What is translational research? This has usually been understood as the process of taking the findings of either basic or clinical research to produce innovation in healthcare settings (see Cooksey, Reference Cooksey2006; Peters, Reference Peters2004; Rutter & Plomin, Reference Rutter and Plomin2009). Sometimes this is misinterpreted as meaning that it is taking the findings of basic science from the bench to the bedside, but that is not correct for two rather different reasons. First, it is a two-way process, and second, the benefits need to be considered with respect to both applications to individual patients and also population-wide gains with respect to prevention (Peters, Reference Peters2004; Rutter & Plomin, Reference Rutter and Plomin2009).
Prevention Implications
Implications for prevention are most easily illustrated by taking two rather different examples (see Academy of Medical Sciences, 2007; Insel & Scholnick, Reference Insel and Scholnick2006; Rutter & Plomin, Reference Rutter and Plomin2009). The first example is the association between smoking and lung cancer. Here the starting point was Doll and Hill's (Reference Doll and Hill1950, Reference Doll and Hill1954) case-control observational studies in humans. This was later followed by a trial in humans, but then it went on to laboratory studies with animals showing the carcinogenic effects in the tars present in cigarette smoke. Since then, the ill effects of smoking have been found to be much wider than lung cancer. It has also been found that, although the greatest effects are found with active smoking, there are also somewhat similar, albeit lesser, effects of passive smoking (in other words, inhalation of smoke produced by other people). It took a surprisingly long time (almost 50 years) before the causal connection was widely accepted. However, it is of course now well understood and has been acted upon.
The second example is fetal alcohol syndrome (see Randall, Reference Randall2001). The starting point here was the clinical observation of a particular pattern of malformations in the offspring of alcoholic mothers. Numerous human studies went on to show the associations with abnormalities and with behavioral development (Gray & Henderson, Reference Gray and Henderson2006). Animal models using mice showed similar craniofacial malformations. In this case, as in the smoking example, the causal inference was greatly strengthened by the combination of human data and animal model findings. However, questions remain. In particular, it is still unclear whether the main risk effect comes from the average level of alcohol consumption over time or rather the peak level, as in binge drinking. This is where animal models could be very helpful.
Experimental Medicine That Bridges Basic and Clinical Science
In recent years the term experimental medicine has come into increasing use as one that bridges basic and clinical science. The key feature is a hypothesis-testing approach that investigates mediating mechanisms with respect to either causation or treatment. Often this involves an interactive approach beginning with some observation that suggests an environmental cause for a disorder. This then goes on to test for environmental mediation and then examines possible biological pathways for that mediation, using animal models to examine these experimentally, returning then to the human situation to determine whether the mechanisms apply to human disease. Often a key methodologic test involves the development of a biological measure likely to be involved in the disease process but which reflects a challenge or stress that gives rise to an immediate response: an intermediate phenotype. One example is provided by Battaglia, Ogliari, D'Amato, and Kinkead's (Reference Battaglia, Ogliari, D'Amato and Kinkead2014) work using carbon dioxide inhalation as a stimulus for hyperventilation, a feature that works as well in mice as it does in humans. The psychopathological outcome of interest is panic attacks, and it would seem that this is on that same pathway. Sometimes this has been called an endophenotype on the grounds that it gets closer to the gene, but that is probably a misleading term in that what is important is that it is on the biological pathway of causal interest. Thus, T cell response has been used as a way of looking at the effects of drugs on AIDS, and this has got nothing to do with the effect on genes; rather, it concerns the effects on the infection.
Other examples are the human imaging studies undertaken by the Weinberg group studying gene–environment interaction. These are discussed in more detail below.
Areas of Scientific Progress and Challenge
Drug action
There has been huge progress in basic science with the understanding of the mechanisms involved in drug actions. These have led to several Nobel Prizes (see Volkow & Swanson, Reference Volkow, Swanson, Rutter, Thapar, Pine, Leckman, Scott and Snowling2008), especially in the imaging studies of the effect of drugs at their cellular targets such as by Kapur, Zipursky, and Remington (Reference Kapur, Zipursky and Remington1999). It might be thought that these findings should have provided a really strong basis for the development of new drugs for mental disorders. Unfortunately, that has not been the case. Most of the drugs now being used to treat mental disorders derive from serendipitous clinical observations and not from basic science discoveries (see Ayd & Blackwell, Reference Ayd and Blackwell1970; Basu, Pereira, & Aitchison, Reference Basu, Pereira, Aitchison, Stein and Wilkinson2007). There clearly is a potential for translational studies in this field (Hyman, Reference Hyman2007; Insel & Scholnick, Reference Insel and Scholnick2006), but there has been little progress so far and the difficulties in discovering really new drugs for mental disorders have led major pharma to withdraw from this field to a considerable extent.
Social cognition
This has been a field of very considerable progress (see Blakemore, Reference Blakemore2008), and again, there would seem to be the possibility of translational progress. Two rather contrasting examples may be given. First, there is the role of the amygdala in psychopathy (Viding & McCrory, Reference Viding, McCrory, Thapar, Pine, Leckman, Scott, Snowling and Taylor2015). This has been shown using a range of quite different research strategies. Clearly there is a role, but questions remain on diagnostic specificity and on whether the findings apply to children who do not also have conduct disorder. Historically, the research has mostly been with adult criminals, but recent research has shown that callous–unemotional traits thought to typify psychopathy can occur in children as well as adults and in those with and without antisocial behavior (see Frick, Ray, Thornton, & Kahn, Reference Frick, Ray, Thornton and Kahn2014; Kumsta, Sonuga-Barke, & Rutter, Reference Kumsta, Sonuga-Barke and Rutter2012).
The second example is provided by the possibility of “broken mirror” neurons as the basis of autism (Kilner & Lemon, Reference Kilner and Lemon2013; Southgate & Hamilton, Reference Southgate and Hamilton2008). The discovery was by Rizzolatti in monkeys of “mirror neurons” that modulate their activity when an individual performs some specific act and also when they observe a similar act performed by somebody else (Gallese, Fadiga, Fogassi, & Rizzolatti, Reference Gallese, Fadiga, Fogassi and Rizzolatti1996). This was a most important discovery and it has been very influential. It led to the suggestion that a damaged mirror neuron system might impair imitation and thereby underlie autism (Iacoboni & Dapretto, Reference Iacoboni and Dapretto2006). Unfortunately, subsequent research has cast doubt on the suggestion (Southgate & Hamilton, Reference Southgate and Hamilton2008). It is a bit too early to say that the issue is closed, but one of the problems is that mirror neurons are scattered rather widely in the brain and they almost certainly have more than one effect (Hickok, Reference Hickok2014).
