The risk to develop depression is largely determined by both genetic and environmental factors, particularly adverse childhood events (Heim, Plotsky, & Nemeroff, Reference Heim, Plotsky and Nemeroff2004). A common polymorphism in the serotonin transporter (5-HTT, SLC6A4) gene, which is the 5-HTT linked polymorphic region (5-HTTLPR), has been shown to moderate the impact of stressful life events on the occurrence of major depression (Caspi et al., Reference Caspi, Sugden, Moffitt, Taylor, Craig and Harrington2003; Karg, Burmeister, Shedden, & Sen, Reference Karg, Burmeister, Shedden and Sen2011; but see also Risch et al., Reference Risch, Herrell, Lehner, Liang, Eaves and Hoh2009). Individuals with the short allele of the 5-HTTLPR polymorphism seem to be especially sensitive to the experience of early life stress (ELS; Karg et al., Reference Karg, Burmeister, Shedden and Sen2011). Yet, the increased sensitivity of short-allele carriers to the environment is not restricted to adverse events, as short-allele carriers show increased benefit from positive, supportive environments (Kaufman et al., Reference Kaufman, Yang, Douglas-Palumberi, Houshyar, Lipschitz and Krystal2004; Pluess, Belsky, Way, & Taylor, Reference Pluess, Belsky, Way and Taylor2010; van IJzendoorn, Belsky, & Bakermans-Kranenburg, Reference van IJzendoorn, Belsky and Bakermans-Kranenburg2012). Thereby, the 5-HTTLPR polymorphism seems to be consistent with the differential susceptibility model, which postulates that Gene (5-HTTLPR) × Environment interactions are for better and worse (Belsky, Bakermans-Kranenburg, & van IJzendoorn, Reference Belsky, Bakermans-Kranenburg and van IJzendoorn2007; Ellis, Boyce, Belsky, Bakermans-Kranenburg, & van IJzendoorn, Reference Bakermans-Kranenburg and van IJzendoorn2011; Homberg & Lesch, Reference Homberg and Lesch2011). The depressogenic ELS × 5-HTTLPR interaction is likely influenced by additional interactions with other genetic and environmental factors (Heiming & Sachser, Reference Heiming and Sachser2010; Uher, Reference Uher2010; Van der Doelen, Kozicz, & Homberg, Reference Van der Doelen, Kozicz and Homberg2013). One example is the epistasis between 5-HTTLPR and polymorphisms in the corticotropin-releasing hormone receptor 1 (CRH1R) gene, which influences the impact of childhood maltreatment on depressive symptoms (Cicchetti, Rogosch, & Oshiri, Reference Cicchetti, Rogosch and Oshri2011; Ressler et al., Reference Ressler, Bradley, Mercer, Deveau, Smith and Gillespie2010). CHR1R is one of two genes encoding a receptor for corticotropin-releasing factor (CRF) or hormone (CRH).
CRF/CRH is a 41 amino acid peptide, which has an initiating and coordinating role in the physiological and behavioral stress response (Sutton, Koob, Le Moal, Rivier, & Vale, Reference Sutton, Koob, Le Moal, Rivier and Vale1982; Vale, Spiess, Rivier, & Rivier Reference Vale, Spiess, Rivier and Rivier1981). The activity of CRF neurons in the paraventricular nucleus (PVN) of the hypothalamus is important in the neurobiology of depression, as these neurons drive the activity of the hypothalamic–pituitary–adrenal (HPA) axis. In depression, a subset of patients exhibit hyperactivity of the HPA axis, resulting in elevated levels of glucocorticoids, which is thought to be driven by the increased activity of the CRF neurons in the PVN (Arborelius, Owens, Plotsky, & Nemeroff, Reference Arborelius, Owens, Plotsky and Nemeroff1999). Furthermore, postmortem studies have found an increased number of CRF neurons and CRF mRNA content in the PVN of depressed subjects (Raadsheer, Hoogendijk, Stam, Tilders, & Swaab, Reference Raadsheer, Hoogendijk, Stam, Tilders and Swaab1994; Raadsheer et al., Reference Raadsheer, Van Heerikhuize, Lucassen, Hoogendijk, Tilders and Swaab1995; Wang, Kamphuis, Huitinga, Zhou, & Swaab, Reference Wang, Kamphuis, Huitinga, Zhou and Swaab2008). In addition, these patients display increased levels of CRF in their cerebrospinal fluid, which can be reduced by treatment with antidepressants or electroconvulsive shocks (Heuser et al., Reference Heuser, Bissette, Dettling, Schweiger, Gotthardt and Schmider1998, Nemeroff et al., Reference Nemeroff, Widerlov, Bissette, Walleus, Karlsson and Eklund1984; Nemeroff, Bissette, Akil, & Fink, Reference Nemeroff, Bissette, Akil and Fink1991). Furthermore, the intracerebroventricular administration of CRF in experimental animals produces responses reminiscent of symptoms of major depression: anhedonia, anxiety, decreased food intake, decreased sexual activity, and disturbed sleep and locomotion (Bale & Vale, Reference Bale and Vale2004; Binder & Nemeroff, Reference Binder and Nemeroff2010). We have previously shown in rats that ELS and 5-HTT genotype interact to program the adult HPA axis, but we did not find altered CRF mRNA levels in the PVN (Van der Doelen et al., Reference Van der Doelen, Deschamps, D'Annibale, Peeters, Wevers and Zelena2014). However, CRF is not only found in the PVN but also expressed in higher order brain regions, including the amygdala and the bed nucleus of the stria terminalis (BNST). Both regulate the activity of the HPA axis, as well as autonomic and behavioral responses to stress (Ulrich-Lai & Herman, Reference Ulrich-Lai and Herman2009; Walker, Miles, & Davis, Reference Walker, Miles and Davis2009). The central amygdaloid nucleus (CeA) and the oval subdivision of the BNST (BNSTov) contain the major populations of CRF neurons in the rat amygdala and BNST, respectively (Merchenthaler, Vigh, Petrusz, & Schally, Reference Merchenthaler, Vigh, Petrusz and Schally1982; Morin, Ling, Liu, Kahl, & Gehlert, Reference Morin, Ling, Liu, Kahl and Gehlert1999; Sterrenburg et al., Reference Sterrenburg, Gaszner, Boerrigter, Santbergen, Bramini and Roubos2012). Modulation of CRF expression in the CeA affects basal and stress-induced anxiety-like behavior in rodents (Callahan, Tschetter, & Ronan, Reference Callahan, Tschetter and Ronan2013; Flandreau, Ressler, Owens, & Nemeroff, Reference Flandreau, Ressler, Owens and Nemeroff2012; Regev et al., Reference Regev, Neufeld-Cohen, Tsoory, Kuperman, Getselter and Gil2011; Regev, Tsoory, Gil, & Chen, Reference Regev, Tsoory, Gil and Chen2012). Furthermore, CRF overexpression in the CeA has been reported to lead to increased CRF mRNA in the PVN and increased stress-induced HPA activity (Callahan et al., Reference Callahan, Tschetter and Ronan2013; Flandreau et al., Reference Flandreau, Ressler, Owens and Nemeroff2012; Keen-Rhinehart et al., Reference Keen-Rhinehart, Michopoulos, Toufexis, Martin, Nair and Ressler2009). In the BNSTov, modulation of CRF expression has been shown to affect conditioned anxiety, as well as depression-like behavior, but not HPA activity (Regev et al., Reference Regev, Neufeld-Cohen, Tsoory, Kuperman, Getselter and Gil2011; Sink et al., Reference Sink, Walker, Freeman, Flandreau, Ressler and Davis2013). Yet, the CRF populations in the CeA and BNSTov seem to have complementary functions in fear conditioning (Walker et al., Reference Walker, Miles and Davis2009) and are responsive to exposure to acute and chronic stress paradigms (Rouwette et al., Reference Rouwette, Klemann, Gaszner, Scheffer, Roubos and Scheenen2011; Sterrenburg et al., Reference Sterrenburg, Gaszner, Boerrigter, Santbergen, Bramini and Elliott2011).
