Since its formalization about three decades ago (Cicchetti, Reference Cicchetti1984; Sroufe & Rutter, Reference Sroufe and Rutter1984), developmental psychopathology has emerged as an increasingly inclusive approach to understanding maladaptive behavior (see, e.g., Cicchetti, Reference Cicchetti, Rolf, Masten, Cicchetti, Nuechterlein and Weintraub1990). As foreshadowed in the writings of its early proponents (e.g., Achenbach, Reference Achenbach1974), developmental psychopathology now bridges multiple scientific disciplines, including psychiatric genetics, child psychiatry, developmental psychology, developmental neuroscience, clinical psychology, and prevention science, which are areas of study that were once independent and in some cases even insular (see Beauchaine, Neuhaus, Brenner, & Gatzke-Kopp, Reference Beauchaine, Neuhaus, Brenner and Gatzke-Kopp2008; Cicchetti, Reference Cicchetti, Beauchaine and Hinshaw2008).
The multidisciplinary perspective embodied in developmental psychopathology follows naturally from recognition that all behaviors, including those that are maladaptive, arise from complex, temporally dynamic interactions between individuals and environments. Accordingly, diathesis–stress models (e.g., Bleuler, Reference Bleuler1963; Gottesman & Shields, Reference Gottesman and Shields1967; Meehl, Reference Meehl1962; Rosenthal, Reference Rosenthal and Rosenthal1963), which were articulated at least a decade before the developmental psychopathology perspective, served as one foundational theme from which the discipline emerged (see, e.g., Beauchaine & Marsh, Reference Beauchaine, Marsh, Cicchetti and Cohen2006; Cicchetti, Reference Cicchetti, Beauchaine and Hinshaw2008). Contemporary transactional models, which are largely unique to developmental psychopathology (e.g., Beauchaine, Hinshaw, & Pang, Reference Beauchaine, Hinshaw and Pang2010; Beauchaine, Klein, Crowell, Derbidge, & Gatzke-Kopp, Reference Beauchaine, Klein, Crowell, Derbidge and Gatzke-Kopp2009; Cicchetti & Toth, Reference Cicchetti and Toth1998; Crowell, Beauchaine, & Linehan, Reference Crowell, Beauchaine and Linehan2009; Dawson, Reference Dawson2008), can actually be viewed as elaborations and extensions of the traditional diathesis–stress framework. Such models acknowledge that vulnerabilities and risk factors operate at many levels of analysis between genes and environments (e.g., epigenetic, endophenotypic, neural, autonomic, emotional, cognitive) and that causal influences often cross levels of analysis, sometimes changing direction in response to endogenous and/or exogenous factors (e.g., Cicchetti, Reference Cicchetti, Beauchaine and Hinshaw2008; Cicchetti & Blender, Reference Cicchetti and Blender2004; Cicchetti & Dawson, Reference Cicchetti and Dawson2002; Cicchetti & Posner, Reference Cicchetti and Posner2005; Mead, Beauchaine, & Shannon, Reference Mead, Beauchaine and Shannon2010). The complexity of these transactional models, as well as the levels of analysis they span, requires a multidisciplinary approach to the study of psychopathology. We therefore cannot expect to understand trajectories of behavioral maladaptation by focusing on any particular level of analysis.
However, our goal in writing this article is not to describe the merits of multidisciplinary or multiple levels of analyses research. Others have done so quite effectively in the past (e.g., Cicchetti, Reference Cicchetti, Beauchaine and Hinshaw2008; Cicchetti & Dawson, Reference Cicchetti and Dawson2002), and some will undoubtedly do so again in this Special Issue. Rather than rearticulate their message, we instead describe the program of research conducted in our lab over the past 12 years or so. Our research is focused primarily on the development of trait impulsivity and its multifinal outcomes, including antisocial and self-injurious behaviors. As our work demonstrates and as we attempt to describe here, heterogeneous and heterotypic developmental trajectories of trait impulsivity cannot be understood when studied at any single level of analysis. Doing so has led to a number of misleading and even faulty conclusions in the past (see Beauchaine et al., Reference Beauchaine, Hinshaw and Pang2010). By studying impulsivity across biological systems (genetic, autonomic, hormonal, neural), psychological constructs (social, affective, motivational), developmental epochs (preschool, middle childhood, adolescence, adulthood), sexes (male, female), and methods of inquiry (self-report, informant report, treatment outcome, cardiovascular, electrophysiological, neuroimaging), we have arrived at a developmental model that we believe captures some of the heterogeneity in outcomes for this highly heritable trait.
As we describe in detail below, one such outcome comprises a developmental trajectory that begins in preschool with hyperactive–impulsive attention-deficit/hyperactivity disorder (ADHD), followed by oppositional and delinquent behavior in grade school, then by criminality and antisocial behavior in adulthood (Beauchaine, Klein, Crowell, Derbidge, & Gatzke-Kopp, Reference Beauchaine, Klein, Crowell, Derbidge and Gatzke-Kopp2009). As Robins (Reference Robins1966) noted nearly 50 years ago, almost all males with antisocial personality disorder (ASPD) follow this developmental trajectory (see also Loeber & Hay, Reference Loeber and Hay1997). However, mere description of antisocial personality development has not resulted in meaningful treatment advances for a condition that costs the US Healthcare system about $25 billion annually in corrections expenditures alone, roughly $200 for each US taxpayer (Bureau of Justice Statistics, 2007).
Our program of research provides an empirical instantiation of the multiple levels of analysis perspective, and it exemplifies the benefits conferred by using any and all available methods needed to understand the development of a complex behavioral trait. Although much remains to be learned about multifinal outcomes of trait impulsivity, we now know much more than we did a decade ago. In addition, we believe that further research in the multiple levels of analysis tradition will continue to advance our understanding of impulsive and antisocial behaviors in the years to come, resulting eventually in improved prevention and intervention programs (see Beauchaine, Neuhaus, et al., 2008).
With this brief discussion in mind, we now describe our research on trait impulsivity as a predisposing, biologically based vulnerability to emerging conduct problems, delinquency, and antisocial personality development. Although some of our work is longitudinal, several of the studies we describe are cross-sectional. This work has been conducted with preschoolers, middle schoolers, adolescents, and young adults. Cross-sectional studies are by nature more limited for informing our understanding of development processes. However, all of the samples we describe were recruited at least in part because they were impulsive; and together they comprise a body of research that provides a coherent picture of how impulsivity interacts with contextual risk to promote the development of antisocial personality and other forms of psychopathology.
Defining Trait Impulsivity
Before continuing, it is necessary to describe our approach to characterizing trait impulsivity. As we have outlined elsewhere, considerable debate exists over whether definitions of impulsivity should be (a) restricted to precise measures on neuropsychological or other cognitive tests (and if so, what specific scales); (b) based on broad factor scores capturing behaviors that cut across functional domains; and/or (c) multifaceted (see Beauchaine & Neuhaus, Reference Beauchaine, Neuhaus, Beauchaine and Hinshaw2008). These debates will undoubtedly continue in the foreseeable future, and we do not wish to review or resolve them here. However, three points are particularly relevant. First, trait measures of impulsivity, defined by DSM (American Psychiatric Association, 1980, 1987, 1994, 2000) derived ADHD scales and closely related constructs (e.g., Achenbach & Edelbrock, Reference Achenbach and Edelbrock1991; Conners, Sitarenios, Parker, & Epstein, Reference Conners, Sitarenios, Parker and Epstein1998), are almost entirely heritable. In reviewing 21 behavioral genetics studies including over 10,000 twin pairs, Willcutt(in press) noted an average heritability coefficient of .85 for ADHD, a value rivaled by no other human behavioral trait except intelligence (see, e.g., Plomin, Pedersen, Lichtenstein, & McClearn, Reference Plomin, Pedersen, Lichtenstein and McClearn1994). These studies strongly suggest that ADHD scales capture a valid construct that can be reliably measured in representative samples.
Second, attempts to operationalize impulsivity based on individual differences in very specific behaviors such as reaction time during verbal tasks (see Oas, Reference Oas1985), errors in maze solving (e.g., Porteus, Reference Porteus1965), perseverative errors during set shifting (e.g., Avila, Cuenca, Félix, Parcet, & Miranda, Reference Avila, Cuenca, Félix, Parcet and Miranda2004), preference for immediate small rewards over delayed larger rewards (e.g., Ainslie, Reference Ainslie1975), and performance on gambling tasks (e.g., Hooper, Luciana, Conklin, & Yarger, Reference Hooper, Luciana, Conklin and Yarger2004) explain at best a modest amount of variance in ADHD scores and are poor predictors of functional outcomes. Although such measures may hold value in identifying specific deficits among individuals and in marking certain subgroups of children with impulse-control problems, they do not capture the highly heritable trait identified by ADHD scales.