Cognitive neuroscience
The study of memory through cognitive neuroscience has been a major growth area and has had some spectacular successes (Baddeley, Reference Baddeley2007; Milner, Squire, & Kandel, Reference Milner, Squire and Kandel1998; Tulving, Reference Tulving2002). The key stimulus was provided by Milner's study (Milner, Corkin, & Teuber, Reference Milner, Corkin and Teuber1968; Scoville & Milner, Reference Scoville and Milner1957) of a patient “HM” who, following surgery to remove the medial temporal lobe and hippocampus, lost all long-term memory skills but retained general intelligence, short-term memory, and new learning. The challenge provided by this startling finding prompted Kandel's Reference Kandel2007 Nobel Prize winning research (using the sea snail) into the molecular mechanisms involved in long-term memory. Functional brain imaging studies in humans (Frith, Reference Frith2007) located the brain areas activated by these functions, and basic science studies of nerve cells in the hippocampus led to the identification of the mechanism of long-term potentiation (Bliss, Collingridge, & Morris, Reference Bliss, Collingridge and Morris2003). The story provides a wonderful example of how the beginning was provided by clinical and human experimental studies. Without doubt, this could lead to translational studies into drugs affecting memory (Kandel, Reference Kandel2007), but that time has not yet quite arrived (but see Redondo et al., Reference Redondo, Kim, Arons, Ramirez, Liu and Tonegawa2014).
Molecular genetics
Currently, there is much excitement over locating genes through genome-wide association studies (GWAS), claiming that this provides invaluable insights into the biological basis of mental disorders (Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014). Thus, a paper in Nature dealing with nearly 33,000 cases and over 113,000 controls found 108 schizophrenia-associated genetic loci. That these were not randomly distributed, were often highly expressed in the brain, and were often related to abnormal glutamatergic synaptic and calcium channel functions means that there are useful leads on what might be looked at further, but evidence on what the genes actually do (with respect to effects on proteins) is still lacking. This cannot come from GWAS, and this means that the translational step to therapeutics is a big one.
The effects found for individual genes were tiny (Rietveld et al., Reference Rietveld, Conley, Eriksson, Esko, Medland and Vinkhuyxen2014) and still quite small when the genes were combined to produce a polygenic score. However, the large sample enabled replication. The enthusiasm for GWAS has to be limited, however, because the research strategy necessarily provides no evidence of what the genes “do” with respect to effects on proteins. There has to be reliance on bioinformatics data on human and animal findings on protein effects, such as in the neuroscience base provided by Croning, Marshall, McLaren, Armstrong, and Grant (Reference Croning, Marshall, McLaren, Armstrong and Grant2009). However, there is as yet no agreement on the requirements specifying what is meant by a biological pathway. The situation is likely to improve in the future, but at present it is unsatisfactory.
The combination of genes to provide a composite polygenic score has been seen by some as a useful way forward for clinical prediction (see Hodgson et al., Reference Hodgson, Uher, Crawford, Lewis, O'Donovan and Keers2014; Iyegbe, Campbell, Butler, Ajnakina, & Sham, Reference Iyegbe, Campbell, Butler, Ajnakina and Sham2014). The concept of multifactorial disorders (this applies to most of child psychiatry) involves the operation of multiple causal influences, both genetic and environmental. Accordingly, the development of polygenic scores is in keeping with multifactorial causation. Nevertheless, the clinical value of polygenic scores has yet to be established (see Rutter & Pickles, Reference Rutter and Pickles2015). In addition, from a biological pathways perspective, there is a huge problem that there is no way of telling which genes of the polygenic index are having particular effects. It cannot be assumed that they will all act on the same pathway. A further problem with all uses of GWAS is that findings are consistent in showing that most genes are pleiotropic, meaning that they have multiple, often diverse, effects. It is quite likely that this will greatly increase the difficulty in identifying biological pathways.
Battaglia has been a pioneer in integrating human and animal studies of carbon dioxide sensitivity in order to look at early risk factors for panic and separation anxiety disorders (Battaglia et al., Reference Battaglia, Ogliari, D'Amato and Kinkead2014). He and his colleagues pointed out that the precise molecular and biological mechanisms through which carbon dioxide hypersensitivity and panic disorder are linked and developed remain unexplained, but the finding that they largely share the same underlying liability and that carbon dioxide hypersensitivity can be experimentally induced by early interference with parent–infant separation can be used to guide the next investigations in the field. This program of research is probably best described as experimental medicine rather than translational research, but clearly it could form the basis for translation as new findings come in.
Gene–environment (G × E) interaction
The neural effects (Hariri et al., Reference Hariri, Mattay, Tessitore, Kolachana, Fera and Goldman2002; Meyer-Lindenberg et al., Reference Meyer-Lindenberg, Buckholtz, Kolachana, Hariri, Pezawas and Blasi2006; Pezawas et al., Reference Pezawas, Meyer-Lindenberg, Drabant, Verchinski, Munoz and Kolachana2005) rely on relevant stimuli to elicit an immediate response using functional and structural brain imaging in conjunction with DNA data on the relevant gene and using a sample of participants free of psychopathology. Differential susceptibility is such that the genes associated with vulnerability in the presence of adversity are also associated with increased susceptibility to positive experiences in the absence of adversity. The G × E focused on acute life stresses, but studies pooling p values using the Liptak–Stouffer test were important in showing that G × E largely referred to the interaction with maltreatment, with only quite marginal effects in the case of acute life events (Karg, Burmeister, Shedden, & Sen, Reference Karg, Burmeister, Shedden and Sen2011). It is also clear that G × E largely affects recurrent or chronic depression rather than acute episodes of depression (Uher et al., Reference Uher, Caspi, Houts, Sugden, Williams and Poulton2011). It is important that maltreatment in childhood has the big G × E effect. This is scarcely surprising in that maltreatment on its own has a strong effect on outcome, but it is also relevant that the effects span several years and hence do not concern any kind of provoking effects at the time of the environmental challenge or stimulus. Some critiques of G × E suggested the findings lack adequate replication, but a careful look at the evidence indicates that the focus has been on acute life events rather than maltreatment and that the failures to replicate are studies that are either biased or lacking good measurement.
The neural effects include amygdala activation and response to fearful stimuli (Hariri et al., Reference Hariri, Mattay, Tessitore, Kolachana, Fera and Goldman2002), the functional connectivity between the subgenual cingulate and the amygdala according to genotype (Pezawas et al., Reference Pezawas, Meyer-Lindenberg, Drabant, Verchinski, Munoz and Kolachana2005), and the genotype effect on anterior cingulate activation during response inhibition (Meyer-Lindenberg et al., Reference Meyer-Lindenberg, Buckholtz, Kolachana, Hariri, Pezawas and Blasi2006). A thorough review of the findings by Caspi, Hariri, Holmes, Uher, and Moffitt, (Reference Caspi, Hariri, Holmes, Uher and Moffitt2010) indicates that G × E is robust in view of both the animal experiments and the human experiments on the neural findings (see Rutter & Pickles, Reference Rutter and Pickles2015).