During a stress response, CRF mRNA levels in the PVN, CeA, and BNSTov are frequently found to be upregulated (Kovács & Sawchenko, Reference Kovács and Sawchenko1996; Ma, Levy, & Lightman, Reference Ma, Levy and Lightman1997; Makino et al., Reference Makino, Shibasaki, Yamauchi, Nishioka, Mimoto and Wakabayashi1999; Rouwette et al., Reference Rouwette, Klemann, Gaszner, Scheffer, Roubos and Scheenen2011). Prominent examples of transcription factors involved in Crf expression are activating protein (AP-1), nerve growth factor induced gene B, and cyclic AMP (cAMP) response element binding protein (Itoi et al., Reference Itoi, Horiba, Tozawa, Sakai, Sakai and Abe1996; Kovács & Sawchenko, Reference Kovács and Sawchenko1996; Murphy & Conneely, Reference Murphy and Conneely1997; Yao, Stenzel-Poore, & Denver, Reference Yao, Stenzel-Poore and Denver2007). The proximal promoter region of Crf contains a cAMP response element, as well as a putative AP-1 binding site (Yao & Denver, Reference Yao and Denver2007). DNA methylation is an epigenetic mechanism that occurs at cytosine–phosphate–guanine (CpG) sites and is associated with the repression of gene transcription (Moore, Le, & Fan, Reference Moore, Le and Fan2013). The epigenetic programming of gene transcription is considered to be a key interface for Gene × Environment (G × E) interactions (Bale et al., Reference Bale, Baram, Brown, Goldstein, Insel and McCarthy2010; Bogdan, Hyde, & Hariri, Reference Bogdan, Hyde and Hariri2013; Meaney, Reference Meaney2010; van IJzendoorn, Caspers, Bakermans-Kranenburg, Beach, & Philibert, Reference van IJzendoorn, Caspers, Bakermans-Kranenburg, Beach and Philibert2010). Exposure to ELS has been shown previously to regulate DNA methylation and transcription of the glucocorticoid receptor gene in the hippocampus of rats (Weaver et al., Reference Weaver, Cervoni, Champagne, D'Alessio, Sharma and Seckl2004) and humans (McGowan et al., Reference McGowan, Sasaki, D'Alessio, Dymov, Labonté and Szyf2009), and the Crf and arginine vasopressin genes in the PVN of mice (Chen et al., Reference Chen, Evans, Liu, Honda, Saavedra and Aguilera2012; Mueller & Bale, Reference Mueller and Bale2008; Murgatroyd et al., Reference Murgatroyd, Patchev, Wu, Micale, Bockmühl and Fischer2009).
A frequently used rodent model of ELS is the prolonged and repeated separation of newborn pups from their mother (maternal separation; Macrì & Würbel, Reference Macrì and Würbel2006; Pryce et al., Reference Pryce, Rüedi-Bettschen, Dettling, Weston, Russig and Ferger2005). Maternal separation has been reported to affect the basal and stress-induced expression of CRF in the PVN, CeA, and BNSTov (Aisa, Tordera, Lasheras, Del Rio, & Ramirez, Reference Aisa, Tordera, Lasheras, Del Río and Ramírez2007; Bravo, Dinan, & Cryan, Reference Bravo, Dinan and Cryan2011; Chen et al., Reference Chen, Evans, Liu, Honda, Saavedra and Aguilera2012; Desbonnet, Garrett, Daly, Mcdermott, & Dinan, Reference Desbonnet, Garrett, Daly, McDermott and Dinan2008; Ladd, Thrivikraman, Huot, & Plotsky, Reference Ladd, Thrivikraman, Huot and Plotsky2005; Pierce, Ryals, Wang, & Christianson, Reference Pierce, Ryals, Wang and Christianson2014; Plotsky et al., Reference Plotsky, Thrivikraman, Nemeroff, Caldji, Sharma and Meaney2005; Veenema, Reber, Selch, Obermeier, & Neumann, Reference Veenema, Reber, Selch, Obermeier and Neumann2008). The brain's serotonin (5-HT) system seems to have a significant impact on the regulation of the Crf gene as well. The modulation of neuronal 5-HT content, for instance, affects CRF mRNA levels in the PVN (Jørgensen, Knigge, Kjaer, Møller, & Warberg, Reference Jørgensen, Knigge, Kjaer, Møller and Warberg2002), while the administration of selective serotonin reuptake inhibitors can reverse stress-induced elevations of Crf transcription (Pan, Hong, Zhang, & Kong, Reference Pan, Hong, Zhang and Kong2013; Stout, Owens, & Nemeroff, Reference Stout, Owens and Nemeroff2002).
Based on the literature described above, we hypothesized that ELS and 5-HTT gene variation interact to affect DNA methylation of the Crf promoter region in the adult rat brain. Because basal and stress-induced expression levels of CRF are expected to be programmed by Crf promoter methylation, this could represent an interesting target for intervening in ELS × 5-HTTLPR-related psychopathology. To test our hypothesis, we used an animal model of the ELS × 5-HTTLPR interaction. Specifically, we performed a Crf promoter DNA methylation assay on PVN, CeA, and BNSTov biopsies of adult heterozygous and homozygous 5-HTT knockout rats (5-HTT +/−, 5-HTT −/−), and their wildtype (5-HTT +/+) littermates exposed to ELS (maternal separation paradigm) or a control treatment. We have performed these analyses for a cohort of rats that was not exposed to any additional stressors (adult naive cohort) and a cohort that was examined for stress coping behavior in the learned helplessness (LH) paradigm (adult stress cohort). Furthermore, to study Crf expression, we performed quantitative real-time PCR analyses for both cohorts, and applied CRF immunohistochemistry to brains derived from the naive cohort.
Methods and Materials
Animals
The experimental procedures were approved by the Committee for Animal Experiments of the Radboud University Nijmegen, The Netherlands, and all efforts were made to minimize animal suffering and to reduce the number of animals used. Serotonin transporter knockout rats (Slc6a4 1Hubr) were generated by N-ethyl-N-nitrosurea (ENU)-induced mutagenesis (Smits et al., Reference Smits, Mudde, Van de Belt, Verheul, Olivier and Homberg2006). Experimental animals (5-HTT +/+, 5-HTT +/−, and 5-HTT −/− rats) were derived from crossing 3-month-old 5-HTT +/− rats that were outcrossed for at least 12 generations with commercial (Harlan, Ter Horst, The Netherlands) wild-type Wistar rats. The pregnant dams were housed in standard polypropylene cages (40 × 20 × 18 cm) with sawdust bedding and ad libitum access to water and rodent chow (Sniff Spezialdiäten, Soest, Germany) in a temperature (21 ± 1 °C) and humidity-controlled room (45%–60% relative humidity), with a 12:12 h light/dark cycle (lights on at 7:00 a.m.). The dams were inspected daily for delivery of pups at 5:00 p.m., and day of birth was designated as postnatal day (PND) 0. At PND 1, two paper towels (22.5 × 24.5 cm) were supplied to the mother for nest construction. Further, the litters were culled to a maximum of 10 pups, with gender ratios in favor of a male majority to maximally 7:3.
ELS
We used repeated and prolonged maternal separation as a model for ELS. Litters were randomly allocated to one of two rearing conditions (from PND 2 to 14): maternal separation for 180 min (MS180) or a control treatment with immediate reunion of mother and pups (MS0). MS180 was started daily between 8:30 and 9:00 a.m., and was performed as follows: the mother was removed from the home cage and placed into an identical cage until the end of the separation period. Pups were then removed from the nest as complete litters and placed into a cage (24 × 15 × 14 cm) with only sawdust bedding, after which they were transferred to an adjacent room. The cages were placed on heat pads, which were set to maintain a bedding temperature of 31–33 °C for PND 2–7 and 29–31 °C for PND 8–14. At the end of the separation period, litters were returned to their home cage by first rolling them in the home cage bedding material and then placing them in the nests. This was followed by reunion with the mothers. During PND 0–22, half of the bedding material of the home cages was refreshed every week. At PND 14, ear punches were taken of the pups for identification and genotyping, which was performed by Kbiosciences (Hoddesdon, UK). The procedure of genotyping has been described previously (Homberg et al., Reference Homberg, Olivier, Smits, Mul, Mudde and Verheul2007). At PND 22, the pups were weaned and housed in groups of two to three littermates of the same sex and rearing, under the same conditions as mentioned above.
