Chronic or severe psychosocial stress in childhood and adolescence is linked to dysregulation in stress-mediating systems, including the hypothalamic–pituitary–adrenal (HPA) axis (Doom & Gunnar, Reference Doom and Gunnar2013; Koss & Gunnar, Reference Koss and Gunnar2018). Dysregulation in cortisol, one of the HPA axis's primary stress-mediating hormones, is associated with the onset of a number of mental and physical health problems (McEwen, Reference McEwen1998). However, most investigations of associations between psychosocial stress, the HPA axis, and mental health focus on diurnal cortisol regulation or cortisol reactivity and recovery in response to stress. It is largely unclear how chronic or severe psychosocial stress during childhood and adolescence prospectively affect overall output of cortisol over time, which may be particularly important in order to understand patterns of cortisol regulation and whether specific patterns predict the onset of psychopathology. The current investigation addresses this gap in the literature by testing whether parental harshness and parental disengagement measured prospectively during childhood and adolescence predict overall cortisol output at 15 years.
Although there is evidence that both heightened and reduced overall cortisol output are associated with a number of mental and physical health problems (Adam et al., Reference Adam, Quinn, Tavernier, McQuillan, Dahlke and Gilbert2017; Dockray, Susman, & Dorn, Reference Dockray, Susman and Dorn2009; Shirtcliff & Essex, Reference Shirtcliff and Essex2008), there is little empirical work that directly investigates whether heightened or reduced overall cortisol output may mediate associations between psychosocial stress and mental health problems. Only one study (White et al., Reference White, Ising, von Klitzing, Sierau, Michel, Klein and Müller-Myhsok2017), to our knowledge, investigated whether overall cortisol output measured via hair (rather than diurnal cortisol levels or cortisol reactivity) may serve as a mediator. This cross-sectional study showed reduced hair cortisol concentrations in maltreated children at 9–16 years of age, compared to nonmaltreated children (White et al., Reference White, Ising, von Klitzing, Sierau, Michel, Klein and Müller-Myhsok2017). Reductions were related to chronicity and type of maltreatment. This reduction in cortisol output mediated the association between maltreatment and externalizing symptoms (White et al., Reference White, Ising, von Klitzing, Sierau, Michel, Klein and Müller-Myhsok2017). It remains unclear, however, whether overall cortisol output can mediate associations between both earlier and later developmental stress and internalizing and externalizing symptomology in adolescence. Here we tested whether overall cortisol output measured via hair (reflecting 1–2 months of overall cortisol secretion) is a statistical mediator between childhood and adolescent parental harshness and disengagement (measured prospectively from age 1 year) and internalizing and externalizing problems at age 15.
Measuring overall cortisol output via hair
Advances in cortisol assessment have led to the development of cortisol assays using hair samples, which can reliably index overall cortisol output over time, retrospectively for at least 3 months (Gao et al., Reference Gao, Stalder, Foley, Rauh, Deng and Kirschbaum2013; Kirschbaum, Tietze, Skoluda, & Dettenborn, Reference Kirschbaum, Tietze, Skoluda and Dettenborn2009). Hair cortisol is often easier to collect than salivary measures of cortisol as it does not rely on careful sample timing and participant compliance with strict collection rules. It also is able to capture overall cortisol exposure over months, providing an integrated measure that captures both day and night secretion. This may provide a better biomarker of brain exposure to cortisol over time, which might be a critical factor in shaping longer term consequences of early-life stress experiences. This method has been validated in recently pregnant women, whose third trimester cortisol outputs are known to be higher (Kirschbaum et al., Reference Kirschbaum, Tietze, Skoluda and Dettenborn2009) and in individuals showing higher hair cortisol concentration following major life stressors (Staufenbiel, Penninx, Spijker, Elzinga, & van Rossum, Reference Staufenbiel, Penninx, Spijker, Elzinga and van Rossum2013). Hair cortisol concentration is also positively correlated with diurnal salivary cortisol concentrations (D'Anna-Hernandez, Ross, Natvig, & Laudenslager, Reference D'Anna-Hernandez, Ross, Natvig and Laudenslager2011; Flom, St. John, Meyer, & Tarullo, Reference Flom, St. John, Meyer and Tarullo2017), though it requires a number of days of saliva collection to reliably see this association, likely because saliva cortisol is more strongly influenced by state-factors that vary day to day (e.g., meals, exercise, sleep patterns, compliance with sampling timing and rules). Thus, it likely provides a better integrated measure of overall secretion.
Stress and overall cortisol output
Chronic stress and contextual factors such as lower socioeconomic status (SES) are associated with overall cortisol output measured via hair in children and adults (Gray et al., Reference Gray, Dhana, Van Der Vyver, Van Wyk, Khumalo and Stein2018; Stalder et al., Reference Stalder, Steudte-Schmiedgen, Alexander, Klucken, Vater, Wichmann and Miller2017). However, evidence is mixed with regard to the direction of these associations, with most studies reporting small positive associations between stress and hair cortisol concentration (Khoury, Enlow, Plamondon, & Lyons-Ruth, Reference Khoury, Enlow, Plamondon and Lyons-Ruth2019). However, there are several studies reporting moderately sized negative associations between stress and hair cortisol concentration (Khoury et al., Reference Khoury, Enlow, Plamondon and Lyons-Ruth2019), including the study by White et al. (Reference White, Ising, von Klitzing, Sierau, Michel, Klein and Müller-Myhsok2017) described above. Timing of adversity is a significant moderator of hair cortisol concentration, with adult trauma showing stronger positive associations with overall cortisol output than childhood trauma (Khoury et al., Reference Khoury, Enlow, Plamondon and Lyons-Ruth2019). Childhood stress is often broadly defined (e.g., maltreatment), and timing is frequently not accounted for in the wider literature on stress and HPA functioning. These issues likely contribute to discrepant findings that have been noted in studies of stress and other HPA measures such as cortisol reactivity or diurnal cortisol pattern. Prospective longitudinal studies, as reported here, could help us understand how factors like the type and timing of adversity influence overall cortisol output.
Threat versus deprivation
Recent theoretical work argues that conceptualizations of early-life stress as a unidimensional construct, with more stress leading broadly to more negative effects, are incomplete. This theoretical work suggests that stress should be conceptualized as having at least two dimensions: threat and deprivation (Humphreys & Zeanah, Reference Humphreys and Zeanah2015; McLaughlin, Sheridan, & Lambert, Reference McLaughlin, Sheridan and Lambert2014; Sheridan & McLaughlin, Reference Sheridan and McLaughlin2014). Threat refers to experiences that threaten one's physical integrity, such as physical abuse, neighborhood violence, or parental harshness. Greater threat exposure is related to atypical emotion processing and hippocampal functioning, as well as reduced ventromedial prefrontal cortex volume and/or thickness (McLaughlin et al., Reference McLaughlin, Sheridan and Lambert2014). Deprivation refers to a lack of expected and needed environmental inputs, as seen with physical or emotional parental neglect/disengagement. These researchers argue, based on both human and non-human animal studies, that there are distinct neurobiological and behavioral signatures that result from experiences of threat versus deprivation. For instance, deprivation is associated with lower gray matter volume, changes in cortical activity, and difficulties with cognition, including executive function and language (McLaughlin et al., Reference McLaughlin, Sheridan and Lambert2014; Sheridan & McLaughlin, Reference Sheridan and McLaughlin2014). Nonhuman animal studies of environmental deprivation and enrichment also demonstrate neurobiological changes in response to the degree of environmental deprivation (Van Praag, Kempermann, & Gage, Reference Van Praag, Kempermann and Gage2000; Walsh, Reference Walsh1981).
These different neurobiological effects of threat versus deprivation may alter receptor distributions and sensitivities in brain regions that modulate HPA regulation, one consequence of which may be altered sensitivity to negative feedback at multiple levels of the HPA axis. This hypothesis has been tested in relation to cortisol reactivity to threat. In the Fragile Families and Child Wellbeing Study (FFCWS), the cohort examined in the current analyses, childhood violence exposure—but not childhood social deprivation—was associated with blunted cortisol reactivity in adolescence (Peckins et al., Reference Peckins, Roberts, Hein, Hyde, Mitchell, Brooks-Gunn and Lopez-Duran2020). Similarly, another study found that exposure to interpersonal violence (threat) was associated with blunted cortisol/ dehydroepiandrosterone (DHEA) reactivity to the Trier Social Stress Test in adolescents after controlling for poverty (which the authors conceptualized as deprivation in the study; Busso, McLaughlin, & Sheridan, Reference Busso, McLaughlin and Sheridan2017). Blunted HPA reactivity mediated the association between threat and externalizing behaviors. Similar mediational work has not yet been done using overall cortisol output (measured by hair cortisol concentration). White et al. (Reference White, Ising, von Klitzing, Sierau, Michel, Klein and Müller-Myhsok2017) tested hair cortisol concentration as a mediator between maltreatment and externalizing problems, but did not separate threat and deprivation within maltreatment. In the paper, early maltreatment and neglect each showed the strongest associations with lower hair cortisol concentration (White et al., Reference White, Ising, von Klitzing, Sierau, Michel, Klein and Müller-Myhsok2017). This hypocortisolism could be a marker of allostatic load following the stress of neglect (McEwen, Reference McEwen1998), as neglect is typically a more chronic stressor occurring over longer time periods than abuse, which can be more intermittent over days and weeks, even if it occurs over long periods of time. This difference in time scales between neglect (deprivation) and abuse (threat) might explain the more frequent finding of hypocortisolism in those who have experienced chronic neglect versus abuse (Fisher, Reference Fisher2017). A recent study by Schalinski, Teicher, and Rockstroh (Reference Schalinski, Teicher and Rockstroh2019) separated maltreatment into neglect and abuse components and reported similar hair cortisol concentration findings. In this study, greater neglect at age 3 was associated with attenuated hair cortisol in adult inpatients with psychiatric conditions compared to patients with low neglect at age 3 and nonmaltreated control participants (Schalinski et al., Reference Schalinski, Teicher and Rockstroh2019). Patients with greater neglect at age 3 and low hair cortisol concentration were the most likely to show high trauma symptoms (Schalinski et al., Reference Schalinski, Teicher and Rockstroh2019).
Research examining overall cortisol output as a mediator between threat and deprivation and later psychopathology would be valuable because overall cortisol secretion and acute reactivity may reflect different regulatory dynamics. Understanding both types of regulatory dynamics may help us better delineate this complex system and more fully characterize how threat and deprivation influence the HPA axis and lead to psychopathology. This may be particularly true in developmental studies as we may see changes in one measure of HPA functioning before another. Tracing the developmental sequence may be critical to fully illuminating the pathways from risk exposures to symptom expression.
Busso et al. (Reference Busso, McLaughlin and Sheridan2017) linked altered acute cortisol reactivity to threat to earlier threat exposure, but not to deprivation, using poverty exposure as a proxy for deprivation. However, poverty is, itself, associated with neighborhood violence, domestic violence, and other types of threat (Evans, Reference Evans2004). We were interested in better isolating the impact of deprivation as defined by a lack of needed care from the environment, so we chose to use measures of social, physical, and emotional parental disengagement as it would not be confounded by threatening experiences often associated with poverty. Previous investigations have statistically separated threat and deprivation, although there are positive correlations between the two measures (Sumner, Colich, Uddin, Armstrong, & McLaughlin, Reference Sumner, Colich, Uddin, Armstrong and McLaughlin2019). In this study, we focused on parental harshness as the measure of threat and parental disengagement as the measure of deprivation. Parental harshness reflects threatening experiences by the parent such as hitting, slapping, and spanking. Parental disengagement reflects a depriving environment, including experiences such as not providing food or medical care, being too drunk or high to care for the child, and not reading to or playing games with the child. Thus, these measures broadly cover experiences of threat and deprivation via parenting.
