Oppositional–defiant and conduct problems (CPs) in childhood are a reliable precursor of most adult mental health problems (Copeland, Shanahan, Costello, & Angold, Reference Copeland, Shanahan, Costello and Angold2009; Kim-Cohen et al., Reference Kim-Cohen, Caspi, Moffitt, Harrington, Milne and Poulton2003). Among children with CPs, high callous–unemotional (CU) traits (lack of concern for others’ feelings, lack of guilt/remorse) specify the developmental precursor to psychopathy and indicate a distinct etiology (Frick & White, Reference Frick and White2008). That is, CPs with high CU traits develop relatively independently of parenting (Kroneman, Hipwell, Loeber, Koot, & Pardini, Reference Kroneman, Hipwell, Loeber, Koot and Pardini2011; Oxford, Cavell, & Hughes, Reference Oxford, Cavell and Hughes2003; Pasalich, Dadds, Hawes, & Brennan, Reference Pasalich, Dadds, Hawes and Brennan2011; Wootton, Frick, Shelton, & Silverthorn, Reference Wootton, Frick, Shelton and Silverthorn1997), and biological determinants of CP may predominate in high CU traits children. Specifically, high CU traits are associated with lower levels of fear (Frick, Lilienfeld, Ellis, Loney, & Silverthorn, Reference Frick, Lilienfeld, Ellis, Loney and Silverthorn1999), decreased responsiveness to negative emotional stimuli (Blair, Morris, Frith, Perrett, & Dolan, Reference Blair, Morris, Frith, Perrett and Dolan1999; Dadds et al., Reference Dadds, Perry, Hawes, Merz, Riddell and Haines2006), and dampened reactivity to emotional stimuli in the amygdala (De Brito et al., Reference De Brito, McCrory, Mechelli, Wilke, Jones and Hodgins2011; Deeley et al., Reference Deeley, Daly, Surguladze, Tunstall, Mezey and Beer2006; Jones, Laurens, Herba, Barker, & Viding, Reference Jones, Laurens, Herba, Barker and Viding2009; Viding et al., in press); and twin studies show relatively high genetic loadings for CU traits (heritability estimates = ~0.67) and CPs in the presence of high CU traits (~0.81), compared to CPs in the absence of CU traits (~0.30; Viding, Blair, Moffitt, & Plomin, Reference Viding, Blair, Moffitt and Plomin2005; Viding, Frick, & Plomin, Reference Viding, Frick and Plomin2007).
A candidate system that may be related to the core characteristics of psychopathy is the oxytocin (OXT) system. OXT is a neuropeptide synthesized in hypothalamic cells that plays crucial roles in parturition and lactation (Gopal Rao, Loffler, Battey, & Hansmann, Reference Gopal Rao, Loffler, Battey and Hansmann1992) as well as in affiliative/prosocial behavior. It can be studied via circulating levels, via polymorphisms in the OXT receptor gene (OXTR), and by manipulating endogenous levels via nasal sprays or injections. Evidence from each of these shows that OXT function is likely to characterize aspects of psychopathy: circulating blood levels are associated with affiliative/prosocial behavior; administration of OXT impacts the perception of emotion, trust, and generosity as well as amygdala function, all known to be impaired in psychopathy (Meyer-Lindenberg, Domes, Kirsch, & Heinrichs, Reference Meyer-Lindenberg, Domes, Kirsch and Heinrichs2011). Further, several common polymorphisms of the OXTR gene are associated with phenotypic variations in affiliation, empathy, and associated neural systems (Ebstein, Knafo, Mankuta, Chew, & Lai, Reference Ebstein, Knafo, Mankuta, Chew and Lai2012). A number of authors have hypothesized a role for OXT in psychopathy (Bora, Yucel, & Allen, Reference Bora, Yucel and Allen2009; Dadds & Rhodes, Reference Dadds and Rhodes2008), and we recently proposed that central OXT influences the balance of central versus basolateral amygdala function in cognitive and emotional processing (Moul, Killcross, & Dadds, Reference Moul, Killcross and Dadds2012). Few molecular genetic studies have stratified CP samples by CU or psychopathic traits (Sakai et al., Reference Sakai, Crowley, Stallings, McQueen, Hewitt and Hopfer2012). However, two recent studies have shown that common polymorphisms of common OXTR single nucleotide polymorphisms are associated with high levels of CU traits in CP samples (Beitchman et al., Reference Beitchman, Zai, Muir, Berall, Nowrouzi and Choi2012; Dadds, Reference Dadds2013).
