Individuals vary in their sensitivity to their environments and experiences. The differential susceptibility and biological sensitivity to context models posit that some children and adolescents are more sensitive to their environments than are other youth, for better and for worse; these individuals are expected to demonstrate more positive outcomes in supportive, advantaged environments, but to develop more problematic behaviors in adverse environments (Belsky, Reference Belsky2016; Boyce, Reference Boyce2016). Conversely, the diathesis stress model predicts that while some children are more vulnerable to the negative effects of adversity, in more positive or supportive environments they do not fare better than other children (Belsky & Pluess, Reference Belsky and Pluess2009; Monroe & Simons, Reference Monroe and Simons1991). These theoretical models have provided the impetus for elucidating biological markers of sensitivity and vulnerability to environmental influence, ranging from measures of autonomic nervous system activity, to brain structure and function, to genetic and epigenetic variation (Boyce, Reference Boyce2016; Guyer, Reference Guyer2020). In this context, researchers have examined biological measures as moderators of a wide range of environmental factors and experiences hypothesized to affect emotional development and mental health, including parenting (Deane et al., Reference Deane, Vijayakumar, Allen, Schwartz, Simmons, Bousman and Whittle2020; Miller et al., Reference Miller, Chocol, Nuselovici, Utendale, Simard and Hastings2013), family functioning (Obradović, Bush, Stamperdahl, Adler, & Boyce, Reference Obradović, Bush, Stamperdahl, Adler and Boyce2010; Schriber et al., Reference Schriber, Anbari, Robins, Conger, Hastings and Guyer2017), trauma (Lahat et al., Reference Lahat, Tang, Tanaka, Lieshout, MacMillan and Schmidt2018; Lin, Kidwell, Kerig, Crowell, & Fortuna, Reference Lin, Kidwell, Kerig, Crowell and Fortuna2021), socioeconomic status (SES) (Obradović, Portilla, & Ballard, Reference Obradović, Portilla and Ballard2016; Sturge-Apple et al., Reference Sturge-Apple, Suor, Davies, Cicchetti, Skibo and Rogosch2016), and peer stress (Eisenlohr-Moul et al., Reference Eisenlohr-Moul, Miller, Giletta, Hastings, Rudolph, Nock and Prinstein2018; Shell, Gazelle, & Faldowski, Reference Shell, Gazelle and Faldowski2014). Some of these studies find evidence for differential susceptibility and biological sensitivity to context (Obradović et al., Reference Obradović, Portilla and Ballard2016; Schriber et al., Reference Schriber, Anbari, Robins, Conger, Hastings and Guyer2017; Sturge-Apple et al., Reference Sturge-Apple, Suor, Davies, Cicchetti, Skibo and Rogosch2016), whereas others provide support for diathesis stress (Eisenlohr-Moul et al., Reference Eisenlohr-Moul, Miller, Giletta, Hastings, Rudolph, Nock and Prinstein2018; Lahat et al., Reference Lahat, Tang, Tanaka, Lieshout, MacMillan and Schmidt2018; Shell et al., Reference Shell, Gazelle and Faldowski2014), or find mixed evidence within the same study (Deane et al., Reference Deane, Vijayakumar, Allen, Schwartz, Simmons, Bousman and Whittle2020; Obradović et al., Reference Obradović, Bush, Stamperdahl, Adler and Boyce2010).
The majority of this literature has focused on susceptibility and vulnerability to environmental adversity, defined as stressful life events and circumstances, rather than on perceived stress. Environmental adversity and perceived stress may be associated with mental health via different pathways (Baldwin, Reuben, Newbury, & Danese, Reference Baldwin, Reuben, Newbury and Danese2019; Monroe, Reference Monroe2008). Aspects of perceived stress are related to, but are not necessarily synonymous with, mental health symptoms (Monroe, Reference Monroe2008); further, the relation between these constructs could vary depending on a moderator. Few studies, however, have considered applying differential susceptibility, biological sensitivity, or diathesis stress perspectives to our understanding of variability in the effects of aspects of perceived stress (e.g., concerns about a stressful event) or activities (e.g., learning about a stressful event) on mental health.
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2, or COVID-19) pandemic offers a unique opportunity for testing the differential susceptibility and diathesis stress hypotheses. As a result of enforced social isolation and distancing during the COVID-19 pandemic, all youth are experiencing significant departures from their normal activities that may have major repercussions for health and well-being. Many families are facing multiple adversities in the context of the COVID-19 pandemic, including financial strain, threats to physical health, and self-quarantining measures. These adversities have the potential to undermine family resources and disrupt family routines and relationships, which, in turn, can compromise child and adolescent well-being (Prime, Wade, & Browne, Reference Prime, Wade and Browne2020). In addition to family adversity, stress and concerns about health and safety and increased media consumption are common during this pandemic; for some individuals, these sources of stress may also cause significant mental health problems (Lau, Griffiths, Choi, & Tsui, Reference Lau, Griffiths, Choi and Tsui2010; Thompson, Garfin, Holman, & Silver, Reference Thompson, Garfin, Holman and Silver2017). For example, increased exposure to media, which may contain sensationalized or speculative stories, can contribute to inaccurate estimations of pandemic-related threat and increased stress and anxiety (Garfin, Silver, & Holman, Reference Garfin, Silver and Holman2020). Taken together, exposure to COVID-19-related family adversity and COVID-19-related stress or concerns may put individuals at risk for experiencing mental health difficulties during the pandemic.
Adolescence is a particularly important developmental period for examining COVID-19-related family adversity and stress, given that school closings and restrictions on in-person interactions with peers likely limit access to important sources of social support and connection (Golberstein, Wen, & Miller, Reference Golberstein, Wen and Miller2020). Nevertheless, COVID-19-related family adversity and stress may be more consequential for some adolescents than for others. It is possible that biological measures of sensitivity or vulnerability moderate the impact of pandemic-related events and experiences on emotional problems. Therefore, in this study we examined whether physiological functioning earlier in adolescence (i.e., prior to the COVID-19 pandemic) moderates the effects of COVID-19 stress (e.g., worried about being infected, reading and talking about COVID-19) and COVID-19 family adversity (e.g., financial strain due to COVID-19, having a family member in self-quarantine) on emotional problems during the pandemic.