Parental harshness and disengagement often co-occur, which makes studying unique effects on HPA activity challenging, particularly in samples that are recruited for severe abuse experiences (e.g., White et al., Reference White, Ising, von Klitzing, Sierau, Michel, Klein and Müller-Myhsok2017). Abuse is associated with higher cortisol levels closer to the onset of abuse, but may lead to down-regulation of the HPA axis in response to repeated cortisol elevations and result in lower levels of cortisol over time (Trickett, Noll, Susman, Shenk, & Putnam, Reference Trickett, Noll, Susman, Shenk and Putnam2010). Thus, we may predict high cortisol levels would be predicted following recent-onset abuse, but lower cortisol levels years later. However, in a diverse sample of urban pregnant women, childhood physical and/or sexual abuse was associated with higher hair cortisol concentration in Black women (Schreier, Enlow, Ritz, Gennings, & Wright, Reference Schreier, Enlow, Ritz, Gennings and Wright2015), suggesting that overall cortisol output may remain high for those who experienced abuse. A meta-analysis suggested that ongoing stress rather than past or absent stress is associated with higher cortisol output measured in hair (Stalder et al., Reference Stalder, Steudte-Schmiedgen, Alexander, Klucken, Vater, Wichmann and Miller2017), which could suggest that individuals exposed to high levels of parental harshness in childhood with higher cortisol output could be experiencing continued stress. Thus, adolescents with high levels of recent parental harshness may have higher hair cortisol than adolescents without recent experiences of parental harshness, though adolescents with past but not recent experiences of parental harshness may show hair cortisol levels similar to or possibly lower than those without these experiences of harshness.
Studies of neglect in children typically report cortisol hyporeactivity to stress and a flattened diurnal cortisol rhythm (Bruce, Fisher, Pears, & Levine, Reference Bruce, Fisher, Pears and Levine2009; Dozier et al., Reference Dozier, Manni, Gordon, Peloso, Gunnar, Stovall-McClough and Levine2006; Koss, Mliner, Donzella, & Gunnar, Reference Koss, Mliner, Donzella and Gunnar2016; Reilly & Gunnar, Reference Reilly and Gunnar2019; van der Vegt, Van Der Ende, Kirschbaum, Verhulst, & Tiemeier, Reference van der Vegt, Van Der Ende, Kirschbaum, Verhulst and Tiemeier2009). These studies usually involve children living in extreme situations such as foster care or children adopted from institutions (i.e., orphanages) into high-resource families. In the current study, we focused on depriving experiences termed parental disengagement, which are typically not as extreme as institutional care. Overall, associations between the HPA axis, parental harshness (threat), and parental disengagement (deprivation) are often not in the same direction, which supports the need to test parental harshness and disengagement separately in studies of HPA activity, particularly in families in the community facing high levels of adversity.
Overall cortisol output and psychopathology
Hair cortisol concentration is associated with several types of psychopathology, with different directions of effects depending on the type of psychopathology. For example, high overall cortisol output is more common in individuals with major depression and higher levels of depressive symptoms, while individuals with posttraumatic stress disorder (PTSD) and externalizing problems typically show decreased overall cortisol output (Rietschel et al., Reference Rietschel, Streit, Zhu, McAloney, Kirschbaum, Frank and Martin2016; Schalinski, Elbert, Steudte-Schmiedgen, & Kirschbaum, Reference Schalinski, Elbert, Steudte-Schmiedgen and Kirschbaum2015; Staufenbiel et al., Reference Staufenbiel, Penninx, Spijker, Elzinga and van Rossum2013; White et al., Reference White, Ising, von Klitzing, Sierau, Michel, Klein and Müller-Myhsok2017). Depression has been associated with higher cortisol reactivity to stress for individuals who have experienced maltreatment (Harkness, Stewart, & Wynne-Edwards, Reference Harkness, Stewart and Wynne-Edwards2011; Heim, Newport, Mletzko, Miller, & Nemeroff, Reference Heim, Newport, Mletzko, Miller and Nemeroff2008) and in dysphoric post-pubertal adolescents (Hankin, Badanes, Abela, & Watamura, Reference Hankin, Badanes, Abela and Watamura2010). However, some studies have reported blunted cortisol reactivity in adolescents with more severe depression regardless of maltreatment history (Harkness et al., Reference Harkness, Stewart and Wynne-Edwards2011), while others show no increases in cortisol in response to negative daily events for depressed compared to nondepressed individuals (Peeters, Nicholson, & Berkhof, Reference Peeters, Nicholson and Berkhof2003). In adolescents, higher levels of morning cortisol may predict onset of major depression (Goodyer, Herbert, Tamplin, & Altham, Reference Goodyer, Herbert, Tamplin and Altham2000), and flatter diurnal cortisol slopes are associated with past depressive episode, a recent depressive episode comorbid with an anxiety disorder, as well as current general distress (Doane et al., Reference Doane, Mineka, Zinbarg, Craske, Griffith and Adam2013).
Findings for associations between hair cortisol and anxiety are mixed. There is some evidence that individuals with generalized anxiety disorder have lower levels of hair cortisol compared to individuals without the disorder (Steudte et al., Reference Steudte, Stalder, Dettenborn, Klumbies, Foley, Beesdo-Baum and Kirschbaum2011), although results are inconsistent across studies and are likely complicated by comorbidities (Steudte-Schmiedgen et al., Reference Steudte-Schmiedgen, Wichmann, Stalder, Hilbert, Muehlhan, Lueken and Beesdo-Baum2017). A higher cortisol awakening response may predict onset of anxiety disorders in adolescents (Adam et al., Reference Adam, Vrshek-Schallhorn, Kendall, Mineka, Zinbarg and Craske2014). Comorbidity in psychopathology, particularly between depressive and anxiety symptoms, may explain some mixed findings in the literature, which is why the current study will examine several types of emotional and behavioral problems in the same model.
Meta-analytic evidence suggests that there is no association between externalizing problems and cortisol reactivity (Alink et al., Reference Alink, van Ijzendoorn, Bakermans-Kranenburg, Mesman, Juffer and Koot2008). However, externalizing problems are associated with a small but significant decrease in diurnal salivary cortisol (Alink et al., Reference Alink, van Ijzendoorn, Bakermans-Kranenburg, Mesman, Juffer and Koot2008). The association between overall cortisol output and symptomology thus appears to be complex, with different associations with differing types of psychopathology, and perhaps shaped by different pathways from early-life adversity to differentially altered HPA axis regulation (perhaps not always visible in simple measures of cortisol levels) to different types of symptom profiles.
Developmental psychopathology framework
Considerations of how the type, timing, severity, and chronicity of stress impact adaptation are central to the developmental psychopathology framework (Cicchetti & Rogosch, Reference Cicchetti and Rogosch2009; Manly, Kim, Rogosch, & Cicchetti, Reference Manly, Kim, Rogosch and Cicchetti2001). Discrepancies in associations between childhood stress and heightened or reduced cortisol levels are puzzling, and these discrepancies are likely due, at least in part, to the heterogeneity and timing of stress, as well as different methods used in each study. If different profiles of childhood risk (i.e., parental harshness vs. disengagement) show unique associations with overall cortisol output over time, which then predict different types of adolescent symptomology (internalizing vs. externalizing), researchers will have a better understanding of how childhood stress can lead to a multitude of different outcomes (multifinality). The use of prospective, rather than retrospective measurements of parental harshness and disengagement, as done in the current study, is critical in efforts to determine if and how the timing of stress may lead to differing neurobiological and behavioral consequences. Careful neurobiological work in humans and in animal models provides evidence for sensitive periods of brain development that vary by brain region and neural networks (Knudsen, Reference Knudsen2004). Differences in the timing of stress, intersecting with sensitive periods, may produce variable impacts on brain regions involved with HPA axis, and particularly on hippocampus and prefrontal cortex, which we know are influenced by development, rich in glucocorticoid receptors, and important in HPA axis regulatory control. These types of timing differences might help explain some of the discrepancies in the stress and HPA literature. The current study assessed whether prospectively measured parental harshness and parental disengagement, and the timing of each, have differential associations with overall cortisol output in adolescence with implications for mental health.
This study seeks to contribute to the developmental psychopathology framework by testing a potential pathway by which childhood stress influences the development of psychopathology in adolescence, which is essential for understanding risk and resilience. Adolescence is a time of increased risk for psychopathology (Andersen & Teicher, Reference Andersen and Teicher2008; Hankin et al., Reference Hankin, Abramson, Moffitt, Silva, McGee and Angell1998; Lewinsohn, Striegel-Moore, & Seeley, Reference Lewinsohn, Striegel-Moore and Seeley2000), so establishing risk pathways across this developmental period is particularly important. By adding overall cortisol output as a potential mediator of associations between childhood and adolescent stress and adolescent mental health, this study will expand knowledge of how stress influences regulatory control of the HPA axis and the development of brain circuits that influence integrated cortisol output and associated behavioral proclivities that get reflected in “symptoms.”
The current study
The primary aim of the study was to test whether parental harshness and disengagement during childhood and adolescence are associated with hair cortisol concentration at 15 years. Our second aim was to test whether hair cortisol concentration at 15 years is a statistical mediator between parental harshness and disengagement during childhood and adolescence and depressive symptoms, anxiety symptoms, and antisocial behaviors at 15 years. A final, exploratory aim was to test whether the timing of parental harshness and disengagement between 1 and 15 years predicts hair cortisol concentration better than the average severity of parental harshness and disengagement from 1–15 years. The prospective assessment of parental harshness and disengagement starting in infancy and the multi-informant, multimodal assessment of psychopathology in adolescence make the current sample, a subset of the FFCWS, ideal for asking these questions. The population of this study was oversampled for unwed mothers, so many more of these youth grew up in poverty and were exposed to more chronic and severe stressors compared to other studies while still having dimensional data on stress (e.g., not just maltreated vs. controls).
We hypothesized that higher cumulative parental harshness from ages 1–15 years would be associated with higher hair cortisol concentration at age 15 based on the majority of papers showing positive associations between stress and overall cortisol output (Khoury et al., Reference Khoury, Enlow, Plamondon and Lyons-Ruth2019). Due to previous research on post-institutionalized and foster care youth experiencing neglect, we predicted that higher cumulative parental disengagement from 1–15 would be associated with lower hair cortisol concentration at age 15 (Dozier et al., Reference Dozier, Manni, Gordon, Peloso, Gunnar, Stovall-McClough and Levine2006; Koss et al., Reference Koss, Mliner, Donzella and Gunnar2016). Based on previous literature, we predicted that higher hair cortisol concentration would be associated with higher depressive symptoms at age 15 and that lower hair cortisol concentration at age 15 would be associated with greater anxiety symptoms and antisocial behaviors at age 15. We hypothesized that there would be three significant indirect pathways: (a) from cumulative parental harshness to higher hair cortisol concentration to higher depressive symptoms, (b) from cumulative parental disengagement to lower hair cortisol concentration to higher anxiety symptoms, and (c) from cumulative parental disengagement to lower hair cortisol concentration to higher antisocial behaviors. We hypothesized that cumulative parental harshness and disengagement rather than parental harshness and disengagement at a specific time point (1, 3, 5, 9, or 15) would be better predictors of overall cortisol output.
