Childhood adversity and anxiety symptoms are associated with a greater risk of developing internalizing disorders, especially anxiety disorders (Hirshfeld-Becker, Micco, Simoes, & Henin, Reference Hirshfeld-Becker, Micco, Simoes and Henin2008; Teicher & Samson, Reference Teicher and Samson2013). Harsh parenting (i.e., hostile, cold, rejection, critical and coercive child-rearing behaviors), though a milder form of adversity, is closely related to high levels of anxiety and worry in childhood (Brown & Whiteside, Reference Brown and Whiteside2008; McLeod, Wood, & Weisz, Reference McLeod, Wood and Weisz2007). Given the significant prevalence of harsh parenting practices in the absence of severe maltreatment and of subclinical anxiety symptoms among youth (Wood, McLeod, Sigman, Hwang, & Chu, Reference Wood, McLeod, Sigman, Hwang and Chu2003), understanding neural correlates of these two risk factors concurrently may have significant public health relevance. The present study aimed to examine how enduring harsh parenting in the absence of severe maltreatment and subclinical anxiety symptoms relate, both independently and in concert, to brain structure in youth.
Several past studies in youth with a history of severe childhood adversity (e.g., physical, emotional, sexual abuse; physical, emotional neglect; institutionalization; domestic violence; trauma, early-life stress, all without posttraumatic stress disorder [PTSD]), have demonstrated lower prefrontal cortex (including orbitofrontal and anterior cingulate cortex) volume and thickness compared to youth unexposed to severe childhood adversity (Baker et al., Reference Baker, Williams, Korgaonkar, Cohen, Heaps and Paul2012; De Brito et al., Reference De Brito, Viding, Sebastian, Kelly, Mechelli, Maris and McCrory2013; Hanson et al., Reference Hanson, Chung, Avants, Shirtcliff, Gee, Davidson and Pollak2010; Hodel et al., Reference Hodel, Hunt, Cowell, Van Den Heuvel, Gunnar and Thomas2015; Kelly et al., Reference Kelly, Viding, Wallace, Schaer, De Brito, Robustelli and Mccrory2013; McLaughlin et al., Reference McLaughlin, Sheridan, Winter, Fox, Zeanah and Nelson2014). Lower prefrontal cortex volume and anterior cingulate cortex volume and thickness have also been associated with higher trauma levels (Edmiston et al., Reference Edmiston, Wang, Mazure, Guiney, Sinha, Mayes and Blumberg2011; Korgaonkar et al., Reference Korgaonkar, Antees, Williams, Gatt, Bryant, Cohen and Grieve2013). Amygdala volume was smaller in youth with a history of childhood adversity, compared to youth without such a history (Edmiston et al., Reference Edmiston, Wang, Mazure, Guiney, Sinha, Mayes and Blumberg2011; Hanson et al., Reference Hanson, Nacewicz, Sutterer, Cayo, Schaefer, Rudolph and Davidson2015; Korgaonkar et al., Reference Korgaonkar, Antees, Williams, Gatt, Bryant, Cohen and Grieve2013). To our knowledge, no study has looked at the anatomy of amygdala and prefrontal cortex in youth who have experienced coercive parenting practices in the absence of severe maltreatment. Nevertheless, extant studies point to possible reductions in prefrontal cortical volume and cortical thinning, as well as amygdala volume reduction, that may be present in youth who have experienced high levels of persistent coercive parenting practices during childhood.
In youth with, compared to without, anxiety disorders, prefrontal, orbitofrontal, or anterior cingulate cortical volumes did not differ (De Bellis et al., Reference De Bellis, Keshavan, Shifflett, Iyengar, Dahl, Axelson and Ryan2002; Liao et al., Reference Liao, Yang, Zhang, He, Song, Jiang and Li2013, Reference Liao, Yang, Zhang, He, Su and Li2014; Milham et al., Reference Milham, Nugent, Drevets, Dickstein, Leibenluft, Ernst and Pine2005; Mueller et al., Reference Mueller, Aouidad, Gorodetsky, Goldman, Pine and Ernst2013). However, orbitofrontal (Jones et al., Reference Jones, Jackson, Chambers, Dabbs, Hsu, Stafstrom and Hermann2015) and rostral anterior cingulate (Suffren, Chauret, Nassim, Lepore, & Maheu, Reference Suffren, Chauret, Nassim, Lepore and Maheu2019) cortex thinning have been observed in youth with anxiety disorders. Further, in youth without any anxiety disorders, but with at least one parent with an anxiety disorder, we have previously reported rostral anterior cingulate cortex thinning compared to youth with no parent with an anxiety disorder (Suffren et al., Reference Suffren, Chauret, Nassim, Lepore and Maheu2019). Others have reported increased ventromedial prefrontal cortex thickness (including prefrontal cortex and rostral anterior cingulate cortex) with increased anxiety levels (Ducharme et al., Reference Ducharme, Albaugh, Hudziak, Botteron, Nguyen, Truong and Karama2013). In addition, amygdala volume is more often reported as smaller (Milham et al., Reference Milham, Nugent, Drevets, Dickstein, Leibenluft, Ernst and Pine2005; Mueller et al., Reference Mueller, Aouidad, Gorodetsky, Goldman, Pine and Ernst2013; Suffren et al., Reference Suffren, Chauret, Nassim, Lepore and Maheu2019); but in some studies observed to be greater (De Bellis et al., Reference De Bellis, Casey, Dahl, Birmaher, Williamson, Thomas and Ryan2000; Jones et al., Reference Jones, Jackson, Chambers, Dabbs, Hsu, Stafstrom and Hermann2015) or no different (Liao et al., Reference Liao, Yang, Zhang, He, Song, Jiang and Li2013, Reference Liao, Yang, Zhang, He, Su and Li2014), in pediatric anxiety disorders, compared to youth without a psychiatric disorder. In youth without anxiety disorder, anxiety symptoms (Juranek et al., Reference Juranek, Filipek, Berenji, Modahl, Osann and Spence2006; Qin et al., Reference Qin, Young, Duan, Chen, Supekar and Menon2014) and exposure to maternal depressive symptomatology (Lupien et al., Reference Lupien, Parent, Evans, Tremblay, Zelazo, Corbo and Séguin2011) seem to be linked to increased amygdala volume. Thus, we expect that subclinical anxiety symptoms in youth will be linked to greater prefrontal cortex thickness and greater amygdala volume.
