Supportive social relationships are important determinants of physical and psychological well-being across the life span. Social partners can buffer the effects of stress not only through tangible and instrumental supports that reduce the experience of stress but also through buffering physiological responses to stress. The social buffering effects on physiological reactivity have been clearly characterized in the relationships between life partners (Ditzen et al., Reference Ditzen, Neumann, Bodenmann, von Dawans, Turner, Ehlert and Heinrichs2007; Kirschbaum, Klauer, Filipp, & Hellhammer, Reference Kirschbaum, Klauer, Filipp and Hellhammer1995) and between infants and their parents (Gunnar & Donzella, Reference Gunnar and Donzella2002). A large body of research supports the role of social support in buffering hypothalamic–pituitary–adrenal (HPA) axis functioning (Gunnar & Hostinar, Reference Gunnar and Hostinar2015), with a particular focus on the downstream effects on neurobehavioral development (Gunnar & Vazquez, Reference Gunnar, Vazquez, Cicchetti and Cohen2006).
Recently, attention has turned to the potential for social relationships to buffer the intergenerational transmission of stress. Adverse childhood experiences (ACEs) have been associated with poor health and psychological outcomes not only in survivors (Anda et al., Reference Anda, Felitti, Bremner, Walker, Whitfield, Perry and Giles2006; Edwards, Holden, Felitti, & Anda, Reference Edwards, Holden, Felitti and Anda2003; Felitti et al., Reference Felitti, Anda, Nordenberg, Williamson, Spitz, Edwards and Marks1998) but also in their offspring (Flory, Bierer, & Yehuda, Reference Flory, Bierer and Yehuda2011; Tomfohr-Madsen, Bayrampour, & Tough, Reference Tomfohr-Madsen, Bayrampour and Tough2016; Yehuda, Bell, Bierer, & Schmeidler, Reference Yehuda, Bell, Bierer and Schmeidler2008). The transmission of psychological, social, and physiological sequela of ACEs from one generation to the next is believed to occur through both social learning (e.g., stress affects the behavior of parents, which is observed and emulated in the next generation) and through biological pathways, such as HPA axis dysregulation (Bowers & Yehuda, Reference Bowers and Yehuda2016). Nevertheless, most studies have examined social learning and few studies have examined the biological transmission of maternal ACEs during gestation, a period when the developmental potential of the next generation is highly susceptible to maternal experience. Because ACEs are associated with changes in maternal HPA axis function during pregnancy (Bublitz, Parade, & Stroud, Reference Bublitz, Parade and Stroud2014; Bublitz & Stroud, Reference Bublitz and Stroud2012; Shea et al., Reference Shea, Streiner, Fleming, Kamath, Broad and Steiner2007), and because maternal HPA axis function during pregnancy is associated with infant HPA axis function (Giesbrecht, Letourneau, & Campbell, Reference Giesbrecht, Letourneau and Campbell2017; O'Connor, Bergman, Sarkar, & Glover, Reference O'Connor, Bergman, Sarkar and Glover2013), it is plausible that maternal stress experienced prior to pregnancy (i.e., ACEs) may influence infant HPA axis function through changes in maternal HPA axis function. If so, maternal HPA axis function would serve as a mediator between maternal ACEs exposure and infant HPA axis function. Furthermore, HPA axis functioning in both adults and infants is sensitive to social buffering, and it is possible that social support during or after pregnancy may buffer or prevent biological indicators of maternal stress from being transmitted to offspring. Nevertheless, human studies demonstrating these links are lacking. The objective of the current study was, therefore, to determine whether maternal HPA axis function is a mediator and perceived social support is a moderator of the association between a maternal history of ACEs and infant HPA axis function.
Early life stress is a strong predictor of HPA axis function in children (Cicchetti & Rogosch, Reference Cicchetti and Rogosch2001), and absent or inadequate social buffering of stress is thought to be a primary mechanism by which adversity “gets under the skin” to affect development (Hostinar, Sullivan, & Gunnar, Reference Hostinar, Sullivan and Gunnar2014). The developing HPA axis is particularly susceptible to stress and also to social buffering, with parents being the primary source of social support (Gunnar, Reference Gunnar1998). For instance, a secure attachment relationship, the product of a history of sensitive, responsive interactions that are reliably supportive, can greatly reduce the increases in cortisol that are frequently observed among infants in response to every day stressors (Dozier, Peloso, Lewis, Laurenceau, & Levine, Reference Dozier, Peloso, Lewis, Laurenceau and Levine2008; Nachmias, Gunnar, Mangelsdorf, Parritz, & Buss, Reference Nachmias, Gunnar, Mangelsdorf, Parritz and Buss1996), or even acute stressors, such as receiving immunizations (Gunnar, Brodersen, Nachmias, Buss, & Rigatuso, Reference Gunnar, Brodersen, Nachmias, Buss and Rigatuso1996). Longitudinal studies suggest that changes in HPA axis function following exposure to ACEs persist over time, and may increase in divergence from HPA axis function in individuals without adversity exposure (Trickett, Noll, Susman, Shenk, & Putnam, Reference Trickett, Noll, Susman, Shenk and Putnam2010; van der Vegt, van der Ende, Kirschbaum, Verhulst, & Tiemeier, Reference van der Vegt, van der Ende, Kirschbaum, Verhulst and Tiemeier2009). Furthermore, stress-related dysregulation of the HPA axis is associated with both physical and mental disease.
Several previous studies have shown that exposure to ACEs can be detected in HPA axis function during pregnancy (Bublitz et al., Reference Bublitz, Parade and Stroud2014; Bublitz & Stroud, Reference Bublitz and Stroud2012; Shea et al., Reference Shea, Streiner, Fleming, Kamath, Broad and Steiner2007). During pregnancy, the placenta secretes large quantities of corticotropin-releasing hormone (CRH; Duthie & Reynolds, Reference Duthie and Reynolds2013; Reis, Fadalti, Florio, & Petraglia, Reference Reis, Fadalti, Florio and Petraglia1999), which stimulates the maternal HPA axis to secrete more cortisol, resulting in a two- to fourfold increase in circulating cortisol by the end of the third trimester (Jung et al., Reference Jung, Ho, Torpy, Rogers, Doogue, Lewis and Inder2011). However, early life adversity, such as experiences of childhood sexual abuse, appears to change the way that the maternal HPA axis adjusts to pregnancy (Bublitz & Stroud, Reference Bublitz and Stroud2012). As a result, the effect of ACEs on the maternal HPA axis function may change with advancing gestation, and because timing of prenatal exposure to maternal cortisol may also have differential effects on the developing fetal systems (Davis & Sandman, Reference Davis and Sandman2010; Ellman et al., Reference Ellman, Dunkel Schetter, Hobel, Chicz-DeMet, Glynn and Sandman2008; Glynn, Wadhwa, Dunkel-Schetter, Chicz-Demet, & Sandman, Reference Glynn, Wadhwa, Dunkel-Schetter, Chicz-Demet and Sandman2001; Vedhara et al., Reference Vedhara, Metcalfe, Brant, Crown, Northstone, Dawe and Smith2012), it is important to examine HPA axis function across pregnancy for the effects of ACEs.
Although the maternal HPA axis represents a plausible biological pathway for the intergenerational transmission of stress, it also suggests a potential intervention target for buffering such intergenerational transmission. For example, Giesbrecht, Poole, Letourneau, Campbell, and Kaplan (Reference Giesbrecht, Poole, Letourneau, Campbell and Kaplan2013) used an ecological momentary assessment study of pregnant women to demonstrate that women who perceived adequate social support from partners during pregnancy secreted less cortisol following report of psychological distress, as indexed by the profile of mood states, compared to women who perceived inadequate support. This finding suggests that maternal experiences of social support reduce HPA axis reactivity to psychological distress and may therefore shield the fetus from the potentially harmful effects of frequent or strong elevations in maternal cortisol. Despite suggestive evidence that the maternal HPA axis is a mechanism by which maternal ACEs become embedded in infant HPA axis function and that such effects may be moderated by social support, no studies to date have tested such moderated mediation models.
Whereas social support during pregnancy may alter maternal HPA axis function and thereby alter the transmission of maternal stress on the developing fetus, it is also known that supportive social relationships during the postpartum period strongly influence reactivity of infant stress response systems. As suggested above, responsive social partners who are reliably supportive may buffer infant physiological responses to stress and over time may thereby “recalibrate” infant HPA axis function (Del Giudice, Ellis, & Shirtcliff, Reference Del Giudice, Ellis and Shirtcliff2011). The quality of the relationships infants have with their caregivers is therefore an important source of stress buffering. What about relationships that do not directly include the infant? For example, does the quality of the relationship between the infant's parents serve as a source of social buffering for the infant? Evidence suggesting that it may, comes from studies showing that supportive relationships between life partners buffers against the intergenerational continuity of harsh and abusive parenting (Conger, Schofield, Neppl, & Merrick, Reference Conger, Schofield, Neppl and Merrick2013), and a supportive partner is also an important predictor of maternal sensitivity (Goldstein, Diener, & Mangelsdorf, Reference Goldstein, Diener and Mangelsdorf1996; Shin, Park, & Mi, Reference Shin, Park and Mi2006). Maternal sensitivity (the ability to accurately recognize infant cues and respond in a timely, accurate, and warm manner; Center on the Developing Child at Harvard University, 2010) not only influences responsivity of the infant HPA axis (Enlow et al., Reference Enlow, King, Schreier, Howard, Rosenfield, Ritz and Wright2014) but also moderates the association between prenatal stress exposure and infant HPA axis reactivity to stress (Grant, McMahon, Reilly, & Austin, Reference Grant, McMahon, Reilly and Austin2010). Nevertheless, it is not known whether partner support buffers the effects of maternal early life stress on infant HPA axis function. The identification of a buffer to the effects of maternal early life stress is potentially valuable because exposure to ACEs is common and because the provision of social support is a potentially modifiable intervention target.