Heart rate variability (HRV) reflecting parasympathetic nervous system (PNS) activity has garnered significant interest as a measure of biological sensitivity or vulnerability. Increased PNS activation promotes rest and perception of the environment as safe, whereas decreases in PNS activity help to mobilize metabolic resources in response to environmental demands (Porges, Reference Porges2007). Consequently, researchers often use HRV at rest and in response to challenging tasks as measures of resting PNS activity and PNS reactivity, respectively; these physiological processes have been linked to social and emotional functioning (Beauchaine, Reference Beauchaine2015; Graziano & Derefinko, Reference Graziano and Derefinko2013; Miller, Reference Miller2018), and have also been found to moderate environmental effects on child and adolescent outcomes (Abaied et al., Reference Abaied, Stanger, Wagner, Sanders, Dyer and Padilla-Walker2018; McLaughlin, Rith-Najarian, Dirks, & Sheridan, Reference McLaughlin, Rith-Najarian, Dirks and Sheridan2015; Obradović et al., Reference Obradović, Bush, Stamperdahl, Adler and Boyce2010; Obradović, Bush, & Boyce, Reference Obradović, Bush and Boyce2011; Skowron, Cipriano-Essel, Gatzke-Kopp, Teti, & Ammerman, Reference Skowron, Cipriano-Essel, Gatzke-Kopp, Teti and Ammerman2014). That is, the strength of the associations between environmental factors and outcomes appears to vary at different levels of resting PNS activity and PNS reactivity, potentially reflecting increased sensitivity or vulnerability to context. Thus, in addition to coordinating responses to challenging events, PNS functioning may play a role in filtering and encoding environmental information (Del Giudice, Ellis, & Shirtcliff, Reference Del Giudice, Ellis and Shirtcliff2011). Although the biological sensitivity to context model specifically focuses on stress reactivity as conferring sensitivity to environmental input (Boyce, Reference Boyce2016), there are reasons to also examine resting PNS measures. For example, considering both resting and reactivity measures increases comparability to past studies of differential susceptibility and diathesis stress that have focused on resting PNS activity, PNS reactivity, or both (Eisenberg et al., Reference Eisenberg, Sulik, Spinrad, Edwards, Eggum, Liew and Hart2012; McLaughlin et al., Reference McLaughlin, Rith-Najarian, Dirks and Sheridan2015; Obradović et al., Reference Obradović, Bush, Stamperdahl, Adler and Boyce2010; Skowron et al., Reference Skowron, Cipriano-Essel, Gatzke-Kopp, Teti and Ammerman2014). In addition, given that sensitivity or vulnerability to context is hypothesized to be trait-like, biological measures should capture stable individual differences. Resting PNS activity generally shows greater stability of individual differences over time than do measures of PNS reactivity (Alkon, Boyce, Davis, & Eskenazi, Reference Alkon, Boyce, Davis and Eskenazi2011; Dollar et al., Reference Dollar, Calkins, Berry, Perry, Keane, Shanahan and Wideman2020). Thus, resting PNS activity could be a candidate measure of sensitivity or vulnerability to context and experience.
It is unclear whether individuals with lower or higher resting PNS activity are more sensitive to their environments. In infancy, higher resting PNS activity may be associated with greater emotional and behavioral reactivity to both positive and negative events (Beauchaine, Reference Beauchaine2001; Porges, Doussard-Roosevelt, Portales, & Suess, Reference Porges, Doussard-Roosevelt, Portales and Suess1994); further, higher resting PNS activity during infancy has been found to moderate the association between attachment relationships and problem behaviors in toddlerhood in a manner that reflects differential susceptibility (Conradt, Measelle, & Ablow, Reference Conradt, Measelle and Ablow2013). Later in development, however, higher resting PNS activity is hypothesized to reflect a capacity for effective emotion regulation (Beauchaine, Reference Beauchaine2015; Calkins & Fox, Reference Calkins and Fox2002) and a higher threshold for arousal (Miller, Kahle, & Hastings, Reference Miller, Kahle and Hastings2017; Miller, Xia, & Hastings, Reference Miller, Xia and Hastings2019) – processes that buffer against the perturbations of challenging experiences. Lower resting PNS activity in adolescents has been found to be associated with stress-related increases in negative emotionality (Scott & Weems, Reference Scott and Weems2014), potentially reflecting a biological diathesis to adversity; further, McLaughlin et al. (Reference McLaughlin, Rith-Najarian, Dirks and Sheridan2015) reported that lower resting PNS activity in adolescents exacerbates stress effects on internalizing symptoms. The broader literature, however, contains inconsistent findings. For example, Eisenberg et al. (Reference Eisenberg, Sulik, Spinrad, Edwards, Eggum, Liew and Hart2012) found that children with lower resting PNS activity are at risk for negative outcomes regardless of family adversity, and other studies have not found evidence that resting PNS activity moderates environmental effects on mental health in children or adolescents (Giletta et al., Reference Giletta, Hastings, Rudolph, Bauer, Nock and Prinstein2017; Shanahan, Calkins, Keane, Kelleher, & Suffness, Reference Shanahan, Calkins, Keane, Kelleher and Suffness2014).
In addition to resting PNS activity, PNS reactivity to challenge has been found to be related to risk and resilience by moderating the effects of environments and experiences on emotional functioning (Del Giudice et al., Reference Del Giudice, Ellis and Shirtcliff2011; Ellis, Boyce, Belsky, Bakermans-Kranenburg, & van Ijzendoorn, Reference Ellis, Boyce, Belsky, Bakermans-Kranenburg and van Ijzendoorn2011). For example, some studies have found that children and adolescents who exhibit greater PNS reactivity, indexed by stronger decreases in HRV corresponding to stronger increases in autonomic arousal, are among the best adjusted when they are living in supportive, relatively low-stress environments, but among the most poorly adjusted when they are living in harsh, stressful environments (Abaied et al., Reference Abaied, Stanger, Wagner, Sanders, Dyer and Padilla-Walker2018; Obradović et al., Reference Obradović, Bush, Stamperdahl, Adler and Boyce2010; Skowron et al., Reference Skowron, Cipriano-Essel, Gatzke-Kopp, Teti and Ammerman2014). Thus, several researchers have posited that greater PNS reactivity increases individuals’ openness to environmental input, which is a core principle of the differential susceptibility and biological sensitivity to context models (Boyce & Ellis, Reference Boyce and Ellis2005; Del Giudice et al., Reference Del Giudice, Ellis and Shirtcliff2011; Ellis et al., Reference Ellis, Boyce, Belsky, Bakermans-Kranenburg and van Ijzendoorn2011). It should be noted, however, that much like research on resting PNS activity, the literature on PNS reactivity as a moderator of environmental input is also mixed. For example, prior studies have found evidence that lower PNS reactivity or PNS activation (i.e., mild decreases or increases in HRV) indicates differential susceptibility to environmental influence (Wagner, Hastings, & Rubin, Reference Wagner, Hastings and Rubin2018), or have produced inconsistent evidence within the same sample depending on the outcome of interest (Obradović et al., Reference Obradović, Bush, Stamperdahl, Adler and Boyce2010) and the measure of environmental adversity (Abaied et al., Reference Abaied, Stanger, Wagner, Sanders, Dyer and Padilla-Walker2018). In addition, although most work in this area has focused on childhood, research suggests that PNS reactivity in adolescence can also moderate environmental effects on emotional functioning (Abaied et al., Reference Abaied, Stanger, Wagner, Sanders, Dyer and Padilla-Walker2018; Diamond, Fagundes, & Cribbet, Reference Diamond, Fagundes and Cribbet2012).