It is important to keep in mind that we cannot infer causality, either in reported literature reviews or in this study. Observed cerebral differences may be consequences of experienced parenting practices and anxiety. However, it is also possible that brain differences are risk markers for both a tendency in children to be more difficult, in parents to become more severe, and in parents and children to be more anxious. In studies previously reported, we did not know if the youth have experienced anxiety and coercive parenting practices, respectively. It is unclear whether anatomical differences reported in the extant literature are directly associated with experienced adversity or anxiety (Bremner et al., Reference Bremner, Vythilingam, Vermetten, Southwick, McGlashan, Nazeer and Charney2003; Kitayama, Quinn, & Bremner, Reference Kitayama, Quinn and Bremner2006; Weniger, Lange, Sachsse, & Irle, Reference Weniger, Lange, Sachsse and Irle2008). The lack of comparison groups of participants with a history of adversity but who do not have anxious symptoms, or who conversely have anxious symptoms without a history of adversity, limits the ability to determine whether observed cerebral differences are linked more specifically to adversity or anxiety (Hart & Rubia, Reference Hart and Rubia2012; McCrory, De Brito, & Viding, Reference McCrory, De Brito and Viding2011). An interaction between parental practices and subclinical anxiety symptoms is particularly interesting considering that these two factors are both important risk factors to develop anxiety disorders later in life.
Importantly, there has been no research on the link between brain anatomy and parental practices and subclinical anxiety symptoms which persist over the course of childhood, specifically between the ages of 2.5 and 9 years. A design in which youth are prospectively followed every year for 7 years allows for identification of youth with distinctive and persistent harsh parenting and anxiety experience over time. Moreover, few studies have examined the association between harsh parenting practices in the absence of severe maltreatment and brain structure anatomy (Merz, Maskus, Melvin, He, & Noble, Reference Merz, Maskus, Melvin, He and Noble2019). Understanding these associations could: (a) provide insights on neurodevelopmental corelates of parental practices and subclinical anxiety symptoms; (b) raise awareness about the importance of reducing the use of coercive parenting practices in the absence of severe maltreatment; (c) implement effective family-based interventions for anxiety symptoms in the context of harsh parenting at a young age; (d) develop interventions that are informed by neural alterations as well as symptoms. If youth with one or more risk factors to develop anxiety disorders have anatomical brain differences similar to those seen in youth who already have anxiety disorders, interventions that reduce anxiety symptoms that also decrease the impact of the risk factors might reduce brain differences. The goal of this study was to investigate the association between harsh parenting, in the absence of severe maltreatment, and subclinical anxiety symptoms, measured yearly from 2.5 to 9 years old. We aimed to study how harsh parenting, in the absence of severe maltreatment, how subclinical anxiety symptoms, and how the interaction between these two factors, are associated with the prefrontal cortical and amygdala anatomy in 12–16 years-old youth who had no history of psychopathology or medication exposure at the time of magnetic resonance imaging (MRI).
Material and Method
Participants
Sample
Participants were recruited in two related cohorts from two prospective longitudinal studies: in 2001, I was 5 years old (Jetté, Desrosiers, & Tremblay, Reference Jetté, Desrosiers and Tremblay1998) and The Quebec Longitudinal Study of Children's Development (Jetté & Des Groseilliers, Reference Jetté and Des Groseilliers2000). The total sample of 2,174 youths born in Quebec, Canada, between 1996 and 1998 (children from the far north, Cree or Inuit regions, and aboriginal reservations excluded) were followed since birth. Youth were evaluated yearly between 2.5 and 9 years old (at 2.5, 3.5, 4, 5, 6, 7, 8, 9 years old). For the purpose of the current study, we narrowed down the cohort to 1,761 possible participants after selecting those for whom data on their anxiety symptoms profile and their parents’ harsh parenting practices were collected at least three times from the time they were 2.5 years old until 9 years old. At least one of those measures had to be taken from one of the last two times they were evaluated, at age 8 or 9 years old.
Measures evaluated over time
Items evaluating youth subclinical anxiety symptoms were selected from the Child Behavior Checklist’ anxious/depressed and emotionally reactive subscales (Achenbach, Reference Achenbach1991). Three items were selected: the child “is nervous, high strung or tense,” “appears fearful or anxious,” “appears worried.” Mothers indicated on a two-point scale ranging from 0 (never) to 2 (often) if their child exhibited these symptoms.
Harsh parenting practices were assessed by the “Hostile/Ineffective” scale, used in a Canadian national longitudinal study of children and youth (Boyle et al., Reference Boyle, Jenkins, Georgiades, Cairney, Duku and Racine2004; StatisticsCanada, 1995) as the “Parental Cognitions and Conduct Toward the Infant” scale (Boivin et al., Reference Boivin, Pérusse, Dionne, Saysset, Zoccolillo, Tarabulsy and Tremblay2005). Items evaluating harsh parenting practices included: “how often do you get angry when you punish your child?”; “in the last 12 months, how often did you spank your child when he/she was difficult?”; “in the last 12 months, when your child broke the rules or did things he/she was not supposed to do, how often did you raise your voice, scold or yell at him/her?”; “in the last 12 months, when your child broke the rules or did things he/she was not supposed to do, how often did you use physical punishment (e.g., shaking)?”. Mothers indicated on a four-point scale ranging from 0 (never) to 4 (always) to what extent given statements described their parenting practices.
The coefficients of internal consistency (which indicate the interrelatedness between items supposedly measuring the same concept) for the child subclinical anxiety symptoms items were weaker at very young ages, and fair to satisfactory from 5 to 9 years old (0.5, 0.6, 0.6, 0.7, 0.7, 0.8, 0.7, 0.8 at 2.5, 3.5, 4, 5, 6, 7, 8, and 9 years old, respectively). Coefficients of internal consistency followed a reversed pattern for maternal harsh parenting practices (0.7, 0.7, 0.6, 0.6, 0.6, 0.5, 0.6, 0.6 at 2.5, 3.5, 4, 5, 6, 7, 8, and 9 years old, respectively). To further demonstrate the interrelatedness among the anxiety items, and among the harsh parenting items, a set of correlations was run between the three anxiety items, and another set was run between the four maternal harsh parenting behavior items, at every time point (2.5, 3.5, 4, 5, 6, 7, 8, and 9 years old). For each time point, youth anxiety symptoms items were correlated (r between .2 and .9, p < .05), and the maternal harsh parenting practices items were also correlated (r between .2 and .9, p < .05). Based on these items, we used an empirically based method that identifies groups of children who follow similar developmental patterns over time (Nagin, Reference Nagin2005) to divide youth among four cells of interest: youth with high levels of harsh parenting and anxiety symptoms, youth with high levels of harsh parenting and low levels of anxiety symptoms, youth with low levels of harsh parenting and high levels of anxiety symptoms, and youth with low levels of harsh parenting and anxiety symptoms, all assessed over time. Details regarding design and procedure of the study are described elsewhere (La Buissonnière-Ariza et al., Reference La Buissonnière-Ariza, Séguin, Nassim, Boivin, Pine, Lepore and Maheu2019).
