Attention-deficit/hyperactivity disorder (ADHD) is one of the most common childhood mental disorders, affecting approximately 5% of children worldwide (Polanczyk, de Lima, Horta, Biederman, & Rohde, Reference Polanczyk, de Lima, Horta, Biederman and Rohde2007). It is characterized by inattention and/or hyperactivity-impulsivity (American Psychiatric Association, 2013), and the median age of first diagnosis is estimated to be around 7–9 years (Kessler et al., Reference Kessler, Amminger, Aguilar-Gaxiola, Alonso, Lee and Ustun2007).
The importance of early onset is emphasized in the ICD-10 classification system, where symptoms of the disorder should be present prior to 7 years of age (World Health Organization, 1993). It is therefore important to understand etiological factors underlying early stages of ADHD. Genetically informative designs, such as the classical twin method (Neale & Cardon, Reference Neale and Cardon2013), offer a framework for quantifying the relative influence of genetic and environmental factors underlying the disorder.
Cross-sectional studies have shown that there are substantial genetic influences underlying early ADHD (Thapar, Cooper, Eyre, & Langley, Reference Thapar, Cooper, Eyre and Langley2013). In a meta-analysis aggregating across 102 studies, the estimated heritability of ADHD in children under 12 years of age was 75% (Polderman et al., Reference Polderman, Benyamin, De Leeuw, Sullivan, Van Bochoven, Visscher and Posthuma2015).
Cross-sectional findings are useful for understanding the importance of genetic and environmental contributions at a given stage in development, but they are of limited help in trying to develop a clearer understanding of the extent to which developmental trajectories are driven by stable or innovative genetic and environmental influences. Such knowledge is important for understanding the developmental processes involved in ADHD because different genetic and environmental factors might contribute to phenotypic variation at different ages. Awareness of these dynamics can direct future research on specific environmental risk factors and risk genes, processes that are poorly understood (Thapar et al., Reference Thapar, Cooper, Eyre and Langley2013; Willoughby, Reference Willoughby2003).
Compared to school age and adolescence, genetically informative longitudinal studies of ADHD symptomatology in early childhood are sparse. In a Dutch twin sample (Rietveld, Hudziak, Bartels, Beijsterveldt, & Boomsma, Reference Rietveld, Hudziak, Bartels, van Beijsterveldt and Boomsma2004), broad heritability, including both additive and dominance genetic effects, was found to be high for both overactivity and attention problems at 3, 7, 10, and 12 years of age, ranging between 70% and 74%. Genetic influences were important for the stability of these problems over time, with cross-time correlations of additive genetic effects ranging from .4 to .7. In a twin sample from England and Wales, ADHD symptoms were also found to be highly heritable at 2, 3, and 4 years of age, with estimates ranging from 78% to 81% (Price et al., Reference Price, Simonoff, Asherson, Curran, Kuntsi, Waldman and Plomin2005). Stability over time was largely attributable to genetic effects. In the same sample Kuntsi, Rijsdijk, Ronald, Asherson, and Plomin (Reference Kuntsi, Rijsdijk, Ronald, Asherson and Plomin2005) investigated ADHD symptoms at 2, 3, 4, 7, and 8 years of age, finding heritability estimates ranging from 76% to 86% and genetic correlations between .3 and .6. There are to date no twin studies on ADHD symptoms in children under age 2, and no knowledge on the stability of genetic risk below this age.
In the current study, our primary goal was to use measures of ADHD symptoms at 1.5, 3, and 5 years of age from a large prospective Norwegian birth cohort to investigate (a) the relative genetic and environmental contributions to ADHD in earlier stages of childhood than available in previous studies, and (b) the stability of genetic and environmental factors underlying early ADHD developmental trajectories. We also explored potential differential genetic and environmental influences in very young boys and girls, and the presence of twin-specific effects by extending the classic twin design with the inclusion of nontwin siblings.
