Timely and effective control of bleeding and prevention of infection are the most common problems in modern warfare. In the absence of validated therapeutic techniques, it is difficult to choose an effective approach to avoid the high mortality caused by bleeding and morbidity caused by infection. Topical hemostatic agents are commonly used first aid agents because of their flexibility and efficacy in shortening the bleeding time. Chitosan (CS), the only alkaline polysaccharide found in nature, has the unique characteristics of inducing akaryocyte agglutination, promoting platelet activation, and activating the complement system.Reference Khor and Lim 1 It also has antibacterial and immunological activity, good biocompatibility, and high levels of biological activity. Celox (MedTrade Products Ltd, Cheshire, United Kingdom) is a proprietary preparation of CS indicated for moderate to severe hemorrhage and currently used for hemostasis in emergency and military settings.Reference Millner, Lockhart and Bird 2 However, CS has poor hydrophilia at neutral pH. Many reports have been made on hydrophobic modifications of CS.Reference Martin, Wilson and Koosha 3 , Reference Chen and Park 4 Various modified CS materials, likely to become a new generation of local hemostatic agents, are now under active development.
Carboxymethyl chitosan (CMCS) is an important, water-soluble derivative of CS whose beneficial biological properties as a drug carrier, delivery material, and hemostatic agent have already been investigated.Reference Chen, Wang and Chen 5 , Reference Liu, Fan and Chen 6 Li et alReference Li, Zhang and Meng 7 prepared amphiphilic self-assembled nanoparticles of oleoyl-CMCS encapsulating rifampicin to be used as a drug delivery system. Wu et alReference Wu, Peng and Han 8 found that CMCS powder increases hemostasis in wounds with venous bleeding and has better histocompatibility than other CS preparations. We first prepared N,O-CMCS powder with different degrees of substitution by using chloroethanoic acid and CS and found that carboxymethyl improves the wettability of the surface of the material and thus increases the hydrophilicity. Coagulation tests in vitro and in vivo have shown that CMCS powder with substitution degrees of 60% to 80% has excellent hemostatic properties.Reference Zhao, Liu and Han 9
The particle size in such powders affects the extent of hemostasis. Various microporous hemostatic materials such as starchReference Humphreys, Castle and Andrews 10 and zeoliteReference Li, Chen and Li 11 have been investigated. For example, Liu et alReference Liu, Bian and Chen 12 prepared porous corn starch by sequential hydrolysis by using hydrochloric acid and a composite enzyme method and achieved a final water-absorbing rate for this material of 156.98%. QuikClot Combat Gauze has a large specific surface area (300 m2/g) and can increase the local concentration of clotting factors because it can quickly absorb large amounts of liquid, inducing platelet aggregation.Reference Motamedi and Sagafinia 13 However, the heat released during liquid absorption might cause tissue damage and increase pain. Liqun et alReference Liqun, Lei, Shuwei and Jiacan 14 developed a mesoporous silicon dioxide microsphere/starch composite hemostatic dressing that has been effective in shortening the bleeding time of skin and liver wounds in rabbits.
CMCS is water-soluble and has good biocompatibility, but few studies have reported on the use of CMCS in microsphere preparations for hemostatic applications. Etamsylate (DIC) is widely used as a hemostyptic agent in clinical practice. It can inhibit the synthesis and activation of prostaglandins for vasoconstriction, reduce capillary permeability, and increase platelet aggregation and adhesion, thus improving hemostasis. We prepared CS and CMCS microspheres (CSMs and CMCSMs, respectively) by using the emulsion cross-linking method and evaluated their effect on hemostasis. CMCSMs loaded with DIC were also studied to determine whether DIC had a synergetic role in hemostasis.
