Because substance use disorders (SUDs) contribute to multiple negative physical and psychosocial outcomes (World Health Organization, 2004), identifying factors that increase risk for substance use problems is important. Among Sher's (Reference Sher1991) proposed and widely studied models that explain the intergenerational transmission of SUDs is the deviance-proneness pathway. In this pathway, children of parents with alcohol use disorders (AUDs) show elevated behavioral undercontrol (i.e., sensation seeking and conduct problems) and receive poor parenting. This combination places them at risk for affiliation with deviant peers and SUDs.
Sher's model (1991) considers both genetic and environmental influences and recognizes that the relations among parenting, peer influences, and SUDs may be influenced by genetic factors. However, these relations are often treated as if they are environmental in nature. We extend the previous literature by examining whether parental monitoring and affiliation with substance-using peers mediate the effects of parental AUD and adolescent polygenic risk on emerging adult SUD. We also test whether polygenic risk moderates the relations between parental monitoring and peer substance use, in predicting problematic substance use in emerging adulthood.
Parent monitoring has often been linked to adolescent substance use outcomes (Chassin, Pillow, Curran, Molina, & Barrera, Reference Chassin, Pillow, Curran, Molina and Barrera1993; White et al., Reference White, McMorris, Catalano, Fleming, Haggert and Abbott2006). Until recently, researchers assumed that information that parents acquired about their children's lives resulted from parents actively seeking out this knowledge. As a result, the term parental monitoring was used to describe how much parents knew about their children's activities and friends. However, Stattin and Kerr (Reference Stattin and Kerr2000) discovered that most of the variance in parental monitoring was explained by adolescent self-disclosure, that is, the extent to which offspring chose to share this information with their parents. In addition, youth self-disclosure, and to a lesser extent parental solicitation of information, predicted changes in adolescent delinquency (Kerr, Stattin, & Burke, Reference Kerr, Stattin and Burke2010). These findings suggest that parental knowledge is a more accurate term than parental monitoring. This distinction is important because the concept of parental monitoring may underestimate the importance of child effects.
The Putative Roles of Parent AUD, Parental Knowledge, and Peer Affiliation
Links within the deviance-proneness pathway have received substantial empirical support. Specifically, parent AUD is associated with poor parent–child relationships and less parental knowledge in younger adolescence (Latendresse et al., Reference Latendresse, Rose, Viken, Pulkkinen, Kaprio and Dick2008), which in turn is related to increased substance use in older adolescence and emerging adulthood (Lac & Crano, Reference Lac and Crano2009). Parents who know more about their children's lives may be better positioned to limit offspring substance use (Dishion, Capaldi, Spracklin, & Li, Reference Dishion, Capaldi, Spracklin and Li1995). Therefore, our first hypothesis was that parental AUD would predict younger adolescent parental knowledge, which would affect older adolescent parental knowledge, in turn influencing risk for emerging adult SUD. In other words, together, younger and older parental knowledge would mediate the effect of parent AUD on emerging adult SUD. See the paths labeled “1” in Figure 1 for a visual depiction of this effect.
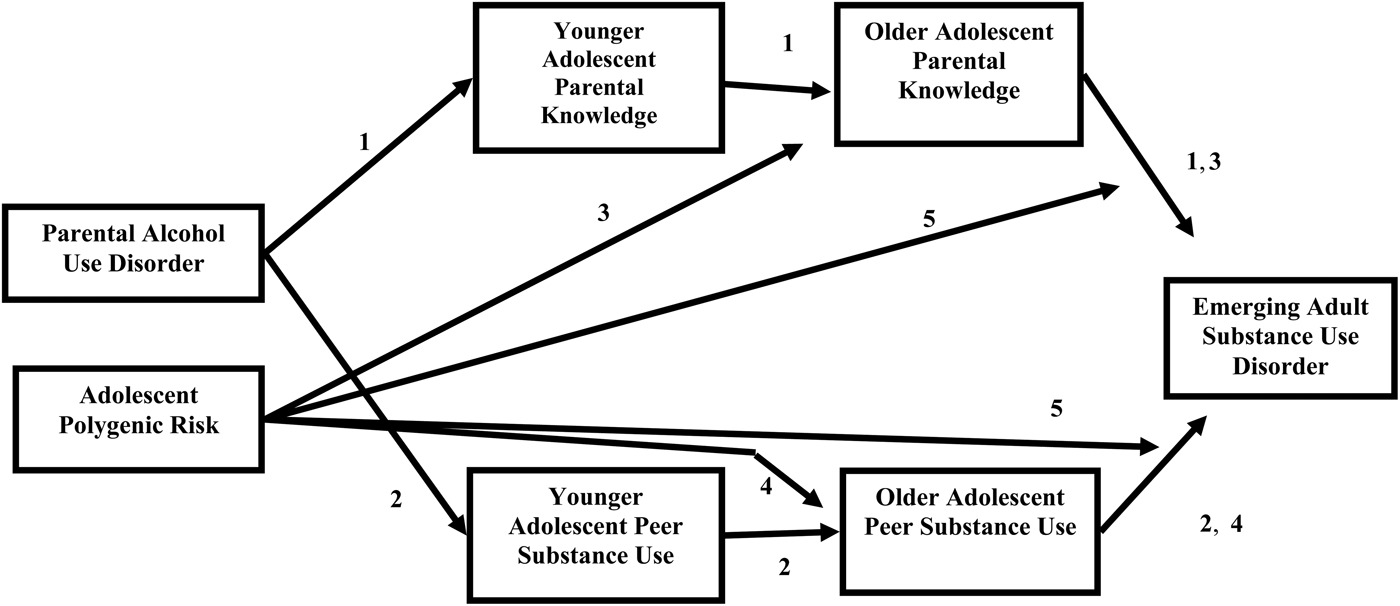
Figure 1. Conceptual model. Paths correspond to the hypothesized effects, with numbers matching the hypotheses listed in the text.
In addition to parental AUD predicting parental knowledge, parental AUD may also influence the characteristics of the adolescent's peer group, which in turn affects risk for SUDs. Parents with AUDs may model for their adolescents regular alcohol misuse with their own friends, thus communicating to their offspring the acceptability of drinking behaviors with one's peer group (White, Johnson, & Buyske, Reference White, Johnson and Buyske2000). Adolescents who believe that regular alcohol misuse with peers is normative and acceptable may be more likely to have friends who also drink and potentially use drugs. Parents with AUDs may also be more likely to tolerate substance use behavior among their children's friends (Abar & Turrisi, Reference Abar and Turrisi2008). Finally, parents with AUDs may be less psychologically and/or physically present and may therefore have less knowledge about their children's friends, making it more difficult to discourage association with a deviant peer group (Latendresse et al., Reference Latendresse, Rose, Viken, Pulkkinen, Kaprio and Dick2008). For all of these reasons, children of parents with AUDs may be more likely to affiliate with a substance-using peer group. Membership in a substance-using peer group may in turn increase risk for SUDs, as these friends provide opportunities for substance use (White et al., Reference White, McMorris, Catalano, Fleming, Haggert and Abbott2006), and normalize and encourage substance use (Dishion & Owen, Reference Dishion and Owen2002). Our second study hypothesis was therefore that parental AUD would predict younger adolescent peer substance use, which would affect older adolescent peer substance use, in turn influencing risk for emerging adult SUD. That is, together, younger and older peer substance use would mediate the effect of parent AUD on emerging adult SUD. See the paths labeled “2” in Figure 1 for a visual depiction of this effect.
The Putative Role of Gene–Environment Correlation
Although there is evidence for direct effects of parental knowledge on offspring substance use outcomes, as well as indirect effects mediated through peer substance use, these pathways may be at least in part genetically determined. Specifically, it may be that adolescents at genetic risk for alcohol and/or drug use disorders may be more likely to engage in deviant behaviors. They may also be unlikely to disclose details about their day-to-day lives to their caregivers (Tilton-Weaver & Marshall, Reference Tilton-Weaver, Marshall, Kerr, Stattin and Engels2008). Their caregivers may in turn withdraw, resulting in less parental knowledge (Dishion, Nelson, & Bullock, Reference Dishion, Nelson and Bullock2004; Kerr & Stattin, Reference Kerr, Stattin, Crouter and Booth2003; Kerr, Statin, & Pakalniskiene, Reference Kerr, Stattin and Pakalniskiene2008). This is an example of an evocative gene–environment effect, such that individuals with particular genotypes evoke particular responses from their environments (Klahr & Burt, Reference Klahr and Burt2014; Plomin, DeFries, & Loehlin, Reference Plomin, DeFries and Loehlin1977). Therefore, differences in parental knowledge may be partially determined by genetic differences between individuals (Plomin, Reiss, Hetherington, & Howe, Reference Plomin, Reiss, Hetherington and Howe1994; Reiss, Neiderhiser, Hetherington, & Plomin, Reference Reiss, Neiderhiser, Hetherington and Plomin2000).