Third, considerable evidence now suggests that trait impulsivity is a predisposing vulnerability to disorders across the externalizing spectrum, including ADHD, oppositional defiant disorder (ODD), conduct disorder (CD), delinquency, alcohol/drug abuse/dependencies, and antisocial personality development (see Beauchaine et al., Reference Beauchaine, Hinshaw and Pang2010). This evidence derives from behavioral genetics studies conducted with large twin samples of children and adults in which a common latent factor accounts for most of the covariation among externalizing constructs (e.g., Krueger et al., Reference Krueger, Hicks, Patrick, Carlson, Iacono and McGue2002; Tuvblad, Zheng, Raine, & Baker, Reference Tuvblad, Zheng, Raine and Baker2009; Young, Stallings, Corley, Krauter, & Hewitt, Reference Young, Stallings, Corley, Krauter and Hewitt2000). This factor, which likely captures trait impulsivity (Beauchaine et al., Reference Beauchaine, Klein, Crowell, Derbidge and Gatzke-Kopp2009, Reference Beauchaine, Hinshaw and Pang2010; Beauchaine & Marsh, Reference Beauchaine, Marsh, Cicchetti and Cohen2006), is almost entirely heritable and confers vulnerability to a wide range of externalizing outcomes as affected individuals mature.
For these reasons, we chose to recruit children with very high scores on the hyperactivity/impulsivity dimension of ADHD in our program of study on trait impulsivity and its developmental sequelae. Accordingly, children and adolescents recruited into the clinical groups of our studies have to meet diagnostic criteria for the hyperactive/impulsive or combined subtypes of ADHD, either on the Diagnostic Interview Schedule for Children (Shaffer, Fisher, Lucas, Mina, & Schwab-Stone, Reference Shaffer, Fisher, Lucas, Mina and Schwab-Stone2000) or on an interviewer-administered dimensionalized checklist of DSM-IV (American Psychiatric Association, 1994) hyperactivity/impulsivity symptoms (Gadow & Sprafkin, Reference Gadow and Sprafkin1997). We did not recruit children with the purely inattentive subtype of ADHD for two reasons. Although inattention often co-occurs with hyperactivity/impulsivity, it is not specific. It is more important that evidence suggests that the purely inattentive subtype of ADHD marks a different disorder than the hyperactive/impulsive and combined subtypes, with distinct clinical correlates and neural substrates (see, e.g., Beauchaine et al., Reference Beauchaine, Hinshaw and Pang2010; Milich, Balentine, & Lynam, Reference Milich, Balentine and Lynam2001).
A Brief Conceptual Overview
As we allude to above and have described in detail elsewhere (Beauchaine, Reference Beauchaine2009; Beauchaine, Gatzke-Kopp, & Mead, Reference Beauchaine, Gatzke-Kopp and Mead2007; Beauchaine, Neuhaus, Zalewski, Crowell, & Potapova, Reference Beauchaine, Neuhaus, Zalewski, Crowell and Potapova2011), much of our work over the past 12 years has focused on (a) identifying neurobiological markers of trait impulsivity (e.g., Beauchaine, Gartner, & Hagen, Reference Beauchaine, Gartner and Hagen2000; Beauchaine & Marsh, Reference Beauchaine, Marsh, Cicchetti and Cohen2006; Crowell et al., Reference Crowell, Beauchaine, Gatzke-Kopp, Sylvers, Mead and Chipman-Chacon2006; Gatzke-Kopp, Reference Gatzke-Kopp2011; Gatzke-Kopp et al., Reference Gatzke-Kopp, Beauchaine, Shannon, Chipman, Fleming and Crowell2009; Sauder, Beauchaine, Shannon, & Gatzke-Kopp, Reference Sauder, Beauchaine, Gatzke-Kopp, Shannon and Aylward2012; Shannon, Sauder, Beauchaine, & Gatzke-Kopp, Reference Shannon, Sauder, Beauchaine and Gatzke-Kopp2009) and (b) determining how neurobiological vulnerabilities interact with environmental risk factors to potentiate the development of psychopathology, particularly externalizing behavior disorders (see, e.g., Crowell, Beauchaine, & Lenzenweger, Reference Crowell, Beauchaine, Lenzenweger, Beauchaine and Hinshaw2008; Crowell et al., Reference Crowell, Beauchaine and Linehan2009; Gatzke-Kopp & Beauchaine, Reference Gatzke-Kopp and Beauchaine2007a; Shannon, Beauchaine, Brenner, Neuhaus, & Gatzke-Kopp, Reference Shannon, Beauchaine, Brenner, Neuhaus and Gatzke-Kopp2007; Szajnberg, Elliott-Wilson, Beauchaine, & Waters, Reference Szajnberg, Elliott-Wilson, Beauchaine and Waters2011). Although multiple routes to delinquency have been articulated (see, e.g., Fryer, Crocker, & Mattson, Reference Fryer, Crocker, Mattson, Beauchaine and Hinshaw2008; Gatzke-Kopp & Shannon, Reference Gatzke-Kopp, Shannon, Beauchaine and Hinshaw2008), it has long been known that antisocial adult males almost invariably traverse a trajectory that begins in toddlerhood with severe ADHD, followed in rough temporal sequence by ODD, affiliation with delinquent peers, CD, substance abuse and dependence, and ASPD (see Beauchaine et al., Reference Beauchaine, Hinshaw and Pang2010; Loeber & Hay, Reference Loeber and Hay1997; Loeber & Keenan, Reference Loeber and Keenan1994; Lynam, Reference Lynam1996, Reference Lynam1998; Robins, Reference Robins1966). However, only about half of preschoolers who exhibit ADHD and oppositionality continue on this pathway to more serious conduct problems in later childhood (Campbell, Shaw, & Gilliom, Reference Campbell, Shaw and Gilliom2000). We are interested in why some highly impulsive children progress to more serious externalizing behavior, whereas others do not. This interest has led us to study the development of emotion regulation/dysregulation, which appears to differentiate between impulsive children who do and do not progress along the heterotypically continuous pathway described above.
Our focus on the development of emotion regulation/dysregulation follows from two well-replicated observations. First, strong emotion regulation skills buffer impulsive, biologically vulnerable children from developing more severe conduct problems, even when they are reared in adverse environments (see, e.g., El Sheikh et al., 2009; Shannon et al., Reference Shannon, Beauchaine, Brenner, Neuhaus and Gatzke-Kopp2007). Second, a large proportion of the variance in emotion regulation, and in biological markers thereof, is determined not by heredity but by family environment (see, e.g., Kupper et al., Reference Kupper, Willemsen, van den Berg, de Boer, Posthuma and Boomsma2004, Reference Kupper, Willemsen, Posthuma, De Boer, Boomsma and De Geus2005; Snyder, Schrepferman, & St. Peter, Reference Snyder, Schrepferman and St. Peter1997). This provides leverage for altering children's emotion regulation capabilities through prevention and intervention, a situation that contrasts with that for trait impulsivity, which is almost entirely heritable (see above).Footnote 1 Teaching families of impulsive children to promote the development of strong emotion regulation skills provides their children with a means of coping with inherited impulsivity. Our recent family intervention work with ADHD preschoolers shows that parents can learn to teach their 4- to 6-year-old children stronger emotion regulation skills (Webster-Stratton, Reid, & Beauchaine, Reference Webster-Stratton, Reid and Beauchaine2011a), with effects that (a) last 1 year posttreatment and (b) are associated with concomitant reductions in aggression and other conduct problems (Webster-Stratton, Reid, & Beauchaine, Reference Webster-Stratton, Reid and Beauchaine2011b).