Methods
Participants
The FFCWS (Reichman, Teitler, Garfinkel, & McLanahan, Reference Reichman, Teitler, Garfinkel and McLanahan2001) is a population-based study of children who were born in large US cities, oversampling for non-marital births. At the beginning of the FFCWS, 42.2% of mothers reported their household income in the past 12 months was less than or equal to $25,000, while 60.5% of mothers reported their household income less than or equal to $50,000. FFCWS families were interviewed at the focal child's birth and again at 1, 3, 5, 9, and 15 years of age. A subset of the FFCWS at the Detroit, Toledo, and Chicago sites underwent a more detailed in-person assessment at 15–17 years (M = 15.3 years) that included clinical assessments, neuroimaging, and collection of biological samples as part of the Study of Adolescent Neural Development (SAND; N = 237). A total of 175 participants provided hair samples at 15 years (the final analytic sample was 171 after removing participants with high cortisol values). Sixty-two participants did not provide hair samples for the following reasons: hair too short to collect (n = 33), youth or parent refused (n = 17), youth had braids, weave, dreadlocks or hairpiece that prevented collection (n = 6), or reason unclear (n = 6). The participants who provided a hair sample at 15 did not differ on maternal marital status at birth, depressive symptoms, anxiety symptoms, or antisocial behaviors (described below), medication use, classification as African American or multiracial versus not African American or multiracial, maternal education, poverty status at birth, and poverty status at 15 compared to participants who did not provide a hair sample, ps > .05. Adolescent females, χ2 (1) = 13.22, p < .001, and adolescents with older mothers at birth, M = 26.2 years versus 24.1 years, t(234) = −2.44, p = .02, were more likely to provide hair samples within the SAND subset. The sample used in these analyses is economically high risk (75.4% of mothers not married at birth; 40.9% reported household incomes below 100% of the poverty line at 15) and historically underrepresented in research (76% African American or Black; see Table 1 for descriptive statistics).
Table 1. Descriptive statistics.

N = 171. Calculated on participants with valid data values for overall cortisol output (within 3 SD of the mean).
Childhood parental harshness/disengagement
Our overarching goal was to create constructs that theoretically match threat and deprivation outlined in previous theoretical work (Humphreys & Zeanah, Reference Humphreys and Zeanah2015; McLaughlin et al., Reference McLaughlin, Sheridan and Lambert2014; Sheridan & McLaughlin, Reference Sheridan and McLaughlin2014). Childhood experiences of parental harshness and disengagement were reported prospectively at ages 1, 3, 5, 9, and 15 years. The mother reported experiences at 1 year, and the primary caregiver reported experiences from 3–9 years. Both the primary caregiver and focal child reported experiences at 15. Variables that closely matched our constructs of interests were the same across the ages 3, 5, and 9 waves. However, variables were different for 1 and 15 years, and new variables were chosen to most closely match parental harshness and disengagement at those ages with the available variables. All variable names, respondents, questions, and response options are included in the supplemental methods including correlations between parental harshness and disengagement at each time point and means and standard deviations of each parental harshness and disengagement composite.
Parental harshness
At age 1 year, parental harshness included frequency of spanking by mother, father, and mother's partner due to evidence from FFCWS that spanking at age 1 is developmentally inappropriate and a risk factor for later child protective services involvement (Lee, Grogan-Kaylor, & Berger, Reference Lee, Grogan-Kaylor and Berger2014). Spanking frequency was reverse-coded so that higher numbers indicate greater spanking frequency (see Supplemental materials for specific variables). This variable was a sum of how often the child was spanked in the past month (0 = never spanked, 4 = spanked every day), which was summed across a total of the three individuals (possible range: 0–12). This sum was standardized to create the age 1 parental harshness variable. At ages 3, 5, and 9, a total of 10 items assessed frequency of parental harshness from the Conflict Tactics Scale corporal punishment and psychological aggression subscales (Straus, Hamby, Finkelhor, Moore, & Runyan, Reference Straus, Hamby, Finkelhor, Moore and Runyan1998), including items such as slapping, shaking, pinching, hitting, spanking, threatening, screaming at, and swearing at the child. Items were re-coded in the following way such that higher numbers indicate greater frequency of parental harshness: 0 = never happened in the past year, 1 = once, 2 = twice, 3 = 3–5 times, 4 = 6–10 times, 5 = 11–20 times, 6 = >20 times. All 10 items were summed within each time point to create parental harshness at 3, 5, and 9 years. These sums were then standardized. Reliability for the 10 items of parental harshness from the Conflict Tactics Scale was α = 0.81 at 3 and 5 years and was α = 0.96 at 9 years. At 15 years, the focal child was asked two questions from the Conflict Tactics Scale about how often their primary caregiver does the following: (a) shouts, yells, screams, swears, or curses at you; (b) hits or slaps you. Replies were 1 = never, 2 = sometimes, and 3 = often. The primary caregiver was also asked the same two questions about their behavior towards the child with the same response options. These four variables were standardized and the mean was calculated. This mean was standardized for the parental harshness at 15 years variable. The cumulative parental harshness variable was a mean of the five standardized parental harshness variables from ages 1–15.
Parental disengagement
The neglect scale of the Conflict Tactics Scale that was measured at 3, 5, and 9 years was identified as being a valid measure of more severe parental disengagement in FFCWS. As this scale was not given at ages 1 and 15 years, we chose items relevant to parental disengagement that were in line with the Conflict Tactics Scale neglect scale described below. Parental disengagement at 1 year was a measure of seven variables chosen among all questions at this time point as being relevant to parental disengagement. Questions include six variables that have been previously used as part of a scale to assess maternal engagement in FFCWS that were also relevant to our measure of parental disengagement (α = .66; Turney & McLanahan, Reference Turney and McLanahan2015) and one to assess lack of food for the child: (a) how often the mother played games like “peek-a-boo” and “gotcha” with the focal child (recoded as 0 = every day, 1 = several times per week, 2 = several times per month, 3 = 1–2 times per month, 4 = never); (b) how often the mother sang songs or nursery rhymes to the child (coded as above); (c) how often the mother read stores to the child (coded as above); (d) how often the mother told stories to the child (coded as above); (e) how often the mother played inside with toys such as blocks or Lego with the child (coded as above); (f) how frequently the mother hugged or showed physical affection toward the focal child (coded as above); (g) whether the child went hungry in the past year (coded as 0 = no, 1 = yes). These variables were standardized, and the mean was disengagement at age 1 year. Disengagement is in part defined as a lack of cognitive stimulation and sensory, motor, linguistic, and social input (McLaughlin, Sheridan, & Nelson, Reference Mclaughlin, Sheridan and Nelson2017), which is in line with what the maternal engagement scale measures (e.g., no hugs, no reading or telling stories to child, no or playing games with child). Particularly at 1 year of age, parental engagement is a main source of cognitive, sensory, linguistic, and social input as it is more difficult for infants to navigate their world without parents. With the addition of the hunger question, this composite represents sensory, cognitive, linguistic, physical, emotional, and social disengagement at 1 year. At ages 3, 5, and 9, a total of five items assessed frequency of disengagement using the neglect scale of the Conflict Tactics Scale (Straus et al., Reference Straus, Hamby, Finkelhor, Moore and Runyan1998), including items such as not being able to express their love to their child, not able to give the child food or medical care, leaving the child alone, and being too drunk or high to care for their child. Items were re-coded in the following way such that higher numbers indicate greater frequency of disengagement: 0 = never happened in the past year, 1 = once, 2 = twice, 3 = 3–5 times, 4 = 6–10 times, 5 = 11–20 times, 6 = >20 times. All five items were summed within each time point to create disengagement at 3, 5, and 9 years. These sums were then standardized. The reliability for the neglect scale from age 3–9 years is low-to-moderate (α = .47 at 3 years; α = .15 at 5 years; α = .56 at 9 years), which is consistent with prior studies and is expected based on skewed distributions and reporting on low-baseline events (Guterman, Lee, Taylor, & Rathouz, Reference Guterman, Lee, Taylor and Rathouz2009; Straus et al., Reference Straus, Hamby, Finkelhor, Moore and Runyan1998). However, the measure does have temporal consistency (r = .26–.36, p < .001, for correlations of the neglect scale from ages 3, 5, and 9 years). Parental disengagement at 15 years was measured by six items, five of which were reported by the primary caregiver, and one reported by the focal child. These items were not part of an established questionnaire but were chosen among all possible questions as relevant to disengagement at 15. Primary caregiver questions on substance use asked about (a) how often drinking interfered with responsibilities in past year; (b) how often the caregiver had problems with people because of drinking in past year; (c) how often illegal drug use interfered with responsibilities in past year; and (d) how often the caregiver had problems with people in the past year due to illegal drug use. Answers were coded as 0 = never, 1 = one time, or 2 = more than one time. Parental substance abuse is a risk factor for poorer offspring mental health and drug and alcohol problems and is also a risk factor for child maltreatment (Osborne & Berger, Reference Osborne and Berger2009; Walsh, MacMillan, & Jamieson, Reference Walsh, MacMillan and Jamieson2003), and the items used in the current study specifically assess functional impairment rather than substance use in general.Footnote 1 The caregiver was also asked whether the youth had had a regular check-up or well visit with a health professional in the past 12 months, which was coded as 1 = yes and 2 = no. The child was asked how often he or she spends time alone in his or her home without an adult present. Answers were coded as 0 = never or sometimes, 1 = often. Being alone at home often can lead to the failure of the parent to provide for their youth's physical and emotional needs (Hymel, Reference Hymel2006). These six items were z scored, and the mean of the z scored items was used for the age 15 disengagement variable. The cumulative parental disengagement variable was a mean of the five standardized disengagement variables from ages 1–15. This conceptualization of disengagement is consistent with previous tests of deprivation, with physical and emotional disengagement as well as food insecurity as measures of deprivation (Sumner et al., Reference Sumner, Colich, Uddin, Armstrong and McLaughlin2019).
Adolescent mental health
Depressive symptoms, anxiety symptoms, and antisocial behavior were assessed using a multi-informant, multimethod latent variable approach. Continuous measures of psychopathology rather than diagnoses of psychopathology were used to better capture the dimensional nature of psychopathology (Cuthbert, Reference Cuthbert2014). Latent variables represent shared variances in the observed variables to measure the underlying concept of interest (Bollen, Reference Bollen2002).
Depressive symptoms
We assessed depressive symptoms at 15 using a latent variable that consisted of the following scales: (a) youth-reported Mood and Feelings Questionnaire (MFQ) total (Wood, Kroll, Moore, & Hararington, Reference Wood, Kroll, Moore and Harrington1995) which asked about the youth's feelings in the past 2 weeks and were rated on a 3-point scale (0 = not true, 1 = sometimes true, 2 = true; α = 0.91); (b) parent-reported MFQ total (α = 0.92); (c) the youth-report Children's Depression Inventory (CDI) total (Saylor, Finch, Spirito, & Bennett, Reference Saylor, Finch, Spirito and Bennett1984) which used 3-point Likert scales to assess which option best described the youth's feelings over the past 2 weeks (α = 0.86); (d) parent-reported CDI total which used 4-point Likert scales (0 = not at all, 1 = sometimes, 2 = often, 3 = most times) (α = 0.84); (e) total current symptom count including subclinical symptoms (0 = not present; 1 = present at subclinical levels; 2 = present at clinical threshold) of Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5) major depressive disorder from the Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS), which was completed by the parent and youth; and (f) total current symptom count of DSM-5 persistent depressive disorder (dysthymia) from the K-SADS. K-SADS scores were determined by clinician ratings on a modified version of the K-SADS (KSADS; Kaufman et al., Reference Kaufman, Birmaher, Brent, Rao, Flynn, Moreci and Ryan1997). The K-SADS is a semi-structured interview that has high test–retest reliability in establishing psychiatric diagnoses and has good validity with standard self-report measures of symptoms such as the Child Behavior Checklist (Kaufman et al., Reference Kaufman, Birmaher, Brent, Rao, Flynn, Moreci and Ryan1997). A trained clinical interviewer, typically a psychology doctoral student or post-baccalaureate staff member, administered the K-SADS separately to the youth and primary caregiver. Interviewers were trained using practice interviews and live supervision of interviews. All diagnostic interviews (100%) were reviewed during weekly team diagnostic meetings that included all clinicians and two faculty investigators who are licensed clinical psychologists with decades of experience with the K-SADS. Final current symptoms counts were derived by consensus of the clinical team using best-estimate procedures (Maziade et al., Reference Maziade, Roy, Fournier, Cliche, Mérette, Caron and Dion1992) based on the parent and child report. Consensus diagnosis is the gold standard reliability check, and since the consensus diagnosis was performed on all cases and for every symptom, there was no need to calculate inter-rater reliability within the sample. The CDI shows good internal consistency and is able to identify children with emotional distress (Saylor et al., Reference Saylor, Finch, Spirito and Bennett1984). The MFQ shows acceptable reliability, is a satisfactory screen for major depressive disorder, and is able to identify clinical remission (Wood et al., Reference Wood, Kroll, Moore and Harrington1995).
