Institutional caregiving (e.g., “orphanage rearing”) is an extreme example of caregiving neglect, broadly characterized by an absence of parenting (Ellis, Fisher, & Zaharie, Reference Ellis, Fisher and Zaharie2004; Gunnar & Quevedo, Reference Gunnar and Quevedo2007; Rutter, Reference Rutter1998). Even in the best of circumstances, this type of rearing is a potent stressor for the human infant. As a consequence of this early exposure, institutional caregiving, even when followed by adoption into families, is commonly associated with emotion dysregulation (both behavioral and physiological) difficulties (Ellis et al., Reference Ellis, Fisher and Zaharie2004; Gee et al., Reference Gee, Gabard-Durnam, Flannery, Goff, Humphreys, Telzer and Tottenham2013; Humphreys et al., Reference Humphreys, Gabard-Durnam, Goff, Telzer, Flannery, Gee and Tottenhamin press; Koss, Hostinar, Donzella, & Gunnar, Reference Koss, Hostinar, Donzella and Gunnar2014; McLaughlin et al., Reference McLaughlin, Sheridan, Tibu, Fox, Zeanah and Nelson2015).
As described elsewhere (Gunnar, Bruce, & Grotevant, Reference Gunnar, Bruce and Grotevant2000; Merz & McCall, Reference Merz and McCall2010; Rutter et al., Reference Rutter, Beckett, Castle, Colvert, Kreppner, Mehta and Sonuga-Barke2007; Smyke et al., Reference Smyke, Koga, Johnson, Fox, Marshall, Nelson and Zeanah2007), caregiving provided by institutions can include a range of privations, from inadequate nutrition, hygiene, and medical care, to inadequate cognitive stimulation, to unstable caregiving. These conditions exceed any normative range of expected caregiving (McLaughlin, Sheridan, & Nelson, Reference McLaughlin, Sheridan and Nelson2017; Tottenham, Reference Tottenham2012a), and therefore, act as a potent stressor on infant brain development (e.g., Gee et al., Reference Gee, Gabard-Durnam, Flannery, Goff, Humphreys, Telzer and Tottenham2013; Gunnar, Frenn, Wewerka, & Van Ryzin, Reference Gunnar, Frenn, Wewerka and Van Ryzin2009; McLaughlin et al., Reference McLaughlin, Sheridan, Tibu, Fox, Zeanah and Nelson2015). Biological systems like the amygdala and hypothalamic–pituitary–adrenal axis (HPA) are particularly sensitive to such stressors due in part to their early development (Gilmore et al., Reference Gilmore, Shi, Woolson, Knickmeyer, Short, Lin and Shen2012; Humphrey, Reference Humphrey1968; Payne, Machado, Bliwise, & Bachevalier, Reference Payne, Machado, Bliwise and Bachevalier2010; Ulfig, Setzer, & Bohl, Reference Ulfig, Setzer and Bohl2003) and to an abundance of stress hormone receptors, which animal models have shown are already present in early postnatal life (Avishai-Eliner, Yi, & Baram, Reference Avishai-Eliner, Yi and Baram1996; Fenoglio, Brunson, Avishai-Eliner, Chen, & Baram, Reference Fenoglio, Brunson, Avishai-Eliner, Chen and Baram2004). Moreover, administration of stress hormones has been causally linked to amygdala hyperactivity in the developing rodent (Baram, Hirsch, Snead, & Schultz, Reference Baram, Hirsch, Snead and Schultz1992). Consistent with the hypothesis that institutional caregiving in infancy is a stressor that can modify amygdala structure and function, alterations to amygdala development have been observed in youth adopted internationally from institutional care (Gee et al., Reference Gee, Gabard-Durnam, Flannery, Goff, Humphreys, Telzer and Tottenham2013; Hanson et al., Reference Hanson, Nacewicz, Sutterer, Cayo, Schaefer, Rudolph and Davidson2015; Maheu et al., Reference Maheu, Dozier, Guyer, Mandell, Peloso, Poeth and Ernst2010; Mehta et al., Reference Mehta, Golembo, Nosarti, Colvert, Mota, Williams and Sonuga-Barke2009; Tottenham et al., Reference Tottenham, Hare, Millner, Gilhooly, Zevin and Casey2011). Amygdala phenotypes following institutional care have been associated with a constellation of behaviors that contribute to emotion dysregulation, including atypical responding to environmental cues of potential threat (e.g., avoidance of eye contact; Tottenham et al., Reference Tottenham, Hare, Millner, Gilhooly, Zevin and Casey2011), enhanced visual attention for threat-related cues (Silvers et al., Reference Silvers, Goff, Gabard-Durnam, Gee, Fareri, Caldera and Tottenham2017), difficulty inhibiting behavior in the context of threat cues (Tottenham et al., Reference Tottenham, Hare, Quinn, McCarry, Nurse, Gilhooly and Casey2010), and increased behavioral problems (Hanson et al., Reference Hanson, Nacewicz, Sutterer, Cayo, Schaefer, Rudolph and Davidson2015).
