Exposure to childhood adversity is common, with more than half of children in the United States experiencing at least one form of adversity by the time they reach adulthood (McLaughlin et al., Reference McLaughlin, Greif Green, Gruber, Sampson, Zaslavsky and Kessler2012). Childhood adversity reflects negative environmental events that are relatively severe or chronic over time, and that are likely to require significant adaptation by a child (McLaughlin, Reference McLaughlin2016). These experiences are strongly associated with risk for most forms of psychopathology in childhood, adolescence, and adulthood (Cicchetti & Toth, Reference Cicchetti and Toth1995; Green et al., Reference Green, McLaughlin, Berglund, Gruber, Sampson, Zaslavsky and Kessler2010; McLaughlin et al., Reference McLaughlin, Green, Gruber, Sampson, Zaslavsky and Kessler2010; Vachon, Krueger, Rogosch, & Cicchetti, Reference Vachon, Krueger, Rogosch and Cicchetti2015). The strong links between childhood adversity and psychopathology have generated considerable interest in identifying mechanisms underlying these associations. One factor that has been argued to underlie many of the downstream consequences of childhood adversity, not only on psychopathology but also on risk behaviors and academic difficulties, is poor executive functioning (EF; Noble, Norman, & Farah, Reference Noble, Norman and Farah2005; Noble, Wolmetz, Ochs, Farah, & McCandliss, Reference Noble, Wolmetz, Ochs, Farah and McCandliss2006; Shonkoff, Reference Shonkoff2012) and atypical structure and function in the frontoparietal brain network that supports EF task performance (Hanson et al., Reference Hanson, Hair, Shen, Shi, Gilmore, Wolfe and Pollak2013; Noble et al., Reference Noble, Houston, Brito, Bartsch, Kan, Kuperman and Sowell2015; Sheridan, How, Araujo, Schamberg, & Nelson, Reference Sheridan, How, Araujo, Schamberg and Nelson2013; Sheridan, Sarsour, Jutte, D'Esposito, & Boyce, Reference Sheridan, Sarsour, Jutte, D'Esposito and Boyce2012). It has been posited that adversity is broadly predictive of deficits in EF and the associated neural circuitry; however, emerging evidence indicates that atypical EF development may occur only following certain forms of environmental adversity and not others (Busso, McLaughlin, & Sheridan, Reference Busso, McLaughlin and Sheridan2016; Lambert, King, Monahan, & McLaughlin, Reference Lambert, King, Monahan and McLaughlin2016; Sheridan, Sarsour, et al., Reference Sheridan, Sarsour, Jutte, D'Esposito and Boyce2012). In the current paper, we present a conceptual model of childhood adversity arguing that poor EF and disruptions in related neural circuitry emerge specifically in the context of environments characterized by a lack of social and cognitive stimulation, which we refer to as deprivation. We examine evidence for this model in two studies of children with high levels of exposure to adversity, which measured EF at multiple levels, including performance on an EF task, neural function in a network of frontoparietal regions that support EF task performance, and problems with EF in daily life.
The prevailing approach for examining the developmental consequences of childhood adversity is a cumulative risk model (Evans, Li, & Whipple, Reference Evans, Li and Whipple2013; Felitti et al., Reference Felitti, Anda, Nordenberg, Williamson, Spitz, Edwards and Marks1998). Cumulative risk models count the number of adversities experienced without regard to the type, chronicity, or severity of the experience and use this risk score as a predictor of outcomes. This approach is at least in part the result of sociopolitical forces shaping our cultural understanding of the co-occurrence of adversity exposures (Barnett, Manly, & Cicchetti, Reference Barnett, Manly, Cicchetti, Cicchetti and Toth1993). This model has been transformative with regard to highlighting the strong links between adversity exposure and developmental outcomes and the importance of preventive intervention with children exposed to multiple adversities. However, cumulative risk models give little guidance with regard to the mechanisms through which adversity increases risk for psychopathology and thus the form of intervention that is likely to be most successful and largely ignore research distinguishing differential impacts and developmental pathways through which adversity comes to impact developmental outcomes (Barnett et al., Reference Barnett, Manly, Cicchetti, Cicchetti and Toth1993). Elsewhere we have articulated an alternative to the cumulative risk model that proposes a set of mechanisms explaining how diverse adverse experiences influence psychopathology (McLaughlin & Sheridan, Reference McLaughlin and Sheridan2016; McLaughlin, Sheridan, Alves, & Lambert, Reference McLaughlin, Sheridan, Alves and Mendes2014; Sheridan & McLaughlin, Reference Sheridan and McLaughlin2014, Reference Sheridan and McLaughlin2016). This alternate approach is based on two principles. Across the range of adverse childhood experiences (e.g., maltreatment, community violence, and lack of educational resources), it is possible to extract core underlying dimensions of adversity that encompass numerous types of experiences that share common features. Two initial dimensions proposed in our model are threat, which encompasses experiences involving harm or threat of harm, and deprivation, which involves an absence of expected inputs from the environment, such as cognitive and social stimulation. Conceptually, these dimensions cut across numerous experiences that share the underlying feature of exposure to threat or deprivation to varying degrees. For example, threat is a core feature of sexual abuse, physical abuse, and community violence exposure, whereas deprivation is a core feature of neglect and institutionalization (low parental education, while not necessarily reflecting deprivation, can serve as an important statistical proxy). Other groups have also argued for the importance of considering subtypes and underlying dimensions of maltreatment and childhood adversity (Humphreys & Zeanah, Reference Humphreys and Zeanah2015; Manly, Cicchetti, & Barnett, Reference Manly, Cicchetti and Barnett1994; Manly, Kim, Rogosch, & Cicchetti, Reference Manly, Kim, Rogosch and Cicchetti2001). Thus, the first principle of our model is conceptually similar to ideas that have long been articulated in the childhood adversity field but are often ignored in current approaches relying on cumulative risk.
The second principle underlying this model is that unique emotional, cognitive, and neurobiological pathways underlie the association of these dimensions of experience with developmental outcomes. In the case of threat, we expect that threatening experiences during childhood alter emotional development in ways that facilitate the rapid identification of potential threats in the environment. Specifically, the presence of early learning experiences involving high degrees of threat will bias the development of cortical and subcortical circuits involved in fear learning and salience processing toward early detection of other environmental threats, leading to changes in emotion perception, attention and memory for emotional stimuli, emotional learning, emotional reactivity, and emotion regulation in response to negative emotional stimuli (McLaughlin & Lambert, Reference McLaughlin and Lambert2017). Existing evidence is consistent with this hypothesis. Children who have experienced physical or sexual abuse exhibit attention biases toward threatening stimuli and are more likely to perceive neutral facial expressions as threatening (Pollak, Cicchetti, Hornung, & Reed, Reference Pollak, Cicchetti, Hornung and Reed2000; Pollak & Sinha, Reference Pollak and Sinha2002; Pollak & Tolley-Schell, Reference Pollak and Tolley-Schell2003), exhibit difficulty discriminating between threat and safety cues in learning paradigms (McLaughlin et al., Reference McLaughlin, Sheridan, Gold, Duys, Lambert, Peverill and Pine2016) and exhibit increased amygdala activation to negative emotional cues (McCrory et al., Reference McCrory, De Brito, Sebastian, Mechelli, Bird, Kelly and Viding2011, Reference McCrory, De Brito, Kelly, Bird, Sebastian, Mechelli and Viding2013; McLaughlin, Peverill, Gold, Alves, & Sheridan, Reference McLaughlin, Peverill, Gold, Alves and Sheridan2015). Difficulties with both explicit and implicit forms of emotion regulation are well documented among children with abuse histories (Kim & Cicchetti, Reference Kim and Cicchetti2010; Kim-Spoon, Cicchetti, & Rogosch, Reference Kim-Spoon, Cicchetti and Rogosch2013) as well as atypical function in neural circuitry supporting these emotion regulation processes (Herringa et al., Reference Herringa, Birn, Ruttle, Burghy, Stodola, Davidson and Essex2013; Marusak, Martin, Etkin, & Thomason, Reference Marusak, Martin, Etkin and Thomason2015; McLaughlin et al., Reference McLaughlin, Peverill, Gold, Alves and Sheridan2015). These disruptions in emotional processing have been linked to multiple forms of psychopathology (see McLaughlin & Lambert, Reference McLaughlin and Lambert2017, for a review).
Our model posits that deprivation influences development through mechanisms that are at least partially distinct from experiences of threat. Deprivation refers to an absence of cognitive and social stimulation and constrained opportunities for learning among children whose interactions with supportive caregivers are limited, as in the case of low parental education, neglect, and institutional rearing. In the case of deprivation, animal models document that a lack of environmental stimulation leads to dramatic increases in synaptic pruning. When rodents are raised in low complexity environments characterized by an absence of stimulation, global decreases in cortical volume and thickness are observed (Bennett, Rosenzweig, Diamond, Morimoto, & Hebert, Reference Bennett, Rosenzweig, Diamond, Morimoto and Hebert1974; Diamond et al., Reference Diamond, Law, Rhodes, Lindner, Rosenzweig, Krech and Bennett1966; Diamond, Rosenzweig, Bennett, Lindner, & Lyon, Reference Diamond, Rosenzweig, Bennett, Lindner and Lyon1972). These global changes reflect reductions in the number of synapses per neuron (Turner & Greenough, Reference Turner and Greenough1985), and in the density, branching, and length of dendritic spines (Globus, Rosenzweig, Bennett, & Diamond, Reference Globus, Rosenzweig, Bennett and Diamond1973; Greenough & Volkmar, Reference Greenough and Volkmar1973; Volkmar & Greenough, Reference Volkmar and Greenough1972). These neural changes are accompanied by deficits in numerous forms of learning and memory (Renner & Rosenzweig, Reference Renner and Rosenzweig1987; Rosenzweig & Bennett, Reference Rosenzweig and Bennett1996). Similarly, when cognitive enrichment and social stimulation is low during early human development, for example, among children raised in institutions with limited caregiver contact, cortical volume and thickness are reduced throughout the cortex (Sheridan, Fox, Zeanah, McLaughlin, & Nelson, Reference Sheridan, Fox, Zeanah, McLaughlin and Nelson2012; McLaughlin et al., 2013). In cases where cognitive deprivation is milder, as in low parental education, we would expect the effects to be similar to those observed following the profound deprivation of institutionalization, but more attenuated and circumscribed. Recent evidence is consistent with this prediction, documenting reductions in cortical thickness and surface area that are widespread across the cortex in children raised in low socioeconomic status (SES) environments (Mackey et al., Reference Mackey, Finn, Leonard, Jacoby-Senghor, West, Gabrieli and Gabrieli2015; Noble et al., Reference Noble, Houston, Brito, Bartsch, Kan, Kuperman and Sowell2015).