The crossover effect according to the context of high adversity versus low adversity is best illustrated by findings from Obradović, Bush, Stamperdahl, Adler, and Boyce (Reference Obradović, Bush, Stamperdahl, Adler and Boyce2010) using cortisol reactivity rather than genes. The notion of differential susceptibility requires further testing, but the evidence in support is steadily growing. Another neat example making use of a randomized controlled trial is provided by Brett et al. (Reference Brett, Humphreys, Smyke, Gleason, Nelson and Zeanah2015) based on the Bucharest study of Romanian children experiencing early institutional care of poor quality in which the follow-up involved placement into well-supervised, high-quality foster homes. What was striking was that those with the genetic risk allele were more vulnerable in the institution but also did better in relation to the foster care (Figure 1).
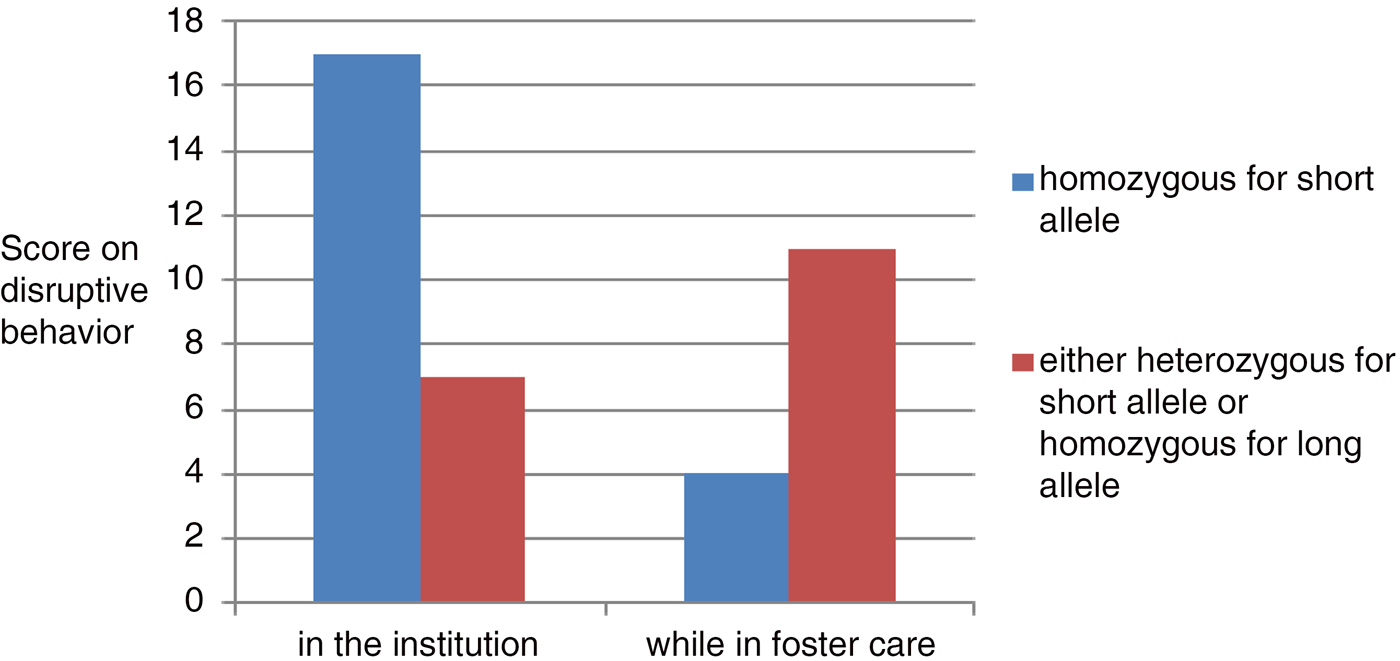
Figure 1. (Color online) Disruptive behavior within the institution and while in foster care (based on Brett et al., Reference Brett, Humphreys, Smyke, Gleason, Nelson and Zeanah2015).
Epigenetics
Epigenetics refers to mechanisms that change genetic effects through influences on gene expression without altering the gene sequence. They mainly operate chemically through DNA methylation or histone modification (Champagne, Reference Champagne2010). The translational interest derives from six main features:
-
1. the mechanisms have been shown to operate across a highly diverse range of organisms;
-
2. the epigenetic changes are evident across a great range of experiences;
-
3. the changes show an interesting mixture of considerable stability over time and yet responsivity to change;
-
4. the changes work through genetics, thereby bridging nature and nurture;
-
5. there is a potential for persistence across the generations; and
-
6. epigenetic findings have been subject to rigorous and searching tests of the causal inference, particularly by Meaney and colleagues (Meaney, Reference Meaney2010; Weaver, Meaney, & Szyf, Reference Weaver, Meaney and Szyf2006).
Epigenetic changes are both tissue specific and developmental phase specific, making human studies during life problematic, although postmortem studies have been informative (McGowan et al., Reference McGowan, Sasaki, Huang, Unterberger, Suderman and Ernst2008, Reference McGowan, Sasaki, D'Alessio, Dymov, Labonté and Szyf2009) and buccal cells have been used to study age effects in humans (Essex et al., Reference Essex, Boyce, Hertzman, Lam, Armstrong and Neumann2013).
Challenges in the translation of epigenetic findings
Despite the strength of the field of epigenetics, there are several important challenges that remain to be tackled (Heijmans & Mill, Reference Heijmans and Mill2012; Rutter, Reference Rutter2012). To begin with, as with most biological studies, the findings concern mean differences between groups. The key question is whether the inferences based on the group findings are robust and valid at an individual level (Rutter, Reference Rutter, Singh, Sinnoff-Armstrong and Sarnlesan2014). That is more of a challenge than many people appreciate. It is a universal finding that a confidence interval around the mean score on any test or marker being investigated is very wide. That necessarily means that the generalization from a difference in mean level between two populations may not work as well when being employed as an individual predictor within any population. However, in addition to that, it is necessary to recognize the complexities of individual variation in multifactorial interplay. The interplay among multiple genetic and multiple environmental factors will vary substantially among individuals with respect to the specific details. The second issue is whether the epigenetic findings can differentiate among different types of adversity. For example, can they differentiate between acute stresses and chronic adversities, between maltreatment and neglect, and can they identify the factors operating at a population level (such as poverty and social disadvantage)? Third, it is necessary to ask whether epigenetic findings can differentiate between individuals who succumb to whatever adversity is being considered and those who show resilience. From a clinical translational perspective, it is a most important distinction. Fourth, it is necessary to ask whether the epigenetic findings predict mental or physical health outcomes. Because most, if not all, meaningful experiences involve epigenetic effects, it is likely that differentiations will be found with mental or physical health outcomes. Nevertheless, it is important to know whether the mental or physical health outcome is predicted better at the epigenetic level or at, for example, the hypothalamus–pituitary–adrenal (HPA) level. It may well be that the HPA axis effects are brought about by epigenetic changes, but that does not mean that the proximal effect is best identified at the epigenetic level.