Latent factor scores were extracted in Mplus and used in the current analyses.Footnote 2 Factor scores were calculated instead of calculating the latent variable within the model due to problems with model fit. There was a high (r > .95) correlation between the child report of the MFQ and the SCARED, which caused the depressive and anxiety symptoms latent variables to be correlated at greater than 1. Extracting latent factor scores in separate models eliminated this problem such that the final depressive and anxiety factor scores were correlated at 0.27, p < .01, in bivariate analyses (Table 2), which did not negatively impact model fit. The model fit for the depressive symptoms confirmatory factor analysis (CFA) was excellent (comparative fit index (CFI) = .99, root mean square error of approximation (RMSEA) = .03, standardized root mean square residual (SRMR) = .03). All individual variables were significantly associated with the latent depressive symptoms variable, p < .01. Standardized factor loadings for each of the variables on the latent depressive symptoms factor follow: MFQ child report (0.54, p < .001), MFQ parent report (0.32, p < .001), CDI child report (0.83, p < .001), CDI parent report (0.33, p < .001), K-SADS major depressive episode (0.36, p < .001), and K-SADS persistent depressive disorder (0.17, p = .004).
Table 2. Correlation table.
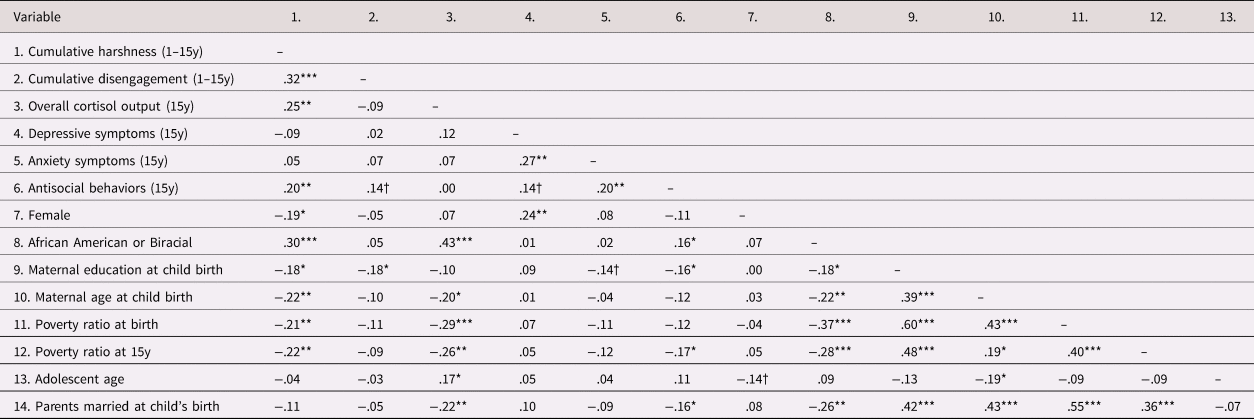
N = 171. Correlations computed using SPSS v25. †p < .10, *p < .05. **p < .01. ***p < .001.
Anxiety symptoms
We assessed anxiety symptoms at age 15 using a latent variable that consisted of the following scales: (a) youth-reported Screen for Child Anxiety Related Emotional Disorders (SCARED) (Hale, Raaijmakers, Muris, & Meeus, Reference Hale, Raaijmakers, Muris and Meeus2005) (α = 0.92); (b) parent-reported SCARED (α = 0.88); (c) anxiety symptoms scale from the Child Behavior Checklist (CBCL; Achenbach & Edelbrock, Reference Achenbach and Edelbrock1991) where items were rated by parents on a 3-point scale (0 = not true, 1 = somewhat or sometimes true, 2 = very true or often true) (α = 0.78); and (d) total current symptom count rated on a 3-point scale (0 = not present; 1 = present at subclinical levels; 2 = present at clinical threshold) of DSM-5 social phobia from the K-SADS. K-SADS scores were determined using the same consensus meetings as for depressive symptoms above. The SCARED demonstrates good internal consistency and discriminant validity between anxiety disorders and other disorders (Birmaher et al., Reference Birmaher, Khetarpal, Brent, Cully, Balach, Kaufman and Neer1997). The CBCL is a widely used measure with good internal consistency, and the anxiety scale of the CBCL predicts DSM-IV diagnoses, demonstrating external validity (Achenbach & Edelbrock, Reference Achenbach and Edelbrock1991; Ferdinand, Reference Ferdinand2008).
Latent factor scores were extracted in Mplus and used in the analyses. Model fit for the anxiety symptoms CFA was excellent (CFI = 1.00, RMSEA = .00, SRMR = .00). The SCARED child and parent report and the CBCL were all significantly associated with the anxiety factor, while the K-SADS had a p value of 0.14. The K-SADS was left in the model due to its theoretical relevance to the construct. Standardized factor loadings for each of the variables on the latent anxiety symptoms factor follow: SCARED child report (0.17, p = .04), SCARED parent report (0.99, p < .001), CBCL (0.60, p < .001), K-SADS (0.11, p = .14).
Antisocial behavior
We assessed age-15 antisocial behavior via a latent variable approach using Mplus (Muthén & Muthén, Reference Muthén and Muthén2014): (a) the aggression scale of the CBCL (α = 0.89) and (b) the rule-breaking scale of the CBCL (α = 0.82); (c) total score (excluding substance use items) from the youth-reported 62-item Self Report of Delinquency (SRD) (Elliott, Huizinga, & Ageton, Reference Elliott, Huizinga and Ageton1985) (α = 0.87); and (d) total current symptom count of DSM-5 conduct disorder and oppositional defiant disorder rated on a 3-point scale (0 = not present; 1 = present at subclinical levels; 2 = present at clinical threshold). KSADS scores were determined using the same consensus meetings as for depressive and anxiety symptoms above. The SRD demonstrates good validity, with individuals who have been arrested or have gone to court showing higher SRD scores than those without these experiences (Huizinga & Elliott, Reference Huizinga and Elliott1986).
Latent factor scores were extracted in Mplus and used in the current analyses. Model fit for the antisocial behavior CFA was excellent (CFI = 1.00, RMSEA = .07, SRMR = .01). Standardized factor loadings for each of the variables on the latent antisocial behavior factor follow: CBCL aggression (0.70, p < .001), CBCL rule breaking (0.76, p < .001), SRD (0.49, p < .001), and K-SADS (0.87, p < .001).
Overall cortisol output via hair
Hair samples were collected via standardized procedures at 15 years. A research assistant (RA) explained the procedure to participants, answered any questions they had to ensure understanding, and obtained consent. Participants then filled out a questionnaire about hair treatments such as straightening, coloring, and frequency of hair washing. A small section of hair at the posterior vertex of the head was tied with thread. The RA then cut the tied strands as close to the scalp as possible without pulling the hair. This procedure was repeated two more times in slightly different locations around the posterior vertex. The collected hair was placed in foil, and the thread was taped to the foil with the scalp-near ends together and clearly labeled. Foil was folded without folding any hair and stored at room temperature until all samples could be shipped together to Dr. Clemens Kirschbaum's lab at Technische Universität Dresden.
A liquid chromatography tandem mass spectrometry-based method was used to quantify hair cortisol concentration in hair samples (Gao et al., Reference Gao, Stalder, Foley, Rauh, Deng and Kirschbaum2013). If there was enough hair volume in 1 cm of hair for the assay, 1 cm was used. If there was not enough volume within a 1 cm segment, 2 cm were used. We chose to include participants with at least 1 cm of hair to avoid biasing the sample, which would have excluded any participants with short hair. Of the 171 with valid cortisol data, 126 samples used 1 cm of hair and 45 samples used 2 cm. Concentrations were calculated as picograms of cortisol per milligram of hair. All hair was washed with isopropanol. Steroid hormones were extracted by methanol incubation from 10 mg whole, nonpulverized hair. A column switching strategy was applied for online solid phase extraction. Then, analyte detection on an AB Sciex API 5000 QTrap mass spectrometer was conducted. Intra- and inter-assay coefficients of variation (CVs) ranged from 3.7 and 9.1%. Quantification limits were below (or equal to) 0.1 pg/mg. All samples were run in a single batch. If lab personnel were concerned about a high or low value and there was enough hair left after the first assay, a second assay was conducted. Cortisol values were ln-transformed for analysis to correct for skewness and values above 3SD were removed (four values total, yielding 171 participants with valid cortisol data).
Data analytic plan
Cumulative (ages 1–15) parental harshness and disengagement model
A correlation table of study variables is shown in Table 2. Longitudinal structural equation modeling with bootstrapping (10,000 iterations) in Mplus was used to test whether hair cortisol concentration across 1–2 months mediated the association between parental harshness and disengagement from 1–15 years and depressive symptoms, anxiety symptoms, and antisocial behavior problems. Model fit was examined (Hu & Bentler, Reference Hu and Bentler1999; Kline, Reference Kline2015) using the CFI (>.93), RMSEA (<.06), and SRMRs (<.08). The following were tested as covariates for parental harshness, disengagement, hair cortisol concentration, depressive symptoms, anxiety symptoms, and antisocial behavior: poverty ratio at focal child's birth (mother's household income/poverty threshold), sex, race/ethnicity (African American or multiracial vs. not African American or multiracial; initial analyses demonstrated that these groups showed higher cortisol levels than other groups), maternal education (1 = less than high school, 2 = high school or equivalent, 3 = some college, 4 = college degree or more), mother's marital status (1 = married or 0 = not married), and mother's age at focal child's birth. For hair cortisol concentration, depressive symptoms, anxiety symptoms, and antisocial behavior, the following covariates were tested in addition to those above: poverty at 15 (1 = 0–49% of poverty line, 2 = 50–99%, 3 = 100–199%, 4 = 200–299%, 5 = 300% +), and current age. Guided by meta-analyses of determinants of hair cortisol concentration in children and adults (Gray et al., Reference Gray, Dhana, Van Der Vyver, Van Wyk, Khumalo and Stein2018; Stalder et al., Reference Stalder, Steudte-Schmiedgen, Alexander, Klucken, Vater, Wichmann and Miller2017), we also tested whether hair treatments such as frequency of hair washing, use of chemical treatment on hair, or straightening, blow drying, and hair curling (1 = uses treatment, 0 = does not use treatment), medication use (0 = no medications or no medications with effects on cortisol, 1 = medication with possible effects on cortisol, and 2 = medication with known effects on cortisol [e.g., inhalers, birth control, psychotropic medications]) (Granger, Hibel, Fortunato, & Kapelewski, Reference Granger, Hibel, Fortunato and Kapelewski2009), length of hair used (1 cm vs. 2 cm), and body mass index (BMI) z score predicted hair cortisol concentration. To enhance model parsimony, a covariate was kept in the model if it showed an association with the variable at p < .10. A covariate that was associated with one variable may not be associated with other variables in the model and paths with p values greater than .10 were removed (final covariates shown in Table 3).