MATERIALS AND METHODS
Materials
CS (degree of deacetylation, 90.6%; molecular weight, 2.7×105) was purchased from Aokang (Shandong, China). CMCS was synthesized in our laboratory (substitution degree, 11.04%; molecular weight, 1.09×104). Liquid paraffin, Span-80, glutaral, and ligroin were purchased from Guangfu Fine Chemical Research Institute (Tianjin, China). Etamsylate freeze-dried powder (purity, 99.5%) was from the National Institute for the Control of Pharmaceutical and Biological Products (China; no. 201103)
Preparation of CSMs and CMCSMs
Samples of CS and CMCS were mixed with 1% acetic acid or distilled water to the concentration of 3% (w/v) and 1% (w/v), respectively. Aliquots of the CS and CMCS solution (10 mL) were added to liquid paraffin (100 mL, containing 2% of Span-80) and agitated at 1200 r/min, at 50°C, until the emulsion was stable. Then, the samples were placed in an ice bath and stirred gently for 30 min. A total of 2 mL of 50% (v/v) glutaral and 30 mL of isopropanol were added to the emulsion, and the mixture was continuously stirred for 1.5 h. CSMs and CMCSMs were centrifuged at 2000×g for 5 min, washed 3 times with ligroin, and vacuum-dried.
Preparation of CMCSMs loaded with DIC
DIC powder was added to CMCS solution (3% or 1%) to a mass ratio of 1:1. The rest of the procedure was as described above.
Obtaining DIC Standard Curve and Determination of Drug Loading Rate and Entrapment Rate
An amount of 10 mg of DIC powder was dissolved in double-distilled water (ddH2O) in a volumetric flask (100 mL) to obtain DIC stock solution. The stock solution was diluted with ddH2O to give standard solutions with concentrations of 4, 10, 20, 40, and 60 µg/mL. The maximum absorbance was detected at 300 nm using an ultraviolet full wavelength scanner. Absorbance of different standard solutions was recorded to make the standard curve.
An aliquot of 20 mg of DIC-CMCSMs was added to 50 mL of ddH2O, sonicated for 30 min, and centrifuged for 5 min at 1200 r/min. The absorbance was measured at a wavelength of 300 nm, and DIC content was calculated from the standard curve. The drug loading rate and entrapment rate were calculated as follows:
where WDIC in microspheres, Wm, and WDIC represent the DIC content after breaking microspheres, the weight of vacuum-dried microspheres, and the total weight of DIC added, respectively.
Morphology and Size Distribution of CMCS Microspheres
The morphology of the CMCS microspheres was examined under an optical microscope and scanning electron microscopy (SEM). Microspheres were dispersed in ligroin and placed on a coverslip with a plastic head dropper. After solvent evaporation, the sample was fixed on the aluminum plate coated with gold and then examined under SEM. The size distribution of the microspheres was measured by dynamic light scattering by using a Zetasizer 3000 Particle Sizer (Malvern, United Kingdom). All dynamic light scattering measurements were performed at a wavelength of 659.0 nm at 25°C.
Swelling Behavior
Each sample of dry CMCS nanospheres (Wdry) was placed in a centrifuge tube containing 10 mL of 0.1 M phosphate-buffered saline (PBS) solution at room temperature. After 24 h, the samples (n=5) were centrifuged at 2000×g for 5 min to remove excess PBS and weighed (W=Wwet + Wtube). The water absorption of the CMCS nanospheres was calculated by using the following equation:
Hemolytic Test
The hemolytic test was carried out by using erythrocyte suspension, prepared from fresh rabbit blood, in 109 mmol/L sodium citrate solution. Samples were divided into 3 groups: materials group (extract CMCS microspheres in normal saline), positive control group (sterile distilled water), and negative control group (normal saline). An amount of 10 mL of each sample was placed in a centrifuge tube and incubated at 37°C for 30 min. An amount of 0.2 mL of erythrocyte suspension was added to the tubes, and the tubes were mixed gently and kept in a water bath at 37°C for 60 min. Then, the samples were centrifuged for 5 min (2500 rpm) and the absorbance was determined at 545 nm. The hemolysis rate was calculated by using the following equation:
Coagulation Time In Vitro
The dynamic clotting time methodReference Yushan, Xin and Yue 15 was applied to evaluate the hemostatic ability of the materials in vitro. Trisodium citrate solution (109 mmol/L) was added to blood at the volume ratio of 1:9 to form a fresh anticoagulant. An aliquot of 200 μL of the fresh anticoagulant was added to 50 mg of each sample. ddH2O (100 mL) was slowly added to each sample of blood at 5, 10, 15, and 20 min after the addition of the anticoagulant. Diluted blood samples were left standing for 10 min, and then optical density values were determined at 540 nm.