Given our interest in how emotion dysregulation promotes progression along the externalizing spectrum from ADHD to more severe conduct problems among trait impulsive children (see Beauchaine et al., Reference Beauchaine, Klein, Crowell, Derbidge and Gatzke-Kopp2009, Reference Beauchaine, Hinshaw and Pang2010), our work examines behavioral outcomes among young children with ADHD (e.g., Webster-Stratton et al., Reference Webster-Stratton, Reid and Beauchaine2011a, Reference Webster-Stratton, Reid and Beauchaine2011b) and both autonomic and central nervous system (CNS) markers of trait impulsivity and emotion dysregulation in preschoolers (e.g., Crowell et al., Reference Crowell, Beauchaine, Gatzke-Kopp, Sylvers, Mead and Chipman-Chacon2006), middle schoolers (e.g., Brenner & Beauchaine, Reference Brenner and Beauchaine2011; Shannon et al., Reference Shannon, Beauchaine, Brenner, Neuhaus and Gatzke-Kopp2007), and adolescents (e.g., Crowell et al., Reference Crowell, Beauchaine, McCauley, Smith, Stevens and Sylvers2005; Sauder et al., 2012). This work includes studies of boys with ADHD, boys with CD (e.g., Beauchaine, Katkin, Strassberg, & Snarr, Reference Beauchaine, Katkin, Strassberg and Snarr2001; Gatzke-Kopp et al., Reference Gatzke-Kopp, Beauchaine, Shannon, Chipman, Fleming and Crowell2009), and girls with borderline personality traits (e.g., Crowell et al., Reference Crowell, Beauchaine, Hsiao, Vasilev, Yaptangco and Linehan2012, in press; Crowell, Beauchaine, McCauley, et al., 2008). Given the focus of this Special Issue, we include some description of our work on borderline personality development given the overlap in etiology and high comorbidity rates between externalizing disorders and borderline pathology (Beauchaine et al., Reference Beauchaine, Klein, Crowell, Derbidge and Gatzke-Kopp2009; Paris, Reference Paris1997). However, we focus primarily on our work with boys on the externalizing trajectory outlined above. Nevertheless, because borderline personality disorder (BPD) is partially defined by pathological impulsivity and resides in the same cluster of Axis II disorders with ASPD, several authors, including us, have speculated that BPD captures an impulsive–antisocial externalizing trajectory more characteristic of females, rather than an etiologically distinct form of psychopathology vis-à-vis ASPD.
To summarize, a core assumption of our work is that impulsivity interacts across development with socialized deficiencies in emotion regulation to potentiate antisocial personality development primarily among boys and borderline personality development primarily among girls. Broadly speaking, this model can be summarized as follows (see Beauchaine et al., Reference Beauchaine, Klein, Crowell, Derbidge and Gatzke-Kopp2009, Reference Beauchaine, Hinshaw and Pang2010):
1. A principal neurobiological substrate of trait impulsivity, which is expressed early in life as ADHD, is mesolimbic dopamine (DA) dysfunction (described in detail below). Many if not most children with the hyperactive/impulsive and combined subtypes of ADHD suffer from reduced tonic mesolimbic DA activation at rest and reduced phasic mesolimbic DA reactivity to reward cues compared with controls.
2. Impulsive children are especially vulnerable to developing more severe externalizing conduct within high-risk family environments where emotional lability is shaped by negative operant reinforcement contingencies.
3. Over time, such reinforcement contingencies result in enduring patterns of emotion dysregulation, culminating in antisocial and borderline personality development among already impulsive individuals.
Detailed theoretical bases of these assumptions have been presented in recent full-length reviews (Beauchaine et al., Reference Beauchaine, Klein, Crowell, Derbidge and Gatzke-Kopp2009, Reference Beauchaine, Hinshaw and Pang2010; Crowell et al., Reference Crowell, Beauchaine and Linehan2009). Interested readers are referred to these articles for more comprehensive accounts of our transactional model (presented in Figure 1), in which specific genetic vulnerabilities, particularly those that affect DA neurotransmission (top panel), interact with high-risk or protective familial environments to result in either the externalizing trajectory outlined above (left panel), or socialized impulsivity (right panel). In the present article, we focus more on how the use of autonomic nervous system (ANS) markers, neuroimaging experiments, and genetic data (peripheral serotonin) have led our research group to our current thinking about the roles of impulsivity and emotion dysregulation in the development of externalizing and borderline pathologies. Nevertheless, where data are available, we describe how biological vulnerabilities interact with environmental risk factors to predict adverse outcomes.
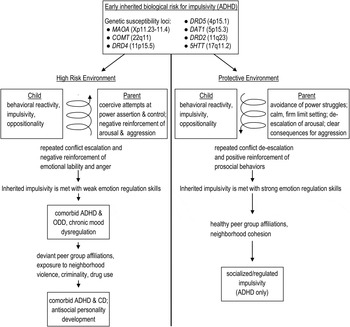
Figure 1. A transactional model of antisocial personality development in which (left) specific genetic vulnerabilities (primarily those affecting dopaminergic neurotransmission) are either amplified by familial reinforcement of emotional lability/emotion dysregulation, resulting in (right) escalation of externalizing behavior across development, or attenuated by development of strong emotion regulation skills, resulting in attention-deficit/hyperactivity disorder. Adapted from “Polyvagal Theory and Developmental Psychopathology: Emotion Dysregulation and Conduct Problems From Preschool to Adolescence,” by T. P. Beauchaine, L. Gatzke-Kopp, and H. K. Mead, Reference Beauchaine, Gatzke-Kopp and Mead2007, Biological Psychology, 74. Copyright 2007 Elsevier. Adapted with permission.
Embedding Genetic Liability Models Into Broader Conceptualizations of Externalizing Conduct
We have already emphasized that externalizing behaviors are multiply-determined outcomes of influences spanning many levels of analysis and that affected individuals evoke and react to environmental risk factors dynamically and transactionally over time. Consistent with the thesis of this Special Issue, capturing this complexity in any single program of research is not possible. It is therefore important that we embed the genetic liability perspective outlined in Figure 1 into (a) a broader conceptual account of emerging externalizing conduct and (b) an extended range of biological vulnerabilities and environmental risk factors for delinquency and antisocial personality development. Although the model presented in Figure 1 presupposes specific genetic vulnerabilities that affect dopaminergic and to a lesser extent serotonergic neurotransmission (see Beauchaine et al., Reference Beauchaine, Klein, Crowell, Derbidge and Gatzke-Kopp2009, Reference Beauchaine, Hinshaw and Pang2010), impulsivity derived from specific patterns of mesolimbic DA activity/reactivity, which may be induced by a broad range of factors known to affect neurodevelopment and functioning, including teratogenic agents, pre- and perinatal stress exposure, and medical complications resulting in hypoxia (see, e.g., Beauchaine et al., Reference Beauchaine, Neuhaus, Zalewski, Crowell and Potapova2011; Fryer et al., Reference Fryer, Crocker, Mattson, Beauchaine and Hinshaw2008; Gatzke-Kopp, Reference Gatzke-Kopp2011; Gatzke-Kopp & Beauchaine, Reference Gatzke-Kopp and Beauchaine2007a; Gatzke-Kopp & Shannon, 2008). Consequently, separating children who are impulsive because of specific genetic vulnerabilities from those who are impulsive partly or wholly because of other etiological factors is not possible in practice (Beauchaine & Neuhaus, Reference Beauchaine, Neuhaus, Beauchaine and Hinshaw2008). In Figure 2 we therefore provide a broadened conceptual model of influences that may shape and/or maintain externalizing behaviors. Consistent with the discussion above, the bottom of this figure represents a range of possible outcomes from ordinary personality variation to Axis II disorders and associated life-long psychopathology. Where an individual falls along this spectrum is moderated by both endogenous (emotion regulation, IQ, etc.) and exogenous (family dynamics, peer influences, gender socialization, etc.) factors. Each of the endogenous vulnerabilities and exogenous risk factors presented are well-established influences in increasing susceptibility to delinquency and antisocial personality development (see, e.g., Beauchaine et al., Reference Beauchaine, Klein, Crowell, Derbidge and Gatzke-Kopp2009; Dishion, McCord, & Poulin, Reference Dishion, McCord and Poulin1999; El-Sheikh et al., Reference El-Sheikh, Kouros, Erath, Keller, Cummings and Staton2009; Lynam et al., Reference Lynam, Caspi, Moffitt, Wikström, Loeber and Novak2000; Mead et al., Reference Mead, Beauchaine and Shannon2010; Meier, Slutske, Arndt, & Cadoret, Reference Meier, Slutske, Arndt and Cadoret2008; Meier, Slutske, Heath, & Martin, Reference Meier, Slutske, Heath and Martin2009; Shannon et al., Reference Shannon, Beauchaine, Brenner, Neuhaus and Gatzke-Kopp2007). However, it is the premise of our work that individuals are differentially susceptible to environmental risk factors and that adversity following environmental risk exposure is most likely for those who are high on trait impulsivity. Thus, the bottom portion of Figure 2 illustrates the concept of multifinality, whereby a single vulnerability (impulsivity) predisposes to a range of heterotypic outcomes, depending on the specific combination and/or sequencing of risk factors incurred over the course of development.