Table 3. Estimates of direct pathways from cumulative parental harshness and parental disengagement from 1–15y to 15y depressive symptoms, anxiety symptoms, and antisocial behaviors.
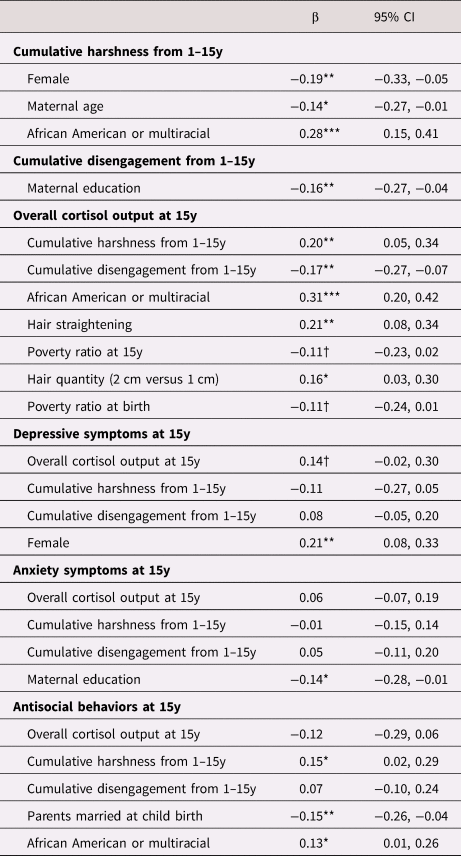
Note. All estimates reported are standardized estimates and 95% confidence intervals (CI) for each of the direct pathways to behavior problems. Dependent variables are in bold with independent variables and associated standardized (β) coefficients. Note. Covariates were included in the final model if they were associated with the model variable at p < .10. †p < 0.10, *p < .05. **p < .01. ***p < .001.
Cross-lagged timing of parental harshness and disengagement model
Secondary analyses were conducted to understand whether parental harshness or disengagement at a particular time point (1, 3, 5, 9, or 15) was driving any associations between parental harshness or disengagement and hair cortisol concentration at 15 years. A cross-lagged model was conducted with parental harshness and disengagement at each of the time points predicting hair cortisol concentration as a mediator and depressive symptoms, anxiety symptoms, and antisocial behaviors as dependent variables. Paths from parental harshness and disengagement at the previous time point were tested, and within-time paths between parental harshness and disengagement were tested. Covariates were pared in the same manner as above, and parental harshness and disengagement variables not associated with hair cortisol concentration, depressive symptoms, anxiety symptoms, and antisocial behaviors were pared in the same way to understand unique associations with parental harshness and disengagement at a particular time point with hair cortisol concentration and mental health symptoms (final covariates shown in Table 4).
Table 4. Estimates of direct pathways in the cross-lagged model from parental harshness and parental disengagement at ages 1–15 years to age 15 depressive symptoms, anxiety symptoms, and antisocial behaviors.

Note. All estimates reported are standardized estimates and 95% confidence intervals (CI) for each of the direct pathways to behavior problems. Dependent variables are in bold with independent variables and associated standardized (β) coefficients. Note. Covariates were included in the final model if they were associated with the model variable at p < .10. †p < 0.10, *p < .05. **p < .01. ***p < .001.
Sex differences and curvilinear analyses
Sex differences in the cumulative model were tested. Curvilinear analyses were conducted in the cumulative model to understand whether high levels of parental harshness or disengagement were associated with either heightened or reduced hair cortisol concentration (quadratic) rather than with only high or low levels (linear). The ln-transformed hair cortisol concentration was centered and squared for this analysis. The model remained the same as the cumulative parental harshness and disengagement from 1–15 model described above except that the squared log-transformed cortisol variable was also added as a mediator in addition to the log-transformed cortisol variable (see Supplemental information).
Results
Parental harshness, disengagement, and hair cortisol concentration
Greater cumulative parental harshness from 1–15 predicted higher hair cortisol concentration at 15 (β = 0.20, 95% CI: 0.05 to 0.34, p = .007), while greater cumulative disengagement from 1–15 predicted lower hair cortisol concentration (β = −0.17, 95% CI: −0.27 to −0.07, p = .001; Figures 1 and 2). This model demonstrated excellent fit (CFI = 1.00, RMSEA = 0.00, SRMR = .04). R2 values for the following key endogenous variables are as follows: hair cortisol concentration (0.33), depressive symptoms (0.08), anxiety symptoms (0.03), and antisocial behavior (0.09).
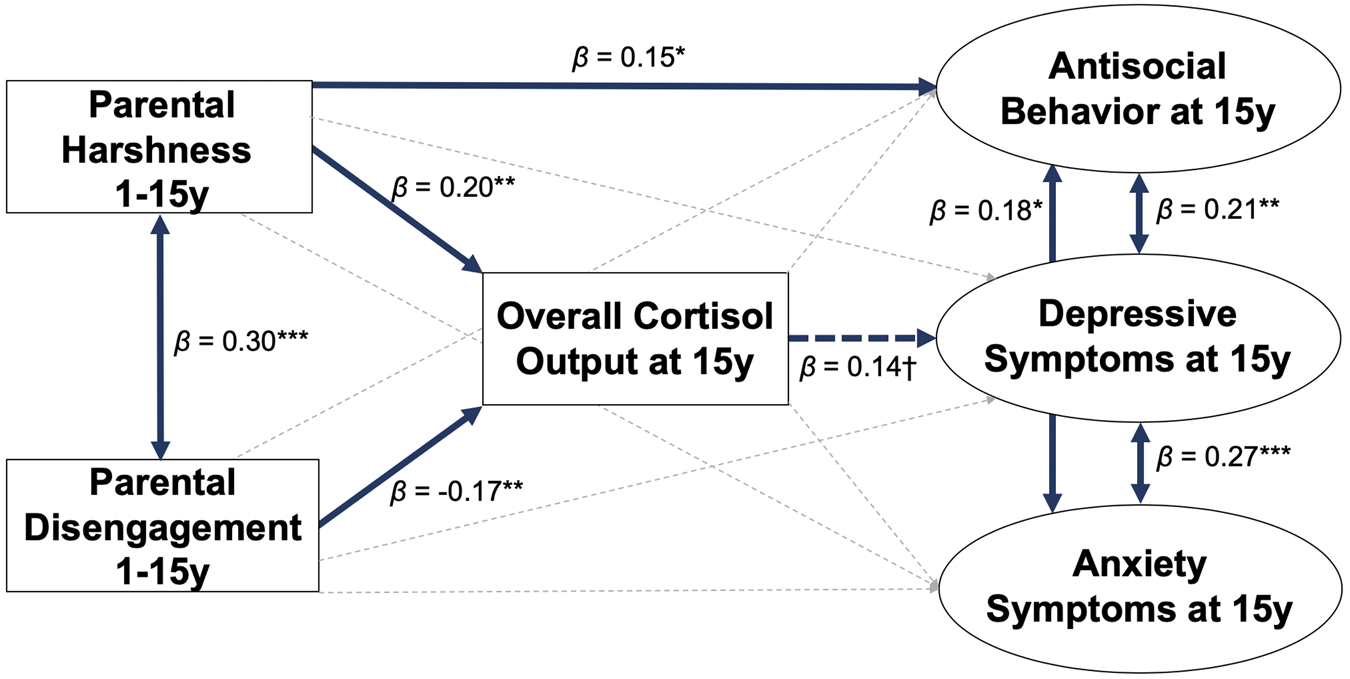
Figure 1. Longitudinal structural equation model testing overall cortisol output at 15 years as a mediator between cumulative parental harshness and disengagement from 1–15 and depressive symptoms, anxiety symptoms, and antisocial behaviors at 15 years. Values presented are standardized coefficients. Solid thick blue lines represent significant pathways (p < .05), dotted thick blue lines represent pathways with p between 0.05 and 0.10, while gray dotted thin lines represent non-significant pathways. ***p < 0.001, **p < 0.01, *p < 0.05, †p < 0.10.
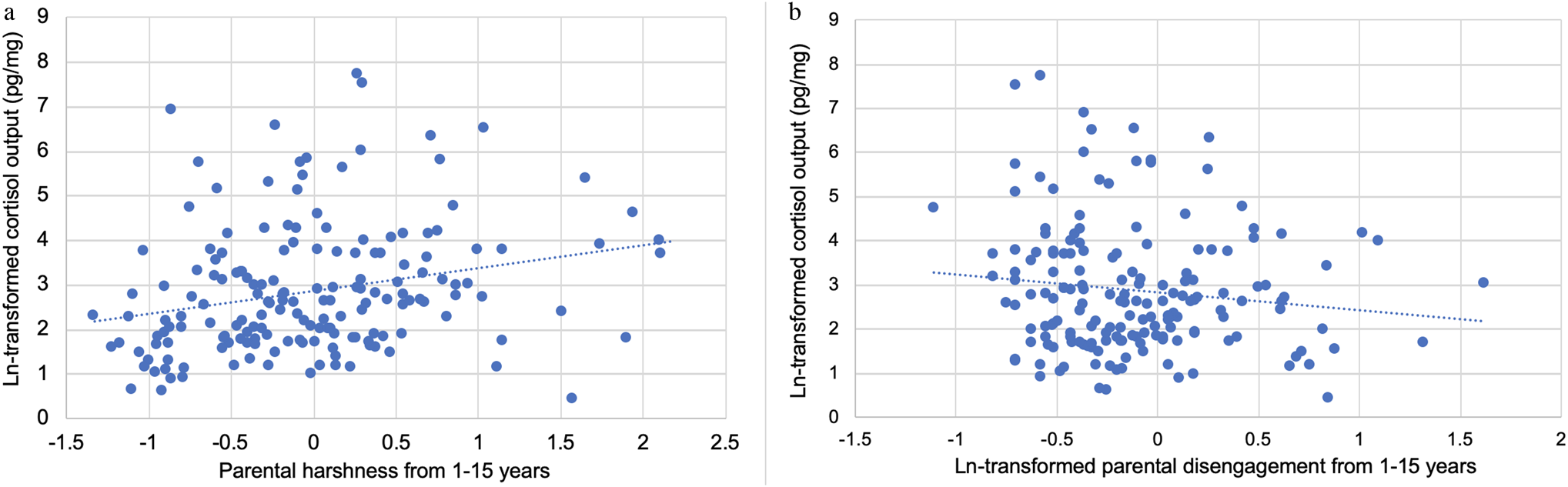
Figure 2. Scatter plot of natural log-transformed hair cortisol concentration with a) parental harshness from 1–15 years (mean of z-scored parental harshness from 1, 3, 5, 9, and 15 years), and b) natural log-transformed (due to skewness) parental disengagement from 1–15 years (mean of z scored parental disengagement from 1, 3, 5, 9, and 15 years).