Assignation to the four cells of this study
We used a developmental trajectory methodology to distribute the 1,761 youths across the four clusters of interest of this study (see Figure 1). This is an empirically based method that identifies groups of children who follow similar developmental patterns over time. A semi-parametric, group-based mixture model was computed using all available data points across time. The model assigns individuals to trajectory groups on the basis of a posterior probability rule (Nagin, Reference Nagin2005). Developmental trajectories were estimated based on youth anxiety symptoms (as evaluated by their mothers) and maternal harsh parenting practices collected when youths were 2.5, 3.5, 4, 5, 6, 7, 8, and 9 years old.

Figure 1. Developmental trajectories groups for the (a) youth anxiety symptoms and (b) maternal harsh parenting practices (N = 1761). Note. The curves represent the values predicted by the model, while the dots represent the values observed for each participant. All trajectories follow a cubic model. The percentages in the legend correspond to the predicted percentage of youths in each group.
The best-fitting model, that is, the model which minimized the Bayesian information criterion (BIC) (Nagin, Reference Nagin2005), for both youth anxiety symptoms and maternal harsh parenting practices was a two-group (high vs. low) solution model. Formation of these two groups (high vs. low) was based on the posterior probability of group membership, that is, on the probability an individual has of belonging to a trajectory group based on observations across assessments. Figure 1a shows the developmental trajectory groups for the youth anxiety symptoms: one group included youths with moderate rates of anxiety symptoms at 2.5 years old, which increased substantially over time, and plateau around the age of 6; the other group included youths with low rates of anxiety symptoms at 2.5 years old, which also increased gradually over time. The average posterior probabilities for the assigned trajectory groups were 0.91 for the moderate-rising anxiety symptoms group, and 0.90 for the low-rising anxiety symptoms group, thereby indicating a good fit of the model (Nagin, Reference Nagin2005). Demonstrating the relevance of the estimated model, the continued increase in anxiety levels observed from 2.5 to 9 years old fits well with previous developmental trajectory results from two population-based longitudinal studies, this Quebec sample showing increases in the level of anxiety symptoms from 1.5 to 5 years old (Côté et al., Reference Côté, Boivin, Liu, Nagin, Zoccolillo and Tremblay2009), and one in the Netherlands showing that the frequency of anxiety increases from 4 to 15 years of age (Bongers, Koot, Van der Ende, & Verhulst, Reference Bongers, Koot, Van Der Ende and Verhulst2003). Figure 1b shows the developmental trajectory groups for maternal harsh parenting practices: one group included youths submitted to moderate-high rates of harsh parenting at 2.5 years old, which moderately decreased over time; the other group included youths submitted to low rates of harsh parenting at 2.5 years old, which decreased less over time. The average posterior probabilities for the assigned trajectory groups were 0.90 for the moderate-high decreasing maternal harsh parenting practices group, and 0.90 for the low-decreasing maternal harsh parenting practices group, thereby indicating a good fit of the model (Nagin, Reference Nagin2005). Demonstrating the relevance of the estimated model, elevated maternal harsh parenting practices towards children of 2.5 years old were recently reported in two population-based longitudinal studies (Forget-Dubois et al., Reference Forget-Dubois, Boivin, Dionne, Pierce, Tremblay and Pérusse2007; Pierce et al., Reference Pierce, Boivin, Frenette, Forget-Dubois, Dionne and Tremblay2010). Moreover, recent findings demonstrated that hostile parenting practices diminished over time, from middle childhood to mid-adolescence, in the general population (Forget-Dubois et al., Reference Forget-Dubois, Boivin, Dionne, Pierce, Tremblay and Pérusse2007; Pierce et al., Reference Pierce, Boivin, Frenette, Forget-Dubois, Dionne and Tremblay2010).
Finally, further validating the distinct anxiety symptoms and harsh parenting trajectories, a repeated measures analysis of variance (ANOVA) with groups (high vs. low anxiety levels) as the between-subjects factor, and time (2.5, 3.5, 4, 5, 6, 7, 8, and 9 years old) as the within-subjects factor, shows that anxiety levels were significantly higher in the high-anxiety group compared to the low-anxiety group at every time point, independently of harsh parenting levels (p < .05). Similarly, an ANOVA with harsh parenting as the between-groups factor (high vs. low harsh parenting levels) revealed that harsh parenting practices were significantly greater in the high-harsh parenting group, compared to low-harsh parenting group, independently of anxiety levels (p < .05).
Developmental trajectories for maternal harsh parenting practices and youth anxiety symptoms were then estimated jointly (using a cross trajectory model) to get an accurate estimation of the proportion of youths in all four groups of interest (see Figure 2). Based on the anxiety levels and parenting practices, the model also gives us the probability of the participants to belong to each one of the four groups. The average posterior probabilities for the assigned trajectory groups were 0.83 for the low harsh parenting–low anxiety group, 0.79 for the high harsh parenting–low anxiety group, 0.77 for the low harsh parenting–high anxiety group, and 0.85 for the high harsh parenting–high anxiety group, thereby indicating a good fit of the model (Nagin, Reference Nagin2005). Youth with the highest probability of belonging to any of these respective four groups were invited to participate in the study first, until each group has reached the target number of 30 participants to perform MRI. A total of 234 families were contacted. Of these, 122 refused to participate and six left the current study. Twelve participants were excluded because they had comorbidities that could affect brain (four had attention-deficit/hyperactivity disorder (ADHD), one had severe dyslexia with central hearing disorder, four had anxiety disorders, one had major depression, and one had consumption disorder). The remaining 94 participants completed the study and MRI (see Figure 2). Study protocol was approved by the CHU Sainte-Justine and IUGM Research Ethics Boards, Montreal, Canada. Participants and their parents gave informed assent and consent, respectively, and were compensated for their participation.

Figure 2. Selection and number of participants at each stage of the research.