The objective of the current study was therefore to determine whether the association between maternal ACEs and infant HPA axis function is mediated by maternal HPA axis function during early and late pregnancy and moderated by prenatal and postnatal social support. We hypothesized that ACEs would be associated with changes in the maternal HPA axis, and these changes would then be associated with increased infant HPA axis reactivity to a stressor. In addition, we hypothesized that prenatal social support would buffer the effects of ACEs on the maternal HPA axis during pregnancy and postnatal social support would buffer the effects of maternal HPA axis function on infant HPA axis reactivity. To test these hypotheses, HPA axis function and social support were repeatedly assessed in pregnant women and maternal social support and infant stress reactivity were assessed at 6 months postpartum.
Method
Participants
Participants were 243 women enrolled in an ongoing prospective cohort study, the Alberta Pregnancy Outcomes and Nutrition (APrON) study, which is a community sample of volunteers recruited from prenatal clinics from 2009 to 2012 (Kaplan et al., Reference Kaplan, Giesbrecht, Leung, Field, Dewey and Bell2012). Women were included if they had a singleton pregnancy, were less than 22 weeks of gestation at the first study visit, and were 18 years of age or older. Women were excluded if they smoked or consumed alcohol during pregnancy, were being treated with a synthetic glucocorticoid, or had known fetal complications at time of study entry. The study time points were as follows: Time 1 (T1; 6–22 weeks gestation, mean = 15.1 weeks +/– 3.5), Time 2 (T2; 27–37 weeks gestation, mean = 32.5 weeks +/– 1.0), and Time 3 (T3; 5–10 months postnatal, mean = 6.1 months +/– 0.7). Gestational age at each time point in pregnancy was determined based on last reported menstrual period and confirmed by at least one ultrasound. All women enrolled in the current study reported being in a heterosexual relationship.
Descriptive information for the study sample is shown in Table 1. The study sample represents a relatively low sociodemographic risk population of women and infants as the majority were mature (mean age 31.3 years), married or in common-law relationships, White, had university-level education, and had middle to upper-middle class household annual income. Fourteen infants included in the study were identified as being born preterm (i.e., <37 weeks gestation). Although the study sample underrepresents young (i.e., under 20) and low-income pregnant women, as compared to a nationally representative sample (Leung, McDonald, Kaplan, Giesbrecht, & Tough, Reference Leung, McDonald, Kaplan, Giesbrecht and Tough2013; Public Health Agency of Canada, 2009), the sample is largely consistent with the sociodemographics of families living within the recruitment region (Calgary, Canada) and may be comparable to low sociodemographic risk families living in Canada and other developed countries.
Table 1. Sociodemographic characteristics for the study sample (n = 243)

Note: SES, socioeconomic status, is a composite z score composed of family income, maternal education, and ethnicity whereby higher values indicate higher sociodemographic risk. ACEs, adverse childhood experiences.
Data collection
Maternal perception of social support received from her partner was assessed at each time point (T1–T3) using a standardized questionnaire. Maternal history of ACEs was assessed using a retrospective self-report measure of adverse experiences prior to the age of 18. Prenatal maternal cortisol was assessed from saliva samples collected by mothers over multiple days within each time point (excluding weekends to rule out potential weekend–weekday difference in stress and diurnal cortisol; Schlotz, Hellhammer, Schulz, & Stone, Reference Schlotz, Hellhammer, Schulz and Stone2004). Women self-collected saliva samples on 2 consecutive days at T1 and T2 (during pregnancy). During the first study visit, participants were instructed on the use of a personal digital assistant (PDA), which was used to facilitate saliva collection at waking, 30 min after waking, 11:00 a.m., and 9:00 p.m. Timing of each assessment was recorded by the PDA, permitting precise modeling of diurnal patterns. Participants were asked to refrain from consuming food, caffeine, citric drinks, and dairy, and to avoid vigorous exercise or brushing teeth in the 30 min prior to saliva collection and to report adherence to these guidelines via questions administered by the PDA after each saliva collection. Responses to the adherence questions on the PDA were used to evaluate whether nonadherence was associated with cortisol levels. Analysis revealed that adherence to the protocol was excellent (85% of samples) and nonadherence was not associated with maternal cortisol levels. Accordingly, adherence indicators were not included in the models as covariates. At the 6-month postnatal assessment (T3), infants underwent a standardized battery of frustration tasks. Saliva samples were collected from the infants at baseline and 20 min postfrustration to assess cortisol reactivity to stress. Prior to data collection, participants provided informed consent to the procedures, which were approved by the University of Calgary Conjoint Health Research Ethics Board.
Measures
Social support effectiveness
Women's perception of social support received from their romantic partner was assessed via the Social Support Effectiveness Questionnaire (SSEQ; Rini, Dunkel Schetter, Hobel, Glynn, & Sandman, Reference Rini, Dunkel Schetter, Hobel, Glynn and Sandman2006), a 35-item measure of emotional, informational, task, and negative support received over the previous 3 months. Within each domain, women were asked to rate on a 5-point scale (a) how well the quantity of support received from her partner matched the amount she wanted, (b) whether she wished the support had differed somehow, (c) how skillful her partner was at providing support, (d) how often it was difficult to solicit support, and (e) if her partner offered support without being asked. Women also rated negative support, or the extent to which a respondent perceived her partner's support as negatively infringing on her own efficacy/self-esteem. Internal consistency of the SSEQ is strong (Cronbach α = 0.87), and it has previously been used to distinguish levels of social support in samples of pregnant women (Giesbrecht et al., Reference Giesbrecht, Poole, Letourneau, Campbell and Kaplan2013; Rini et al., Reference Rini, Dunkel Schetter, Hobel, Glynn and Sandman2006; Stapleton et al., Reference Stapleton, Dunkel Schetter, Westling, Rini, Glynn, Hobel and Sandman2012). Total scores of the scale can range from 0 to 80, with higher scores indicating more effective support.
Maternal history of ACEs
The Adverse Childhood Experiences is a 10-item questionnaire (Felitti et al., Reference Felitti, Anda, Nordenberg, Williamson, Spitz, Edwards and Marks1998) that assesses early life adversity in three domains: abuse (emotional, physical, and sexual), neglect (physical and emotional), and household challenges (mother treated violently, substance abuse, mental illness, parental separation/divorce, and incarcerated household member). The questionnaire is a widely used measure that has demonstrated good reliability and internal consistency (α = 0.81; Bruskas & Tessin, Reference Bruskas and Tessin2013) as well as adequate test–retest reliability, κ = .64; 95% confidence interval [.36, .60]; Dube, Williamson, Thompson, Felitti, & Anda, Reference Dube, Williamson, Thompson, Felitti and Anda2004). In the current study, the occurrence of individual ACEs was summed to create the ACE scores (range: 0–9). Because scores of 4 or more occurred infrequently, and in keeping with previous methods (e.g., Anda et al., Reference Anda, Felitti, Bremner, Walker, Whitfield, Perry and Giles2006), scores above 4 were recoded to a value of 4 so that the final ACEs scores ranged from 0 to 4.
Prenatal maternal cortisol
After self-collection according to the schedule described above, saliva samples were stored in home freezers (1–3 days) until they could be shipped on freezer packs to the lab, where they were stored at –60 °C at the university laboratory until they were shipped frozen to Salimetrics, State College, Pennsylvania, for assay. Several HPA axis parameters were calculated from the saliva samples. The cortisol awakening response (CAR) was calculated using the trapezoid method for area under the curve increase (AUCi) as a measure of the morning increase in cortisol (Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, Reference Pruessner, Kirschbaum, Meinlschmid and Hellhammer2003). To ensure that our measure of the CAR was valid, samples were excluded if they were taken more than 15 minu after waking (for the waking sample) or more than 50 min after waking for the second sample (Okun et al., Reference Okun, Krafty, Buysse, Monk, Reynolds, Begley and Hall2010). A total of 14 CAR samples (1 from early pregnancy and 13 from late pregnancy) were not available for data analyses. Because the AUCi is dependent on the amount of time between baseline and the +30 min sample, CAR was standardized to reflect 30 min of output in order to account for individual differences in the time between the waking and waking +30 min samples. Total daytime cortisol over the day was estimated as area under the curve with respect to ground (AUCg; Pruessner et al., Reference Pruessner, Kirschbaum, Meinlschmid and Hellhammer2003). As with the AUCi, AUCg is sensitive to the total amount of time between the waking and bedtime samples, and because individuals had different amounts of time between these samples, AUCg was standardized to the average time between waking and 9:00 p.m. samples (853 min). The diurnal cortisol slope was calculated as ([9:00 p.m. – waking] / time in hours) to describe the decline in cortisol concentration per hour across the day (Fekedulegn et al., Reference Fekedulegn, Andrew, Burchfiel, Violanti, Hartley, Charles and Miller2007).