Investigators have not yet considered the role of PNS functioning in moderating the associations of pandemic-related stress and adversity with emotional functioning during a pandemic. In the current study we addressed this gap by leveraging psychophysiological data obtained in early adolescence, approximately four years prior to the COVID-19 pandemic, to examine whether PNS functioning moderates the effects of COVID-19 stress and COVID-19 family adversity on current emotional functioning. If we identified statistically significant interaction effects involving COVID-19 stress or COVID-19 family adversity with measures of resting PNS activity or PNS reactivity, we then evaluated whether the interactions were more consistent with the diathesis stress or the differential susceptibility models. Both theoretical models would predict that the associations of COVID-19 stress and COVID-19 family adversity with emotional problems will be stronger for some adolescents based on their PNS functioning. The models differ, however, in their predictions of an ordinal (diathesis stress) versus a disordinal (i.e., crossover) interaction effect (differential susceptibility). Specifically, diathesis stress predicts that those with a particular pattern of PNS functioning (e.g., relatively low resting PNS activity) will experience higher levels of emotional problems under conditions of high COVID-19 stress or high COVID-19 family adversity, but not lower levels of emotional problems under condition of low COVID-19 stress or low COVID-19 family adversity. In contrast, differential susceptibility predicts that those with a particular pattern of PNS functioning (e.g., relatively high PNS reactivity) will experience higher levels of emotional problems under conditions of higher COVID-19 stress or higher COVID-19 family adversity, but also lower levels of emotional problems under conditions of lower COVID-19 stress or lower COVID-19 family adversity. It is important to note that although our focus on COVID-19 stress and COVID-19 family adversity is similar to prior biological sensitivity to context studies that considered high versus low levels of adversity (Obradović et al., Reference Obradović, Bush and Boyce2011, Reference Obradović, Bush, Stamperdahl, Adler and Boyce2010), the absence of COVID-19 stress or COVID-19 family adversity does not necessarily imply positive experiences during the pandemic. It is possible that the range of experiences assessed in our study may increase the likelihood of finding evidence for diathesis stress instead of differential susceptibility and biological sensitivity to context models.
Method
Participants and procedures
As part of a larger study on the effects of early life stress on neurodevelopment during puberty, participants 9–15 years of age were recruited from the San Francisco Bay Area and assessed between 2013 and 2019 (Time 1) when we measured resting HRV and HRV reactivity to a social stress task. At study entry, participants had to be fluent in English and have no history of major medical illness or neurological disorder. Given that the parent study included neuroimaging, any contraindication to magnetic resonance imaging (e.g., claustrophobia, braces) was also an exclusion criterion. Participants reflected the racial/ethnic (46% White; 20% Multiracial; 16% Asian; 7% Hispanic/Latinx; 5% Black; 6% Other) and socioeconomic distributions of the San Francisco Bay Area (median annual family income at study entry = $100 K–$150 K, range from $5 k to $10 K to greater than $150K).
Between 3 April and 28 April 2020, approximately 3 weeks after shelter-in place was implemented in the Bay Area on 17 March 2020, participants completed online questionnaires assessing their exposure to and the impact of the COVID-19 crisis on their behaviors and emotional well-being (Time 2). Of the 214 participants who were part of the larger parent study, 170 provided HRV data at Time 1. HRV data were not available for all 214 participants because we added the psychophysiological assessment to our study protocol after we had started data collection at Time 1. At Time 2, 103 participants completed the COVID-19 survey; 87 of these participants also had HRV data at Time 1. Thus, the current analyses included 87 adolescents (47 girls; mean age at Time 1 = 12.28 years, SD = 1.34, range = 9.47–15.73; mean age at Time 2 = 16.50 years, SD = 1.32, range = 13.82–19.98 years). Participants who were included in the current analyses did not differ significantly from those with incomplete data with respect to age at study entry, sex, or race/ethnicity (all ps > .286); however, participants with complete data came from households with higher annual family income compared to those without complete data (t[192] = 3.21, p = .002). Because of the focus of the parent study on puberty, boys and girls were matched on pubertal stage by design. Given that girls typically reach sexual maturity earlier than boys (Dorn, Dahl, Woodward, & Biro, Reference Dorn, Dahl, Woodward and Biro2006), boys in the current sample were slightly older than girls at both time points (mean difference at Time 1 = 0.83 years, t[85] = 3.03, p = .003; mean difference at Time 2 = 0.59 years, t[85] = 2.14, p = .035). This study protocol was approved by the Institutional Review Board at our university. Consent was obtained from parents of participants who were minors and directly from participants if they were older than 18 years. All participants were compensated for their time.
Measures
Heart rate variability at Time 1: Resting baseline and stress reactivity
At Time 1, 1.5 to 6.5 years prior to the COVID-19 assessment (mean interval = 4.23 years, SD = 1.28), we obtained electrocardiogram (ECG) data during a resting baseline task and a modified version of the Trier Social Stress Test (TSST) (Kirschbaum, Pirke, & Hellhammer, Reference Kirschbaum, Pirke and Hellhammer1993). To assess HRV at rest, adolescents were instructed to sit quietly and relax for 5 min. The TSST was administered following the resting baseline period. An examiner started a story and instructed adolescents that over the next 5 min they should prepare an exciting ending to the story. Adolescents were told that a judge would come in to evaluate their story based on content and memorization. After the 5-min speech preparation, adolescents presented their stories in front of a judge. Following the 5-min speech presentation, the judge instructed adolescents to complete a 5-min serial subtraction task. The judge interrupted adolescents when they made a mistake, took notes, and maintained a neutral expression throughout the speech presentation and subtraction tasks.