In parallel to these neurodevelopmental disruptions, deprivation in cognitive stimulation will also influence multiple domains of cognitive development, including EF. The general link between parental SES and cognitive development in children has often been attributed to differential exposure to cognitively stimulating experiences and opportunities for learning. Children born to better educated parents are likely to have more formal and informal educational opportunities beginning at an early age, live in houses with more books where parents speak more often and in more complex ways to their children, and are more likely to experience an enriched educational environment when they enter school relative to children of parents with less education (Duncan & Brooks-Gunn, Reference Duncan and Brooks-Gunn1999; Evans, Reference Evans2004). Lack of early learning opportunities is thought to directly drive neurocognitive development; for example, low linguistic complexity in parental speech predicts poor child language development (Hoff, Reference Hoff2003). Several randomized control trials have provided experimental support for the this pathway by demonstrating that enhanced access to learning opportunities through early educational programs, increased access to learning materials such as books, and increased parent–child interactions have positive long-term effects for cognitive development among children growing up in low-SES families (Campbell et al., Reference Campbell, Conti, Heckman, Moon, Pinto, Pungello and Pan2014; Muennig, Schweinhart, Montie, & Neidell, Reference Muennig, Schweinhart, Montie and Neidell2009; Reynolds, Reference Reynolds1994; Reynolds, Temple, Robertson, & Mann, Reference Reynolds, Temple, Robertson and Mann2001). The neural pathways outlined above are also likely to play a key role in shaping cognitive development in children exposed to deprived early environments. Reductions in cortical volume and thickness are likely to yield deficits in higher order cognitive functions such as EF, which requires coordinated function of multiple areas of association cortex, most notably late-developing areas of the brain such as the prefrontal and superior parietal cortices (D'Esposito et al., Reference D'Esposito, Detre, Alsop, Shin, Atlas and Grossman1995; Finn et al., Reference Finn, Minas, Leonard, Mackey, Salvatore, Goetz and Gabrieli2016; Finn, Sheridan, Kam, Hinshaw, & D'Esposito, Reference Finn, Sheridan, Kam, Hinshaw and D'Esposito2010; Kharitonova, Winter, & Sheridan, Reference Kharitonova, Winter and Sheridan2015; Nomura et al., Reference Nomura, Gratton, Visser, Kayser, Perez and D'Esposito2010; Peverill, McLaughlin, Finn, & Sheridan, Reference Peverill, McLaughlin, Finn and Sheridan2016). Together, atypical neural and cognitive development among children exposed to early deprivation are associated with later risk for externalizing psychopathology (Machlin et al., Reference Machlin, McLaughlin and Sheridan2017; McLaughlin et al., 2013; Tibu et al., Reference Tibu, Sheridan, McLaughlin, Nelson, Fox and Zeanah2015).
One of the primary arguments for the cumulative risk model is that children who encounter adversity often experience multiple adversities that are challenging to disentangle. Population-representative data suggest that adversities are co-occurring, with children experiencing one adversity often exposed to several others (McLaughlin et al., Reference McLaughlin, Greif Green, Gruber, Sampson, Zaslavsky and Kessler2012). The co-occurrence of adversities means that to isolate the unique associations of particular dimensions of adversity with developmental outcomes, it is critical to measure both dimensions and adjust for them simultaneously. This approach thus isolates the aspects of, for example, low SES that are associated with deprivation from co-occurring experiences of community violence or exposure to violence in the home. Although this approach is frequently used to examine the associations of threat exposures, such as abuse, with neurocognitive function over and above the influence of deprivation, such as low SES or neglect (Manly et al., Reference Manly, Cicchetti and Barnett1994, Reference Manly, Kim, Rogosch and Cicchetti2001; McLaughlin et al., Reference McLaughlin, Peverill, Gold, Alves and Sheridan2015; Pollak et al., Reference Pollak, Cicchetti, Hornung and Reed2000), the contrasting controls are not often applied: the association of low parental SES with neural structure/function, cognitive task performance, and psychopathology are typically examined without assessments of or control for exposure to abuse or other forms of interpersonal violence (e.g., Hanson et al., Reference Hanson, Hair, Shen, Shi, Gilmore, Wolfe and Pollak2013; Hanson, Chandra, Wolfe, & Pollak, Reference Hanson, Chandra, Wolfe and Pollak2011; Noble et al., Reference Noble, Norman and Farah2005, Reference Noble, Houston, Brito, Bartsch, Kan, Kuperman and Sowell2015; Noble, McCandliss, & Farah, Reference Noble, McCandliss and Farah2007; Sheridan, Sarsour, et al., Reference Sheridan, Fox, Zeanah, McLaughlin and Nelson2012). This limits the ability of most current research on adverse childhood experiences to identify differences in the impact of deprivation and threat on neurocognitive function.
Yet, it is clear that these dimensions are separable. Distinct associations of abuse and neglect on emotion perception and other developmental domains are well documented (Manly et al., Reference Manly, Cicchetti and Barnett1994, Reference Manly, Kim, Rogosch and Cicchetti2001; Pollak et al., Reference Pollak, Cicchetti, Hornung and Reed2000). For example, abused children are more likely to classify facial emotion as anger, whereas neglected children experience global difficulties discriminating between distinct emotional expressions with no specific response bias for anger (Pollak et al., Reference Pollak, Cicchetti, Hornung and Reed2000). In addition, we have previously demonstrated that childhood threat experiences in the form of exposure to interpersonal violence are associated with increased emotional reactivity and recruitment of the amygdala when viewing negative scenes, difficulty discriminating between threat and safety cues, physiological responses to stress consistent with a threat response, and cortical thinning in emotional control structures such as the ventromedial prefrontal cortex over and above the effects of parental education or poverty, neither of which were associated with any of these outcomes after adjusting for abuse (Busso et al., Reference Busso, McLaughlin and Sheridan2016; Gold et al., Reference Gold, Sheridan, Peverill, Busso, Lambert, Alves and McLaughlin2016; Lambert et al., Reference Lambert, King, Monahan and McLaughlin2016; McLaughlin et al., Reference McLaughlin, Peverill, Gold, Alves and Sheridan2015, Reference McLaughlin, Sheridan, Gold, Duys, Lambert, Peverill and Pine2016). In addition, in one study, low parental SES selectively predicted performance on a cognitive control task after controlling for exposure to interpersonal violence (Lambert et al., Reference Lambert, King, Monahan and McLaughlin2016), whereas violence exposure was unrelated to cognitive control. Together, these studies provide initial evidence that the impact of deprivation may be separable from the impact of threat even in relatively small samples and lend preliminary support for the overall conceptual model.
Here we provide an empirical test of our conceptual model across multiple levels of analysis. In Study 1 we examine the association of two exposures on the hypothesized deprivation dimension (neglect and low parental education) and two exposures on the hypothesized threat dimension (community violence exposure and abuse) with parent reports of EF difficulties in daily life. We predicted that severity of exposure to deprivation would predict EF after controlling for severity of exposure to threat. We further predicted that threat exposure, regardless of severity, would not predict EF after controlling for exposure to deprivation.
In Study 2 we present the strongest test of the theory by directly contrasting the impact of a relatively mild form of deprivation, low parental education, and a relatively severe form of threat, abuse, as predictors of neural activation and behavioral performance in an EF task. Specifically, we measured WM and inhibition using a spatial WM/filtering task that allowed us to examine neural recruitment associated with WM (i.e., recruitment during encoding and maintenance of to-be-remembered stimuli in conditions of high vs. low load) and inhibition (i.e., recruitment during encoding and maintenance when distractors are present vs. absent during encoding), two of the canonical functions that comprise EF, in the same task. We expected that lower parental education would be associated with poorer performance on WM and inhibitory control measures of task performance, controlling for exposure to abuse. In addition, consistent with previous findings and our theoretical model, we expected that low parental education would be associated with inefficient recruitment (increased recruitment in the context of similar task performance) in the network of frontoparietal regions that support WM/filtering and that these associations would persist after adjustment for exposure to abuse. Finally, we did not expect abuse exposure to be associated with any of these behavioral and neural outcomes after controlling for parental education.
Method Study 1
Sample
Participants were 168 adolescents ages 13–17 years (M = 14.91, SD = 1.36, 56% male). Participants were recruited into this study from schools, after-school programs, medical clinics, and the general community in Boston and Cambridge, Massachusetts, between July 2010 and November 2012. Recruitment was aimed at a sample with variability in exposure to adversity. As such, recruitment specifically targeted communities with high levels of community violence and clinics that served a low-SES catchment area. Data collection occurred as a part of a larger study that involved measurement of physiological responding during a laboratory stressor and assessment of psychopathology through participant report of symptoms. Data on the physiological measures from this larger study have been published elsewhere (McLaughlin, Sheridan, Alves, et al., Reference McLaughlin, Sheridan, Alves and Mendes2014). Exclusion criteria included psychiatric medication use with the exception of stimulant medications for attention-deficit/hyperactivity disorder, active substance use disorder, major developmental or genetic disorders, and being non-English speaking. Parental education was missing for 8 participants, parent report of EF was missing for 9 participants, and child report of community violence was missing for 1 participant. Thus, for analyses including all variables the final N = 156. All procedures were approved by the institutional review board at Boston Children's Hospital.