Variations in single nucleotide polymorphism frequencies are unlikely to explain large variance in the psychopathy phenotype (Viding et al., Reference Viding, Hanscombe, Curtis, Davis, Meaburn and Plomin2010), and several complementary strategies are needed. First, genetic variations in OXTR should be enriched with data on circulating levels of OXT. Plasma levels are known to correlate with important state and trait behaviors (Ebstein et al., Reference Ebstein, Knafo, Mankuta, Chew and Lai2012; Meyer-Lindenberg et al., Reference Meyer-Lindenberg, Polin, Kohn, Holt, Egan and Weinberger2001), and with genetic variations in the OXTR gene (Feldman et al., Reference Feldman, Zagoory-Sharon, Weisman, Schneiderman, Gordon and Maoz2012) that are associated with psychopathy. Lower peripheral levels are generally associated with lower trust, affiliation, and higher psychopathology; however, the opposite is also found (Hoge, Pollack, Kaufman, Zak, & Simon, Reference Hoge, Pollack, Kaufman, Zak and Simon2008; Jansen et al., Reference Jansen, Gispen-De Wied, Wiegant, Westenberg, Lahuis and Van Engeland2006; Mitchell et al., Reference Mitchell, Smid, Troelstra, Wever, Ziegler and Beech2012).
Second, the function of the OXTR gene is influenced by epigenetic variations or gene expression. Methylation is an epigenetic process by which gene transcription is “repressed” because of the specific binding of methyl molecules to “islands” of regions of DNA where a cytosine nucleotide occurs next to a guanine nucleotide in the linear sequence of bases along its length, linked together by phosphate binding (CpG sites). In mammals, 70% to 80% of CpG cytosines are methylated and can be triggered by a variety of biological and environmental stimuli (Meaney & Ferguson-Smith, Reference Meaney and Ferguson-Smith2010). Increased methylation is generally associated with reduced messenger RNA (mRNA) signaling and the resulting protein products. It is important that there is increasing recognition that important environmental processes, such as quality of parenting and abuse history, can lead to methylation of genes critical for neurodevelopment (Meaney & Szyf, Reference Meaney and Szyf2005; van IJzendoorn, Bakermans-Kranenburg, & Ebstein, Reference van IJzendoorn, Bakermans-Kranenburg and Ebstein2011). Further, some DNA methylation states appear to be reversible (Dulac, Reference Dulac2010; Meaney & Ferguson-Smith, Reference Meaney and Ferguson-Smith2010). Methylation of the OXTR gene is a likely candidate for predicting changes in overall OXT function associated with developmental psychopathology (Gregory et al., Reference Gregory, Connelly, Towers, Johnson, Biscocho and Markunas2009).
Research into methylation of the OXTR gene is in its infancy but has generated important leads (Kumsta, Hummel, Chen, & Heinrichs, in press). In the human OXTR gene, there is a CpG island from 140 base pair upstream to 2338 base pair downstream of the transcription start site. Kusui and colleagues (Reference Kusui, Kimura, Ogita, Nakamura, Matsumura and Koyama2001) investigated whether the methylation state of this region affects the transcription of the OXTR gene. HepG2 derived from human hepatoblastoma, in which OXTR gene transcription was suppressed, was treated with a demethylating agent. OXTR mRNA was significantly increased in a dose-dependent manner. They then compared the effect of in vivo methylation of the CpG island on transcriptional activity of OXTR. The reporter gene activity of expression was suppressed by 70% by methylation of the CpG island. The deletion of the methylated segment reduced the suppression to 32%. These results indicate that the methylation of the CpG island in the human OXTR gene promoter suppresses its transcription (at least in liver) and provides the first evidence of functional significance of the methylated region. Gregory et al. (Reference Gregory, Connelly, Towers, Johnson, Biscocho and Markunas2009) showed that methylation of the two CpG sites in the island described by Kusui et al. (Reference Kusui, Kimura, Ogita, Nakamura, Matsumura and Koyama2001) was increased in a sample of children with autism and that the methylation was associated with a 20% reduction in OXTR mRNA. Jack, Connelly, and Morris (Reference Jack, Connelly and Morris2012) showed that methylation of CpG sites with the region are associated with the regulation of neural processes associated with the social interpretation of ambiguous stimuli.