ECG data were recorded at a sampling rate of 1000 Hz using the Biopac MP150 system and AcqKnowledge acquisition software (Biopac Systems, Goleta, CA). We preprocessed ECG data from the resting baseline and TSST periods using ANSLab software (Blechert, Peyk, Liedlgruber, & Wilhelm, Reference Blechert, Peyk, Liedlgruber and Wilhelm2016). This included visually inspecting the ECG data for artifacts based on improbable interbeat intervals, and editing ECG data by manually adding and removing R-peaks when appropriate. Within the resting baseline task and the speech presentation and subtraction portions of the TSST, we computed the root mean square of successive differences (RMSSD) as a time-domain measure of HRV reflecting parasympathetic activity (Laborde, Mosley, & Thayer, Reference Laborde, Mosley and Thayer2017). Prior studies suggest that RMSSD is relatively unaffected by respiration compared to other measures of HRV (Hill & Siebenbrock, Reference Hill and Siebenbrock2009; Penttilä et al., Reference Penttilä, Helminen, Jartti, Kuusela, Huikuri, Tulppo and Scheinin2001). We computed HRV for the entire durations of the resting baseline task, speech presentation, and subtraction portions of the TSST (5 min each). Five minutes is considered the conventional minimum recording time for reliably estimating RMSSD (Shaffer & Ginsberg, Reference Shaffer and Ginsberg2017). All participants had complete HRV data. Resting baseline HRV was subtracted from the averaged HRV from the speech presentation and subtraction portions of the TSST to create change scores reflecting HRV reactivity. Negative change scores, or decreases in HRV from rest to the TSST (average of speech presentation and subtraction portions of TSST), reflect greater HRV reactivity. On average, adolescents demonstrated decreases in HRV from rest to the TSST task (mean difference = −15.50, t[86] = 3.94, p < .001; 68 of 87 participants had negative HRV change scores). Higher resting HRV was associated with greater decreases in HRV from rest to TSST (r[85] = −.34, p = .001).
COVID-19 assessment at Time 2: COVID-19 stress
We administered the youth self-report version of the Coronavirus Health Impact Survey (CRISIS v. 0.2; https://github.com/nimh-mbdu/CRISIS). The CRISIS was designed to assess the impact of life changes during the COVID-19 pandemic on behavior and well-being. Participants in our sample reported experiencing many of the same life changes due to the COVID-19 pandemic (e.g., all participants reported their schools being closed in the last two weeks). No participants reported being diagnosed, or having a family member diagnosed, with COVID-19. Only three participants reported knowing someone outside of their family who was diagnosed with COVID-19. Currently, there is no standard system for scoring the CRISIS survey. We focused on CRISIS items assessing concerns about infection and well-being, and amount of exposure to information about COVID-19. This focus fits with recent work highlighting pandemic-related concerns and media consumption as sources of stress (Garfin et al., Reference Garfin, Silver and Holman2020; Holmes et al., Reference Holmes, O'Connor, Perry, Tracey, Wessely, Arseneault and Bullmore2020). We constructed COVID-19 stress scores using CRISIS items that were selected a priori. Specifically, adolescents rated on a 5-point scale how worried they had been over the past two weeks about being infected, having a family member or friend being infected, their own physical and mental health being affected by COVID-19 (all ranging from 1 = not at all to 5 = extremely), and how often they had been reading or talking about COVID-19 (ranging from 1 = never to 5 = most of the time) (Cronbach's α = .77). We averaged responses to five items (selected a priori) from the “Coronavirus/COVID-19 Health/Exposure Status” section of the CRISIS v. 0.2 survey (items 19 through 23 in CRISIS v. 0.2 or items 6 through 11 in CRISIS v. 0.3) to compute a total score for COVID-19 stress.
COVID-19 assessment at Time 2: COVID-19 family adversity
Adolescents reported on whether any of the following events happened to their family members because of COVID-19: fallen ill physically (2% of participants endorsed), hospitalized (1% of participants endorsed), put into self-quarantine with symptoms (5% of participants endorsed), put into self-quarantine without symptoms (e.g., due to possible exposure; 8% of participants endorsed), lost job (9% of participants endorsed), reduced ability to earn money (31% of participants endorsed), and passed away (no participants reported experiencing this event). We summed responses (0 = no, 1 = yes; item 19 in CRISIS v. 02 and in CRISIS v. 03) to compute a total score for COVID-19 family adversity. Sixty two percent of participants did not endorse any of these events happening to family members because of COVID-19.
COVID-19 assessment at Time 2: Emotional problems
Adolescents rated on a 5-point scale their experiences of anxiety, worry, loneliness, fatigue, sadness, anhedonia, irritability, restlessness, negative thoughts, and difficulties with concentration and focus over the past two weeks (Cronbach's α = .88). We reverse coded the items “how happy versus sad were you?” and “how much were you able to enjoy your usual activities?”. We then averaged responses to the 10 items of the “Emotions/Worries (Past Two Weeks)” section of the CRISIS (items 66 through 75 in CRISIS v. 0.2 or items 73 through 82 in CRISIS v. 0.3) to yield a total score for emotional problems.
Internalizing problems at Time 1: Youth self-report
At Time 1, participants reported on their own internalizing problems over the preceding 6 months using the Youth Self-Report (YSR; Achenbach, Reference Achenbach1991). Internalizing problems were defined as the sum of the anxious/depressed, somatic complaints, and withdrawn subscales. The YSR has been used in previous studies of internalizing problems and has good psychometric properties (Ebesutani, Bernstein, Martinez, Chorpita, & Weisz, Reference Ebesutani, Bernstein, Martinez, Chorpita and Weisz2011).
Statistical analyses
We conducted two regression analyses to examine whether baseline HRV and HRV reactivity assessed at Time 1 moderated the association between COVID-19 stress and current emotional problems assessed at Time 2. We repeated these analyses to examine whether HRV measures moderated the association between COVID-19 family adversity and emotional problems. We controlled for the additive effects of interval between Time 1 and Time 2, age at Time 2, and sex. All variables were centered prior to forming interaction terms. Statistically significant interactions were probed by examining the effect of COVID-19 stress or COVID-19 family adversity on emotional problems at 1 SD above and below the mean of baseline HRV or HRV reactivity. We applied a proportion-affected index to quantify whether interaction effects were disordinal (consistent with the differential susceptibility model) or ordinal (consistent with the diathesis stress model) (Roisman et al., Reference Roisman, Newman, Fraley, Haltigan, Groh and Haydon2012). In our study, the proportion-affected index identifies the proportion of individuals in our sample who fall on the low COVID-19 stress side of the crossover point. To compute the proportion-affected index, we first estimated the value of COVID-19 stress where regression lines cross over by dividing −b 2 by b 3, where b 2 is the regression beta weight for HRV and b 3 is the beta weight for the interaction of HRV and COVID-19 stress. We then identified the proportion of the sample with COVID-19 stress values to the left of the estimated crossover point. We interpreted proportions closer to .50 (i.e., roughly half of the sample falling below where regression lines intersect) as evidence for a disordinal interaction favoring differential susceptibility because the effect is roughly equally represented at both low and high COVID-19 stress. We interpreted proportions closer to 0 (i.e., majority of the sample falling above where regression lines intersect) as evidence for an ordinal interaction favoring diathesis stress because the effect is primarily represented at high, but not low, COVID-19 stress. The proportion-affected index relies on the assumption that the predictor variable is normally distributed; we did not use this index to assess interaction effects involving COVID-19 family adversity given the nonnormal distribution of these data (62% of our sample were assigned a score of 0 for COVID-19 family adversity). To further test for differential susceptibility in significant interaction effects, we conducted additional analyses testing the simple slopes for HRV at high and low levels of COVID-19 stress (i.e., 1 SD above and below the mean of COVID-19 stress) and at high and low levels of COVID-19 family adversity (i.e., 1 SD above the mean of COVID-19 family adversity and no reported COVID-19 family adversity). We interpreted differential significant associations between emotional problems and HRV at high and low levels of COVID-19 stress as evidence favoring differential susceptibility.
