Internalizing and externalizing behaviors in early childhood can place children on developmental trajectories toward clinically significant levels of symptoms later in life (Beesdo et al., Reference Beesdo, Bittner, Pine, Stein, Höfler, Lieb and Wittchen2007; Coie & Dodge, Reference Coie, Dodge, Damon and Eisenberg1998; Copeland, Shanahan, Costello, & Angold, Reference Copeland, Shanahan, Costello and Angold2009; Goodwin, Fergusson, & Horwood, Reference Goodwin, Fergusson and Horwood2004; Kovacs & Devlin, Reference Kovacs and Devlin1998; Keiley, Lofthouse, Bates, Dodge, & Pettit, Reference Keiley, Lofthouse, Bates, Dodge and Pettit2003; Luby, Gaffrey, Tillman, April, & Belden, Reference Luby, Gaffrey, Tillman, April and Belden2014), suggesting that levels of internalizing and externalizing behaviors early in life are useful predictors of later risk. Internalizing behaviors – distress responses characterized by fearfulness, withdrawal, anxiety, and somatic complaints (Achenbach, Reference Achenbach1966; Achenbach & Edelbrock, Reference Achenbach and Edelbrock1978) – constitute some of the most common forms of psychopathology (Kessler, Chiu, Demler, & Walters, Reference Kessler, Chiu, Demler and Walters2005; Luby et al., Reference Luby, Heffelfinger, Mrakotsky, Brown, Hessler, Wallis and Spitznagel2003; Merikangas et al., Reference Merikangas, He, Burstein, Swanson, Avenevoli, Cui and Swendsen2010) and show some stability throughout the life span (Carter et al., Reference Carter, Wagmiller, Gray, McCarthy, Horwitz and Briggs-Gowan2010). On the contrary, externalizing behaviors – overt distress responses characterized by aggression, hyperactivity, defiance, and destructive behaviors (Achenbach, Reference Achenbach1991; Campbell, Reference Campbell1995) – are common early in life and tend to show strong declines throughout the childhood years (Coie & Dodge, Reference Coie, Dodge, Damon and Eisenberg1998). Studies probing early onset of both types of behaviors have pointed to the parent–child relationship as a key predictor of concurrent levels and longitudinal trajectories (Hollenstein, Granic, Stoolmiller, & Snyder, Reference Hollenstein, Granic, Stoolmiller and Snyder2004; Leve, Kim, & Pears, Reference Leve, Kim and Pears2005; McLeod, Wood, & Weisz, Reference McLeod, Wood and Weisz2007; Rapee, Reference Rapee2012; Wu & Lee, Reference Wu and Lee2020). For example, one study with kindergarteners found that more rigid parent–child interactions were associated with more child externalizing and internalizing behaviors concurrently, and predicted growth of externalizing behaviors over a 1-year period (Hollenstein et al., Reference Hollenstein, Granic, Stoolmiller and Snyder2004). Early childhood is a particularly important time for identifying risk trajectories towards psychopathology (Dougherty et al., Reference Dougherty, Leppert, Merwin, Smith, Bufferd and Kushner2015; Wakschlag et al., Reference Wakschlag, Roberts, Flynn, Smith, Krogh-Jespersen, Kaat and Davis2019) and represents a developmental stage when children still depend heavily on their parents to serve as primary emotion socialization agents (Denham, Bassett, & Wyatt, Reference Denham, Bassett, Wyatt, Grusec and Hastings2007). Given the predictive role of preschool-age internalizing and externalizing behaviors on later psychopathology and the role that the parent–child relationship plays in the emergence and maintenance of symptoms, a more in-depth exploration of parent–child predictors of trajectories of internalizing and externalizing behaviors in this period is warranted. An explicit exploration of parent–child interactions may help to identify targets for the mitigation of risk for psychiatric disorders in early childhood.
Parent–child synchrony
There is growing evidence that parent–child interactions from early in life are reciprocally shaped by both parent and child (Feldman, Reference Feldman2007a, Reference Feldman2007b; Lunkenheimer, Hamby, Lobo, Cole, & Olson, Reference Lunkenheimer, Hamby, Lobo, Cole and Olson2020; Lunkenheimer, Olson, Hollenstein, Sameroff, & Winter, Reference Lunkenheimer, Olson, Hollenstein, Sameroff and Winter2011; Murray et al., Reference Murray, De Pascalis, Bozicevic, Hawkins, Sclafani and Ferrari2016), and that these bidirectional influences cannot be fully accounted for by examining each member of the dyad independently (Granic, Reference Granic, Lewis and Granic2000; Moore et al., Reference Moore, Powers, Bass, Cohn, Propper, Allen and Lewinsohn2013). Thus, parent–child interaction measures are a promising avenue for identifying dyadic-level predictors of psychopathology trajectories early in life as they offer unique information about the parent–child relationship. Parent–child synchrony refers to a dyadic pattern of interaction by which behavioral and biological states are coordinated within the dyad, often through co-regulated interactions characterized by contingent responding and social reciprocity (Condon & Sander, Reference Condon and Sander1974; Feldman, Reference Feldman2012; Harrist & Waugh, Reference Harrist and Waugh2002). Parent–child synchrony is evident from infancy (Condon & Sander, Reference Condon and Sander1974; Feldman, Greenbaum, & Yirmiya, Reference Feldman, Greenbaum and Yirmiya1999; Ham & Tronick, Reference Ham and Tronick2009), supports attachment and bond formation within the parent–child dyad (Fleming, O'Day, & Kraemer, Reference Fleming, O'Day and Kraemer1999; Leclère et al., Reference Leclère, Viaux, Avril, Achard, Chetouani, Missonnier and Cohen2014), and remains a beneficial index of adaptive social interactions throughout the life span (e.g., Helm, Sbarra, & Ferrer, Reference Helm, Sbarra and Ferrer2014; Yaniv et al., Reference Yaniv, Salomon, Waidergoren, Shimon-Raz, Djalovski and Feldman2021). Research has consistently demonstrated that stronger positive parent–child behavioral synchrony is an important predictor of better self-control, increased communicative competence, higher empathy and moral internalization, and fewer behavioral problems in both cross-sectional and longitudinal works (Feldman, Reference Feldman2007a, Reference Feldman2012; Feldman et al., Reference Feldman, Greenbaum and Yirmiya1999; Harrist & Waugh, Reference Harrist and Waugh2002; Im-Bolter, Anam, & Cohen, Reference Im-Bolter, Anam and Cohen2015; Jaffe et al., Reference Jaffe, Beebe, Feldstein, Crown, Jasnow, Rochat and Stern2001; Kochanska, Aksan, Prisco, & Adams, Reference Kochanska, Aksan, Prisco and Adams2008; Lindsey, Cremeens, Colwell, & Caldera, Reference Lindsey, Cremeens, Colwell and Caldera2009; Scholtes, Lyons, & Skowron, Reference Scholtes, Lyons and Skowron2020). For example, Im-Bolter et al. (Reference Im-Bolter, Anam and Cohen2015) found that children aged 6–10 years with clinical levels of behavioral problems (a combined measure of internalizing and externalizing behaviors) showed significantly lower parent–child behavioral synchrony during play compared to a nonclinical group.