Range of Proximal Psychosocial Experiences Showing a Mental Health Risk
It is usual to differentiate between proximal and distal risk experiences. By proximal is meant those that have a more immediate risk effect and by distal those that are better conceptualized as broad experiences impinging on populations, with their presence creating a liability to the more immediate proximal risk factors.
The range of proximal psychosocial risk experiences carrying a substantial risk for psychopathology is large (Danese & McCrory, Reference Danese, McCrory, Thapar, Pine, Leckman, Scott, Snowling and Taylor2015; Glaser, Reference Glaser, Thapar, Pine, Leckman, Scott, Snowling and Taylor2015; Jenkins, Madigan, & Arseneault, Reference Jenkins, Madigan, Arseneault, Thapar, Pine, Leckman, Scott, Snowling and Taylor2015). Thus, they include physical, sexual, and emotional abuse and also physical and emotional neglect. Parental mental disorder would ordinarily be included in proximal risk factors because they impinge on individuals, but the risk could lie as much in the associated features as in the depression as such. For example, parental mental disorder often exhibits itself to other people as hostility or irritability, and it may be involved in abuse or neglect. Institutional deprivation has been much studied and it is clear that it has major risk effects, but there is still some uncertainty as to how far these extend from an institutional rearing as such or rather from the grossly depriving circumstances experienced in some institutions but not in others. Family conflict and disruption of parent–child relationships and/or interaction is another risk experience as is scapegoating of individuals and exposure to domestic violence. Acute life stresses involving loss, humiliation, or conflict are another group of risk experiences that may or may not be associated with chronic adversity. Bullying has been seen as an unpleasant experience for a long time, but it is only in the last decade or so that there has been much systematic research into its risk effects both at the time and later (see, e.g., Arseneault et al., Reference Arseneault, Cannon, Fisher, Polanczyk, Moffitt and Caspi2011; Takisawa, Danese, Maughan, & Arseenault, Reference Takizawa, Danese, Maughan and Arseneault2015). This recent work has been indistinctive in using genetically sensitive designs in order to test for environmental mediation and using physical measures such as blood inflammation biomarkers to examine the possible biological embedding of the experiences.
From a translational perspective, it is necessary to recognize that the risk features are rarely single and distinct and that, therefore, it is important to try to disentangle the most important mediating risk mechanisms. A further key query with respect to translation is that there is a need to determine the direction of causal influences. For example, are the inflammation findings associated with bullying a consequence of bullying or, rather, a consequence of the patterns of psychopathology brought about by the bullying? Longitudinal studies are helpful provided that there are repeated measurements of both the risk experience and the biological features. That is because it will sort out which came first. In addition, however, more experimental approaches may also be required.
Key Concepts and Challenges in Relation to Stress
Toxicity
A key question is whether stress resides in the experience itself or the physiological response to the experience (see Shonkoff, Reference Shonkoff2010). A tripartite typology has been suggested. At the positive end there is “positive” stress, a concept reflecting a recognition that challenge is a normal part of healthy development (Rutter & Rutter, Reference Rutter and Rutter1992). There is then what is called “tolerable” stress, which is meant to refer to a time-limited physiological state in which protective relationships allow the stress-response system to recover. In addition, there is “toxic” stress, which is thought to disrupt brain architecture, affect other organ systems, and lead to lastingly lower thresholds for responsiveness. On the face of it, this sounds a potentially useful way of thinking about stress, but it lacks a quantification of stress that is separate from its effects. It is also misleading in its incorporation of neglect into a stress framework. Obviously, neglect is a serious hazard, but whether it operates by the same mechanism as stress is questionable.
Allostatic load
Allostatic load refers, not to stress, but to the body's physiological response to stress and adversity (McEwen & Lasley, Reference McEwen and Lasley2002). In practice, this is indexed by features such as systolic blood pressure, urinary cortisol, catecholamines, waist/hip ratio, and high-density lipoprotein. In short, it is not a measure of stress as such, in that it refers to an imbalance in systems that promote adaptation. This may come about through lack of exercise or an unhealthy lifestyle or social isolation, as well as through stressful experiences. It is a more justifiable, and useful, concept than toxic stress in that it is objectively measureable. However, a key limitation is that the imbalance in systems may come about through a wide variety of features, many of which are not appropriately conceptualized as “stressful.”
Chronicity and the HPA axis
Both human and animal studies have shown elevated glucocorticoid levels following acute stress, but prolonged repeated stress leads to hypocortisolism and a blunted HPA response to the stressor (Gunnar & Vazquez, Reference Gunnar, Vazquez, Cicchetti and Cohen2006; Loman & Gunnar, Reference Loman and Gunnar2010). Fisher, van Ryzin, and Gunnar (Reference Fisher, van Ryzin and Gunnar2011) found that maltreated children also showed this latter pattern (i.e., blunted HPA axis response) in relation to placement changes, but this was substantially prevented by attachment-based caregiver interventions.
Objective or subjective stress
It might be thought that objective measures of stress would provide better predictions of health than subjective ones, but the reverse has been found to be the case, at least with respect to social standing (Cohen, Alper, Adler, Treanor, & Turner, Reference Cohen, Alper, Adler, Treanor and Turner2008; Gianaros et al., Reference Gianaros, Horenstein, Cohen, Matthews, Brown and Flory2007; Singh-Manoux, Marmot, & Adler, Reference Singh-Manoux, Marmot and Adler2005). Nancy Adler developed a subjective self-assessment of personal social status, using a ladder rating. Two separate studies showed that this had superior predictive power for health outcomes, and a third study (by a different research group) showed that it also was associated with stronger biological embedding. The findings provide a reminder of the importance of the personal processing of experiences.
Why Is It Necessary to Test Causal Inferences?