Contemporary models of developmental psychopathology (e.g., Beauchaine & Zisner, Reference Beauchaine and Zisner2017) posit that these latent indicators of subcortical vulnerabilities contribute to the transdiagnostic risk for mental health difficulties involving emotion dysregulation, including elevated anxiety symptoms (discussed in Beauchaine, Reference Beauchaine2015; Cole, Hall, & Hajal, Reference Cole, Hall, Hajal, Beauchaine and Hinshaw2013). Accordingly, increased trait anxiety symptoms have been observed following institutional care, which have correlated with alterations in subcortical structures including the amygdala (e.g., Gee et al., Reference Gee, Gabard-Durnam, Flannery, Goff, Humphreys, Telzer and Tottenham2013; Green et al., Reference Green, Goff, Gee, Gabard-Durnam, Flannery, Telzer and Tottenham2016; Silvers et al., Reference Silvers, Lumian, Gabard-Durnam, Gee, Goff, Fareri and Tottenham2016). Of particular note is separation anxiety, a common outcome of caregiving instability and early stress exposure (Pine & Cohen, Reference Pine and Cohen2002; Rapee & Szollos, Reference Rapee and Szollos2002), which although observed in childhood, can persist into adolescence (Gee et al., Reference Gee, Gabard-Durnam, Flannery, Goff, Humphreys, Telzer and Tottenham2013; Green et al., Reference Green, Goff, Gee, Gabard-Durnam, Flannery, Telzer and Tottenham2016; Humphreys et al., Reference Humphreys, Gleason, Drury, Miron, Nelson, Fox and Zeanah2015).
Despite this elevated risk for separation anxiety symptoms, there is notable heterogeneity in outcomes following institutional care (Gee et al., Reference Gee, Gabard-Durnam, Flannery, Goff, Humphreys, Telzer and Tottenham2013; Green et al., Reference Green, Goff, Gee, Gabard-Durnam, Flannery, Telzer and Tottenham2016; Humphreys et al., Reference Humphreys, Gleason, Drury, Miron, Nelson, Fox and Zeanah2015; Tottenham, Reference Tottenham2012b). Consistent with the concept of developmental multifinality, adverse caregiving experiences are followed by large individual differences in mental health outcomes (Doom & Cicchetti, Reference Doom, Cicchetti, Harkness and Hayden2018). For instance, two adolescents with early institutional care experiences may exhibit very different developmental trajectories, and these differences have been linked to age of adoption (e.g., Castle et al., Reference Castle, Groothues, Bredenkamp, Beckett, O'Connor and Rutter1999; Fox, Almas, Degnan, Nelson, & Zeanah, Reference Fox, Almas, Degnan, Nelson and Zeanah2011; Green et al., Reference Green, Goff, Gee, Gabard-Durnam, Flannery, Telzer and Tottenham2016; Loman, Wiik, Frenn, Pollak, & Gunnar, Reference Loman, Wiik, Frenn, Pollak and Gunnar2009), to genetic differences (Drury et al., Reference Drury, Gleason, Theall, Smyke, Nelson, Fox and Zeanah2012), and to postadoption factors (e.g., Vantieghem et al., Reference Vantieghem, Gabard-Durnam, Goff, Flannery, Humphreys, Telzer and Tottenham2017). Individual differences have been noted across several types of caregiving adversities in the resilience literature (including maltreatment, parent psychopathology/substance abuse, and other wide-ranging early adversities; Cicchetti & Rogosch, Reference Cicchetti and Rogosch1997; Martel et al., Reference Martel, Nigg, Wong, Fitzgerald, Jester, Puttler and Zucker2007; Masten, Best, & Garmezy, Reference Masten, Best and Garmezy1990; Masten & Tellegen, Reference Masten and Tellegen2012), and this literature often highlights factors extrinsic to the child that promote resilience (e.g., presence of a supportive adult). However, mechanisms within the individual (i.e., intraindividual factors) have also been noted, and identifying these mechanisms remains an area of active research (e.g., Blair & Raver, Reference Blair and Raver2016; Curtis & Cicchetti, Reference Curtis and Cicchetti2007; Masten & Barnes, Reference Masten and Barnes2018; Raver, McCoy, Lowenstein, & Pess, Reference Raver, McCoy, Lowenstein and Pess2013; Vantieghem et al., Reference Vantieghem, Gabard-Durnam, Goff, Flannery, Humphreys, Telzer and Tottenham2017).