This sample was racially and ethnically diverse: 40.8% of the sample identified as White (n = 69), 18.34% as Black (n = 31), 17.8% as Hispanic/Latino (n = 30), 7.7% as Asian (n = 13), and 14.8% as biracial or other (n = 25). Approximately one-third of the sample (40.1%, n = 63) was from single-parent households; 26.8% (n = 42) were living below the poverty line. See Table 1 for means and standard deviations of relevant study variables.
Table 1. Means and standard deviations of threat and deprivation variables
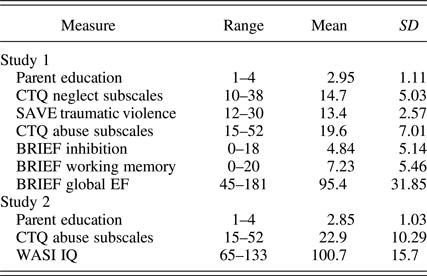
Note: CTQ, Child Trauma Questionnaire; SAVE, Scale for Adolescent Violence Exposure; BRIEF, Behavioral Rating Inventory of Executive Function; WASI IQ, Wechsler Abbreviated Scale of Intelligence.
Abuse and neglect
Child maltreatment was assessed using the Childhood Experiences of Care and Abuse (CECA) interview and the Child Trauma Questionnaire (CTQ). The CECA assesses multiple aspects of caregiving experiences, including physical and sexual abuse. Interrater reliability for maltreatment reports is excellent, and multiple validation studies suggest high agreement between siblings on reports of maltreatment (Bifulco, Brown, & Harris, Reference Bifulco, Brown and Harris1994; Bifulco, Brown, Lillie, & Jarvis, Reference Bifulco, Brown, Lillie and Jarvis1997). The CTQ is a self-report measure that assesses physical, sexual, and emotional abuse and physical and emotional neglect in childhood (Bernstein, Ahluvalia, Pogge, & Handelsman, Reference Bernstein, Ahluvalia, Pogge and Handelsman1997; Scher, Stein, Asmundson, McCreary, & Forde, Reference Scher, Stein, Asmundson, McCreary and Forde2001). The CTQ has excellent psychometric properties including internal consistency, test–retest reliability, and convergent and discriminant validity with interviews and clinician reports of maltreatment (Bernstein et al., Reference Bernstein, Fink, Handelsman, Foote, Lovejoy, Wenzel and Ruggiero1994). We used a composite of the abuse subscales, which include physical, sexual, and emotional abuse, as one measure of exposure to significant threat, and a composite of the neglect subscales, which include physical and emotional neglect, as one measure of lack of exposure to species-expectant caregiver inputs (i.e., deprivation). The abuse subscale includes physical abuse items, such as “I got hit so hard by someone in my family that I had to see a doctor or go to the hospital,” sexual abuse items, such as “Someone tried to touch me in a sexual way, or tried to make me touch them,” and emotional abuse items, such as “People in my family called me things like stupid, lazy, or ugly.” This scale had excellent internal consistency in our sample (α = 0.88). The neglect subscale includes physical neglect items, such as “There was always someone to take me to the doctor if I needed it,” and emotional neglect items, such as “My family was a source of strength and support.” The internal consistency of the neglect subscale was also good (α = 0.81).
The sample included high levels of exposure to maltreatment. For example, a total of 38.2% of the sample experienced abuse, based on either reporting physical or sexual abuse during the interview or having a score on any of the three CTQ abuse subscales above a previously identified threshold (Walker et al., Reference Walker, Unutzer, Rutter, Gelfand, Saunders, VonKorff and Katon1999). No participant was currently experiencing maltreatment, and the proper authorities were contacted in cases where we had safety concerns. The CECA was used to determine the presence/absence of abuse, but does not provide severity ratings. As such, we use the CTQ as our primary measure of abuse and neglect severity in all analyses.
Parental education
Parental education was measured by asking the parent or caregiver who attended the study visit with the adolescent participant about the highest degree they had earned and the highest degree their partner earned (if they had one). Parental education for the parent with the highest educational attainment was coded for analysis on the following scale: high school or less (1; n = 24, 15%), some college attendance without a degree or a degree from a 2-year professional school (2; n = 31, 19.4%), college degree (3; n = 34, 21.3%), graduate degree (4; n = 71, 42%). The range of parental education was thus 1 to 4 (M = 2.95, SD = 1.11).
Community violence
Community violence exposure was measured using the Screen for Adolescent Violence Exposure. This measure is composed of three subscales: indirect violence exposure (hearing about violence in the community), traumatic violence exposure (direct experiences of community violence), and physical/verbal abuse. We used the traumatic violence subscale to index participant's direct exposure to community violence. This subscale includes items such as “I have been jumped.” The range of scores on this subscale was 12 to 30 (M = 13.4, SD = 2.6). The Screen for Adolescent Violence Exposure has excellent psychometric properties including good reliability and validity as well as correlations with objective neighborhood-level crime data (Hastings & Kelley, Reference Hastings and Kelley1997). This scale had moderately good internal consistency in our sample (α = 0.77).
Parent ratings of EF
Parent ratings of EF were measured using the Behavior Rating Inventory of Executive Function Parent Form (Gioia, Isquith, Guy, & Kenworthy, Reference Gioia, Isquith, Guy and Kenworthy2000). This questionnaire asks parents to rate their child on everyday behavioral examples of EF. Here, we focus on the subscales for WM, inhibition, and a global composite of EFs. Daily WM is measured with questions such as “My child has trouble remembering things, even for a few minutes.” Daily inhibitory control is measured with questions such as “My child interrupts others.” The global composite comprises these two core domains of EF along with additional subsidiary domains of EF (e.g., monitoring and organization).
Data analysis
We included several covariates in our analysis for demographic variables that were associated with our predictor variables. Age was positively associated with the neglect and abuse subscales of the CTQ, but not community violence or parental education (ps > .13). Sex was associated with community violence but not parental education, abuse, or neglect (ps > .13). Males were more likely to report direct exposure to community violence than females. Given the strength and direction of these associations, age, gender, and race/ethnicity were controlled for in all subsequent analyses.
Associations between our four indicators of childhood adversity (abuse, community violence, low parental education, and neglect) are reported using Pearson's correlation (Table 2). Next, the independent associations of these four predictor variables with parent reports of child inhibition, WM, and global EF are examined using ordinary least squares regression controlling for age and gender. Finally, we examine the unique associations of our deprivation indicators (low parental education and neglect) with parent reports of EF, by controlling for abuse exposure and community violence in addition to demographic covariates.
Table 2. Bivariate correlations among all variables considered in analysis
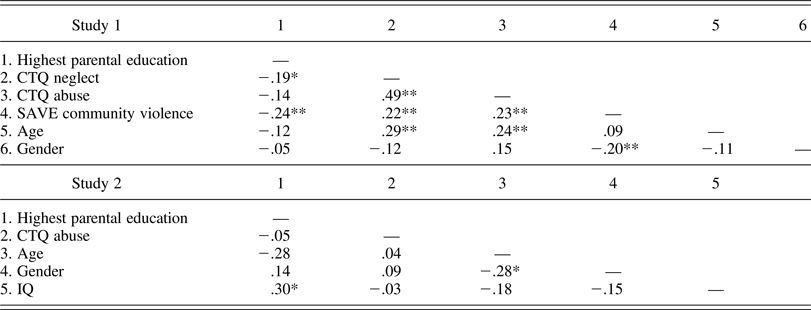
Note: CTQ, Child Trauma Questionnaire; SAVE, Scale for Adolescent Violence Exposure.
*p < .05. **p < .01.
Results
Associations between deprivation and threat
Table 1 provides descriptive statistics for all study variables, and Table 2 shows bivariate correlations. As has been reported in other samples (Jackson et al., Reference Jackson, Thompson, Christiansen, Colman, Wyatt, Buckendahl and Peterson1999), parental education was significantly but only moderately correlated with neglect and traumatic community violence and was not associated with abuse exposure. Neglect was significantly associated with abuse and community violence. Finally, community violence and abuse were significantly correlated. In sum, increased parental education was associated with reductions in exposure to neglect and community violence but not abuse. Abuse was positively associated with degree of exposure to community violence and neglect.
Parent report of EF
After controlling for demographic covariates, parental education was significantly associated with parent report of child inhibitory control (β = –0.22, p = .007) but not WM or global EF (ps > .15). As parental education increased, child problems with inhibition decreased. Neglect severity was also significantly associated with parent report of inhibitory control (β = 0.27, p = .001) and global EF (β = 0.24, p = .003), but was not associated with WM (p = .14). As neglect severity increased, child problems with global EF and inhibition also increased. Abuse was associated with parent report of inhibitory control (β = 0.17, p = .04) but not WM or global EF (ps > .25). Community violence exposure was not associated with parent report of inhibitory control, WM or global EF (all ps > .4).
Next, we adjust for abuse and community violence while examining the impact of parental education or neglect on EF and vice versa (see Table 3). Significance and direction of associations between both parental education and neglect and child inhibition were unchanged after adding controls for both abuse and community violence exposure to the model. Similarly, the direction and significance of the association between neglect and child global EF was robust to controls for both abuse and community violence exposure. In contrast, controlling for parental education and neglect made the association between abuse and parent report of inhibitory control nonsignificant.