Hair cortisol concentration and mental health
In the same model, higher hair cortisol concentration at 15 was not significantly associated with depressive symptoms (β = 0.14, 95% CI: −0.02 to 0.30, p = .08), anxiety symptoms (β = 0.06, 95% CI: −0.07 to 0.19, p = .36) or antisocial behaviors (β = −0.12, 95% CI: −0.29 to 0.06, p = .18). None of the indirect paths from cumulative parental harshness or disengagement to depressive symptoms, anxiety symptoms, or antisocial behaviors through hair cortisol concentration were statistically significant, ps > .05. Cumulative parental harshness from 1–15 directly predicted greater age 15 antisocial problems (β = 0.15, 95% CI: 0.02 to 0.29, p = .03) above other paths in the model. Parental harshness and disengagement showed positive covariance (β = 0.30, 95% CI: 0.15 to 0.44, p < .001). Depressive symptoms were positively associated with anxiety symptoms (β = 0.27, 95% CI: 0.12 to 0.41, p < .001), and antisocial behaviors (β = 0.21, 95% CI: 0.06 to 0.36, p = .006). Anxiety symptoms were positively associated with antisocial behaviors (β = 0.18, 95% CI: 0.02 to 0.34, p = .03).
Timing
Results of the timing analysis demonstrated that parental harshness and disengagement at two time points were most highly associated with hair cortisol concentration at 15 years. Greater disengagement at 1 year was associated with lower hair cortisol concentration (β = −0.13, 95% CI: −0.20 to −0.05, p = .002), and greater parental harshness at 15 years was associated with higher hair cortisol concentration (β = 0.14, 95% CI: 0.02 to 0.26, p = .02; Figure 3). Greater disengagement at 1 year was directly associated with greater depressive symptoms at 15 years (β = 0.11, 95% CI: 0.003 to 0.22, p = .04). Greater disengagement at 3 years (β = 0.20, 95% CI: 0.03 to 0.38, p = .02) and at 15 years (β = 0.17, 95% CI: 0.02 to 0.31, p = .02) was associated with greater anxiety at 15 years, though greater parental harshness at 3 years was associated with lower anxiety at 15 years (β = −0.21, 95% CI: −0.38 to −0.05, p = .01). Greater parental harshness at 15 years was directly associated with more antisocial behaviors at 15 years (β = 0.40, 95% CI: 0.23 to 0.56, p < .001). This model demonstrated good fit (CFI = 0.94, RMSEA = 0.03, SRMR = .06). R2 values for the key endogenous variables are as follows: hair cortisol concentration (0.32), depressive symptoms (0.10), anxiety symptoms (0.08), and antisocial behavior (0.22). These findings suggest that disengagement at 1 year and parental harshness at 15 years may be driving the associations of ages 1–15 parental harshness and disengagement with hair cortisol concentration at 15 years. Results of the model also suggest that parental harshness at 15 years may be driving the association between ages 1–15 parental harshness and greater antisocial behaviors at 15 years.
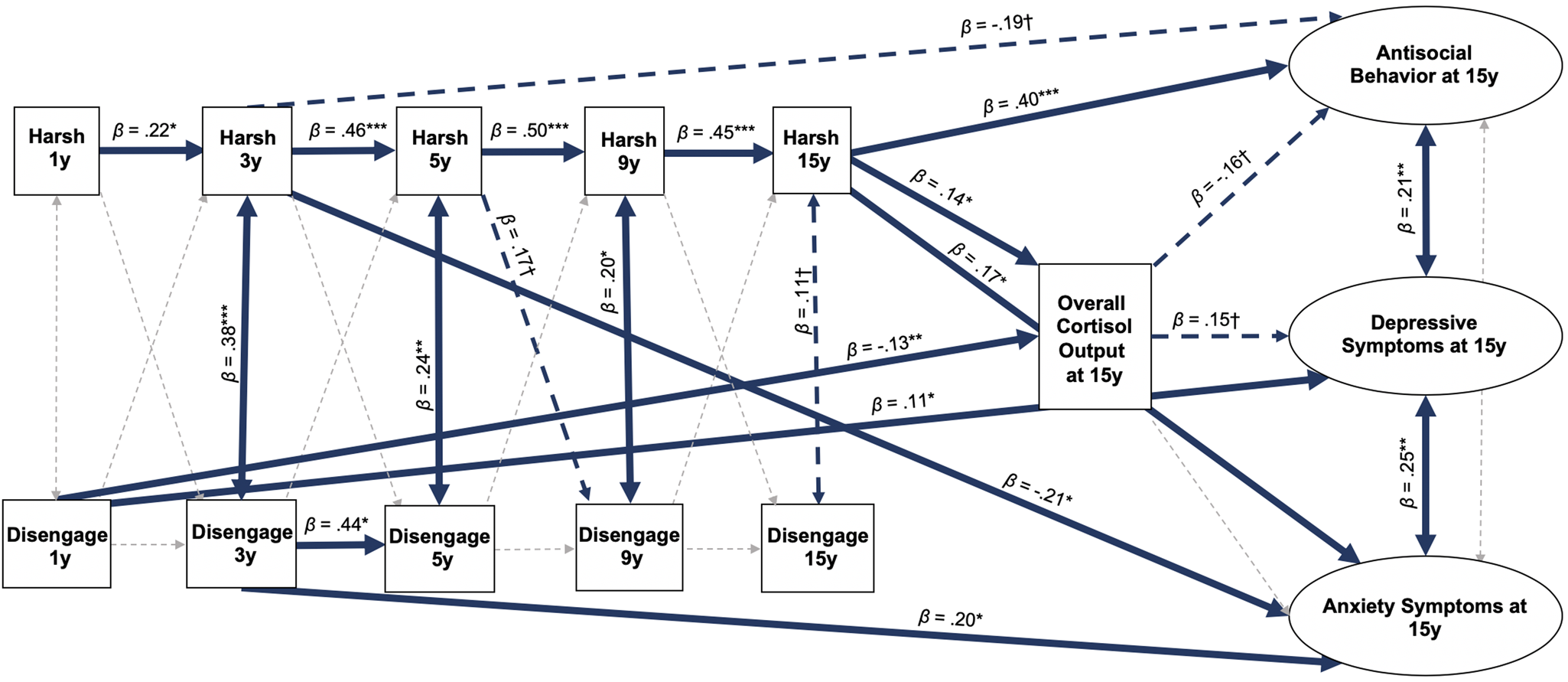
Figure 3. Cross-lagged structural equation model testing overall cortisol output at 15 years as a mediator between parental harshness and parental disengagement at 1, 3, 5, 9, and 15 years and depressive symptoms, anxiety symptoms, and antisocial behaviors at 15 years. Values presented are standardized coefficients. Solid thick blue lines represent significant pathways (p < .05), dotted thick blue lines represent pathways with p between 0.05 and 0.10, while gray dotted thin lines represent nonsignificant pathways. ***p < .001, **p < .01, *p < .05, †p < .10. Harsh = Parental harshness; Disengage = Parental disengagement.
Sex differences
Models where the five main pathways (parental harshness and disengagement to cortisol, cortisol to depressive symptoms, anxiety symptoms, and antisocial behaviors) were constrained between males and females one at a time, each in separate models, were compared to a model where all paths were free to vary by sex suggested that there were no differences in model fit, all p > .05. Thus, there were no significant sex differences between any of the main paths in the model (see Supplemental information).
Curvilinear analyses
As standardized regression estimates cannot be interpreted in the same way for power terms, the unstandardized results were used. These analyses revealed no curvilinear associations between hair cortisol concentration and parental harshness from 1–15, p = .33, or disengagement from 1–15, p = 0.09. There were no curvilinear associations between hair cortisol concentration and depressive symptoms, p = .78, anxiety symptoms, p = .84, or antisocial behaviors, p = .88. There were also no increases in R2 values for any of the mental health variables with the addition of the squared cortisol value, and the R2 value for the squared cortisol value was not statistically significant. This evaluation of R2 values suggests that the addition of the quadratic cortisol term did not improve model fit (Cohen, Cohen, West, & Aiken, Reference Cohen, Cohen, West and Aiken2003) (see Supplemental information for more model results).
Discussion
The current study demonstrated that cumulative parental harshness from ages 1–15 years was associated with higher overall cortisol output at 15, while cumulative parental disengagement from 1–15 predicted lower overall cortisol output at 15. Higher hair cortisol concentration at 15 (representing overall cortisol output over the past 1–2 months) was not significantly associated with depressive symptoms, anxiety symptoms, or antisocial behaviors at 15 years. There was a timing effect such that age 15 parental harshness was particularly associated with higher hair cortisol concentration, while greater parental disengagement at age 1 uniquely predicted lower hair cortisol concentration at age 15. These findings add to knowledge of how different types of childhood stress are associated with overall cortisol output. The longitudinal nature of the study and prospective assessment of parental harshness and disengagement make these results particularly informative, given known discrepancies between prospective and retrospective reports of maltreatment (Baldwin, Reuben, Newbury, & Danese, Reference Baldwin, Reuben, Newbury and Danese2019).
Most studies of psychosocial stress and hair cortisol concentration show positive associations (Khoury et al., Reference Khoury, Enlow, Plamondon and Lyons-Ruth2019; Staufenbiel et al., Reference Staufenbiel, Penninx, Spijker, Elzinga and van Rossum2013), which are consistent with our finding of greater cumulative parental harshness from ages 1–15 predicting higher hair cortisol concentration at 15. Our analyses of timing effects suggest that age 15 parental harshness may have stronger associations with hair cortisol concentration than parental harshness at other time points, pointing to adolescence—or the past year—as periods during which parental harshness may shape adolescent HPA functioning. This finding is in line with the idea that a harsh and threatening environment over time may produce more occasions for the HPA axis to respond to and recover from stressors, which may shape the HPA axis and receptors over time to affect cortisol output at age 15. A harsh environment may also shape the brain's responses to threat. For example, high levels of threat may lead to changes in threat detection and threat circuitry (Dannlowski et al., Reference Dannlowski, Kugel, Huber, Stuhrmann, Redlich, Grotegerd and Baune2013; Pollak & Tolley-Schell, Reference Pollak and Tolley-Schell2003), which could lead individuals to selectively attend to negative stimuli, process neutral stimuli as threatening, and respond accordingly. There is preliminary evidence that threat exposure is associated with increased stress perception in females, which is then associated with higher morning cortisol levels (LoPilato et al., Reference LoPilato, Addington, Bearden, Cadenhead, Cannon, Cornblatt and Tsuang2019), suggesting a possible mechanism by which threat may lead to heightened cortisol secretion. Heightened threat may also alter emotion processing such that individuals may have more negative emotions or trouble regulating emotion (Kim & Cicchetti, Reference Kim and Cicchetti2010; Kim et al., Reference Kim, Evans, Angstadt, Ho, Sripada, Swain and Phan2013). A chronically harsh and threatening environment could lead to individuals having difficulties with returning to baseline after responding to frequent stressors which could lead to higher cortisol output. These more regular perturbations may also lead to problems with the system's regulatory dynamics, which could lead to a chronic stress response if glucocorticoid receptors remain occupied (Gunnar & Vazquez, Reference Gunnar, Vazquez, Cicchetti and Cohen2006; Gunnar, Doom, & Esposito, Reference Gunnar, Doom, Esposito, Lamb and Lerner2015). Heightened threat has been associated with changes in the hippocampus, ventromedial prefrontal cortex, and sometimes the amygdala (Gold et al., Reference Gold, Sheridan, Peverill, Busso, Lambert, Alves and McLaughlin2016; Hanson et al., Reference Hanson, Nacewicz, Sutterer, Cayo, Schaefer, Rudolph and Davidson2015), all of which have indirect inputs to the HPA axis and could lead to chronic activation and higher cortisol output (Herman et al., Reference Herman, McKlveen, Ghosal, Kopp, Wulsin, Makinson and Myers2016; Ulrich-Lai & Herman, Reference Ulrich-Lai and Herman2009). There is evidence that high cortisol output over time can lead to down-regulation of the HPA axis at one or more levels of the system as an adaptation (De Bellis et al., Reference De Bellis, Chrousos, Dorn, Burke, Helmers, Kling and Putnam1994). Thus, it could be that continued parental harshness may be associated with lower cortisol output later in life in these same adolescents that are currently showing higher cortisol output.