An evocative effect of genetic risk on parental knowledge may also change with development. Estimates of genetic influences on parenting, and gene–environment correlation specifically, appear to increase across adolescence (Avinun & Knafo, Reference Avinum and Knafo2013). Changing also is the amount of time adolescents spend with their family versus outside of the home, with older adolescents spending more time away from their parents and siblings, compared to younger adolescents (Crosnoe & Johnson, Reference Crosnoe and Johnson2011). As adolescents gain increasing autonomy, they are more able to make decisions that are consistent with their genotypes. Therefore, the association between genetic risk for alcohol and/or drug use disorders and evoked parental knowledge may increase across adolescence. Because of the potentially increasing ability of individuals to influence their parenting environments across adolescence, we were particularly interested in the impact of parental knowledge during older adolescence on later risk for SUD. Thus, we tested whether polygenic risk predicted parental knowledge both in younger and older adolescence, and whether older adolescent parental knowledge predicted risk for emerging adult SUD. Our third study hypothesis was that older adolescent parental knowledge would mediate the effect of polygenic risk on SUD. See the paths labeled “3” in Figure 1 for a visual depiction of this effect.
In Sher's (Reference Sher1991) deviance-proneness pathway, the effects of parenting on SUD are mediated through affiliation with substance-using peers. Although studies consistently support the importance of these peer influences, peer group affiliation may reflect genetic risk also common to risk for SUD. Adolescents at high genetic risk for SUDs may be more likely to select friends who enjoy drinking alcohol and using drugs. This may reflect either an active gene–environment correlation (in which the individual's own genes make him/her seek certain environments) or an evocative gene–environment correlation (in which an individual's own genes increase the chance that he/she will evoke particular behaviors in others; Dishion & Owen, Reference Dishion and Owen2002; Fowler et al., Reference Fowler, Shelton, Lifford, Rice, McBride and Ivan2007; Scarr & McCartney, Reference Scarr and McCartney1983).
There is evidence of genetic influences on peer affiliation. Twin studies find that genetic influences explain up to 37% of the variance in peer delinquency/substance use outcomes in late adolescence and emerging adulthood, but may explain only 3% of the variance in these outcomes in early adolescence (Beaver et al., Reference Beaver, Shutt, Boutwell, Ratchford, Roberts and Barnes2009; Fowler et al., Reference Fowler, Shelton, Lifford, Rice, McBride and Ivan2007; Iervolino et al., Reference Iervolino, Pike, Manke, Reiss, Hetherington and Plomin2002). In addition, Fowler, Settle, and Christakis (Reference Fowler, Settle and Christakis2011) reported that there was a significant positive relation between each adolescent's genotype and his/her peers’ genotype (controlling for age, sex, and ethnicity), as measured by the dopamine D2 receptor (DRD2) gene single nucleotide polymorphism (SNP) rs1125394. Chassin et al. (Reference Chassin, Lee, Cho, Wang, Agrawal and Sher2012) found that offspring of parents with AUDs were more likely to have a particular genetic makeup on μ-opioid receptor M1 gene (OPRM1) rs1799971, which predicted peer substance use for males, but not for females (Chassin et al., Reference Chassin, Lee, Cho, Wang, Agrawal and Sher2012).
Although there is some evidence that genetic effects influence peer group affiliations (e.g., Christakis & Fowler, Reference Christakis and Fowler2014), the research is not unequivocal. As stated, one explanation for this variability is that the studies finding significant genetic influences on peer substance use were conducted with older samples. During and across adolescence, individuals spend less time with family members and more time outside the home, compared to during earlier developmental periods (Crosnoe & Johnson, Reference Crosnoe and Johnson2011). Therefore, as individuals begin to have more control over the people with whom they socialize, the relation between genetic risk for SUDs and choice of a substance-using peer group would be expected to increase (Kendler et al., Reference Kendler, Jacobsen, Gardner, Gillespie, Aggen and Prescott2007; Salvatore et al., Reference Salvatore, Aliev, Bucholz, Agrawal, Hesselbrock and Hesselbrock2014). Genetic influences on peer substance use are therefore likely to be stronger in older adolescence, compared to younger adolescence. Older adolescence may also be the ideal time to examine peer influences on substance use outcomes, because these effects are strongest during this developmental period (Steinberg & Monahan, Reference Steinberg and Monahan2007). Accordingly, the current study examined whether genetic risk significantly predicted affiliations with substance-using peers in younger and older adolescence, and whether older adolescent peer substance use influenced risk for SUD. Our fourth study hypothesis was therefore that older adolescent peer substance use would mediate the effect of genetic risk on SUD. See the paths labeled “4” in Figure 1 for a visual depiction of this effect.
The Putative Role of Gene × Environment Interaction
We have described the role of gene–environment correlation in the relations among parent knowledge, peer affiliation, and emerging adult SUDs. However, there is also literature to suggest a role for Gene × Environment interaction. Many studies report that genetic effects are stronger at higher levels of environmental risk, and environmental effects are stronger at higher levels of genetic risk (Miranda et al., Reference Miranda, Reynolds, Ray, Justus, Knopik and McGeary2012; Salvatore et al., Reference Salvatore, Aliev, Bucholz, Agrawal, Hesselbrock and Hesselbrock2014). Environments that provide less opportunity for substance use suppress the behavioral manifestations of genetic risk, whereas environments that allow for more substance use opportunities amplify the behavioral manifestations of genetic risk (Hicks, South, DiRago, Iacano, & McGue, Reference Hicks, South, DiRago, Iacano and McGue2009). These diathesis–stress interactions have been found for substance use outcomes in twin studies (Agrawal et al., Reference Agrawal, Balasubramanian, Smith, Pamela, Bucholz and Heath2010), as well as in studies using measured genes (Dick et al., Reference Dick, Wang, Plunkett, Aliev, Hinrichs and Bertelsen2007; Miranda et al., Reference Miranda, Reynolds, Ray, Justus, Knopik and McGeary2012). Accordingly, our fifth and final study hypothesis was that older adolescent parental knowledge and peer substance use would exert larger effects on offspring emerging adult SUDs for those at higher levels of polygenic risk for SUDs. See the paths labeled “5” in Figure 1 for a visual depiction of these effects.
Measuring Genetic Risk: Creating a Polygenic Risk Score
In order to test these questions, we created a literature-based score based on prior research and theory, which was formed to meaningfully represent interplay between genetic risk and study variables. In attempting to create a score measuring literature-based polygenic risk, SNPs were chosen from theoretically plausible receptor systems that have been found to be related to risk for alcohol and/or drug use disorders in at least two prior studies. A search was conducted using the terms “alcohol use disorder,” “drug use disorder,” “alcohol misuse,” or “drug misuse” with “SNP” or “polymorphism” via Google Scholar, PubMed, and HuGE Navigator. The results of this literature search yielded SNPs from the dopamine (DRD2), opioid (OPRM1, prodynorphin [PDYN]), GABA (GABA receptor subunit alpha-2 [GABRA2]), drug metabolism (alcohol dehydrogenase 4 [ADH4]), and cannabinoid (endocannabinoid receptor 1 [CNR1]) systems. Genes within these systems are associated with pleasure derived from using substances, and more punitive effects of discontinuing substance use (Brady & Sinha, Reference Brady and Sinha2005; Costa, Giagnoni, & Colleoni, Reference Costa, Giagnoni and Colleoni2000; Koob, Reference Koob1992).