Adult stress
For this study, we have made use of two cohorts of male 5-HTT +/+, 5-HTT +/−, and 5-HTT −/− rats that underwent ELS exposure. Cohort 1 was left undisturbed until sacrifice (PND 85–95) and was therefore naive in terms of adult stress exposure. Cohort 2 was a group of rats (PND 77–116) that underwent inescapable foot shocks for 2 days, followed 24 hr later by a shuttle box escape test (LH paradigm). The exposure to inescapable foot shocks induces a deficit in active coping behavior in a subset of vulnerable rats, as measured by escape latencies in the shuttle box escape test (Vollmayr & Henn, Reference Vollmayr and Henn2001). Previously, we reported that the LH paradigm differentially affects coping behavior in the current model of ELS × 5-HTT Genotype interaction. Specifically, we found that 5-HTT +/− rats were especially sensitive to showing successful predictive adaptation to a putative match between early and adult life environmental conditions, which was expressed as a reduced latency to escape stress (Van der Doelen et al., Reference Van der Doelen, Kozicz and Homberg2013).
Tissue collection and preparation
Cohort 1 was split into a group of rats that was sacrificed by decapitation and a group of rats that was sacrificed by perfusion. Of Cohort 2, all rats were sacrificed by decapitation. For decapitations, rats were taken from their home cage into a separate room and decapitated within 10 s. Immediately after decapitation, the brains were isolated, frozen in aluminum foil on dry ice, and stored at −80 °C. In a cryostat (−15 °C), the brains were prepared in 420 µm thick coronal slices in order to obtain punches from the anterodorsal part of the bed nucleus of the stria terminalis (Bregma +0.24 and −0.18 mm), the PVN (Bregma −1.32 and −1.74 mm), and the CeA (Bregma −1.72 and −2.14 mm). The punch from the anterodorsal part of the bed nucleus of the stria terminalis contains the BNSTov, the major site of CRF expression within the BNST (Merchenthaler et al., Reference Merchenthaler, Vigh, Petrusz and Schally1982; Morin et al., Reference Morin, Ling, Liu, Kahl and Gehlert1999; Sterrenburg et al., Reference Sterrenburg, Gaszner, Boerrigter, Santbergen, Bramini and Roubos2012). The brain areas were bilaterally punched out with a Miltex 1.0 mm biopsy puncher (Integra Miltex, York, PA), collected in sterile vials, immediately placed on dry ice, and stored at −80 °C. Half of the punches were used for RNA isolation, the other half for the isolation of genomic DNA. The punches were distributed by systematic randomization, accounting for lateralization and Bregma position. Representative images of punched sections and group sizes are available in the online-only supplementary material. For transcardial perfusion, rats received an intraperitoneal injection of sodium pentobarbital (50 mg/kg body weight). The perfusion was performed with phosphate buffered saline (PBS, pH 7.4) and a clamp on the abdominal aorta, followed by fixation of the brain with 4% paraformaldehyde in PBS. The fixated brains were immediately isolated, postfixated in fresh 4% paraformaldehyde, and transferred to a 30% sucrose solution in PBS. The sucrose solution was refreshed every 2 days until the brains were completely submerged. Then the brains were frozen by use of dry ice and were cut in 25 µm thick coronal slices on a freezing microtome (Microm, Walldorf, Germany). The sections were stored in a sterile antifreeze solution (0.05 M PBS, 30% ethylene glycol, 20% glycerol) at −20 °C.
DNA methylation assay
The DNA methylation assay was performed as described previously (Sterrenburg et al., Reference Sterrenburg, Gaszner, Boerrigter, Santbergen, Bramini and Elliott2011). Briefly, genomic DNA was isolated from punches of the BNSTov, PVN, and CeA by use of the DNeasy blood and tissue kit (Qiagen, Valencia, CA) and according to the manufacturer's instructions. Bisulfite conversion of the DNA samples and pyrosequencing of the promoter region and the initial part of exon 1 of the Crf gene were performed by EpigenDX (Hopkinton, MA; Kim, Hwang, Kim, & Kang, Reference Kim, Hwang, Kim and Kang2007). The Crf promoter region contains important CpG-containing regulatory sites, such as binding sites for transcription factors as cAMP response element binding protein and AP-1 (Yao et al., Reference Yao, Stenzel-Poore and Denver2007).