Parental disengagement at 1 year showed negative associations with hair cortisol concentration at 15. This finding is consistent with literature on post-institutionalized and foster care youth who show decreased output of cortisol (Dozier et al., Reference Dozier, Manni, Gordon, Peloso, Gunnar, Stovall-McClough and Levine2006; Koss et al., Reference Koss, Mliner, Donzella and Gunnar2016; van der Vegt et al., Reference van der Vegt, Van Der Ende, Kirschbaum, Verhulst and Tiemeier2009). Similarly, a recent meta-analysis reported that child maltreatment is associated with low morning cortisol output, which could partially contribute to lower overall secretion (Bernard, Frost, Bennett, & Lindhiem, Reference Bernard, Frost, Bennett and Lindhiem2017). The negative association is also in line with a recent paper demonstrating that maltreatment in childhood is associated with lower overall cortisol output in middle childhood to adolescence (White et al., Reference White, Ising, von Klitzing, Sierau, Michel, Klein and Müller-Myhsok2017). This study showed high rates of neglect (63.7%) in their maltreated sample, which is consistent with our findings for disengagement in this investigation (White et al., Reference White, Ising, von Klitzing, Sierau, Michel, Klein and Müller-Myhsok2017). Thus, neglect may be driving the findings of lower cortisol output in their sample. Our findings are also in line with a recent study demonstrating that greater neglect at 3 years was the best predictor of attenuated hair cortisol concentration in adult inpatients with psychiatric conditions (Schalinski et al., Reference Schalinski, Teicher and Rockstroh2019). The absence of expected sensitive and responsive caregiving may be a common theme among these similar HPA findings for neglected, foster care, and post-institutionalized youth (Bruce et al., Reference Bruce, Fisher, Pears and Levine2009; Fisher, Reference Fisher2017). This type of care may precede neurobiological changes that produce low levels of cortisol output. Hypocortisolism in these samples may also be a marker of allostatic load (McEwen, Reference McEwen1998), and the long period of time between parental disengagement at 1 year and lower cortisol output at 15 years may be needed for the HPA axis to down-regulate to produce lower cortisol output following early disengagement.
There are multiple potential pathways from early disengagement to low cortisol output years later. First, there are complex genetic and epigenetic processes through which early experience may shape adult cognitive and emotional regulatory processes, and their impact on risk for psychopathology (Turecki, Ota, Belangero, Jackowski, & Kaufman, Reference Turecki, Ota, Belangero, Jackowski and Kaufman2014), and these effects may be partly mediated by the HPA axis, with its dense receptor distribution in relevant brain regions like the hippocampus and prefrontal cortex (McEwen & Morrison, Reference McEwen and Morrison2013; Wang et al., Reference Wang, Van Heerikhuize, Aronica, Kawata, Seress, Joels and Lucassen2013). Why this would differ between parental disengagement and parental harshness experiences in not immediately clear, but it is possible that disengagement elicits specific behavioral, cognitive, and socioemotional adaptations that help children survive and navigate in their deprived environments. These adaptations could include simultaneous and linked changes in HPA axis regulatory control, leading to reduced overall cortisol secretion and alterations within hippocampal-prefrontal circuits involved in cognitive-emotional processing. Early disengagement may have particularly strong associations with the HPA axis as the absence of expected environmental input—in this case, a caregiver—to buffer stress reactivity before the infant is able to regulate their own biological and behavioral responses (Fisher, Reference Fisher2017). Diminished HPA responses may be an evolutionarily adaptive way of conserving metabolic resources in the face of stress even if diminished responses may have other negative consequences (Fisher, Reference Fisher2017). The consequences of these HPA alterations may have profound effects behaviorally and physiologically, with implications for risk for psychiatric disruptions (Holi, Auvinen-Lintunen, Lindberg, Tani, & Virkkunen, Reference Holi, Auvinen-Lintunen, Lindberg, Tani and Virkkunen2006). Low cortisol may be a predictor of future behavior and mental health problems even if there were no significant associations between cortisol and antisocial behavior at 15. In the current study, parental harshness and disengagement were correlated (r = .32), which is expected considering there is often co-occurrence of different stressors for the same individual. The model allowed us to account for this correlation to be able to identify unique associations between parental harshness, parental disengagement, hair cortisol concentration, and mental health. The zero-order correlations demonstrated a significant positive correlation between parental harshness from 1–15 and hair cortisol concentration at 15, while there was a negative (though not statistically significant) association between disengagement from 1–15 and hair cortisol concentration. There appears to be a suppressor effect where the negative but not statistically significant association between parental disengagement and hair cortisol concentration becomes statistically significant when added to the model with parental harshness and other variables. We hypothesize that this suppressor effect may result because only the aspects of disengagement that are not associated with parental harshness are associated with lower cortisol output. Including both parental harshness and disengagement together in the same model controls for the overlap between the variables, leading to suppression effects that increase the regression weights (Paulhus, Robins, Trzesniewski, & Tracy, Reference Paulhus, Robins, Trzesniewski and Tracy2004).
Another possible explanation for the differential associations of parental harshness and disengagement with overall cortisol secretion is that the experience of parental harshness and disengagement likely occurs on different time scales. For example, parental disengagement may be a more chronic, daily occurrence, while parental harshness may be a more unpredictable, intermittent occurrence that occurs as a single event or a string of individual events such as making threats or hitting the child intermittently. It could be that having a chronic stressor versus a repeated unpredictable stressor is what leads to differences in overall cortisol output. The finding that parental harshness at 15 years is associated with higher cortisol output is also consistent with the hypothesis that recent unpredictable or intermittent stress is associated with increases in cortisol output. Maltreatment that starts early in life and is chronic is predicted to transition from producing high cortisol output right away to downregulation of the HPA axis leading to low cortisol output over time. This down-regulation of the HPA axis following early-life parental disengagement, which may be a chronic early-life stressor, is consistent with our finding that parental disengagement in infancy rather than in recent years is associated with lower cortisol output. Down-regulation of cortisol output may be a marker of allostatic load (McEwen, Reference McEwen1998), and it may require longer intervals of time to see hypocortisolism.
The HPA axis has inputs from a number of limbic and cortical structures that help to regulate output of stress mediators. Early neglect has been associated with lower grey matter volume and changes in cortical activity (McLaughlin et al., Reference McLaughlin, Sheridan and Lambert2014), which could indirectly change inputs to the HPA axis leading to low cortisol output across the day and night (Herman, Ostrander, Mueller, & Figueiredo, Reference Herman, Ostrander, Mueller and Figueiredo2005, Reference Herman, McKlveen, Ghosal, Kopp, Wulsin, Makinson and Myers2016; Ulrich-Lai & Herman, Reference Ulrich-Lai and Herman2009). Our data suggest that parental disengagement specifically during infancy was associated with low cortisol output. This could be due to the stage of rapid brain development occurring at the time of parental disengagement. Circuits involving the hippocampus and amygdala that are being sculpted by early experience are simultaneously involved in behavioral and emotional adaptations and HPA axis regulation. These circuits may be particularly sensitive to early parental disengagement. There is also evidence in childhood that disruptions in caregiver attachment may be another pathway to lower cortisol output (Dozier, Peloso, Lewis, Laurenceau, & Levine, Reference Dozier, Peloso, Lewis, Laurenceau and Levine2008). Mouse models of early-life stress include a protocol that limits nesting materials provided to the dam, which then leads to higher stress and more aberrant and fragmented maternal behaviors by the dam (Rice, Sandman, Lenjavi, & Baram, Reference Rice, Sandman, Lenjavi and Baram2008). This model has been associated with impaired HPA regulation in both dams and pups (Baram et al., Reference Baram, Davis, Obenaus, Sandman, Small, Solodkin and Stern2012; Ivy, Brunson, Sandman, & Baram, Reference Ivy, Brunson, Sandman and Baram2008; Rice et al., Reference Rice, Sandman, Lenjavi and Baram2008). It will be helpful for translational researchers to use the findings in the animal literature to help disentangle why parental harshness versus disengagement (i.e., threat versus deprivation) may lead to different neuroendocrine and behavioral effects in both offspring and caregivers.
Although the association between hair cortisol concentration and age 15 depressive symptoms was positive (β = 0.14), as predicted based on previous literature (Dettenborn et al., Reference Dettenborn, Muhtz, Skoluda, Stalder, Steudte, Hinkelmann and Otte2012; Staufenbiel et al., Reference Staufenbiel, Penninx, Spijker, Elzinga and van Rossum2013; Wei et al., Reference Wei, Sun, Zhao, Yang, Liu, Lin and Ma2015), it was not statistically significant (p = .08). Slower recovery of cortisol levels to baseline following a stressor and potentially higher baseline output of cortisol in the afternoon are reported in samples with depression (Burke, Davis, Otte, & Mohr, Reference Burke, Davis, Otte and Mohr2005). High morning cortisol can predict subsequent depression in adolescents (Goodyer et al., Reference Goodyer, Herbert, Tamplin and Altham2000). As with parental disengagement, adaptations important to survival in harsh environments may involve simultaneous changes in regulatory control of both stress hormones and cognitive-emotional processes, via their shared circuitry involving the hippocampus and prefrontal cortex, creating linkages across time, for those subjected to parental harshness, between later cortisol output, behavioral adaptations, and depressive symptoms. A tendency towards a depressive-withdrawn state may well be adaptive when trying to avoid parental harshness, but at the cost of a vulnerability to a depressive disorder later in life. As the association between hair cortisol concentration and depressive symptoms was not statistically significant, hair cortisol concentration could not statistically mediate the association between parental harshness from 1–15 and depressive symptoms at 15. However, this finding suggests that while parental harshness and parental disengagement may be predictive of hair cortisol output, hair cortisol levels do not appear to be predictive of mental health 1–2 months later. It is important to report these null indirect effects as they may guide researchers to examining other mediators by which stress from 1–15 years is associated with mental health. Researchers may also find that hair cortisol output following parental harshness and disengagement may mediate associations with outcomes such as physical health or may be associated with mental health over different time scales than those examined here. It is also likely that more nuanced measures of HPA axis function will be needed to tease out its true role in connecting stress exposures to health outcomes. Hair cortisol is an integrated measure that captures both day and night cortisol secretion over weeks to months, and though it shows promise as potentially informative, its failure to demonstrate mediation here does not mean that the HPA axis is not a key mediator. Replication of this finding is needed in larger samples to test whether there is a true null association between hair cortisol and mental health at age 15 or if more power is needed to detect a small effect. Research using multiple measures of HPA regulation and reactivity in relation to parental harshness and disengagement at different developmental time points is needed.