While behavioral synchrony measures are the most commonly used measures of parent–child synchrony, there is work to suggest that biological measures of parent–child synchrony offer complementary information about parent–child interactions. Studies examining the coordination of biological states – termed biological synchrony – often find similar links to those reported in behavioral studies (Feldman, Reference Feldman2007c; Kalomiris & Kiel, Reference Kalomiris and Kiel2018; Lunkenheimer, Tiberio, Skoranski, Buss, & Cole, Reference Lunkenheimer, Tiberio, Skoranski, Buss and Cole2018; Suveg et al., Reference Suveg, Braunstein West, Davis, Caughy, Smith and Oshri2019). For example, in a study with preschoolers and their mothers (Lunkenheimer et al., Reference Lunkenheimer, Tiberio, Skoranski, Buss and Cole2018), lower parent–child autonomic synchrony (as measured via respiratory sinus arrhythmia [RSA], an index of parasympathetic activation) during periods of mild stress and free play was associated with a higher risk for behavioral problems (both internalizing and externalizing behaviors). Interestingly, this period of mild stress resulted in weaker autonomic synchrony compared with the period of free play. Importantly, these contexts were also differentially associated with internalizing and externalizing behaviors. While children's externalizing behaviors were associated with weaker autonomic synchrony during the stressful context as well as the free play context, children's internalizing behaviors were associated with weaker autonomic synchrony during a period of free play only, suggesting that context might play a role in these associations (Lunkenheimer et al., Reference Lunkenheimer, Tiberio, Skoranski, Buss and Cole2018). In other studies, weaker positive parent–child synchrony in cortisol concentration and weaker autonomic synchrony (i.e., RSA synchrony) have also been linked with higher levels of internalizing behaviors in childhood (Kalomiris & Kiel, Reference Kalomiris and Kiel2018; Suveg et al., Reference Suveg, Braunstein West, Davis, Caughy, Smith and Oshri2019). One additional study by Suveg et al. (Reference Suveg, Braunstein West, Davis, Caughy, Smith and Oshri2019) found that preadolescents with high levels of internalizing behaviors showed negative RSA synchrony with their mothers during a modified Trier Social Stress Task (Kirschbaum, Pirke, & Hellhammer, Reference Kirschbaum, Pirke and Hellhammer1993), while preadolescents with low levels of internalizing behavior showed positive RSA synchrony, suggesting that parent–child synchrony might be disrupted in dyads with a child high in internalizing behaviors. These results suggest that there is growing evidence that disrupted patterns of parent–child synchrony are linked with more internalizing and externalizing behaviors across childhood, and that parent–child biological synchrony might be a particularly important predictor of internalizing trajectories. While much more research is needed, results from these and other similar studies (e.g., Smith et al., Reference Smith, Jones, Charman, Clackson, Mirza and Wass2019) suggest that strong parent–child synchrony might not be universally adaptive and that, in some cases, high parent–child synchrony might increase risk for behavioral problems. For example, stronger parent–child synchrony in the context of strongly negative interactions is likely to increase risk for significant behavioral problems in children already showing signs of behavioral problems or at high risk of developing them. Thus, while positive forms of parent–child behavioral synchrony are likely to be universally positive, it is still unclear at what point in the spectrum (from typically developing to clinically impaired) and under which contexts (e.g., mildly negative vs. highly negative interactions) parent–child physiological synchrony becomes a risk rather than a protective factor.
Neural synchrony
While most research on parent–child synchrony has focused on behavioral and physiological measures, recent work has started to explore the synchronization of neural responses (often referred to as hyperscanning) as a way to assess the neural underpinnings of dyadic coordination during in vivo interactions (Azhari et al., Reference Azhari, Leck, Gabrieli, Bizzego, Rigo, Setoh and Esposito2019; Hoyniak et al., Reference Hoyniak, Quiñones-Camacho, Camacho, Chin, Williams, Wakschlag and Perlman2021; Miller et al., Reference Miller, Vrtička, Cui, Shrestha, Hosseini, Baker and Reiss2019; Montague et al., Reference Montague, Berns, Cohen, McClure, Pagnoni, Dhamala and Apple2002; Nguyen et al., Reference Nguyen, Schleihauf, Kayhan, Matthes, Vrtička and Hoehl2020a, Reference Nguyen, Schleihauf, Kayhan, Matthes, Vrtička and Hoehl2020b; Quiñones-Camacho et al., Reference Quiñones-Camacho, Fishburn, Belardi, Williams, Huppert and Perlman2021; Reindl, Gerloff, Scharke, & Konrad, Reference Reindl, Gerloff, Scharke and Konrad2018). This synchronization of neural activation has been theorized to facilitate the formation of bonds and shared mental states (Redcay & Schilbach, Reference Redcay and Schilbach2019; Wheatley, Kang, Parkinson, & Looser, Reference Wheatley, Kang, Parkinson and Looser2012) by helping optimize internal models of complex dynamic environments, enhancing memory and attention to the interacting partner, while reducing the cognitive resources that need to be engaged during the social interaction (Macrae, Duffy, Miles, & Lawrence, Reference Macrae, Duffy, Miles and Lawrence2008; Miles, Nind, & Macrae, Reference Miles, Nind and Macrae2009, Reference Miles, Griffiths, Richardson and Macrae2010). Emerging evidence suggests that increased parent–child neural synchrony in typically developing children might play an important role in children's healthy development (Miller et al., Reference Miller, Vrtička, Cui, Shrestha, Hosseini, Baker and Reiss2019; Quiñones-Camacho et al., Reference Quiñones-Camacho, Fishburn, Camacho, Hlutkowsky, Huppert, Wakschlag and Perlman2019b; Reindl et al., Reference Reindl, Gerloff, Scharke and Konrad2018). To illustrate this, one study of 5- to 9-year-olds found that stronger positive parent–child neural synchrony in the prefrontal cortex (PFC) during cooperation was linked with better emotion regulation in both the parent and the child, and mediated the association between parent and child emotion regulation (Reindl et al., Reference Reindl, Gerloff, Scharke and Konrad2018). In another study, with preschoolers and their mothers, communicative reciprocity (i.e., turn-taking) during a conversation was associated with higher neural synchrony (Nguyen et al., Reference Nguyen, Schleihauf, Kayhan, Matthes, Vrtička and Hoehl2020a, Reference Nguyen, Schleihauf, Kayhan, Matthes, Vrtička and Hoehl2020b). Thus, research to date suggests that neural synchrony of the PFC might be an underlying biological mechanism for dyadic attunement facilitating behavioral synchronization and socialization processes more broadly. While work on parent–child neural synchrony has started to explore synchrony under various contexts such as cooperation conditions (Miller et al., Reference Miller, Vrtička, Cui, Shrestha, Hosseini, Baker and Reiss2019; Nguyen et al., Reference Nguyen, Schleihauf, Kayhan, Matthes, Vrtička and Hoehl2020a), passive video watching (Azhari et al., Reference Azhari, Leck, Gabrieli, Bizzego, Rigo, Setoh and Esposito2019), conversation (Nguyen et al., Reference Nguyen, Schleihauf, Kayhan, Matthes, Vrtička and Hoehl2020b), or has compared cooperation versus competition conditions (Reindl et al., Reference Reindl, Gerloff, Scharke and Konrad2018), little research has explored how emotionally salient interactional contexts might influence parent–child neural synchrony. This is particularly important when thinking about possible links between parent–child neural synchrony and psychopathology as contexts that require some level of active emotion co-regulation may be more informative for predicting risk for psychopathology. We know from research on child development that parent–child physiological synchrony during contexts with varied regulatory demands differentially relate to child outcomes (e.g., Lunkenheimer et al., Reference Lunkenheimer, Tiberio, Skoranski, Buss and Cole2018; Quiñones-Camacho et al., Reference Quiñones-Camacho, Fishburn, Camacho, Hlutkowsky, Huppert, Wakschlag and Perlman2019b). Thus, it is possible that measures of neural synchrony during varying tasks/contexts would also differentially relate to child outcomes. Demonstrating the utility of assessing parent–child neural synchrony during contexts with varying regulatory demands, a recent study with 4- to 5-year-olds found that lower parent–child neural synchrony during a period of play following a frustration induction was associated with higher child irritability – a transdiagnostic marker of both externalizing and internalizing behaviors (Quiñones-Camacho et al., Reference Quiñones-Camacho, Fishburn, Camacho, Hlutkowsky, Huppert, Wakschlag and Perlman2019b). This study offers, to our knowledge, the only evidence to date that parent–child neural synchrony is linked with early markers of risk for psychopathology.