Coagulation Test In Vivo
Twenty-one rats (200 to 250 g, from Animal Experimental Center of WJ, Tianjin, China) were anesthetized with 40 mg/kg of sodium pentobarbital. A hepatic injury model was created to examine coagulation in vivo according to Matsuoka.Reference Matsuoka, Hildreth and Wisner 16 An incision was made along the midline abdominal muscles, and the excess peritoneal fluid was wiped with cotton gauze. Using a scalpel, the liver was transected at 2 cm above the inferior border; 200 mg of the tested material was then deposited onto the site of injury, and the timing commenced. The time at the end of bleeding was recorded. The animal experiments were in accordance with the governmental guidelines for the care and use of laboratory animals and were approved by the Academy of Military Medical Sciences Ethics Committee, China.
Statistical Analysis
SPSS 10.0 was used to test the significant differences among the 3 groups (IBM Corp, Armonk, NY). Data were presented as means ± standard errors. In all cases, the results were considered statistically different at P<0.05.
RESULTS
Effect of Different Concentrations of CS (CMCS) on Microsphere Particle Size
CSMs and CMCSMs with different particle sizes were prepared by using 3% (w/v) and 1% (w/v) CS (or CMCS) solution. Optical microscope and SEM images of CSMs (CMCSMs) are shown in Figure 1. Microspheres prepared with 3% CS solution (Figure 1A) were of unequal sizes and often an incomplete spherical shape. Microspheres prepared with 1% CS solution (Figure 1B) were smaller and completely spherical. CMCSMs with different diameters showed the same trend (Figure 1C, 1D). The mean diameters of the particles shown in Figure 1A, B, C, and D were 3561.3 nm, 1284.8 nm, 2375.0 nm, and 940.6 nm, respectively.