There was no association between age 15 hair cortisol concentration and anxiety symptoms in the current investigation. This is inconsistent with some evidence in the literature that anxiety is associated with decreased overall cortisol output (Staufenbiel et al., Reference Staufenbiel, Penninx, Spijker, Elzinga and van Rossum2013). However, there are inconsistencies in the literature in regard to associations between anxiety and long-term versus short-term cortisol output (hair cortisol concentration versus diurnal and reactivity measures) (Staufenbiel et al., Reference Staufenbiel, Penninx, Spijker, Elzinga and van Rossum2013). In general, there is little relationship, acutely, between cortisol levels and subjectively experienced anxiety (Dickerson & Kemeny, Reference Dickerson and Kemeny2004), but links to more chronic types of anxiety are less well studied. One of the challenges is that anxiety is a heterogeneous phenomenon with multiple different types of associated disorders that may have different neurobiological underpinnings. Findings in the current study may also be impacted by the adolescent age of assessment.
Interestingly, although not statistically significant, there was a negative association (β = −0.12) between hair cortisol concentration and age 15 antisocial behavior, which is the direction we would expect based on previous literature. Although this sample has more participants than many studies with hair cortisol concentration measurement, there still may not be enough power to detect significant effects for weaker associations. For example, the low hair cortisol concentration and externalizing behavior association in the maltreatment study by White et al. (Reference White, Ising, von Klitzing, Sierau, Michel, Klein and Müller-Myhsok2017) had a sample size of over 500. This association may become more pronounced over time such that low cortisol output could be a predictor of later antisocial behaviors. This analysis also does not consider the contributions of other hormones that may interact with cortisol or vary by early experiences or type of psychopathology. However, in the current study, we conclude that there is no statistically significant association between hair cortisol concentration and antisocial behaviors at 15 years.
Additional potential contributors to our findings include the role of sleep and health behaviors in shaping cortisol output. Hair cortisol concentrations are determined by both daytime and nighttime cortisol secretion. Cumulative daytime cortisol secretion as measured in saliva samples collected daily (from waking to bedtime) for 30 days only explains 36% of the variance in hair cortisol concentration in the centimeter of hair thought to capture that same month's cortisol secretion (Short et al., Reference Short, Stalder, Marceau, Entringer, Moog, Shirtcliff and Buss2016). Other factors are likely in play, but nocturnal secretion rates may account for some of the unexplained variance, since they contribute to hair cortisol concentrations, but are not captured in daytime saliva collections. Sleep disruptions can influence nocturnal secretion rates (Leproult, Copinschi, Buxton, & Van Cauter, Reference Leproult, Copinschi, Buxton and Van Cauter1997), and a large percentage of daily cortisol secretion occurs during the early morning rise that begins well before awakening (Hirotsu, Tufik, & Andersen, Reference Hirotsu, Tufik and Andersen2015). If sleep patterns are altered, either chronically by early stress exposures or acutely by recent stressors, this could contribute to alterations in nocturnal cortisol secretion, which could then be reflected in hair cortisol concentrations. There is some evidence that chronic abuse can contribute to sleep disruption (McPhie, Weiss, & Wekerle, Reference McPhie, Weiss and Wekerle2014). Further work is needed to explore sleep disturbances as a potential mediator between parental disengagement or parental harshness and adolescent cortisol secretion rates and patterns. Similarly, physical activity and dietary intake can each lead to changes in cortisol output (Gerber et al., Reference Gerber, Jonsdottir, Kalak, Elliot, Pühse, Holsboer-Trachsler and Brand2013; Gibson et al., Reference Gibson, Checkley, Papadopoulos, Poon, Daley and Wardle1999), and both exercise and dietary habits may well be shaped by earlier stress exposures (Hanson & Chen, Reference Hanson and Chen2006; Michels et al., Reference Michels, Sioen, Braet, Eiben, Hebestreit, Huybrechts and De Henauw2012). Future studies should also examine these types of potential behavioral mediators in efforts to identify pathways from developmental experiences to later in life HPA axis functioning.
There are limitations of the study that should be considered. First, parental disengagement and parental harshness were only reported by the mother or primary caregiver from 1–9 years. Due to social desirability biases and potential concerns about legal ramifications, caregivers likely underreport harsh or disengaged behaviors. Thus, the rates of parental harshness and disengagement in this sample are most likely an underreport of true experiences, and associations may be stronger than we were able to detect in this study, especially due to evidence that associations between maltreatment and cortisol are stronger for agency-referred samples (Bernard et al., Reference Bernard, Frost, Bennett and Lindhiem2017). It would be important to confirm these findings with objective reports of child maltreatment. Sexual abuse was not included in our parental harshness measure, which limits generalizability of the findings to emotional and physical dimensions of parental harshness. Further, we created broad measures of parental harshness and disengagement in this study rather than specific and validated measures of maltreatment. Though our goals were to make constructs that theoretically match threat and deprivation (Humphreys & Zeanah, Reference Humphreys and Zeanah2015; McLaughlin et al., Reference McLaughlin, Sheridan and Lambert2014; Sheridan & McLaughlin, Reference Sheridan and McLaughlin2014) and to capture harsh and disengaged parent behavior dimensionally, the weakness of these measures is that by combining several idiographic measures, our work is less likely to be directly replicated by others. Thus, our findings may be specific to this sample and study. Second, hair was collected at the same visit as the age 15 symptomology variables. Hair cortisol concentrations in one or two centimeters of hair reflect secretion rates over the prior 1–2 months, which is before the symptomology variables were measured. However, far more temporally intensive measurement would be needed, of both cortisol and symptoms, to track their relationships to each other over time, and that was not feasible in a large-scale, long-term longitudinal study like this. Similarly, parental harshness and disengagement measures administered at 15 years either probed experiences in the past year or asked about an unspecified period (e.g., How often does your primary caregiver hit or slap you?). The exact temporal sequence cannot be established from our data. Third, the challenge with using data from studies spanning over a decade is that the original purpose of the study was not exactly in line with the goals of the current analysis. The funding and data collection challenges of collecting data on parental harshness and disengagement prevented more frequent collection of self-report, parent-report, and biological measures in the overall study. Thus, we attempt in this paper to leverage the available prospective data in a high-risk sample to be able to test approximate periods of risk. Another limitation is that hair samples were only collected once in this study and on a subset of participants. Although assessing cortisol output via hair was not well established when the study began, future studies should incorporate multiple measures of overall cortisol output across development to better assess the possible role of HPA functioning in associations between parental harshness, parental disengagement, and psychopathology. Finally, factor loadings for some of the scales included in the depression and anxiety factors were low, and, as seen in many studies, there was more within-reporter correlation than within-construct correlation (De Los Reyes & Kazdin, Reference De Los Reyes and Kazdin2005), which could have influenced our inability to fit the latent factors together in the model.
Strengths of the study include the prospective nature of the parental harshness and disengagement assessments. With retrospective reports of childhood stress in adolescence and adulthood, those reports may be biased by current emotions and psychopathology, memory failures, or neuroticism. Likewise, it is extremely difficult to accurately understand timing effects of stress in early childhood using retrospective recall because adolescents and adults generally cannot remember specific events as early as 1 and 3 years of age. Thus, the prospective nature of the FFCWS makes questions of long-term timing effects more addressable. Though the measurements were fixed in this dataset, they fall at ideal time periods that are in line with known sensitive periods of brain development from infancy through early childhood. Negative experiences from 0–5 years have shown associations with HPA functioning and mental health years later during adolescence and adulthood (Gunnar, DePasquale, Reid, & Donzella, Reference Gunnar, DePasquale, Reid and Donzella2019; Humphreys, Fox, Nelson, & Zeanah, Reference Humphreys, Fox, Nelson and Zeanah2017; Koss et al., Reference Koss, Mliner, Donzella and Gunnar2016; van der Vegt et al., Reference van der Vegt, Van Der Ende, Kirschbaum, Verhulst and Tiemeier2009; White et al., Reference White, Ising, von Klitzing, Sierau, Michel, Klein and Müller-Myhsok2017). Similarly, middle childhood and recent negative experiences are associated with adolescent HPA function and mental health (Bosch et al., Reference Bosch, Riese, Reijneveld, Bakker, Verhulst, Ormel and Oldehinkel2012; Ge, Conger, & Elder Jr, Reference Ge, Conger and Elder2001; Rao, Hammen, Ortiz, Chen, & Poland, Reference Rao, Hammen, Ortiz, Chen and Poland2008; Shirtcliff & Essex, Reference Shirtcliff and Essex2008). Further, a proximal measure of overall cortisol output at 15 years is a strength given higher variability in mood during early adolescence compared to later adolescence (Maciejewski, van Lier, Branje, Meeus, & Koot, Reference Maciejewski, van Lier, Branje, Meeus and Koot2015). Thus, cortisol output 1–2 months preceding the time of assessment may be more predictive of symptoms than cortisol output at an early time point, though this hypothesis should be tested empirically. As a result, the study allows us to better understand sensitive periods of risk using prospective measurement of parental harshness and parental disengagement.
Another strength is the focus on understudied populations, with high representation of low-income and racial/ethnic minority families. The FFCWS oversampled for unwed mothers and their children, who are at higher risk for mental and physical health problems due to lower SES and the myriad of stressors associated with poverty. Even within this high-risk sample, we found that parental harshness and disengagement had differential impacts on hair cortisol concentration. This result highlights the heterogeneity of risk in families even within high-risk populations. Future research should investigate how this heterogeneity, including the type and timing of stress exposure, impacts development. Even in this sample, parental harshness and disengagement were only modestly correlated (r = .32), which allowed us to somewhat disentangle their separate contributions. Finally, we were able to use a validated instrument to measure adolescent psychopathology and included both caregivers and youth as reporters. Thus, we are confident that our depressive symptoms, anxiety symptoms, and antisocial behavior constructs provide a good measure of adolescent mental health in this model. This study design is methodologically stronger than those assessing mediation with only cross-sectional data as we have data from 1–15 years of age. Thus, this study should be weighed more heavily than analyses with only cross-sectional data and retrospective reports of parental harshness and parental disengagement.
The results of this study inform our understanding of the biology that may underlie the association between psychosocial stress and psychopathology. The literature has documented both positive and negative associations between cortisol and stress, and careful considerations of type of stress may be crucial to how heterogeneity and timing of stress may lead to different cortisol patterning. Parental harshness and parental disengagement may have unique characteristics that may be processed differently by the brain, leading to different inputs to the HPA axis. This difference in overall cortisol output between parental harshness and disengagement may help to clarify why there have been discrepant findings in the stress and cortisol literatures. Thus, these results may help researchers characterize stress in ways that may make more sense of discrepant biological findings in maltreated populations. Inconsistent results are notoriously common in cortisol studies, at least partly because measurement of cortisol levels does not capture the regulatory changes within this complex system that are likely the actual mediators of interest. The studies that will resolve this issue will have to follow large cohorts longitudinally over development using more sensitive measures of regulatory changes within the HPA axis. Ideally, we would like to identify specific epigenetic changes and alterations in glucocorticoid receptor distribution and sensitivity, which cannot yet be discerned from collection of saliva and hair. These cortisol patterns following parental harshness or parental disengagement may be specific to adolescence, or they may be seen in childhood and adulthood. Ongoing follow-up of this cohort and careful longitudinal work in other cohorts will help us to disentangle these developmental associations. These findings further support the hypothesis that HPA axis functioning may be differently affected by timing and type of maltreatment, though the implications for adolescent mental health are unclear.
Supplementary Material
The supplementary material for this article can be found at https://doi.org/10.1017/S0954579420000954
Acknowledgements
We thank the families for their participation in the study. We would like to acknowledge the following grants that supported this work: F32HD088029 (PI: Doom), K01HL143159 (PI: Doom), R01MH10376 (PI: Monk), T32HD007109 (McLoyd & Monk), R01HD36916 (PI: McLanahan), R25HD074544, and a Doris Duke Fellowship for the Promotion of Child Well-Being (Recipient: Hein).
Conflicts of Interest
None.