It is sometimes supposed that it is a matter of scientific pedantry to suggest that statistical associations do not necessarily imply causation. However, that is not the case because common sense and science differ in recognizing the crucial necessity to consider and examine alternative explanations. Only science does this systematically (Rutter & Solantaus, Reference Rutter and Solantaus2014). Cognitive impediments may cause misinterpretations of clinical observations (Lilienfeld, Ritschel, Lynn, Cautin, & Latzman, Reference Lilienfeld, Ritschel, Lynn, Cautin and Latzman2014), meaning that expectations based on personal experience may give rise to misinterpretations of clinical observations. That is an additional reason for testing causal influences. Thus, the need arises from the evidence from comparisons of experimental and nonexperimental findings in that the latter often lead to systematic sizable biases (Duncan, Magnuson, & Ludwig, Reference Duncan, Magnuson and Ludwig2004). The awareness for the need to test causal inferences provides an impetus for the development of a range of different strategies to test for causal effects. A natural experiment is defined as a design that pulls apart variables that ordinarily go together (Thapar & Rutter, Reference Thapar, Rutter, Thapar, Pine, Leckman, Scott, Snowling and Taylor2015). Basically, there are four main sorts of designs that have been developed. First, there are those intended to circumvent genetic mediation; second, there are designs to avoid selection bias; third, there are designs to identify key environmental risk features; and fourth, there is the one-and-only design available to deal with unmeasured confounders, namely, the regression discontinuity design. The regression discontinuity design is a truly ingenious strategy, but it has very strict requirements and there have not been many instances in which it has been valid to apply it. However, it was used with great effect by Cahan and Cohen (Reference Cahan and Cohen1989) to contrast cognitive gains resulting from schooling and cognitive gains resulting from maturation (Figure 2). The findings showed a school effect on all the cognitive measures, but these were greatest for scholastic achievement (as would be expected) and least for crystallized abilities that are not direct taught.

Figure 2. (Color online) Effects of children's age and schooling on cognitive performance (based on Cahan & Cohen, Reference Cahan and Cohen1989).
Developmental researchers have made surprisingly little use of animal models, but it is clear that they do allow for experimental manipulation of experiences that can be very informative in testing the causal inference. We give some examples below.
Randomized controlled trials have a place because they do constitute the design with the highest internal validity. However, with respect to testing the causal inference, they do have the substantial limitation resulting from the need to extrapolate backward in time. Thus, for example, there is evidence that electric convulsive therapy is an effective treatment for severe psychotic depression. It is little used these days because of the availability of active medication, but it still has some use. The problem with causation, however, is that the evidence would seem to suggest that the cause of depression is a lack of electric shocks.
Finally, there are human experiments that make use of an intermediate phenotype resulting in an immediate response that operates on the same biological pathway as the phenotype of interest (Battaglia et al., Reference Battaglia, Ogliari, D'Amato and Kinkead2014). Their role in the study of gene–environment interaction has already been discussed, and there can be no doubt that it played an important role in validating the reality of gene–environment interaction.
Natural Experiments
Just three examples of natural experiments will be given to illustrate the approaches and the issues that need to be considered in their use.
Assisted conception design
Rice et al. (Reference Rice, Harold, Boivin, Hay, Van Den Bree and Thapar2009) had the ingenious idea that assisted conception provided a special opportunity to find a test for environmental mediation versus genetic mediation risk effects associated with prenatal exposure. Observational studies had consistently shown that prenatal smoking exposure was associated with an increased risk of attention-deficit/hyperactivity disorder in the offspring even after controlling for what seemed to be all the relevant possible confounding factors. Their techniques, such as sperm donation, involved no note of the genetic link between mother and child because the ovary was still provided by the mother. Others, such as egg donation, however, did cut the link between mother and child. The first step was to examine the reduction in offspring birth weight of related and unrelated offspring because all the evidence, including animal models, had shown that there was a true environmentally mediated effect of exposure to mother's smoking during the pregnancy in lowering birth weight. Figure 3 illustrates this and shows that the effects were as strong in unrelated as in related pregnancies. If that had not been the case, it would have cast doubt on the validity of the design, but that was not a problem. The same comparison was then made in relation to the offspring's antisocial behavior as shown in Figure 4. Findings here are entirely different. In the unrelated pregnancy there was no effect of smoking, whereas there was a substantial effect of smoking in the related pregnancy in increasing the risk of antisocial behavior. The same design by the same researchers was done with respect to the outcome of attention-deficit/hyperactivity disorder, and the findings were exactly the same. Whereas it is quite likely that there were social selection factors in the use of assisted conception methods, it is not anywhere near as likely that there would be a difference between sperm donation and egg donation. However, that is a possible confound that needs to be taken into account once the relevant evidence is available (which it has not been up to now).

Figure 3. Reduction in offspring birth weight in genetically related and unrelated offspring of prenatal maternal smokers and nonsmokers (based on Rice et al., Reference Rice, Harold, Boivin, Hay, Van Den Bree and Thapar2009).

Figure 4. Increased offspring antisocial behavior in related, but not unrelated, offspring of prenatal maternal smokers and nonsmokers (based on Rice et al., Reference Rice, Harold, Boivin, Hay, Van Den Bree and Thapar2009).
Discordant monozygotic twin design
A second, somewhat different, way of dealing with the possibility of genetic mediation is to provide a contrast within monozygotic pairs who, by definition, do not differ genetically. This design was used by Caspi et al. (Reference Caspi, Moffitt, Morgan, Rutter, Taylor and Arseneault2004) in order to examine the effects of maternal negativism on children's behavior problems. Like many of the best natural experiment designs, additional steps were taken to ensure validity. Maternal negativism was assessed on the basis of observed and systematically rated maternal behavior, and behavior problems were assessed through teacher ratings, meaning that there was complete separation of the two measures. In addition, maternal negativism was assessed before the children entered school, and the behavior was assessed 2 years later after the child was at school. What this meant is that the correlations over time spanned a period where there was a major change of environment (from home to school). The essence of the design then is to take pairs of twins in which one twin was exposed to high maternal negativism and the other twin was not. In other words, the design was one that was loaded in favor of not finding environmental mediation. It follows the expectation that the onus is on researchers to attempt to disprove their own hypotheses before publishing (National Research Council, 2002). The findings provided clear-cut evidence in favor of an environmentally mediated effect, which was of sufficient size to be clinically meaningful. There were two potential threats to the validity of the comparison that had to be considered. First, what was it that led mothers to have such different responses to two twins that both looked alike and in most respects behaved alike? Caspi et al. (Reference Caspi, Moffitt, Morgan, Rutter, Taylor and Arseneault2004) sought to answer that question, but without any findings that provided a persuasive answer. Second, the other threat is that although monozygotic twins are the same in terms of genes they possess, they need not be the same in terms of gene expression (Fraga et al., Reference Fraga, Ballestar, Paz, Ropero, Setien and Ballestar2005).