Continuing this line of inquiry, the present study examined whether working memory (WM) acts as an intraindividual resilience factor to mitigate the link between early institutional caregiving and separation anxiety during late childhood and adolescence. Previous work has shown that WM mediates the link between early institutional care and attention-deficit/hyperactivity symptoms in 12-year-olds (Tibu et al., Reference Tibu, Sheridan, McLaughlin, Nelson, Fox and Zeanah2016). This effect of WM on cognitive functions is perhaps intuitive given its function to “temporarily store […] information as part of the performance of complex tasks” (Baddeley, Reference Baddeley1992). However, perhaps less intuitively, WM and other closely related functions (e.g., inhibitory control and attention control), have also been associated with affective symptoms and emotion regulation (Beauchaine & Thayer, Reference Beauchaine and Thayer2015; Etkin & Schatzberg, Reference Etkin and Schatzberg2011; Schmeichel & Demaree, Reference Schmeichel and Demaree2010; Schmeichel, Volokhov, & Demaree, Reference Schmeichel, Volokhov and Demaree2008), and have been causally linked with reducing anxiety symptoms (Hadwin & Richards, Reference Hadwin and Richards2016; Sari, Koster, Pourtois, & Derakshan, Reference Sari, Koster, Pourtois and Derakshan2016). Mechanistically, it has been argued that better WM translates into being more “adept at juggling multiple streams of information,” which then facilitates better management of emotional responding (Schmeichel & Demaree, Reference Schmeichel and Demaree2010, p. 742). In the current study, we investigated whether WM moderated the association between early institutional caregiving and separation anxiety symptoms during late childhood and adolescence. We used a modified version of an “object-in-place” task that requires memory of novel locations of four objects over an 8-s delay and subsequently reporting the location of a cued item. This type of task has been shown to rely on the hippocampus and medial prefrontal cortex in animal models of WM (Bachevalier & Nemanic, Reference Bachevalier and Nemanic2008; Barker & Warburton, Reference Barker and Warburton2015; Kim, Delcasso, & Lee, Reference Kim, Delcasso and Lee2011). Of note, these regions have previously been associated with resilience against anxiety symptoms in previously institutionalized (PI) samples (e.g., Gee et al., Reference Gee, Gabard-Durnam, Flannery, Goff, Humphreys, Telzer and Tottenham2013; Silvers et al., Reference Silvers, Lumian, Gabard-Durnam, Gee, Goff, Fareri and Tottenham2016).
WM skills have also routinely been associated with individual differences in diurnal cortisol production; in particular, better WM skills have been associated with higher morning cortisol levels in adults and children (Erickson, Drevets, & Schulkin, Reference Erickson, Drevets and Schulkin2003; Maldonado et al., Reference Maldonado, Fernandez, Trianes, Wesnes, Petrini, Zangara and Ambrosetti2008). This association may be the result of the intimate neurohormonal relationship between cortisol and corticohippocampal circuits underlying WM skills (see Eichenbaum, Reference Eichenbaum2000; Jay et al., Reference Jay, Rocher, Hotte, Naudon, Gurden and Spedding2004), which are rich with cortisol receptors and interact with the HPA axis (Buchanan, Tranel, & Adolphs, Reference Buchanan, Tranel and Adolphs2006; Jay et al., Reference Jay, Rocher, Hotte, Naudon, Gurden and Spedding2004; Lupien & Lepage, Reference Lupien and Lepage2001; Oei, Everaerd, Elzinga, van Well, & Bermond, Reference Oei, Everaerd, Elzinga, van Well and Bermond2006; Roozendaal, Reference Roozendaal2002). Morning cortisol levels have also been linked with anxiety symptoms, although the direction of effects has been mixed (O'Donovan et al., Reference O'Donovan, Hughes, Slavich, Lynch, Cronin, O'Farrelly and Malone2010; Vreeburg et al., Reference Vreeburg, Zitman, van Pelt, Derijk, Verhagen, van Dyck and Penninx2010). Of note, diurnal cortisol production often exhibits atypical patterns (i.e., blunted morning cortisol) following institutional care when measured in young children (Gunnar & Quevedo, Reference Gunnar and Quevedo2007). However, at older ages this pattern appears to alleviate for some PI youth in the transition to adolescence (Flannery et al., Reference Flannery, Gabard-Durnam, Shapiro, Goff, Caldera, Louie and Tottenham2017; Quevedo, Johnson, Loman, Lafavor, & Gunnar, Reference Quevedo, Johnson, Loman, Lafavor and Gunnar2012). If there is an association between morning cortisol and WM, then individual differences in morning cortisol measured in late childhood and adolescence might be associated with better WM performance and lower separation anxiety.
The goal of the present study was to investigate individual differences in separation anxiety following early institutional care by examining whether WM skills moderated the link between early care and separation anxiety. In addition, we examined the associations between morning WM, separation anxiety, and cortisol during the late childhood/adolescent period, given the earlier findings that cortisol is linked with WM and may become more typical in adolescence for those who experienced early institutional caregiving.
Method
Participants
One hundred and ten youth (M age = 12.9 years old, SD = 2.2; range = 10–17); 70 female/40 male) were drawn from a larger community-based study examining behavioral and neurobiological outcomes following early institutional care. Forty-two participants with a history of institutional caregiving (PI) who were later adopted into families and 68 participants with no history of institutional caregiving who had always lived with their parent(s) (comparison group) participated in the current study. Youth in the PI group were recruited via local international adoption agencies and family networks. Youth in the comparison group were recruited via flyer advertisements within the surrounding community or from state birth records. Youth in the comparison group were only included if parents indicated that they had no psychiatric, neurological, or learning disorder diagnosis. Estimated IQ was assessed with the Wechsler Abbreviated Scale of Intelligence (Wechsler, Reference Wechsler1999; two subtest form), and there were no significant group differences in estimated IQ, F (1, 109) = 0.004, p = .95, partial η2 = 10−5, PI M = 105.79, SD = 14.45; comparison M = 104.53, SD = 13.28. The mean age of placement in institutional care for PI participants was 9.49 months (median = 0.2; SD = 24.18; range = 0–126) and the mean age of adoption was 32.86 months (median = 15; SD = 37.45; range = 2–168). PI youth were internationally adopted from East Asia (55%), Eastern Europe (38%), Central America (5%), and India (2%). The protocol was approved by the university institutional review board. Parents provided informed consent for youth participation, and youth provided written assent.