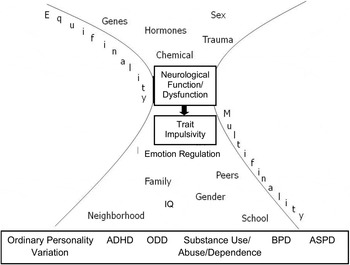
Figure 2. A broadened model of externalizing conduct in which neural vulnerability interacts with contextual risk to produce externalizing spectrum behaviors. In this model, no assumptions are made about specific sources (e.g., genetic, epigenetic, allostatic) of neural dysfunction. Nevertheless, neural deficiencies primarily in the mesolimbic dopamine system (described in text) provide the principal mechanism through which impulsivity is expressed. Adapted from “The Canary in the Coal Mine: The Sensitivity of Mesolimbic Dopamine to Environmental Adversity During Development,” by L. M. Gatzke-Kopp, Reference Gatzke-Kopp2011, Neuroscience and Biobehavioral Reviews, 35. Copyright 2011 Elsevier. Adapted with permission.
Figure 2 expands on heterogeneity of biological vulnerabilities as well. Trait impulsivity derives from individual differences in neural functioning, particularly in the mesolimbic DA system (see above). Tonic activity and phasic reactivity of midbrain and forebrain DA neurons is presumed to arise not only from genes but also from hormones, teratogens, and maladaptive developmental contexts, all of which may be further moderated by sex and allostatic processes (see Beauchaine et al., Reference Beauchaine, Klein, Crowell, Derbidge and Gatzke-Kopp2009, Reference Beauchaine, Neuhaus, Zalewski, Crowell and Potapova2011; Gatzke-Kopp, Reference Gatzke-Kopp2011; Mead et al., Reference Mead, Beauchaine and Shannon2010). Thus, the upper portion of Figure 2 illustrates the concept of equifinality, whereby multiple biological vulnerabilities can result in “tuning” of the developing mesolimbic DA system in a manner that increases trait impulsivity (see Beauchaine et al., Reference Beauchaine, Neuhaus, Zalewski, Crowell and Potapova2011; Gatzke-Kopp, Reference Gatzke-Kopp2011). When both equifinality and multifinality are at play in the development of a complex behavioral trait such as impulsivity, the number of possible individual developmental trajectories becomes immense. This is especially challenging when we focus on specific vulnerabilities (top) and risk factors (bottom) at the widest levels of the hourglass, which illustrates the vast conceptual distance between, for instance, particular genotypes and specific diagnostic classes. When enrolling participants into studies based on these specific diagnostic classes, the expectation that individuals will be characterized by a high frequency of a particular genetic allele fails to consider the vast etiological heterogeneity likely to be present within the diagnostic phenotype. Similarly, selection for specific allelic variants is unlikely to yield diagnostic homogeneity given a nearly unlimited number of intervening developmental experiences. This may be the primary reason why candidate alleles in molecular genetics research account for only a small fraction of the variance in externalizing (and most other) phenotypes (see Beauchaine et al., Reference Beauchaine, Hinshaw and Pang2010).
Our research is founded in part on the premise that the evaluation of neural function may advance our understanding of trait impulsivity and emerging externalizing behavior. It is at this point in Figure 2 where the conceptual association between impulsivity and vulnerability to delinquency is most narrow and thus reduces the extent of potentially unaccounted for influences. For instance, exposure to teratogenic agents, early developmental stress exposure, or possession of specific allelic variants impose vulnerability to developing externalizing behavior only insofar as they affect neurological function. Furthermore, if multiple etiological factors produce similar patterns of neural function/dysfunction (equifinality), the original source(s) may be functionally moot. Accordingly, our work has focused largely on surrogate markers of neurobiological function that are suitable for research with humans and considered to index activity in dopaminergically rich brain regions. Following from the discussion above, particularly regarding trait impulsivity as a vulnerability to a range of externalizing outcomes, we believe this perspective will enhance our understanding of Biological Vulnerability × Environment Risk Factor interactions by better characterizing the transactions between individual susceptibilities and contextual insults, eventually refining approaches to prevention and intervention.
More on Central DA Responding and Trait Impulsivity
One challenge we have faced in contributing to and translating the extensive body of research on dopaminergic neural substrates of impulsivity is identifying a biological marker that is suitable for research with humans across a broad age range. Functional neuroimaging provides the spatial resolution necessary for assessing limbic regions associated with impulsivity, yet it is difficult to use with very young participants. Thus, our research regularly incorporates psychophysiological markers of autonomic activity that are reflective of dopaminergic neural networks that underlie approach motivation (see Beauchaine, Reference Beauchaine2009; Brenner & Beauchaine, Reference Brenner and Beauchaine2011; Brenner, Beauchaine, & Sylvers, Reference Brenner, Beauchaine and Sylvers2005).
It has long been known that impulsive individuals, including those with ADHD, ODD, CD, ASPD, and various addictive disorders, respond differently to rewards than controls. For example, in various monetary incentive paradigms, males with these disorders perseverate in responding for reward for longer periods of time than their peers both (a) when contingencies change and they begin to lose rather than win money and (b) when monetary incentives are discontinued entirely (e.g., Giancola, Peterson, & Pihl, Reference Giancola, Peterson and Pihl2006; Matthys, van Goozen, Snoek, & van Engeland, Reference Matthys, van Goozen, Snoek and van Engeland2004). A principal CNS substrate of aberrant reward responding is underactivation in the ventral striatum, a phylogenically old network of neural structures rich in dopaminergic projections and known to subserve approach motivation in mammals. Several lines of research indicate that this dopaminergic network is less responsive to reward, including monetary incentives, among impulsive individuals than among controls (see Durston, Reference Durston2003; Gatzke-Kopp & Beauchaine, Reference Gatzke-Kopp, Beauchaine, Coch, Dawson and Fischer2007b; Gatzke-Kopp et al., Reference Gatzke-Kopp, Beauchaine, Shannon, Chipman, Fleming and Crowell2009; Sagvolden, Johansen, Aase, & Russell, Reference Sagvolden, Johansen, Aase and Russell2005). Consistent with theories of central and autonomic underarousal (e.g., Gatzke-Kopp, Raine, Loeber, Stouthamer-Loeber, & Steinhauer, Reference Gatzke-Kopp, Raine, Loeber, Stouthamer-Loeber and Steinhauer2004), we have argued that those with impulse control disorders across the externalizing spectrum engage in excessive reward-seeking behaviors in part to upregulate a persistently underactive mesolimbic DA system, which is experienced as an aversive, irritable mood state (e.g., Laakso et al., Reference Laakso, Wallius, Kajander, Bergman, Eskola and Solin2003).
Cardiac Preejection Period (PEP) Reactivity to Reward: A Peripheral Marker of Central DA Responding?
Several sources of evidence now suggest that cardiac PEP, a sympathetic nervous system (SNS) mediated, systolic time interval spanning, left ventricular depolarization to the onset of ejection of blood into the aorta, marks striatal DA responding specifically during approach behaviors, including those elicited by monetary incentives (Brenner & Beauchaine, Reference Brenner and Beauchaine2011; Brenner et al., 2005). Cardiac PEP shortens through β-adrenergic mechanisms when the SNS is activated. The argument that PEP shortening marks central DA reactivity during conditions of reward is based on several observations. Behavioral approach requires energy mobilization, a function served by the SNS to meet metabolic demands. In addition, increases in cardiac output required for motivated behavior are mediated by SNS-induced changes in the contractile force of the left ventricle (Sherwood, Allen, Obrist, & Langer, Reference Sherwood, Allen, Obrist and Langer1986). Finally, direct infusions of DA agonists into striatal structures produce SNS-mediated increases in cardiac output (van den Buuse, Reference van den Buuse1998), similar to those observed when normal controls participate in reward tasks (see Brenner et al., Reference Brenner, Beauchaine and Sylvers2005). This set of observations suggests that reduced SNS-linked cardiac reactivity to incentives may mark attenuated DA responding. This argument is supported further by research indicating that PEP shortening among controls is specific to conditions of reward, and it is not observed during extinction or mood induction in well-controlled experiments (Brenner et al., Reference Brenner, Beauchaine and Sylvers2005; Richter & Gendolla, Reference Richter and Gendolla2009).