Infant cortisol reactivity
Infant cortisol reactivity was assessed after exposure to a standardized stressor. The stressor involved the toy retraction, toy barrier, and arm restraint tasks from the Laboratory Temperament Assessment Battery (Goldsmith & Rothbart, Reference Goldsmith and Rothbart1996), which elicit cortisol responses in infants (Laurent, Ablow, & Measelle, Reference Laurent, Ablow and Measelle2012). One saliva sample was collected at baseline (after acclimatization to the lab but before onset of the stressors) and the second sample was collected at 20 min poststressor (which accommodates the delay between exposure to a stressor and peak HPA axis response; Kirschbaum & Hellhammer, Reference Kirschbaum and Hellhammer1999) using a synthetic swab (Salimetrics Children's Swab, Salimetrics, State College, PA), which has been validated for the collection of cortisol (Bright, Frick, Out, & Granger, Reference Bright, Frick, Out and Granger2014). To avoid saliva contamination by breast or cow's milk, participants were asked to refrain from feeding their infant in the 30 min prior to coming to the lab or during the laboratory procedure. In cases where feeding occurred within the 30 min before sample collection, water was used to rinse the infant's mouth, and then samples were collected 10 min after rinsing. Sufficient saliva samples for the cortisol assay were obtained from all infants in the current study. Saliva samples were stored at –60 °C at the university laboratory until they were shipped frozen to Salimetrics, State College, Pennsylvania. Infant cortisol reactivity was calculated as AUCi from baseline to poststress (Pruessner et al., Reference Pruessner, Kirschbaum, Meinlschmid and Hellhammer2003).
Cortisol assay
All maternal and infant samples were assayed for salivary cortisol using the Salimetrics enzyme immunoassay. It has a lower limit of sensitivity of 0.007 μg/dl, standard curve range from 0.012 to 3.0 μg/dl, and average intra- and interassay coefficients of variation 3.5% and 5.1%, respectively. Method accuracy, determined by serial dilution are 100.8% and 91.7%. A random 25% of maternal and 10% of infant samples were assayed in duplicate to confirm reliability; the intra-assay coefficient of variation was 4.2% and 3.5% for maternal and infant samples, respectively. Mean values from duplicate samples were used for analysis.
Covariates
In order to rule out potential confounders as the basis for any observed effects, and based upon previous studies, a number of covariates were selected a priori for inclusion in the analyses. Gestational age at each prenatal assessment was included to account for individual differences in the exact timing of the prenatal assessments. Infant sex was included as a covariate because sex differences in the effects of prenatal exposures on infant outcomes are frequently observed (Giesbrecht, Letourneau, Campbell, & APrON Study Team, Reference Giesbrecht, Letourneau and Campbell2017; Mueller & Bale, Reference Mueller and Bale2007; Sandman, Glynn, & Davis, Reference Sandman, Glynn and Davis2013), as well as to account for differences in maternal HPA axis regulation during pregnancy as a function of fetal sex (Giesbrecht, Campbell, Letourneau, & APrON Study Team, Reference Giesbrecht, Campbell and Letourneau2015). Finally, given the association between preterm birth and altered HPA axis function (Grunau et al., Reference Grunau, Haley, Whitfield, Weinberg, Yu and Thiessen2007), gestational age at birth was included as a covariate in the analyses.
Additional covariates were investigated for inclusion in the analyses by conducting bivariate correlations with the primary study variables. Sociodemographic characteristics (i.e., socioeconomic status [SES], parity, and maternal age) were investigated as potential covariates given that they have previously been shown to be related to HPA axis function, ACEs, and social support (Cohen, Doyle, & Baum, Reference Cohen, Doyle and Baum2006; Kivlighan, DiPietro, Costigan, & Laudenslager, Reference Kivlighan, DiPietro, Costigan and Laudenslager2008; Lupien, King, Meaney, & McEwen, Reference Lupien, King, Meaney and McEwen2001; Madigan, Wade, Tarabulsy, Jenkins, & Shouldice, Reference Madigan, Wade, Tarabulsy, Jenkins and Shouldice2014; Metzler, Merrick, Klevens, Ports, & Ford, Reference Metzler, Merrick, Klevens, Ports and Ford2017).
A composite SES variable was created by summing z scores of self-reported family income (1 = <$20,000/year, 2 = $20,000–$39,999, 3 = $40,000–$69,000, 4 = $70,000–$99,999, 5 = ≥$100,000), maternal education (1 = less than high school, 2 = high school, 3 = trade or technical school, 4 = undergraduate degree, 5 = graduate degree), and ethnicity (0 = non-White, 1 = White). The distribution of the composite SES scores for the study sample were negative skewed, and thus the scores were reflected and log transformed, as recommended (Tabachnick & Fidell, Reference Tabachnick and Fidell2012). Final composite SES scores were then mean centered with higher values indicating greater sociodemographic risk (i.e., lower annual income, less education, and non-White). SES was included as a covariate because it was associated with maternal CAR at T1, r (235) = .188, p = .004, and social support at T2, r (236) = –.149, p = .021.
Parity was also included as a covariate because it was associated with total maternal cortisol output at T1, r (241) = –.145, p = .024, maternal CAR at T1, r (240) = –.142, p = .027, and social support at T2, r (241) = –.166, p = .010. Maternal age did not meet criteria for inclusion as a covariate as it was not associated with any of the primary study variables (p < .05).
Data analytic strategy
Consistent with our overall aim to determine whether maternal cortisol is a mechanism by which maternal ACEs become embedded in infant development and to determine whether social support moderates this pathway, we assessed a series of moderation and moderated mediation models. Given that associations between maternal HPA axis function and infant stress reactivity may differ as a function of timing during pregnancy, separate models were tested for early and late pregnancy. Regression analyses were conducted to determine which aspects of maternal HPA axis function (i.e., total cortisol output, CAR, and diurnal cortisol slope) in pregnancy were associated with ACEs and if any of these associations were conditional on prenatal social support. Similarly, regression analyses were conducted to determine which aspects of maternal HPA axis function in pregnancy were associated with infant cortisol reactivity and if any of these associations were conditional on postnatal social support. Significant moderation (interaction) effects were probed by examining the conditional effects for two-way interactions between predictors (Aiken & West, Reference Aiken and West1991). The Johnson–Neyman technique was used to derive regions of significance (ROS) for the conditional effects of the prenatal predictors at varying levels of social support (Aiken & West, Reference Aiken and West1991; Johnson & Neyman, Reference Johnson and Neyman1936).
Next we assessed moderated mediation models for variables that met criteria for testing mediation. Potential models met criteria to test for mediation if the direct or conditional pathway from ACEs to prenatal maternal HPA axis function and the direct or conditional pathway from maternal HPA axis function to infant cortisol reactivity were both significant, as determined by the models described above. The moderated mediation analyses were conducted according to the steps outlined by Hayes and Preacher (Reference Hayes, Preacher, Hancock and Mueller2013) and using PROCESS (Hayes, Reference Hayes2012), a regression-based analytic tool implemented in SPSS. A bootstrapping procedure (n = 10,000 bootstrap resamples) and product of coefficients approach was used to estimate bias-corrected 95% confidence intervals, as described by Preacher and Hayes (Hayes, Reference Hayes2013; Preacher & Hayes, Reference Preacher and Hayes2004, Reference Preacher and Hayes2008). This approach accounts for the nonnormality and/or asymmetry in the indirect effect (Hayes & Preacher, Reference Hayes, Preacher, Hancock and Mueller2013), and it balances power and validity considerations (i.e., Type 1 and Type 2 errors; Hayes, Reference Hayes2013). Mediation is supported when the confidence interval (CI) does not contain zero. To aid with interpretability, the following variables were mean centered prior to analyses: social support, gestational age at prenatal assessment, gestational age at birth, and SES.
Results
Missing value analysis
Two hundred forty-three women completed the T1 (early pregnancy) assessment, 231 women completed the T2 (late pregnancy) assessment, and 243 women and infants completed the T3 (6 months postpartum) assessment.
Preliminary analyses
Bivariate correlations were conducted to gain a preliminary understanding of the associations between the study variables (Table 2). There was a positive association between maternal ACEs and maternal CAR in early pregnancy, r = .22, p < .001. Maternal ACEs were not associated with diurnal cortisol slope or total maternal cortisol output in early or late pregnancy. Total maternal cortisol output in early pregnancy was associated with higher infant cortisol reactivity at 6 months of age, r = .13, p = .04. Neither maternal ACEs nor social support was associated with infant cortisol reactivity.