In examining SNPs from these receptor systems specifically, the SNP rs1800497 within the gene DRD2 is associated with increased risk for higher frequency of binge drinking and frequency of and problems associated with substance misuse among adolescents (Brody, Chen, & Beach, Reference Brody, Chen and Beach2013; Foley et al., Reference Foley, Loh, Innes, Williams, Tannenberg and Harper2004). Research has also found associations between rs3762894 (ADH4) and adverse effects of alcohol use (e.g., flushing; Liu et al., Reference Liu, Zhou, Hodgkinson, Yuan, Shen and Mulligan2011; MacGregor et al., Reference MacGregor, Lind, Bucholz, Hansell, Madden and Richter2008). In addition, prior work has found support for the association between the SNP rs1049353 (CNR1) within the cannabinoid system and unpleasant effects following discontinuation of alcohol use, as well as cannabis dependence (Hartman et al., Reference Hartman, Hopfer, Haberstick, Rhee, Crowley and Corley2009; Preuss, Koller, Zill, Bondy, & Soyka, Reference Preuss, Koller, Zill, Bondy and Soyka2003). Research also found associations between GABRA2 SNP rs11503014 and subjective response to cocaine and heroin addiction (Enoch, Reference Enoch, Hodgkinson, Yuan, Shen, Goldman and Roy2010; Smelson et al., Reference Smelson, Yu, Buyske, Gonzalez, Tischfield and Deutsch2012). Finally, the SNP rs609148 (in high linkage disequilibrium [LD]Footnote 1 with rs558025), rs495491 (in high LD with rs510769), rs548646 (in high LD with rs660756) within the genes OPRM1 and PDYN are associated with increased rewarding effects of alcohol, as well as alcohol use and drug (i.e., opioid) misuse in adolescents and adults (Ehlers, Lind, & Wilhelmsen, Reference Ehlers, Lind and Wilhelmsen2008; Shabalina et al., Reference Shabalina, Zaykin, Gris, Ogurtsov, Gauthier and Shibata2009; Zhang et al., Reference Zhang, Luo, Kranzler, Lappalainen, Yang and Krupitksy2006). By creating a polygenic risk score using SNPs from theoretically plausible receptor systems, the current study is able to interpret significant main and interaction effects involving this gene score. See Table 1 for more information on included SNPs.
Table 1. SNPs used to create polygenic risk
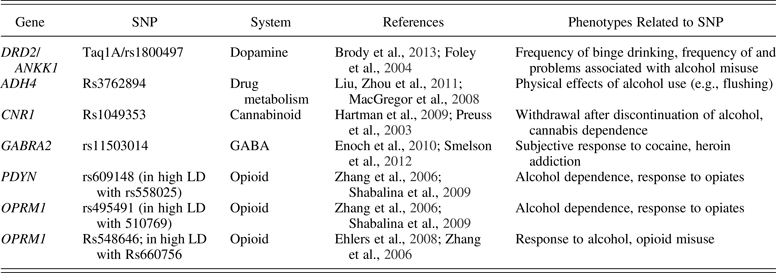
Note: SNP, Single nucleotide polymorphism; SUD, substance use disorder; GABA, gamma-aminobutyric acid.
In general, these genetic effects on alcohol and drug use disorders have explained increasing amounts of variance as individuals move from adolescence into adulthood (Dick et al., Reference Dick, Wang, Plunkett, Aliev, Hinrichs and Bertelsen2007; Kendler et al., Reference Kendler, Chen, Dick, Maes, Gillespie and Neale2012). This trend may occur because developmentally limited deviance and drinking during adolescence masks genetic risk. These findings may also be explained by the fact that, as individuals age, they are able to exercise freedom to make decisions consistent with their genetic risk, compared to earlier developmental periods when adults in their lives might make these decisions for them. Because of this trend in the literature, and in order to provide a stricter test of environmental influences, the current study examines predictors of emerging adult SUD.
Present Study
In summary, the current study had five main study hypotheses. First, we hypothesized that together, younger and older parental knowledge would mediate the effect of parental AUD on emerging adult SUD, and second, that younger and older peer substance use would mediate this effect. Third, we predicted that older adolescent parental knowledge would mediate the effect of adolescent genetic risk for SUDs on propensity for developing a SUD (no such hypothesis is made about younger adolescent parental knowledge). Fourth, we predicted that older (but not younger) adolescent peer substance use would mediate the effect of adolescent genetic risk on propensity for developing a SUD. Fifth, we predicted that the effects of older adolescent parental knowledge and peer substance use on emerging adult SUD would be stronger for those at higher level of genetic risk. We examined these hypotheses after controlling for earlier levels of outcome variables, as well as ancestry, gender, and age.
Method
The original study
Participants were a subsample from a larger longitudinal study of familial alcoholism in a large metropolitan area in the Southwest United States. There have been six waves of data collection, with Wave 1 beginning in 1988, Wave 2 in 1989, Wave 3 in 1990, Wave 4 in 1995, Wave 5 in 2000, and Wave 6 in 2005. The total sample at Wave 1 consisted of 454 adolescents, 246 of whom had at least one biological alcoholic parent who was also a custodial alcoholic parent (i.e., child of an alcoholic). The remaining 208 were controls, matched on neighborhood, child's age, number of parents in the household, and ethnicity, who had no biological or custodial alcoholic parents. At Wave 1 of the study, families in which one or more caregivers met criteria for an AUD reported lower levels of education. However, families with and without parent AUD were comparable on family income and the likelihood of a parent being unemployed. Adolescents and their families were interviewed annually for 3 years for the first three waves, and then 5, 10, and 15 years later for the last three waves.
In terms of sociodemographic characteristics in the larger sample, the average total yearly household income of original participants in 1988 was $43,000. The range in reported household incomes was $6,000 to $180,000, with about 10% of families reporting household incomes of $22,000 or less a year. In addition, on average, mothers and fathers completed high school or had taken some college classes. The overwhelming result was that most (80%) families reported that both biological parents lived at home with the children. The large proportion of two-biological parent families was created by the selection criterion that the alcoholic parent be both biological and custodial and then matching nonalcoholic and alcohol samples in family structure. Other adolescents lived with only their biological mother (7% of the sample), biological father (1%), or some other relative (12%). Additional details of sample recruitment and representativeness are reported elsewhere (Chassin, Barrera, Bech, & Kossak-Fuller, Reference Chassin, Barrera, Bech and Kossak-Fuller1992).
The current subsample
The current study used data from Waves 1–5 of the larger parent project. Of the 454 Wave 1 adolescent participants, 266 supplied genetic data. Of these 266, there were 5 cases in which the call rate was unacceptable, resulting in 261 remaining participants. Of these 261, 7 reported ethnicities other than non-Hispanic Caucasian or Hispanic, and were eliminated. The resulting 254 comprised the subsample for the current study.
Participants who were included in the subsample (N = 254) were compared to those excluded (N = 200). Of the 254 adolescents included in current study analyses, 120 (47.2%) had at least one parent with an AUD and 134 (52.8%) did not. In addition, 120 (47.2%) attended at least some college. There were no differences between those included and excluded on age, ethnicity, education level achieved by the offspring (i.e., emerging adults, between the ages of 18 and 25), younger or older adolescent mother or offspring report of parental knowledge, younger or older adolescent peer substance use, or on younger adolescent own substance use. Those who met inclusion criteria and who were included in the subsample were less likely to be children of alcoholics, male, and to meet criteria for a lifetime SUD between the ages of 18 and 25. However, the magnitude of these significant differences was small,Footnote 2 suggesting minimal bias in terms of sample characteristics.
Measures
Age bands
Age bands were created in order to limit the age heterogeneity of participants at the times when study variables were examined. The first age band captured peer substance use, adolescent substance use, and parental knowledge when the adolescent was between ages 11 and 14 (mean age = 12.93; “younger adolescence”). The second age band captured peer substance use and parental knowledge when adolescents were between ages 15 and 17 (mean age = 15.93; “older adolescence”).Footnote 3 The third age band captured emerging adult SUD when the individual was between ages 18 and 25 (mean age = 21.22). Descriptive statistics for study variables are presented in Table 2.
Table 2. Descriptive statistics
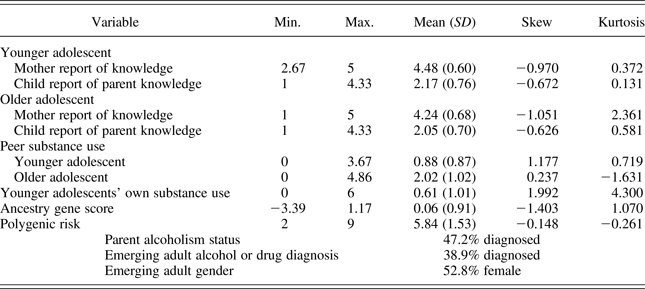
Adolescent gender
A dummy code indicating gender (52.8% female) was used as a covariate (0 = female, 1 = male).