Research on PEP responding to incentives has accumulated over the last decade in studies of samples ranging in age from preschool to adulthood (Beauchaine et al., Reference Beauchaine, Katkin, Strassberg and Snarr2001; Beauchaine, Hong, & Marsh, Reference Beauchaine, Hong and Marsh2008; Brenner & Beauchaine, Reference Brenner and Beauchaine2011; Brenner et al., Reference Brenner, Beauchaine and Sylvers2005; Bubier & Drabick, Reference Bubier and Drabick2008; Crowell et al., Reference Crowell, Beauchaine, Gatzke-Kopp, Sylvers, Mead and Chipman-Chacon2006; Mead et al., Reference Mead, Beauchaine, Brenner, Crowell, Gatzke-Kopp and Marsh2004; Richter & Gendolla, Reference Richter and Gendolla2009). These samples have included individuals with ADHD, ODD, CD, and antisocial personality traits. In each of our studies, male externalizers exhibited less PEP reactivity to reward than controls. In most of our studies, no PEP reactivity to incentives was evident in the externalizing groups. The consistency of these findings across the broad span of development investigated suggests that the neural substrates of impulsivity are evident as early as age 4 among children who exhibit externalizing symptoms. This would be expected if (a) disorders across the externalizing spectrum, including ADHD, share a heritable etiological substrate (see above) and (b) PEP reactivity to incentives marks the biobehavioral expression of this trait. It is worth reemphasizing that such findings parallel consistent neuroimaging results showing attenuated mesolimbic reactivity to incentives among boys with ADHD, both with and without CD (see, e.g., Durston, Reference Durston2003).
Finally, in some of our most recent research, PEP nonreactivity to incentives assessed at ages 8–12 predicted escalation of alcohol and other substance use 3 years later in a sample of 206 at-risk youths (Brenner & Beauchaine, Reference Brenner and Beauchaine2011). Thus, not only does PEP responding to incentives mark concurrent risk for externalizing outcomes but it is also emerging as a promising biomarker of prospective risk as well, which is precisely what might be expected of a peripheral index of central DA dysfunction. The relation between PEP reactivity to incentives at ages 8–12 and risk for future alcohol use is presented in Figure 3.

Figure 3. The association between age and probability of alcohol use in the past year for at-risk participants scoring below the 25th percentile and above the 75th percentile on PEP reactivity to incentives (N = 206). Greater sympathetic nervous system responding is marked by preejection period (PEP) shortening. Thus, those scoring below the 25th percentile exhibited less PEP shortening than those scoring above the 75th percentile. Adapted from “Cardiac Pre-Ejection Period Reactivity and Psychiatric Comorbidity Prospectively Predict Substance Use Initiation Among Middle-Schoolers: A Pilot Study,” by S. L. Brenner and T. P. Beauchaine, 2011, Psychophysiology, 48. Copyright 2011 Wiley Blackwell. Adapted with permission.
Emotion Regulation as a Moderator of Externalizing Vulnerability
As noted above and illustrated in Figure 2, trait impulsivity does not determine antisocial personality development. Rather, it confers susceptibility to more severe behavior problems when coupled with other vulnerabilities and risk factors. Important among such vulnerabilities is deficient emotion regulation. Emotion regulation comprises processes through which emotional experience and expression are shaped, whether volitionally or automatically, in the service of adaptive behavior (Thompson, Reference Thompson and Thompson1990). According to this definition, emotion dysregulation might best be described as a pattern of emotional experience and/or expression that interferes with appropriate goal directed behavior. In most forms of psychopathology, whether externalizing or internalizing, some form of negative emotion (sadness, panic, rage, anxiety) is experienced either too intensely or too enduringly to be adaptive (Beauchaine et al., Reference Beauchaine, Gatzke-Kopp and Mead2007). Emotion dysregulation is therefore a broad rather than specific risk factor for psychopathology (see Beauchaine, Reference Beauchaine2001; Beauchaine et al., Reference Beauchaine, Gatzke-Kopp and Mead2007).
Much has been learned about the CNS substrates of emotion regulation in the past two decades. Neural structures that subserve emotion regulation include the amygdala, the septohippocampal system, and the ventromedial prefrontal cortex (vmPFC; see Beauchaine et al., Reference Beauchaine, Neuhaus, Zalewski, Crowell and Potapova2011; Goldsmith, Pollak, & Davidson, Reference Goldsmith, Pollak and Davidson2008). The vmPFC in particular inhibits amygdala activation when negative emotions are suppressed volitionally. It is important that lesions to the vmPFC impair ANS responses to emotional stimuli (Verbane & Owens, Reference Verbane and Owens1998). Elegant theoretical models have also been articulated regarding the modulatory functions of certain brainstem nuclei, particularly the nucleus ambiguous, on emotional experience and expression (see Porges, Reference Porges1995, Reference Porges2001, Reference Porges2003, Reference Porges2007). These nuclei serve as final common pathways via the vagus nerve from the CNS to the cardiovascular system.
Respiratory Sinus Arrhythmia (RSA) and Emotion Regulation
In his polyvagal theory, Porges (Reference Porges1995, Reference Porges2001, Reference Porges2003, Reference Porges2007) argues that dynamic regulation of autonomic reactivity is a phylogenic precondition of social affiliation and is therefore a function of lower brain networks that are evolutionarily old and structurally similar across mammalian species, including primates. These brain networks exert inhibitory peripheral control over target organs through the parasympathetic nervous system (PNS). Inhibition of cardiac function in particular is exerted via the PNS through the vagus (10th cranial) nerve. In the past decade, it has become increasingly clear that emotion regulation capabilities are marked by vagal efference to the heart, which can be quantified by RSA—oscillatory increases and decreases in heart rate across the respiratory cycle (see Beauchaine, Reference Beauchaine2001; Beauchaine et al., Reference Beauchaine, Gatzke-Kopp and Mead2007; Porges, Reference Porges1995, Reference Porges2007). Rigorous experimental work shows that under appropriate stimulus conditions, RSA indexes neural traffic through the vagus nerve (see Ritz, Reference Ritz2009), which provides a physiological mechanism for rapid acceleration and deceleration of cardiac output in response to environmental (including social) demands (Porges, Reference Porges1995). Since publication of Porges’ (1995) polyvagal theory, a consistent body of research has emerged linking deficiencies in RSA to emotion dysregulation and various forms of psychopathology (see, e.g., Ahs, Sollers, Furmark, Fredrikson, & Thayer, Reference Ahs, Sollers, Furmark, Fredrikson and Thayer2009; Asmundson & Stein, Reference Asmundson and Stein1994; Beauchaine, Reference Beauchaine2001, Reference Beauchaine2012; Beauchaine et al., Reference Beauchaine, Katkin, Strassberg and Snarr2001, Reference Beauchaine, Gatzke-Kopp and Mead2007; Crowell et al., Reference Crowell, Beauchaine, McCauley, Smith, Stevens and Sylvers2005, Reference Crowell, Beauchaine, Gatzke-Kopp, Sylvers, Mead and Chipman-Chacon2006; Hastings et al., Reference Hastings, Nuselovici, Utendale, Coutya, McShane and Sullivan2008; Neuhaus, Beauchaine, & Bernier, Reference Neuhaus, Beauchaine and Bernier2011; Porges, Reference Porges2007; Rottenberg, Reference Rottenberg2007; Rottenberg, Salomon, Gross, & Gotlib, Reference Rottenberg, Salomon, Gross and Gotlib2005; Rottenberg, Wilhelm, Gross, & Gotlib, Reference Rottenberg, Wilhelm, Gross and Gotlib2002; Thayer, Friedman, & Borkovec, Reference Thayer, Friedman and Borkovec1996; Vasilev, Crowell, Beauchaine, Mead, & Gatzke-Kopp, Reference Vasilev, Crowell, Beauchaine, Mead and Gatzke-Kopp2009). As we have reviewed elsewhere, low baseline RSA and/or excessive RSA withdrawal in response to emotionally evocative stimuli have been linked to conduct problems, trait hostility, eating disorders, anxiety disorders, depression, and panic, among other adverse outcomes (see Beauchaine, Reference Beauchaine2001).