Materials and procedure
WM
WM was assessed behaviorally using a modified “object-in-place” task. Participants were tested individually in a private room in the laboratory on a single visit. Participants were instructed to memorize the locations of four white schematic objects (apple, backpack, envelope, and hat) on a black screen within a 2 × 2 grid for 6000 ms (Figure 1). All four schematic images were presented simultaneously, and their locations changed for each new trial. These presentations were followed by a delay period of 3000 ms (central fixation on screen). After this delay period, there was an additional delay period for 2000 ms during which the question, “Where was the [object],?” together with an object (e.g., apple) were presented, followed by an additional delay period of 3000 ms (i.e., total delay = 8000 ms). Participants were then presented with a blank 2 × 2 grid, where they indicated (via keyboard press) the location of the specific object (e.g., apple) to the best of their ability. This response period was followed by an intertrial interval of 3000 ms. There was a total of 10 trials in the task. Accuracy was computed as the percentage of correct trials.

Figure 1. (a) Illustration of working memory task. (b) Group means on the working memory task. Circles are individual participants. PI, previous institutional care.
Separation anxiety
Separation anxiety was assessed via parent report (Screen for Child Anxiety Related Disorders—Parent Report; Birmaher et al., Reference Birmaher, Brent, Chiappetta, Bridge, Monga and Baugher1999). This is a 41-item questionnaire designed for ages 8–18 years old, which has shown good internal consistency (e.g., Cronbach's α for separation anxiety = 0.81) and test–retest validity (e.g., interclass correlations range: .70–.90; Birmaher, Reference Birmaher1997; Wren et al., Reference Wren, Berg, Heiden, Kinnamon, Ohlson, Bridge and Bernal2007). In the current sample, similar good internal consistency was observed for the separation anxiety subscale (Cronbach's α = 0.83). We focused on the continuous scores from the separation anxiety subscale as previous research suggests a robust association between early institutional care and elevated separation anxiety scores (e.g., Gee et al., Reference Gee, Gabard-Durnam, Flannery, Goff, Humphreys, Telzer and Tottenham2013).
Morning cortisol
Although the current analyses focused on morning levels only, diurnal salivary cortisol was measured across 2 consecutive (or close to consecutive) days (for analyses of the full diurnal cycle, see Flannery et al., Reference Flannery, Gabard-Durnam, Shapiro, Goff, Caldera, Louie and Tottenham2017). Parents were asked to collect saliva across four different times of the day (at wake-up, 45 min after wake-up, at 5 p.m., and at 8 p.m.) for 2 days. Parents were provided with saliva collection kits with four tubes, cotton sticks, and a daily saliva diary. Participants were asked to chew on a piece of Trident® sugarless gum and then place a sorbette in their mouth to absorb saliva (Dabbs, Reference Dabbs1991; Salimetrics, State College, PA). Saliva samples were either mailed back in or brought in by families. Attempts were made to obtain home saliva samples as close to in-lab testing as possible (time difference M = 47.5, SD = 123.74 days; median = 13 days). Samples were stored in a locked freezer at –20 °C until they were mailed over dried ice to Technical University Dresden to be processed by Dr. Clemens Kirschbaum's Biological Psychology Laboratory. Salivary cortisol concentrations were measured in singlet using commercially available chemiluminescence-immunoassays with high sensitivity. The interassay coefficient for cortisol was below 8% (Kirschbaum & Hellhammer, Reference Kirschbaum and Hellhammer2000). Because several studies have shown that PI samples are likely to differ most in morning values of cortisol (e.g., Flannery et al., Reference Flannery, Gabard-Durnam, Shapiro, Goff, Caldera, Louie and Tottenham2017; Koss, Mliner, Donzella, & Gunnar, Reference Koss, Mliner, Donzella and Gunnar2016), the current study focused on morning values; this was computed by taking the natural log of the mean of the morning collections (wake-up and wake-up+45 min) across the 2 days. No extreme observe values were found within this sample; therefore, all data points were included.
Statistical analysis
Statistical analyses were performed in SPSS (version 24; SPSS Inc., USA), including the PROCESS macro (Hayes, Reference Hayes2018), and JASP (https://jasps-stats.org). All analyses included the covariates of age and sex of participants. No data points were excluded in analyses; any missing data points are missing because the data were not able to be obtained. If data were missing for any variable, analyses including that variable dropped listwise.