Table 3. Associations of threat and deprivation with parent-reported executive functioning a
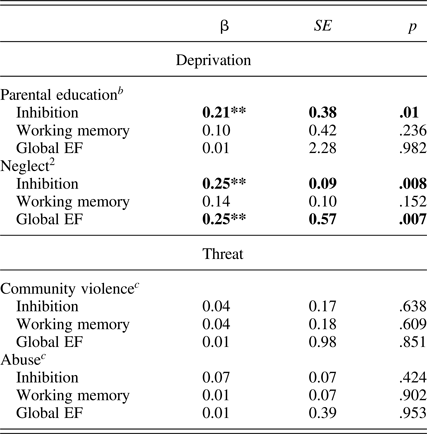
Note: EF, executive functioning.
a Linear regressions controlling for age, gender.
b These models additionally include controls for community violence and abuse.
c These models additionally include controls for parental education and neglect.
*p < .05. **p < .01.
Conclusions
We examined neglect and low parental education, examples of exposure to deprivation, a lack of exposure to scaffolded socioemotional and cognitive learning opportunities (McLaughlin, Sheridan, & Lambert, Reference McLaughlin, Sheridan and Lambert2014; Sheridan & McLaughlin, Reference Sheridan and McLaughlin2014), as well as abuse and community violence, which constitute exposure to threat, as predictors of EF in adolescents. We predicted that these two dimensions of adversity exposure would differentially predict EF. Our findings are consistent with the predictions of our conceptual model regarding the impact of these dimensions of adversity on EF. We observed that parent report of child EF was predicted by neglect and parental education but not abuse or community violence, despite the fact that a significant proportion of our sample reported fairly severe exposure to abuse and community violence. Associations between parental education and neglect and EF were robust to controls for abuse, community violence, age, and sex. These findings provide clear support of our predictions, but several key limitations must be considered.
First, in Study 1 we examined parent reports of children's EF in daily life. For exposures such as neglect, it is possible that parents are unreliable reporters of their child's cognitive ability, because they are less involved in or attentive to their child's daily experiences. Second, unlike our measures of threat exposure that directly assess exposure to interpersonal violence in the home and community, our measures of deprivation reflect markers that increase risk for, but do not directly assess, a lack of cognitive stimulation and learning opportunities in the home and school environment. In particular, the CTQ neglect scale measures disparate aspects of neglect (both low warmth and a lack of meeting children's physical needs), but does not specifically query cognitive stimulation and learning experiences. Given these limitations, the use of the neglect subscale on the CTQ as an index of the deprivation dimension constitutes only a preliminary assessment of the overall hypotheses.
To address these limitations, in Study 2 we employ an objective measure of child EF and do not rely on parent report. We measure EF using child behavior on a WM/filtering task and neural recruitment in the service of WM and selective encoding of task relevant stimuli, but not distractors (i.e., inhibition). We examine associations between a mild form of deprivation, low parental education, and these EF outcomes. While parental education itself does not measure learning opportunities for the child, parental education is strongly correlated with these experiences, predicting exposure to complex linguistic input, number of words spoken every day, learning materials in the home, and formal and informal educational opportunities (Duncan & Brooks-Gunn, Reference Duncan and Brooks-Gunn1999; Evans, Reference Evans2004; Hackman, Farah, & Meaney, Reference Hackman, Farah and Meaney2010; Hoff, Reference Hoff2003). Finally, in a strong test of our model, we examine associations between parental education and neurocognitive measures of WM and inhibition while controlling for a particularly severe form of threat: abuse exposure.
Method Study 2
Sample
Participants were 51 adolescents ages 13.75–20.23 (M = 17.04, SD = 1.5, 61% female). Forty of these participants were recruited from the previous study and were specifically chosen based on their level of violence exposure. Eleven additional subjects were recruited into the study using similar methods to those described for Study 1, because we were unable to recruit enough participants from Study 1 to complete MRI scanning to ensure adequate statistical power. These two groups of participants did not differ in age, race, abuse exposure, or parental education (all ps > .27), although the group of newly recruited participants were more likely to be female compared to the participants recruited from the larger behavioral study, t (49) = 2.45, p = .03. The sample was racially and ethnically diverse: 23.5% of the sample identified as Latino, 27.5% as White, 25.5% as Black, 9.8% as Asian, and 11.8% as “other.” See Table 1 for means and standard deviations of relevant study variables.
Exclusion criteria included psychiatric medication use with the exception of stimulant medications for attention-deficit/hyperactivity disorder (discontinued 24 hr before the scan), metal orthodontics unsuitable for MRI, claustrophobia incompatible with entering the MRI machine, active substance use disorder, major developmental or genetic disorders, and being non-English speaking. The results from other aspects of this study have been published elsewhere (McLaughlin et al., Reference McLaughlin, Peverill, Gold, Alves and Sheridan2015; Peverill et al., Reference Peverill, McLaughlin, Finn and Sheridan2016). Parents were not willing to provide information about their educational attainment for five participants. The final sample for all analyses including parental education was N = 46. All procedures were approved by the institutional review board at Boston Children's Hospital.
Deprivation and threat
Parental education and abuse exposure were assessed using identical methods to those described for Study 1. The range of parental education (from the parent or caregiver with the highest educational attainment) was 1 (high school or less) to 4 (graduate degree; M = 2.8, SD = 1.03). The highest degree earned for 13% of parents in the sample was a high school degree, 21.7% had some college, 32.6% had a college degree, and 32.6% had a graduate degree. A total of 17 participants (33.3%) had experienced abuse using the threshold described in Study 1.
IQ
We administered the matrix reasoning subscale of the Wechsler Abbreviated Scale of Intelligence; t scores on this subscale were used for all participants as an index of IQ. The range of IQ was 65 to 133 (M = 100.7, SD = 15.8). The Wechsler Abbreviated Scale of Intelligence was administered by trained research assistants during the study visit, which preceded scanning. IQ testing was not completed due to time constraints with 6 participants. IQ was marginally associated with parental education, r (41) = .30, p = .06, but not abuse exposure, r (45) = –.14, p = .36. Given the high level of missing data on IQ, analyses were run with and without IQ as a covariate, and when including IQ changed the direction or significance of results; this is reported.
WM/filtering task
A delayed match-to-sample WM task with and without distractors was administered during functional magnetic resonance imaging (fMRI) scanning (see Figure 1). This task was modeled after an existing filtering task designed for adults with some modifications (McNab & Klingberg, Reference McNab and Klingberg2008). Participants first viewed a cue, either a square or a trapezoid. This cue lasted 3–5 s and indicated if there would be distractors present on the subsequent trial. The specific shape indicating distractors was counterbalanced across subjects. Following the cue, an encoding screen was presented for 1 s. During encoding, participants viewed an array of 16 circles presented in a circle around a centrally located fixation cross with red or yellow stars with eyes in 2 or 4 of the circles (see Figure 1). Participants were told to remember the location of the stars. There were four conditions: low load, where participants saw two red stars; high load, where participants saw four red stars; distraction, where participants saw four stars (two red and two yellow) and were cued to ignore the yellow stars; and high load-two color trials, where participants saw two red and two yellow stars and were cued to remember the locations of all stars. A delay period lasting 2, 3, or 4 s followed encoding. During the delay period, participants saw a fixation cross and needed to maintain in memory the location of the stars viewed during encoding. Following the delay, participants viewed the same 16-circle array for 2 s. One circle had a question mark in it, and participants indicated with a button press if that question mark was in the same location as one of the stars to be remembered. The next trial began 3 or 4 s after the probe ended. Participants completed scanning in four functional runs lasting 9 min each. Each run contained 10 trials of each condition, for a total of 160 trials per subject.
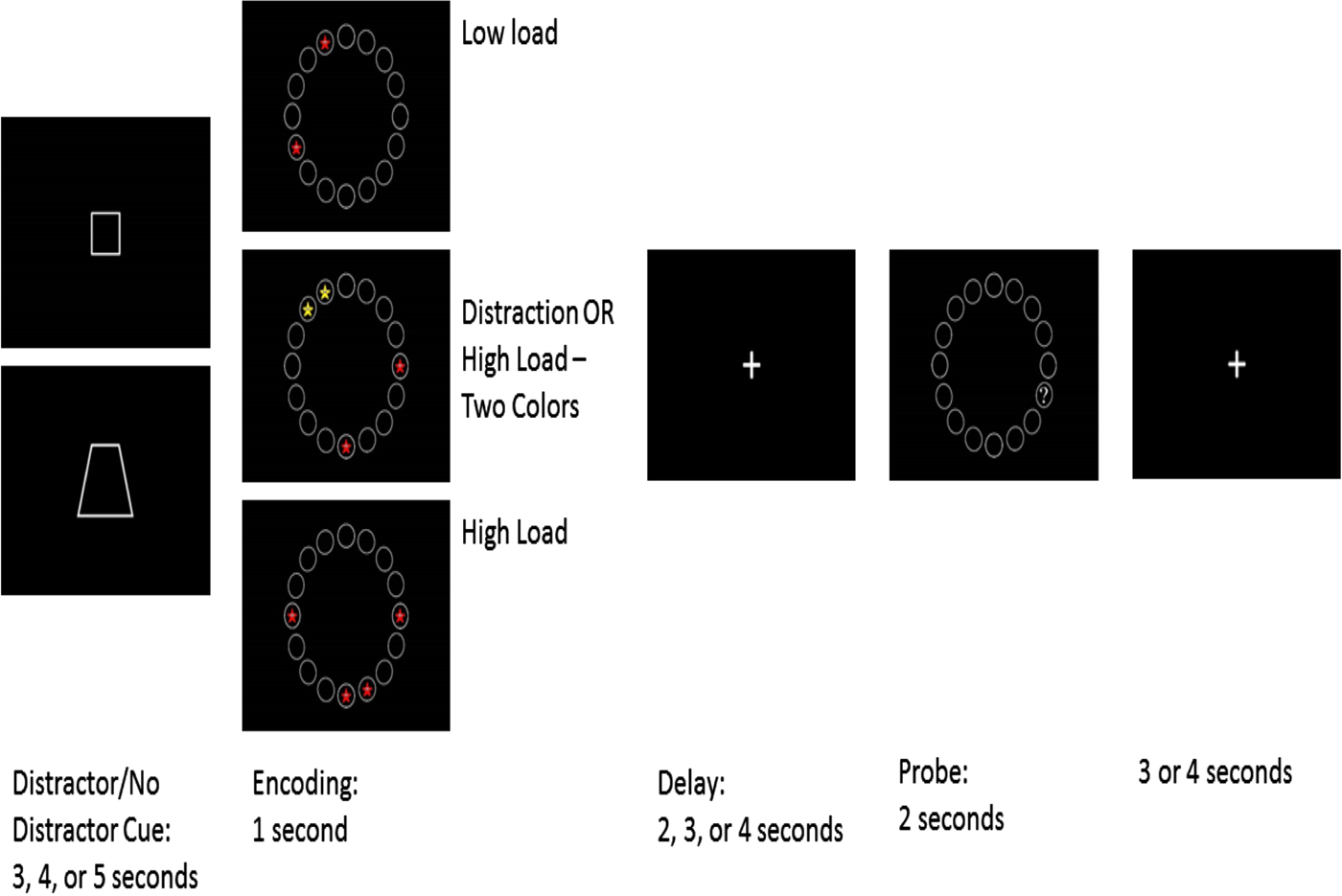
Figure 1. (Color online) Working memory filtering task. In this spatial delayed match to sample task adapted from McNab and Klingberg (Reference McNab and Klingberg2008), participants were given 1 s to remember either two or four stars (encoding). On 25% of the trials, they were instructed prior to encoding to ignore two yellow stars while remembering the location of two red stars (cue period). After a 2-, 3-, or 4-s delay (delay) where they viewed a white fixation crosshair, they were shown a screen with a single question mark (probe). They pressed one button to indicate if that question mark was in the same place as a star to be remembered and another to indicate that it was not. Stimuli were presented in four runs lasting approximately 9 min each.