Results
Descriptive statistics and correlations are presented in Table 1. Girls reported more COVID-19 stress (t[85] = 2.50, r = .26, p = .015) and recent emotional problems (t[85] = 3.84, r = .38, p < .001) than did boys. In addition, adolescents who reported more COVID-19 stress or COVID-19 family adversity also reported higher levels of recent emotional problems (r[85] = .45, p < .001 and r[85] = .26, p = .015, respectively). HRV measures were not significantly correlated with either COVID-19 stress, COVID-19 family adversity, or emotional problems (all ps > .12).
Table 1. Descriptive statistics and correlations

Note. ***p < .001, **p < .01, *p < .05. T1 = Time 1; T2 = Time 2; HRV = heart rate variability.
Resting HRV moderates the association between COVID-19 stress and emotional problems
Table 2 presents the regression model testing whether the association between COVID-19 stress and emotional problems was moderated by earlier resting HRV. The interaction effect was statistically significant, even after controlling for interval between Time 1 and Time 2, age at Time 2, and sex (see Figure 1). The association between COVID-19 stress and recent emotional problems was significantly stronger for adolescents who previously had higher resting HRV (β = .72, p < .001) than for adolescents who had resting HRV levels at the sample mean (β = .40, p < .001). Conversely, the association between COVID-19 stress and emotional problems was not statistically significant for adolescents who had lower resting HRV (β = .08, p = .602). The estimated crossover point of the interaction effect was at a value of 2.30 for COVID-19 stress. 37% of the sample reported COVID-19 stress lower than this value. Roisman et al. (Reference Roisman, Newman, Fraley, Haltigan, Groh and Haydon2012) suggested that researchers question whether interaction effects were more consistent with diathesis stress than with differential susceptibility when a proportion-affected index was less than 16%. Our proportion-affected index was greater than 16%, indicating that the interaction effect was consistent with the differential susceptibility model. To further test for differential susceptibility, we investigated whether resting HRV was differentially associated with emotional problems at high and low levels of COVID-19 stress by treating COVID-19 stress as the moderator variable. For adolescents who reported high levels of COVID-19 stress, higher resting HRV was associated with more severe emotional problems (β = .43, p = .027). At low levels of COVID-19 stress, the negative association between resting HRV and emotional problems was not statistically significant (β = −.21, p = .159).

Figure 1. Time 1 resting heart rate variability (HRV) moderates the association between time 2 COVID-19 stress and emotional problems. Note. The data points follow a color gradient. Lower resting HRV values are presented as lighter in color and higher resting HRV values are presented as darker in color.
Table 2. Regression models testing resting HRV × COVID-19 stress and HRV reactivity × COVID-19 stress predicting emotional problems
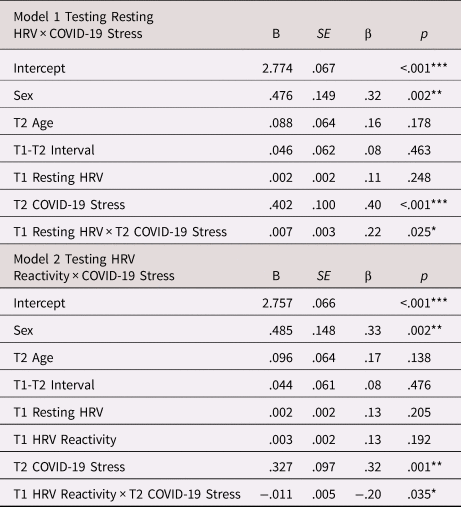
Note. ***p < .001, **p < .01, *p < .05. B = unstandardized coefficient; β = standardized coefficient; T1 = Time 1; T2 = Time2; HRV = heart rate variability. Males were coded as −0.5 and females were coded as 0.5 (Kraemer & Blasey, Reference Kraemer and Blasey2004).
HRV reactivity moderates the association between COVID-19 stress and emotional problems
Table 2 presents the regression model testing whether the association between COVID-19 stress and emotional problems was moderated by earlier HRV reactivity. This model included resting HRV in addition to the covariates described above. The significant interaction between HRV reactivity at Time 1 and subsequent COVID-19 stress followed a similar pattern to the interaction involving resting HRV (see Figure 2). That is, the association of COVID-19 stress and emotional problems was significantly stronger for adolescents who had previously exhibited greater HRV reactivity (i.e., greater decreases in HRV from rest to TSST as represented by lower change scores) (β = .72, p = .001) than it was for adolescents who had demonstrated HRV reactivity at the sample mean (β = .32, p = .001); the association between COVID-19 stress and emotional problems was not statistically significant for adolescents who had previously demonstrated HRV augmentation (i.e., higher change scores reflecting a modest increase in HRV from baseline to the TSST) (β = −.07, p = .745). The estimated crossover point of the interaction effect was at a value of 2.79 for COVID-19 stress. 61% of the sample had COVID-19 stress lower than this value. Thus, our proportion-affected index was well above the 16% cutoff proposed by Roisman et al. (Reference Roisman, Newman, Fraley, Haltigan, Groh and Haydon2012), indicating that the interaction effect was consistent with the differential susceptibility model. To further test for differential susceptibility, we investigated whether HRV reactivity was differentially associated with emotional problems at high and low levels of COVID-19 stress by treating COVID-19 stress as the moderator variable. For adolescents who reported high levels of COVID-19 stress, HRV reactivity was not significantly associated with emotional problems (β = −.26, p = .245). At low levels of COVID-19 stress, HRV augmentation was associated with more severe emotional problems (β = .52, p = .007).

Figure 2. Time 1 heart rate variability (HRV) reactivity moderates the association between time 2 COVID-19 stress and emotional problems. Note. +1 SD on HRV reactivity represents increases in HRV from rest to the trier task (i.e., HRV augmentation). −1 SD on HRV reactivity represents stronger decreases in HRV from rest to the trier task (i.e., high reactivity). The data points follow a color gradient. Negative HRV reactivity values are presented as lighter in color and positive HRV reactivity values are presented as darker in color.