Table 2. Bivariate correlations and descriptive statistics for the primary study variables
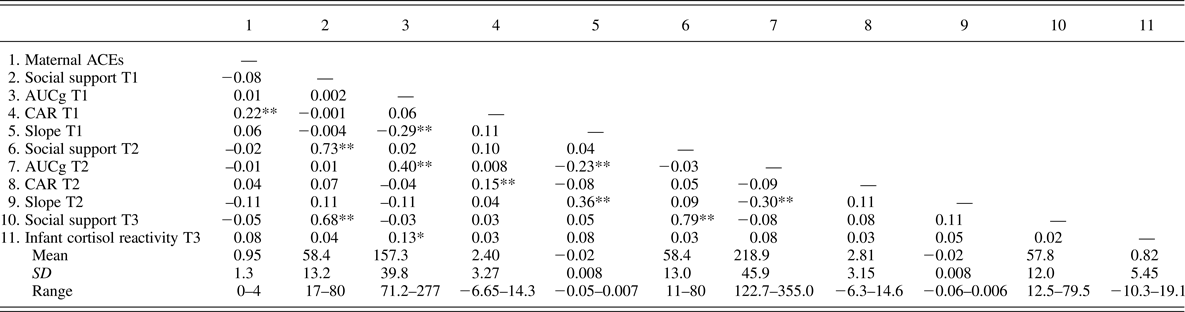
Note: Means, standard deviations, and ranges displayed are for raw data values (not mean centered). ACEs, adverse childhood experiences. AUCg, area under the curve from ground (a measure of total daytime cortisol secretion). CAR, cortisol awakening response. Slope, diurnal cortisol slope. T1, Time 1 (early pregnancy). T2, Time 2 (late pregnancy). T3, Time 3 (6 months postnatal). *p < .05. ** p ≤ .01.
Direct and conditional associations between maternal ACEs and maternal HPA axis function during pregnancy
Early pregnancy (T1)
The direct and conditional associations between maternal ACEs and HPA axis function in early pregnancy are displayed in Table 3.
Table 3. Direct and conditional associations between maternal ACEs and maternal hypothalamic–pituitary–adrenal axis function during early pregnancy

Note: ACEs, adverse childhood experiences. AUCg, area under the curve from ground (a measure of total daytime cortisol secretion). CAR, cortisol awakening response. Slope, diurnal cortisol slope. T1, Time 1 (early pregnancy). SES, socioeconomic status, is a composite z score composed of family income, maternal education, and ethnicity whereby higher values indicate higher sociodemographic risk. *p < .05. **p ≤ .01.
Total daytime cortisol
There was no association between maternal ACEs and total daytime cortisol, B = 1.18, p = .56, and no conditional association as a function of social support, B = –0.003, p = .98.
CAR
There was a positive association between ACEs and maternal CAR in early pregnancy, B = 0.50, p = .003, that was not conditional on social support, B = –0.001, p = .96.
Diurnal slope
There was no association between ACEs and maternal diurnal slope, B = 0.0001, p = .75, and no conditional association based on level of social support, B = 0.00003, p = .36.
Late pregnancy (T2)
The direct and conditional associations between maternal ACEs and HPA axis function in late pregnancy are displayed in Table 4.
Table 4. Direct and conditional associations between maternal ACEs and maternal hypothalamic–pituitary–adrenal axis function during late pregnancy

Note: ACEs, adverse childhood experiences. AUCg, area under the curve from ground (a measure of total daytime cortisol secretion). CAR, cortisol awakening response. Slope, diurnal cortisol slope. T2, Time 2 (late pregnancy). SES, socioeconomic status, is a composite z score composed of family income, maternal education, and ethnicity whereby higher values indicate higher sociodemographic risk. *p < .05. **p ≤ .01.
Total daytime cortisol
The association between maternal ACEs and total daytime cortisol in late pregnancy was conditional on level of social support, B = –0.36, p = .04 (Figure 1a). The Johnson–Neyman ROS test indicated a positive association between ACEs and total cortisol output when late pregnancy levels of prenatal social support were below the 5th percentile, which corresponds to scores below 31 on the SSEQ. No association was present at higher levels of prenatal social support.
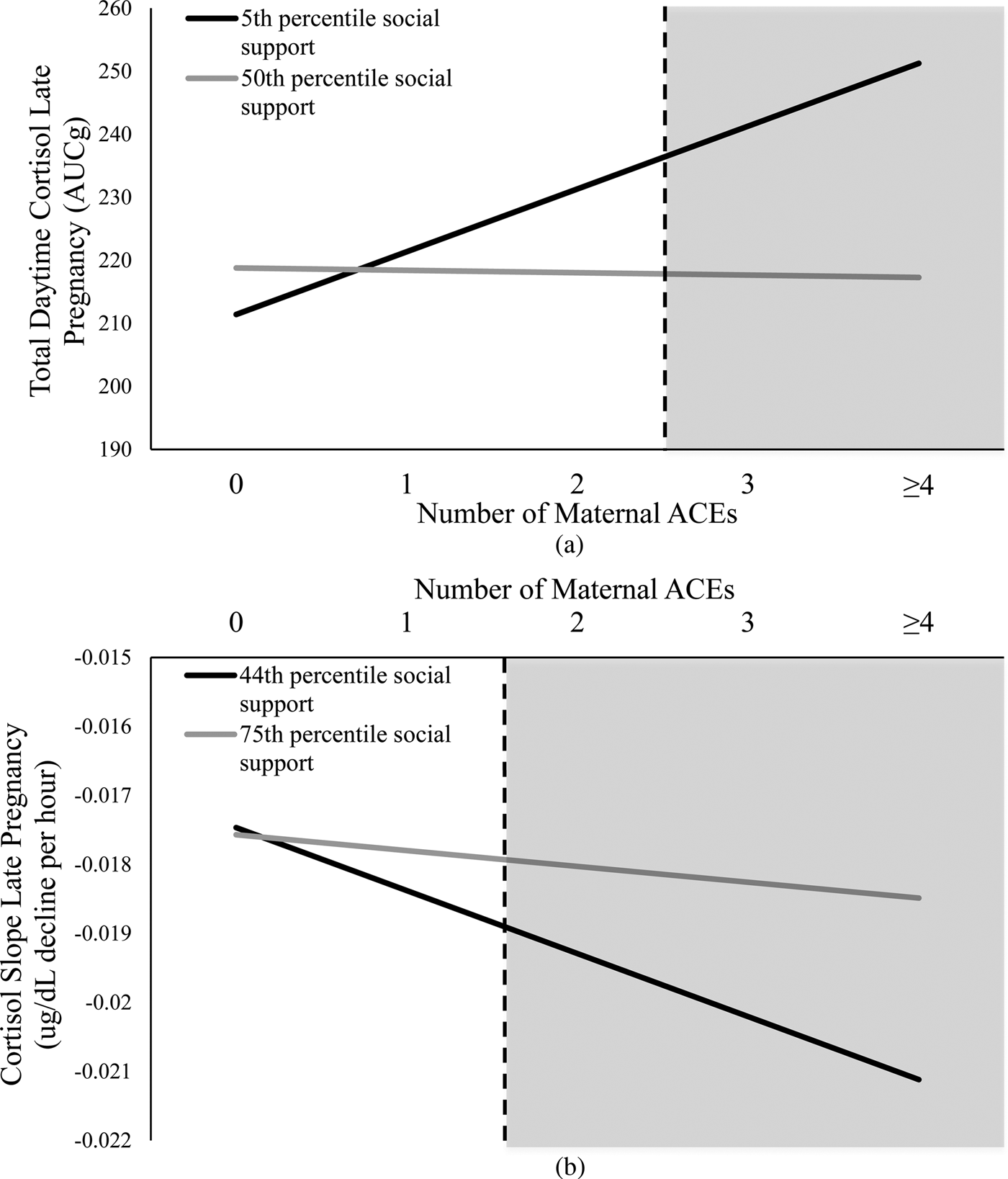
Figure 1. The association between maternal ACEs, total daytime cortisol output, and cortisol slope in late pregnancy. (a) At low levels of prenatal social support (i.e., <5th percentile), there was a positive association whereby higher ACEs were associated with higher cortisol output. (b) At low levels of prenatal social support (i.e., <44th percentile), there was a negative association whereby higher ACEs were associated with a more negative (i.e., steeper) diurnal slope. The dotted lines indicate the number of maternal ACEs above which the effect of low social support differentially affects maternal total daytime cortisol levels and cortisol slope in late pregnancy. ACEs, adverse childhood experiences.
CAR
There was no association between ACEs and CAR in late pregnancy, B = 0.09, p = .60, and no conditional association that depended on level of social support, B = –0.01, p = .34.
Diurnal slope
The association between maternal ACEs and diurnal cortisol slope in late pregnancy was conditional on level of social support, B = 0.0001, p = .05 (Figure 1b). The Johnson–Neyman ROS test indicated a negative association between ACEs and diurnal cortisol slope when late pregnancy levels of prenatal social support were below the 44th percentile, which corresponds to scores below 58 on the SSEQ. The negative association indicates that the cortisol slope becomes steeper (i.e., more negative) with increasing number of ACEs. No association was present at higher levels of prenatal social support.
Direct and conditional associations between maternal prenatal HPA axis function and infant cortisol reactivity
Early pregnancy (T1)
The direct and conditional associations between maternal HPA axis function in early pregnancy and infant cortisol reactivity are displayed in Table 5.