Although some have suggested that impulsivity is a direct manifestation of emotion dysregulation, the two traits derive from very different etiological and neural substrates, as reviewed above. Moreover, behavioral genetics studies indicate that impulsivity is almost entirely heritable (e.g., Willcutt, Reference Willcutt and Barchin press), whereas individual differences in RSA are determined by both heredity and the environment (e.g., Kupper et al., Reference Kupper, Willemsen, Posthuma, De Boer, Boomsma and De Geus2005). Experimental research also indicates that emotion dysregulation, which is marked by low RSA and excessive RSA reactivity, is largely socialized within families (Beauchaine et al., Reference Beauchaine, Gatzke-Kopp and Mead2007, Reference Beauchaine, Klein, Crowell, Derbidge and Gatzke-Kopp2009; Crowell et al., in press; Snyder, Edwards, McGraw, Kilgore, & Holton, Reference Snyder, Edwards, McGraw, Kilgore and Holton1994; Snyder et al., Reference Snyder, Schrepferman and St. Peter1997). Accordingly, impulsivity and emotion regulation are represented as distinct behavioral constructs in Figures 1 and 2. In order to assess both impulsivity and emotion regulation at the autonomic level, in addition to collecting PEP reactivity to incentives, we also assess RSA both at baseline and in response to emotionally evocative (usually sadness-inducing) stimuli. It is interesting that in contrast to attenuated PEP reactivity to reward, which is evident as early as age 4 (see above), attenuated baseline RSA and excessive RSA withdrawal to emotion evocation are only observed among older conduct-disordered middle schoolers and adolescents (Beauchaine et al., Reference Beauchaine, Katkin, Strassberg and Snarr2001; Beauchaine, Hong, et al., 2008; Mead et al., Reference Mead, Beauchaine, Brenner, Crowell, Gatzke-Kopp and Marsh2004). Neither RSA nor RSA reactivity discriminate externalizing preschoolers with ADHD and ODD from controls (Crowell et al., Reference Crowell, Beauchaine, Gatzke-Kopp, Sylvers, Mead and Chipman-Chacon2006).
At first we found this perplexing because preschool children with ADHD and ODD are at high risk for later conduct problems and delinquency (Campbell et al., Reference Campbell, Shaw and Gilliom2000). However, we now believe the distinction may have important implications for understanding the developmental progression of externalizing behavior. About 50% of preschool children with ADHD never develop more serious externalizing conduct (see above). It is important that emotion regulation is undergoing critical development across this age range (see, e.g., Cole & Hall, Reference Cole, Hall, Beauchaine and Hinshaw2008; Cole, Martin, & Dennis, Reference Cole, Martin and Dennis2004), and differences in RSA (a peripheral biomarker of emotion regulation; see above) appear to emerge over time between children who develop versus fail to develop effective emotion regulation skills (Derbidge, Beauchaine, & Mead, Reference Derbidge, Beauchaine and Mead2011). This interpretation is consistent with research demonstrating that ADHD progresses to more serious conduct problems only among children within families where emotional lability is negatively reinforced (e.g., Patterson, DeGarmo, & Knutson, Reference Patterson, DeGarmo and Knutson2000). Accordingly, our current thinking is that impulsivity may be “regulated,” expressed as pure ADHD, or “dysregulated,” expressed as more serious externalizing outcomes, depending on emotion regulation strategies that are socialized through recurring parent–child interactions (Beauchaine et al., Reference Beauchaine, Hinshaw and Pang2010). In the case of externalizing preschoolers, it may be too soon for familial negative reinforcement processes to have fully shaped emotional lability, with consequential deficiencies in RSA (see Beauchaine et al., Reference Beauchaine, Gatzke-Kopp and Mead2007). We have also found that adolescents characterized only by ADHD demonstrate less extreme RSA withdrawal to emotion evocation than adolescents with both ADHD and CD (Beauchaine et al., Reference Beauchaine, Katkin, Strassberg and Snarr2001). In contrast, adolescents with only ADHD do not differ from those with ADHD and CD on neural measures of reward reactivity (Gatzke-Kopp et al., Reference Gatzke-Kopp, Beauchaine, Shannon, Chipman, Fleming and Crowell2009). Taken together, this line of research supports the notion that trait impulsivity, which is derived from CNS deficiencies in reward processing, represents a core component of externalizing vulnerability, yet acquired deficiencies in emotion regulation facilitate progression along the externalizing spectrum.
This research highlights the benefits of considering transactional relations between biological vulnerabilities and contextual risk, rather than viewing each as independent contributors to adversity. Only by conceptualizing our work within a broad developmental model, which capitalizes on research conducted at one level of analysis (i.e., family socialization processes) to inform the interpretation of research conducted at another level of analysis (i.e., biological), do we gain a more comprehensive understanding of emerging psychopathology.
Our work with adolescent girls at risk for borderline personality development also demonstrates the need to account for interactions between biological vulnerabilities and familial risk factors in predicting behavioral adjustment. For example, among self-injuring adolescent girls, we uncovered a significant interaction between platelet serotonin and the quality of dyadic discussions with mothers in predicting self-harm, including cutting, overdoses, and suicide attempts (see Crowell, Beauchaine, McCauley, et al., 2008; Crowell, Beauchaine, & Lenzenweger, Reference Crowell, Beauchaine, Lenzenweger, Beauchaine and Hinshaw2008; Crowell et al., Reference Crowell, Beauchaine, McCauley, Smith, Stevens and Sylvers2005). Girls with low levels of peripheral serotonin tended toward self-harm regardless of dyadic negativity with their mothers. However, girls with high peripheral serotonin were only at risk when dyadic interactions were highly negative. It is important that dyadic negativity and platelet serotonin levels were unrelated and accounted for only 23% and 3% of the variance in self-harm behaviors, respectively. Yet in conducting our research across multiple levels of analysis by including a Serotonin × Dyadic Negativity interaction term into the model, we accounted for an astounding 64% of the variance in self-harm. Had we measured only dyadic negativity, we would have vastly underestimated its importance in predicting self-harm. Had we measured only platelet serotonin, we would have concluded it was unrelated to self-harm. We showed more recently that (a) self-injuring and non-self-injuring girls with equivalent levels of depression are differentiated from one another by electrodermal responding (Crowell et al., 2012), a peripheral biomarker of trait impulsivity (low responding)/trait anxiety (high responding; see Beauchaine et al., Reference Beauchaine, Katkin, Strassberg and Snarr2001; Katkin, Reference Katkin1965), and (b) self-injuring girls’ RSA reactivity and parental invalidation of their emotions interact to predict self-harm (Crowell, Beauchaine, Potapova, et al., in press). In cases such as these, where important endogenous and exogenous influences on emergent psychopathology interact across levels of analysis, failure to capture their conjoint effects leads us to incorrect conclusions about etiology and can have detrimental effects on the development of appropriately targeted interventions (see also Beauchaine et al., Reference Beauchaine, Hinshaw and Pang2010; Beauchaine, Neuhaus, et al., 2008).
RSA as a Moderator of Externalizing Vulnerability
Given that emotion dysregulation and associated RSA deficiencies confer risk for psychopathology, especially among impulsive children and adolescents, well-socialized emotion regulation skills, reflected both behaviorally and in high RSA, should buffer children from some of the adverse effects of trait impulsivity. Our research demonstrated the buffering effects of RSA on the relations between paternal ASPD and adolescent conduct problems (Shannon et al., Reference Shannon, Beauchaine, Brenner, Neuhaus and Gatzke-Kopp2007). Children with low baseline RSA tend toward conduct problems regardless of paternal ASPD symptoms, whereas children high in RSA are partially protected from their father's antisociality. Moreover, an accumulating literature links high RSA to children's positive adjustment in the face of diverse familial risk factors for psychopathology, including interparental conflict, parental drinking, and parental divorce (El-Sheikh, Reference El-Sheikh2005; El-Sheikh, Harger, & Whitson, Reference El-Sheikh, Harger and Whitson2001; Katz & Gottman, Reference Katz and Gottman1995).