Resting HRV moderates the association between COVID-19 family adversity and emotional problems
Table 3 presents the regression model testing whether the association between COVID-19 family adversity and emotional problems was moderated by earlier resting HRV. The significant interaction between resting HRV at Time 1 and subsequent COVID-19 family adversity followed a different pattern than the interactions involving COVID-19 stress (see Figure 3). The positive association of COVID-19 family adversity and emotional problems was significant only for those adolescents who previously had lower resting HRV (β = .41, p = .003), but not for adolescents who had higher resting HRV (β = −.21, p = .278) or resting HRV levels at the sample mean (β = .07, p = .444).Footnote 1 In analyses treating COVID-19 family adversity as the moderator variable, the association between resting HRV and emotional problems did not reach statistical significance at either high (β = −.41, p = .085) or at low levels of reported COVID-19 family adversity (β = .14, p = .180).

Figure 3. Time 1 resting heart rate variability (HRV) moderates the association between Time 2 COVID-19 family adversity and emotional problems. Note. The data points follow a color gradient. Lower resting HRV values are presented as lighter in color and higher resting HRV values are presented as darker in color.
Table 3. Regression models testing resting HRV × COVID-19 family adversity and HRV reactivity × COVID-19 family adversity predicting emotional problems

Note. ***p < .001, **p < .01, *p < .05. B = unstandardized coefficient; β = standardized coefficient; T1 = Time 1; T2 = Time2; HRV = heart rate variability. Males were coded as −0.5 and females were coded as 0.5 (Kraemer & Blasey, Reference Kraemer and Blasey2004).
HRV reactivity did not interact significantly with COVID-19 family adversity to predict emotional problems.
Sensitivity analyses
As recommended by Keller (Reference Keller2014), we tested additional models that included two-way interactions between covariates and HRV, COVID-19 stress, and COVID-19 family adversity in order to properly control for covariates as confounding variables. With the exception of the interaction between interval and COVID-19 family adversity (p = .032), these interactions effects were not statistically significant (all ps > .167). In addition, these models yielded results similar to those obtained in our prior analysis for interactions between HRV and COVID-19 stress and family adversity, although the interaction effect with HRV reactivity and COVID-19 stress was reduced to a trend (p = .071).
To test whether the resting HRV and HRV reactivity by COVID-19 stress interactions were uniquely predictive of emotional problems, we conducted a post hoc regression analysis that included both interaction terms in the same model. This analysis resulted in both interaction effects becoming statistically non-significant predictors of emotional problems (β = .17 and β = −.13, p = .110 and p = .194 for the resting HRV and HRV reactivity interaction effects, respectively).
It is possible that adolescents who were more prone to internalizing problems prior to the pandemic were also more prone to COVID-19 stress. Thus, COVID-19 stress may be a manifestation of or proxy for adolescents’ mental health problems rather than an independent construct. In this context, therefore, we conducted additional analyses to investigate associations among T1 internalizing problems (prior to the pandemic), T2 emotional problems (during the pandemic), and COVID-19 stress. T1 internalizing problems were positively associated with emotional problems during the pandemic, although this association did not reach statistical significance (r = .18, p = .089). T1 internalizing problems, however, were not associated with COVID-19 stress (r = .04, p = .736) or COVID-19 family adversity (r = .03, p = .755). To determine whether interaction effects on emotional problems during the pandemic were present after adjusting for prior internalizing problems, we conducted regression analyses including T1 internalizing problems as a covariate. The previously observed interaction effects involving HRV and COVID-19 stress, and HRV and COVID-19 family adversity, on emotional problems remained statistically significant when adjusting for T1 internalizing problems (all p < .045).
Discussion
The ongoing COVID-19 pandemic is an unprecedented crisis that has implications for adolescent mental health. We have previously documented in this sample that adolescents reported experiencing greater emotional problems during the pandemic relative to the three months prior to the pandemic (Chahal, Kirshenbaum, Miller, Ho, & Gotlib, Reference Chahal, Kirshenbaum, Miller, Ho and Gotlib2021). Concerns about safety and repeated exposure to information about COVID-19 are widespread and may contribute to mental health difficulties (Garfin et al., Reference Garfin, Silver and Holman2020; Holmes et al., Reference Holmes, O'Connor, Perry, Tracey, Wessely, Arseneault and Bullmore2020; Thompson et al., Reference Thompson, Garfin, Holman and Silver2017). In the current study we found that adolescents who reported worrying more about being infected, worrying more about the health of friends and family, worrying more about the impact of COVID-19 on their own health, and who read and talked more about COVID-19, also reported experiencing more severe recent emotional problems. Disruptions to family processes, such as increased financial strain and social isolation, are also common during the COVID-19 pandemic and are impacting youth well-being (Prime et al., Reference Prime, Wade and Browne2020). We found that adolescents with family members who experienced financial insecurity, physical health concerns, and self-quarantining during the COVID-19 pandemic reported more severe recent emotional problems. We hypothesized, however, that COVID-19 stress and COVID-19 family adversity are more consequential for some adolescents than for others. We tested whether PNS functioning assessed in adolescence 1.5–6.5 years prior to the COVID-19 pandemic moderated the associations of COVID-19 stress and COVID-19 family adversity with emotional problems during the pandemic. We found novel evidence that resting PNS activity and PNS reactivity interact with COVID-19 stress in a manner consistent with the differential susceptibility and biological sensitivity to context models (Boyce & Ellis, Reference Boyce and Ellis2005; Ellis et al., Reference Ellis, Boyce, Belsky, Bakermans-Kranenburg and van Ijzendoorn2011). Specifically, adolescents who exhibited higher resting PNS activity prior to the pandemic reported the lowest levels of emotional problems in the context of experiencing low COVID-19 stress but the highest levels of emotional problems in the context of experiencing high COVID-19 stress. We also observed this pattern for adolescents who previously exhibited greater PNS reactivity as indicated by stronger decreases in HRV to a stressor. In contrast, there was no association between COVID-19 stress and emotional problems during the pandemic for adolescents who exhibited lower resting PNS activity or less PNS reactivity. Conversely, we found that resting PNS activity, but not PNS reactivity, interacted with COVID-19 family adversity in a manner consistent with the diathesis stress model (Belsky & Pluess, Reference Belsky and Pluess2009; Monroe & Simons, Reference Monroe and Simons1991). Adolescents who exhibited lower resting PNS activity prior to the pandemic appeared to be vulnerable to the adverse effects of COVID-19 family adversity on emotional problems during the pandemic.