Results
Descriptive data
WM scores were collected from 110 youth, and accuracy scores ranged from 30% to 100%. There were no group differences in WM accuracy scores, F (1, 110) = 0.03, p = .87, partial η2 = .00; PI M = 82%, SD = 17% and comparison M = 84%, SD = 17% (see Figure 1). We followed this analysis with a Bayesian analysis of covariance model in JASP to compute whether the data were more likely under the null versus the alternative hypothesis. The Bayes factor indicated that the data were 4.26 times more likely to be observed under the null hypothesis than they were under the alternative hypothesis. In other words, this analysis is consistent with there being no significant difference between groups in these WM scores. There was a main effect of age, F (1, 110) = 7.72, p < .01, partial η2 = .07, such that WM scores correlated positively with age. There were no other main effects of interactions for WM scores. Separation anxiety scores were obtained from 101 of these 110 youth. As anticipated based on the recruitment strategy of the comparison group, there were group differences in separation anxiety scores, F (1, 101) = 6.42, p < .015, partial η2 = .06, such that PI adolescents (M = 4.03, SD = 3.83) had higher values than the comparison group (M = 2.02, SD = 2.73). Sixty-four percent of the youth in the PI group were above the cutoff for clinical concern on the Screen for Child Anxiety Related Disorders—Parent Report. There was also a main effect of age, F (1, 101) = 11.00, p < .001, partial η2 = .10, such that younger participants had higher separation anxiety scores. There were no other main effects or interactions for separation anxiety scores. Morning levels of salivary cortisol were obtained from 80 of these 110 youth. There was a nonsignificant trend for group, F (1,80) = 3.15, p = .08, partial η2 = .04, in the direction of the PI group having lower morning cortisol levels (PI M = 2.76 nmol/L natural logged, SD = 0.40; comparison M = 2.89 nmol/L natural logged, SD = 0.54). There was a main effect of sex, F (1, 80) = 6.81, p < .015, partial η2 = .08 (female M = 2.96 nmol/L, SD = 0.45; male M = 2.68 nmol/L, SD = 0.51). There were no other main effects or interactions.
Table 1 shows the bivariate correlations between WM scores, separation anxiety scores, and morning cortisol levels for each group. As shown, these three variables were significantly correlated within the PI group, but not in the comparison group.
Table 1. Within-group Pearson correlation matrices for study variables

*p < .05. **p < .001.
Moderation analysis: WM
To test whether WM moderated the association between caregiving group and separation anxiety scores, a PROCESS model (#1) in SPSS was used. Group (PI vs. comparison) was entered as the independent variable, with WM as the moderator, and separation anxiety symptoms as the dependent variable, controlling for age and sex. The overall model was significant, F (5, 95) = 7.82, p < 10−4, R 2 = .29). In addition, the Group × WM interaction accounted for a significant proportion of the variance in separation anxiety scores, ΔR 2 = .05, ΔF (1, 95) = 6.39, p < .015, β = –8.67, 95% confidence interval (CI) [–15.48, –1.86]. As illustrated in Figure 2, post hoc linear regressions showed that higher WM scores were associated with lower separation anxiety scores in the PI group, β = –0.41, t (39) = –2.89, p < .01, ΔR 2 = .15, but not in the comparison group, β = –0.12, t (62) = –0.92, p = .36, ΔR 2 = .01. This analysis controlled for sex and age; sex did not account for any significant variance in separation anxiety, β = 0.54, t (101) = 0.87, p = .38, and as expected and described earlier, age was associated with separation anxiety scores, β = –0.03, t (101) = –2.81, p < .01.

Figure 2. Interaction between group and working memory scores on separation anxiety scores. Residual scores are plotted, controlling for age and sex. PI, previous institutional care.
Because WM could be associated with IQ, an additional analysis accounting for estimated IQ retested this moderation. A general linear model confirmed that WM and estimated IQ were highly associated with each other, F (1, 109) = 25.72, p < 10−5, partial η2 = .20. Therefore, the PROCESS moderation was repeated, but this time including estimated IQ as a covariate. Even when controlling for estimated IQ, the Group × WM interaction remained significant, ΔR 2 = .05, ΔF (1, 93) = 6.09, p < .015, β = –8.52, 95% CI [–15.37, –1.66].
Associations with morning cortisol
As WM scores, separation anxiety scores, and morning cortisol levels were all correlated with each other in the PI group (see Table 1), but not in the comparison group, we tested a moderated mediation (PROCESS model #59) with morning cortisol as the independent variable, separation anxiety scores as the dependent variable, and WM scores as the mediator, controlling for age and sex. Caregiving group was treated as the moderator of the entire mediation. As shown in Figure 3, this analysis showed that there was a significant moderated mediation (index = –1.48, SE [boot] = 1.08), and the 95% CI [–4.91, –0.15] did not include 0. This analysis indicated that the association between morning cortisol and separation anxiety scores was statistically mediated by WM in the PI youth.