Current study
The goal of the current study was to examine behavioral and neural parent–child synchrony as predictors of longitudinal changes in internalizing and externalizing behaviors across the preschool period. In particular, we extended findings from previous work by exploring whether neural synchrony during a frustration induction and a period of play predicted trajectories of internalizing and externalizing behaviors across a 1.5-year period. Moreover, we explored whether similar associations between synchrony and symptoms would be present when using measures of behavioral versus neural synchrony. We hypothesized that there would be modest decreases in internalizing and externalizing behaviors throughout this period, consistent with previous studies that have investigated trajectories of internalizing and externalizing behaviors in this age range (Bub, McCartney, & Willett, Reference Bub, McCartney and Willett2007; Coie & Dodge, Reference Coie, Dodge, Damon and Eisenberg1998; Fanti & Henrich, Reference Fanti and Henrich2010; Schappin, Wijnroks, Venema, & Jongmans, Reference Schappin, Wijnroks, Venema and Jongmans2018). Based on previous work showing that internalizing and externalizing behaviors are differentially associated with parent–child physiological synchrony based on task context (e.g., mild stress vs. free play; Lunkenheimer et al., Reference Lunkenheimer, Tiberio, Skoranski, Buss and Cole2018), we hypothesized that higher levels of parent–child neural synchrony in our community sample during a frustration period would predict lower initial levels and a greater rate of change (i.e., greater decreases) in externalizing behaviors, and that higher levels of parent–child neural synchrony during the period of play following the frustration induction would predict lower initial levels and a greater rate of change (i.e., greater decreases) in internalizing behaviors. Lastly, we explored whether behavioral synchrony would show similar associations with internalizing and externalizing symptoms as did neural synchrony.
Method
Participants
In total, 151 preschoolers (4–5 years old at Time 1; M = 4.85 years, SD = .6) and a caregiver (144 mothers) took part in a longitudinal study designed to explore the neural underpinnings of emotional development and the emergence of psychopathology in the preschool period (e.g., Quiñones-Camacho et al., Reference Quiñones-Camacho, Fishburn, Camacho, Wakschlag and Perlman2019a). In the current study, data from four time points were used – an initial visit when the children were 4–5 years old and three other time points that were separated by 6 months; thus Time 1 = 0 months, Time 2 = 6 months after the initial visit, Time 3 = 12 months after the initial visit, and Time 4 = 18 months after the initial visit (these time points are henceforth referred to as T1, T2, T3, and T4, respectively). Families were excluded from participating in the study if the parents reported having already sought clinical services for their child or if their child had any current or past psychiatric diagnosis at the first time point. Children were also excluded if they had a neurological disorder, a history of loss of consciousness, sensory impairments (e.g., epilepsy, cerebral palsy, autism spectrum disorder), or significant intellectual disability. The study was approved by the Institutional Review Board and all families consented before participation in the study. Parents reported on their child's race. The racial breakdown was 68% White, 23% Black or African American, 6% Biracial, 2% Asian American, and 1% Native American or Pacific Islander. Children were identified as being 95% non-Hispanic. Household income was reported as follows: 70 families (47%) reported as an income of less than US$60,000/year, 55 families (36%) reported an income of $61,000–$120,000/year, and 26 families (17%) reported an income higher than $121,000/year.
Child internalizing and externalizing behaviors
Parents completed the Child Behavior Checklist (CBCL) (Achenbach, Reference Achenbach1991) at all four time points. The CBCL is a widely used assessment of problem behavior in children. Given the focus of the study, we used the internalizing and externalizing behavior scales. We used two versions of the CBCL – one version that has been validated for ages 1.5–5 years and another for ages 6–18 years. Parents were asked to complete the version corresponding to their child's age at the time of assessment. In both versions, parents rate their child's behavior using a 3-point Likert scale (0 = not true; 1 = somewhat true; 2 = very true). Given our interest in explicitly mapping longitudinal changes in internalizing and externalizing behaviors in a community sample, we used raw scores rather than age- and gender-normalized t scores for analyses. The psychometric properties of the CBCL have been previously demonstrated (Achenbach, Reference Achenbach1991; Achenbach, Dumenci, & Rescorla, Reference Achenbach, Dumenci and Rescorla2001). Reliability for the internalizing subscale was good in our sample (T1: 1.5–5 years version, α = .82; T2: 1.5–5 years version, α = .81; 6–18 years version, α = .86; T3: 1.5–5 years version, α = .85; 6–18 years version, α = .83; T4: 1.5–5 years version, α = .82; 6–18 years version, α = .81). As was the reliability for the externalizing subscale (T1: 1.5–5 years version, α = .91; T2: 1.5–5 years version, α = .92; 6–18 years version, α = .80; T3: 1.5–5 years version, α = .91; 6–18 years version, α = .90; T4: 1.5–5 years version, α = .90; 6–18 years version, α = .88). Means, standard deviations, and ranges for each time point are listed in Table 1. Using CBCL standardized scores, at T1, 16 children (11%) were in the borderline or clinical range for internalizing and 10 children (7%) were in this range for externalizing. At T2, 16 children (11%) were in this range for internalizing and 16 (11%) for externalizing. T3 scores were in a similar range – 18 children (12%) for internalizing and 20 (13%) for externalizing – as were the scores at T4 – 16 children (11%) for internalizing and 19 (13%) for externalizing. Nine children were missing CBCL data at T2, 10 were missing data at T3, and 11 were missing data at T4. All children had data at T1 and 148 children had data for at least two time points. All missing CBCL data occurred due to missing a visit in the longitudinal study.