Adolescent ethnicity/ancestry
A factor score of SNPs reflecting Hispanic ancestry was used as a covariate. Scores were derived from 37 ancestry marker SNPs, which, in previous literature, have differentiated Hispanics from non-Hispanic Caucasians (Tian et al., Reference Tian, Hinds, Adler, Lee, Pahl and Silva2007). These were coded to ensure that higher scores indicated more Caucasian and less Hispanic ancestry, with scores of 0, 1, and 2 reflecting low, medium, and high levels of Caucasian ancestry, respectively. After trichotomizing, a principal components analyses of these 37 SNPs indicated that the first component explained 18.99% of the variance, with only an additional 3.36% and 3.11% accounted for by the second and third, respectively. The first component had an eigenvalue of 7.025, and the second through ninth components had eigenvalues between 1.243 and 1.020. Based on these findings, analyses used one component. Of the 37 ancestry marker SNPs, 32 loaded on this one component, with loadings at least as large as 0.3 or –0.3. These 32 SNPs were included in a factor analysis in Mplus, and these factor scores significantly correlated with self-reported ethnicity (r = .868, p < .001). Mplus fit statistics for the one-factor model showed good fit, root mean square error of approximation (RMSEA) = 0.025, comparative fit index (CFI) = 0.943, standard root mean square residual = 0.027.
Adolescent age
Self-reported age at age band 1 (age 11–14) was used as a covariate in predicting younger adolescent parental knowledge, peer substance use, and substance use (all at ages 11–14). The mean age at age band 1 was 12.93.
Parent alcohol abuse/dependence
Parent lifetime alcohol abuse/dependence diagnoses were obtained with a computerized version of the Diagnostic Interview Schedule (version III; Robins, Helzer, Croughan, & Ratcliff, Reference Robins, Helzer, Croughan and Ratcliff1981). Parents who were not interviewed were diagnosed based on spousal report using the Family History—Research Diagnostic Criteria (FH-RDC; Endicott, Andreason, & Spitzer, Reference Endicott, Andreason and Spitzer1975). The parent alcohol abuse/dependence variable was measured at the beginning of the original study when the adolescents were between the ages of 11 and 15 (i.e., generally corresponding to the age when “younger adolescent” constructs were examined in the current study).
Analyses (χ2 = 23.884) indicated that those with one parent with an AUD (p < .01) or two parents with AUDs (p < .001) were more likely to develop a SUD, compared to those whose parents had not had an AUD. However, there were no differences in risk for offspring SUD between those with one versus two parents with an AUD (ns). There were also no differences in risk as a function of the gender of the parent with the AUD either among male offspring (χ2 = 2.269, ns) or female offspring (χ2 = 1.375, ns). Specifically, 59.1% of males with a father with an AUD, 50% of males with a mother with an AUD, and 66.7% of males with a mother and a father with an AUD met criteria for a SUD. In addition, 46.8% of females with a father with an AUD, 40% of females with a mother with an AUD, and 66.7% of females with a mother and a father met criteria for a SUD. Accordingly, parent AUD was treated as a dichotomous variable, either absent in both parents (0; 52.8% of sample) or present in one or two parents (1; 47.2% of sample).Footnote 4
Parental knowledge
Mother and offspring report of knowledge about the adolescent's behavior (ages 11–14, “younger adolescence,” and 15–17, “older adolescence,”) was assessed via three items designed by project staff. These items assess the extent to which the parents talked with the adolescent about his/her plans for the day, had a “pretty good idea” about the adolescent's interests and whereabouts, and generally knew the people with whom he/she associated (range = 1–5; higher scores indicate more knowledge). These items have been shown to have good predictive validity in terms of their associations with peer substance use and offspring substance use (Chassin et al., Reference Chassin, Pillow, Curran, Molina and Barrera1993). The Cronbach α values for mother and offspring report of knowledge were 0.78 and 0.60 in younger adolescence and 0.82 and 0.63Footnote 5 in older adolescence, respectively.
Peer substance use
Adolescent report of substance use in the peer group (ages 11–14 and 15–17) was assessed with the mean of six items adapted from the Monitoring the Future Questionnaire (Johnston, O'Malley, & Bachman, Reference Johnston, O'Malley and Bachman1988). These items assessed how many of their friends drink alcohol, smoke marijuana, or take other illicit drugs occasionally and regularly (range = 0–5; 0 = none and 5 = all). The Cronbach α values were 0.92 in younger adolescence and 0.94 in older adolescence.
SUD
Emerging adult (ages 18–25) diagnoses of lifetime SUD were obtained from a computerized version of the Diagnostic Interview Schedule (Robins et al., Reference Robins, Helzer, Croughan and Ratcliff1981). Dichotomous dummy-coded variables compared participants meeting lifetime criteria for alcohol or drug abuse or dependence (38.9%) with those who did not (61.1%). Of those meeting criteria for alcohol or drug abuse or dependence, 70.5% met criteria for alcohol or drug dependence, and the remaining 29.5% met criteria for only abuse of one or more substances.
To assess a precursor of this outcome at ages 11–14 (and thus establish prospective prediction), a variable was created to reflect the highest frequency of alcohol or drug use in younger adolescence. These items assessed the highest frequency of use of alcohol, marijuana, amphetamines, barbiturates, tranquilizers, hallucinogens, cocaine, opiates, and inhalants (range = 0–7; 0 = never and 7 = every day).
Polygenic risk
DNA extraction and plating were performed at the Department of Psychiatry at Washington University School of Medicine, and samples were genotyped at the Washington University Genome Sequencing Center. Illumina Golden Gate Technology was used to design a set of 1,536 SNPs for genotyping. Checks were conducted to detect Mendelian inconsistencies, incorrect gender assignments, and potentially unclear relatedness. SNPs with low call rates (<95%) and deviations from Hardy–Weinberg equilibrium (p < 10–6) were eliminated.
To create literature-based polygenic risk, a literature review was conducted to identify previous studies associating available SNPs in the dopamine, GABA, cannabinoid, alcohol effects, and opioid systems (or SNPs that were in high LD with available SNPs), and SUD. Inclusion was based on finding associations between the SNP and SUD in at least two prior studies and not on its association with the phenotype of interest in this sample (see Table 1 for a list). In addition, in order to be included in this literature-based score, prior literature had to agree on the allele that confers greater risk for SUD. When prior work (between two and six studies) disagreed about the risk allele direction, findings from studies including those most similar to current study participants (e.g., in terms of ethnicity, being from a high-risk community sample) were prioritized. These criteria resulted in seven SNPs that were used to create this polygenic risk score.
Using prior literature to determine the direction of risk, SNPs were coded additively to indicate low, medium, and high levels of genetic risk (0, 1, 2) based on the number of risk alleles inherited. The seven scores were then summed, as is standard practice (Morrison et al., Reference Morrison, Bare, Chambless, Ellis, Malloy and Kane2007).Footnote 6 This score was related to emerging adult SUD in the zero-order correlations (r = .128, p < .05). In terms of its main effect on SUD in study models, this score was significantly or marginally significantly predictive (βs were 0.203 and 0.233, p < .1 and p < .05, respectively). This score explained 1.63% of the variance in emerging adult SUD.Footnote 7
Although this polygenic risk score only explained 1.63% of the variance in emerging adult SUD, others have commented on the problem of “missing heritability.” That is, there is a large gap between the heritability of a trait and the variance accounted for by measured genetic associations (Plomin & Simpson, Reference Plomin and Simpson2013). The magnitude of variance explained by this risk score is consistent with other studies utilizing polygenic risk scores to predict substance use outcomes in adolescents and young adults (e.g., variance explained ranging from 0.5%–2%; Davis & Loxton, Reference Davis and Loxton2013; Derringer et al., Reference Derringer, Krueger, Dick, Aliev, Grucza and Saccone2012).
In further support of this method, seven SNPs that were not included in the polygenic risk were randomly chosen from the remaining SNPs. These SNPs were added together, used to create a polygenic score, and tested as a predictor of this same phenotype. These SNPs were coded such that the direction of effect for each SNP on SUD was positive, with scores of 0, 1, and 2 reflecting low, medium, and high levels of genetic risk, respectively. This random gene score did not predict emerging adult SUD (β = –0.062, ns). In addition, it explained 0.02% of the variance, which is less than the variance explained by the current study's polygenic risk score. This analysis increases our confidence in the current study's measure of polygenic risk.