Table 5. Direct and conditional associations between maternal hypothalamic–pituitary–adrenal axis function during early pregnancy and infant cortisol reactivity at 6 months

Note: AUCg, area under the curve from ground (a measure of total daytime cortisol secretion). CAR, cortisol awakening response. Slope, diurnal cortisol slope. T1, Time 1 (early pregnancy). T3, Time 3 (6 months postnatal). SES, socioeconomic status, is a composite z score composed of family income, maternal education, and ethnicity whereby higher values indicate higher sociodemographic risk. *p < .05. **p ≤ .01.
Total daytime cortisol
The positive association between total daytime cortisol output in early pregnancy and infant cortisol reactivity at 6 months of age, B = 0.02, p = .02, was not conditional on level of postnatal social support, B = –0.0001, p = .92.
CAR
The association between CAR in early pregnancy and infant cortisol reactivity was conditional on level of postnatal social support, B = 0.02, p = .04 (Figure 2a). The Johnson–Neyman ROS test indicated a positive association between CAR in early pregnancy and infant cortisol reactivity at levels of postnatal social support above the 92nd percentile, which corresponds to scores above 74 on the SSEQ. No association was present at lower levels of postnatal social support.
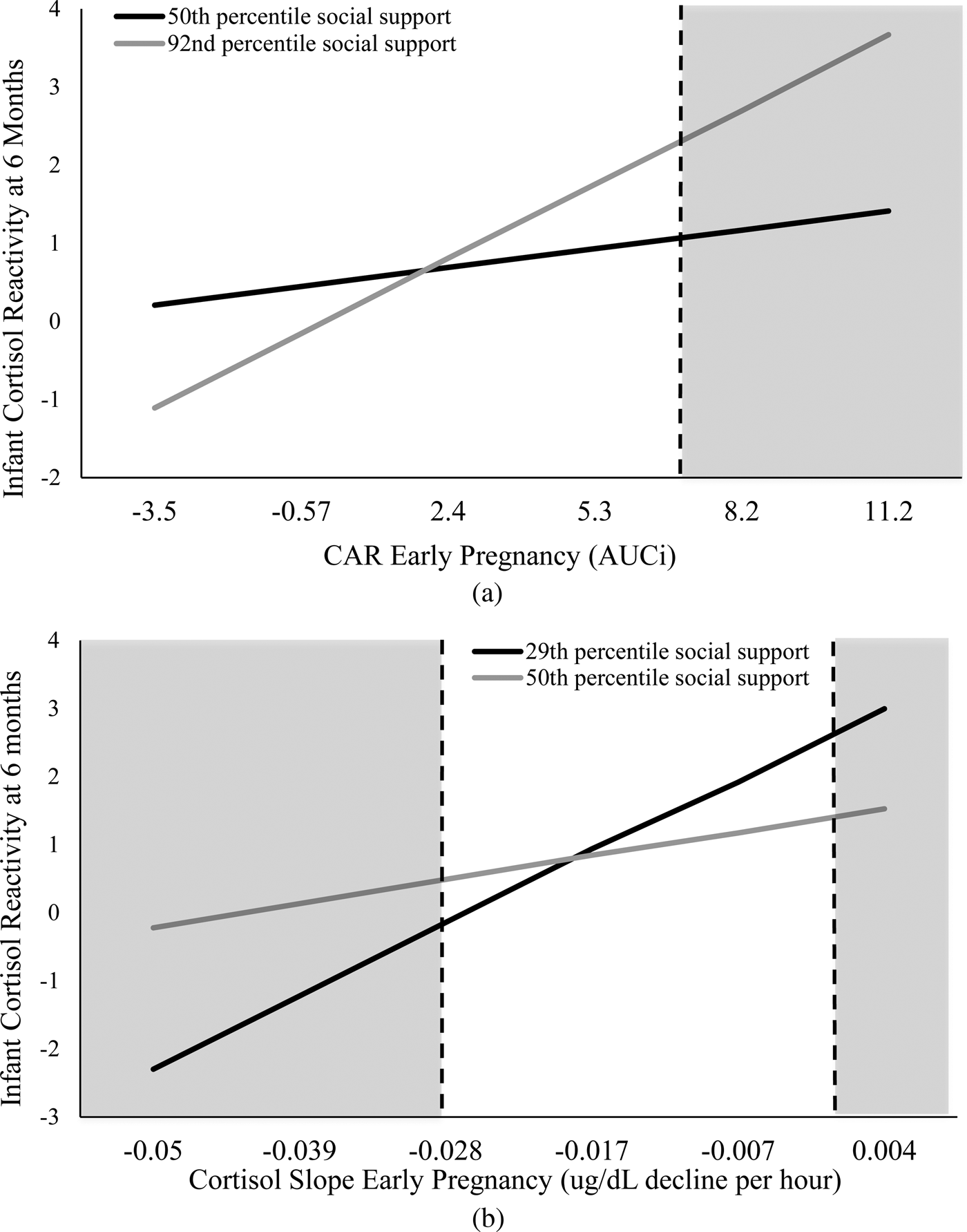
Figure 2. The association between infant cortisol reactivity, maternal CAR, and maternal diurnal slope in early pregnancy. (a) At high levels of postnatal social support (i.e., above the 92nd percentile), there was a positive association whereby higher CAR was associated with higher infant cortisol reactivity. (b) At low levels of postnatal social support (i.e., below the 29th percentile), the association between maternal cortisol slope and infant cortisol reactivity was positive such that more positive (i.e., flatter) diurnal slopes were associated with increased infant cortisol reactivity. The dotted lines indicate the levels of maternal CAR and diurnal slope above which and below which the effect of high and low social support, respectively, differentially affects infant cortisol reactivity at 6 months of age. CAR, cortisol awakening response.
Diurnal slope
The association between diurnal cortisol slope in early pregnancy and infant cortisol reactivity was also conditional on levels of postnatal social support, B = –8.21, p = .03 (Figure 2b). The Johnson–Neyman ROS test indicated a positive association between diurnal cortisol slope in early pregnancy and infant cortisol reactivity at levels of postnatal social support below the 29th percentile, which corresponds to scores below 51 on the SSEQ. The positive association indicates that as the slope becomes flatter (i.e., more positive), infant cortisol reactivity increases. No association was present at higher levels of postnatal social support.
Late pregnancy (T2)
The direct and conditional associations between maternal HPA axis function in late pregnancy and infant cortisol reactivity are displayed in Table 6.
Table 6. Direct and conditional associations between maternal hypothalamic–pituitary–adrenal axis function during late pregnancy and infant cortisol reactivity at 6 months

Note: AUCg, area under the curve from ground (a measure of total daytime cortisol secretion). CAR, cortisol awakening response. Slope, diurnal cortisol slope. T2, Time 2 (late pregnancy). T3, Time 3 (6 months postnatal). SES, socioeconomic status, is a composite z score composed of family income, maternal education, and ethnicity whereby higher values indicate higher sociodemographic risk. *p < .05.
Total daytime cortisol
The association between total daytime cortisol output in late pregnancy and infant cortisol reactivity was conditional on levels of postnatal social support, B = –0.001, p = .04 (Figure 3). The Johnson–Neyman ROS test indicated a positive association between total cortisol output in late pregnancy and infant cortisol reactivity at levels of postnatal social support below the 29th percentile, corresponding to scores below 51 on the SSEQ. No association was present at higher levels of postnatal social support.

Figure 3. The association between total daytime cortisol output in late pregnancy and infant cortisol reactivity. At low levels of postnatal social support (i.e., <29th percentile), the association was positive such that higher cortisol was associated with higher infant cortisol reactivity. The dotted line indicates the level of maternal total daytime cortisol output in late pregnancy below which the effect of low social support differentially affects infant cortisol reactivity at 6 months of age.
CAR
The association between CAR in late pregnancy and infant cortisol reactivity, B = 0.06, p = .63, was not conditional on level of postnatal social support, B = 0.008, p = .43.
Diurnal slope
Similarly, the association between diurnal cortisol slope in late pregnancy and infant cortisol reactivity, B = 34.9, p = .43, was not conditional on level of postnatal social support, B = 3.62, p = .34.
Indirect and conditional indirect associations between maternal ACEs and infant cortisol reactivity
Early pregnancy (T1)
In early pregnancy, the only potential mediator that satisfied criteria to test for mediation was maternal CAR. Mediation analyses indicated there was a conditional indirect association between ACEs and infant cortisol reactivity through CAR, which was moderated by postnatal social support, B = 0.008, 95% CI [0.0001, 0.024] (Figure 4). Maternal ACEs were associated with increased CAR in early pregnancy, B = 0.50, p = .003, and the association between CAR in early pregnancy and infant cortisol reactivity was conditional on level of postnatal social support, B = 0.02, p = .04. Specifically, the association between CAR and infant cortisol reactivity was positive at higher levels of postnatal social support. Thus, at higher levels of postnatal social support, increased maternal ACEs were associated with increased infant cortisol reactivity through increased CAR in early pregnancy.

Figure 4. Conditional indirect effect of maternal ACEs on infant cortisol reactivity through maternal CAR in early pregnancy. Maternal ACEs were associated with increased CAR in early pregnancy, which then had a conditional association with increased infant cortisol reactivity. The association between CAR and infant cortisol reactivity was positive at higher levels of postnatal social support. Gestational age at the Time 1 assessment, infant sex, gestational age at birth, parity, and socioeconomic status were included as covariates in the model. ACEs, adverse childhood experiences. CAR, cortisol awakening response. *p < .05. **p ≤ .01.