Table 1. Descriptive statistics and correlations among variables of interest

Note: T1 = Time 1 (0 months), T2 = Time 2 (6 months after initial visit), T3 = Time 3 (12 months after initial visit), and T4 = Time 4 (18 months after initial visit). Values in bold = p < .05.
a Age in months.
Parent–child synchrony task
The current study used data from the Disruptive Behavior Diagnostic Observation Schedule: Biological Synchrony (DB-DOS: BioSync) (Quiñones-Camacho et al., Reference Quiñones-Camacho, Fishburn, Camacho, Hlutkowsky, Huppert, Wakschlag and Perlman2019b) during the initial in-lab visit. The DB-DOS: BioSync was adapted from the validated DB-DOS (Wakschlag et al., Reference Wakschlag, Hill, Carter, Danis, Egger, Keenan and Briggs-Gowan2008), which was designed to elicit variations in emotional and behavioral regulation and to assess parent–child dyads’ ability to co-regulate across contexts with varying demands. The modified version of the DB-DOS used for this study was developed to fit the task requirements of functional near-infrared spectroscopy (fNIRS) and other biological measures (e.g., minimization of movement). The DB-DOS: BioSync consists of two contexts – a “frustration” induction context always followed by an unstructured play context. The frustration context consisted of a period of 10 min in which the dyads were instructed to complete challenging tangram puzzles together as fast as they could while refraining from playing with attractive toys left next to the table. The puzzles consisted of seven flat geometric shapes that were combined to form a larger shape (e.g., a cat). This frustration context consisted of four blocks of solving up to five puzzles within a 2-min window, followed by a 15-s inter-block interval. To motivate the dyads to work together to complete the puzzles, they were told that they would receive a prize if they completed the task. To further increase the frustrating nature of this context, the puzzles were too difficult for the child's developmental stage, they were given 1:45 min instead of the expected 2:00 min, and they were shown a countdown clock on a screen indicating how much time they had left. The “frustration” context was followed by the “play” context, which also consisted of 10 min divided into four blocks of 2 min, followed by a 15 s inter-block interval. During this period, the dyads were told they could play with the attractive toys that had been originally placed next to them. Each block was an opportunity for the dyad to add a new toy.
fNIRS data acquisition and preprocessing
A NIRScout fNIRS system (NIRx Medical Technologies LLC, Glen Head, NY, USA) was used to collect noninvasive optical imaging (i.e., fNIRS) data using a continuous-wave system at T1. fNIRS data were also collected at T3 (these data are not included in this article). Light was emitted at 760 nm and 850 nm from eight LED light sources and measured by four photodiode light detectors, resulting in 10 measurement channels per wavelength. Optical signals were collected at 15.625 Hz. Sensors were mounted on a neoprene head cap, with a source–detector distance of 2.9–3.1 cm. The head caps were placed following the international 10–20 coordinate system for both the parent and the child, with the dorsomedial sources over AF3/AF4 and the ventromedial sources over Fp1/Fp2 (Figure 1). This placement resulted in the probe extending over the middle frontal gyrus and inferior frontal gyrus of each hemisphere of the PFC, registered to the Colin27 brain atlas (Holmes et al., Reference Holmes, Hoge, Collins, Woods, Toga and Evans1998). When necessary, hair was manually separated under the optodes to improve signal detection. Out of the original 151 subjects, 117 dyads had usable fNIRS data (for both members of the dyad) at T1; data loss was due to computer errors, poor contact of the sensors with the scalp, or too much movement in either the parent or the child.

Figure 1. Probe configuration visualized on the surface of the scalp after registration to the Colin27 brain atlas. Green lines represent measurement channels.
Preprocessing of the fNIRS data and activation analyses were carried out in MATLAB (MathWorks, Natick, MA USA) using the NIRS Brain AnalyzIR toolbox (Santosa, Zhai, Fishburn, & Huppert, Reference Santosa, Zhai, Fishburn and Huppert2018). The raw fNIRS intensity signals were first converted to changes in optical density. These optical density signals were then motion-corrected using the temporal derivative distribution repair method (Fishburn, Ludlum, Vaidya, & Medvedev, Reference Fishburn, Ludlum, Vaidya and Medvedev2019). This method utilizes a robust regression approach to remove large fluctuations in the optical density signal (attributed to motion artifacts) while retaining smaller fluctuations (attributed to hemodynamic activity). The motion-corrected signals were then resampled to 4 Hz to reduce computational overhead for the synchrony analyses, and a high-pass filter (cutoff of 0.01 Hz and filter order of four) was used to remove slow drifts in the signal. The optical density signals were then converted to oxygenated hemoglobin concentration using the modified Beer–Lambert law.
Quantification of neural synchrony
Parent–child neural synchrony was defined as the concurrent lateral PFC activation of the parent and the child during the “frustration” and “play” contexts separately. Timings were first standardized across participants. Then, the signals were whitened by eliminating the temporal autocorrelations using an autoregressive model. This was done as serial correlations are a common source of noise in fNIRS data and can inflate correlation estimates (Santosa, Aarabi, Perlman, & Huppert, Reference Santosa, Aarabi, Perlman and Huppert2017). There is evidence that serial correlations in time series data can artificially inflate functional connectivity estimates from wavelet transform coherence or Pearson correlations, and that this increased false discovery rate can be controlled by using a robust correlation approach with temporally whitened signals (Santosa et al., Reference Santosa, Aarabi, Perlman and Huppert2017). We thus chose this approach for our analyses. The Bayesian information criterion was used to choose the order of the autoregressive model from a minimum value of one to a maximum of 32 – a value of 20 has been shown to be sufficient (Santosa et al., Reference Santosa, Aarabi, Perlman and Huppert2017). A robust regression approach was then used to calculate robust correlation coefficients between participants (Shevlyakov & Smirnov, Reference Shevlyakov and Smirnov2011). Parent–child neural synchrony was then quantified using the Fisher r-to-z transformation of the absolute value of the robust correlation coefficient. This was done for all possible channel pairs. Reciprocal connections were enforced to reduce the number of unique connections and thus prevent multiple comparisons corrections from being overly conservative.