CRF expression
The expression of CRF (mRNA, protein) was studied by the use of quantitative real-time PCR (brain punches, Cohorts 1 and 2) and immunohistochemistry (fixated brain sections, Cohort 1). A detailed description of both techniques is available in the online-only supplementary material.
Statistical analysis
All statistical tests have been carried out using SPSS (version 20, IBM corporation, Armonk, NY). The results are presented as the mean with the standard error of the mean. The reaction time/polymerase chain reaction 2−ΔCt data have been expressed as a ratio compared to the average of the MS0-wild-type group. All data have been examined with factorial analysis of variance (ANOVA). If a significant main effect of genotype or interaction (Genotype × Early Life Stress) was found, post hoc testing was performed. For the correlational analysis between CRF mRNA and DNA methylation at the 12 CpG sites as well as the average CpG methylation, Bonferroni correction was applied for every brain area (p < .05/13 = p < .003846). Furthermore, one-tailed Spearman correlation was performed because of the consensus of a negative relationship between DNA methylation and transcriptional activity (Moore et al., Reference Moore, Le and Fan2013) and because the measure of DNA methylation is derived from an ordinal, nonlinear scale (percentage of methylated versus unmethylated PCR clones). Statistical significance was set at p < .05.
Results
DNA methylation of the CRF gene promoter region
We examined the DNA methylation of the promoter region of the Crf gene, which contains important sites for the regulation of transcription. All but one of the examined CpGs are found upstream of the transcription start site of the gene, indicated by a minus sign (online-only supplementary Figure S.1). CpG −226 resides in the middle of a cAMP response element site, while CpG −79 is located in a putative AP-1 binding site (Yao & Denver, Reference Yao and Denver2007). Furthermore, CpG −36, CpG −33, and CpG −15 surround the gene's TATA box, a sequence 24–30 base pairs from the Crf transcription start site, which recruits RNA polymerase II and transcription factors (Lee & Young, Reference Lee and Young2000; supplementary Figure S.1).
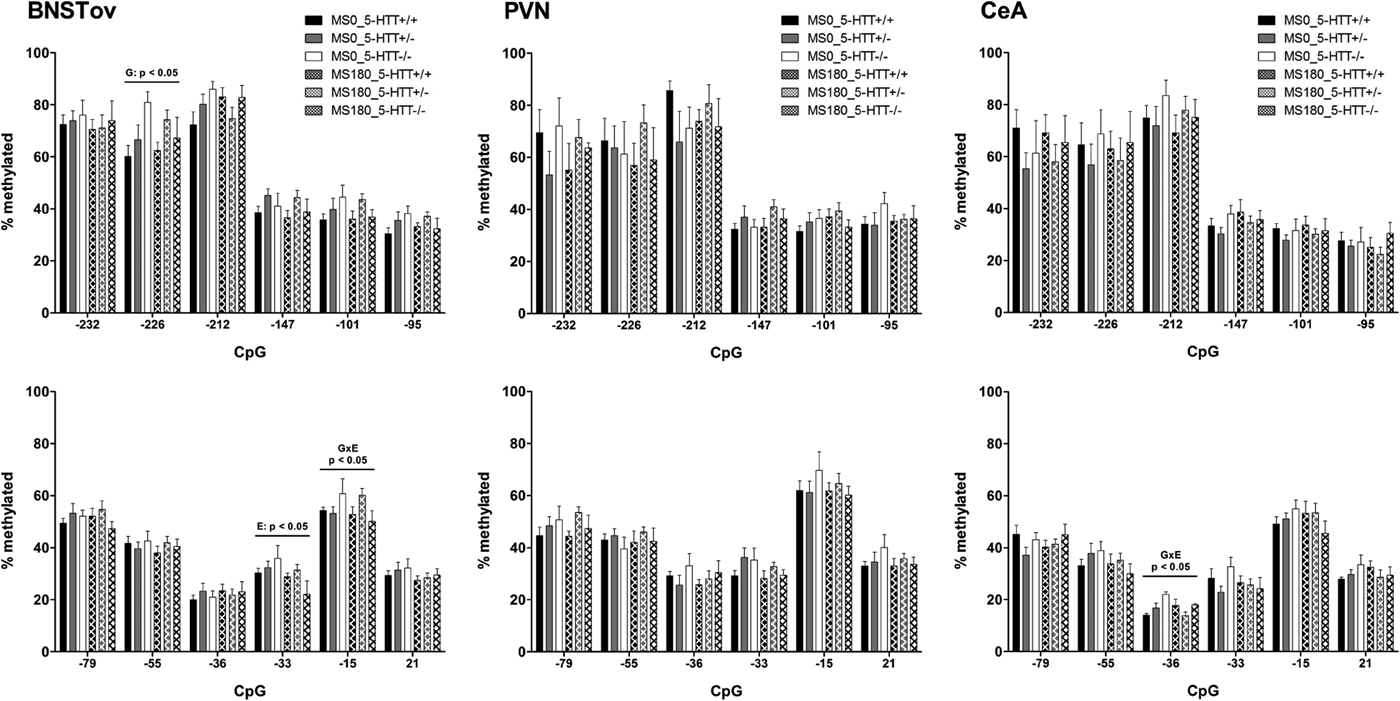
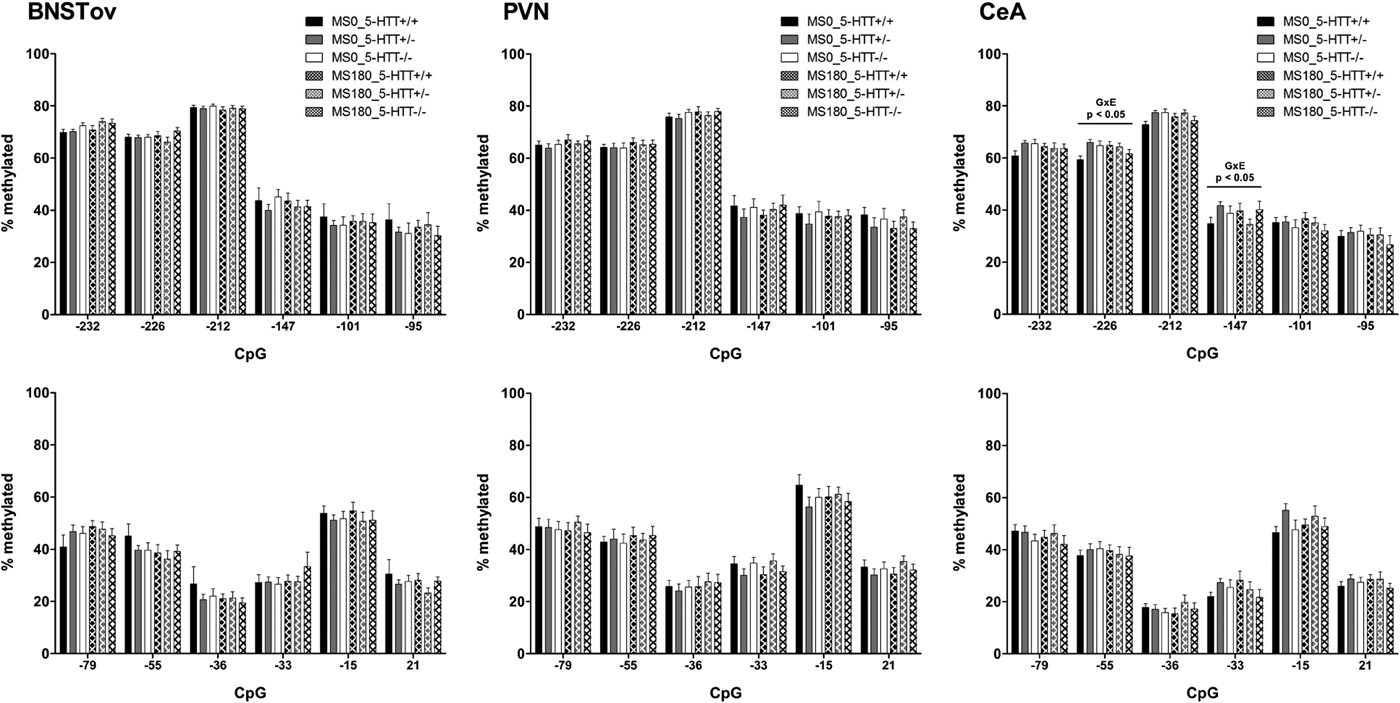
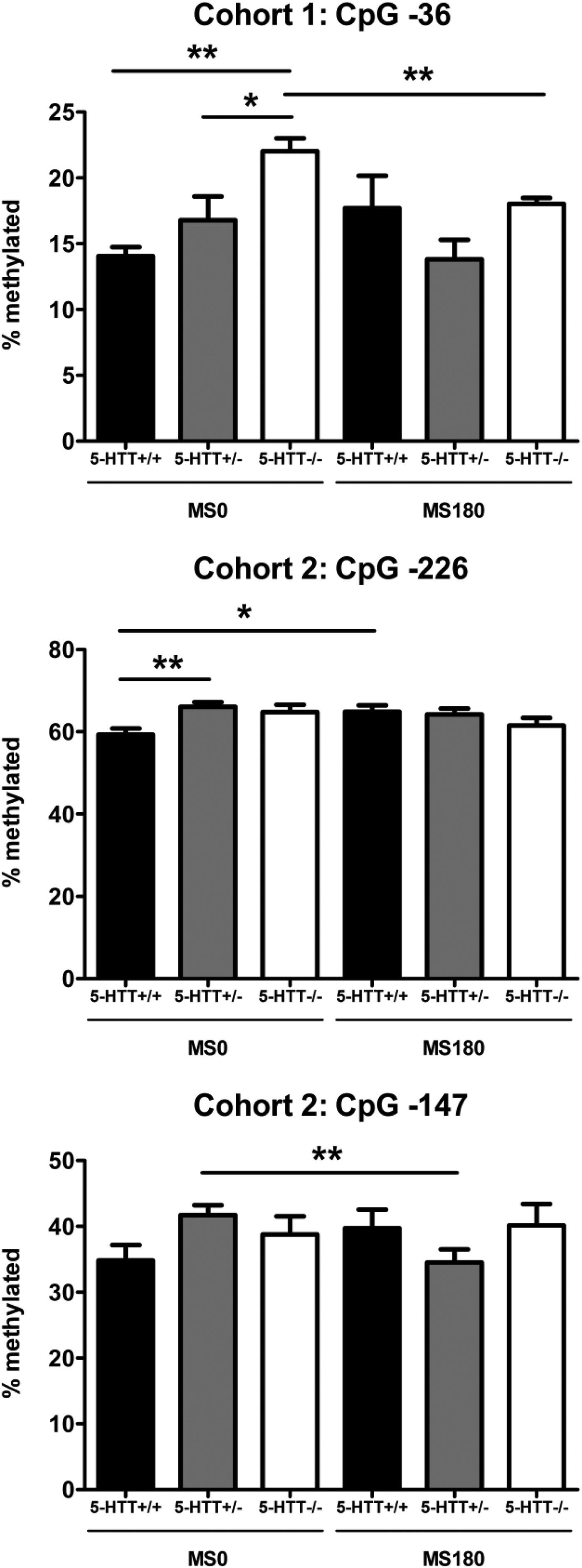
Overall, the DNA methylation levels of the promoter region of the Crf gene were found to be highly similar in the two cohorts and across the BNSTov, PVN, and CeA (Figures 1–2). In all three brain areas, DNA methylation was consistently the highest for CpG −232, CpG −226, and CpG −212 (60%–80%) compared to the downstream CpGs (30%–40%), with the exception of CpG −15 (50%–60%). For both cohorts, DNA methylation levels of the Crf promoter in the CeA were significantly affected by the interaction between ELS and 5-HTT genotype (Figures 1–2). In Cohort 1, methylation of CpG −36 was found to be significantly affected by the G × E interaction, F (2, 37) = 3.42, p < .05 (Figure 3). Post hoc analysis showed that 5-HTT −/− rats exhibit a higher degree of CpG −36 DNA methylation compared to 5-HTT +/− (p < .05) and 5-HTT +/+ (p < .01) rats under control conditions (MS0). With a history of ELS exposure, this 5-HTT genotype difference is no longer present, and this leads to a downregulation of CpG −36 methylation in 5-HTT −/− rats (p < .01; Figure 3). In Cohort 2, the methylation of CpG −226, F (2, 87) = 4.18, p < .05, and CpG −147, F (2, 87) = 3.60, p < .05, were significantly affected by the ELS × 5-HTT Genotype interaction (Figure 3). Here, post hoc analysis showed a significantly higher degree of CpG −226 methylation in 5-HTT +/− rats compared to 5-HTT +/+ rats (p < .01) under control conditions (MS0). With a history of ELS exposure, this 5-HTT genotype difference is no longer present, and this leads to an upregulation of CpG −226 methylation in 5-HTT +/+ rats (p < .05). Furthermore, CpG −147 methylation was found to be selectively decreased in 5-HTT +/− rats by exposure to ELS (p < .01; Figure 3).