Inclusion/exclusion criteria and measures at the time of MRI
MRI was performed only once when the youth were between 12 and 16 years old, in the remaining 94 participants from the birth cohort. Inclusion criteria were defined by absence of medical illness; any past or current psychiatric disorders, as determined by a semistructured psychiatric evaluation conducted with Kiddie Schedule for Affective Disorders and Schizophrenia (Kaufman et al., Reference Kaufman, Birmaher, Brent, Rao, Flynn, Moreci and Ryan1997); treatment for psychiatric illness (pharmacological or behavioral); past or current abuse; past head injury or trauma; contraindications for MRI (e.g., braces); IQ score < 70, as assessed by Peabody Picture Vocabulary Test – Revised (Dunn & Dunn, Reference Dunn and Dunn1981). The socioeconomic status (SES) of each participant was also measured and assessed using Hollingshead two-factor index scale (Hollingshead & Redlich, Reference Hollingshead and Redlich1958; Miller & Salkind, Reference Miller and Salkind2002) and three components (parental education, employment, and income); pubertal stage assessed using Tanner puberty stage self-administered scale (Duke, Litt, & Gross, Reference Duke, Litt and Gross1980; Morris & Udry, Reference Morris and Udry1980). Current anxiety symptoms were measured through the Screen for Child Anxiety Related Emotional Disorders – Revised (Martin & Gosselin, Reference Martin and Gosselin2012). The parenting questionnaire used to evaluate current harsh parenting practices was the same as the one used to determine harsh parenting trajectories from 2.5 to 9 years old (Boivin et al., Reference Boivin, Pérusse, Dionne, Saysset, Zoccolillo, Tarabulsy and Tremblay2005; Boyle et al., Reference Boyle, Jenkins, Georgiades, Cairney, Duku and Racine2004; Pierce et al., Reference Pierce, Boivin, Frenette, Forget-Dubois, Dionne and Tremblay2010).
Image acquisition, processing, and analysis
Structural neuroimaging was performed for 94 healthy youth from the birth cohort without anxiety disorder (12–16 years old) at the Geriatric University Institute of Montreal (IUGM, Montreal, Canada). All scans were performed on a 3 Tesla MRI scanner (Magnetom Tim Trio, Siemens) equipped with a standard head coil. Whole-brain, high-resolution, T1-weighted anatomical images were acquired using a MPRAGE sequence (repetition time [TR] = 2300 ms, echo time [TE] = 2.98 ms, flip angle = 9°, matrix size = 256 × 256 mm, voxel size = 1 × 1 × 1 mm3, MRI - field of view [FOV] = 256 mm, 176 slices). Two-dimensional DICOM files of each brain were organized into volumetric three-dimensional files using MRIcron software package (http://www.mccauslandcenter.sc.edu/mricro/mricron/). After removing participants with motion artifacts (ghosting, blurring), a total of 88 youths were included in analyses: 21 High harsh parenting/High anxiety symptoms youth; 22 High harsh parenting/Low anxiety symptoms youth; 23 Low harsh parenting/High anxiety symptoms youth; and 22 Low harsh parenting/Low anxiety symptoms youth.
Voxel-based morphometry (VBM) processing and analysis (Ashburner, Reference Ashburner2009; Ashburner & Friston, Reference Ashburner and Friston2000) were performed using SPM8 (Wellcome Department of Imaging Neuroscience, London, UK; http://www.fil.ion.ucl.ac.uk/spm/software/spm8) and MATLAB 7.10.0 (R2009a; MathWorks, Natick, MA, USA). All T1-weighted anatomical images were manually reoriented to place the anterior commissure (AC) at the origin of the three-dimensional Montreal Neurological Institute (MNI) space. The images were then segmented into gray matter, white matter, and cerebrospinal fluid (Ashburner & Friston, Reference Ashburner and Friston2005). These segmentations were then inspected for their quality. A diffeomorphic nonlinear registration algorithm (diffeomorphic anatomical registration through exponentiated lie algebra toolbox – DARTEL) (Ashburner, Reference Ashburner2007) was used to spatially normalize the segmented images and generate a study-specific brain template. The resulting images were then spatially normalized into the MNI space; normalized, and warped to an isotropic voxel size of 1.5 mm × 1.5 mm × 1.5 mm; modulated by the Jacobian transformed tissue probability maps (to obtain volume differences rather than concentration differences in gray matter) and then smoothed with an 10-mm full-width half-maximum (FWHM) isotropic Gaussian kernel.
FreeSurfer (version 5.1; http://surfer.nmr.mgh.harvard.edu), a fully automated surface-based pipeline, was used to assess cortical thickness (Koolschijn & Crone, Reference Koolschijn and Crone2013; Raznahan et al., Reference Raznahan, Shaw, Lalonde, Stockman, Wallace, Greenstein and Giedd2011; Wierenga, Langen, Oranje, & Durston, Reference Wierenga, Langen, Oranje and Durston2014). T1 images were processed into a common stereotactic space, in which cortical thickness values can be derived on a participant-by-participant basis (Fischl, Reference Fischl2012). Cortical segmentation procedure involves the assignment of a neuroanatomical label to each voxel in a MRI volume using voxel intensity, a probabilistic atlas estimated from a manually labeled training set, and Bayesian classification rules (Fischl et al., Reference Fischl, Salat, Busa, Albert, Dieterich, Haselgrove and Dale2002). This technique was previously shown to be comparable in accuracy to manual tracing (Fischl et al., Reference Fischl, Salat, Busa, Albert, Dieterich, Haselgrove and Dale2002; Morey et al., Reference Morey, Petty, Xu, Pannu Hayes, Wagner Ii, Lewis and McCarthy2009). Cortical thickness was automatically quantified within FreeSurfer on a vertex-by-vertex basis by computing the average shortest distance between the white matter boundary and the pial surface (i.e., the cerebral spinal fluid boundary) at each point on the cortex (Fischl & Dale, Reference Fischl and Dale2000).
Based on a priori hypotheses of associations between gray matter volume and thickness, and early-life adversity and anxiety, in two regions (prefrontal cortices, including orbitofrontal cortex and rostral anterior cingulate cortex) and amygdala, we adopted an independent region of interest (ROI) approach. For VBM analyses, three ROIs were defined using the automated anatomical labeling (AAL) WFU Pick-Atlas toolbox: bilateral prefrontal cortex (including orbitofrontal cortex and anterior cingulate cortex), right and left amygdala. For VBM ROI analysis, we corrected for multiple comparisons using small-volume correction (SVC) in each ROI, with a Gaussian random field threshold set at α = 0.05, corrected, and an extent of at least 10 contiguous voxels (see Mueller et al., Reference Mueller, Aouidad, Gorodetsky, Goldman, Pine and Ernst2013). Significant peak voxels (p < .05, family-wise error rate [few]-corrected) were extracted and analyzed with SPSS v.20 (Armonk, NY) (see Mueller et al., Reference Mueller, Aouidad, Gorodetsky, Goldman, Pine and Ernst2013). For FreeSurfer analysis, cortical thickness of rostral anterior cingulate cortex and of medial and lateral orbitofrontal cortex were extracted and analyzed with SPSS v.20 (Armonk, NY). Moreover, a whole-brain VBM analysis was performed for the reader's interest, but was not discussed. For whole-brain VBM analysis, we corrected for multiple comparisons using small-volume correction, with a Gaussian random field threshold set at α = 0.05, corrected, and an extent of at least 10 contiguous voxels. Significant peak voxels (p < .05, Family-Wise Error correction (FWE)-corrected) were reported.