Methods
Participants
The sample came from the population-based Norwegian Mother and Child Cohort Study (MoBa), a prospective birth cohort including pregnancies between 1999 and 2008 (Magnus et al., Reference Magnus, Birke, Vejrup, Haugan, Alsaker, Daltveit and Knudsen2016). Participants were recruited following a routine ultrasound examination offered to all pregnant women in Norway at week 17–18 during pregnancy. The total sample now includes approximately 114,500 children, 95,000 mothers, and 75,000 fathers. Approximately 41% of eligible women participated. Version 9 of the quality-assured MoBa data files were used, released in 2015. Written informed consent was obtained from all participants upon recruitment. The MoBa study has been granted a license from the Norwegian Data Inspectorate, and the present study was approved by the Regional Committee for Medical Research Ethics.
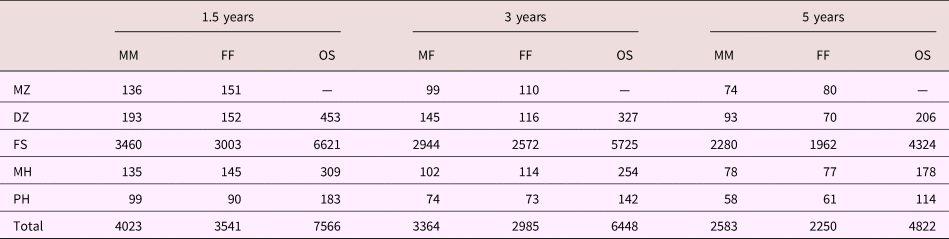
For the analyses in the current study, we included responses obtained when children were 1.5, 3, and 5 years of age. The sample comprised 302,406 responses from 27,818 siblings nested in 15,704 sibling pairs. Out of the total number of sibling pairs, 306 were monozygotic twins, 855 were dizygotic twins, 13,518 were full siblings, 627 were maternal half-siblings, and 398 were paternal half-siblings. When the number of siblings in a family was greater than two, we selected two siblings at random to be included for analysis. Nontwin siblings differ in age, but the data collection scheme ensured that assessments were always obtained at the same biological age. Table 1 cross-tabulates sample size by type of relationship, sex, and age.
Table 1. Number of sibling pairs cross-tabulated by age, sex, and relationship

Note: MM, male-male. FF, female-female. OS, opposite sex. MZ, monozygotic twins. DZ, dizygotic twins. FS, full siblings. MH, maternal half-siblings. PH, paternal half-siblings. Dash signifies value not available.
Classification of sibling relationships
For 387 of the same-sex twin pairs, zygosity was obtained by genotype testing. Maternal reports on zygosity were obtained using questionnaire items for all twin pairs. A logistic regression model, regressing genotype classifications on the questionnaire items, was fitted to the twin pairs having both measurements. The fitted model was used to classify the twin pairs that had not been genotyped, based on the questionnaire responses. The discrepancy between classification by questionnaire and genotyping had an expected misclassification rate of <2%. These questionnaire items have also previously been shown to classify correctly more than 97% of twin pairs (Magnus, Berg, & Nance, Reference Magnus, Berg and Nance1983). Nontwin siblings were classified according to their familial relationship as registered in the MoBa study.
Measures
At each assessment, maternal responses to a short form of the Child Behavior Checklist for preschool children (CBCL; Achenbach & Rescorla, Reference Achenbach and Rescorla2001) were obtained. CBCL is a standardized questionnaire assessing behavioral and emotional problems in children. In the current study, we used the DSM-oriented attention-deficit/hyperactivity problems subscale, which has been shown to perform well when validated against other CBCL scales as well as clinical diagnoses (Nakamura, Ebesutani, Bernstein, & Chorpita, Reference Nakamura, Ebesutani, Bernstein and Chorpita2009). Two of the six scale items were not available at the first measurement occasion. Mothers rated the appropriateness of statements regarding their child's behavior using three mutually exclusive category options: (1) not appropriate, (2) sometimes appropriate, and (3) appropriate.