Equal numbers of all durations for cue, delay, and intertrial interval were present across each condition and run (each cue and delay duration was used in 33% of trials, and each intertrial interval duration in 50% of trials). Red stars in the encoding phase were placed in a pseudorandom fashion such that they were distributed evenly across available spaces. In distraction and high load-two color trials, yellow stars regularly occurred in one of four patterns, which were counterbalanced by subject between high-load two color and distraction conditions. A recognition test given immediately postscan showed no sensitivity to the presence of these patterns, indicating that pattern learning was not a significant factor in performance. In 56% of trials, the probe was presented in a target location (match trials). On trials with a distractor present, the probe was presented in the location of a distractor for 31% of trials. For trials where the probe was presented in a previously empty circle (nonmatch trials), the probe was located one space away from a filled circle in 90% of trials.
Prior to scanning, participants were given instructions on how to complete the task and the meaning of the distraction cue. Participants practiced the task and were quizzed as to the meaning of the distraction cues prior to completing the in the scanner. To ensure that all participants had equally good knowledge of the meaning of the “distractor” cue and the “nondistractor” cue, all participants were quizzed on the meaning of different cues directly prior to imaging the task. They were allowed to continue once they correctly identified if the square or trapezoid indicated that there would be a distractor present on a trial at least two times in a row.
Image acquisition
Scanning was performed on a 3-Tesla Siemens Tim Trio scanner at the Harvard Center for Brain Science using a 32-channel head coil. Anatomical scans (T1-weighted multiecho MPRAGE volumes) were acquired for coregistration with fMRI (repetition time = 2530 ms, echo time = 1640–7040 μs, flip angle = 7°, field of view = 220 mm, 176 slices, in-plane voxel size = 1 mm3). To reduce motion-related artifacts a navigator echo was used prior to scan acquisition, which compares slices to this echo online and permits up to 20% of slices be reacquired.
Blood oxygen level dependent (BOLD) signal during functional runs was acquired using a gradient-echo T2*-weighted echo planar imaging sequence. Thirty-two 3 mm thick slices were acquired parallel to the AC-PC line (repetition time = 2s, echo time = 30 ms, flip angle = 90°, bandwidth = 2240 Hz/Px, echo spacing = 0.51 ms, field of view = 216 mm, matrix size = 64 × 64). Two hundred seventy volumes were acquired for each of four functional runs. Prior to each scan, four images were acquired and discarded to allow longitudinal magnetization to reach equilibrium. An online prospective motion correction algorithm (PACE) was used to reduce the effect of motion artifacts.
Image processing
Preprocessing and analysis of fMRI data was performed in Nipype, a platform that implements analysis tools from multiple software packages using the Python programming language (Gorgolewski et al., Reference Gorgolewski, Burns, Madison, Clark, Halchenko, Waskom and Ghosh2011). fMRI preprocessing included a four-dimensional spatial realignment and slice-time correction (Roche, Reference Roche2011) followed by spatial smoothing (6 mm full width at half-maximum) implemented in FSL. Data were inspected for artifacts using the RapidART library in Nipype; single point outlier regressors were generated for any volume in which scan to scan motion of any center point of a cuboid drawn around the brain exceeded 1.5 mm or in which overall image intensity was more than 3 SD from the mean. Six rigid-body motion regressors were included as nuisance covariates in person-level models. Person and group-level models were estimated in FSL. A component-based anatomical noise correction method was used to reduce noise associated with physiological fluctuations (Behzadi, Restom, Liau, & Liu, Reference Behzadi, Restom, Liau and Liu2007). Following estimation of person-level models, the resulting contrast images were normalized into standard anatomical space, and anatomical coregistration of the functional data with each participant's T1-weighted image was performed using Advanced Normalization Tools software (Avants et al., Reference Avants, Tustison, Song, Cook, Klein and Gee2011).
To identify regions of interest based on structurally defined boundaries, each participant's T1-weighted images were automatically segmented and parcellated using FreeSurfer (Destrieux, Fischl, Dale, & Halgren, Reference Destrieux, Fischl, Dale and Halgren2010; Fischl et al., Reference Fischl, Salat, Busa, Albert, Dieterich, Haselgrove and Dale2002). FreeSurfer morphometric procedures have demonstrated good test–retest reliability across scanner manufacturers and field strengths (Han et al., Reference Han, Jovicich, Salat, van der Kouwe, Quinn, Czanner and Fischl2006). In addition, these procedures have been successfully used in studies of children as young as age 4 (Ghosh et al., Reference Ghosh, Kakunoori, Augustinack, Nieto-Castanon, Kovelman, Gaab and Fischl2010).
Data analysis
Behavioral task response was indexed using accuracy. Reaction time was not considered a measure of performance on this task because of the long delay between encoding and probe. This is consistent with previous studies showing weak associations between performance and reaction time using the delayed match-to-sample task (Sheridan, Hinshaw, & D'Esposito, Reference Sheridan, Hinshaw and D'Esposito2010). Simple associations between abuse exposure, parental education, and task performance are reported controlling for age and gender. Next, ordinary least squares regressions where accuracy on high load trials was predicted by parental education (1–4 indicating highest level of education attained) controlling for low load trial accuracy, abuse exposure, age, gender, and race. All analyses were run with and without IQ as a covariate.
fMRI analysis
Regressors were created by convolving a boxcar function of phase duration and amplitude one with the standard hemodynamic response function for each phase of the task: cue, encoding + delay, and probe. Cue was modeled separately for “distractor instruction” (i.e., cues that indicated participants were in a distraction trial) and “no distractor instruction” (i.e., cues that indicated all other trials) trials. The encoding + delay period was modeled as a single regressor, and all four encoding + delay conditions were modeled separately (low load single color, low load with distractors, high load single color, and high load two colors). Probe was also modeled separately for each of these four conditions. Using FSL FLAME, a general linear model was constructed to estimate the association between variation in BOLD signal and task demands across time for each subject, prior to normalization. Individual-level estimates of BOLD activity were submitted to group-level random effects models that contrasted activity across conditions. We defined contrasts to examine the effect of load (high load > low load) at encoding + delay, the effect of distraction (distraction > low load) at encoding + delay, and the effect of the distractor instruction (distractor instruction > no distractor instruction) at cue. We used a stringent cluster-level correction threshold of z > 2.3, p < .01 in FSL. This cluster-level correction threshold in FSL has been shown in recent simulations to not be associated with dramatic inflation of either false positive or false negative findings (Eklund, Nichols, & Knutsson, Reference Eklund, Nichols and Knutsson2016). We examined differences in BOLD response during contrasts of interest as a function of parental education controlling for abuse, age, and gender. For each trial phase, accurate and inaccurate trials were modeled separately; only accurate trials were used to examine neural activity at the whole-brain level.
The main effect of task manipulations (load, distractors) in this data set has been reported elsewhere (Peverill et al., Reference Peverill, McLaughlin, Finn and Sheridan2016). Briefly, increased load resulted in increased recruitment bilaterally in the intraparietal sulcus (IPS), medial frontal gyrus (MFG), and anterior cingulate cortex (ACC) during the encoding and delay period. The presence of distracting stimuli during encoding resulted in increased activation of the IPS and inferior frontal gyrus (IFG) during the encoding and delay period.