A 2×2 analysis of covariance (ANCOVA) with harsh parenting (high vs. low) and anxiety symptoms (high vs. low) levels as the between-subjects factors, and age, sex, SES, and total brain volume as covariates of no interest, was performed in SPSS v.20 (Armonk, NY). Bonferroni's corrections for multiple comparisons were applied with a significance level of p < .01 (p < .05/5 number of comparisons) to cortical and subcortical volume and thickness (orbitofrontal cortex volume, medial, and lateral orbitofrontal cortex thickness, rostral anterior cingulate cortex thickness, and amygdala volume). When an Anxiety×Parenting interaction was significant, findings were decomposed using pairwise comparisons with a Bonferroni correction for multiple comparisons in SPSS v.20 (Armonk, NY). For all significant results, we calculated and reported effect sizes (Cohen's partial η²) (Cohen, Reference Cohen1973) and post hoc power (calculated using G*Power 3.1) (Faul, Erdfelder, Lang, & Buchner, Reference Faul, Erdfelder, Lang and Buchner2007) in addition to p values.
Results
Participants
As we can see in Table 1, groups differed significantly in age and SES. Although there were no significant group differences in terms of sex and total brain volume, these variables were used as covariates of no interest in cerebral analysis, with age and SES, to ensure that they did not account for any of the findings, as these variables were previously reported to be linked with brain anatomy (Burgaleta, Johnson, Waber, Colom, & Karama, Reference Burgaleta, Johnson, Waber, Colom and Karama2014; Casey, Jones, & Somerville, Reference Casey, Jones and Somerville2011; Hanson et al., Reference Hanson, Hair, Shen, Shi, Gilmore, Wolfe and Pollak2013; Welborn et al., Reference Welborn, Papademetris, Reis, Rajeevan, Bloise and Gray2009). IQ was not included as a covariate to avoid over-correction because of its significant correlation with SES (r =.26, p = .02). Analyses were performed with and without IQ as covariate, and results were the same. Brain differences were also the same with parental education levels instead of Hollingshead two factors index as a covariate to represent SES.
Table 1. Demographic and clinical characteristics of youth, separately for high and low harsh parenting practices, and high and low anxiety levelsa

Note: N, number of participants; PPVT-R, Peabody Picture Vocabulary Test – Revised; SES, socioeconomic status; SCARED-R, Screen for Child Anxiety Related Emotional Disorders – Revised; A, Main effect of anxiety; H, Main effect of Harsh parenting; A×H, interaction between anxiety and harsh parenting levels.
a Means and standard deviations are reported.
b Chi-squares for quantitative measures.
c Two-way ANOVAs with harsh parenting (high vs. low) and anxiety (high vs. low) levels as the between-subjects factors.
d Hollingshead 2 factors index: higher score corresponds to lower socioeconomic status
e TBV was calculated as the sum of the volumes of gray matter and white matter.
f Participants High harsh parenting practices/High anxiety were significantly older than the High harsh parenting practices/Low anxiety (F 1,84 = 5.37, p = .023) and Low harsh parenting practices/High anxiety participants (F 1,84 = 4.90, p = .03).
As expected, the ANOVAs comparing current harsh parenting and anxiety levels revealed that current levels of harsh parenting was significantly higher in the high harsh parenting relative to the low harsh parenting adolescents (p = .001) and that current anxiety levels, as measured by the Screen for Child Anxiety Related Emotional Disorders – Revised (parent ratings), were higher in the high anxiety compared to the low anxiety adolescents (p = .008). No differences between high anxiety and low anxiety adolescents were observed when analyzing the child scores collected using the Screen for Child Anxiety Related Emotional Disorders – Revised (child ratings). It is important to note that youth anxiety symptoms levels (parent ratings) were not correlated to harsh parenting practices levels (r = .1, p = .32).
Main effect of parenting practices
Regions of interest (VBM volumes and FreeSurfer cortical thickness)
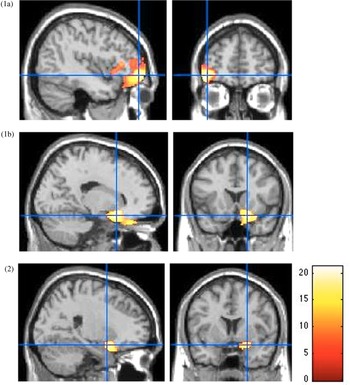
As shown in Figure 3, VBM analysis revealed smaller orbitofrontal cortex volumes in the right medial part [18, 16.5, −18] (F 1,80 = 23.28, p < .001, FWE-corrected, k = 299, partial η² = 0.23, power = 0.99) and left lateral part [−42, 52.5, −9] (F 1,80 = 33.52, p < .001, FWE-corrected, k = 1956, partial η² = 0.30, power = 0.99), and smaller right amygdala volume [21, 4.5, −18] (F 1,80 = 15.69, p < .001, FWE-corrected, k = 105, partial η² = 0.16, power = 0.98) in youth with high levels of harsh parenting over time, relative to low scorers on harsh parenting dimension.