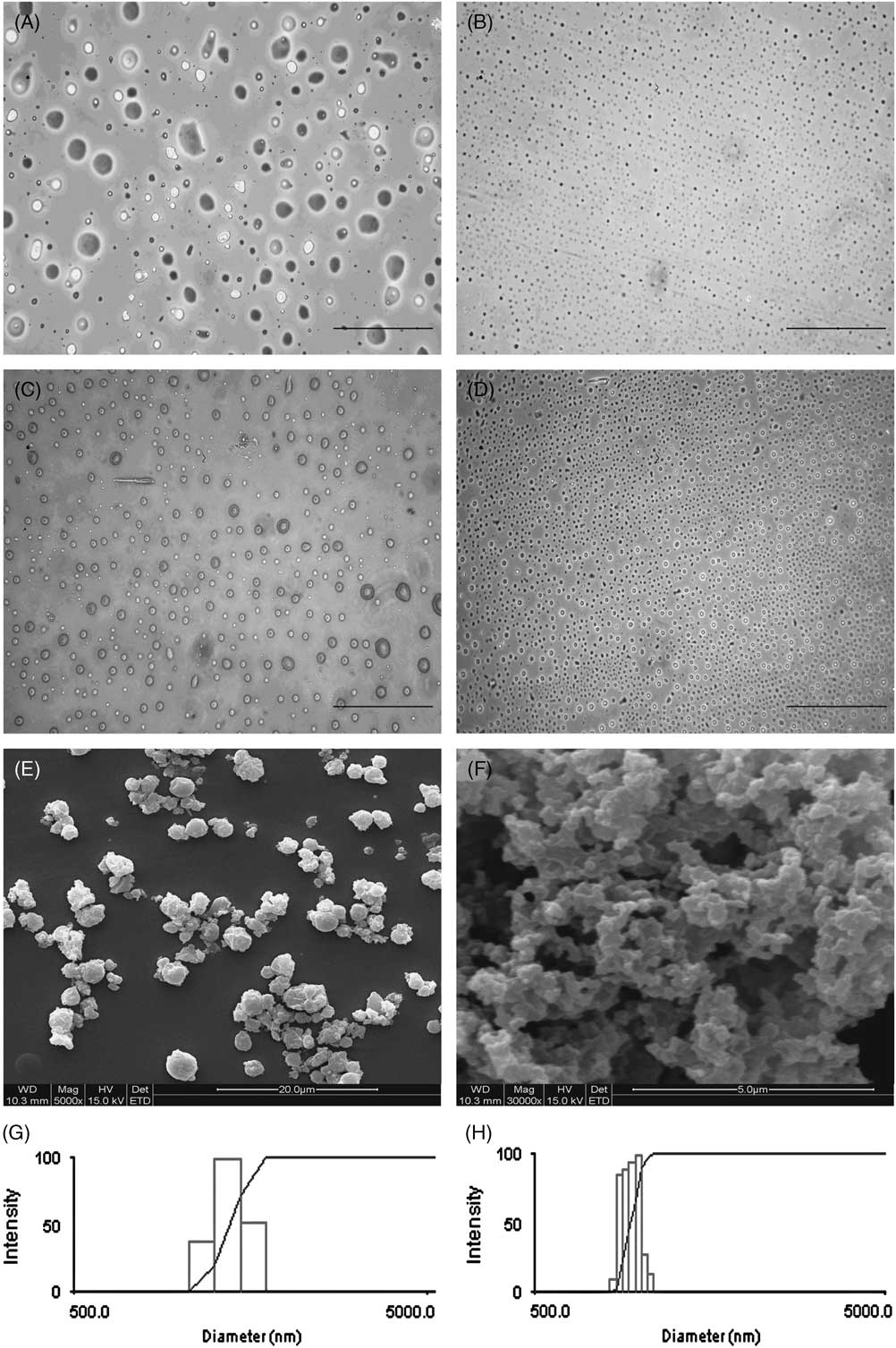
Figure 1 Optical Microscope and Scanning Electron Microscope Images of CSMs and CMCSMs. Abbreviations: CMCSM, carboxymethyl chitosan microspheres; CS, chitosan; CSMs, chitosan microspheres. (A) CSMs prepared with 3% CS solution; (B) CSMs prepared with 1% CS solution; (C, E) CMCSMs prepared with 3% CMCS solution; (D, F) CMCSMs prepared with 1% CMCS solution (magnification 400×). Unequal sizes and incomplete spherical shapes (A, C, E) were seen when the microspheres were prepared with 3% solutions. Both CSMs and CMCSMs prepared with 1% solution looked uniform and consisted of narrow, small particles (B, D, and F). (G) and (H) show the size distributions of CSMs and CMCSMs, respectively (made using 1% solutions).
For the sake of brevity, we will refer to the particles prepared by using 3% (w/v) and 1% (w/v) CS or CMCS solution as 1% CSMs (CMCSMs) and 3% CSMs (CMCSMs), respectively.
Drug Loading and Entrapment Efficiency of DIC-Loaded CMCS Microspheres
Ultraviolet absorption of DIC and CMCS was determined by using the full spectrum scan (Figure 2A, B). DIC exhibited intensive bands at wavelengths of 300.5 nm and 220.7 nm, and CMCS at 233.2 nm. To avoid overlapping, the concentration of DIC in DIC-loaded CMCS microspheres was determined at the wavelength of 300.5 nm. The absorbance versus concentration plot for DIC was linear within the range of 4 to 60 µg/mL; the linear regression equation was A=1.2467C + 0.0482 (n=5), and the correlation coefficient was 0.9998. Using the standard curve and formulas 1 and 2, we calculated the drug loading for DIC-CMCS (made by using 3% solution) and DIC-CMCS (made by using 1% solution) as 13.09% and 5.95%; the entrapment efficiency was 16.58% and 8.27%, respectively.