Late pregnancy (T2)
In late pregnancy, only total maternal daytime cortisol output met criteria to test for mediation. There was a conditional indirect effect of ACEs on infant cortisol reactivity that was mediated by total daytime cortisol output in late pregnancy and moderated by both prenatal and postnatal social support B = 0.0004, 95% CI [0.00001, 0.0013] (Figure 5). Specifically, the association between maternal ACEs and total daytime cortisol output was conditional on level of prenatal social support, B = –0.36, p = .04, whereby greater ACEs exposure was associated with higher total cortisol output in pregnant women, but only when levels of prenatal social support were low. The association between total daytime cortisol output and infant cortisol reactivity was conditional on postnatal social support, B = –0.001, p = .05, such that higher maternal daytime cortisol output was associated with greater infant cortisol reactivity when levels of postnatal social support were low. Thus, at low levels of prenatal and postnatal social support, greater ACEs exposure was associated with increased infant cortisol reactivity through increased maternal daytime cortisol output during late pregnancy.
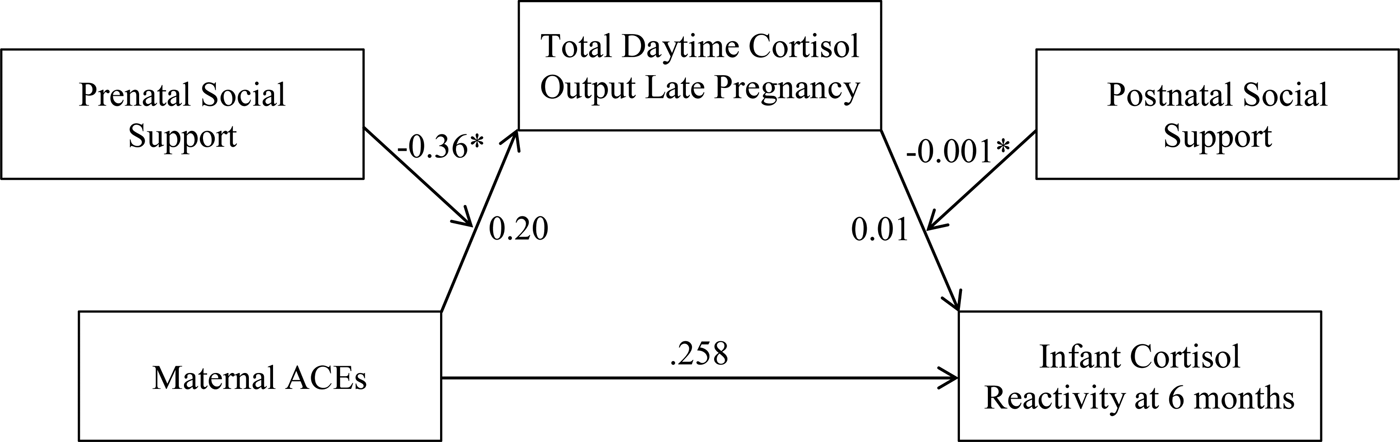
Figure 5. Conditional indirect association between maternal ACEs and infant cortisol reactivity via total maternal daytime cortisol output (AUCg). At low levels of prenatal social support, maternal ACEs were associated with higher total cortisol output. At low levels of postnatal social support, higher cortisol output was associated with higher infant cortisol reactivity. Gestational age at the Time 2 assessment, infant sex, gestational age at birth, and socioeconomic status were included as covariates in the model. ACEs, adverse childhood experiences. AUCg, area under the curve with respect to ground. *p < .05.
Sensitivity analyses
Given that preterm birth has previously been associated with infant HPA axis function, the analytic models were rerun after excluding 14 infants born less than 37 weeks of gestation. All models yielded the same findings as reported above, with the exception of one change. In the final model (see Figure 5), the moderating effect of postnatal social support was reduced to a nonsignificant effect, B = –0.001, p = .06, and therefore also the overall index of moderated mediation was reduced to a nonsignificant effect, B = 0.0004, 95% CI [–0.000004, 0.0013].
Discussion
This study found evidence to support the hypotheses that some aspects of maternal HPA axis function are a mechanism by which maternal ACEs are transmitted to infant development, and that social support in both the prenatal and postnatal periods buffers this cascade. Most of the effects were observed when levels of social support were low, suggesting that when women experience adequate or satisfactory levels of social support from their partners, the transmission of early life experiences to offspring is greatly attenuated. Our findings are in line with previous studies showing that supportive partners reduce psychophysiological response to acute stressors in nonpregnant women (Heinrichs, Baumgartner, Kirschbaum, & Ehlert, Reference Heinrichs, Baumgartner, Kirschbaum and Ehlert2003) and psychological distress in pregnant women (Giesbrecht et al., Reference Giesbrecht, Poole, Letourneau, Campbell and Kaplan2013). Whereas previous studies have shown only the value of social support for buffering current experiences of stress, our findings extend the scope of social support by suggesting that it can “reach back” into histories of stress to reduce their influence on current functioning. Such apparently retroactive effects may operate via epigenetic mechanisms whereby the original biological commitments are recalibrated by current psychosocial circumstances (Bowers & Yehuda, Reference Bowers and Yehuda2016). The expected outcomes of this epigenetic recalibration include improved psychological adjustment and reduction in physiological and emotional responses to stressful events. The current findings highlight the social sensitivity of the HPA axis and suggest the utility of social relationships as an intervention target to reduce the effects of maternal prenatal stress on infant outcomes and the need for further research to describe the mechanisms by which supportive social relationships revise histories of adversity.
Social buffering of the maternal HPA axis during pregnancy
As expected, maternal ACEs were associated with increased total daytime cortisol output, but only in women who reported lower perceived social support from their partners. This conditional association was identified only in the latter half of pregnancy (after 27 weeks gestation), which may indicate that prenatal social support modulates the way in which the maternal HPA axis adjusts to pregnancy. During pregnancy, the placenta secretes an ever increasing amount of cortisol (resulting from increased placental CRH; Duthie & Reynolds, Reference Duthie and Reynolds2013; Reis et al., Reference Reis, Fadalti, Florio and Petraglia1999), which is believed to be important for maturation of fetal tissues (Garbrecht, Klein, Schmidt, & Snyder, Reference Garbrecht, Klein, Schmidt and Snyder2006) and setting the timing of parturition (Sandman et al., Reference Sandman, Glynn, Schetter, Wadhwa, Garite, Chicz-DeMet and Hobel2006). Nevertheless, relatively larger elevations in cortisol from middle to late pregnancy have been associated with adverse outcomes such as preterm birth and low birthweight (Field et al., Reference Field, Hernandez-Reif, Diego, Figueiredo, Schanberg and Kuhn2006), as well as accelerated cognitive development within the first year of life (Davis & Sandman, Reference Davis and Sandman2010). Although the latter finding may appear beneficial, accelerated brain development is also associated with exposure to maternal depression during gestation, and this early development may occur at the expense of extended brain plasticity, affecting cognitive and behavioral outcomes over the life span (Lebel et al., Reference Lebel, Walton, Letourneau, Giesbrecht, Kaplan and Dewey2015). Relatively larger increases in cortisol during gestation may therefore increase the risk for poor developmental outcomes in offspring. Our findings indicate that women with lower perceived support have relatively larger increases in cortisol over gestation (as determined by total daytime cortisol production), suggesting increased risk for poor birth and child development outcomes. Nevertheless, in women who perceive adequate levels of social support, the increase in total cortisol production is reduced, and therefore risks to offspring may also be reduced.
Despite the robust pregnancy-related changes in total cortisol production during pregnancy, the diurnal cortisol pattern, including the CAR and diurnal slope, are largely preserved (de Weerth & Buitelaar, Reference de Weerth and Buitelaar2005). The “normality” of the CAR and diurnal slope during pregnancy suggest that it may be possible to interpret patterns of HPA axis function during pregnancy similar to the way such patterns are interpreted in nonpregnant humans.
Maternal ACEs were associated with elevated CAR in early pregnancy, but this association was not moderated by prenatal social support. The CAR is a complex biological phenomenon that is both highly heritable and influenced by many environmental and situational factors including age, gender, female reproductive factors (e.g., menstrual cycle phase and oral contraceptive use), physical/mental health, and stress (Fries, Dettenborn, & Kirschbaum, Reference Fries, Dettenborn and Kirschbaum2009; Wüst, Federenko, Hellhammer, & Kirschbaum, Reference Wüst, Federenko, Hellhammer and Kirschbaum2000). A positive association between ACEs and CAR has been observed across multiple studies, including women with a history of childhood sexual abuse (Bublitz et al., Reference Bublitz, Parade and Stroud2014; Bublitz & Stroud, Reference Bublitz and Stroud2012), and in nonpregnant women who experienced childhood maltreatment (Gonzalez, Jenkins, Steiner, & Fleming, Reference Gonzalez, Jenkins, Steiner and Fleming2009) or childhood trauma (Lu et al., Reference Lu, Gao, Wei, Wu, Liao, Ding and Li2013). Taken together, it appears that ACEs leave an enduring biological signature of stress on the CAR that persists into pregnancy and that, as shown here, is robust against the buffering effects of social support.