In addition to the whole-brain analysis, we investigated the association between parental education and recruitment of three a priori defined regions of interest (ROIs) in the prefrontal cortex: the MFG, the ACC, and the IFG. The MFG was encoded as the frontal middle gyrus, the ACC as the anterior cingulate gyrus, and the IFG as the frontal inferior gyrus–opercularis region using the Destrieux atlas in FreeSurfer 5.3 (Destrieux et al., Reference Destrieux, Fischl, Dale and Halgren2010). These regions were selected given their known involvement in WM and inhibition (described below), and their recruitment in the whole sample during the load and distractor manipulations within our task. The MFG is widely implicated in the performance of delayed match to sample WM tasks (D'Esposito et al., Reference D'Esposito, Detre, Alsop, Shin, Atlas and Grossman1995; Miller & D'Esposito, Reference Miller and D'Esposito2005) and is particularly considered necessary for the reliable encoding and maintenance of items to be remembered (Goldman-Rakic, Reference Goldman-Rakic1996). The ACC is understood to play a critical role in cognitive control, the ability to selectively attend and respond to task-relevant stimuli, a process which is commonly elicited by both WM maintenance and tasks with enhanced inhibitory demands, such as encoding relevant stimuli in the presence of irrelevant distractors (Botvinick, Cohen, & Carter, Reference Botvinick, Cohen and Carter2004; Carter et al., Reference Carter, Braver, Barch, Botvinick, Noll and Cohen1998). The IFG, in particular the right IFG, is commonly activated when inhibitory control demands are high. Inhibitory control is the ability to selectively attend and respond to relevant versus irrelevant information, a cognitive process that would be required to encode and maintain relevant items in memory when distractors are present (Aron, Reference Aron2007; Aron, Robbins, & Poldrack, Reference Aron, Robbins and Poldrack2014).
Thus, consistent with previous literature and our own observations in this data set of increased recruitment of the MFG, ACC, and IFG during WM encoding, we examine the association between adversity exposure and activation in these ROIs during the encoding and maintenance period. We examine the association between activation of these regions and adversity exposure for both the high > low load and the distractor present > low load contrasts. Because we have no a priori reason to expect differences in associations for the right and left hemispheres of the brain, we report on average activation in the right and left hemispheres for both the MFG and the ACC. Because activation in the service of inhibitory control is commonly right-lateralized, we examine activation only in the right hemisphere for the right IFG.
Results
Task-related effects on behavior
Increasing WM load from two to four items significantly impacted accuracy, t (50) = 5.06, p < .001. Average accuracy for trials with a load of two items was 77% and 71% for trials with a load of four items. Introducing distractors did not significantly impair accuracy. Average accuracy for trials with a load of two items with distractors present was 78% compared to 77% for trials with a load of two items but no distractors, t (50) = 0.12, p = .9.
Deprivation, threat, and task-related behavior
Table 1 provides descriptive statistics for all study variables, and Table 2 shows bivariate correlations. Parental education level was significantly associated with accuracy on trials involving high WM load (i.e., memory for four items relative to two items; β = –0.16, p = .03) after controlling for age and gender. As parental education increased, accuracy increased on high relative to low load trials. In contrast, abuse severity was not associated with accuracy on trials involving high WM load (β = –0.005, p = .95) after controlling for demographics. When IQ was included as an additional covariate the pattern and significance of these results was unchanged.
Next we examined the association between adversity exposure and WM performance on high versus low load trials with both forms of adversity exposure in the same model. Including abuse exposure did not change associations between parental education and WM performance (Table 4) and the association between abuse exposure and WM performance remained nonsignificant in this model.
Table 4. Associations of threat and deprivation with and effect of working memory load on brain and behavior

Note: MFG, medial frontal gyrus; ACC, anterior cingulated cortex; IFG, inferior frontal gyrus.
a These models additionally include controls for community violence and abuse.
b These models additionally include controls for parental education and neglect.
*p < .05. **p < .01.
There was no significant association between parental education or abuse exposure on accuracy on trials involving distractors (memory on distractor trials relative to low load trials) with or without controls for IQ (ps > .5). Because the main effect of distractors on memory across the whole group was negligible, we also examined the association between these exposure variables and performance on distractor trials without controlling for low load performance. These associations were also nonsignificant (ps > .5).
In sum, parental education but not abuse exposure was strongly associated with WM task performance; this association was strongest at high WM load and was unchanged by including controls for IQ or abuse exposure. Abuse was unrelated to WM performance regardless of controls for parental education.
Deprivation, threat, and neural activation
Here we report on the associations between parental education and abuse severity with neural recruitment on the WM task in a whole-brain analysis and in three a priori defined ROIs (MFG, ACC, and IFG).
Whole brain
Parental education was significantly associated with BOLD signal in one cluster spanning the medial and lateral superior parietal cortex, including the precuneus (Miinnesota Neurological Institute [MNI] coordinates: X = –4, Y = –68, Z = 52, z = 3.5, p < .001), the superior parietal lobule (SPL; MNI coordinates: X = –8, Y = –60, Z = 48, z = 3.4, p < .001), and the IPS (MNI coordinates: X = –14, Y = –60, Z = 52, z = 2.41, p < .01), during the encoding and delay period from the contrast of high > low load, after controlling for age, gender, and abuse severity (Figure 2) such that lower parental education was associated with greater response in these areas. No changes in BOLD signal were associated with parental education for distractor > low load trials for any task period.
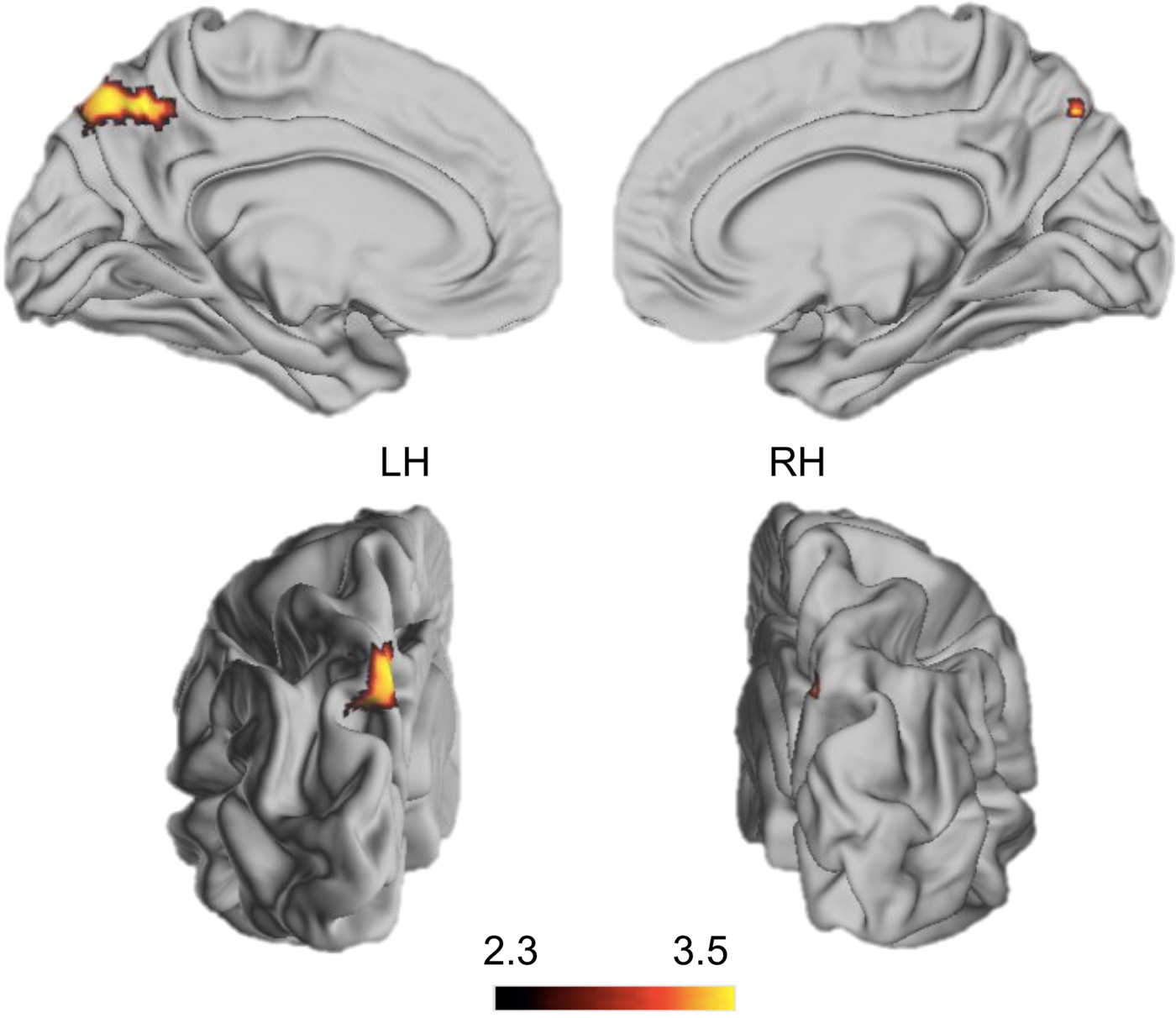
Figure 2. (Color online) Whole-brain associations with parental education. Associations of parental education with neural response to trials involving high > low working memory load. Regions with greater blood oxygen level dependent activation during high > low working memory load as parental education decreased. Cluster-level correction to a cluster level p = .05 was applied in FSL with z > 2.3, p < .01 as our voxel-level threshold. Severity of abuse exposure, age, and sex were included as nuisance regressors in all analyses.
No changes in BOLD signal were associated with abuse severity for any task period or contrast.
ROIs
BOLD activation in three prefrontal regions were examined separately.
MFG
BOLD activity in the MFG for high > low load trials during the encoding and maintenance period was significantly associated with parental education (β = –0.37, p = .03) controlling for age and gender. Including IQ as a covariate did not change the magnitude or significance of this association. As parental education increased, MFG recruitment during the high relative to low WM load condition decreased (Figure 3). Abuse exposure was unrelated to MFG recruitment in the service of WM for high > low load trials with or without controls for parental education (ps > .6). Neither abuse exposure nor parent education were significantly associated with activation in the MFG during encoding and maintenance for distractor > low load trials with or without controlling for IQ (all ps > .6).

Figure 3. Activation in regions of interest and with parental education. Associations of parental education with neural recruitment during high > low working memory load in prefrontal cortex regions of interest: anterior cingulate cortex (ACC), medial frontal gyrus (MFG), and right inferior frontal gyrus (rIFG). Regions of interest were defined structurally using FreeSurfer (see Methods section for details). Parameter estimates were extracted for the contrast of high > low working memory load. Severity of abuse exposure, age, and sex were included as nuisance regressors in all analyses.