The Importance of Stimulus Conditions
It is common for social scientists to misguidedly equate behavioral constructs and behavioral traits such as impulsivity with psychophysiological markers such as cardiac PEP. When this error is made, we expect the biological marker, in this case PEP, to discriminate between impulsive and nonimpulsive children, regardless of stimulus conditions (Beauchaine, Reference Beauchaine2009). Yet our data indicate clearly that PEP reactivity is not observed during extinction or emotion evocation in impulsive individuals or controls. Instead, it is only during reward that consistent group differences emerge (see Brenner & Beauchaine, Reference Brenner and Beauchaine2011; Brenner et al., Reference Brenner, Beauchaine and Sylvers2005). Our choice to use stimulus conditions of reward to assess PEP responding as a marker of central DA responding/impulsivity is based on strong theoretical considerations regarding the function of SNS-linked cardiac reactivity during approach behaviors, as explained in detail above (Beauchaine, Reference Beauchaine2001; Beauchaine et al., Reference Beauchaine, Katkin, Strassberg and Snarr2001, Reference Beauchaine, Gatzke-Kopp and Mead2007). The same argument applies to RSA reactivity as a marker of emotion dysregulation and emotional lability (see also Hastings et al., Reference Hastings, Nuselovici, Utendale, Coutya, McShane and Sullivan2008). Here, we expect maximal differentiation between emotionally dysregulated individuals and controls during conditions of emotion evocation, not during conditions of reward. Our data outlined above support this supposition. Nevertheless, behavioral scientists continue to expect group differences in psychophysiological markers across all of their stimulus conditions. When they do not, null findings are interpreted as either failures to replicate or, worse yet, as nonexistence of links between biological systems important to self-regulation and the behavioral traits they subserve. Thus, atheoretical choices of stimulus conditions lead not only to confusion in the literature (Beauchaine, Reference Beauchaine2009) but also to incorrect conclusions about the influences of variables across levels of analysis. We should therefore select our stimuli carefully, based on specific links between physiological processes and behavioral outcomes that we seek to study (see also Cole et al., Reference Cole, Martin and Dennis2004; Goldsmith & Davidson, Reference Goldsmith and Davidson2004).
Neuroimaging and Trait Impulsivity Among Adolescents
Although our research using PEP as a peripheral biomarker of central DA responding to reward is grounded strongly in theory and has advanced our understanding of the neurobiology of trait impulsivity, it is obviously advantageous to assess neural reactivity more directly when possible. Accordingly, we have used magnetic resonance imaging to evaluate the links between both brain function and brain structure in theoretically relevant regions of interest (ROIs) during specific stimulus conditions, including reward and extinction, among adolescents, who find it easier than young children to endure 1-hr experimental sessions (structural + functional) without excessive movement. From this work, several new findings linking brain structure and function to externalizing outcomes deserve consideration.
First, ADHD adolescents, with and without comorbid CD, differ in anterior cingulate cortex (ACC) activation during extinction of previously rewarded behaviors (Gatzke-Kopp et al., Reference Gatzke-Kopp, Beauchaine, Shannon, Chipman, Fleming and Crowell2009). As expected, controls recruit striatal regions when responding to reward, but they shift neural activity forward to the ACC when reward is removed. This is consistent with the role of the ACC in error monitoring and decision making (see Holroyd & Coles, Reference Holroyd and Coles2002). Put simply, the ACC helps us detect changing reinforcement contingencies, providing for appropriate adjustment of behavior to new stimulus conditions (among other functions). In contrast to controls, boys with externalizing behavior disorders show no shift forward in neural reactivity from striatal regions to the ACC when reward ceases.
This work is important because it extends our understanding of associative learning processes, including those invoked by reward, across distributed neural networks that span functional levels of analysis. Complex behavioral traits such as trait impulsivity and emotion regulation typically do not derive from single source nuclei but rather from interactions between integrated brain networks (see Goldsmith & Davidson, Reference Goldsmith and Davidson2004; Goldsmith et al., Reference Goldsmith, Pollak and Davidson2008). Consistent with this perspective, we have also identified both top-down and bottom-up deficiencies in functional connectivity between striatal regions and the ACC among externalizing adolescents (Shannon et al., Reference Shannon, Sauder, Beauchaine and Gatzke-Kopp2009). Thus, not only is the ACC underactive among boys with ADHD and/or CD during extinction, but also patterns of neural communication between the ACC and phylogenically older brain structures implicated in reward responding appear to be compromised.
We have also found structural irregularities in mesolimbic ROIs associated with reward processing (see above), septohippocampal ROIs associated with trait anxiety (see Gray & McNaughton, Reference Gray and McNaughton2000), and anterior cingulate ROIs associated with error monitoring/decision making (see above) among boys with externalizing behavior disorders (Sauder, Beauchaine, Gatzke-Kopp, Shannon, & Aylward, Reference Sauder, Beauchaine, Gatzke-Kopp, Shannon and Aylwardin press). Perhaps it is more important that none of these findings were expressed as main effects. Rather, Externalizing × Internalizing Symptom interactions accounted for individual differences in gray matter density in each region, whereby boys with ADHD/CD and comorbid internalizing symptoms showed smaller reductions in gray matter than individuals with externalizing psychopathology alone. Externalizing boys with comorbid anxiety/depression showed gray matter densities that were similar to those of controls. In contrast, boys with externalizing symptoms alone showed the largest reductions in gray matter in each region. These findings may indicate a neural substrate of long-noted behavioral protective effects of anxiety among children with externalizing disorders. In brief, symptoms of anxiety predict better response to certain treatments among children with ADHD and CD (Jensen et al., Reference Jensen, Hinshaw, Kraemer, Lenora, Newcorn and Abikoff2001) and externalizing youth with comorbid anxiety are less aggressive, regarded less negatively by peers, and experience fewer police contacts than youth with CD alone (Walker et al., Reference Walker, Lahey, Russo, Frick, Christ and McBurnett1991). Our findings indicate neuroanatomical correlates of these protective effects among externalizing youth, providing cross levels of analysis support for theoretical models in which trait anxiety dampens excessive approach behaviors, including aggression (e.g., Beauchaine, Reference Beauchaine2001).
Dissociation of Expressive and Psychophysiological Responding to Emotion Evocation and Conduct Problems
In addition to our extensive work on transactional models of emerging antisocial and borderline traits (see above; Beauchaine et al., Reference Beauchaine, Klein, Crowell, Derbidge and Gatzke-Kopp2009; Crowell et al., Reference Crowell, Beauchaine and Linehan2009; Gatzke-Kopp, Reference Gatzke-Kopp2011), we have also conducted multiple levels of analysis research evaluating desynchrony of expressive and psychophysiological responses to emotion-eliciting stimuli among boys with conduct problems (Marsh, Beauchaine, & Williams, Reference Marsh, Beauchaine and Williams2008). In this work, we evaluated time-linked correspondence of sad facial expressions and autonomic reactivity while boys with CD and controls watched an empathy-eliciting film. As expected following from contemporary models of both empathy and emotional responding more broadly (e.g., Eisenberg & Eggum, Reference Eisenberg, Eggum, Decety and Ickes2009), controls exhibited synchrony between sad facial expressions and autonomic reactivity, as indexed by reduced SNS (lower skin conductance level, lengthened cardiac PEP) and increased PNS (higher RSA) activity. In contrast, even though boys with CD exhibited equivalent levels of facial sadness compared to controls, no correspondence between these expressions and measures of autonomic reactivity was observed (see Figure 4). Moreover, lower correspondence between facial expressions and PEP reactivity predicted externalizing symptom severity.

Figure 4. The associations among (top) facial expressions of sadness and skin conductance level (SCL), (middle) preejection period (PEP), and (bottom) respiratory sinus arrhythmia (RSA) for (dashed lines) boys with disruptive behavior disorders and (solid lines) controls. Facial sadness ranged from 0 (no sadness) to 3 (highest level of sadness) for SCL and from 0 (no sadness) to 6 (highest level of sadness) for PEP and RSA because of the different temporal resolutions of measures. Adapted from “Dissociation of Sad Facial Expressions and Autonomic Nervous System Responding in Boys With Disruptive Behavior Disorders,” by P. Marsh, T. P. Beauchaine, and B. Williams, 2008, Psychophysiology, 45. Copyright 2008 Wiley Blackwell. Adapted with permission.
Taken together, these results highlight the need to evaluate a broadened range of emotional response systems than is typically examined in studies focused on either psychophysiological or expressive components of emotional responding. By considering multiple response systems, we discriminated between boys with behavior problems and their peers. Had we examined any particular emotional response system in isolation, these patterns would not have been evident.