Figure 1. Cohort 1: DNA methylation of the promoter region of the Crf gene. DNA methylation levels of the Crf promoter in the paraventricular nucleus (PVN), the oval subdivision of the bed nucleus of the stria terminalis (BNSTov), and the central amygdala (CeA) of serotonin transporter (5-HTT) homozygous knockout (5-HTT −/−), heterozygous knockout (5-HTT +/−), and wild-type (5-HTT +/+) rats exposed to daily 3-h separations (MS180) or a control treatment (MS0) from postnatal day 2 to 14. DNA methylation was assessed for 11 cytosine–phosphate–guanine (CpG) sites upstream of the transcription start site (e.g., −232, −226) and one CpG in exon 1 (+21) of the Crf gene. In the CeA, methylation of CpG −36 showed a significant regulation by 5-HTT Genotype × ELS interaction (G × E: p < .05). In the BNSTov, CpG −226 methylation was found to be significantly affected by 5-HTT genotype (G: p < .05), CpG −33 methylation by early life stress (ELS, E: p < .05), and CpG −15 methylation by 5-HTT Genotype × ELS interaction (G × E: p < .05). In the PVN, DNA methylation of the Crf promoter region was not found to be significantly affected by ELS, 5-HTT genotype, or their interaction.

Figure 2. Cohort 2: DNA methylation of the promoter region of the Crf gene. DNA methylation levels of the Crf promoter in the paraventricular nucleus (PVN), the oval subdivision of the bed nucleus of the stria terminalis (BNSTov), and the central amygdala (CeA) of serotonin transporter (5-HTT) homozygous knockout (5-HTT −/−), heterozygous knockout (5-HTT +/−), and wild-type (5-HTT +/+) rats exposed to daily 3-h separations (MS180) or a control treatment (MS0) from postnatal day 2 to 14. In addition, this cohort of rats was exposed on 3 consecutive days to footshocks in a learned helplessness paradigm in adulthood (Van der Doelen et al., Reference Van der Doelen, Kozicz and Homberg2013). DNA methylation was assessed for 11 cytosine–phosphate–guanine (CpG) sites upstream of the transcription start site (e.g., −232, −226) and one CpG in exon 1 (+21) of the Crf gene. In the CeA, methylation of CpG −226 and CpG −147 showed a significant regulation by ELS × 5-HTT Genotype interaction (G × E: p < .05). In the BNSTov and PVN, DNA methylation of the Crf promoter region was not found to be significantly affected by ELS, 5-HTT genotype, or their interaction.

Figure 3. Central amygdala methylation levels of cytosine–phosphate–guanine (CpG) −36 (Cohort 1), CpG −226, and CpG −147 (Cohort 2) of the Crf gene of serotonin transporter (5-HTT) homozygous knockout (5-HTT −/−), heterozygous knockout (5-HTT +/−), and wild-type (5-HTT +/+) rats exposed to daily 3-h separations (MS180) or a control treatment (MS0) from postnatal day 2 to 14. Cohort 1 was not exposed to stress in adulthood (adult naive group), while Cohort 2 was exposed on 3 consecutive days to footshocks in a learned helplessness paradigm (adult stress group; Van der Doelen et al., Reference Van der Doelen, Kozicz and Homberg2013). Methylation of CpG −36 (Cohort 1), CpG −226, and CpG −147 (Cohort 2) was found to be significantly regulated by the interaction of ELS and 5-HTT genotype, F (2, 37) = 3.42, p < .05, F (2, 87) = 4.18, p < .05, F (2, 87) = 3.60, p < .05, respectively. Post hoc analysis of CpG −36 methylation (Cohort 1) showed that 5-HTT −/− rats exhibit a higher degree of DNA methylation compared to 5-HTT +/− and 5-HTT +/+ rats under control conditions (MS0). With a history of ELS exposure (MS180), this 5-HTT genotype difference is no longer present, and this leads to a downregulation of CpG −36 methylation in 5-HTT −/− rats. Post hoc analysis of CpG −226 methylation (Cohort 2) showed that 5-HTT +/− rats display a significantly higher degree of DNA methylation compared to 5-HTT +/+ rats under control conditions (MS0). With a history of ELS exposure (MS180), this 5-HTT genotype difference is no longer present, and this leads to an u-regulation of CpG −226 methylation in 5-HTT +/+ rats. Post hoc analysis of CpG −147 methylation (Cohort 2) showed that ELS selectively induces a downregulation of DNA methylation at this CpG site in 5-HTT +/− rats. *p < .05, **p < .01.
In the BNSTov of Cohort 1, DNA methylation levels were found to be affected at three different CpG sites: CpG −226, CpG −33, and CpG −15 (Figure 1). In Cohort 2, however, DNA methylation of the Crf promoter in the BNSTov was not found to be significantly affected (Figure 2). For Cohort 1, factorial ANOVA revealed that DNA methylation at CpG −226 was significantly affected by 5-HTT genotype, F (2, 36) = 3.68, p < .05, while CpG −33 methylation was found to be decreased by ELS exposure (MS0 > MS180), F (1, 36) = 5.04, p < .05. DNA methylation at CpG −15 was found to be significantly affected by the ELS × 5-HTT Genotype interaction, F (2, 36) = 3.62, p < .05. For CpG −226, post hoc analysis showed that 5-HTT −/− rats display significantly higher methylation levels compared to 5-HTT +/+ rats (p < .05). For CpG −33 and CpG −15, post hoc testing did not reveal any significant differences between the individual experimental groups. For the PVN, DNA methylation of the Crf promoter region was not found to be significantly affected by ELS, 5-HTT genotype, or their interaction (Figures 1–2).
CRF expression
Of Cohort 1, we have previously published the qRT-PCR analysis of CRF mRNA levels in the PVN, for which we did not find any significant effect of ELS, 5-HTT genotype, or their interaction (Van der Doelen et al., Reference Van der Doelen, Deschamps, D'Annibale, Peeters, Wevers and Zelena2014). Here, we report that CRF mRNA levels in the BNSTov and CeA of Cohort 1, and CRF mRNA levels in the BNSTov, PVN, and CeA of Cohort 2 are also not significantly regulated by ELS, 5-HTT genotype, or their interaction (online-only supplementary Figures S.2–S.3). In addition, we performed immunohistochemistry to assess CRF expression at the protein level in Cohort 1. In the BNSTov and CeA, the number and sum of square differences (SSD) of both CRF-ir neurons and CRF-ir boutons were not found to be significantly regulated by ELS, 5-HTT genotype, or their interaction in the factorial ANOVA analysis (online-only supplementary Figures S.4–S.5). For the PVN, however, we found a significant effect of 5-HTT genotype on the number, F (2, 21) = 6.06, p < .01, as well as the SSD, F (2, 21) = 3.79, p < .05, of CRF-ir neurons. Post hoc analysis indicated that 5-HTT −/− rats display an increased number of CRF-ir neurons in the PVN compared to 5-HTT +/+ rats (p < .01). In contrast, 5-HTT −/− rats showed a lower SSD of CRF-ir neurons in the PVN compared to 5-HTT +/+ rats, but according to post hoc analysis, this difference was not significant (p = .07). In contrast to 5-HTT genotype, ELS and the ELS × 5-HTT Genotype interaction were not found to have a significant impact on the number and SSD of CRF-ir neurons in the PVN (online-only supplementary Figure S.6).
Correlational analysis: Crf promoter methylation and CRF mRNA levels
For both Cohorts 1 and 2, the levels of CRF mRNA and DNA methylation of the promoter region of the Crf gene were measured in the same subset of animals, which enables an analysis of the correlation between transcription and DNA methylation of Crf. The analysis was performed by combining Cohort 1 and 2 with all the 12 individual CpGs in the Crf promoter region as well as the average methylation of the CpG sites. Bonferroni correction for multiple testing was applied (p < .05/13 = p < .0038).