ACC
BOLD activity in the ACC for high > low load trials during encoding and delay was significantly associated with parental education (β = –0.46, p = .002). The direction and significance of this association was not changed by controlling for IQ. As parental education increased, MFG recruitment during the high relative to low WM load condition decreased (Figure 3). Abuse exposure was unrelated to ACC recruitment in the service of WM with and without controlling for IQ (ps > .8). Neither abuse exposure nor parent education was significantly associated with activation in the ACC during encoding and maintenance for distractor > low load trials with our without controlling for IQ (all ps > .4).
IFG
BOLD activity in the right IFG was unrelated to parental education or abuse exposure with or without controlling for IQ regardless of the condition examined (all ps > .11).
Conclusions
We observed an association between WM and parental education but not abuse. We observed this association using multiple measures of WM, including accuracy on a delayed match to sample task for high relative to low load trials and neural recruitment in regions known to support WM performance during the encoding and delay period for high relative to low load trials. Associations between parental education and neural recruitment in the superior parietal cortex were identified using a whole-brain cluster-corrected analysis. These observations were complimented by identification of associations between parental education and activation in a priori selected regions in the prefrontal cortex. These associations with parental education were robust to controls for age, gender, IQ, and abuse exposure. In contrast, abuse exposure was not associated with task performance or neural recruitment despite a significant level of exposure to severe physical and sexual abuse in this sample.
This pattern of associations is consistent with our previously hypothesized model (McLaughlin et al., Reference McLaughlin, Sheridan, Winter, Fox, Zeanah and Nelson2014; Sheridan & McLaughlin, Reference Sheridan and McLaughlin2014, Reference Sheridan and McLaughlin2016) and extends the findings of Study 1 to include a more objective assessment of EF. We proposed that exposure to deprivation, or a lack of social and cognitive stimulation and learning opportunities, would be negatively associated with EF, whereas exposure to threat would not. We operationally defined deprivation as low parental education and threat as exposure to abuse. Across many studies, low parental education is predictive of reductions in cognitive stimulation and both formal and informal learning opportunities (Duncan & Brooks-Gunn, Reference Duncan and Brooks-Gunn1999). However, some children of parents with very little education have access to a rich and complex set of learning opportunities, meaning that parental education is merely a proxy for deprivation exposure. Using this kind of proxy measure is a limitation of these findings, and future research should couple in-depth measures of the home environment with the rich neurocognitive assessments used here. In contrast to parental education, child abuse is a clear and severe exposure to threat. Thus, this test of our model pitted a relatively mild form of risk for deprivation (parental education) against a relatively severe exposure to threat (abuse) in predicting objectively measured WM performance and neural recruitment. That we observe predicted relationships constitutes strong preliminary evidence for the deprivation and threat model.
Discussion
Exposure to childhood adversity dramatically increases risk for psychopathology in childhood, adolescence, and adulthood (Green et al., Reference Green, McLaughlin, Berglund, Gruber, Sampson, Zaslavsky and Kessler2010; McLaughlin et al., Reference McLaughlin, Green, Gruber, Sampson, Zaslavsky and Kessler2010). Understanding the pathways through which adversity increases risk for psychopathology has the potential to increase the efficacy of preventive interventions through the targeting of specific mechanisms in these pathways. To date, the cumulative risk model has been the prevailing approach to conceptualizing childhood adversity. This model fails to distinguish between different types of adversity that might influence development through distinct mechanisms, assuming that disruptions in the physiological stress response are the primary mechanism explaining psychopathology and other negative health outcomes associated with adversity (Evans, Li, & Whipple, Reference Evans, Li and Whipple2013; Shonkoff, Reference Shonkoff2012; Shonkoff, Boyce, & McEwen, Reference Shonkoff, Boyce and McEwen2009). Elsewhere, we have proposed an alternative to the cumulative risk model. This model posits that within the construct of childhood adversity exist at least two dimensions of environmental experience that can be differentiated from one another: deprivation and threat (McLaughlin & Sheridan, Reference McLaughlin and Sheridan2016; McLaughlin, Sheridan, & Lambert, Reference McLaughlin, Sheridan and Lambert2014; Sheridan & McLaughlin, Reference Sheridan and McLaughlin2014, Reference Sheridan and McLaughlin2016). Our model posits that the neurodevelopmental consequences of deprivation and threat are at least partially distinct. Specifically, our model argues that exposure to threat has primary influences on neural systems underlying emotional reactivity and regulation, particularly of negative stimuli, and “hot” affectively laden forms of cognition. In contrast, we hypothesize that deprivation primarily influences neural circuitry underlying “cold cognition,” including complex cognitive processes such as EFs. Here we provide an empirical test of this deprivation hypothesis across multiple levels of analysis.
First, we predicted that exposure to deprivation would predict poor performance on an EF task, a pattern of inefficient neural recruitment during this task, and greater problems in using EF in daily life. We found strong support for these hypotheses. In Study 1, children who experienced neglect were rated by their parents as experiencing numerous problems with applying EF skills in everyday life and, in particular, in situations that require inhibitory control. Children whose parents had low educational attainment similarly exhibited problems in applying inhibitory control skills in daily life. In Study 2, children's performance on a WM task improved linearly as parent education increased, particularly as the WM demands increased on the task. Moreover, parent education was negatively associated with neural activation in the superior parietal cortex, a region strongly linked to WM and to EF more broadly (Todd & Marois, Reference Todd and Marois2004), during trials involving high WM demands in a whole-brain analysis, correcting for multiple comparisons. Children whose parents had less education exhibited a less efficient pattern of neural recruitment in the superior parietal cortex on these trials; they exhibited more activation in the superior parietal cortex and performed less well on the task. In a region of interest analysis similar associations were observed in two regions of prefrontal cortex known to be involved in WM and EF. Children whose parents had less education also exhibited a less efficient pattern of neural recruitment in the MFG and ACC on trials with high relative to low WM demands. Second, we expected that these associations would be robust to controls for exposure to adverse experiences reflecting threat. This hypothesis was also supported. In all cases, the associations of neglect and low parental education with EF outcomes persisted after adjustment for co-occurring exposure to threat, including experiences of abuse and community violence. Third, we anticipated that threat exposure would have no relation to these measures of EF. This hypothesis was also supported in both studies. Exposure to environmental threats involving physical and sexual abuse and direct experiences of interpersonal violence in the community were not associated with any measure of EF in either study after adjustment for co-occurring experiences of deprivation.
We have defined experiences of deprivation as environments that provide little exposure to social and cognitive stimulation and learning opportunities. In the case of neglect, these opportunities are reduced because access to caregivers and caregiver investment is minimal. In the case of low SES, a reduction in the degree of exposure to complex cognitive stimuli (e.g., complex language, books, and informal and formal learning opportunities) is a well-documented correlate of low parental education (Britto & Brooks-Gunn, Reference Britto and Brooks-Gunn2001; Gormley, Gayer, Phillips, & Dawson, Reference Gormley, Gayer, Phillips and Dawson2005; Howard, Martin, Berlin, & Brooks-Gunn, Reference Howard, Martin, Berlin and Brooks-Gunn2011; Raikes et al., Reference Raikes, Pan, Luze, Tamis-LeMonda, Brooks-Gunn, Constantine and Rodriguez2006). We hypothesize that a lack of enriched learning opportunities intersects with the typical neurodevelopmental process during early childhood to reduce the degree to which children are prepared for future cognitive tasks. For infants and young children, early learning opportunities happen primarily in the context of caregiver interactions. Caregivers direct child attention to important stimuli in the environment through child-directed speech and facial displays (Gratier et al., Reference Gratier, Devouche, Guellai, Infanti, Yilmaz and Parlato-Oliveira2015; Harder, Lange, Hansen, Væver, & Køppe, Reference Harder, Lange, Hansen, Væver and Køppe2015; Pelucchi, Hay, & Saffran, Reference Pelucchi, Hay and Saffran2009). These early interactions shape and support the development of basic associative learning mechanisms and attentional control, which in turn are the building blocks of more complex cognitive functions including numerous aspects of EF (Healey, Gopin, Grossman, Campbell, & Halperin, Reference Healey, Gopin, Grossman, Campbell and Halperin2010). By school entry, differences in the quantity and quality of parental interactions are associated with school readiness (Britto & Brooks-Gunn, Reference Britto and Brooks-Gunn2001), and interventions that target this early childhood period have pervasive impacts on child outcomes (Muennig et al., Reference Muennig, Schweinhart, Montie and Neidell2009; Reynolds, Reference Reynolds1994; Reynolds et al., Reference Reynolds, Temple, Robertson and Mann2001). By middle childhood, socioeconomic differences in exposure to linguistic stimuli predict neural function and performance on novel learning tasks (Sheridan, Sarsour, et al., Reference Sheridan, Sarsour, Jutte, D'Esposito and Boyce2012). Consistent with the importance of early learning experiences to brain development, here we demonstrate that two factors associated with decreased exposure to early learning in the context of caregiver interactions, neglect and low parental education, are associated with EF during adolescence.