Sex Differences in the Development of Antisocial Behavior
As outlined in detail above, the trajectory followed by most males who develop ASPD is well characterized, with rich specification of transactional relations among susceptibility variables including genes, psychobiological processes, familial socialization mechanisms, peer influences, and cultural factors. In contrast, efforts to delineate developmental pathways to and transactional models of externalizing conduct among females are much more recent (e.g., Crowell at al., 2009). Among these, we articulated a shared liability model of antisocial and borderline personality development in which inherited impulsivity interacts with environmental risk factors to confer differential risk for impulsive aggression and mood lability among males versus impulsive self-injury and mood lability among females (Beauchaine et al., Reference Beauchaine, Klein, Crowell, Derbidge and Gatzke-Kopp2009). Although full articulation of our shared liability model is beyond the scope of this paper, it follows from observations that ASPD and BPD (a) are often experienced among male and female offspring, respectively, from the same families (Goldman, D'Angelo, & DeMaso, Reference Goldman, D'Angelo and DeMaso1993); (b) are often comorbid in clinical samples (Becker, McGlashan, & Grilo, Reference Becker, McGlashan and Grilo2006); (c) are both defined largely by impulse control problems (Paris, Reference Paris1997); (d) carry significant risk for depression and suicide (American Psychiatric Association, 2000); and (e) are often characterized by traumatic experiences early in childhood (Lyons-Ruth, Reference Lyons-Ruth2008; Norden, Klein, Donaldson, Pepper, & Klein, Reference Norden, Klein, Donaldson, Pepper and Klein1995). Furthermore, ASPD and BPD share several susceptibility genes (see Beauchaine et al., Reference Beauchaine, Klein, Crowell, Derbidge and Gatzke-Kopp2009), and similar socialization mechanisms of emotional lability have been observed in the families of those with the disorders (e.g., Crowell et al., Reference Crowell, Beauchaine, Hsiao, Vasilev, Yaptangco and Linehanin press; Patterson et al., Reference Patterson, DeGarmo and Knutson2000). Accordingly, in recent years we have conceptualized antisocial and borderline personality development as multifinal outcomes of parallel etiologies. However, much work remains before models of borderline personality development reach the level of specification seen in contemporary models of antisocial personality development, as described above.
Future Directions in Work on Biology × Environment Interactions
Following from our discussion thus far, it has become increasingly clear that certain biological vulnerabilities interact with contextual risk to potentiate psychopathology (see also Bubier, Drabick, & Breiner, Reference Bubier, Drabick and Breiner2009). In addition to psychophysiological markers of vulnerability, several recent Gene × Environment interactions have been reported in the etiology of externalizing behavior disorders, particularly among males. The most renowned of these was reported by Caspi et al. (Reference Caspi, McClay, Moffitt, Mill, Martin and Craig2002), who found an interaction between child maltreatment and a polymorphism in the monoamine oxidase A (MAOA) gene in predicting antisocial behavior. Males who experienced maltreatment and inherited the low MAOA activity genotype were at far greater risk of developing antisocial behavior than those who experienced maltreatment but did not inherit the low MAOA activity genotype.
Despite the importance of such Gene × Environment interaction studies in elucidating the effects of context in the expression of behavioral phenotypes (see also Gottlieb, Reference Gottlieb1998), the model illustrated in Figure 2 identifies a large conceptual distance between any single vulnerability (including molecular genetic) identified in the upper portion of the hourglass and any single behavioral outcome identified in the lower portion of the hourglass. As a result, associations between genetic variation and behavioral outcomes are difficult to identify and notoriously inconsistent across attempts to replicate. In contrast, biological processes, which more proximally mark current neuropsychological function (see Beauchaine, Reference Beauchaine2009), may offer a more useful level of analysis for considering how vulnerabilities interact with environments (Crowell, Beauchaine, et al., Reference Beauchaine, Hinshaw, Gatzke-Kopp, Beauchaine and Hinshaw2008; Raine, Reference Raine2002). Nonheritable biological vulnerabilities including those incurred by epigentic changes to genome structure, allostatic changes in the operating ranges of vital biological systems, and other influences (see Beauchaine et al., Reference Beauchaine, Neuhaus, Zalewski, Crowell and Potapova2011; Gatzke-Kopp, Reference Gatzke-Kopp2011), can lead to similar neurobiological function (see above). Thus, when recruiting participants based on psychopathological or other behavioral traits, vast heterogeneity in etiological risk always weakens our ability to detect effects of particular genes, despite the possibility that the gene has a strong influential effect on a subset of the participants in the sample. Similarly, the selection of participants based on the possession of specific allelic variants may mark only weak effects on complex psychological outcomes (see Gottlieb, Reference Gottlieb1998) because of the vast number of intervening contextual factors that affect emerging phenotypes. Therefore, (a) maximum homogeneity of trait vulnerabilities for externalizing behavior are most likely to be found at the neural level (although this in no way precludes the potential for etiological hetereogeneity at the neural level; see Gatzke-Kopp et al., Reference Gatzke-Kopp, Greenberg, Fortunato and Coccia2012 [this issue]) and (b) research including variables spanning the greatest conceptual distance in Figure 2 will be propelled by the application of detailed theoretical models that help bridge large developmental gaps.
Multiple levels of analysis research is also important because biological vulnerabilities and environmental risk factors are often synergistic rather than additive in conferring susceptibility to psychopathology (Crowell, Beauchaine, et al., Reference Beauchaine, Hinshaw, Gatzke-Kopp, Beauchaine and Hinshaw2008; Raine, Reference Raine2002). Significant Biology × Environment interactions are often observed in cases where each variable in isolation evidences only a weak association if any with outcome (Beauchaine, Hinshaw, et al., 2008). As we describe above, for example, in a recent study of biological and behavioral correlates of self-injury among adolescent females, a Peripheral Serotonin × Dyadic Negativity interaction accounted for 64% of the variance in self-injurious behaviors, including suicide attempts, even though the combined main effects accounted for only about 25% of the variance in the same outcome (Crowell, Beauchaine, McCauley, et al., Reference Crowell, Beauchaine, McCauley, Smith, Vasilev and Stevens2008). Similarly, in the Caspi et al. (Reference Caspi, McClay, Moffitt, Mill, Martin and Craig2002) study described above, the MAOA genotype explained less than 1% of the variance in antisocial behavior. However, the joint effects of maltreatment and genotype explained about 65%. Had only main effects been assessed, the authors would have incorrectly concluded that the MAOA genotype was unrelated to antisocial behavior and only maltreatment mattered.
In addition to such moderating effects, mediational models linking genes, neural responses, and behavior are now emerging. Although it is now well understood that genes do not affect behavior directly (see, e.g., Beauchaine, Hinshaw, et al., 2008), only recently have direct meditational models begun to be tested to establish specific gene → brain → behavior mechanisms. In one such example, Buckholtz et al. (Reference Buckholtz, Callicott, Kolachana, Hariri, Goldberg and Genderson2008) recently reported that stronger neural coupling between the amygdala and vmPFC mediated links between MAOA polymorphisms and personality. Mediational models specifying neural processes through which genes affect behavior are a critical scientific development. Such models take us one step closer to understanding the overwhelming complexities and trajectories of behavioral dysfunction illustrated in Figure 2. Specification of causal pathways through which genes affect behavior holds promise to answer critical questions that behavioral scientists have been pondering for generations.
Concluding Remarks
In this article, we summarized our thinking about how trait impulsivity may be amplified or attenuated across development by socialization mechanisms that occur within families. As noted, this work may have implications for the development of more effective prevention and intervention strategies (see also Beauchaine, Neuhaus, et al., 2008). Our developmental model implies that interventions that focus on teaching strong emotion regulation skills to impulsive children and their parents may prevent the development of conduct problems and borderline traits as children mature. Such interventions already appear to be effective among externalizing children (see, e.g., Beauchaine et al., Reference Beauchaine, Webster-Stratton and Reid2005), even preschoolers (Webster-Stratton et al., Reference Webster-Stratton, Reid and Beauchaine2011a, Reference Webster-Stratton, Reid and Beauchaine2011b).
It is important that our work also illustrates the potential weaknesses of interventions that target single environmental processes. Our broadened conceptual model outlined in Figure 2 emphasizes the importance of carefully considering individual-level vulnerability in susceptibility to psychopathology. Although extensive research has documented mechanisms through which peer interactions promote deviance (e.g., Dishion et al., Reference Dishion, McCord and Poulin1999), which has important implications for treatment, broadened conceptualizations of antisocial personality development indicate the selectivity of this risk factor for specific individuals. Although prevention programs aimed at altering deviant peer processes may reduce probabilistic risk for exacerbation of externalizing symptoms, these programs do not alter individual vulnerability and may therefore not affect the development of other externalizing behaviors, such as future drinking (see, e.g., Brenner & Beauchaine, Reference Brenner and Beauchaine2011). Through careful use of ANS and CNS markers we are learning increasingly more about the brain bases of behavior and behavioral change. We believe that studying the emergence of externalizing psychopathology across multiple levels of analysis will prove critical in furthering our understanding and in formulating better targeted and therefore more effective prevention and intervention programs.