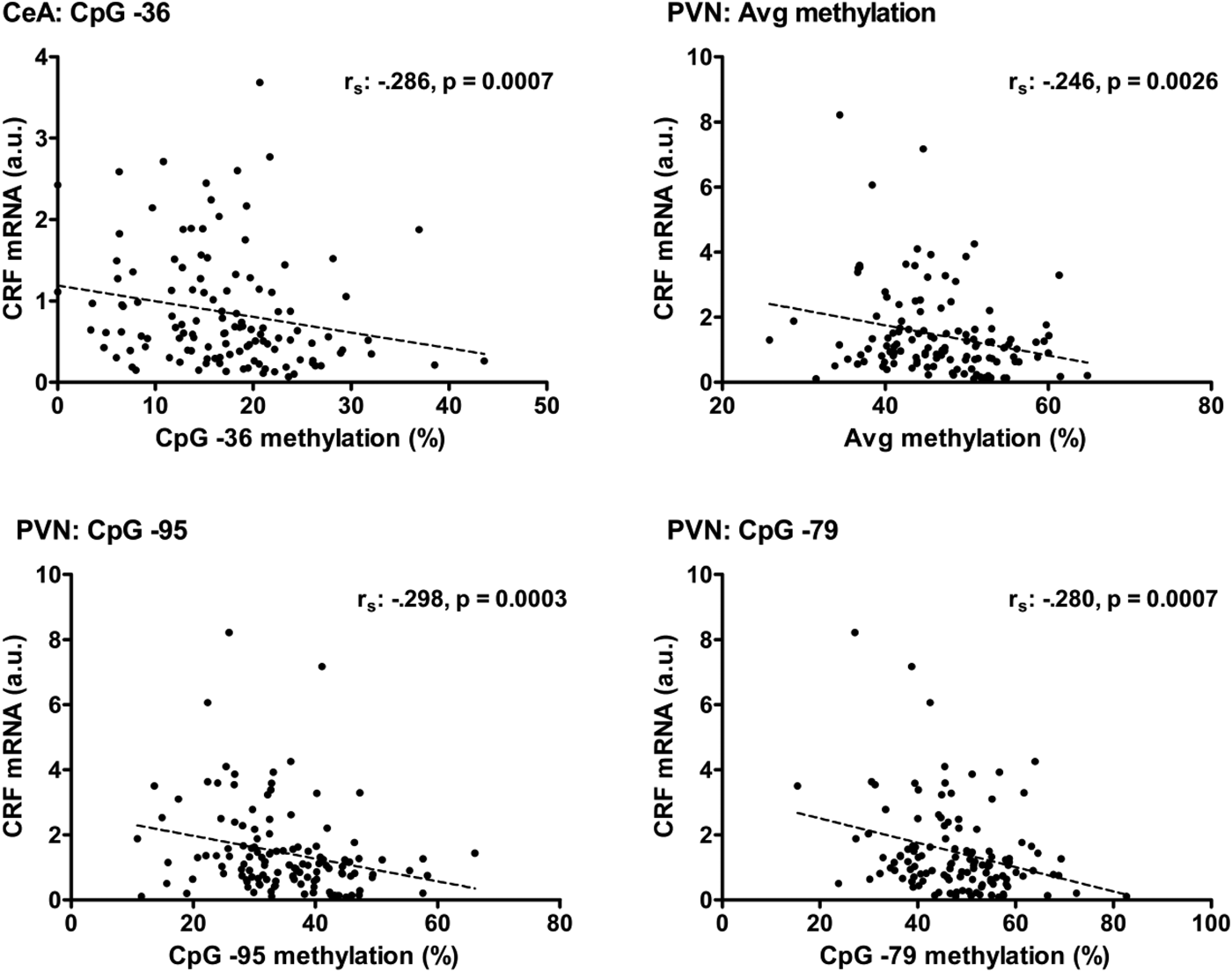
For the CeA, a negative correlation between CRF mRNA levels and DNA methylation at CpG −36 was found to be highly significant (r s = −.286, p = .0007; Figure 4). The methylation of CpG −33, CpG −15, CpG +21, and the average methylation of all CpGs also showed a negative correlation with CRF mRNA levels in the CeA (p < .05), but these correlations were not significant after Bonferroni correction.

Figure 4. Correlations between DNA methylation of the Crf promoter and CRF mRNA levels in the central amygdala (CeA) and paraventricular nucleus of the hypothalamus (PVN). In the CeA, the negative relationship (r s = −.286) between CRF mRNA and methylation of the cytosine–phosphate–guanine (CpG) −36 site in the promoter region of the Crf gene was found to be significant (p = .0007). In the PVN, a significant negative relationship was found between CRF mRNA levels and the average methylation of the Crf promoter (r s = −.246, p .0026) as well as the methylation of the individual CpG sites CpG −95 (r s = −.298, p = .0003) and CpG −79 (r s = −.280, p = .0007).
For the PVN, negative correlations between CRF mRNA levels and DNA methylation at CpG −95 (r s = −.298, p = .0003), CpG −79 (r s = −.280, p = .0007), as well as the average methylation of all CpGs (r s = −.246, p = .0026) survived Bonferroni correction (Figure 4). One might consider that the extreme values of PVN CRF mRNA levels drive the significance of the correlations. Nevertheless, these correlations remained significant with omission of the extremes of the CRF mRNA levels (p ≤ .0031). In addition, methylation of CpG −232, CpG −212, CpG −101, CpG −55, CpG −36, and CpG +21 seemed to contribute (p < .05) to the significant negative correlation between CRF mRNA levels and DNA methylation of the Crf promoter in the PVN. For the BNSTov, no significant correlations between CRF mRNA levels and DNA methylation of the Crf gene promoter region were identified.
Correlational analysis: CRF mRNA levels and stress coping behavior
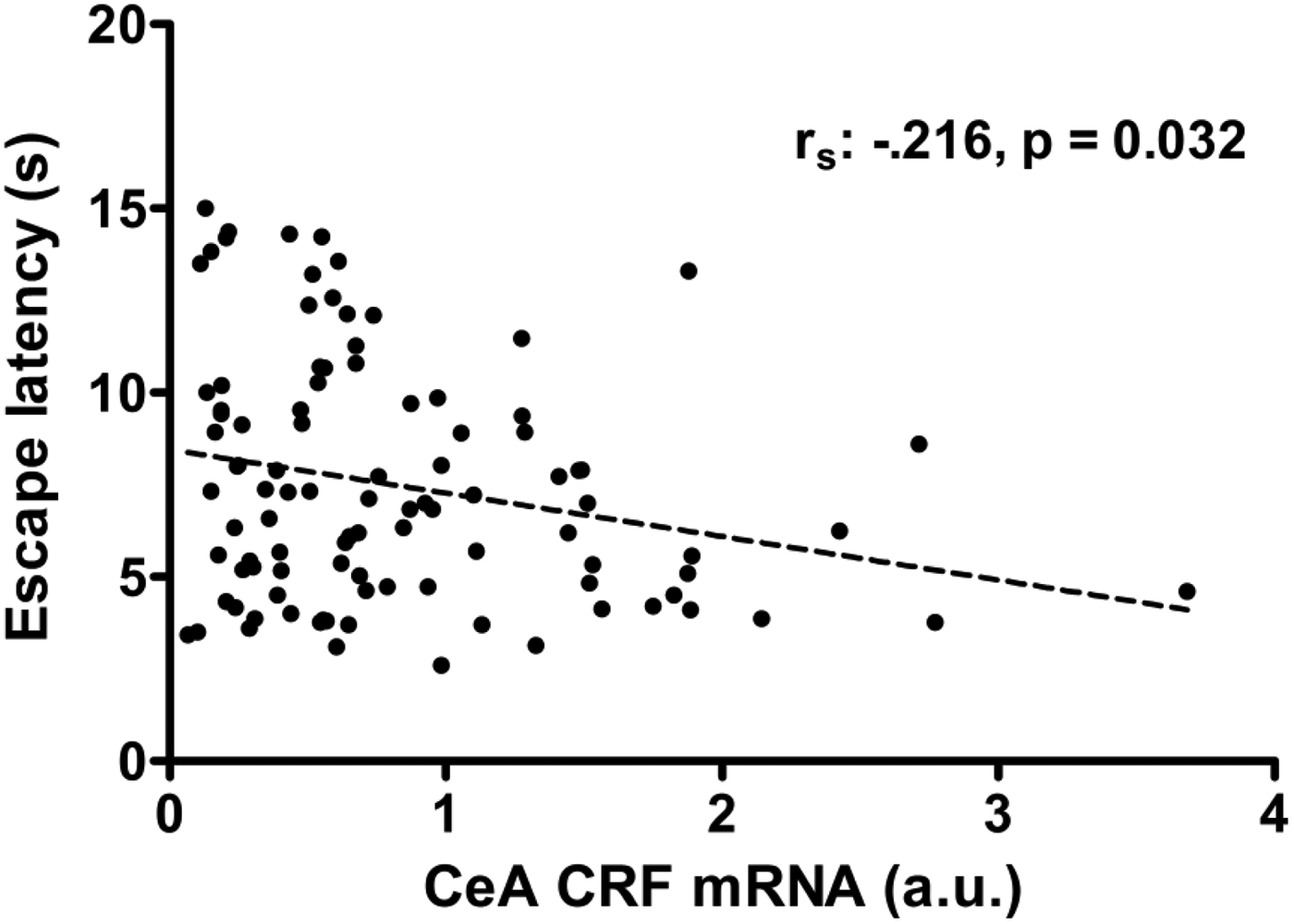
Of Cohort 2, we have previously reported altered stress coping behavior (escape latencies) as a function of ELS and 5-HTT genotype after previous exposure to inescapable shocks (LH paradigm; Van der Doelen et al., Reference Van der Doelen, Kozicz and Homberg2013). Several studies have indicated the functional involvement of CeA-CRF neurons in the behavioral expression of LH (Hammack, Cooper, & Lezak, Reference Hammack, Cooper and Lezak2012; Homberg & Contet, Reference Homberg and Contet2009). Here, a significant ELS × 5-HTT Genotype interaction was found for Crf promoter methylation in the CeA of both Cohort 1 and 2, which was furthermore significantly related to the expression of CRF mRNA in the CeA. Therefore, we hypothesize that CeA CRF mRNA levels could be involved in the coping behavior that was observed in the escape test of the LH paradigm in Cohort 2. In support of this hypothesis, we found that higher CeA CRF mRNA levels were associated with lower escape latencies (r s = −.216, p = .032; Figure 5).