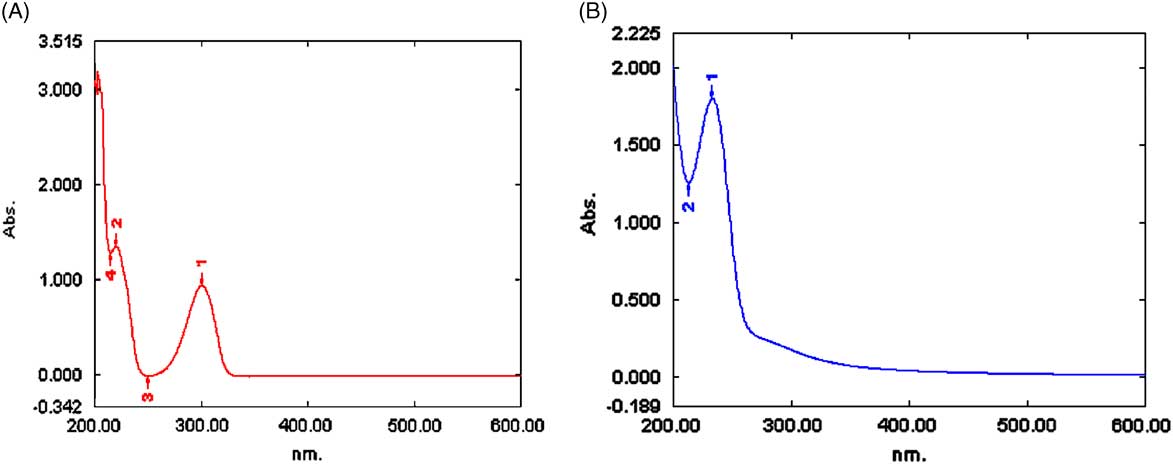
Figure 2 Full Spectrum Scan. (A) Etamsylate (DIC). (B) Carboxymethyl chitosan (CMCS).
CMCSMs Swelled More Than CSMs and CMCSMs (Made by Using 3% Solution) Had the Highest Swelling Rate
Immersing the microspheres in PBS resulted in an increase in mass within 24 hours, as shown in Figure 3. The increase for CMCSMs was 3-fold that for CSMs (P<0.05). Pure CMCSMs had higher absorption than drug-loaded CMCSMs (P<0.05). Larger particles had higher swelling rate than the small spheres. The swelling rate of 3% CMCSMs was 1684%.
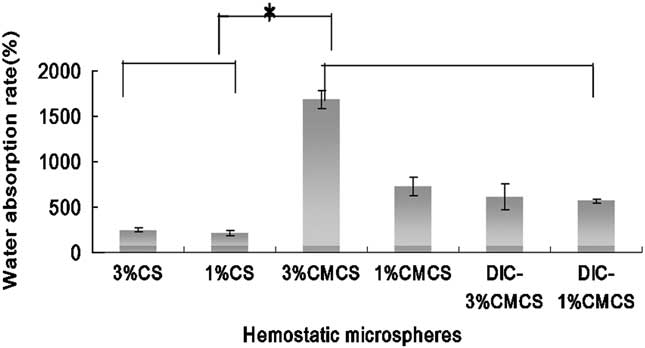
Figure 3 Swelling Behavior of Hemostatic Microspheres. Abbreviations: CMCS, carboxymethyl chitosan; CS, chitosan; DIC, etamsylate. CMCS microspheres (3%) had the highest swelling rate, whereas CS microspheres (3% and 1%) had the lowest.