Designs that deal with social selection include those that provide universal exposure to risk and those that involved universal removal of risk
The examples of universal exposure to risk are best exemplified by the Dutch famine during World War II and the somewhat comparable Chinese famine (St. Clair et al., Reference St. Clair, Xu, Wang, Yu, Fang and Zhang2005). In the Dutch famine (Stein, Susser, Saenger, & Marolla, Reference Stein, Susser, Saenger and Marolla1975), the whole population was exposed to the famine. There were very few opportunities for individuals to escape exposure. The exposure to famine during pregnancy was associated with increase in the risk of schizophrenia later. The possibility of this being a valid environmentally mediated effect was suggested by the fact that there was a parallel increase in the rate of congenital malformations (McLellan, Susser, & King, Reference McLellan, Susser and King2006). However, more recently, epigenetic findings provided the possibility of a further test of environmental mediation. The comparison here is between siblings who were exposed during in utero and those who were not (because they were either too young or too old). What the findings showed was that epigenetic effects were evident only in those exposed during the pregnancy period (Tobi et al., Reference Tobi, Slagboom, van Dongen, Kremer, Stein and Putter2012). Once more, it is the added tests that provide the study with its major strength.
The best example of the removal of risk is shown by the Native American reservation experiment reported by Costello, Compton, Keeler, and Angold (Reference Costello, Compton, Keeler and Angold2003). When a casino is set up on an Native American reservation, US federal law requires that a specified proportion of the funds be distributed to the Native Americans living on the reservation. They do not have to ask for it, and they do not have to deserve it; it is just provided as a matter of right. By good fortune, the casino was set up in the middle of the Great Smoky Mountains longitudinal study and Jane Costello was astute enough to recognize the wonderful natural experiment opportunity that was thereby provided. The first test was whether the changes over time in levels of poverty were the same or different among Native Americans and others. The findings showed that the fall in levels of poverty was largely confined to those Native Americans who were living on the reservation (as would be expected). Some of the inhabitants were not poor either before or after the casino was set up and some were poor both before and after the casino was established. The interest, however, lay not in either of those two groups but rather in the group whose financial circumstances changed in relation to the building of the casino. The research findings showed that setting up the casino was accompanied by the significant reduction in behavioral problems when the casino establishment was associated with a reduction in poverty. Of course, it raises the question as to what were the elements that were associated with this change. It meant a move away from the protection provided by the universal exposure design. However, what the findings did suggest was that it was changes in the interaction between parents and their children that were responsible. In other words, it was not the direct effect of income level that seemed crucial but rather what this was associated with in terms of family functioning. The study was also distinctive in involving a follow-up, and broadly speaking, this provided the same pattern of findings (Costello, Erkanko, Copeland, & Angold, Reference Costello, Erkanko, Copeland and Angold2010). Costello et al. (Reference Costello, Erkanko, Copeland and Angold2010) reported a follow-up to age 21 years. The findings were important in showing that the benefits of the income supplement as a result of the setting-up of the casino persisted into early adult life, whereas a supplement received only in adulthood had no impact on adult psychopathology. The natural experiment provided a powerful means of testing the effects of the income supplement, but with respect to generalizing to other populations, it is important to note that the supplement was very large (US ~$9,000 a year by 2006). It is also notable that although the design provided a powerful test of the causal inference, it was less informative on how and why the poverty supplement worked in the way that it did. This is often the case with natural experiments. That is, they are quite powerful in pointing to whether something happened, but they are frequently less informative on the mediating influences.
Animal Models
Animal models have been widely used as examples of basic science that provide a starting point for considering translation. The best animal models for studying environmental effects had their origin in either clinical observations or theories applicable to clinical features. For example, this is evident in Hubel and Weisel's (Reference Hubel and Wiesel2005) research into the effects of binocular visual input on the growth of the visual cortex. This had been prompted by Von Senden's (Reference Von Senden1960) observation of the deficits found following removal of children's congenital cataracts. It is noteworthy that there was a careful design of experimental conditions. Thus, this dealt with the suturing of cats’ eyelids in order to limit visual input. It led to a detailed study of possible mediating mechanisms. Thus, Hubel and Weisel showed that the cortical effects related more to competition between the eyes than to simple disuse. In keeping with the characteristics of the best animal models, Hubel and Weisel went on to provide a detailed study of the brain processes involved. This work is a good example of one that was prompted by human observations but then produced findings that fed back into implications for the human situation. Thus, the findings on an early sensitive period for the cortical effects prompted actions to intervene early with strabismus (squints) in young children.
Enrichment and deprivation effect
The starting point here was that laboratory rats given formal problem-solving training showed neurochemical changes in the cerebral cortex. Rosenzweig, Krech, Bennett, and Diamond (Reference Rosenzweig, Krech, Bennett and Diamond1962) went on to test whether these was a function of enrichment and deprivation effects by an experimental comparison of three groups of rats. The first group was reared in a large cage in a group of 10 to 12 animals with a variety of stimulus objects that changed daily. This comprised the enrichment condition. The second group was reared in a standard colony situation with 3 animals in a cage; this was the control condition. The third group was reared in single housing, comprising the deprivation condition.
The initial study was done with juveniles because of a general assumption at that time that the effects would only be found at a time of maximal brain growth. Later studies, dealing with adult rats, showed that the pattern in adults was similar to that found in juveniles (although the effects were greater in the young; Rosenzweig & Bennett, Reference Rosenzweig and Bennett1996). The research undertaken by Rosenzweig's group considered not only behavioral effects of the different rearing conditions but also the cortical effects of the different patterns of rearing.
Many years later, interest in the topic rose again with respect to the London taxi driver study (Maguire, Woollett, & Spiers, Reference Maguire, Woollett and Spiers2006). This hit the headlines because it showed taxi drivers who undertook the very intensive task of “learning the knowledge” that generally took some 3 years and that required the learning of the routes all over London including landmarks as well as street names. Findings showed that taxi drivers, when compared with bus drivers, had an enlargement of the posterior hippocampus associated with a diminished size of the anterior hippocampus. Two key questions arose with respect to these findings. First, were the effects caused by the intensive learning experience or were these already present in taxi drivers before they undertook “the knowledge”? Woollett, Spiers, and Maguire (Reference Woollett, Spiers, Maguire, Happé and Frith2010) approached this by determining what happened when taxi drivers retired. The findings showed that the pattern differences remained after retirement but were greatly reduced. In other words, the effects were enduring but they were not permanent. Second, the other query was whether one could assume that this was a causal effect. The retirement findings partially answered that question and certainly went in the direction of pointing to a likely causal effect. However, the matter was taken further by a parallel study by other investigators looking at the effects of intensive musical training. At first, findings were cross sectional (like the taxi driver study), but then there was an intervention study (Hyde et al., Reference Hyde, Lerch, Norton, Forgeard, Winner and Evans2009), which showed that the changes followed the training and did not precede it. These findings brought out another feature, which was that whereas the taxi driver findings applied to the hippocampus, these findings applied to the cortex and hence the assumption that only the hippocampus could change in adult life was wrong. A different study looking at jugglers (Draganski & May, Reference Draganski and May2008) similarly showed that training in juggling brought about changes in the cortex.
Putting these findings together as a whole serves as a reminder that there are always questions that need to be asked as to when the researcher knew enough from the basic research to move on to translating the findings. It is clear that this is a matter of judgment but it is nevertheless an important issue (see Academy of Medical Sciences, 2007).