Parent–child neural synchrony
The significance of the neural synchrony analyses was estimated via permutation testing with random dyads (e.g., parent of dyad B with child of dyad D). This approach allowed us to confirm that the synchrony was driven by a dyad's active interaction rather than being driven by two people completing similar tasks. Neural synchrony was calculated between all possible subject pairs to determine the appropriate null distribution of neural synchrony values. Due to some data loss, there were neural synchrony values for 117 concurrent parent–child dyads and 27,144 nonconcurrent (null) parent–child dyads $( N_{{\rm null}} = [ ( N_{{\rm subject}}^2 -N_{{\rm subject}}) /2] -N_{{\rm dyad}})$![]() . Permutation testing was then conducted to calculate the p value associated with each concurrent dyad's neural synchrony value by calculating the proportion of values from null pairings that were equal to or greater than the observed value, for example $\hat{p} = [ \sum ( Z_{{\rm null}} \ge Z_{{\rm observed}}) + 1] /( N + 2)$
. Permutation testing was then conducted to calculate the p value associated with each concurrent dyad's neural synchrony value by calculating the proportion of values from null pairings that were equal to or greater than the observed value, for example $\hat{p} = [ \sum ( Z_{{\rm null}} \ge Z_{{\rm observed}}) + 1] /( N + 2)$![]() . The constant terms were chosen to guarantee that the resulting p values would be between zero and one. After this, adjusted Z values were calculated from the estimated p values using an inverse cumulative density function for the standard normal distribution. One dyad had an adjusted Z value that was over four standard deviations and was removed from analyses. Another dyad had usable data but was excluded due to a child's brain abnormality identified via magnetic resonance imaging at a later time point. Adjusted Z values were submitted to a mixed-effects model with task condition modeled as a fixed effect and dyad ID modeled as a random effect. The presence of parent–child neural synchrony was assessed for each condition using the t contrast corresponding to a one-sample t test. Lastly, the corresponding p values were corrected for multiple comparisons by calculating the Benjamini–Hochberg false discovery rate-corrected p value (Benjamini & Hochberg, Reference Benjamini and Hochberg1995) across all unique channel pairs. Both conditions resulted in significant neural synchrony compared to the null distribution, and no significant differences in neural synchrony emerged between conditions; for a full description of the neural synchrony findings, which are not described further in this paper, please refer to Quiñones-Camacho et al. (Reference Quiñones-Camacho, Fishburn, Camacho, Hlutkowsky, Huppert, Wakschlag and Perlman2019b). In order to include parent–child neural synchrony as a predictor in the latent growth models, we extracted synchrony values for each context from a channel pair that showed the strongest effect in the mixed-effects model (i.e., peak channel) to be used for further analyses. This channel generally corresponds to the right dorsolateral PFC. This allowed us to reduce the number of parameters estimated from using all significant channel pairs and allowed for a closer comparison of the neural and behavioral synchrony models.
. The constant terms were chosen to guarantee that the resulting p values would be between zero and one. After this, adjusted Z values were calculated from the estimated p values using an inverse cumulative density function for the standard normal distribution. One dyad had an adjusted Z value that was over four standard deviations and was removed from analyses. Another dyad had usable data but was excluded due to a child's brain abnormality identified via magnetic resonance imaging at a later time point. Adjusted Z values were submitted to a mixed-effects model with task condition modeled as a fixed effect and dyad ID modeled as a random effect. The presence of parent–child neural synchrony was assessed for each condition using the t contrast corresponding to a one-sample t test. Lastly, the corresponding p values were corrected for multiple comparisons by calculating the Benjamini–Hochberg false discovery rate-corrected p value (Benjamini & Hochberg, Reference Benjamini and Hochberg1995) across all unique channel pairs. Both conditions resulted in significant neural synchrony compared to the null distribution, and no significant differences in neural synchrony emerged between conditions; for a full description of the neural synchrony findings, which are not described further in this paper, please refer to Quiñones-Camacho et al. (Reference Quiñones-Camacho, Fishburn, Camacho, Hlutkowsky, Huppert, Wakschlag and Perlman2019b). In order to include parent–child neural synchrony as a predictor in the latent growth models, we extracted synchrony values for each context from a channel pair that showed the strongest effect in the mixed-effects model (i.e., peak channel) to be used for further analyses. This channel generally corresponds to the right dorsolateral PFC. This allowed us to reduce the number of parameters estimated from using all significant channel pairs and allowed for a closer comparison of the neural and behavioral synchrony models.
Parent–child behavioral synchrony
Instances of parent–child behavioral synchrony were assessed during both contexts of the DB-DOS: BioSync task. Behavioral synchrony was a global code and was defined as the amount of time the dyad spent engaged in mutually responsive and co-regulated interactions via shared attention, reciprocal communication, eye contact, and coordinated behaviors (the coding scheme can be found in Supplementary Material 1). Every second of each of the contexts (separately) was coded as being either synchronous or asynchronous. A synchronous code indicated that the dyad engaged in a mutually responsive and co-regulated interaction during that second of the interaction. Specifically, each second of the interaction was coded as synchronous if the dyad showed reciprocal communication, eye contact, and coordinated behaviors with directed gaze during that period. Before the first synchrony code was given, the dyad had to exchange three verbal or behavioral turns. Since our coding of behavioral synchrony was a single global score, waiting for the dyad to exchange three verbal or behavioral turns before coding the interaction as synchronous ensured that the dyad was actually engaging in reciprocal responding, which is necessary to establish synchrony and is likely to take more than 1 s. Behavioral synchrony continued to be coded until there was a break in the reciprocal exchanges (e.g., the dyad did not show any reciprocal responding for more than 3 s). After a period of asynchrony, parent–child dyads could regain synchrony by engaging in coordinated and reciprocal interactions for at least 3 s. These individual second-by-second measures were summed to create a general behavioral synchrony score for each context (i.e., the total time spent in synchrony during the frustration and play contexts). Videos were coded offline. Of the 151 dyads who participated in the study, only 127 had codable videos (missingness was due to problems with the video camera or audio). Parent–child behavioral synchrony was coded by six trained research assistants who did not interact with the dyad during the visit. Behavioral synchrony training comprised of an initial conceptual grounding, followed by coding for eight master tapes to 0.80 reliability (kappa) of the master codes. After this, coders were assigned new videos to code. Reliability was calculated on 20% of data for all codable videos and was acceptable (kappa =.81). There were no significant differences in behavioral synchrony between conditions.
Data analysis plan
To examine the association between parent–child neural synchrony and change in internalizing and externalizing behaviors across early childhood, we used latent growth curve (LGC) modeling. LGC analysis allows for the modeling of change over time in internalizing and externalizing behaviors while also allowing for investigation of between-person variability in change and predictors of rate of change (McArdle & Epstein, Reference McArdle and Epstein1987). LGC modeling was done using Mplus version 8.4 (Muthén & Muthén, Reference Muthén and Muthén1998–2017). Internalizing and externalizing behavior data from the CBCL across the four time points were used to estimate latent intercept and slope factors. The latent intercept factor was centered at T1, making its interpretation equivalent to the level of internalizing (or externalizing) behaviors at T1. The latent slope factor represents the rate of change from T1 to T4 (T4 being 1.5 years after T1). Variances for the latent intercept and slope factors reflect the presence of individual differences in initial levels and rate of change (for latent intercept and slope, respectively). After an initial growth model was established, models were fitted for parent–child neural synchrony during frustration and play, separately. Specifically, the single neural synchrony score extracted for each condition (described at the end of the parent-child neural synchrony section) was entered as a predictor of the intercept and slope in the LGC model. All models were estimated using full information maximum likelihood and model fit was determined via examination of the chi-square (χ2), the comparative fit index (CFI), the Tucker–Lewis index (TLI), the root mean squared error of approximation (RMSEA), and the standardized root mean square residual (SRMR). Standard guidelines were used to estimate good model fit, such as nonsignificant χ2, CFI and TLI values higher than .95, and RMSEA and SRMR values smaller than .06 (Hu & Bentler, Reference Hu and Bentler1998).