Data analytic strategy
To reduce nonessential multicollinearity, continuous variables were centered (Cohen, Cohen, West, & Aiken, Reference Cohen, Cohen, West and Aiken2003). Covariates were adolescent ancestry, gender, and age band 1 age (age 11–14). Because there were both continuous and categorical dependent variables models, we used the weighted least squares estimator with mean and variance adjustments (WLSMV), which computes ordinary least squares parameter estimates for continuous outcomes and probit parameter estimates for categorical outcomes. Because the WLSMV estimator provides probit regression estimates, which cannot be converted to odds ratios (as logit regression estimates can), there are no odds ratios in this document despite the prediction of a dichotomous outcome (Cohen et al., Reference Cohen, Cohen, West and Aiken2003; Muthén & Muthén, Reference Muthén and Muthén1998–2011). However, the categorical is function in Mplus was used, indicating to the program that the outcome variable is a categorical variable. WLSMV uses a four-stage process to estimate missing data, with the first two stages using full information at maximum likelihood estimation (Muthén & Satorra, Reference Muthén and Satorra1995), and then techniques similar to pairwise deletion are used in the last two steps. Prior work suggests that full information maximum at likelihood produces unbiased estimates under the missing at random assumption (Enders & Bandalos, Reference Enders and Bandalos2001).
Missing data on endogenous variables were estimated as a function of the observed exogenous variables under the missingness at random assumption (Schafer & Graham, Reference Schafer and Graham2002). As Shafer and Graham point out, there is no way to test whether missing at random holds in a data set without following up with nonresponders. However, it is possible to examine whether earlier values on a construct predict missingness on that same construct at the next time point (which would suggest a missing not at random pattern). Using this method, we did not find a significant association between young adolescent parental knowledge and older adolescent missing parent knowledge data. We also did not find a relation between young adolescent peer substance use and older adolescent missing peer substance use data. In addition, there was no association between younger adolescent substance use and missing substance use diagnosis in emerging adulthood. Therefore, we conclude that our data are missing at random.Footnote 8
Models were assessed for goodness of fit using the chi-square goodness of fit test statistic, CFI ≥ 0.95, RMSEA ≤ 0.08, and weighted root mean square residual (WRMR) < 0.90 (Hu & Bentler, Reference Hu and Bentler1999; Yu & Muthén, Reference Yu and Muthén2002). The mediated effects of parental alcoholism and polygenic risk on emerging adult SUDs through parental knowledge and peer substance use were tested using the Model Indirect and Sobel statements in Mplus (Muthén & Muthén, Reference Muthén and Muthén1998–2010).
Regression diagnostics
Because Mplus does not yield regression diagnostics, models were estimated in ordinary least squares and logistic regression using SPSS to examine the influence of outliers on results. No abnormalities were detected; therefore, no cases were deleted from the analyses.
Results
Correlations
Table 3 gives the zero-order pearson (between two continuous variables), tetrachoric (two dichotomous variables), and biserial (dichotomous and continuous variables) correlations. In terms of covariates, according to mother report, older adolescents at the first age band (i.e., ages 11–14) have mothers who know less about their lives, and are more likely to drink alcohol and have friends who use substances. Compared to males, females have parents who know more about their lives (by mother report), and more friends who use substances in younger adolescence. However, males are more likely to meet criteria for age 18–25 SUD. Those of greater Caucasian ancestry have mothers who know more about their lives in older adolescence (by child report).
Table 3. Correlations between study variables
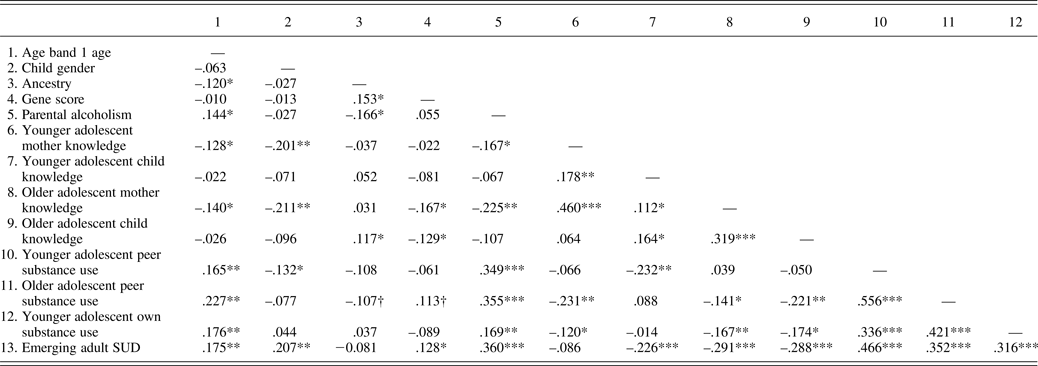
Note: N = 254, although exact n varies across reporter. SUD, Substance use diagnosis. Gender is coded 0 = female, 1 = male. Ancestry score is coded such that higher scores mean more Caucasian ancestry. Parental alcoholism, 0 = non-child of alcoholic (COA), 1 = COA; SUD, 0 = no diagnosis, 1 = diagnosis.
†p < .1. *p < .05. **p < .01. ***p < .001.
In terms of predictors, offspring of parents with AUDs have parents who know less about their lives (by mother report), are more likely to have friends who use substances, and are more likely to meet criteria for SUD. Higher scores on polygenic risk were significantly associated with less adolescent and mother-reported knowledge in older (but not younger) adolescence, and were significantly associated with greater risk for emerging adult SUD.
More younger adolescent substance use was generally associated with less adolescent mother- and child-reported knowledge. Younger adolescent substance use also conferred risk for later association with substance-using peers and emerging adult SUD. Greater younger adolescent peer substance use was associated with less younger adolescent child-reported knowledge, greater younger adolescent substance use, and emerging adult SUD. Finally, less mother- (in younger and older adolescence) and child-reported knowledge (in older adolescence) in older and younger adolescence were associated with older adolescent peer substance use, as well as emerging adult SUD.
Final study models
Separate models using mother and adolescent report of parental knowledge were estimated. Because of the hypotheses about polygenic risk predicting parental knowledge and peer substance use across developmental periods, paths from this polygenic risk score to younger and older adolescent parental knowledge and peer substance use were estimated. Prior work (Keller, Reference Keller2014) suggests that confounders may exert significant influences on outcomes via interactions with genetic and environmental a prior predictors. Therefore, covariate by covariate and covariate by predictor interactions were estimated, and when not significant, were dropped from final models.
The models using mother and adolescent report of knowledge (N = 254) showed good fit to the data (mother report: RMSEA = 0.065, CFI = 0.805, WRMR = 0.796, see Table 4; adolescent report: RMSEA = 0.078, CFI = 0.728, WRMR = 0.888, see Table 5). In terms of covariate effects, in both the mother report and adolescent report of knowledge models, more younger adolescent knowledge was related to older adolescent knowledge, and more younger adolescent peer use conferred risk for increased older adolescent peer use. In the mother-report model, more younger adolescent own substance use predicted increased risk for more older adolescent peer use. In addition, in both models, males were more likely to meet criteria for a SUD in emerging adulthood. Mothers reported that they knew more about the lives of their daughters, compared to their sons, in younger adolescence, but adolescent report did not yield this same trend. Finally, more younger adolescent substance use was associated with decreased parent knowledge in older adolescence, according to adolescent (but not mother) report.
Table 4. Results of longitudinal model using mother report of parental knowledge (N = 254)
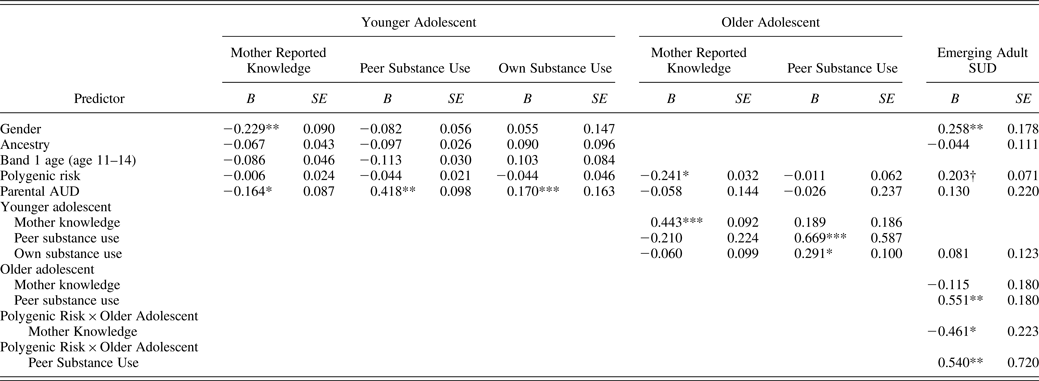
Note: B, Standardized regression coefficient; AUD, alcohol use disorder. Parental AUD is coded 0 for children of nonalcoholics and 1 for children of alcoholics. Gender is coded 0 for females and 1 for males.