There is increasing evidence that changes in epigenetic regulation of gene expression are naturally sensitive to critical developmental periods (Szyf & Bick, Reference Szyf and Bick2013). Thus, maturation, especially pubertal changes, is likely to be critical to understanding associations between methylation and complex traits such as psychopathy. Considerable research in animals shows that androgens and central/peripheral OXT undergo interdependent changes around puberty (Insel, Young, Witt, & Crews, Reference Insel, Young, Witt and Crews1993). Sex hormones are also known to trigger promoter region methylation in several genes associated with neurodevelopment and complex illnesses (Kaminsky, Wang, & Petronis, Reference Kaminsky, Wang and Petronis2006). In terms of the psychopathy phenotype, a wealth of evidence indicates that levels and the nature of aggression, antisocial behavior, and related traits, such as empathy and risk taking, undergo dramatic changes at puberty (Moffitt, Reference Moffitt1993; Sisk & Foster, Reference Sisk and Foster2004). The ideal design for research trying to map the development of antisocial behavior and psychopathy would be longitudinal studies of the changing phenotype in relation to gene expression and OXT function through puberty. In the meantime, the use of cohorts stratified by puberty is a reasonable starting place.
We report here on a first pilot test of epigenetic methylation and functional levels of OXT in the development of psychopathy. We hypothesized that in CP male children, CU traits would be associated with lower circulating OXT and increased methylation of the promoter region of the OXTR gene. Given the primitive state of the literature on age and OXT, we tested a null hypothesis that these associations would apply equally across pre- and postpubescent groups.
Methods
Participants
Ethics approval was obtained from the University of New South Wales. Participants were a sample of males referred for the treatment of CPs who (a) met formal criteria for DSM-IV diagnosis and severity rating (0–6, where >3 = frank diagnosis; American Psychiatric Association, 1994) of CPs (oppositional–defiant disorder or conduct disorder) using the Diagnostic Interview Schedule for Children, Adolescents, and Parents structured interview (Holland & Dadds, Reference Holland and Dadds1997), (b) were aged from 4 to 16 years, (c) had no major neurological/physical illness, (d) IQ > 70, and (e) had no diagnosis or features of autism.
Our original ethics approval was for a sample volume of blood sufficient only to extract DNA. Approval to take sufficient blood to extract DNA and assay for circulating OXT came later. As such, the sample sizes for specific biological measures varied as follows: male children with all diagnostic and CU trait measures and any biological measure (N = 156; the demographics for this feeder sample are provided in a later section), a subsample with methylation (n = 98), OXT blood levels (n = 95), and both methylation and OXT blood levels (n = 37). The distribution of puberty by age using the Pubertal Development Scale (Petersen, Crockett, Richards, & Boxer, Reference Petersen, Crockett, Richards and Boxer1988) showed that substantial numbers of the sample started reporting signs of pubertal development at 9+ years, so we split at 8.5 years, resulting in two groups: 4–8 years and 9–16 years. The specific sample sizes used in each analysis are described in the relevant sections.
Diagnostic procedures and measure
Diagnoses were made using DSM-IV criteria by the assessing psychiatrist/psychologist using the Diagnostic Interview Schedule for Children, Adolescents, and Parents (Holland & Dadds, Reference Holland and Dadds1997) with parents and the child for those older than 8 years. Diagnoses were checked by having a second diagnostic team make an independent diagnosis. Kappa agreements on primary and secondary diagnoses were always >0.7. The level of CU traits was measured using the Antisocial Process Screening Device (Frick & Hare, Reference Frick and Hare2001) and the prosocial subscale of the Strengths and Difficulties Questionnaire (Goodman, Reference Goodman1997). This system produces reliable indices and has been extensively validated (Dadds, Frost, Fraser, & Hawes, Reference Dadds, Frost, Fraser and Hawes2005; Dadds & Hawes, Reference Dadds and Hawes2006; Dadds & Rhodes, Reference Dadds and Rhodes2008). The DSM-5 proposal for a CU specifier to the diagnosis of conduct disorder (Frick & Moffitt, Reference Frick and Moffitt2010) suggests that CU traits be evident across settings; thus, we collected reports from mothers, fathers, teachers, and for children >9 years (mothers 92.4%, fathers 39.0%, teachers 51.1%, and youths 27.2%). All of these had good reliability (range α = 0.77–0.90), and correlations of mothers to other raters were r = .570 and p < .001 for father, r = .219 and p < .001 for teacher, and r = .344 and p < .01 for youths. The top one-third of CU trait scores was used to designate “high CU,” consistent with prevalence estimates in CP samples (Frick & Moffitt, Reference Frick and Moffitt2010). Adversity for the child was measured using the Quality of the Family Environment (Rey et al., Reference Rey, Singh, Hung, Dossetor, Newman and Plapp1997), a clinician rating scale of the lowest quality of family environment to which the child was exposed during a substantial period (at least 1 year) before the age of 12. Ratings were made by a second naive clinician on a subset of cases (r = .96).