Statistical analysis
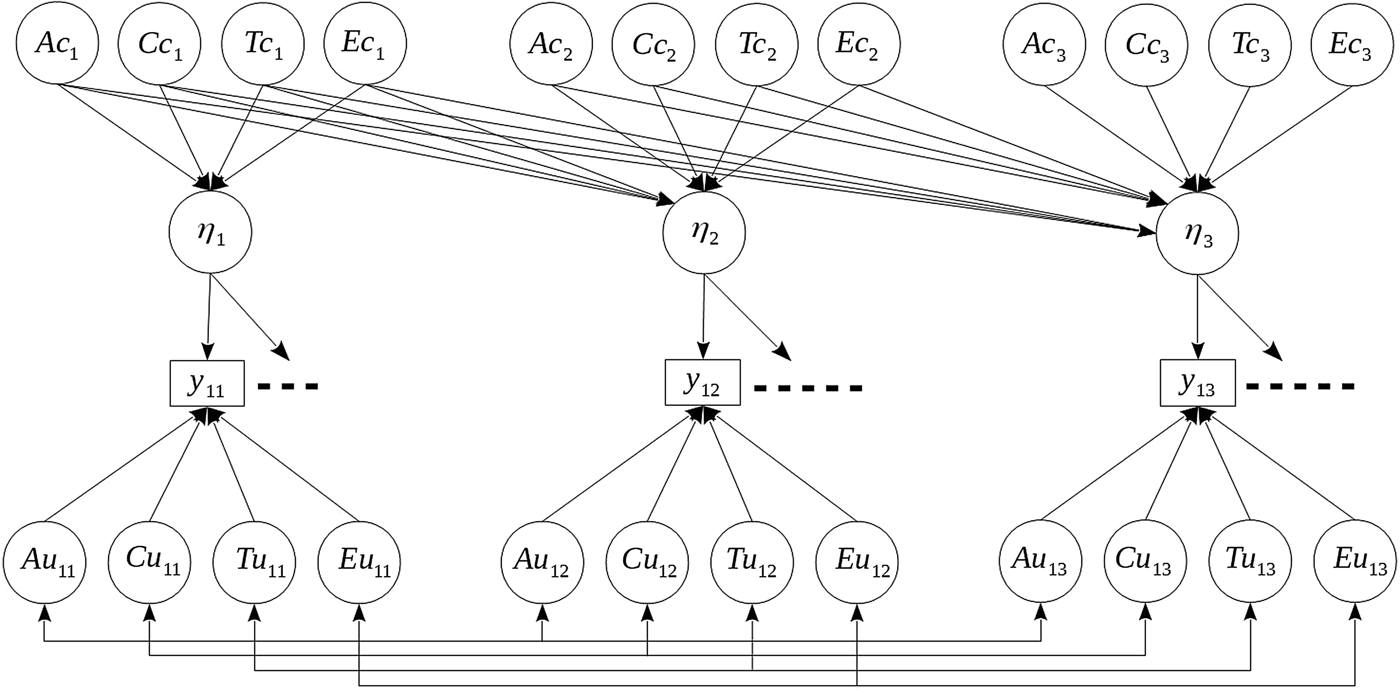
We used structural equation models to estimate genetic and environmental contributions to development across time. Figure 1 illustrates key components of the statistical models (see online-only Supplementary Material for a more detailed description). For each consecutive time point, we defined a common factor (η) indexing a latent trait that accounts for the observed associations among the items. From this latent variable perspective, the observed items are viewed as indicators of a single underlying ADHD construct, and variance in common to the items is separated from variance specific to the items.

Figure 1. Path-diagram showing key components of the longitudinal models for a single sibling in a sibling-pair. Latent ADHD levels at 1.5, 3 and 5 years of age are represented by circles labeled η 1-3. Observed variables are represented by rectangles. The horizontal dots indicate observed indicator variables not depicted in the figure, but which have similar structure. Latent ADHD levels are explained by genetic and environmental latent variables Ac, Cc, Tc and Ec, whereas observed variables are also explained by latent variables Au, Cu, Tu and Eu. All directional and undirectional paths are freely estimated parameters in the models, except for the first factor loading associated with each common factor, which was fixed to one for identification purposes.