†p < .1. *p < .05. **p < .01. ***p < .001.
Table 5. Results of longitudinal model using adolescent report of parental knowledge (N = 254)
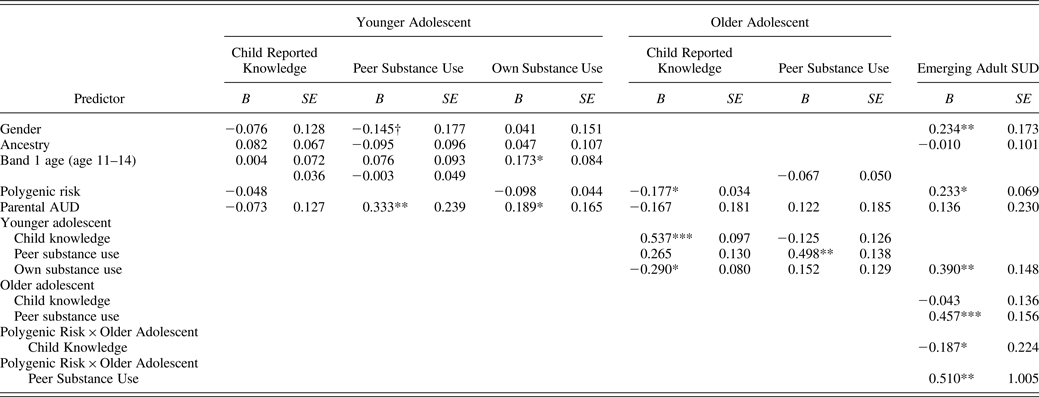
Note: B, Standardized regression coefficient; AUD, alcohol use disorder. Parental AUD is coded 0 for children of nonalcoholics and 1 for children of alcoholics. Gender is coded 0 for females and 1 for males.
†p < .1. *p < .05. **p < .01. ***p < .001.
Hypothesized effects
The first hypothesis posited that together, younger and older adolescent parental knowledge would mediate the effect of parental AUD on emerging adult SUD. Findings indicate that parental AUD was associated with mother report, but not adolescent report, of younger adolescent parental knowledge. For both mother report and adolescent report, younger adolescent knowledge predicted older adolescent knowledge. The effect of older adolescent parental knowledge on emerging adult SUD was significant for those at high levels of genetic risk. The indirect effect of parent AUD on SUD through younger adolescent and older adolescent parental knowledge was significant for those at high levels of genetic risk using mother report (confidence interval [CI] = 0.016–0.061, p < .05). This indirect effect was not significant for those at medium (CI = –0.020 to 0.053) or low levels (CI = –0.046 to 0.081) of parental knowledge using mother report. This indirect effect was nonsignificant for those at high (CI = –0.001 to 0.046), medium (CI = –0.014 to 0.021), and low levels of genetic risk (CI = –0.054 to 0.075) when using adolescent report. Therefore, our first hypothesis was supported in mother report (for those at high genetic risk) but not in adolescent report.
The second hypothesis was that peer substance use would mediate the effect of parental AUD on emerging adult SUD. For models using both mother report and adolescent report of knowledge, parental AUD was associated with greater likelihood of associating with younger adolescent substance-using peers. Having more younger adolescent substance-using peers was associated with greater affiliation with older adolescent substance-using peers. Finally, older adolescents with more substance-using peers who used substances were more likely to meet criteria for a SUD in emerging adulthood, with this effect particularly strong for those at highest and medium levels of genetic risk. This indirect effect of parent AUD on SUD through younger and older adolescent peer substance use was significant for those at highest (mother report: CI = 0.109–0.809, p < .01; adolescent report: CI = 0.028–0.394, p < .01), medium (mother report: CI = 0.106–0.775, p < .05; adolescent report: CI = 0.015–0.321, p < .05), and lowest levels of genetic risk (mother report: CI = 0.102–0.741, p < .05; adolescent report: CI = 0.011–0.203, p < .05).
The third hypothesis posited that older adolescent parental knowledge would mediate the effect of genetic risk on emerging adult SUD. For both mother report and adolescent report, the effect of polygenic risk on older (but not younger) adolescent parental knowledge was significant. In addition, the effect of older adolescent parental knowledge on emerging adult SUD was significant for those at highest, but not medium or low, levels of genetic risk. The indirect effect of the genetic risk score on SUD through older adolescent parental knowledge was significant when examining the effect of parental knowledge on SUD at highest levels of genetic risk (mother report: CI = 0.018–0.115, p < .05; adolescent report: 0.001–0.045, p < .05). This indirect effect was not significant at medium (mother report: CI = –0.020–0.053; adolescent report: CI = –0.014–0.023) or low levels of genetic risk (mother report: CI = –0.076–0.065; adolescent report: CI = –0.075–0.051).
The fourth study hypothesis was that older adolescent peer substance use would mediate the effect of genetic risk on emerging adult SUD. In both models, the effect of polygenic risk on older adolescent peer substance use was nonsignificant, but peer substance use predicted emerging adult SUD, and this effect was particularly strong for those at highest and medium levels of genetic risk. This indirect effect was nonsignificant for those at highest (mother report CI = 0.091–0.084, ns; adolescent report CI = –0.095 to 0.052, ns), medium (mother report CI = –0.089 to 0.081, ns; adolescent report CI = –0.076 to 0.039, ns), and lowest levels of genetic risk (mother report CI = –0.085 to 0.079, ns; adolescent report CI = –0.034 to 0.018, ns).
The fifth and final hypothesis was that the effect of older adolescent parental knowledge and older adolescent peer substance use on risk for SUD would be stronger for those at highest levels of genetic risk. In both models, polygenic risk interacted with knowledge to predict emerging adult SUD. Specifically, less mother- (β = –0.351, p < .05) and adolescent-reported (β = –0.213, p < .05) parent knowledge conferred greater risk for SUD, but only for those at highest levels of genetic risk. There was no relation between mother- (β = –0.115, ns; β = 0.081, ns) or adolescent-reported (β = –0.043, ns; β = 0.096, ns) knowledge and risk for SUD for those at medium or low levels of risk, respectively. See Figures 2–5 for graphical depictions of and regions of significance for these interactions involving genetic risk and parental knowledge.

Figure 2. (Color online) Interaction between mother-reported parental knowledge and genetic risk to predict substance use disorder (N = 254).

Figure 3. (Color online) Regions of significance (p < .05) for interaction between parental knowledge and genetic risk to predict substance use disorder (mother report of knowledge model). Regression coefficients are nonsignificant at values of the moderator falling within the region (–3.14 to 1.17; gene score mean = 0, SD = 1.53). However, because very few individuals have gene scores below –3.14, it can be concluded that the simple slope of substance use disorder regressed on parental knowledge is significantly different from zero (p < .05) for values of genetic risk at or above 1.17, which is about 0.75 SD above the mean.

Figure 4. (Color online) Interaction between adolescent-reported parental knowledge and genetic risk to predict substance use disorder (N = 254).

Figure 5. (Color online) Regions of significance (p < .05) for interaction between parental knowledge and genetic risk (adolescent report of knowledge model). Regression coefficients are nonsignificant at values of the moderator falling within the region (i.e., –3.11 to 1.47; gene score mean = 0, SD = 1.53). However, because very few individuals have gene scores below –3.11, it can be concluded that the simple slope of substance use disorder regressed on parental knowledge is significantly different from zero (p < .05) for values of genetic risk at or above 1.47, which is about 0.9 SD above the mean.