Effects of separation in rhesus monkeys
Harry Harlow's monkey studies (Harlow & Zimmermann, Reference Harlow and Zimmermann1959) compared monkeys reared on so-called cloth mothers versus ones reared on so-called wire mothers. Harlow had a provocative style of reporting his findings and was very insistent about indicating that all of this was concerned with mother “love.” Bowlby, much impressed by Harlow's findings, made use of them in his trilogy on attachment (Bowlby, Reference Bowlby1969, Reference Bowlby1973, Reference Bowlby1980). However, at about the same time, Hinde looked at the separation in rhesus monkeys with a focus that was distinctive in using a more normal range of situations and with an interest in individual differences (Hinde & McGinnis, Reference Hinde and McGinnis1977; Hinde & Spencer-Booth, Reference Hinde and Spencer-Booth1970).
There were three main findings that were important with respect to human implications. First, it was found that infant distress was a function of both preseparation and contemporaneous mother–infant relationships. Second, the changes in mother–infant interaction were largely dependent on the mother. Third, infants showed much less postseparation distress and a more normal mother–infant interaction when they themselves were removed to a strange place for the same time period. The findings were important in pointing to the greater importance of disturbed maternal behavior than any direct effect of separation per se. Hinde was careful to point out that it could not be assumed that the relative role of the mother and the infant would be the same in humans as found in rhesus monkeys. However, it did seem likely that a shift of focus was needed from separation per se to a history of relationships.
Some years later, Harris, Brown, and Bifulco (Reference Harris, Brown and Bifulco1986) took up the issues and identified the crucial role of lack of adequate parental care as the key mediator between the risk effects of loss of parenting in childhood and adult depression (see Figure 5). What they found was that the quality of parental care was a predictor even in the absence of a loss of a parent, but the reverse did not apply. Fergusson, Horwood, and Lynskey (Reference Fergusson, Horwood and Lynskey1992) examined much the same issue except that their focus was on adult antisocial behavior as the outcome of interest. They found that whereas family conflict and discord were associated with offending behavior even after taking into account the effects of degree of family change, family change was not associated with offending once the level of family discord had been taken into account (see Figure 6).
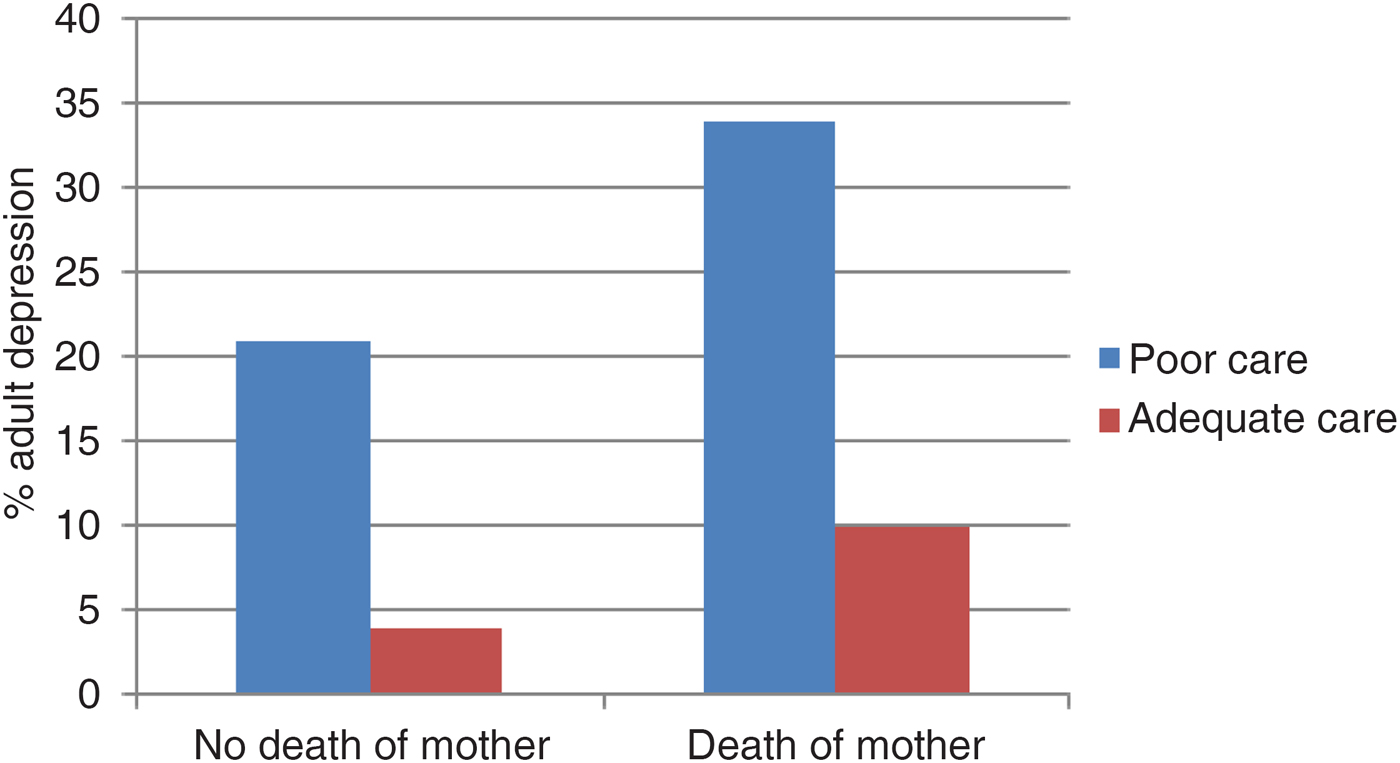
Figure 5. (Color online) Death of mother, quality of parental are, and adult depression (based on Harris et al., Reference Harris, Brown and Bifulco1986).

Figure 6. (Color online) Delinquency, family discord, and family change (data from Fergusson et al., Reference Fergusson, Horwood and Lynskey1992).
This body of evidence, combining human research and animal models, underlines the crucial importance of identifying what is the key risk mediator before moving on to implement translational activities. The historical example where translation activities went woefully wrong was the blaming of crime on “broken homes.” Barbara Wootton in a scathing critique (Reference Wootton1959) made much the same point that is being made here; namely, it is easy to make mistakes in the identification in the key causal element.
Epigenetic Effects of the Environment on Gene Expression
Meaney's studies of the effects of the environment on gene expression (Meaney, Reference Meaney2010) started with his observations that lactating mother rats varied greatly in the extent to which they licked and groomed their offspring and showed archback nursing. The differences were stable over the first week and were not associated with differences in the overall amount of contact. Moreover, these differences were associated with variations in dopamine in a particular region of the brain, and the variations in maternal behavior were associated with individual differences in the offspring's behavior and response to stress. This led to a series of ingenious, well-controlled experimental studies to answer questions on mediating mechanisms.