Figure 5. Cohort 2: Negative correlation of escape latencies with central amygdala (CeA) corticotropin-releasing factor (CRF) mRNA levels (r s = −2.16, p = .032). The rats of Cohort 2 were exposed to two sessions of inescapable foot shocks, followed 24 h later by a shock escape test where escape latencies were automatically recorded. Two hours after the onset of the escape test, the rats were sacrificed, the brains were isolated and later CRF mRNA levels in micropunches of the CeA were determined.
Discussion
In this study, we report that ELS and 5-HTT genotype interact in the CeA to program DNA methylation of the Crf promoter region in two independent cohorts. Furthermore, methylation of CpG −36, which immediately precedes an essential transcription factor binding site (TATA box), was found to show a highly significant negative correlation with CRF mRNA levels in the CeA. Yet, we did not find ELS × 5-HTT Genotype alterations in the expression of CRF (mRNA, protein), suggesting the involvement of additional (epigenetic) mechanisms in the regulation of CRF expression. Furthermore, we found correlative evidence suggesting that CeA CRF neurons could be involved in stress coping behavior in our ELS × 5-HTT Genotype model. Specifically, CeA CRF mRNA levels were found to negatively correlate with escape latencies in rats that were subjected to the LH paradigm, which has high face and predictive validity for the translation to clinical depression (Hammack et al., Reference Hammack, Cooper and Lezak2012; Pryce et al., Reference Pryce, Azzinnari, Spinelli, Seifritz, Tegethoff and Meinlschmidt2011).
By applying the LH paradigm to our ELS × 5-HTT Genotype model, we have previously reported that exposure to ELS is associated with increased expression of adaptive, active coping behavior (Van der Doelen et al., Reference Van der Doelen, Deschamps, D'Annibale, Peeters, Wevers and Zelena2013). The LH paradigm consisted of exposure to inescapable shock stress for 2 days, and an escape test on the third day in the same context where rats could now escape the foot shocks. Because inescapable shock exposure induces an escape deficit (passive coping) in vulnerable rats, active versus passive coping behavior is quantified by measuring escape latencies in the escape test. When stratifying for 5-HTT genotype, we found that the adaptive effect of ELS exposure was only significant for 5-HTT +/− rats (Van der Doelen et al., Reference Van der Doelen, Deschamps, D'Annibale, Peeters, Wevers and Zelena2013). These behavioral findings support the recently postulated match/mismatch hypothesis, which states that exposure to ELS is not necessarily pathological but can be used to adaptively respond to future stress exposure (Champagne, De Kloet, & Joëls, Reference Champagne, De Kloet and Joëls2009; Gluckman, Hanson, & Beedle, Reference Gluckman, Hanson and Beedle2007; Nederhof & Schmidt, Reference Nederhof and Schmidt2012). The increased sensitivity of 5-HTT +/− rats to benefit from a positive, adaptive match between the early and adult life environment is also in support of the differential susceptibility hypothesis (Belsky et al., Reference Belsky, Jonassaint, Pluess, Stanton, Brummett and Williams2009; Ellis et al., Reference Ellis, Boyce, Belsky, Bakermans-Kranenburg and van IJzendoorn2011). In humans, 5-HTTLPR short-allele carriers have been shown to exhibit increased vulnerability to develop psychopathology following exposure to adverse environments (Caspi et al., Reference Caspi, Sugden, Moffitt, Taylor, Craig and Harrington2003; Karg et al., Reference Karg, Burmeister, Shedden and Sen2011). In addition, short-allele carriers show increased sensitivity to negative environments compared to long–long homozygotes by displaying stronger physiological responses to stress (Alexander et al., Reference Alexander, Kuepper, Schmitz, Osinsky, Kozyra and Hennig2009; Gotlib, Joorman, Minor, & Hallmayer, Reference Gotlib, Joormann, Minor and Hallmayer2008), increased attentional bias to negatively valenced stimuli (Pergamin-Hight, Bakermns-Kranenburg, van IJZendoorn, & Bar-Haim, Reference Pergamin-Hight, Bakermans-Kranenburg, van IJzendoorn and Bar-Haim2012), and higher levels of neuroticism and negative emotionality (Lesch et al., Reference Lesch, Bengel, Heils, Sabol, Greenberg and Petri1996; Pluess et al., Reference Pluess, Velders, Belsky, van IJzendoorn, Bakermans-Kranenburg and Jaddoe2011; Stein, Schork, & Gelernter, Reference Stein, Schork and Gelernter2008). Whereas the diathesis–stress hypothesis focuses solely on the adverse consequences of genetic predispositions, the differential susceptibility hypothesis provides a theoretical framework to explain the evolutional survival of “risk” genes by emphasizing them as “plasticity” genes with greater sensitivity to adverse as well as positive environmental events (Bakermans-Kranenburg & van IJzendoorn, Reference Bakermans-Kranenburg and van IJzendoorn2011; Belsky et al., Reference Belsky, Bakermans-Kranenburg and van IJzendoorn2007; Ellis et al., Reference Ellis, Boyce, Belsky, Bakermans-Kranenburg and van IJzendoorn2011). The 5-HTLLPR short-allele is very common, with an allele frequency of approximately 40% in Caucasians (Eisenberg & Hayes, Reference Eisenberg and Hayes2011; Lesch et al., Reference Lesch, Bengel, Heils, Sabol, Greenberg and Petri1996; Noskova et al., Reference Noskova, Pivac, Nedic, Kazantseva, Gaysina and Faskhutdinova2008), and short-allele carriers have repeatedly been shown to display increased benefit from positive environments in comparison to long–long homozygotes (Kaufman et al., Reference Kaufman, Yang, Douglas-Palumberi, Houshyar, Lipschitz and Krystal2004; Pluess et al., Reference Pluess, Belsky, Way and Taylor2010; van IJzendoorn et al., Reference van IJzendoorn, Belsky and Bakermans-Kranenburg2012).
In the current study, we found a neural correlate (CeA CRF mRNA levels) of coping behavior and, thus, as pointed out above, differential susceptibility. We found that ELS and 5-HTT genotype interact to affect DNA methylation of the Crf promoter specifically in the CeA. Of the investigated CpG sites, CpG −36 showed a highly significant negative correlation with CeA CRF mRNA levels, which itself showed a correlation with coping behavior (escape latencies) in the LH paradigm.