Participants gave blood at local pathology collection clinics, and DNA extraction rates were >95%. Samples were genotyped using iPLEX Gold™ primer extension followed by mass spectrometry analysis on the Sequenom MassARRAY system (Sequenom, San Diego, CA) by the Australian Genome Research Facility (http://www.agrf.org.au/). OXTR methylation levels were quantified for blood samples only using the EpiTYPER assay on the Sequenom MassARRAY system at the Australian Genome Research Facility. Methylation was assayed for 11 CpG dinucleotides within a CpG island (Chr3:BP 8810680 to 8810890: February 2009 GRCh37/hg19 build) at the promoter region of the OXTR gene as described by Gregory et al. (Reference Gregory, Connelly, Towers, Johnson, Biscocho and Markunas2009): left primer, aggaagagagGGTGTAGGTAGTTGGGTGTTAAGTA; right primer, cagtaatacgactcactatagggagaaggctCTATTCCCAAACCCTAACATAAACA; and Amplicon 5′ start, chr3:8810923; Amplicon 3′ end, -chr3:8810650; all assayed CpGs: chr3:8810890, chr3:8810875, chr3:8810863, chr3:8810856, chr3:8810833, chr3:8810808/8810798, chr3:8810775, chr3:8810734, chr3:8810709, chr3:8810700, and chr3:8810682/8810680. Silent signals occurred for four dinucleotides and the data for a further one was considered unreliable, leaving six CpG dinucleotides.
The reliability of the methylation assays was checked for each CpG site. Epitect control DNA samples (Qiagen) known to be fully methylated and fully unmethylated were assessed in parallel to the study DNA to determine the upper and lower limits of detection for each assay. Methylation levels were measured at >92% and <7% for fully methylated and fully unmethylated DNA, respectively.
Previous research has variously looked at methylation at individual CpG sites as well as mean methylation levels across sites (Szyf & Bick, Reference Szyf and Bick2013). Little is known about the convergence of sites within the CpG island of OXTR. Thus, we started by looking at the possibility of reducing the number of tests to be conducted by assessing the convergence of the six dinucleotides into a single measure. Using SPSS scale to produce the Cronbach α indices of convergence, all six resulted in poor convergence (Cronbach α = 0.50). There was reasonable convergence for four dinucleotides to a single scale (Cronbach α = 0.66). These final dinucleotides used were 8810863, 8810833, 8810798/8810808, and 8810700.
In terms of the individual CpG sites, the final six dinucleotides and the proportion methylation for each is shown in Table 1. The extent of methylation across the CpG islands was consistent with previous research: the mean percentage of methylation ranged from 7.3% to 74.3% in this CP sample, compared to 12.2% to 65.8% in controls and 35.1% to 68.1% in the autism probands from the Gregory et al. study (2009).
Table 1. The proportion of methylation for each of the six CpG dinucleotides used in the study
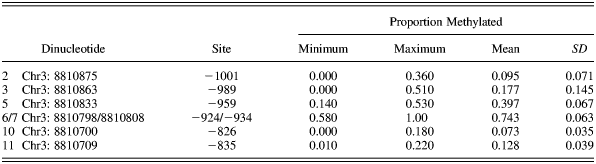
Note: The site position is according to the translation start site (+1). CpG, regions of DNA where cytosine and guanine nucleotides occur next to each other and are linked by phosphate binding.
For plasma OXT, 4 ml of blood was collected using EDTA acid tubes. Samples were mixed well and kept on ice before spinning at 3000 rpm for 5–10 min. Plasma was poured off and kept frozen at –40°C until analysis. Extraction of OXT was performed in the Department of Behavioural and Molecular Neurobiology (Prof. Inga Neumann, University of Regensburg) using cold acetone ether. OXT levels were determined by radioimmunoassay in the laboratory of Prof. R. Landgraf at the Max-Planck Institute of Psychiatry. The intraassay coefficient of variation was 7%–19%, and the minimum level of detection was 0.5 pg. The cross-reactivity with other related peptides including vasopressin was <0.7%.
Results
Statistical significance levels for each comparison were adjusted to maintain p = .05 across multiple comparisons using Bonferroni methods. Demographic and diagnostic data on the full sample are shown in Table 2. We checked the equivalence of the full sample and subsamples for which OXT plasma levels, methylation and both OXT and methylation measures were available. There were no differences on demographic or diagnostic variables on the variables tested and shown in Table 2. We also checked the equivalence of the high and low CU groups, and Table 2 shows data split by CU traits. There were no differences between the groups on these measures; however, the need for covariates did arise in specific analyses, especially OXT blood levels, and these are described in the relevant sections below. If the use of the covariates did not alter the substance of the findings, they were omitted from the final results presented. Data for OXT blood levels showed the expected positive skew and, thus, were transformed into logNat scores.