Higher resting PNS activity and higher PNS reactivity may characterize adolescents who are more attuned to and affected by high and low levels of COVID-19 stress. In contrast, lower resting PNS activity and lower PNS reactivity may buffer adolescents from the negative effects of these experiences. These results corroborate previous findings that PNS functioning may serve as a measure of differential susceptibility (Abaied et al., Reference Abaied, Stanger, Wagner, Sanders, Dyer and Padilla-Walker2018; Conradt et al., Reference Conradt, Measelle and Ablow2013; Obradović et al., Reference Obradović, Bush, Stamperdahl, Adler and Boyce2010; Skowron et al., Reference Skowron, Cipriano-Essel, Gatzke-Kopp, Teti and Ammerman2014), and extend these findings to experiences during the ongoing COVID-19 pandemic. However, some prior studies that have examined the interaction between PNS functioning and context have found evidence for the opposite effects in adolescence – that lower, not higher, resting PNS activity moderates susceptibility to family experiences for better or for worse (Cai & Tu, Reference Cai and Tu2020), or that lower resting PNS activity or higher PNS reactivity represents a diathesis (Li, Sturge-Apple, Martin, & Davies, Reference Li, Sturge-Apple, Martin and Davies2019; McLaughlin et al., Reference McLaughlin, Rith-Najarian, Dirks and Sheridan2015). One explanation for our finding is that the COVID-19 pandemic is a unique experience that is difficult to compare with the stressors on which prior studies have focused, and thus may interact differently with PNS functioning. For example, harsh family environments and parental psychopathology may be negatively associated with general concern for others (Miller & Hastings, Reference Miller, Hastings, Laible, Carlo and Padilla-Walker2019), but concern for friends and family is one of the most frequently reported concerns during the pandemic (Mertens, Gerritsen, Duijndam, Salemink, & Engelhard, Reference Mertens, Gerritsen, Duijndam, Salemink and Engelhard2020). In this context, our COVID-19 assessment asked adolescents how worried they were about family and friends becoming infected. PNS activity reflects regulation of metabolic output that is implicated in a wide range of psychological processes and developmental outcomes (Porges, Reference Porges2007). Effective emotion regulation associated with high resting PNS activity could be protective in the context of certain adverse experiences, such as exposure to maltreatment or community violence (McLaughlin et al., Reference McLaughlin, Rith-Najarian, Dirks and Sheridan2015). In contrast, other processes associated with high resting PNS activity, such as increased social and attentional engagement, may reflect increased sensitivity to experiences of low and high COVD-19 stress.
In contrast to our findings that high resting PNS activity and high PNS reactivity indicated differential susceptibility to the effects of COVID-19 stress, we found that lower resting PNS activity may indicate a vulnerability to emotional problems related to family adversity during the COVID-19 pandemic. Adolescents with moderate or higher resting PNS activity appeared to be protected from the adverse effects of COVID-19 family adversity on mental health. These findings fit with research suggesting that lower resting PNS activity represents a diathesis for psychopathology in the context of adversity (McLaughlin et al., Reference McLaughlin, Rith-Najarian, Dirks and Sheridan2015). Low resting PNS activity may indicate a reduced capacity for emotion regulation (Beauchaine, Reference Beauchaine2015) or lower threshold for increasing arousal (Miller et al., Reference Miller, Xia and Hastings2019) that, when coupled with COVID-19 family adversity, may elevate risk for emotional problems.
One interpretation of our inconsistent findings for COVID-19 stress and COVID-19 family adversity is that these two variables reflect different kinds of challenges during the pandemic. Our measures of COVID-19 stress and COVID-19 family adversity were not correlated with each other, suggesting that pandemic-related concerns and media exposure are independent from the pandemic-related adverse experiences of families. Some patterns of PNS functioning may indicate which adolescents are particularly sensitive to the presence or absence of COVID-19 stress, whereas other patterns of PNS functioning may indicate which adolescents are particularly vulnerable to the presence or absence of COVID-19 family adversity. Our findings are consistent with prior work reporting evidence for both differential susceptibility and diathesis stress in the same study depending on outcome and environmental measure (Richards et al., Reference Richards, Arias Vásquez, von Rhein, van der Meer, Franke, Hoekstra and Hartman2016). An alternative interpretation of our findings is that the distribution and range of our COVID-19 family adversity measure increased the likelihood of finding evidence for diathesis stress instead of differential susceptibility and biological sensitivity to context. For example, almost two-thirds of our adolescents did not endorse any COVID-19 family adversity, which precluded us from testing a proportion-affected index. It is possible that using a measure of COVID-19 family adversity that followed a normal distribution and included positive family events during the pandemic would have yielded findings more consistent with differential susceptibility and biological sensitivity to context. Thus, our findings should be interpreted with caution.
We should note that resting HRV and HRV reactivity did not significantly moderate the association between COVID-19 stress and emotional problems in analyses that controlled for both interaction effects. Consistent with prior studies (Dollar et al., Reference Dollar, Calkins, Berry, Perry, Keane, Shanahan and Wideman2020; El-Sheikh, Reference El-Sheikh2005), resting HRV and HRV reactivity were correlated in our sample (i.e., higher resting HRV was associated with greater decreases in HRV to stress). This correlation may have contributed to our findings when resting HRV and HRV reactivity were considered in separate regression models, and to our nonsignificant findings when they were included in the same regression model. Taken together, it is not clear from our findings whether resting HRV or HRV reactivity is the more robust moderator of the relation between COVID-19 stress and emotional problems. Nevertheless, our findings do indicate that resting HRV, not HRV reactivity, moderates the association between family adversity during COVID-19 and emotional problems.
It is also important to note that the association between COVID-19 stress and recent emotional problems was in the moderate range, and that COVID-19 stress was not associated with earlier internalizing symptoms. These findings provide support for the perspective that our measure of COVID-19 stress was not confounded with mental health symptoms. To our knowledge, our study is the first to use the differential susceptibility or diathesis stress frameworks in interpreting sensitivity and vulnerability to the effects of subjective worries on mental health. Here, we focused on COVID-19 stress, but extending these theoretical frameworks beyond environmental adversity to considering other aspects of stress is a promising future direction in general.