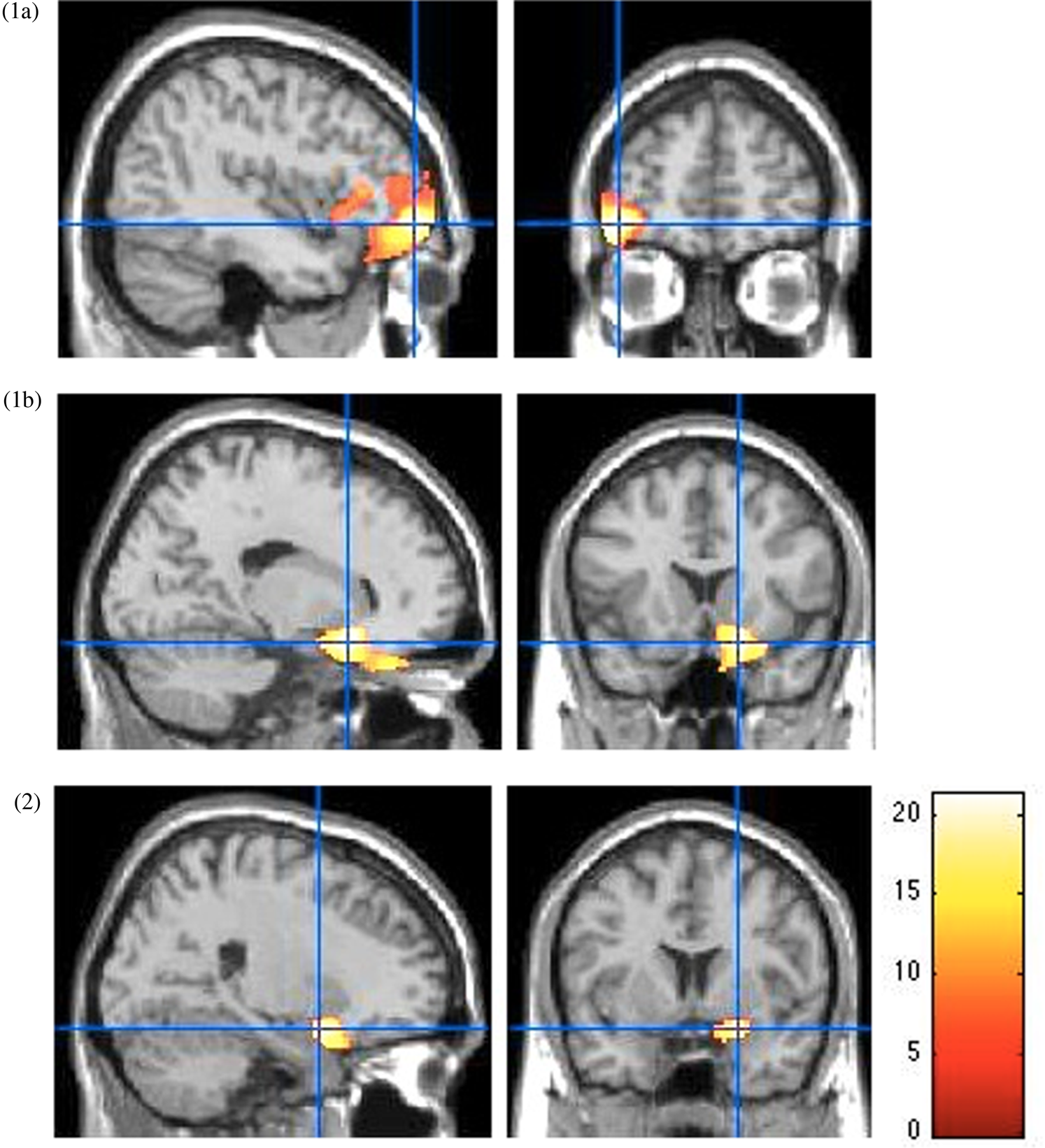
Figure 3. Smaller prefrontal cortex and amygdala volumes in high, compared to low scorers on the harsh parenting dimension. Main effect of parenting for the lateral orbitofrontal cortex (1a), the medial orbitofrontal cortex (1b) and the amygdala (2) from the voxel-based morphometry (VBM) analysis. All p < .05, family-wise error rate (few)-corrected at a peak level. Color bar shows F statistics. Results are displayed on a Montreal Neurological Institute (MNI) T1 brain template, at a threshold of p < .005.
Whole-brain analysis (VBM volume)
Whole-brain VBM analyses revealed one clusters, in the left lateral orbitofrontal cortex, significantly smaller in youth with high levels of harsh parenting over time, relative to low scorers on harsh parenting dimension: [−42, 53, −9] (F 1,80 = 33.33, p = .004, FWE-corrected, k = 49399, partial η² = 0.31, power = 0.99).
Main effect of subclinical anxiety symptoms
There were no significant results related to subclinical anxiety levels.
Parenting by anxiety symptoms interaction
Regions of interest (VBM volumes and FreeSurfer cortical thickness)
In VBM analysis, a Parenting×Anxiety interaction emerged in left amygdala [-28.5, −6, −15] (F 1,80 = 12.13, p < .001, FWE-corrected, k = 303, partial η² = 0.23, power = 0.99). In youth with low subclinical anxiety symptoms levels, high harsh parenting practices levels were associated with smaller amygdala volume (F 1,80 = 7.31, p < .01). In youth with high subclinical anxiety symptoms levels, high harsh parenting practices levels were associated with greater amygdala volume (F 1,80 = 7.31, p < .01). In youth with low harsh parenting levels, high subclinical anxiety symptoms levels were associated with smaller amygdala volume (F 1,80 = 5.16, p < .05). In youth with high harsh parenting levels, high subclinical anxiety symptoms levels were associated with greater amygdala volume (F 1,80 = 5.16, p < .05) (see Figure 4).

Figure 4. Significant Parenting×Anxiety interactions for (a) left rostral anterior cingulate cortex (ACC) thickness from FreeSurfer analysis and (b) left amygdala volume from voxel-based morphometry (VBM) analysis. * p < .05, family-wise error rate (few)-corrected at a peak level for VBM results and Bonferroni corrected for FreeSurfer and VBM analysis. Color bar shows F statistics. Results are displayed on a Montreal Neurological Institute (MNI) T1 brain template, at a threshold of p < .005 for VBM results. Anx, Anxiety; HP, Harsh parenting.
FreeSurfer cortical thickness measurements revealed a Parenting×Anxiety interaction in the left rostral anterior cingulate cortex thickness (F 1,80 = 6.47, p = .01, partial η² = 0.08, power = 0.78). In youth with low subclinical anxiety symptoms levels, high harsh parenting practices levels were associated with greater anterior cingulate cortex thickness (F 1,80 = 3.9, p = .05); the association was nonsignificant for youth with high subclinical anxiety symptoms levels. In youth with low harsh parenting levels, high subclinical anxiety symptoms levels were associated with greater anterior cingulate cortex thickness (F 1,80 = 12.26, p = .001); the association was nonsignificant for youth with high harsh parenting levels (see Figure 4).
Whole-brain analysis (VBM volume)
There were no significant whole-brain volume differences concerning Parenting×Anxiety interaction.
Discussion
This study compared gray matter volume and cortical thickness in healthy youth (12–16 years old) who experienced high or low harsh parenting practices over time and high or low subclinical anxiety symptoms levels over time, between 2.5 and 9 years old. The key findings from this study are the smaller prefrontal cortex and amygdala volumes among youth who have experienced early high levels of harsh parenting over time during childhood, compared to those who have not. The second most important findings are significant interactions between harsh parenting and subclinical anxiety symptoms levels for amygdala volume and rostral anterior cingulate cortex thickness. Youth with high subclinical anxiety symptoms, compared to youth with low subclinical anxiety symptoms, presented (a) lower amygdala volume and (b) greater rostral anterior cingulate cortex thickness, when harsh parenting was low; greater amygdala volume without significant rostral anterior cortex thickness difference, when harsh parenting was high. Youth with high harsh parenting over time, compared to youth with low harsh parenting, presented (a) lower amygdala volume and (b) greater rostral anterior cingulate cortex thickness, when subclinical anxiety symptoms were low; greater amygdala volume without significant rostral anterior cortex thickness difference, when subclinical anxiety symptoms were high.