Our primary interest focused on decomposing the variance/covariance of the common factors into additive genetic (Ac), shared environment (Cc), special twin environment (Tc), and unique environment (Ec) sources of variance. Because we extend the traditional twin design by also including nontwin siblings, twin effects were included to investigate whether there were any excess similarity attributable to twins, compared to regular siblings (Neale & Cardon, Reference Neale and Cardon2013). This specification further allowed us to separate measurement error from unique environmental effects, which could lead to biased estimates of biometrical variance components if not accounted for (Van den Berg, Glas, & Boomsma, Reference Van den Berg, Glas and Boomsma2007). Although our analyses focus on the latent constructs of ADHD, the same decomposition was applied to the item-specific residuals, in order to allow for possible cross-time and cross-sibling correlations not accounted for by the common factors. The residual parameters are not reported on here, but included in the online-only Supplementary Material. We put no restrictions on the structure of the genetic and environmental factors across time points, and for the common factors, parameterize their contributions as a Cholesky decomposition (Neale & Cardon, Reference Neale and Cardon2013). Under this parameterization, the common factors at each age are explained by genetic and environmental effects both being shared with the preceding age as well as new independent innovative effects present only at the following age. This approach is developmentally informative because it allows for the investigation of the possible emergence of new sources of etiological influences contributing to variation in ADHD not present at preceding ages, and is thus informative with respect to the second aim of our study.
The following within-pair covariance structures for the latent genetic and environmental variables were specified for the different groups of siblings. Monozygotic twins were assumed to share all additive genetic (A), shared environment (C), and special twin environment (T) effects. Dizygotic twins were assumed to share 50% of the A effects and all C and T effects. Full siblings were assumed to share 50% of the A effects and all C effects. Maternal half-siblings were assumed to share 25% of the A effects and all C effects. Paternal half-siblings were assumed to share 25% of the A effects.
We investigated two models. First, we fitted a model allowing the effects of the biometrical latent variables associated with the common factors (Ac, Cc, Tc, and Ec) to differ across males and females (M1). Second, we set the effects of all biometrical latent variables associated with the common factors equal across males and females (M2). We report the Bayesian information criterion (BIC) as an index of relative model quality, as this criteria has been shown to perform well for complex biometric models and large sample sizes (Markon & Krueger, Reference Markon and Krueger2004). The total number of parameters was a count of all fixed parameters in the models. Models were estimated using OpenMx (Neale et al., Reference Neale, Hunter, Pritikin, Zahery, Brick, Kirkpatrick and Boker2016). We included all sibling pairs with a response to at least one item from at least one sibling.
Results
Descriptives
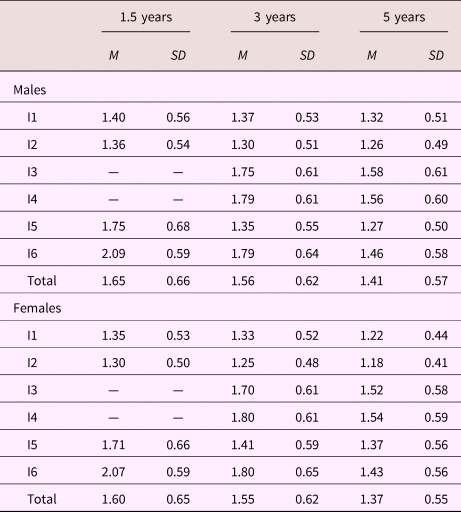
Descriptive summaries for all ADHD items are presented in Table 2. On average, females were scored lower by their mothers than males, and for both sexes mean levels for all items declined with increasing age. Standard deviations were roughly similar across sex and age.
Table 2. Means and standard deviations for all items cross-tabulated by age and sex
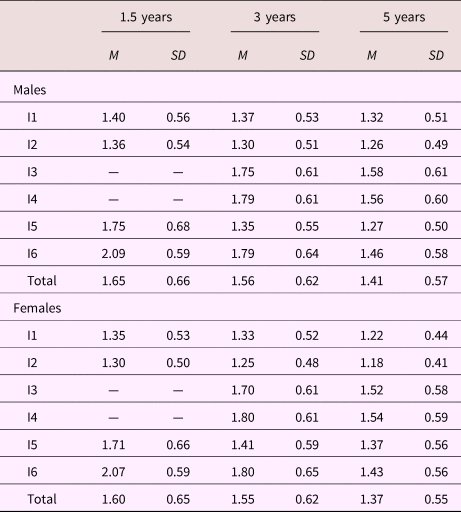
Note: Values in the rows labeled Total represent the average over items. M, mean. SD, standard deviation. Dash signifies value not available.