First, Meaney raised the question as to whether the effects in the offspring were of genetic or environmental origin. Cross-fostering designs were used to tackle this question, and findings clearly showed that the effects were the result of the rearing environment. Second, another question he raised was how the effects were transmitted in a way that persisted into adult life. It was found that the development of the endocrine system was lastingly altered through tissue-specific effects on gene expression. Third, the last question was whether the effects were reversible in later life. His use of a drug to reverse the methylation effect was found to change the endocrine response to stress.
Once more, it is necessary to consider what can be generalized from these findings to the human situation. There are commentators who have wanted to seek a human equivalent of licking and grooming in archback nursing, but that would be the wrong level of generalization. It is not at all clear what would be the human equivalent, and what is important is the fundamentally basic demonstration that environments brought about epigenetic effects that led to chemical changes in methylation and histone modification. What is quite striking is how general these findings are across widely different species (Rutter, Reference Rutter2012).
Biology of Environmental Effects
Although much of the attention has focused on the epigenetic effects of environmental changes, they actually go much more broadly than that. Rutter and Azis-Clauson (Reference Rutter, Azis-Clauson, Thapar, Pine, Leckman, Scott, Snowling and Taylor2015) reviewed the findings with respect to both prenatal and postnatal features. So far as prenatal features were concerned, attention was focused on alcohol, smoking, cocaine, and the relative importance of depression/anxiety versus antidepressants being used to treat the emotional disturbance. It is an important practical point for clinicians dealing with depression/anxiety in pregnant women to know whether the risks are greater from the emotional disturbance or from the use of antidepressants to treat it. This is a key translational issue, and the evidence is so far rather inconclusive although balance seems to suggest that it is safer to continue with treatment rather than to stop it. Nevertheless, it does raise the query as to whether equal benefits could come from psychological treatments rather than pharmacological ones. Other prenatal features that are important are subnutrition, exposure to testosterone, and exposure to maternal stress.
Postnatal features of importance include allostatic overload as a result of prolonged HPA activity, telomere length, inflammation, reduced gray matter volume and reduced brain growth, dysregulation of the HPA axis, epigenetic effects, cortical chemistry effects, and gene–environment interaction effects on brain structure and function. Of course, these are not mutually exclusive alternatives, but translational considerations means that there needs to be identification of which are most crucial in which circumstances for which people.
Does Biological Embedding Help With Respect to Translation?
The answer has to be a clear cut “yes” because it provides evidence on the possible biological pathways that may be involved in the persistence of environmental effects. The answer is also positive because the findings indicate possible targets for intervention. However, there is the evidence that biological embedding probably occurs with most significant experiences and not just with those that are damaging. It is most crucial as well that the physiological changes involved may be adaptive in many circumstances. Three examples may serve to illustrate the key translational questions that arise.
The HPA axis
The usual response to a new acute stressor is a rise in cortisol, representing an urgent adaptive fight or flight response (Loman & Gunnar, Reference Loman and Gunnar2010; McEwen & Lasley, Reference McEwen and Lasley2002; Sapolsky, Reference Sapolsky2004). The hypocortisolism associated with chronic adversity is often described as HPA dysregulation, but it could be that it is adaptive when prolonged adversity does not benefit from a fight/flight response. Chronic hypercortisolism is known to be damaging. Translational issues require consideration of when it would be appropriate to intervene and when it would not be appropriate.
Epigenetics
As discussed above, epigenetic changes are chemical, and different administered chemicals can stop the methylation process that is involved. The translational problem is that it would not be good to shut down all the methylation, and at least up to now, there is no straightforward way to target just the methylation process that you want to inhibit.
Inflammation
Inflammation sounds as if it is a bad thing to have, and hence there has been the use of anti-inflammatory treatments. Their use is clearly desirable when inflammation is causing damage. The key caveat, however, is that inflammation is part of the immune system that orchestrates the body's defenses against infection and injury. In the short term, this is protective, but in the long term, it can lead to damaging overactivation. Thus, the key questions are whether inflammation in some particular case is a response to maltreatment or a risk factor for depression or both. Is it adaptive or maladaptive in the particular circumstances being considered? Should anti-inflammatory drugs be used to prevent depression? These questions have been tackled by Danese et al. (Reference Danese, Moffitt, Pariante, Ambler, Poulton and Caspi2008), as shown in Figure 7. The extreme left-hand column indicates that the effect size was very low when the depressed only group was compared with the control group. The middle column shows that the effect was somewhat greater when the maltreated only group was compared with the control group but the right-hand column shows that the effect was greatest when the individual had both depression and had experienced maltreatment. Thus far, it is not at all clear what this means, but it indicates the need for caution when considering what mechanism might be involved in the combined effect of depression and maltreatment. It seems clear that depression on its own has a very small effect, but why does the combination of maltreatment and depression have the greatest effect?

Figure 7. (Color online) Differences in the effect size of the inflammation factor (based on data from Danese et al., Reference Danese, Moffitt, Pariante, Ambler, Poulton and Caspi2008).
How Should We Proceed From Here?
Shonkoff, Richter, Gaag, and Bhutta (Reference Shonkoff, Richter, Gaag and Bhutta2012) have argued that the time has come to incorporate the biological successes into a new era of increasingly effective early childhood policy and practice. They were careful to point out that, because hard evidence has still to be obtained, hypotheses are required in moving ahead, but they were surely right to argue for a life span approach beginning in early childhood.
Rutter and Plomin (Reference Rutter and Plomin2009) concurred with that, in stating that there is a great need to move from scientific findings to health benefits, but they emphasized the interactive two-way approach that is involved with a focus on prevention as much as on treatment. In addition, they suggested that although there were only a few opportunities for a direct translational approach at the moment, there is a much greater need, and opportunity, for hypothesis-based bridging studies.
It is sometimes thought, or implied, that translation is so obviously a good thing that it ought to be proceeded with as soon as possible. However, there are penalties for acting too soon and for not acting soon enough (Academy of Medical Sciences, 2007). Rutter and Solantaus (Reference Rutter and Solantaus2014) discussed six cases where translation went awry because people treated science and common sense as equivalent, which they are not. It was argued that translation needs to be based on top-quality science, and even the best science needs to take account of the need for multiple research strategies and multiple evaluations. Merton (Reference Merton1973) argued that good science required an attitude of skepticism, a norm of questioning, challenging, and considering alternative counterexplanations. The US National Research Council (2002) and the British Academy (2010) put forward the same message.
In this review, several examples were discussed of cases in which there was too ready an acceptance of research that required further validation before acceptance. The value of natural experiments and animal models was emphasized together with the need for human experiments where they were possible.