Passive coping responses across a variety of rodent stress models have been associated with increased activity of 5-HT neurons in the dorsal raphe nucleus (DRN; Hammack et al., Reference Hammack, Cooper and Lezak2012; Valentino, Lucki, & Van Bockstaele, Reference Valentino, Lucki and Van Bockstaele2010; but see also Wood et al., Reference Wood, Zhang, Reyes, Lee, Van Bockstaele and Valentino2013). The DRN is densely innervated by CRF-containing fibers, which are in part derived from the CeA (Lowry, Rodda, Lightman, & Ingram, Reference Lowry, Rodda, Lightman and Ingram2000; Peyron, Petit, Rambon, Jouvet, & Luppi, Reference Peyron, Petit, Rampon, Jouvet and Luppi1998; Retson & Van Bockstaele, Reference Retson and Van Bockstaele2013). Pharmacological manipulations have shown that CRF can both stimulate and inhibit the activity of DRN 5-HT neurons, via CRF receptor 1 (CRF1R) and CRF receptor 2 (CRF2R), respectively (Valentino et al., Reference Valentino, Lucki and Van Bockstaele2010; Waselus, Nazzaro, Valentino, & Van Bockstaele, Reference Waselus, Nazzaro, Valentino and Van Bockstaele2009). CRF has high affinity for CRF1R, but only low affinity for CRF2R, in contrast to the CRF peptide family members urocortin 1, 2, and 3, which have therefore been proposed to be the endogenous ligands for CRF2R (Hsu & Hsueh, Reference Hsu and Hsueh2001; Lewis et al., Reference Lewis, Li, Perrin, Blount, Kunitake and Donaldson2001; Reyes et al., Reference Reyes, Lewis, Perrin, Kunitake, Vaughan and Arias2001; Vaughan et al., Reference Vaughan, Donaldson, Bittencourt, Perrin, Lewis and Sutton1995). Given the correlation we identified here of CeA CRF mRNA levels with increased active coping (decreased escape latencies), we hypothesize that increased CeA-CRF input to the DRN could strengthen CRF1R over CRF2R activation associated with lower DRN 5-HT activity and increased active coping responses. There is likely an optimum to increased CeA-CRF input though, as increasingly higher doses of CRF would start to activate CRF2R (Hammack, Pepin, DesMarteau, Watkins, & Maier, Reference Hammack, Pepin, DesMarteau, Watkins and Maier2003; Hammack et al., Reference Hammack, Schmid, LoPresti, Der-Avakian, Pellymounter and Foster2003). Furthermore, the consequences of increased DRN 5-HT neuron activity depend on the recruited forebrain projections, because CeA-stimulated 5-HT release in the medial prefrontal cortex has actually been associated with reduced passive coping behavior (Forster et al., Reference Forster, Feng, Watt, Korzan, Mouw and Summers2006, Reference Forster, Pringle, Mouw, Vuong, Watt and Burke2008).
It should be pointed out that although DNA methylation (CpG −36) correlated significantly with CRF mRNA levels in the CeA, only the adult naive cohort showed G × E regulation of CpG −36, while the adult stress cohort showed G × E regulation of CpG −226 and CpG −147. Furthermore, CeA CRF mRNA levels were not significantly affected by the interaction of ELS and 5-HTT genotype, and CpG −36 methylation did not show a significant correlation with escape latencies, unlike CeA CRF mRNA levels. Therefore, whereas our findings are suggestive of an ELS × 5-HTT Genotype effect on CeA Crf promoter methylation that moreover could possibly be linked to stress coping behavior, it is clear that our results also warrant replication as well as further experimentation to connect the dots between Crf promoter methylation, expression of CRF, and behavioral changes that are relevant for psychopathology.
In contrast to the CeA, DNA methylation of the Crf promoter region was not consistently found to be affected by the ELS × 5-HTT Genotype interaction in the BNSTov and PVN. This is not entirely unexpected, because previous studies have shown that chronic stress exposure (early/adult life) can differentially affect DNA methylation of the Crf promoter in the BNSTov, PVN, and CeA (Mueller & Bale, Reference Mueller and Bale2008; Sterrenburg et al., Reference Sterrenburg, Gaszner, Boerrigter, Santbergen, Bramini and Elliott2011). A recent study suggests that the use of more prolonged maternal separations could have induced ELS × 5-HTT Genotype alterations of Crf promoter methylation in the PVN (Chen et al., Reference Chen, Evans, Liu, Honda, Saavedra and Aguilera2012). In the BNSTov of the adult naive cohort, we observed higher methylation of CpG −226 in 5-HTT −/− rats compared to 5-HTT +/+ rats (5-HTT genotype effect), which was not present in Cohort 2. This is most likely explained because of a general downregulation of CpG −226 methylation by the repeated exposure to foot shock stress, becaues chronic stress has previously been shown to lead to a decrease in Crf CpG −226 methylation in the BNSTov (Sterrenburg et al., Reference Sterrenburg, Gaszner, Boerrigter, Santbergen, Bramini and Elliott2011). In line with the absence of consistent alterations of Crf promoter methylation in the BNSTov and PVN, we did not find significant effects of ELS × 5-HTT Genotype interaction on CRF expression in these areas, although DNA methylation of the Crf promoter methylation did show a significant negative correlation with CRF mRNA levels in the PVN.
From this study, together with previous studies that have employed a similar maternal separation paradigm, the picture emerges that the genetic background (strain) of rats modulates the effects of ELS exposure on CRF expression in the BNSTov, PVN, and CeA, supporting the importance of considering G × E interactions in psychopathology. For Long–Evans rats, it has been reported that ELS induces increased CRF expression in the BNST, PVN, and CeA (Francis, Diorio, Plotsky, & Meaney, Reference Francis, Diorio, Plotsky and Meaney2002; Huot, Gonzalez, Ladd, Thrivikraman, & Plotsky, Reference Huot, Gonzalez, Ladd, Thrivikraman and Plotsky2004; Plotsky et al., Reference Plotsky, Thrivikraman, Nemeroff, Caldji, Sharma and Meaney2005; but also see Ladd et al., Reference Ladd, Thrivikraman, Huot and Plotsky2005). In Sprague–Dawley and Wistar rats, however, CRF expression in the BNST/PVN/CeA is unaltered or even lower following exposure to ELS (this study, as well as Bravo et al., Reference Bravo, Dinan and Cryan2011; Chen et al., Reference Chen, Evans, Liu, Honda, Saavedra and Aguilera2012; Desbonnet et al., Reference Desbonnet, Garrett, Daly, McDermott and Dinan2008; but see also Aisa et al., Reference Aisa, Tordera, Lasheras, Del Río and Ramírez2007). Together, these studies suggest that the effects of ELS (maternal separation) on the basal expression levels of CRF in the adult brain are moderated by the genomic differences between rat strains. However, it should be noted that this concerns only a limited number of studies, of which none have directly compared rat strains for their sensitivity to ELS-induced alterations in CRF expression.
Technical note
It should be noted that in the current study, as well as in previous studies (Chen et al., Reference Chen, Evans, Liu, Honda, Saavedra and Aguilera2012; Elliott, Ezra-Nevo, Regev, Neufeld-Cohen, & Chen, Reference Elliott, Ezra-Nevo, Regev, Neufeld-Cohen and Chen2010; Mueller & Bale, Reference Mueller and Bale2008; Sterrenburg et al., Reference Sterrenburg, Gaszner, Boerrigter, Santbergen, Bramini and Elliott2011), DNA methylation of the Crf promoter has been examined in micropunches of brain areas. Becauses these samples contain a large fraction of non-CRF expressing cells, the resulting DNA methylation patterns are “condensed” and the effects of ELS, 5-HTT genotype, and their interaction are likely underestimated.
Conclusions
In conclusion, we report here that ELS and 5-HTT genotype interact to affect DNA methylation of the Crf promoter region in the CeA. Furthermore, methylation of CpG −36 was found to significantly correlate with CRF mRNA levels in the CeA, suggesting that there is a functional relationship between DNA methylation and Crf transcription. In addition, we found that CeA CRF mRNA levels correlated with behavioral resilience to inescapable stress. Therefore, our findings are suggestive of an ELS × 5-HTT Genotype effect on CeA Crf promoter methylation, which is possibly linked to stress coping behavior. As discussed above, our results warrant replication, as well as further experimentation to connect the dots between Crf promoter methylation, expression of CRF, and behavioral changes that are relevant for psychopathology.
Supplementary Materials
The supplementary materials for this article can be found online at http://journals.cambridg.org/dpp.