CMCSMs Caused No Hemolytic Reaction and Could Be Suitable as Hemostatic Agents
In hemolytic tests, the hemolytic rate for the negative control group was 0% and the rate for the positive control group was 100%. The rates for samples of CSMs, CMCSMs, and DIC-loaded CMCSMs were 1.57%, 1.33%, and 0.98%, respectively, all of which were less than the 5% specified in ISO. We concluded that CMCSMs caused no hemolytic reaction, and their properties fulfilled the requirements for hemolytic characteristics of biomaterials.
Effect of Particle Diameter, Swelling Behavior, and DIC Loading on Hemostasis
We investigated the effect of CS powder, CS microspheres (3% and 1%), CMCS microspheres (3% and 1%), and DIC-loaded CMCS microspheres (3% and 1%) on hemostasis by using dynamic coagulation experiments and coagulation tests in vivo. The comparisons between the effects of CS powder, 3% CSMs, and 3% CMCSMs on dynamic clotting time are shown in Figure 4A. Cross-linked CSMs slowed hemostasis, whereas CMCSMs promoted it, indicating that the hydrophilic modification of CS was important for its efficacy in hemostasis. Comparing the results for different microsphere groups (Figure 4B) also supported that conclusion. Both 3% and 1% CMCSMs showed better clotting capability than CSMs. Furthermore, CMCSMs (3%) with a relatively large diameter had a stronger effect on hemostasis than did CMCSMs with a small diameter, indicating that although small CMCSMs have a relatively larger surface area, they lose some swelling capability. In case of CSMs, their poor hydrophilia hampers water absorption, but smaller CS microspheres have larger surfaces, increasing the contact area with water to a certain extent and thus enhancing the hemostatic capability. Clotting curves for CMCSMs loaded with DIC were almost the same as those for unaltered CMCSMs, indicating that DIC had no effect on clotting (Figure 4C).
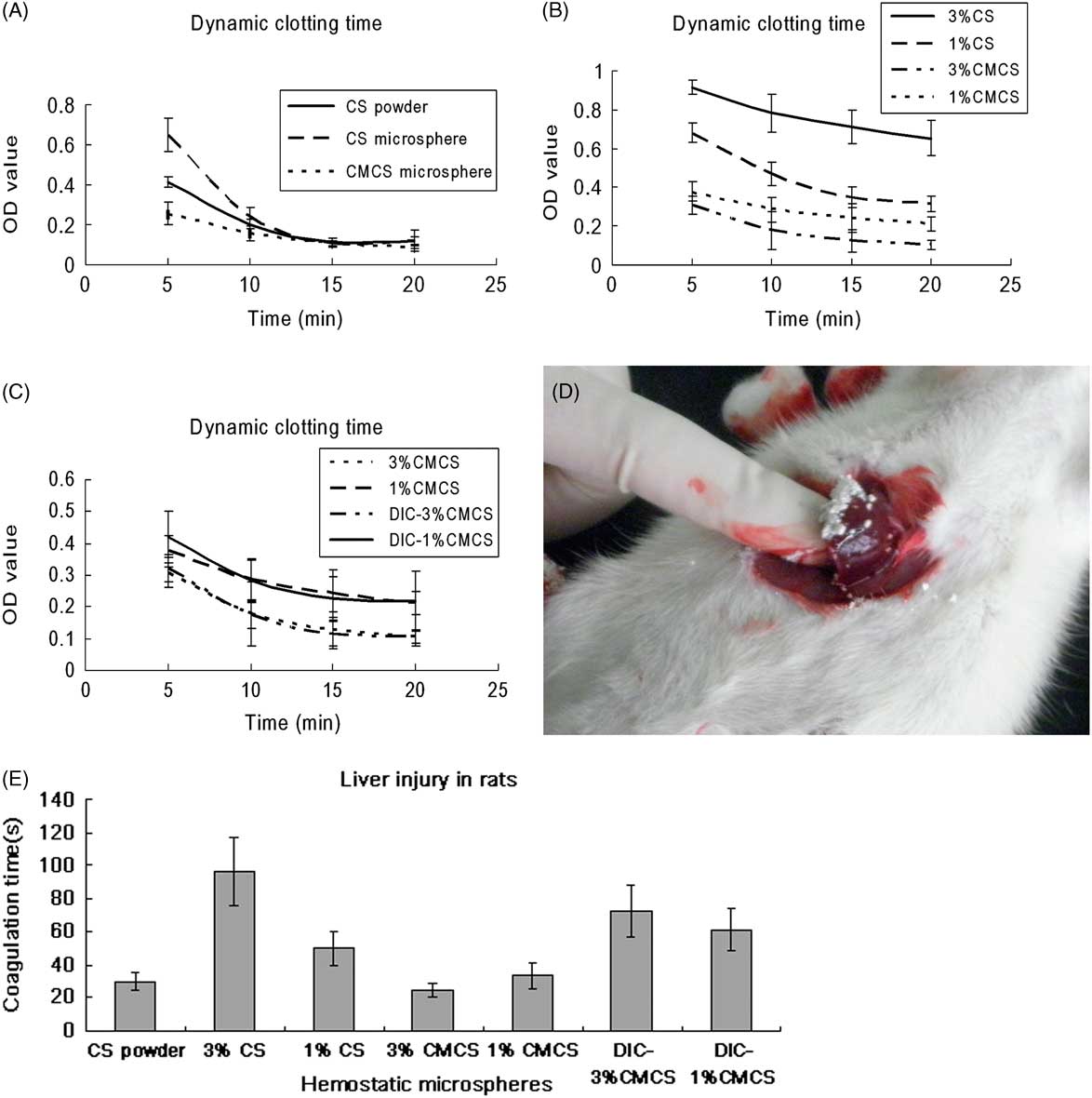
