noted that corixids from Bembridge Marls are only represented by hemelytra. If one presumes that water boatmen are autochthonous then their hemelytra could be transported by local currents. It is also possible that a single hemelytron of the water strider (Gerridae) from the Sedgwick Museum, Cambridge, belongs to the modern genus Gerris (Pl. 14, fig. 1), which are almost exclusively confined to stagnant (lentic) freshwater, such as pools, ponds, and smaller lakes. Some species inhabit temporary pools and these are usually long-winged (Andersen Reference Andersen1973).
2. Acknowledgements
We wish to thank Dr Andrew J. Ross, National Museum of Scotland, Edinburgh (formerly Natural History Museum, London) and Dr Edmund A. Jarzembowski, Maidstone Museum, Maidstone for the opportunity to study collection of fossil Hemiptera from the Bembridge Marls and valuable discussions during preparation of this paper. Many thanks to Phil Crabb, Harry Taylor and Kevin Webb (NHMUK) for photographs. YAP wish to express deep gratitude to Prof. Viktor B. Golub (Voronezh State University, Russia) for helpful discussion on the family Tingidae, Mr Dmitri V. Vasilenko, who supported digitalising the illustrations and Dr E. D. Lukashevich who provided some photographs of the Heteroptera fossils for this work.