Results linked to high levels of harsh parenting over time (regardless of anxiety levels) are in agreement with literature on child severe adversity (Baker et al., Reference Baker, Williams, Korgaonkar, Cohen, Heaps and Paul2012; De Brito et al., Reference De Brito, Viding, Sebastian, Kelly, Mechelli, Maris and McCrory2013; Edmiston et al., Reference Edmiston, Wang, Mazure, Guiney, Sinha, Mayes and Blumberg2011; Hanson et al., Reference Hanson, Chung, Avants, Shirtcliff, Gee, Davidson and Pollak2010, Reference Hanson, Nacewicz, Sutterer, Cayo, Schaefer, Rudolph and Davidson2015; Hodel et al., Reference Hodel, Hunt, Cowell, Van Den Heuvel, Gunnar and Thomas2015; Kelly et al., Reference Kelly, Viding, Wallace, Schaer, De Brito, Robustelli and Mccrory2013; Korgaonkar et al., Reference Korgaonkar, Antees, Williams, Gatt, Bryant, Cohen and Grieve2013; McLaughlin et al., Reference McLaughlin, Sheridan, Winter, Fox, Zeanah and Nelson2014). It has been suggested that smaller amygdala and prefrontal cortex volumes in maltreated children (implicated in emotional processing and regulation, respectively), could be associated with a variety of behavioral problems (e.g., impulsive, anxious, and depressive behaviors) and poorer social functioning, and may be related to increased psychiatric vulnerability (De Brito et al., Reference De Brito, Viding, Sebastian, Kelly, Mechelli, Maris and McCrory2013; Edmiston et al., Reference Edmiston, Wang, Mazure, Guiney, Sinha, Mayes and Blumberg2011; Hanson et al., Reference Hanson, Chung, Avants, Shirtcliff, Gee, Davidson and Pollak2010; Kelly et al., Reference Kelly, Viding, Wallace, Schaer, De Brito, Robustelli and Mccrory2013; Korgaonkar et al., Reference Korgaonkar, Antees, Williams, Gatt, Bryant, Cohen and Grieve2013).
Results from interactions between the two factors (harsh parenting and subclinical anxiety symptoms) levels are particularly interesting. Greater rostral anterior cingulate cortex thickness in youth with high anxiety and youth with high harsh parenting over time (compared to those with low anxiety and low harsh parenting over time, respectively) is significant only when the second risk factor is low. This is in accordance with the study of Ducharme et al. (Reference Ducharme, Albaugh, Hudziak, Botteron, Nguyen, Truong and Karama2013) in youth without an anxiety disorder (Ducharme et al., Reference Ducharme, Albaugh, Hudziak, Botteron, Nguyen, Truong and Karama2013). In that longitudinal study, ventromedial prefrontal cortex thickness (including the rostral anterior cingulate cortex) was increased along with subclinical anxiety levels between 15 and 22 years (Ducharme et al., Reference Ducharme, Albaugh, Hudziak, Botteron, Nguyen, Truong and Karama2013).
Ducharme et al. (Reference Ducharme, Albaugh, Hudziak, Botteron, Nguyen, Truong and Karama2013) proposed two hypotheses to explain increased medial prefrontal cortex thickness with subclinical anxiety level in healthy youth: (a) this is a biological compensatory mechanism for an overly active limbic system, potentially preventing onset of mood and anxiety disorders (Ducharme et al., Reference Ducharme, Albaugh, Hudziak, Botteron, Nguyen, Truong and Karama2013). (b) Knowing that prefrontal cortex thickness normal development is to decrease during adolescence (Mills, Goddings, Clasen, Giedd, & Blakemore, Reference Mills, Goddings, Clasen, Giedd and Blakemore2014), this increase would be related to a delayed maturation of these structure with anxiety symptoms. This maturational delay could be a precursor to a pathological decrease in cortical thickness associated with risk or onset of a disorder (Ducharme et al., Reference Ducharme, Albaugh, Hudziak, Botteron, Nguyen, Truong and Karama2013). A recent longitudinal study in 120 youth has also demonstrated a link between negative maternal behavior and attenuated cortical thinning in prefrontal cortex regions (Whittle et al., Reference Whittle, Simmons, Dennison, Vijayakumar, Schwartz, Yap and Allen2014), which is consistent with the second hypothesis advanced by Ducharme et al. (Reference Ducharme, Albaugh, Hudziak, Botteron, Nguyen, Truong and Karama2013).
Greater anterior cingulate cortex thickness linked to high levels of harsh parenting over time (without anxiety) and to high levels of subclinical anxiety symptoms over time (without harsh parenting) could therefore represent a compensatory mechanism to avoid development of an internalized disorder and / or a delay in this brain structure maturation. Absence of thicker anterior cingulate cortex in youth who have two risk factors (i.e., who have a more severe condition) in this study, and smaller rostral anterior cingulate cortex thickness in youth with subclinical anxiety symptoms and at least one of whose parents had anxiety disorder (Suffren et al., Reference Suffren, Chauret, Nassim, Lepore and Maheu2019), could thus be explained by an absence of compensatory mechanism and/or by the beginning of an anterior cingulate cortex thickness decrease, which makes these youth closer to develop an anxiety disorder. Longitudinal studies will be required to answer this question.
Amygdala volume in youth with high anxiety and youth with high harsh parenting (compared to those with low anxiety and low harsh parenting, respectively) is significantly smaller when the second risk factor is low and greater when the second risk factor is high. This result could explain discrepancies observed in studies on amygdala volume linked to anxiety and adversity (Mueller et al., Reference Mueller, Aouidad, Gorodetsky, Goldman, Pine and Ernst2013). Lack of comparison with a group of participants with a history of adversity but who do not have anxious symptoms, or who conversely have anxious symptoms without a history of adversity, limits the ability to determine whether observed cerebral differences are linked more specifically to adversity or anxiety in the literature (Hart & Rubia, Reference Hart and Rubia2012; McCrory et al., Reference McCrory, De Brito and Viding2011). These results are in accordance with studies showing increased amygdala volume with subclinical anxiety symptoms (Juranek et al., Reference Juranek, Filipek, Berenji, Modahl, Osann and Spence2006; Qin et al., Reference Qin, Young, Duan, Chen, Supekar and Menon2014) and exposure to maternal depressive symptomatology (Lupien et al., Reference Lupien, Parent, Evans, Tremblay, Zelazo, Corbo and Séguin2011).
Whittle et al. (Reference Whittle, Dennison, Vijayakumar, Simmons, Yücel, Lubman and Allen2013) reported delayed amygdala development linked to maltreatment, and accelerated amygdala development in youth who have experienced maltreatment and who have developed psychopathology, compared to youth who have not developed psychopathology (Whittle et al., Reference Whittle, Dennison, Vijayakumar, Simmons, Yücel, Lubman and Allen2013). However, we do not know about youth with subclinical anxiety levels in that study. These results suggest that, during adolescence, the amygdala is larger when youth are at high risk to develop a disorder and smaller when youth have a disorder. It seems important to follow these youth in coming years in order to answer this question.