Figure 4 Clotting Time In Vitro and In Vivo. Abbreviations: CMCS, carboxymethyl chitosan; CMCSM, carboxymethyl chitosan microspheres; CS, chitosan; CSMs, chitosan microspheres; DIC, etamsylate. (A) through (C) are dynamic clotting plots in vitro; (D) and (E) show clotting time in liver injury in rats. Cross-linking CSMs slowed hemostasis, whereas CMCSMs accelerated hemostasis in comparison with CS powder (A); large CMCSMs (3%) showed the best hemostatic effect among all the microspheres (B); CMCSMs loaded with DIC had no effect on hemostasis (D); CMCSMs had the shortest coagulation time (E) in the hepatic injury model (D).
To determine clotting time in vivo, a hepatic injury model was established (Figure 4D). Both CS powder and CMCSMs took less than 40 s to stop the bleeding, whereas CSMs or CMCSMs loaded with DIC took longer, as shown in Figure 4E. This pattern was inconsistent with the clotting pattern in vitro, except that in both cases the smaller CS microspheres had a stronger effect than the larger ones.
DISCUSSION
In this study, we analyzed the effect of 2 different types of microspheres (of different hydrophobicity, particle size, and with or without drug loading) on blood coagulation. We found that the swelling capability of the biomaterial and the surface area of the particles strongly affect coagulation, whereas DIC loading had little effect.
CS and its derivatives are versatile biomaterials recently recognized as the new generation of local hemostatic agents.Reference Ong, Wu and Moochhala 17 Owing to the simplicity of application and their rapid effect on hemostasis, these agents are widely used in general surgery and in military settings. CMCS is an important derivative with a hydrophilic functional group introduced to form a modified CS with better solubility.Reference Luo, Teng and Wang 18 Water content in human blood is around 83%; hydrophilic materials have a low contact angle with water that favors blood permeation and initiates the clotting cascade. Our clotting time results in vitro and in vivo demonstrated that CMCSMs were associated with shorter clotting time than CSMs.