Model comparison
First, we compared the most general model, where the effects of all biometrical latent variables were allowed to differ across males and females (M1), to the constrained model where the effects of the biometrical latent variables associated with the ADHD factors were constrained to equality for males and females (M2). The best (lowest) BIC value was obtained under the constrained model (see Table 3). We therefore focus on results from the constrained model. A complete list of parameter estimates from both models is presented in the online-only Supplementary Material.
Table 3. Summary statistics from the two fitted models

Note: NR, number of responses. NO, number of independent observations. NP, number of parameters. DF, degrees of freedom. –2LL, –2 times log-likelihood. BIC, Bayesian information criterion. M1, model with different biometrical structure for males and females. M2, model with equal biometrical structure for males and females.
Genetic and environmental effects
Over the ages examined, the latent ADHD factor was moderately stable. The correlation between 1.5 years and 3 years was .59. The correlation between 3 years and 5 years was .65. The correlation between 1.5 years and 5 years was .44. In the following biometric analysis, we decompose these longitudinal covariance patterns into genetic and environmental components.
Age-specific contributions
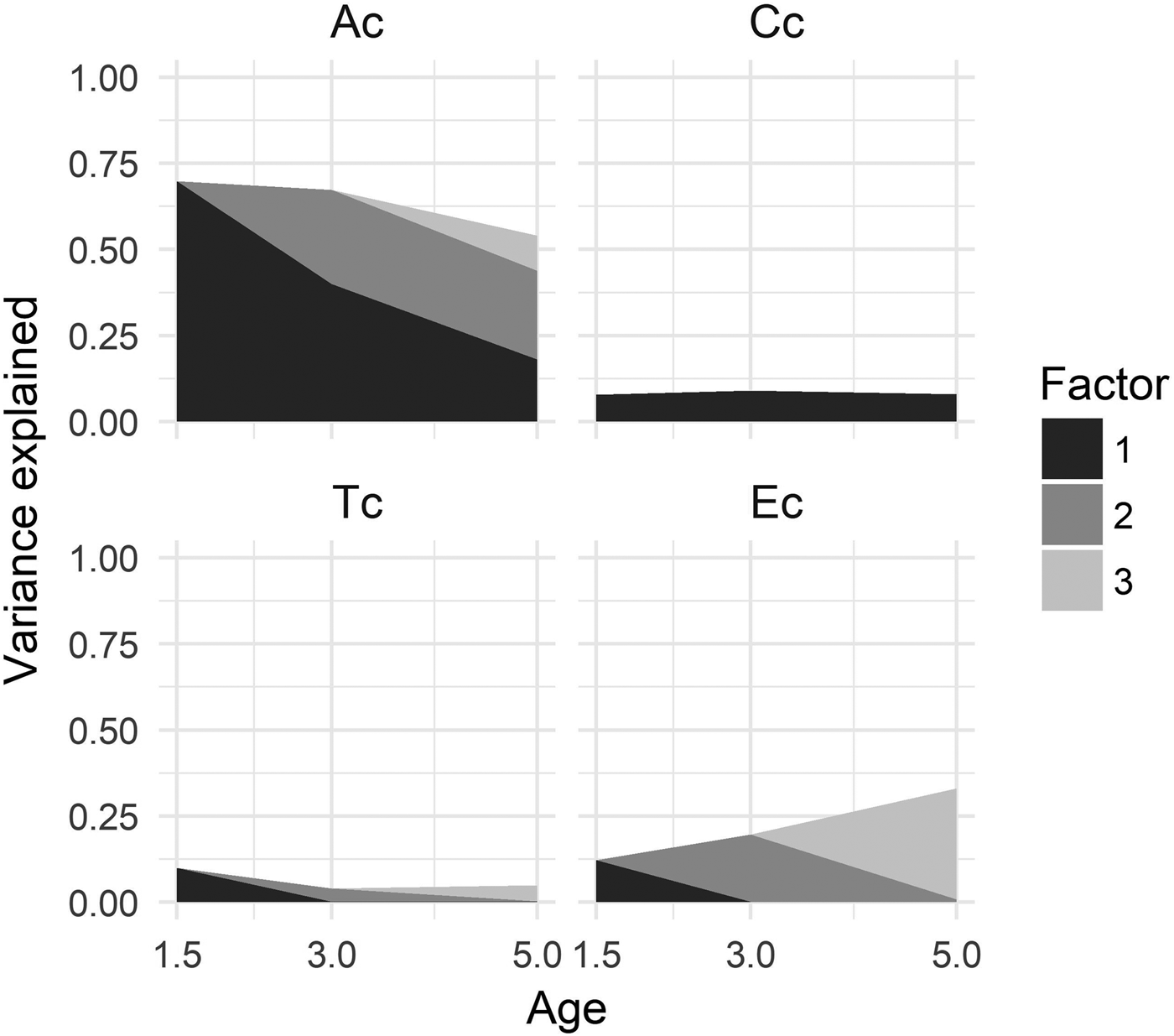
The upper lines in Figure 2 illustrate the relative contributions to variation in the latent ADHD factors, for the sum of genetic and environmental factors at each age. Below we report the percentage of variation attributable to the cumulative influence of the latent biometric variables.
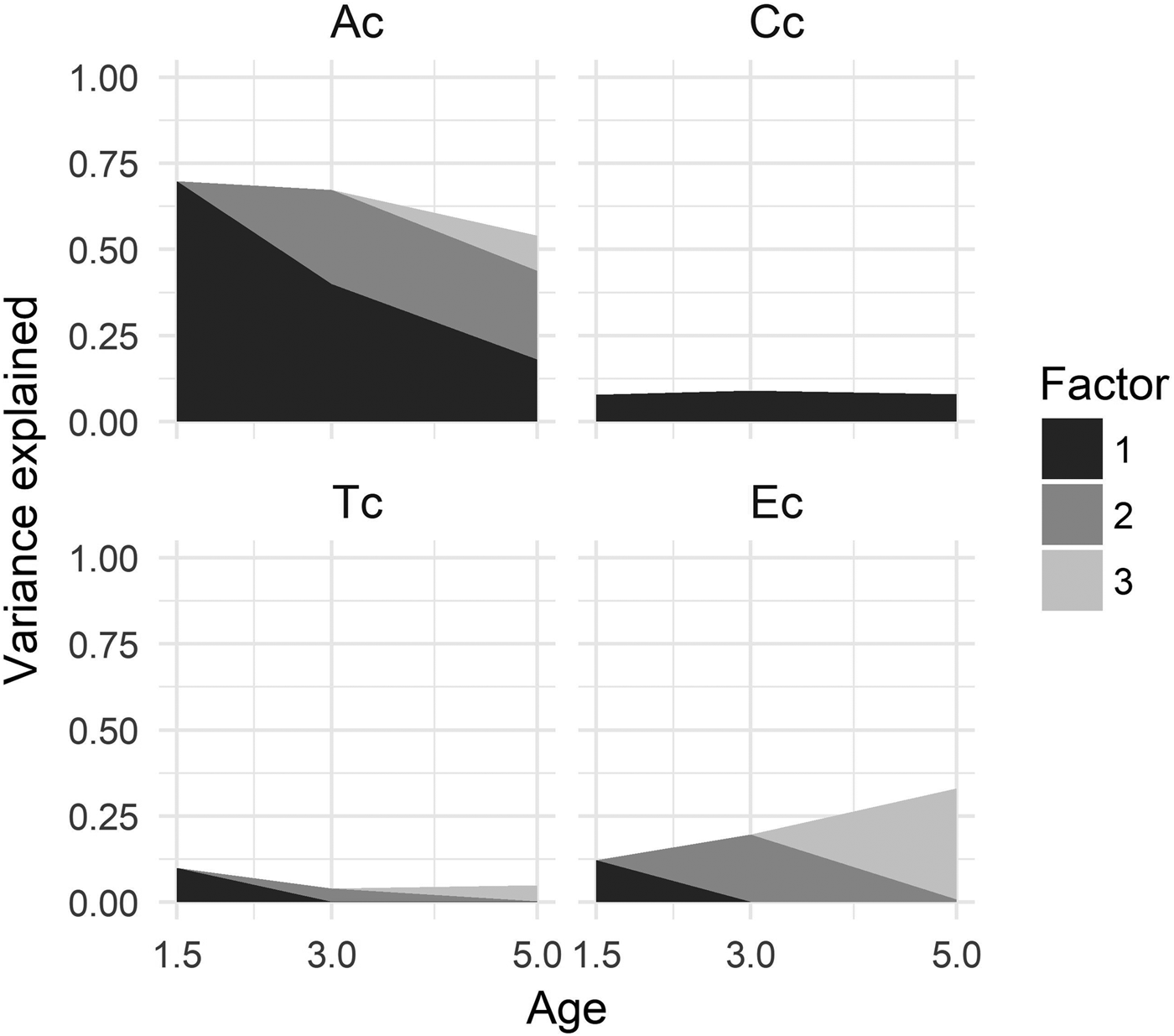