Table 2. Demographic and diagnostic severity data on full sample split by high versus low CU groups
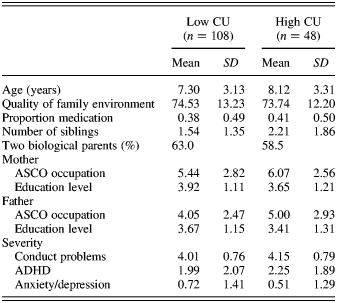
Note: Education level: 1 = primary school, 5 = university; ASCO occupation: 1 = highest professional, 10 = lowest. CU, callous–unemotional traits; ASCO, Australian Standard Classification of Occupations; ADHD, attention-deficit/hyperactivity disorder.
Relationship of CU traits to methylation levels
The first hypothesis concerned the relationship of CU traits and age groups to methylation levels. Methylation levels can reflect exposure to environmental adversity (Meaney & Szyf, Reference Meaney and Szyf2005) and medication usage, so we first examined correlates of methylation that might need to be considered. Using a comprehensive range of measures of parenting quality (Quality of the Family Environment), parental psychopathology, and socioeconomic adversity, we were unable to find any significant relationships to environmental adversity or with level of comorbid symptoms. Thus the association of CU and mean methylation levels (from the four convergent sites) were tested with an analysis of variance with CU and age groups as predictors. Sample sizes are shown in Figure 1. There was an interaction between CU and age group, F (1, 93) = 9.644, p = .003, which is represented in the left panel of the figure. Planned contrasts within age groups showed that in the younger group, methylation did not vary by level of CU traits (p = .377); whereas, methylation was significantly higher in the older high-CU trait group (p = .022) than in the older low-CU trait group. Contrasts within each CU group showed that for the low CU group, there was a trend toward lower methylation in the older children (p = .054). In the high CU group, there was a trend toward higher methylation in the older group (p = .066).
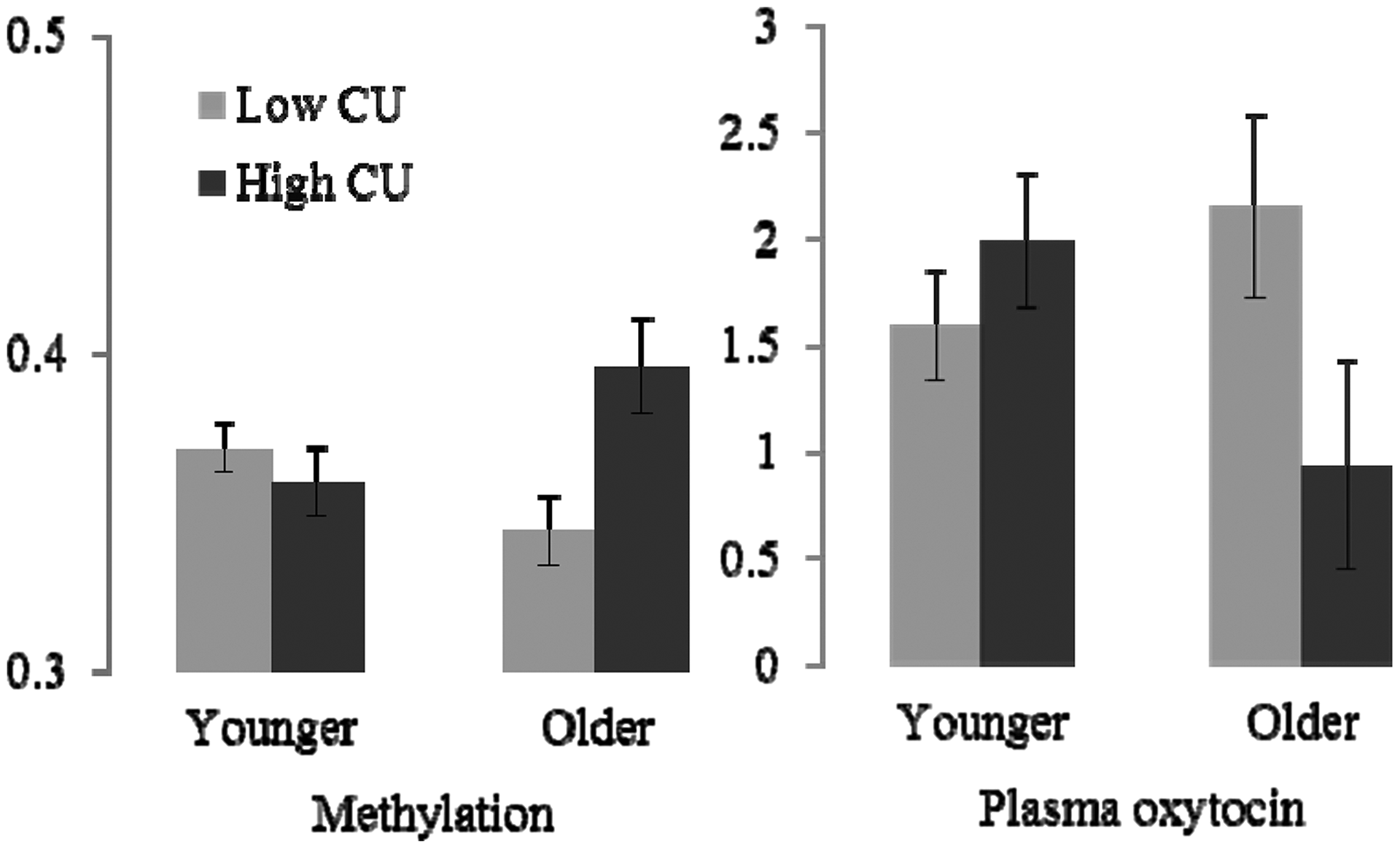
Figure 1. The mean proportion of methylation levels and natural log oxytocin (OXT) plasma levels (pg/ml) by callous–unemotional (CU) traits and age group. Younger = 4–8 years; older = 9–16 years. Error bars indicate the standard error of the mean. Sample sizes for methylation (N = 98): younger, low CU (n = 45), high CU (n = 20); older, low CU (n = 21), high CU (n = 12). Sample sizes for OXT (N = 95): younger, low CU (n = 36), high CU (n = 18); older, low CU (n = 29), high CU (n = 12).