Results
Preliminary results
Descriptive statistics and correlations among the study variables are shown in Table 1. There were no sex differences in any of our variables of interest (t values < .983, p values > .325). Age at the initial visit was also not associated with any of the variables of interest (r values < −.141, p values > .095). As expected, internalizing behaviors were correlated across all four time points (r values > .519, p values < .001), as were externalizing behaviors r values > .575, p values < .001).
Trajectory of internalizing and externalizing behaviors
Internalizing behaviors
A linear growth model showed excellent fit for internalizing behaviors, χ2 (5) = 4.839, p = .436, CFI = 1.000, TLI = 1.000, RMSEA = .000, SRMR = .031, and was an improvement over a random intercept only model, χ2 (8) = 19.171, p = .014, CFI = .958, TLI = .969, RMSEA = .096, SRMR = .070. Fit indices and parameter estimates are provided in Table 2. The mean for the intercept (corresponding to internalizing behaviors at T1) was significant, indicating that initial levels of internalizing behaviors were significantly different from zero. The linear slope was also significant and negative, indicating that, on average, children showed a linear decrease in internalizing behaviors over the four time points. The variances for both the intercept (p < .001) and slope (p = .005) were significant, indicating significant variability across children in their initial levels and rate of change in internalizing behaviors, supporting the addition of predictors to the model.
Table 2. Parameter estimates, standard errors, and fit indices for latent growth curve (LGC) models for children's internalizing behaviors with neural synchrony during frustration and play as predictors

Note: B = unstandardized coefficient, SE = standard error, CFI = comparative fit index, TLI = Tucker–Lewis index, RMSEA = root mean squared error of approximation, SRMR = standardized root mean square residual. Values in bold = p < .05.
Externalizing behaviors
A linear growth model showed adequate fit for externalizing behaviors, χ2 (5) = 16.723, p = .005, CFI = 0.967, TLI = 0.961, RMSEA = .125, SRMR = .059, and was an improvement over a random intercept only model, χ2 (8) = 47.255, p < .001, CFI = .890, TLI = .918, RMSEA = .180, SRMR = .115. Fit indices and parameter estimates are presented in Table 3. The mean for the intercept (corresponding to externalizing behaviors at T1) was significant, indicating that initial levels of externalizing behaviors were significantly different from zero. The linear slope was also significant and negative, indicating that, on average, children showed a linear decrease in externalizing behaviors over the four time points. The variances for the intercept was significant (p < .001), while the variance for the slope was marginal (p = .066), indicating some variability across children in their initial levels and rate of change in externalizing behaviors.
Table 3. Parameter estimates, standard errors, and fit indices for latent growth curve (LGC) models for children's externalizing behaviors with neural synchrony during frustration and play as predictors

Note: B = unstandardized coefficient, SE = standard error, CFI = comparative fit index, TLI = Tucker–Lewis index, RMSEA = root mean squared error of approximation, SRMR = standardized root mean square residual. Values in bold = p < .05.
Neural synchrony as a predictor of internalizing and externalizing trajectories
Internalizing behaviors
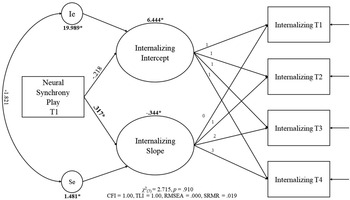
Two models were fitted by adding each neural synchrony measure (i.e., frustration and play) to the linear growth model. The model for neural synchrony during “frustration” resulted in a similar fit as the model with no predictors, χ2 (7) = 2.327, p = .940, CFI = 1.00, TLI = 1.00, RMSEA = .000, SRMR = .016. However, neural synchrony during this context did not predict initial levels or rate of change in internalizing behaviors. The second model with “play” as the predictor (Figure 2) also showed a similar fit as the model with no predictors, χ2 (7) = 2.715, p = .910, CFI = 1.00, TLI = 1.00, RMSEA = .000, SRMR = .019. Neural synchrony during “play” was a predictor of the rate of change only (b = .317, p = .027), suggesting that stronger parent–child neural synchrony during a period of play was associated with a greater rate of change in internalizing behaviors; in this case, a more marked decrease over the four time points.

Figure 2. Latent growth curve (LGC) model with parent–child neural synchrony during play predicting children's internalizing trajectories. Bold values with an asterisk represent significant unstandardized parameters. Ie = Error of the intercept; Se = error of the slope.
Externalizing behaviors
Two additional models were fitted by adding each neural synchrony measure (i.e., frustration and play) to the linear growth model. The model for neural synchrony during “frustration” resulted in a better fit than the model with no predictors, χ2 (7) = 12.802, p = .077, CFI = 0.980, TLI = 0.971, RMSEA = .085, SRMR = .056. However, neural synchrony during this context did not predict initial levels or rate of change in externalizing behaviors. The second model with “play” as the predictor also showed an improved fit from the model with no predictors, χ2 (7) = 12.303, p = .091, CFI = 0.982, TLI = 0.974, RMSEA = .081, SRMR = .056. Neural synchrony during “play” also did not predict rate of change.
Follow-up analyses with behavioral synchrony
Internalizing behaviors
To assess whether the associations observed between internalizing behaviors and parent–child neural synchrony could be explained by behavioral synchrony, an additional set of models with behavioral synchrony added as a second predictor was fitted. A model including behavioral and neural synchrony for the “play” period was fitted for internalizing behaviors first; this model showed a good fit, χ2 (9) = 9.059, p = .432, CFI = 1.000, TLI = 1.000, RMSEA = .008, SRMR = .032. Behavioral synchrony marginally predicted initial level (b = −.008, p = .071) and neural synchrony still significantly predicted rate of change (b = .364, p = .014). A similar model was then fitted for internalizing behaviors with behavioral and neural synchrony during the “frustration” context as predictors. This model showed a good fit, χ2 (9) = 6.489, p = .690, CFI = 1.000, TLI = 1.000, RMSEA = .000, SRMR = .031. However, neither synchrony measure predicted initial levels or rate of change (p values > .350).