Our finding that ACEs were associated with a steeper daytime cortisol slope was unexpected, and the fact that this association was significant only at lower levels of social support (negative association) was doubly surprising. Studies of nonpregnant adults have typically reported a positive relationship between social functioning and daytime cortisol slope (i.e., individuals with more supportive social relationships have a steeper cortisol slope; Adam & Gunnar, Reference Adam and Gunnar2001; Karb, Elliott, Dowd, & Morenoff, Reference Karb, Elliott, Dowd and Morenoff2012) suggesting that flatter daytime slopes are a nonoptimal pattern of HPA axis functioning. Furthermore, current understanding of the daytime cortisol slope is that steeper is better than flatter because steeper slopes are associated with better physical health and psychological adjustment (Adam et al., Reference Adam, Quinn, Tavernier, McQuillan, Dahlke and Gilbert2017). As it is unlikely that lower perceived social support has a beneficial effect on maternal HPA axis function, we speculate that the observed steepening of the diurnal slope in women reporting ACEs and low prenatal social support in our study may represent a compensatory mechanism of the maternal HPA axis to downregulate overall cortisol secretion to protect the fetus from elevated cortisol levels. This is consistent with the finding that the daytime slope becomes increasingly steeper as a function of advancing gestation (Thomas, Magel, et al., Reference Thomas, Magel, Tomfohr-Madsen, Madigan, Letourneau, Campbell and Giesbrecht2017) and with the fact that women with greater number of ACEs had higher overall cortisol levels. In contrast, progressive steepening of the diurnal cortisol slope toward the end of pregnancy in women with low social support may represent a failure of the maternal HPA axis to adjust appropriately to the changing needs of the mother and fetus as pregnancy processes (Murphy & Clifton, Reference Murphy and Clifton2003). The nature of the associations between maternal early life adversity and HPA axis are complex and require further research; however, the current study clearly shows that maternal perception of partner support alters these associations.
Supportive social relationships may alter the effects of early life adversity on maternal HPA axis functioning during pregnancy in a number of ways. Social support is a resilience factor in terms of psychological adjustment and well-being in adulthood following childhood maltreatment. For example, Runtz and Schallow (Reference Runtz and Schallow1997) found that 55% of the variance in psychological adjustment of male and female college students who experienced childhood maltreatment was attributable to social support. Social support, and specifically self-esteem and stress appraisal support, also moderate the association between childhood sexual abuse and the development of posttraumatic stress disorder in adulthood (Hyman, Gold, & Cott, Reference Hyman, Gold and Cott2003). Moreover, psychological adjustment is important because it has direct effects on HPA axis function. For example, pregnant women reporting higher social support have better psychological adjustment and secrete lower levels of cortisol in response to psychological distress compared to women receiving inadequate social support (Giesbrecht et al., Reference Giesbrecht, Poole, Letourneau, Campbell and Kaplan2013). To summarize, social support may buffer the effects of ACEs on maternal HPA axis function by improving psychological adjustment and reducing physiological and emotional responses to stressful events.
In light of the key role that social support may play in buffering the intergenerational transmission of stress, it is important to consider a number of factors that may contribute to individual differences in how women perceive social support. Doyle and Cicchetti (Reference Doyle and Cicchetti2017) have advanced an organizational perspective on social development in the context of the early caregiving environment that describes several factors regulating perception of social closeness and social adjustment. Specifically, adverse early caregiving environments may lead to deficits in social–emotional competencies (e.g. depression and dissociative symptoms) that then contribute to social maladaptation in adulthood (e.g., erratic behaviors that are sometimes withdrawn, sometimes solicitous, sometimes controlling, and sometimes hostile). Perceptions of social partners are based, in part, on mental representations that are established early in life and inform the organization of social relationships throughout life. The existing evidence of adult social adjustment following severe early life adversity (e.g., institutional care) indicates that some adults follow Doyle and Cicchetti's proposed pathway, whereas others demonstrate resilience, suggesting that opportunities for reorganization of mental representations and social adaptations allow for opportunities to recover. Because social support has potential as an intervention target to disrupt the intergenerational transmission of stress, more longitudinal research is needed to better understand how early adversity shapes social expectations and perceptions in intimate relationships and what factors shift the organization of social relationships toward healthy adjustment or maladjustment. In the current study, ACEs had essentially no correlation with maternal perception of social support (see Table 2), indicating that experiences of adversity were not overtly associated with a bias toward negative perception of her partner. This suggests that adversity and social perception are not confounded in our data; however, it will be important in future work to determine the ways in which adverse experiences in the general population may constrain or bias perception of social support.
Social buffering of infant HPA axis
Individual differences in the size of the maternal CAR, the steepness of the diurnal slope, and the overall amount of cortisol increase during pregnancy are associated with “programming” of the fetal HPA axis (Davis, Glynn, Waffarn, & Sandman, Reference Davis, Glynn, Waffarn and Sandman2011; Giesbrecht, Letourneau, & Campbell, Reference Giesbrecht, Letourneau and Campbell2017; Gutteling, de Weerth, & Buitelaar, Reference Gutteling, de Weerth and Buitelaar2004, Reference Gutteling, de Weerth and Buitelaar2005). Here we extend these findings by demonstrating a buffering role of postnatal social support on the association between prenatal cortisol exposure and infant HPA axis function. Specifically, at low levels of postnatal social support, a flatter maternal diurnal cortisol slope and a flatter CAR in early pregnancy, and higher total cortisol output in late pregnancy, were all associated with increased infant cortisol reactivity. No such associations were observed at higher levels of social support. These findings suggest that relationships not directly involving the infant can buffer the effects of maternal cortisol on infant HPA axis function.
Postnatal social support may buffer infant HPA axis reactivity by supporting the mother–infant relationship. Greater perceived social support has been associated with greater maternal sensitivity (Goldstein et al., Reference Goldstein, Diener and Mangelsdorf1996; Shin et al., Reference Shin, Park and Mi2006), which is an important predictor of infant behavioral and physiological responses to stress (Enlow et al., Reference Enlow, King, Schreier, Howard, Rosenfield, Ritz and Wright2014; Thomas, Letourneau, et al., Reference Thomas, Letourneau, Bryce, Campbell and Giesbrecht2017). Maternal sensitivity buffers infant distress to a stressful procedure, and this effect is noticeably stronger among infants exposed to maternal prenatal anxiety (Grant et al., Reference Grant, McMahon, Reilly and Austin2010), suggesting that the quality of the mother–infant relationship is a significant source of resilience. Similar findings have been observed for the negative association between prenatal cortisol exposure and infant cognitive development, which was greatly reduced by secure infant–mother attachment (Bergman, Sarkar, Glover, & O'Connor, Reference Bergman, Sarkar, Glover and O'Connor2010). In addition to supporting the mother–infant relationship, fathers who are perceived as more supportive social partners may also be more directly involved in parenting practices, which has beneficial effects on maternal well-being and sensitivity and on infant stress responsivity. For example, paternal involvement in caregiving is an important predictor of child social, behavioral, and psychological outcomes (Sarkadi, Kristiansson, Oberklaid, & Bremberg, Reference Sarkadi, Kristiansson, Oberklaid and Bremberg2008), and father negativity has been associated with greater increases in infant cortisol responses to emotional challenge at 7 months of age (Mills-Koonce et al., Reference Mills-Koonce, Garrett-Peters, Barnett, Granger, Blair and Cox2011).
Timing effects
The findings suggest that social support had different associations with components of the maternal HPA axis function in early and late pregnancy. Exposures to the maternal CAR and diurnal slope in early pregnancy were moderated by social support, whereas in late pregnancy it was only total cortisol exposure that was moderated by social support. Early pregnancy is when the infant HPA axis is forming and becoming functional (Gitau, Fisk, Teixeira, Cameron, & Glover, Reference Gitau, Fisk, Teixeira, Cameron and Glover2001). Therefore, the fact that it was the pattern (i.e., CAR and diurnal slope) of maternal cortisol secretion in early pregnancy, as opposed to the total amount of cortisol, which was moderated by social support, suggests that the pattern of maternal cortisol secretion may have relatively more salience to the postnatal organization of infant HPA axis function when postnatal maternal social support is low (i.e., the postnatal environment makes relatively few “adjustments” to infant HPA axis function). In late pregnancy, the maternal diurnal patterns may be more difficult for the fetus to detect because the maternal HPA axis is hyperactive in response to placenta secretion of CRH. Social buffering of the total maternal cortisol production in late pregnancy may therefore be an important resilience factor related to birth outcomes (e.g., length of gestation; Entringer, Buss, Andersen, Chicz-DeMet, & Wadhwa, Reference Entringer, Buss, Andersen, Chicz-DeMet and Wadhwa2011), which are themselves related to reactivity of the infant HPA axis. Taken together, these findings suggest that social support may operate in different ways at different points of pregnancy and on different aspects of maternal HPA axis function to influence development of the infant HPA axis.