There are health and social inequalities in the effects of the COVID-19 pandemic (Holmes et al., Reference Holmes, O'Connor, Perry, Tracey, Wessely, Arseneault and Bullmore2020), and our findings should be interpreted in the context of our sample of adolescents who resided in the San Francisco Bay Area and who, on average, came from economically advantaged families. In many of these families, parents may have had the privilege of working from home rather than continuing “essential work,” thus decreasing their likelihood of exposure to COVID-19. Nevertheless, almost 10% of the adolescents in our sample reported having a family member lose a job due to COVID-19, and almost one third of the adolescents reported having a family member earn less money. Financial strain during COVID-19 could have downstream consequences for adolescents’ access and exposure to resilience- or risk-promoting factors. Access to technology is another important contextual factor that likely underlies disparities in vulnerability to the adverse consequences of the COVID-19 pandemic, such as social isolation (Beaunoyer, Dupéré, & Guitton, Reference Beaunoyer, Dupéré and Guitton2020). In our sample, 99% of adolescents reported having access to the internet, and 94% of adolescents reported attending classes online during school closures. Thus, the majority of adolescents in our sample may have had the ability to interact with peers and teachers online during enforced social distancing and school closures. The degree of spreading of COVID-19 throughout one's local community may also affect COVID-19 stress. The Bay Area experienced high daily positivity rates of COVID-19 testing (percent of positive tests out of total tests performed, averaged over the prior 7 days) during our assessment. For example, between 3 April and 28 April 2020, the positive testing rate in Santa Clara County ranged from 3.87% to 11.60% (for more information see https://sccgov.org/sites/covid19/Pages/dashboard-testing.aspx). Adolescents who live in regions characterized by less spreading of COVID-19 may report fewer concerns, fewer COVID-19-related challenges to family members, or fewer emotional problems than what we observed in our sample.
We should note several limitations of this study. First, some research on biological sensitivity to context suggests that the direction of interaction effects depends on the specific task used to assess physiological reactivity (Obradović et al., Reference Obradović, Bush and Boyce2011). Thus, a different pattern of findings might have emerged had we used a different task at Time 1. Conversely, given that individual differences in PNS reactivity are modestly stable across tasks (Calkins & Keane, Reference Calkins and Keane2004), our assessment of PNS reactivity to social stress may reflect sensitivity to challenging experiences in general. Second, we leveraged psychophysiological data 1.5–6.5 years prior to our assessment during the COVID-19 pandemic. Although prior longitudinal studies have found modest to moderate stability of individual differences in resting and reactivity measures of PNS functioning (Calkins & Keane, Reference Calkins and Keane2004; Dollar et al., Reference Dollar, Calkins, Berry, Perry, Keane, Shanahan and Wideman2020; Gatzke-Kopp & Ram, Reference Gatzke-Kopp and Ram2018), roughly half of the variance in these measures may be attributable to state- rather than trait-level factors (Gatzke-Kopp & Ram, Reference Gatzke-Kopp and Ram2018). Thus, measures of PNS functioning taken closer in time to the COVID-19 assessment may have been stronger moderators in our analyses. Third, although we examined whether PNS functioning prior to the pandemic prospectively moderated the association between COVID-19 stress and emotional problems, our COVID-19 assessment was cross-sectional, limiting our ability to infer causality. Fourth, we used self-report methods to assess both COVID-19 stress and emotional problems, and the extent to which shared method variance contributed to our findings is not clear. Fifth, we examined high versus low COVID-19 stress and high versus low COVID-19 family adversity (as well as no exposure vs. exposure to COVID-19 family adversity), similar to previous work on biological sensitivity to context that considered high versus low levels of family adversity or marital conflict (Obradović et al., Reference Obradović, Bush and Boyce2011, Reference Obradović, Bush, Stamperdahl, Adler and Boyce2010). While the absence of COVID-19 stress does not necessarily imply the presence of positive experiences, which are important for fully testing the differential susceptibility and biological sensitivity to context models, the observed disordinal interaction effects for COVID-19 stress nevertheless favor these models over the diathesis stress model, which would predict ordinal interactions. Future research should consider positive experiences during the pandemic, such as access to social support and contact and increased time spent outdoors (Roubinov, Bush, & Boyce, Reference Roubinov, Bush and Boyce2020). Further, the majority of COVID-19 family adversity that was endorsed in our sample was related to loss of income. It is unclear from our results whether HRV significantly interacts with other types of family adversity that are less common and more severe, such as having a family member pass away due to COVID-19. Fifth, the proportion-affected index suggested that the effects of the interaction of HRV and COVID-19 stress were consistent with differential susceptibility and biological sensitivity to context models. Contrary to what these models would predict, however, we did not find statistically significant associations between HRV and emotional problems at both high and low levels of COVID-19 stress; further, HRV was not significantly associated with emotional problems at either high or low levels of COVID-19 family adversity. The inconsistencies between these findings and the proportion-affected index are likely due to low statistical power (Roisman et al., Reference Roisman, Newman, Fraley, Haltigan, Groh and Haydon2012). Lastly, we defined HRV reactivity as the difference between the averages of HRV during the baseline and TSST tasks. Although this is the most common approach for measuring stress reactivity, researchers are increasingly considering within-task changes in HRV in an effort to model reactivity in a more dynamic fashion (Fortunato, Gatzke-Kopp, & Ram, Reference Fortunato, Gatzke-Kopp and Ram2013; Miller et al., Reference Miller, Chocol, Nuselovici, Utendale, Simard and Hastings2013; Miller, Nuselovici, & Hastings, Reference Miller, Nuselovici and Hastings2016; Obradović & Finch, Reference Obradović and Finch2017). To our knowledge, dynamic measures of HRV have not been used in moderator analyses, but this may be a promising direction for future research examining diathesis stress, differential susceptibility, and biological sensitivity to context models.
The COVID-19 pandemic is a unique period of stress and uncertainty that will undoubtedly have significant implications for adolescent mental health. We found that adolescents who reported high COVID-19 stress or high COVID-19 family adversity also reported more emotional problems during the pandemic. Nevertheless, COVID-19 stress and COVID-19 family adversity may be more consequential for some adolescents’ mental health than for others. Adolescents who exhibited higher resting PNS activity or higher PNS reactivity prior to the pandemic reported the highest and lowest levels of emotional problems in the context of high and low COVID-19 stress, respectively. Conversely, adolescents who exhibited lower resting PNS activity or lower PNS reactivity were buffered from the association between COVID-19 stress and emotional problems. Our analysis of COVID-19 family adversity led to a different pattern of findings; adolescents who exhibited lower resting PNS activity prior to the pandemic reported more severe emotional problems in the context of high COVID-19 family adversity. Collectively, these findings are important in supporting and extending the differential susceptibility, biological sensitivity to context, and diathesis stress models to adolescent mental health during the COVID-19 pandemic.
Acknowledgments
We thank Rachel Weisenburger and Johanna Walker for their assistance with data collection and organization, as well as the families who participated in this study.
Funding Statement
This research was supported by the National Institutes of Health (R37MH101495 to IHG, T32MH019908 to Allan Reiss (funding JGM), F32MH120975 to RC, K01MH117442 to TCH), the Stanford University Precision Mental Health and Integrated Diagnostics Center (PHIND to IHG, JSK, and TCH), the Stanford Center on Longevity (funding JGM), and the Fonds de Recherche du Québec – Santé (FRQS/MSSS Resident Physician Health Research Career Training Program to AJG).
Conflicts of Interest
None.