Externalizing behaviors
Two final models were then fitted for externalizing behaviors. The model including behavioral and neural synchrony for the “play” context showed adequate fit, χ2 (9) = 16.589, p = .056, CFI = 0.972, TLI = 0.956, RMSEA = .093, SRMR = .066. Behavioral synchrony predicted initial levels of externalizing behaviors (b = −.020, p = .002); neural synchrony did not predict initial levels of rate of change (p values > .319). The last model, using behavioral and neural synchrony during the “frustration” condition as predictors of externalizing behaviors, also showed adequate fit, χ2 (9) = 19.018, p = .107, CFI = 0.963, TLI = 0.943, RMSEA = .107, SRMR = .069. As with the previous model, behavioral synchrony during the “frustration” condition predicted initial levels of externalizing behaviors (b = −.018, p < .001), but neural synchrony did not predict initial levels of rate of change (p values > .265).Footnote 1
Discussion
The current study examined behavioral and neural forms of parent–child synchrony as predictors of trajectories of internalizing and externalizing behaviors across the preschool period. As expected, and consistent with previous studies (e.g., Bub et al., Reference Bub, McCartney and Willett2007; Fanti & Henrich, Reference Fanti and Henrich2010; Schappin et al., Reference Schappin, Wijnroks, Venema and Jongmans2018), both internalizing and externalizing behaviors decreased over the 1.5-year period, with more sharp decreases in externalizing behaviors, indicative of a developmentally normative transition towards greater self-regulation (Thompson & Meyer, Reference Thompson, Meyer and Gross2007). As we hypothesized, parent–child synchrony during the “play” context predicted modest decreases in internalizing behaviors, but this was only true for our measure of neural synchrony. For externalizing behaviors, we failed to find associations with neural synchrony. However, behavioral synchrony emerged as a predictor of initial levels of externalizing symptoms even when considering a neural measure of parent–child synchrony for the same context. Our findings serve as evidence of the role of parent–child neural synchrony – in particular neural synchrony of the PFC – as an important buffer against internalizing psychopathology. The fact that these findings were specific to a period of play following a frustration induction, which served as a period of recovery from the frustrating context (rather than during the frustration induction per se), supports previous work on the importance of recovery periods for understanding the regulation of distress in children (Kahle, Miller, Lopez, & Hastings, Reference Kahle, Miller, Lopez and Hastings2016; Quiñones-Camacho et al., Reference Quiñones-Camacho, Fishburn, Camacho, Hlutkowsky, Huppert, Wakschlag and Perlman2019b; Santucci et al., Reference Santucci, Silk, Shaw, Gentzler, Fox and Kovacs2008). This is particularly important as both internalizing and externalizing disorders are linked with emotion dysregulation. Our findings offer some neural support for the role that parent–child co-regulation following a period of distress plays in the emergence and maintenance of internalizing behaviors in early childhood. This is particularly meaningful given that we focused on parent–child neural synchrony of the PFC – an area not only associated with emotion regulation (e.g., Lévesque et al., Reference Lévesque, Joanette, Mensour, Beaudoin, Leroux, Bourgouin and Beauregard2004; Ochsner, Bunge, Gross, & Gabrieli, Reference Ochsner, Bunge, Gross and Gabrieli2002), but also a region that undergoes substantial development during the preschool years (Diamond, Reference Diamond, Stuss and Knight2002), and a region that has been found in previous studies on parent–child neural synchrony to partially mediate links between parent and child emotion regulation (Reindl et al., Reference Reindl, Gerloff, Scharke and Konrad2018).
Our finding that neural synchrony during a period of play following a frustration induction was related to internalizing behaviors, but not neural synchrony during the frustration period, is not completely surprising as previous work has shown that positive interactions following challenging dyadic interactions are particularly critical predictors of positive child outcomes (Ham & Tronick, Reference Ham and Tronick2009; Quiñones-Camacho et al., Reference Quiñones-Camacho, Fishburn, Camacho, Hlutkowsky, Huppert, Wakschlag and Perlman2019b; Tronick, Reference Tronick2007). Previous work has found that returning to synchrony after a distressing event is a particularly important indicator of adaptive parent–child interactions (Ham & Tronick, Reference Ham and Tronick2009; Scholtes et al., Reference Scholtes, Lyons and Skowron2020). It is possible that this is the case because the period of play immediately following the challenging and mildly frustrating task allowed the dyad to return to a more positive interactive state, thus serving as a period of repair from the dyadic stress generated during the frustration context. This is likely to be driven by the fact that the play context offered the opportunity for parents to socialize emotion regulation and for children to practice regulating their negative affect in the absence of a distressing stimulus or event. For children with higher internalizing behaviors, periods of distress might be particularly hard to navigate and the negative emotions elicited by distressing events might be sustained for longer periods of time in comparison to peers with lower levels of internalizing behaviors. As such, positive interactions following distressing events may be particularly critical opportunities for parent–child co-regulation. Higher synchrony in this context would then allow children to stay attuned to the parent, facilitating parent–child co-regulation, allowing the child to navigate the sustained negative emotions more easily and thus increasing the child's confidence in their ability to cope with negative emotions. Over time, this could support an increase in self-regulatory competence, decreasing the risk for later psychopathology. There is behavioral (e.g., Feldman et al., Reference Feldman, Greenbaum and Yirmiya1999; Kochanska et al., Reference Kochanska, Aksan, Prisco and Adams2008), physiological (e.g., Feldman, Reference Feldman2007b), and, to a lesser extent, neural (Reindl et al., Reference Reindl, Gerloff, Scharke and Konrad2018) work demonstrating that parent–child synchrony supports the development of adaptive self-regulation. Our work extends those findings by demonstrating the role that parent–child neural synchrony plays in internalizing trajectories.
While neural synchrony of the PFC emerged as an important predictor of internalizing trajectories, the same was not true for externalizing trajectories. However, given that the growth models showed a better fit for internalizing behaviors and that the variance in the slope of externalizing was marginal, this result should be interpreted with caution. It is possible that we did not find a link between neural synchrony and externalizing behaviors because there was not sufficient variability in the trajectories of externalizing behaviors to find this effect. It is also possible that higher parent–child neural synchrony during a period of play following a frustrating episode is particularly important in the context of internalizing behaviors, as it might be indicative of factors, such as a lack of behavioral withdrawal on the part of the child, that might be protective. Given that a tendency to withdraw is a common characteristic of children high in internalizing behaviors (Achenbach, Reference Achenbach1991), being able to continue to interact with their parent after a frustrating episode might allow children to continue to engage in co-regulation with their parents, reducing their distress and serving as a protective factor against increasing internalizing trajectories. However, given that withdrawing from interactions is not common in externalizing disorders, children showing high levels of externalizing behaviors might not benefit to the same extent from higher parent–child neural synchrony during periods of repair. Furthermore, given that neural synchrony facilitates social interactions by helping reduce the cognitive burden necessary for enhanced memory and attention to an interacting partner (Macrae et al., Reference Macrae, Duffy, Miles and Lawrence2008), it is possible that increased parent–child neural synchrony might be particularly useful in the context of internalizing behaviors as it might facilitate modification of children's internal models of difficult social interactions, resulting in a decreased need to withdraw during distressing events and allowing for greater parent–child co-regulation. This, in turn, would result in greater changes in self-regulation and an increased sense of control over time, potentially explaining why neural synchrony was associated with the slope (i.e., change) and not the initial levels of internalizing behaviors.