Given the significant relationship of CU and age to mean methylation levels in the four convergent CpG sites, we proceeded to test the significance of each of the six CpG sites individually. There were several reasons for this. Recall that we reduced the methylation scores across the six candidate CpG sites to a single index of mean methylation levels using four convergent sites; convergence was less than optimal, however, and there is evidence that CpG sites vary in their influence on gene transcription and behavioral phenotypes for OXTR (Gregory et al., Reference Gregory, Connelly, Towers, Johnson, Biscocho and Markunas2009; Kumsta et al., in press; Kusui et al., Reference Kusui, Kimura, Ogita, Nakamura, Matsumura and Koyama2001) and more generally (Szyf & Bick, Reference Szyf and Bick2013). We also wanted to compare our results with Gregory et al. (Reference Gregory, Connelly, Towers, Johnson, Biscocho and Markunas2009), who reported on specific sites.
Using a multivariate analysis of variance, there was a nominal main effect for CU traits for CpG5 chr3:8810833, F (1, 89) = 5.410, p = .022, and interactions of age group and CU traits at two sites: CpG3 chr3:8810863, F (1, 89) = 4.027, p = .048, and CpG5 chr3:8810833, F (1, 89) = 10.447, p = .002. Only the latter was significant using a Bonferroni correction. Planned contrasts showed that the source of the interaction for CpG5 was differences between low- and high-CU trait groups within the older group (younger: low CU, M = 0.409, SE = 0.009; high CU, M = 0.394, SE = 0.015; older: low CU, M = 0.364, SE = 0.014; high CU, M = 0.442, SE = 0.018). Gregory et al. (Reference Gregory, Connelly, Towers, Johnson, Biscocho and Markunas2009) found that this site differentiated autism from control probands in methylation levels in cortex tissue but not in blood. Their site –934 (Chr3:8810809) consistently differentiated autism from controls across all tissue sources and proband combinations. This site did not differentiate high versus low CU traits in this study.