Previous studies have observed timing effects of prenatal cortisol exposure on a variety of infant outcomes (Davis et al., Reference Davis, Glynn, Waffarn and Sandman2011; Davis & Sandman, Reference Davis and Sandman2010; Ellman et al., Reference Ellman, Dunkel Schetter, Hobel, Chicz-DeMet, Glynn and Sandman2008; Vedhara et al., Reference Vedhara, Metcalfe, Brant, Crown, Northstone, Dawe and Smith2012; Yehuda et al., Reference Yehuda, Engel, Brand, Seckl, Marcus and Berkowitz2005), and these findings indicate that timing of exposures may be an important determinant of their specific effects. Our findings are in agreement with these previous studies, but suggest that the susceptibility of the fetal HPA axis to maternal HPA axis function during pregnancy may be modified by postnatal social “recalibration” of the infant HPA axis. Furthermore, the different effects of social support in early and late pregnancy suggest that the time-specific effects of maternal cortisol on the infant HPA are not fixed during gestation but remain open to modification in the postnatal period. The adaptations made by the fetus in response to exposures appear to be “initial commitments” that can be revised based upon the social qualities of the postnatal environment. Although it is obviously not possible for postnatal social support to “go back” and reset HPA axis function, it may be possible for the social environment to modify the developmental decisions that were made during gestation through epigenetic changes within glucocorticoid receptors, which then alter HPA axis function. Such changes have been observed in rodent (Weaver et al., Reference Weaver, Cervoni, Champagne, D'Alessio, Sharma, Seckl and Meaney2004) and human studies (McGowan et al., Reference McGowan, Sasaki, D'Alessio, Dymov, Labonté, Szyf and Meaney2009)
Our findings highlight the importance of exposure timing in programming of infant HPA axis function. It is important to note, however, that our study time points characterizing early and late gestation are broad and therefore provide little specificity with regard to exposure timing of greatest relevance to infant HPA axis function. Nevertheless, our findings demonstrate the significance of examining not only overall levels of cortisol during pregnancy but also diurnal patterns of cortisol, as there appears to be differential programming effects of these cortisol indices in early versus late pregnancy. Future studies should attempt to further delineate the windows during which the fetal HPA axis may be most vulnerable to prenatal cortisol exposures and social buffering by measuring these associations with greater frequency during pregnancy, particularly very early in pregnancy. Denser sampling windows across pregnancy may help to identify sensitive periods during which interventions may be optimally effective in reducing the intergenerational transmission of stress.
Social buffering of the intergenerational transmission of ACEs
ACEs leave an enduring signature of stress within HPA axis physiology that persists into pregnancy (Bublitz et al., Reference Bublitz, Parade and Stroud2014; Bublitz & Stroud, Reference Bublitz and Stroud2012; Shea et al., Reference Shea, Streiner, Fleming, Kamath, Broad and Steiner2007), and this ongoing instantiation of early life experience in the HPA axis during pregnancy is a potential mechanism for the intergenerational transmission of stress. The mediator analyses conducted in the current study suggest that perceived social support during and after pregnancy modulates the effects of such transmission. We observed associations between maternal exposure to ACEs and increased infant cortisol reactivity through both the CAR and total daytime cortisol output, but these associations were conditional on social support. The association between ACEs and increased infant cortisol reactivity was mediated by increased maternal CAR in early pregnancy, but this mediation effect was significant only at higher levels of postnatal social support. In contrast, the mediated effect for total daytime cortisol was significant at low levels of prenatal and postnatal social support. In both cases, maternal exposure to ACEs was associated with increased infant cortisol reactivity, suggesting intergenerational transmission of maternal stress via the maternal HPA axis during pregnancy. This transmission, however, was apparent only at low (for total daytime cortisol output) and very high (for CAR) levels of postnatal social support. The latter finding was not expected, and as we discuss below, such findings encourage a broader view of the implications of increased stress reactivity.
Recent theoretical advancements in the conceptualization of stress reactivity suggest that greater reactivity is associated with bivalent outcomes and is neither bad nor good from a health and development perspective. According to biological sensitivity to context theory (Boyce & Ellis, Reference Boyce and Ellis2005), heightened stress reactivity reflects not only exaggerated arousal under challenge but more generally an increased biological sensitivity to context, with potential for negative health effects when combined with adversity but positive effects under conditions of support. For instance, infants scoring high on measures of negative emotionality (a purported developmental risk factor) have lower self-control at age 2 when the quality of mother–infant interaction is low, but greater self-control when the quality of mother–infant interaction is high, even when compared to children with less negativity as infants (Feldman, Greenbaum, & Yirmiya, Reference Feldman, Greenbaum and Yirmiya1999). This notion of biological sensitivity to context may help to explain why we observed that elevated infant cortisol reactivity was indirectly associated with maternal history of ACEs under conditions of both low and high levels of social support. It is possible that a maternal history of ACEs primes infant stress systems to be more vigilant and adaptable to the environment, meaning that these children have the potential for better or worse developmental outcomes depending on the quality of the environment. It may therefore be more useful to think of the intergenerational transmission of stress as increasing biological sensitivity to context, rather than as increasing developmental risk itself. The findings of the current study highlight that the social context regulates the way in which the underlying physiology translates into developmental outcomes. Based upon biological sensitivity theory, we predict that children born to mothers with early life adversity should have greater variability in outcomes, and those outcomes should be more strongly linked to the postnatal environment than the outcomes of children born to mothers without early life adversity. Such hypotheses can be tested in follow-up work with the current cohort.
Study strengths and limitations
This study has several strengths, including a relatively large sample size, the availability of cortisol data across gestation (i.e., from 6 to 36 weeks), and repeated assessment of women during pregnancy. To our knowledge, the current study is the first to evaluate social support as a buffer for the intergenerational transmission of ACEs via its impact on maternal HPA axis function during pregnancy and infant HPA axis function. Our prospective investigation of prenatal psychobiological mechanisms that are involved in the intergenerational transmission of stress is a unique and significant strength of the current study.
Nevertheless, there are several limitations that warrant consideration. First, the majority of participants in this study represent a relatively low sociodemographic risk sample of women as most participants were married or in common-law relationships, White, had university-level education, and had relatively high household annual income. As such, it may not be appropriate to generalize the findings to sociodemographically diverse or non-White populations. Despite the demographic homogeneity, a substantial proportion of women reported a history of at least one ACE (43.6%), which, consistent with other studies (e.g., Felitti et al., Reference Felitti, Anda, Nordenberg, Williamson, Spitz, Edwards and Marks1998), indicates that ACEs are pervasive, even among populations of low sociodemographic risk. Furthermore, the fact that the observed associations could be detected in a low sociodemographic risk sample demonstrates that the effects of ACEs on the maternal and infant HPA axes persist despite the apparent social and economic advantages of the women in this study. Nevertheless, the findings highlight the need to examine these associations in higher sociodemographic risk groups where the severity of ACEs is likely higher and the quality of the maternal social environment may be lower. Second, we did not enroll women who reported consuming tobacco, nonprescription drugs, or alcohol during pregnancy, which may have (a) excluded women with the least social support, as women in the general population who receive the least effective social support also tend to engage in the poorest health behaviors during pregnancy (Hobel, Goldstein, & Barrett, Reference Hobel, Goldstein and Barrett2008; Hoffman & Hatch, Reference Hoffman and Hatch1996); and (b) excluded women with exposure to more severe forms of early life adversity, given the well-documented associations between ACEs and poor health behaviors (Anda et al., Reference Anda, Croft, Felitti, Nordenberg, Giles, Williamson and Giovano1999; Dube, Anda, Felitti, Edwards, & Croft, Reference Dube, Anda, Felitti, Edwards and Croft2002; Dube et al., Reference Dube, Felitti, Dong, Chapman, Giles and Anda2003). Third, it is important to note that this study focused exclusively on male-to-female romantic partner support. Although partner support is thought to be one of the most significant sources of social support during adulthood and particularly during pregnancy (Rini et al., Reference Rini, Dunkel Schetter, Hobel, Glynn and Sandman2006), other sources of support such as family members may have more salience in other cultural contexts (Campos et al., Reference Campos, Dunkel Schetter, Abdou, Hobel, Glynn and Sandman2008). Fourth and finally, this study utilized cortisol, the end product of the HPA axis, as an indicator of HPA axis function during pregnancy. Although this is a reasonable approach, it neglects other biomarkers of HPA axis function (such as adrenocorticotropic hormone or CRH), which could lend insight into specific aspects of HPA axis function that are associated with exposure to ACEs and buffering by social support. Further study is needed specifically with regard to placental CRH to determine what role the placenta may play in transducing the effects of ACEs and social support to the fetus.
Conclusion
In summary, we report data showing that maternal HPA axis function during pregnancy mediates the effects of maternal early life adversity on infant cortisol reactivity and that this cascade was moderated by social support. The data provide evidence supporting a direct biological mechanism for the intergenerational transmission of stress that may be in addition to social learning processes (e.g., the intergenerational continuity of harsh and abusive parenting; Conger et al., Reference Conger, Schofield, Neppl and Merrick2013). Furthermore, we show how social processes may also serve as a buffer to the biological cascade from mother to infant. These findings contribute to a growing evidence base that increasingly underscores the importance of early intervention efforts for children exposed to ACEs and the value of interventions that include a physiological component (Graham et al., Reference Graham, Yockelson, Kim, Bruce, Pears and Fisher2012).