It is noteworthy that the patterns for behavioral and neural synchrony were different, with behavioral synchrony associated with the intercept of internalizing (marginally) and externalizing behaviors, whereas neural synchrony was associated with the slope for internalizing only. There are various reasons for why this might be the case. For example, while our measure of neural synchrony represented the level of coordination within the dorsolateral PFC of parent and child neural activity throughout the task, our behavioral measure considered parent and child behaviors as part of a single measure, thus representing a dyadic-level global approach to parent–child behaviors. While we cannot conclude from the results of this study exactly why behavioral and neural synchrony showed different patterns of associations with internalizing and externalizing behaviors, our finding that behavioral and neural synchrony were differentially linked with child outcomes is not entirely unexpected as other studies have found similar differences between biological and behavioral measures of synchrony (Suveg, Shaffer, & Davis, Reference Suveg, Shaffer and Davis2016; Woltering, Lishak, Elliott, Ferraro, & Granic, Reference Woltering, Lishak, Elliott, Ferraro and Granic2015). It is possible that our measure of behavioral synchrony was not able to fully capture the type of parent–child dynamics that were driving neural synchrony during the play context. More research is needed, however, to fully understand how behavioral and neural synchrony relate to each other across interactional contexts. This is particularly important as the associations between behavioral and neural synchrony with problem behaviors differed between internalizing and externalizing disorders. A more systematic approach to assessing behavioral and neural forms of parent–child synchrony is needed to clarify whether neural synchrony is only linked to internalizing behaviors or if the associations between neural synchrony and externalizing and behaviors are dependent on context. Nonetheless, our study demonstrates the utility of using measures of neural synchrony as a viable approach to assessing biological underpinnings of the parent–child interaction, offering information beyond what can be captured from behavioral synchrony measures alone. It is possible that parent–child neural synchrony is only associated with internalizing behaviors, even when other interactional contexts are considered. If this is the case, this provides further evidence of the ways in which internal models in internalizing disorders are developed and modified early in life though parent–child interactions. This has substantial implications for our understanding of early psychopathology and the parent–child relationship more broadly.
While our study has several notable strengths, such as the multi-wave assessment of internalizing and externalizing behaviors during preschool years and our use of both behavioral and neural measures of parent–child synchrony, some limitations should be noted. First, while it is important to take dimensional approaches to early psychopathology as this allows for a greater understanding of trajectories towards clinical disorders, internalizing behaviors in our sample were generally at or below the subclinical threshold. The CBCL also has floor effects at the lower end of the normal–abnormal dimensional spectrum (Kaat et al., Reference Kaat, Blackwell, Estabrook, Burns, Petitclerc, Briggs-Gowan and Wakschlag2018). However, there was still significant variability in internalizing behaviors, as suggested by the significant variances for both intercept and slope. Second, and relatedly, we used two different versions of the CBCL throughout this study, meaning that some of the questions included in the internalizing and externalizing subscales changed from the initial visit to later visits. However, due to the heterotypic continuity of psychopathology across childhood, it is critical to use appropriate measures to capture the different developmental manifestations of psychopathology. Changing versions of the CBCL as children became older ensured we were able to capture these changing developmental manifestations. In addition, while our use of a neural measure of parent–child synchrony was a notable strength and provided important information about the neural underpinnings of co-regulation, we do not have detailed coding of the specific co-regulatory behaviors used during the task or detailed coding of the dyads’ affective responding. There is evidence that adaptive parent–child interactions are not monotonously synchronous (Tronick, Reference Tronick2007), and that periods of asynchrony during negative interactions and periods of repair are adaptive (Scholtes et al., Reference Scholtes, Lyons and Skowron2020). Thus, exploration of parent–child behavioral synchrony using a more thorough coding approach is necessary to fully disentangle the patterns of behavioral and affective synchrony that are most adaptive. Given that the current study only included a neural synchrony measure at T1, we were unable to test whether trajectories of internalizing (and externalizing) behaviors influence parent–child neural synchrony at later time points; it is likely that the association between parent–child neural synchrony and child psychopathology is bidirectional and that higher child symptoms influence patterns of dyadic synchrony at later time points. Lastly, fNIRS is a neuroimaging technique that is restricted to the measurement of cortical regions and our analyses focused on neural synchrony within the PFC. Thus, we were limited in the emotion-related regions and networks that we could probe and in our ability to address whether neural synchrony across regions or within regions of the PFC would show similar associations. Moreover, because we did not have anatomical MRI data for each child at T1 and did not have three-dimensional digitizer data available to confirm optode placement, it is possible that the peak channel measured activity of slightly different regions within the larger right PFC area. However, given the limitations of other neuroimaging techniques for measuring neural activity during in vivo social interactions, our study still represents a notable advancement over previous neuroimaging studies on parent–child interactions and child psychopathology. Moreover, our analyses showing that neural synchrony within the PFC was linked with trajectories of preschool psychopathology extends work on the role of the PFC for social processes and its implications for child outcomes.
While research on parent–child neural synchrony is still emerging, findings from this line of work seem promising for identifying biological mechanisms for the parent–child co-regulation of distress, which in turn may help clarify dyadic-level biological correlates of risk for psychopathology. However, before truly clarifying the utility of these measures, a more careful exploration of interactional context will be necessary. This will be needed alongside a more careful exploration of the role of age (using both concurrent and longitudinal approaches), an explicit consideration of parental psychopathology, and direct comparison of neuroimaging modalities (e.g., EEG vs. fNIRS), as well as comparisons of neural and physiological measures of synchrony will be needed. In addition, while work on parent–child neural synchrony and early psychopathology in community samples is necessary for understanding transitions from normative to clinical behaviors, the results might change for children in the more clinical/severe range; in actuality, there is reason to believe that these associations would look different in samples of children and/or parents with high symptomatology. For example, in a study by Suveg et al. (Reference Suveg, Braunstein West, Davis, Caughy, Smith and Oshri2019), preadolescents high in internalizing symptoms showed negative RSA synchrony with their mothers while preadolescents with low internalizing symptoms showed positive RSA synchrony. Although we are not aware of any data, to date, that has focused on parent–child neural synchrony in high-risk groups, we have reason to believe, based on work on physiological measures, that the most adaptive patterns of parent–child neural synchrony might be different in dyads with higher levels of symptoms.
Although substantial work is still needed, the findings from this study advance our understanding of the role of the neurobiological underpinnings of parent–child co-regulation of emotion on trajectories of internalizing and externalizing behaviors during the preschool years. As such, our study offers initial evidence of the longitudinal implications of the parent–child synchronization of neural responses on psychopathology trajectories and underscores the importance of considering the biological mechanisms for parent–child co-regulation as a potential target for the mitigation of risk for internalizing disorders in early childhood.
Supplementary Material
The Supplementary Material for this article can be found at https://doi.org/10.1017/S0954579421000468.
Acknowledgments
We thank Lisa M. Bemis, Christina O. Hlutkowsky and the undergraduate research assistants of the Laboratory for Child Brain Development for their help in data collection. We also thank the children and families who participated in the study.
Funding Statement
This work was supported by the National Institutes of Health (R01-MH107540, PI: Perlman). Laura E. Quiñones-Camacho and Caroline P. Hoyniak were supported by the National Institute of Mental Health (NIMH T32 MH100019-06; PIs: Barch and Luby).
Conflicts of Interest
None.