Relationship of CU traits to peripheral OXT levels
Next, we examined the relationship of CU traits and age group to plasma OXT levels. Sample sizes for these analyses are shown in Figure 1, and it should be recalled that this sample is overlapping but comprises different children from those described for the methylation analyses above. We examined correlates of plasma OXT levels that might need to be considered. There were small correlations (all borderline significance: .05 < p < .1) between OXT levels and with complexity of parenting system (absence of biological parents; presence of alternative caregivers, e.g., stepparents or grandparents, = high OXT), severity of comorbid ADHD, and use of stimulant medication (= lower OXT). The use of these as covariates did not affect the analysis of variance results and are thus not reported. There was a significant interaction between age group and CU traits group, F (1, 89) = 5.421, p = .002. Figure 1 shows the interaction. Planned contrasts within age groups showed that OXT levels did not vary by CU traits (p = .174) in the younger group, whereas OXT levels were significantly lower in the high-CU trait group (p = .039) in the older group. Contrasts within CU groups showed that age group had no effect on OXT levels (p = .148) for the low CU group, but older children showed significantly higher levels of OXT (p = .044) in the high CU group.
The direct test of the association of methylation and OXT levels
The above analyses show that high CU traits in the older children are associated with both higher methylation levels of two CpG sites, and lower circulating OXT levels. This suggests that the degree of methylation is directly associated with lower circulating OXT levels; however, in order to conduct our tests with maximum statistical power, we used overlapping but different samples of children with methylation and OXT measures available. The association needs to be tested directly. Thus, we concluded by looking at correlations between methylation levels and OXT in the subsample for which we had both measures (n = 37; younger, n = 28; older, n = 9), albeit with the caution that numbers are greatly reduced for these analyses. Given its strongest association to CU traits, we restricted the analyses to methylation levels at the CpG5 site. In the full sample, there was no significant correlation between methylation levels and circulating OXT (r = –.122, ns). Similarly in the younger children, there was no significant correlation (r = .184, ns). In the older children there was a significant negative association (r = –.598, p = .05). The correlations in the younger and older groups were significantly different from each other (z = 1.970, p = .048).
Discussion
Early-onset behavioral problems are a robust predictor of a range of adult problems. The presence of high CU traits, a developmental correlate of adult psychopathy, specifies a relatively homogenous subgroup. High CU traits are associated with low environmental susceptibility, and much attention has been drawn to the biological aspects of core characteristics, such as low empathy and poor emotion recognition. There is tentative evidence that impairments in the OXT system may characterize these deficits, and recent studies have shown that high CU traits are associated with variations in common polymorphisms of the OXTR gene (Beitchman et al., Reference Beitchman, Zai, Muir, Berall, Nowrouzi and Choi2012; Dadds, Reference Dadds2013; Malik, Zai, Abu, Nowrouzi, & Beitchman, Reference Malik, Zai, Abu, Nowrouzi and Beitchman2012). We tested a hitherto unexplored aspect of the predicted association between OXT function and CU traits by testing plasma OXT levels and epigenetic changes to the promoter region of the OXTR gene.
The results show that multiple variations in the OXTR system characterize the early signs of psychopathy. High levels of CU traits were associated with methylation and OXT levels differently across age groups. In younger males, CU traits were generally not associated with levels of circulating OXT and methylation. High CU traits in older males were associated with higher levels of methylation of the specific CpG sites in the receptor gene; in a related sample, we also showed that high CU traits in older children were associated with lower levels of circulating OXT. In a smaller sample of children who had measures of methylation and circulating OXT levels, we confirmed that increased methylation was associated with lower circulating levels; however, this was again true only for the older group.
We propose that increased methylation may be indexing an overall down regulation or dampening of the OXT system in these older, roughly “pubertal” male subjects. The specific reason for differences in the age groups is not known. It is known that the nature and level of CPs vary considerably between prepubescent and pubescent males (Moffitt, Reference Moffitt1993). Further, there is some evidence that the specific impairments in empathy that characterize high CU traits may also undergo age-related transformations (Dadds et al., Reference Dadds, Hawes, Frost, Vassallo, Bunn and Hunter2009). Thus, it is possible that variations in the phenotype and its measurement are contributing to the observed differences. As noted, however, epigenetic changes are highly sensitive to developmental changes and periods for a host of biological and environmental reasons. Further research may benefit from using longitudinal designs to look more carefully at methylation levels and the relationship of OXT function to empathy and CPs through this transition point.
In the meantime, it is useful to ask why these age-related changes may occur. First, it should be noted that we cannot assume that the increased methylation is driving the CU traits. A possible explanation for methylation levels varying only in the older children is that the CU traits may be driving the changes. Persistent high levels of CU traits through childhood might result in a range of behaviors and interpersonal experiences, such as minimal intimacy and eye contact with attachment figures (Dadds et al., Reference Dadds, Allen, Oliver, Faulkner, Legge and Moul2012), that downregulate various aspects of the OXT system and lead to suppression of the OXTR through increased methylation and other epigenetic processes. Thus, increased methylation may be an outcome of high CU traits resulting in a more permanent change to the function of the OXT system. Second, we cannot exclude the possibility that there are different processes involved in the development and measurement of the CU construct in children versus adolescents. Third, it is possible that there is a genuine effect of puberty whereby changes in hormones, such as testosterone, long known to be associated with puberty and aggression, are influencing the epigenetic changes in the OXTR system for the high CU group. Evidence from the animal literature suggests that this may be a viable interpretation of our results (Arsenijevic & Tribollet, Reference Arsenijevic and Tribollet1998; Insel et al., Reference Insel, Young, Witt and Crews1993).