These early learning exposures likely also shape neural structure and function through the typical neurodevelopmental process of synaptic pruning (Huttenlocher, Reference Huttenlocher1998, Reference Huttenlocher2003). Specifically, an absence of complex social and cognitive inputs in early development leads to accelerated cortical thinning in animal models of deprivation (Bennett et al., Reference Bennett, Rosenzweig, Diamond, Morimoto and Hebert1974; Diamond et al., Reference Diamond, Law, Rhodes, Lindner, Rosenzweig, Krech and Bennett1966, Reference Diamond, Rosenzweig, Bennett, Lindner and Lyon1972). Recent work in humans suggests that early deprivation, both institutional rearing and low parental SES, is similarly associated with reductions in cortical thickness and surface area throughout the cortex (Mackey et al., Reference Mackey, Finn, Leonard, Jacoby-Senghor, West, Gabrieli and Gabrieli2015; Noble et al., Reference Noble, Houston, Brito, Bartsch, Kan, Kuperman and Sowell2015). Accelerated thinning in regions that support EFs should produce a pattern of inefficient neural recruitment in these areas during tasks that tap these types of complex cognition. We find strong support for this idea in the current study. Specifically, in a whole-brain cluster-level corrected analysis, we observe differences in recruitment of the SPL for high relative to low spatial WM load by parental education. Recruitment of the SPL was negatively associated with parental education, such that adolescents whose parents had less education exhibited stronger and more widespread activation in this region, but performed less well. This pattern is striking as the SPL is sensitive to the number of items held in WM (Todd & Marois, Reference Todd and Marois2004); thus, increased activation in this task in the context of poorer performance is clearly an inefficient pattern of activation. The SPL is part of the frontoparietal task control network and commonly coactivates with the dorsolateral prefrontal and anterior cingulate cortex in support of EF tasks (Michalka, Rosen, Kong, Shinn-Cunningham, & Somers, Reference Michalka, Rosen, Kong, Shinn-Cunningham and Somers2016; Szczepanski, Konen, & Kastner, Reference Szczepanski, Konen and Kastner2010). Consistent with these findings, in an ROI analysis, we found analogous differences in neural recruitment as a function of parental education. Specifically, children whose parents had less education exhibited greater recruitment of the MFG and ACC during trials involving high WM load. Together with the behavioral results, we find strong support for a selective association of low parental education (a relatively mild marker of deprivation) with EF performance and neural recruitment, whereas abuse (a relatively severe marker of threat) has no association with either performance or neural recruitment.
Although not directly investigated in the current paper, elsewhere we have shown, along with other labs, that violence or threat exposure is associated with alterations in numerous forms of emotional processing over and above the effects of co-occurring deprivation. Children exposed to interpersonal violence and abuse exhibit patterns of information processing that are biased toward the identification of anger (Pollak et al., Reference Pollak, Cicchetti, Hornung and Reed2000), including faster attentional engagement and delayed attentional disengagement from anger (Pollak & Tolley-Schell, Reference Pollak and Tolley-Schell2003), interpret ambiguous social situations as threatening (Lansford et al., Reference Lansford, Malone, Dodge, Crozier, Pettit and Bates2006), exhibit atypical patterns of threat-safety discrimination in fear conditioning paradigms (McLaughlin et al., Reference McLaughlin, Sheridan, Gold, Duys, Lambert, Peverill and Pine2016), demonstrate magnified emotional reactions to negative cues, including elevated amygdala responses (McCrory et al., Reference McCrory, De Brito, Sebastian, Mechelli, Bird, Kelly and Viding2011, Reference McCrory, De Brito, Kelly, Bird, Sebastian, Mechelli and Viding2013; McLaughlin et al., Reference McLaughlin, Peverill, Gold, Alves and Sheridan2015), and have difficulty modulating responses to negative emotional stimuli both explicitly and implicitly (Heleniak, Jenness, Stoep, McCauley, & McLaughlin, Reference Heleniak, Jenness, Stoep, McCauley and McLaughlin2016; Herringa et al., Reference Herringa, Birn, Ruttle, Burghy, Stodola, Davidson and Essex2013; Kim & Cicchetti, Reference Kim and Cicchetti2010). These patterns reflect an emotional processing system that is highly attuned to the identification of potential threats in the environment and generates amplified emotional reactions to such threats that are difficult to modulate effectively. In all cases, associations of threat exposure with these patterns of emotional processing are robust to controls for markers of deprivation. In the few studies that examined this directly, exposure to deprivation was not associated with these markers of emotional processing after adjustment for threat (Busso et al., Reference Busso, McLaughlin and Sheridan2016; Lambert et al., Reference Lambert, King, Monahan and McLaughlin2016).
These findings have implications both for the conceptualization of adversity and for understanding the neurodevelopmental mechanisms linking diverse forms of childhood adversity to the onset of psychopathology. First, our findings challenge the cumulative risk approach to studying developmental psychopathology following exposure to childhood adversity. The cumulative risk approach has been pivotal in highlighting the public health importance of childhood adversity and provides a useful screening tool for identifying children with high levels of environmental adversity who may be particularly likely to benefit from intervention (Evans et al., Reference Evans, Li and Whipple2013; Felitti et al., Reference Felitti, Anda, Nordenberg, Williamson, Spitz, Edwards and Marks1998). However, our findings demonstrate clearly that the assumptions of the cumulative risk model fall short when applied to developmental pathways. In particular, the cumulative risk approach assumes that all forms of adversity have additive and similar influences on developmental processes. This assumption is implicit in creating a cumulative risk score, whereby exposure to abuse is coded as present or absent, poverty is coded as present or absent, neglect is coded as present or absent, and so on. Associating the cumulative total of all adversities experienced with neurocognitive measures or other developmental outcomes assumes that each adversity included in the risk score will have a similar (and additive) effect on the outcome in question. Our findings highlight the serious limitations of such an approach by documenting clear specificity in the associations of particular forms of adversity, but not others, with EFs. Together, these findings argue strongly against the use of cumulative risk models in studying the developmental consequences of childhood adversity. Although more differentiated approaches to childhood adversity have been advocated by others for years (Barnett et al., Reference Barnett, Manly, Cicchetti, Cicchetti and Toth1993; Manly et al., Reference Manly, Cicchetti and Barnett1994, Reference Manly, Kim, Rogosch and Cicchetti2001) cumulative risk models remain commonplace.
Second, our findings identify a potential mechanism of multifinality with regard to psychopathology in children who encounter adversity. Strong associations of numerous forms of childhood adversity with most commonly occurring forms of psychopathology are well documented, including anxiety, depression, substance abuse, and behavior problems (Cicchetti & Toth, Reference Cicchetti and Toth1995; Green et al., Reference Green, McLaughlin, Berglund, Gruber, Sampson, Zaslavsky and Kessler2010; McLaughlin et al., Reference McLaughlin, Green, Gruber, Sampson, Zaslavsky and Kessler2010, Reference McLaughlin, Greif Green, Gruber, Sampson, Zaslavsky and Kessler2012). Identifying mechanisms explaining this multifinality is critical for both theory and intervention. Here, we document that disruptions in EF and frontoparietal function, previously identified as transdiagnostic risk factors (Goodkind et al., Reference Goodkind, Eickhoff, Oathes, Jiang, Chang, Jones-Hagata and Etkin2015), may be a core pathway that explains the links between forms of adversity involving deprivation and a wide range of mental health outcomes. Poor EF has been observed commonly in individuals with externalizing (Morgan & Lilienfeld, Reference Morgan and Lilienfeld2000; Willcutt, Doyle, Nigg, Faraone, & Pennington, Reference Willcutt, Doyle, Nigg, Faraone and Pennington2005) and internalizing problems (Olley, Malhi, & Sachdev, Reference Olley, Malhi and Sachdev2007; Snyder, Reference Snyder2013; Wagner, Müller, Helmreich, Huss, & Tadić, Reference Wagner, Müller, Helmreich, Huss and Tadić2015). In contrast, the high rates of psychopathology observed in children exposed to abuse and other forms of interpersonal violence are likely to be explained through alternate pathways, including atypical patterns of emotional processing (Kim & Cicchetti, Reference Kim and Cicchetti2010; McLaughlin, Reference McLaughlin2016). Overall, this suggests that although numerous forms of psychopathology are common in children exposed to many types of adversity, the pathways that lead to those outcomes may be distinct. Greater work is clearly needed to chart these pathways and to identify the most promising targets for intervention.
A number of limitations of this work are important to consider. In Study 1, we relied on parent report of everyday behaviors reflecting EF. Such reports may reflect underlying variation in EF, but they may also reflect personality and contextual factors that influence children's behavior. In contrast, these types of behaviors provide an ecologically valid assessment of how variation in EF influences a child's behavior in the real world. We addressed this limitation in Study 2 by including a task-based measure of EF. In Study 2, we used parent education as a proxy of deprivation and did not directly measure the degree of stimulation in children's environments. Future research on this model should couple in-depth measures of the home environment with the rich neurocognitive assessments used here to provide a more direct test of our hypotheses about deprivation. In addition, we did not include a comprehensive assessment of all types of deprivation and threat experiences in Study 2. As noted above, focusing on parental education provides a conservative test of our deprivation hypothesis as this is a proxy for relatively mild deprivation. In contrast, Study 1 focused on a range of experiences, including both abuse and exposure to violence in the community as indicators of threat and both exposure to neglect and low parental education as indicators of deprivation. Finally, we were unable to assess chronicity and timing of exposures to adversity in this study. Previous work indicates that more severe, chronic, and early exposure to maltreatment is likely to specifically impact development of inhibitory control and WM (Cowell, Cicchetti, Rogosch, & Toth, Reference Cowell, Cicchetti, Rogosch and Toth2015).
Despite these limitations, we provide evidence across multiple levels of analysis for specificity in the associations of deprivation but not threat with EF performance and neural recruitment across two samples with high variability in exposure to adversity. Our findings add to the growing body of evidence suggesting that distinct dimensions of environmental experience are encompassed within the construct of childhood adversity. Here, we provide support for the importance of distinguishing between experiences reflecting threat from those reflecting deprivation when examining their influences on neurocognitive development. In particular, we find strong support for specificity in the association of low parental education and neglect, markers of deprivation in exposure to cognitively enriching environments, with EFs and the neural systems that support EF. The strong associations of EFs with experiences involving deprivation and the absence of association with experiences involving threat indicates the presence of meaningful variation in the neurodevelopmental consequences of distinct forms of childhood adversity. Understanding this variation is critical to inform the development of effective interventions to prevent the downstream consequences of adversity.