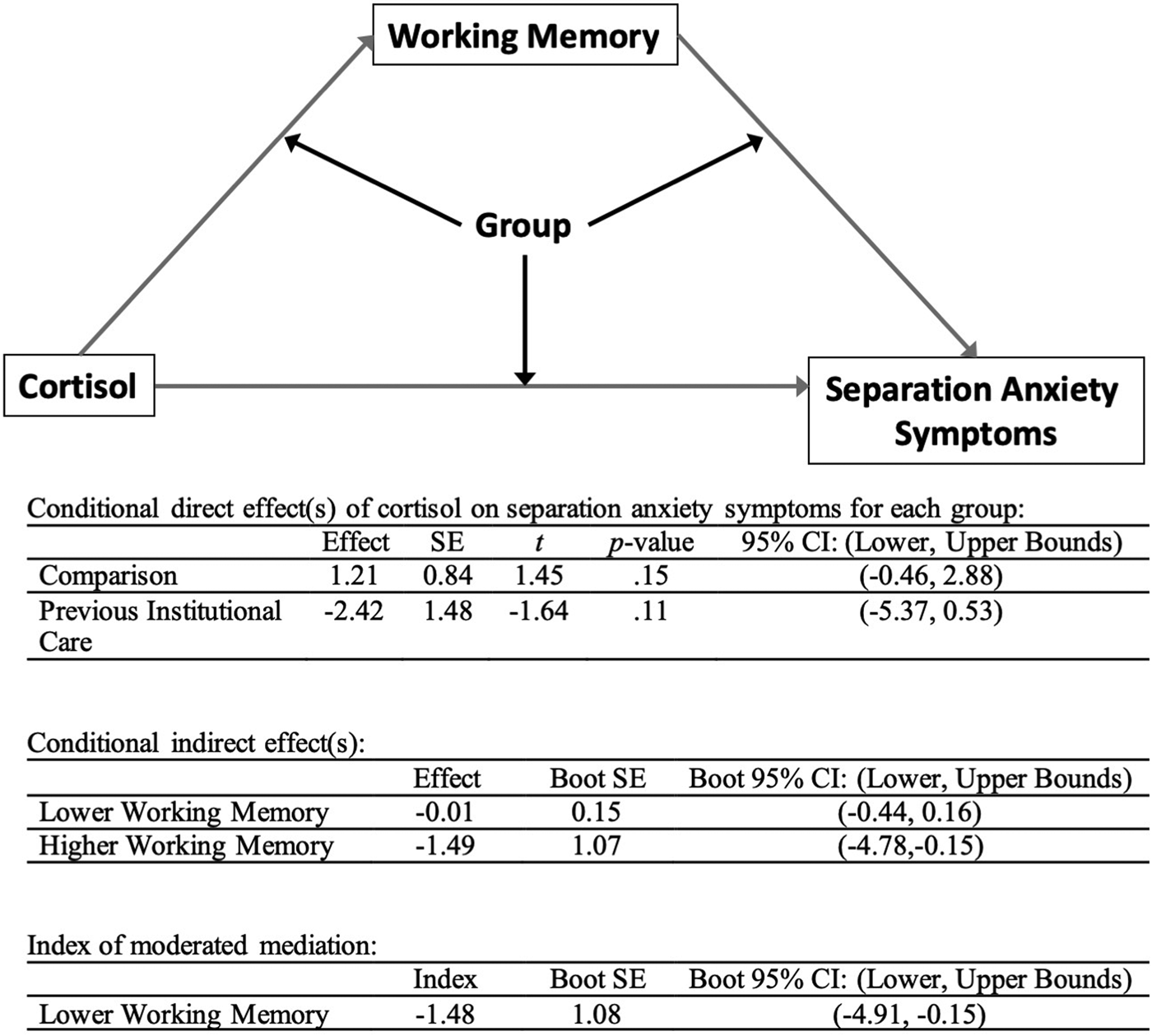
Figure 3. Moderated mediation. For PI youth, the association between morning cortisol and separation anxiety scores was mediated by working memory scores. PI, previous institutional care.
Supplemental analyses of WM and separation anxiety scores
Although our hypothesis predicted that WM would moderate the association between early caregiving and separation anxiety, the measures in this study were collected at (roughly) the same visit, making it difficult to conclude directionality of effects; therefore, we also tested alternative models. We found that neither (a) the reverse moderation (i.e., separation anxiety moderating the association between group and WM, via PROCESS model #1 in SPSS), F (1, 95) = 1.19, p = .28, ΔR 2 = .01, nor (b) the mediation model (i.e., WM as mediator of the association between group and separation anxiety, via PROCESS model #4 in SPSS), point estimate = 0.09 [0.19], 95% CI [–0.17, 0.72], were significant. However, we did find evidence that separation anxiety scores indirectly mediated the association between group and WM scores, F (4, 96) = 4.52, p < .005 (via PROCESS model #4 in SPSS), accounting for 16% of additional variance (controlling for age and sex). The 95% CI for the indirect effect of separation anxiety was near, but did not contain, zero, point estimate = –0.02 [0.02], 95% CI [–.07, –.001]. This finding could mean that although there was no group difference in WM scores (and thus no direct path between group and WM scores), separation anxiety symptoms partially accounted for some of the differences in WM scores in the PI youth.
Correlations with caregiving timing variables
We used separate linear regressions to test for associations between the primary variables in this study (i.e., WM, morning cortisol, and separation anxiety scores) and institutional care timing variables (age placed in institutional care, age adopted, time in institutional care, and time with family), controlling for current age and sex. There were only statistical trends for time in institutional care to be associated with WM (β = –0.31, t = –2.03, p = .051, ΔR 2 = .09) and separation anxiety scores (β = 0.05, t = 1.78, p = .08, ΔR 2 = .07), such that longer stays in institutional care were associated with lower WM scores and higher separation anxiety scores, but these associations did not reach statistical significance. When WM and time in institutional care were entered into the same regression with separation anxiety as the dependent variable, only WM was significantly associated with separation anxiety (β = –8.06, t = –2.41, p < .025), whereas time in institutional care was not (β = 0.026, t = 0.94, p = .35). There were no other significant associations with caregiving timing variables (smallest p > .3).