In terms of the functional properties of specific methylation sites in the OXTR gene, the current study identified both overlap with and differences to the specific sites found previously for autism. We found CpG site chr3:8810833 differentiated low from high CU; Gregory et al. (Reference Gregory, Connelly, Towers, Johnson, Biscocho and Markunas2009) found that this site differentiated autism from control probands in methylation levels in cortex tissue but not in blood samples. Their site –934 (Chr3:8810809) consistently differentiated autism from controls across all tissue sources and proband combinations. This site did not differentiate high versus low CU traits in this study. This overlap but differentiation is consistent with the respective phenotypes, which show commonalities in terms of empathy deficits defined broadly but differences in terms of their specific expression in cognitive versus affective domains (Blair, Reference Blair2008; Jones, Happé, Gilbert, Burnett, & Viding, Reference Jones, Happé, Gilbert, Burnett and Viding2010). It is important that we ensured the current results were not due to any covariation of CU traits and autism features. The results are likely to reflect the well-documented relationship of the OXT system to empathic function and may differentiate early forms of empathy deficit in psychopathy versus autism.
Research into the functional significance of the epigenetic changes in the OXTR gene is in its infancy. However, the review by Kumsta et al. (in press) shows that changes to methylation levels in the promoter region CpG island are associated with reductions in mRNA signaling of the gene (Kusui et al., Reference Kusui, Kimura, Ogita, Nakamura, Matsumura and Koyama2001) and related to phenotypes of autism (Gregory et al., Reference Gregory, Connelly, Towers, Johnson, Biscocho and Markunas2009) and social cognition (Jack et al., Reference Jack, Connelly and Morris2012). The increases in methylation associated with high CU in the older samples were 44% compared to 36% in the low CU group for CpG5 and 24.7% compared to 12.2% for CpG3. These increases are smaller than those reported by Gregory et al. (Reference Gregory, Connelly, Towers, Johnson, Biscocho and Markunas2009), who found an increase of 18% methylation in CpG5 in cortex tissue associated with autism. It is impossible to say what the likely impacts of these increases are for functional changes to mRNA expression, OXTR frequency, distribution and functionality, and associated OXT function and behavioral phenotypes. We note that the significant negative correlation between methylation of CpG5 and circulating OXT levels in the older males does point to functional links of some significance.
Limitations
There are several limitations of this study that should be noted. Sample size is a limitation, and replication of our results is crucial. Methylation was measured from plasma DNA, and the extent to which this is indicative of brain levels is not clear (van IJzendoorn et al., Reference van IJzendoorn, Bakermans-Kranenburg and Ebstein2011). With regard to OXT plasma levels, blood was taken once at a set time in the morning in a pathology collection center. It was not taken in response to a well-defined psychological event, such as physical affection. Future research could improve upon our design by collecting blood at multiple times and looking at change in plasma levels in response to events chosen for their relevance to OXT function in empathy, affiliation, and so on. Finally, the sample was limited to males, and it is unwarranted to generalize the findings to females given the well-documented gender differences in both OXT function (Carter, Reference Carter2007) and CPs (Zoccolillo, Reference Zoccolillo1993).
Future research
Recent enthusiasm has grown for the use of exogenous OXT for improving trust, emotion recognition, empathy, and related skills for various psychiatric conditions (Liu, McErlean, & Dadds, Reference Liu, McErlean and Dadds2012). However, the likely role of modifications in the OXT system and CU traits and psychopathy are unknown. As with our previous findings with regard to OXTR polymorphisms, the current results could be indicative of a role for OXT in interventions for psychopathy. Specifically, the current findings for methylation and its relationship to plasma levels raise the possibility that biological or environmental processes that lead to epigenetic changes, and the putative repression of the OXTR system, could be identified. There are some indications that some DNA methylation states are reversible (Dulac, Reference Dulac2010; Meaney & Ferguson-Smith, Reference Meaney and Ferguson-Smith2010). In the meantime, research is needed to identify the processes associated with these epigenetic changes as well as to provide more precise specification of their functional significance.