The size of microspheres is also an important factor affecting coagulation. The particle size is conversely proportional to the specific surface area of the material. When the blood comes into contact with a material, platelets aggregate and accelerate blood clotting.Reference Liu, Bian and Chen 12 Starch microspheres have an excellent swelling behavior and the large surface area of the microspheres effectively absorbs water and gathers clotting components, thus accelerating the endogenous clotting cascade. Arista hemostatic powder (produced by Medafor Company, Minneapolis, MN) consists of potato starch microspheres and is a good hemostatic agent in clinical applications.Reference Kheirabadi, FieldRidley and Pearson 19 , Reference Murat, Le and Ereth 20 The inorganic oxide zeolite hemostat QuikClot has good hemostatic properties, mainly due to its large specific surface area.Reference Arnaud, Tomori and Carr 21 HuReference Hu, Ying and Depo 22 demonstrated in his coagulation experiments that superfine powder of Xuejie is superior to the conventional powder. We prepared CS microspheres by using an emulsion cross-linking method, in which the volume ratio of the oil and water phase, the agitation speed, and the concentration of CS in the water phase affect the diameter of the microspheres. With other conditions held constant, the diameters of the microspheres were controlled by changing the concentration of CS in the water phase. The particle sizes of CSMs measured in the test were 3561.3 nm and 1284.8 nm at concentrations of 3% and 1%, respectively. The in vitro clotting time for the small CSMs was shorter, indicating that increasing the surface area of the material can accelerate blood clotting. However, emulsion cross-linking fixed the surface polymer network and reduced the elastic properties. Microspheres with the larger particle size had a lower clotting capability than that of CS powder. Therefore, polymer cross-linking might inhibit blood clotting.
Increasing hydrophilicity and expanding the contact area might promote blood clotting. We demonstrated that CMCSMs (3%) had good optical swelling and the shortest clotting time in vitro and in vivo. The water content of human blood is around 83%; hydrophilic materials have a low water contact angle, which helps the blood to permeate the surface of the material, and thus initiates the clotting cascade. Hemostatic agents reinforce multiple mechanisms leading to hemostasis, by improving the initial coagulation process, thrombosis, and (or) preventing fibrinolysis. Many experiments have shown that CMCS can be applied to improve hemostasis and accelerate wound healing.Reference Pusateri, McCarthy and Gregory 23 , Reference Fu, Han and Dong 24 However, the hemostatic mechanism of CMCS is still unclear. By the method of etherification, carboxymethylation of CS occurs first in the primary hydroxyl (C6-OH) due to the strong electronegativity of oxygen atom, and a small part of the reaction occurs in -NH2 (total substitutional degree can not reach 200%), which indicated that both carboxyl group and amino group exists in one molecule. As a result, CMCS belongs to quadripole polymer and has the characteristic of polyelectrolyte to agglutinate proteins. The blood proteins are between 1 and 100 nm in size and have colloidal properties; charge is a very important factor in colloidal aggregation. Plasma does not contain tissue factor III,Reference Mackman and Taubman 25 which is necessary for extrinsic coagulation; CMCS activates the intrinsic coagulation involving factor VIIa.Reference Busani, Cavazzuti and Marietta 26
However, the swelling behavior and clotting capability of CMCSMs (1%) is inferior to that of the CMCSMs (3%) examined in our study. One possible reason is that the cross-linked CMCSMs have lower swelling capability despite their increased surface area. As a result, the hemostatic effect is suppressed. Similarly, drug-loaded CMCSMs did not affect the clotting time in vitro and in vivo, and might even have had the opposite effect. Because the small DIC molecules occupy some parts of the polymer, they might result in weakened swelling.
In summary, enhancing the hydrophilicity of the material and expanding the contact area with the blood helps to accelerate coagulation. CMCSMs have excellent swelling capability and large surface areas; they are promising candidates for the development of new hemostatic agents. The application of cross-linking may hamper swelling behavior and decrease clotting capability. Other methods used to prepare monodisperse hollow microspheresReference Liu, Yan and Zhang 27 might help to further develop micro and nano biomaterials as hemostatic agents.
Funding
Supported by Tianjin Research Program of Application Foundation and Advanced Technology (15JCYBJC18600), Logistics Research Project (WHKL-17, WHTD201303).