Discussion
The neglect inherent to institutional caregiving is a potent stressor for the developing infant that significantly increases the risk for later mental health problems. However, the concept of multifinality predicts large individual differences in outcome. The first aim of this study was to test the hypothesis that during late childhood/adolescence, WM would moderate the association between early adverse caregiving (i.e., institutional care) and separation anxiety scores. Support was found for this hypothesis, such that PI youth with higher WM scores had lower separation anxiety scores than other PI youth. That is, although the average separation anxiety score was high for youth with a history of early institutional care, there were significant individual differences (i.e., multifinality), and these individual differences in separation anxiety scores were explained by WM. The current findings raise the possibility that WM is an intraindividual resilience factor that protects against separation anxiety following caregiving adversity.
Contemporary models of developmental psychopathology have argued that cortically mediated processes, including WM, are part of the latent structure that contribute to individual differences in emotion regulation (e.g., Beauchaine & Zisner, Reference Beauchaine and Zisner2017). These processes are described as exerting “top-down” regulatory influences on affect-related subcortical systems. There is much empirical support for this model from neuroimaging studies in humans as well as nonhuman animal studies (e.g., Etkin, Prater, Hoeft, Menon, & Schatzberg, Reference Etkin, Prater, Hoeft, Menon and Schatzberg2010; Lieberman et al., Reference Lieberman, Eisenberger, Crockett, Tom, Pfeifer and Way2007; Mitchell et al., Reference Mitchell, Nakic, Fridberg, Kamel, Pine and Blair2007; Ochsner, Bunge, Grosss, & Gabrieli, Reference Ochsner, Bunge, Gross and Gabrieli2002; Quirk & Beer, Reference Quirk and Beer2006). Although neuroimaging was not collected during the WM task in the current paper, previous work has shown that prefrontal-amygdala and prefrontal-hippocampal functional connectivity are associated with reduced separation anxiety in PI youth (Gee et al., Reference Gee, Gabard-Durnam, Flannery, Goff, Humphreys, Telzer and Tottenham2013; Silvers et al., Reference Silvers, Lumian, Gabard-Durnam, Gee, Goff, Fareri and Tottenham2016). Taken together with these past findings, the behavioral findings of the current study are consistent with models of emotion regulation that emphasize interactions between multiple levels (e.g., cortical and subcortical) of brain development (e.g., Beauchaine & Zisner, Reference Beauchaine and Zisner2017).
We also examined WM and separation anxiety associations with morning cortisol as WM and mental health have both independently been associated with higher morning cortisol in previous studies (Cicchetti & Rogosch, Reference Cicchetti and Rogosch2007; Maldonado et al., Reference Maldonado, Fernandez, Trianes, Wesnes, Petrini, Zangara and Ambrosetti2008). In the present study, morning cortisol levels, separation anxiety scores, and WM were correlated with each other only in the PI sample. A test of moderated mediation showed that for youth with a history of institutional care, higher morning cortisol levels were associated with higher WM scores, and both were associated with lower separation anxiety scores. The sample size in this study is small for testing complex interactions, and therefore, this full model should be considered with caution. However, the correlations between cortisol, WM scores, and separation anxiety that only existed in the PI group motivated our testing the full model. Although blunted morning cortisol levels have been repeatedly observed in young children with a PI history, adolescents with a history of PI have exhibited more normative morning cortisol levels (Flannery et al., Reference Flannery, Gabard-Durnam, Shapiro, Goff, Caldera, Louie and Tottenham2017; Gunnar & Quevedo, Reference Gunnar and Quevedo2007), which were associated with more time living with adoptive families (Flannery et al., Reference Flannery, Gabard-Durnam, Shapiro, Goff, Caldera, Louie and Tottenham2017). These findings are consistent with the hypothesis that the developing HPA axis is amenable to the ameliorative effects of subsequent stable family environments (Flannery et al., Reference Flannery, Gabard-Durnam, Shapiro, Goff, Caldera, Louie and Tottenham2017; O'Connor, Rutter, Beckett, Keaveney, & Kreppner, Reference O'Connor, Rutter, Beckett, Keaveney and Kreppner2000).