In addition, in both models, polygenic risk interacted with older adolescent peer substance use to predict emerging adult SUD. Specifically, in both mother- and adolescent-report models, more older adolescent peer substance use increased risk for emerging adult SUD. However, this effect was stronger in both models when examining those at highest (β = 0.684, p < .001; β = 0.636, p < .001) and medium levels of genetic risk (β = 0.551, p < .001; β = 0.457, p < .001), compared to those at lowest levels of genetic risk (β = 0.447, p < .05; β = 0.303, p < .05), respectively.Footnote 9 See Figures 6–9 for graphical depictions of and regions of significance for these interactions involving genetic risk and peer substance use.

Figure 6. (Color online) Interaction between peer substance use and genetic risk to predict substance use disorder (coefficients from mother report of parenting model; N = 254).

Figure 7. (Color online) Regions of significance (p < .05) for interaction between peer substance use and genetic risk (mother report of knowledge model). Regression coefficients are nonsignificant at values of the moderator falling outside the region (–1.89 to 4.93; gene score mean = 0, SD = 1.53). However, because very few individuals have gene scores above 4.93, it can be concluded that the simple slope of substance use disorder regressed on peer substance use is significantly different from zero (p < .05) for values of genetic risk at or below –1.89, which is about 1.25 SD below the mean.

Figure 8. (Color online) Interaction between peer substance use and genetic risk to predict substance use disorder (coefficients from adolescent report of parenting model; N = 254).

Figure 9. (Color online) Regions of significance (p < .05) for interaction between peer substance use and genetic risk (adolescent report of knowledge model). Regression coefficients are nonsignificant at values of the moderator falling outside the region (–1.91 to 3.99; gene score mean = 0, SD = 1.53). However, because very few individuals have gene scores above 3.99, it can be concluded that the simple slope of substance use disorder regressed on peer substance use is significantly different from zero (p < .05) for values of genetic risk at or below –1.91, which is about 1.25 SD below the mean.
Alternative analyses: Substituting dependence for abuse/dependence
Because DSM substance abuse diagnoses may overdiagnose disorder, we tested models that restricted emerging adult SUD to dependence only, which produced few changes in findings. In both mother- and adolescent-report models with this substitution, gender no longer predicted substance dependence. In the mother-report model, an additional finding was detected. Specifically, more younger adolescent peer substance use conferred risk for less older adolescent parental knowledge (β = –0.421, p < .05).
Discussion
The current study had five hypotheses. First, we hypothesized that together, younger and older parental knowledge would mediate the effect of parental AUD on emerging adult SUD. Second, we predicted that younger and older peer substance use would mediate the effect of parental AUD on emerging adult SUD. Third, we hypothesized that older adolescent parental knowledge would mediate the effect of adolescent genetic risk for SUDs on propensity for developing a SUD (no such hypothesis was made about younger adolescent parental knowledge). Fourth, we hypothesized that older adolescent peer substance use would mediate the effect of adolescent genetic risk on propensity for developing a SUD (no such hypothesis was made about younger adolescent peer substance use). Fifth, we predicted that the effects of older adolescent parental knowledge and peer substance use on emerging adult SUD would be stronger for those at higher level of genetic risk.
In terms of the first study hypothesis, findings showed that younger and older parental knowledge together mediated the effect of parent AUD on risk for SUD for those at highest levels of genetic risk using mother report of knowledge. Compared to parents without alcohol disorders, parents with AUDs may have less knowledge about their offspring's activities and interests both because they are inconsistently involved in monitoring and also because their children may be difficult to monitor because of their genetic risk (Klahr & Burt, Reference Klahr and Burt2014; Latendresse et al., Reference Latendresse, Rose, Viken, Pulkkinen, Kaprio and Dick2008). The fact that this effect of parent AUD on mother knowledge was significant over and above young adolescent substance use and genetic risk suggests that caregivers do play a unique role in obtaining information about the life of their adolescents (over and above how potentially difficult the adolescent is to monitor). In addition to parent AUD predicting younger adolescent mother-reported parental knowledge (which in turn influenced older adolescent knowledge), less older adolescent parental knowledge predicted greater risk for a SUD among offspring at higher genetic risk. This association was only significant for those at highest levels of genetic risk, which is consistent with a diathesis–stress Gene × Environment interaction in which adverse environments have the most negative effects on the most vulnerable individuals.
That this link between parent AUD and adolescent report of knowledge (and in turn the indirect effect to predict emerging adult SUD) was nonsignificant may be partially attributable to adolescents reporting the knowledge that their parents collectively provided (i.e., not separated by mother and father). Thus, the nondisordered parent may compensate for the parent with an AUD. Therefore, when adolescents were asked about parental knowledge, they may have estimated it by averaging across parents, and this may have obscured the link between parental AUD and parental knowledge when using adolescent report.
In terms of the second study hypothesis, together, younger and older peer substance use mediated the effect of parental AUD on emerging adult SUD (for those at all levels of genetic risk). Specifically, children of alcoholic parents were more likely to have friends who used substances, which increased their risk for SUDs. Parents with SUDs are more likely to model substance use, are less likely to limit offspring drinking, and are more likely to have behaviorally undercontrolled children (Abar & Turrisi, Reference Abar and Turrisi2008; Sher, Reference Sher1991). All of these factors increase the chance that children of alcoholic parents would associate with deviant peers (Hicks, Krueger, Iacano, McGue, & Patrick, Reference Hicks, Krueger, Iacano, McGue and Patrick2004; Kendler et al., Reference Kendler, Chen, Dick, Maes, Gillespie and Neale2012).
There is a large literature suggesting that peer substance use increases risk for later substance problems, as substance-using friends provide access and opportunity for substance use and influence norms that promote substance use (Borsari & Carey, Reference Borsari and Carey2001; Dishion & Owen, Reference Dishion and Owen2002). We replicated and extended this finding by demonstrating that the association between peer substance use and later SUD is still present over and above earlier levels of adolescents’ own substance use and a polygenic risk score. This finding is important in the context of younger and older adolescent peer substance use together mediating the effect of parental AUD on emerging adult AUD. Specifically, these findings suggest that children of parents with AUDs are at risk for associating with substance-using peers because of some mechanism beyond their own initial levels of substance use, and being genetically at risk for substance misuse. Peer substance use did not mediate the effect of genetic risk on SUDs.
In terms of the third study hypothesis, older adolescent parental knowledge mediated the effect of polygenic risk on risk for SUDs, with the effect of parental knowledge on SUDs only significant for those at highest levels of genetic risk. In addition, the finding that polygenic risk for SUDs was related to older, but not younger, adolescent parental knowledge is consistent with previous literature suggesting that gene–environment effects on substance use, as well as evocative gene–environment correlations become stronger across adolescence (Salvatore et al., Reference Salvatore, Aliev, Bucholz, Agrawal, Hesselbrock and Hesselbrock2014). As individuals move through adolescence, they are more likely to make decisions that are consistent with their genotypes, with these choices affecting their caregivers and home environments. For instance, adolescents at higher genetic risk for SUDs may be more easily able to engage in deviant behaviors as they age, and as a result, may withdraw from their caregivers. Parents may in turn then have less knowledge about these adolescents’ lives. The fact that this relation between genetic risk and parental knowledge was significant even when parental AUD was in the model suggests that over and above parents’ mental and physical presence in their children's lives, offspring risk for SUD influences the amount of knowledge that caregivers have. This suggests a unique role of adolescent self-disclosure on parental knowledge.
This trend of individuals at high genetic risk sharing less with their caregivers across adolescence likely does not describe those at medium and lower genetic risk, which may explain why mean levels of parental knowledge in this sample only decrease slightly across adolescence. The fact that this mediation effect (i.e., parental knowledge mediating the impact of polygenic risk on emerging adult SUD) was replicated across mother report and child report of knowledge suggests that it is robust. Although consistent with an evocative gene–environment effect, it should be noted that this finding could also be explained by passive gene–environment correlation. It may be that parents at higher genetic risk for SUDs have less knowledge about their children's lives, and are likely to have children who are also at higher risk for SUDs. Future research should examine whether this association between adolescents’ genetic risk and parental knowledge is better explained by parents’ genetic risk.