It is important to keep in mind that we cannot infer causality here, because of correlational and cross-sectional design of this study. Even if structural abnormalities could be the result of early-life stress, it could also be a pre-existing risk factor for vulnerability to adversity exposure, psychological trauma and stress-related disorders that would be induced by genetics (Gilbertson et al., Reference Gilbertson, Shenton, Ciszewski, Kasai, Lasko, Orr and Pitman2002; Lupien, McEwen, Gunnar, & Heim, Reference Lupien, McEwen, Gunnar and Heim2009). Even if the animal literature tends to confirm that some structural abnormalities follow stress exposure (Qin et al., Reference Qin, Young, Duan, Chen, Supekar and Menon2014; Yan, Reference Yan2012), future studies in humans should try to extend longitudinal studies across the human lifespan.
Lack of data on the mother during pregnancy (e.g., alcohol, drugs, stress) and on parents’ psychiatric health are also limits to this study, since we know that these factors influence brain and emotional development (Jones et al., Reference Jones, Dufoix, Laplante, Elgbeili, Patel, Chakravarty and Pruessner2019; Kolb, Harker, Mychasiuk, de Melo, & Gibb, Reference Kolb, Harker, Mychasiuk, de Melo and Gibb2017; Suffren et al., Reference Suffren, Chauret, Nassim, Lepore and Maheu2019). These factors should be taken into account in future studies.
Finally, parenting and anxiety data are parent-reported data, which raise the question of social desirability (Boivin et al., Reference Boivin, Pérusse, Dionne, Saysset, Zoccolillo, Tarabulsy and Tremblay2005) and parental perception of their own parenting and their child anxiety level (Gordo et al., Reference Gordo, Oliver-Roig, Martínez-Pampliega, Elejalde, Fernández-Alcantara and Richart-Martínez2018). Some studies support reliability of measure reported by the mother (Windham et al., Reference Windham, Rosenberg, Fuddy, McFarlane, Sia and Duggan2004), and trajectories observed in our sample correspond to trajectories of parenting observed in another sample (Lansford et al., Reference Lansford, Criss, Dodge, Shaw, Pettit and Bates2009). Moreover, only participants who had a persistent level over time were recruited, which may suggest that even though coercive parenting practices and/or anxiety levels were generally underestimated, those who reported a higher level actually had a higher level compared to those who reported a lower level. Moreover, parental perceptions of their own parenting and of their child anxiety level can be biased. For example, anxious parents may have negative perceptual or interpretive biases, may be inaccurately critical of their own parenting and see more anxiety behaviors in their children (Gordo et al., Reference Gordo, Oliver-Roig, Martínez-Pampliega, Elejalde, Fernández-Alcantara and Richart-Martínez2018). This may explain, in part, the absence of cerebral differences related to child anxiety levels in this study. It would have been interesting to add longitudinal measures of parenting and anxiety self-reported by youth.
Another limitation concerns VBM method. There are some inherent limitations to that method. In particular, data must be perfectly spatially aligned (Bookstein, Reference Bookstein2001). In addition, the smoothing and blurring steps can be misleading when it comes to detect differences in small structures like amygdala (Grodin, Lin, Durkee, Hommer, & Momenan, Reference Grodin, Lin, Durkee, Hommer and Momenan2013).
To conclude, this study has replicated and extended the relation between adversity (high levels of harsh parenting over time here), and smaller volumes of prefrontal cortex structures and amygdala to youth who did not experience severe adversity, who did not have anxiety disorder and who did not take medication. More interestingly, this study showed significant interactions between parental practices and subclinical anxiety symptoms concerning rostral anterior cingulate cortex thickness and amygdala volume. Youth with high subclinical anxiety symptoms, compared to youth with low subclinical anxiety symptoms, presented (a) lower amygdala volume and (b) greater rostral anterior cingulate cortex thickness, when harsh parenting was low; greater amygdala volume without significant rostral anterior cortex thickness difference, when harsh parenting was high. Youth with high harsh parenting, compared to youth with low harsh parenting, presented (a) lower amygdala volume and (b) greater rostral anterior cingulate cortex thickness, when subclinical anxiety symptoms were low; greater amygdala volume without significant rostral anterior cortex thickness difference, when subclinical anxiety symptoms were high. These results highlight the importance of taking into account these two risk factors and not just one when looking at the emergence of internalized disorders. Since we know that these structural abnormalities can contribute to an increase in psychiatric vulnerability later in life (McCrory, De Brito, & Viding, Reference McCrory, De Brito and Viding2012), following these youth in the coming years may help identify brain markers of risk and resilience to psychopathology in adulthood.
Acknowledgments
We are grateful to the parents and children and the Research Unit on Children's Psychosocial Maladjustment (RUCPM), staff for longitudinal data collection, management, and analysis (in particular Charles-Édouard Giguère and Hélène Paradis) of the QLSCD cohorts. We also thank Karine Leno Ancellin for her proofreading and English language corrections.
Funding Statement
This research was supported by grants from the Canadian Institutes of Health Research (CIHR; #MOP-97983, # MOP-44072 and #HDF-70335), the Quebec Government's Ministry of Health, the Fonds Québécois de la Recherche sur la Société et la Culture (FQRSC), Canada's Social Science and Humanities Research Council (SSHRC), the University of Montreal, and University Laval. The Quebec Longitudinal Study of Child Development was made possible thanks to the funding provided by the Fondation Lucie et André Chagnon, the Institut de la Statistique du Québec, the Ministère de l’Éducation et de l'Enseignement supérieur, the Ministère de la Famille, the Institut de recherche Robert-Sauvé en santé et en sécurité du travail, the Centre Hospitalier Universitaire Sainte-Justine, and the Ministère de la Santé et des Services sociaux du Québec. Source: Data compiled from the final master file ‘E3-E9’ from the Quebec Longitudinal Study of Child Development (2000–2006) © Gouvernement du Québec, Institut de la statistique du Québec. FSM received both a CIHR New Investigator and Fonds de recherche en santé du Québec (FRSQ) Junior 1 early career Awards. SS received PhD fellowships from the FQRSC and the Foundation of Stars/Research Center of the Ste-Justine University Hospital. VLB received PhD fellowships from the CIHR and the FRSQ. JRS is supported by the Fonds Monique Gaumond pour la Recherche en Maladies Affectives. MB holds a Tier 2 Canada Research Chair. The funding sources had no involvement in study design, collection, analysis, and interpretation of data, writing of the manuscript, and decision to submit the manuscript for publication.
Conflicts of Interest
None.