In the current study, we did not observe any obvious deficits in WM associated with PI history, as both comparison and PI youth performed with high accuracy (~83%). On the surface, this finding deviates from previous work identifying poor WM following institutional care (Bick, Zeanah, Fox, & Nelson, Reference Bick, Zeanah, Fox and Nelson2018; Hostinar, Stellern, Schaefer, Carlson, & Gunnar, Reference Hostinar, Stellern, Schaefer, Carlson and Gunnar2012; Tibu et al., Reference Tibu, Sheridan, McLaughlin, Nelson, Fox and Zeanah2016). There are several possible reasons for this difference. The task used in the current study may require less WM than those used in previous studies, which would obfuscate any potential group differences. However, even though potentially an easier task, individual differences in performance nonetheless did show associations with individual differences in separation anxiety scores in the PI group. Another important difference may be related to participant characteristics. The estimated IQ in the current sample of youth with a history of institutional care was higher than those in previous reports. This difference is important as previous work has shown that controlling for IQ removes group differences (PI vs. comparison) in adolescent WM (Bick et al., Reference Bick, Zeanah, Fox and Nelson2018). In the current sample, estimated IQ was correlated with WM scores. However, WM continued to moderate the link between early caregiving group and separation anxiety even when controlling for IQ, indicating that the WM measured in the current study made a unique contribution to separation anxiety over and above IQ. Moreover, a previous study that controlled for IQ nonetheless identified differences following institutional care in an executive function battery, which included a WM task (Hostinar et al., Reference Hostinar, Stellern, Schaefer, Carlson and Gunnar2012). However, this assessment occurred during the preschool period only 1 year following adoption into families. Therefore, it is possible that alterations in WM due to institutional care might exhibit recovery over time as children grow up with their adoptive families. In addition, it is unlikely that WM was simply an index of general proxy for better mental health because we did not find group differences, and the test of reverse moderation (i.e., separation anxiety moderating the link between institutional care and WM) was not significant. In the context of the current findings, it is possible that stable postadoption family environments might also benefit WM and separation anxiety performance (e.g., Humphreys, et al., Reference Humphreys, Gleason, Drury, Miron, Nelson, Fox and Zeanah2015; Merz, McCall, Wright, & Luna, Reference Merz, McCal, Wright and Luna2013), perhaps through improving HPA axis activity. This subsequent exposure to stable caregiving may have particular benefits for the relatively late developing neurobiology that underlies WM skills (Kwon, Reiss, & Menon, Reference Kwon, Reiss and Menon2002; von Allmen, Wurmitzer, & Klaver, Reference von Allmen, Wurmitzer and Klaver2014).
WM mediated the link between morning cortisol and separation anxiety, but this entire path was moderated by caregiving group. In other words, the associations between WM, cortisol, and separation anxiety only existed for the PI youth. One explanation for this observed moderation might be that the comparison group had levels of separation anxiety that were too low, and therefore lacked enough variance to reveal any within-group associations, whereas the PI group had separation anxiety scores that were more likely to be in the clinical concern range. Another possibility is that these processes are supported by different neurobiological mechanisms in the PI versus comparison youth (i.e., a “behavioral phenocopy”; Church, Petersen, & Schlaggar, Reference Church, Petersen and Schlaggar2010). There is precedent for this idea; it has been shown that despite similar behavioral performance, youth with a history of institutional care (vs. those without this PI history) can engage different neurobiology (Silvers et al., Reference Silvers, Lumian, Gabard-Durnam, Gee, Goff, Fareri and Tottenham2016). Of note, these neurobiological regions identified in the Silvers et al. study included the hippocampus and medial prefrontal cortex, which have been shown to be involved in the “object-in-place” type of WM task used in this study (Bachevalier & Nemanic, Reference Bachevalier and Nemanic2008; Barker & Warburton, Reference Barker and Warburton2015).
The present results should be considered within the context of its limitations. There is little information about preadoption experiences of the participants. While this is a common challenge in samples that include individuals with a PI history, it should be considered in balance with the scientific advantage of being able to examine development following a highly significant and temporally bound form of early caregiving adversity. In addition, there was a trend for WM scores to be negatively associated with time in institutional care, consistent with findings that institutional care is causally associated with WM (Bick et al., Reference Bick, Zeanah, Fox and Nelson2018). Youth in the comparison group were enrolled if their parent indicated no history of psychiatric or neurologic illness. This comparison group (not a “control” group) was selected to provide information about WM behavior in the absence of illness, but this enrollment strategy also limits our ability to generalize findings from this “very healthy” comparison group and can exaggerate group differences (although the PI youths’ scores were still very high relative to expected norms). Therefore, future tests of replication should include a comparison sample that is more representative of the general population (i.e., including samples with psychopathology). Finally, the data presented in this paper were obtained at a single time point, which tempers any conclusions about directionality of effects. Our analyses showed that an alternative model also was significant, whereby separation anxiety mediated the association between early caregiving group and WM. If this mediation were replicated in a longitudinal design, the findings would be consistent with the hypothesis that early alterations to affective processes can produce cascading effects on later developing cortical regions (see Tottenham & Gabard-Durnam, Reference Tottenham and Gabard-Durnam2017).
Results from this study demonstrate that PI youth with better WM had decreased levels of separation anxiety and higher morning cortisol levels, suggesting a potential therapeutic target for youth struggling with adversity-related mental health challenges. Although this conclusion requires additional confirmation, these findings highlight individual differences that contribute to multifinal outcomes following early adverse caregiving. Overall these results provide additional evidence of intraindividual factors during late childhood/adolescence that can mitigate the link between early caregiving adversity and mental health outcomes.
Financial support
This research was supported by in part by Grant R01MH091864 from the National Institute of Mental Health. The content is solely the responsibility of the authors and does not necessarily represent the views of funding agencies.
Acknowledgments
We would like to thank Dr. Lila Davachi for task consultation and Dr. Willem Frankenhuis for statistical advice.