The fact that polygenic risk was related to older adolescent (but not younger adolescent) parental knowledge, whereas parental AUD was related to younger adolescent mother-reported knowledge, suggests that there are multiple mechanisms that influence parental knowledge. It may be that in the beginning of adolescence, the extent to which mothers try to know about their offspring's lives is important. Parents with an AUD may engage in less monitoring, have less positive relationships with their children, and therefore be less aware of their adolescents’ activities. In later adolescence, however, adolescents at higher genetic risk may make risky decisions consistent with their genotypes, and may disclose less to their caregivers (Haworth et al., Reference Haworth, Wright, Luciano, Martin, Geus and Beijsterveldt2009; Scarr & McCartney, Reference Scarr and McCartney1983). In this way, older adolescents may exert more control over how much information their parents have. Although much work has been done examining how parents obtain information about their offspring's lives (e.g., Stattin & Kerr, Reference Stattin and Kerr2000), less research has examined how these mechanisms change across development.
In examining the fourth study hypothesis, we tested whether older adolescent peer substance use mediated the effect of polygenic risk on emerging adult SUD. The polygenic risk score was unrelated to peer substance use, although peer substance use conferred risk for emerging adult SUD (for those at all levels of genetic risk). Therefore, older adolescent peer substance use did not mediate this effect. The finding that polygenic risk was not associated with peer substance use is inconsistent with active and evocative gene–environment correlation. The literature examining genetic influences on an individual's choice of peer group is mixed, with stronger effects appearing in older samples and, in some cases, only among males (Beaver et al., Reference Beaver, Shutt, Boutwell, Ratchford, Roberts and Barnes2009; Chassin et al., Reference Chassin, Lee, Cho, Wang, Agrawal and Sher2012; Iervolino et al., Reference Iervolino, Pike, Manke, Reiss, Hetherington and Plomin2002). This age trend may appear because as individuals age, they gain freedom to associate with those whose behaviors are more consistent with their genotypes. A post hoc analysis of the current data showed that the correlation between polygenic risk and emerging adult peer substance use was significant (zero-order correlation r = .157, p < .05). As individuals move out of adolescence and into emerging adulthood, they may have greater control over the people with whom they socialize (active gene–environment correlation) and/or may be more vulnerable to deviant peer groups (evocative gene–environment correlation).
In examining the fifth and final study hypothesis, we did find evidence for genetic risk moderating the effects of older adolescent parental knowledge as well as older adolescent peer substance use, on emerging adult SUD. In terms of the interaction between parental knowledge and genetic risk, for those at highest levels of genetic risk, less parental knowledge was associated with higher risk of SUD. For those at medium and low levels of genetic risk, there was no relation between parental knowledge and SUD.
Many genetically informed studies have found evidence for a fan-shaped interaction, in which genetic influences are stronger at higher levels of environmental risk and environmental influences are stronger at higher levels of genetic risk (for a review, see Dick, Reference Dick2011). Specifically, interactions between parental knowledge and genetic risk have predicted alcohol use and misuse, such that the genotype exerts a stronger influence in environments with less parental knowledge (Miranda et al., Reference Miranda, Reynolds, Ray, Justus, Knopik and McGeary2012; Salvatore et al., Reference Salvatore, Aliev, Bucholz, Agrawal, Hesselbrock and Hesselbrock2014). These studies suggest that risky environments are most predictive of adverse outcomes for those who are most genetically vulnerable. The fact that this interaction effect is consistent with previous research and was obtained over and above gene–environment correlation increases confidence in its validity. It also extends the evidence to clinical SUD outcomes and makes it less likely that parental knowledge is simply a marker of genetic risk for SUDs in the prediction of SUD.
Similar to the case of parental knowledge, genetic risk and older adolescent peer substance use interacted to predict emerging adult SUD. Although the simple slopes for peer substance use on emerging adult SUD were significant at all levels of genetic risk, this effect was more pronounced at highest and medium levels of genetic risk. This finding is consistent with literature finding stronger effects of deviant peer affiliations on substance use phenotypes for those at higher levels of genetic risk (Miranda et al., Reference Miranda, Reynolds, Ray, Justus, Knopik and McGeary2012; Salvatore et al., Reference Salvatore, Aliev, Bucholz, Agrawal, Hesselbrock and Hesselbrock2014). As with the interaction between parental knowledge and genetic risk, this finding suggests that adverse environments have the most negative effects for those most vulnerable individuals. The fact that this interaction holds over and above gene–environment correlation increases confidence in its validity. This finding also adds to existing literature by establishing that peer substance use exerts a prospective effect on emerging adult clinical SUD outcomes, with this effect increasing for those at highest levels of genetic risk for SUD.
It is important to consider these findings in the context of prevention and intervention. First, because polygenic risk predicted older adolescent but not younger adolescent parental knowledge, parenting programs may think about educating caregivers about increasing offspring effects on their parenting behaviors as their adolescents age. Parenting programs might also continue to encourage parents to take active steps to learn about their adolescents’ lives. Although it is recognized that many adolescents thrive on increasing autonomy during this time, it is important for parents of older adolescents to engage with their adolescents and solicit information from them in order to decrease the likelihood of later SUD. Some researchers have found that parental knowledge and accessibility during adolescence prospectively predicted quantity and frequency of drinking during the first year of college (Abar & Turrisi, Reference Abar and Turrisi2008; Turrisi & Ray, Reference Turrisi and Ray2010).
Second, the finding that, together, younger and older peer substance use mediated the effect of parent AUD on SUD suggests that peers uniquely influence risk for SUD. Intervention work should emphasize to parents with AUDs the importance of peer relationships for their adolescents. These interventions should target counteracting the harmful, normalizing messages that substance-using peers communicate to adolescents about alcohol and drugs.
Third, the findings that late adolescent parental knowledge and peer substance use exerted stronger prospective effects on emerging adult SUD for those at higher levels of genetic risk are also noteworthy. These findings suggest that intervention efforts that increase parental solicitation of information and counteract the harmful messages that substance-using peers communicate in late adolescence may be especially important in reducing risk for emerging adult SUD among those at greater propensity for alcohol and drug misuse.
Although the current findings contribute to the literature by delineating the roles of parental knowledge and peer substance use in the development of clinical SUD while considering gene–environment correlation, there are also limitations to consider. First, parent genotype was not measured, so the extent to which parents’ genetic risk influences parenting could not be tested. Prior research has found evidence for significant passive gene–environment effects (Rice, Lewis, Harold, & Thapar, Reference Rice, Lewis, Harold and Thapar2013). Second, in creating the polygenic risk score, we assumed that SNP effects were linear, that each SNP did not interact with others, and that each SNP did not moderate main effects in different ways, which some have previously found (Salvatore & Dick, Reference Salvatore and Dick2015). Third, the effect of this polygenic risk score, as well as other effects of measured genes on outcomes, is typically small (Bierut, Reference Bierut2011) and the sample included relatively few participants. Therefore, the current study may have been underpowered to detect main effects, and even more underpowered to detect interaction effects (Dick et al., Reference Dick, Agrawal, Keller, Adkina, Aliev and Monroe2015). Fourth, a three-item measure of parental knowledge may be less stable and reliable than a measure including more items. Study findings would have also been strengthened by the use of observational measures of parenting and/or defiant peer affiliation (e.g., Granic, Dishion, Hollenstein, & Patterson, Reference Granic, Dishion, Hollenstein and Patterson2003), instead of relying on self-report of these constructs. In addition, we only included mother report and child report of knowledge, so these findings may not generalize to findings using father report of parenting. These findings may also not generalize to families who are not of Caucasian or Hispanic ethnicity. The current subsample (N = 254) is also at lower risk (i.e., less likely to be male, have a parent with an AUD, and meet criteria for a SUD) compared to those who were excluded from the current study analyses (N = 200). Although these differences were small in magnitude, current study findings should be interpreted with caution. Fifth, there are major biological and social differences between 11- and 14-year-olds and between 18- and 25-year-olds (including exposure to substance-using peers), yet individuals within these two age bands were grouped together, potentially obscuring some of the relations among constructs.
In summary, the current study found that parental AUD predicted younger adolescent parental knowledge using mother report, and genetic risk predicted older adolescent parental knowledge, suggesting that there are multiple pathways that influence parental knowledge about their offspring's lives. Moreover, over and above gene–environment correlation, less parental knowledge and more peer substance use predicted greater risk for SUDs, with these effects being stronger for those at higher levels of genetic risk. Finally, this study replicated previous research in finding that peer substance use mediated the effect of parental AUD on SUD and extends this literature by demonstrating this effect over and above polygenic risk. Taken together, these findings support the ideas that evocative gene–environment effects increase with age across adolescence and that some of the most important environmental risk factors for SUDs exert nonuniform effects that vary across level of genetic propensity.
