Figure 2. Proportion of total variance in the ADHD factors accounted for by genetic and environmental factors from 1.5 to 5 years of age. The black area represents the first factor accounting for variation from 1.5 to 5 years, the grey area represents the second factor accounting for variation from 3 to 5 years, and the light grey area represents the third factor accounting for variation at 5 years.
Our analysis indicated that genetic factors by comparison were the most important source of variation at each age. The age-specific point estimates of heritability showed a slight decrease across development, estimated at 70% at 1.5 years, 67% at 3 years, and 54% at 5 years of age. Contributions from shared environmental effects were marginal, but stable across all ages, estimated at 8% at 1.5 years, 9% at 3 years, and 8% at 5 years of age.
There was a minor contribution from twin-specific effects at 1.5 years of age, estimated at 10%. Only 4% and 5% of variation was attributable to twin effects at 3 years and 5 years of age, respectively. However, there was a trend of increasing proportions of unique environmental effects across age, accounting for 12% at 1.5 years, 20% at 3 years, and 33% at 5 years of age.
Innovation
The shaded areas in Figure 2 represents the estimated relative contributions to variation in the latent ADHD measure from each of the consecutive genetic and environmental factors at each age. We report percentage of variation attributable to the successive etiological factors across age.
The first genetic factor (Ac1), beginning at 1.5 years, accounted for 40% of the total variation in the ADHD factor at 3 years and 18% of the variation at 5 years of age. The second genetic factor (Ac2), beginning at 3 years, accounted for 27% of the variation at 3 years and 26% of the variation at 5 years of age. The third genetic factor (Ac3), unique to ADHD at 5 years, accounted for only 10% of the variation. These estimates imply a genetic correlation between 1.5 years and 3 years of .77, between 1.5 years and 5 years of .58, and between 3 years and 5 years of .89.
There was no evidence suggesting a presence of innovation of shared environmental effects across development. The first shared environmental factor (Cc1), beginning at 1.5 years, accounted for all variation attributable to C effects at any of the ages (i.e., 8%, 9%, and 8%).
There was no overlap in contributions from twin effects at any of the subsequent ages. Consequently, the percentage of variation accounted for by twin effects at each age, as reported above, are attributable to effects unique to the specific age.
Unique environmental effects were largely specific to each assessment age. The first environmental factor (Ec1), beginning at 1.5 years, accounted for none of the environmental effects at 3 years and 5 years of age. The second environmental factor (Ec2), beginning at 3 years, accounted for only 1% of variation at 5 years of age. The third environmental factor (Ec3), representing effects specific to 5 years of age, accounted for 32% of the variation.
Discussion
The primary aim of the current study was to investigate the relative contributions of genetic and environmental influences on ADHD symptoms in early childhood, and to characterize the dynamics of these factors through early development. For each of the investigated ages, we found that a substantial proportion of variation was attributable to additive genetic effects whereas environmental effects unique to the individual mainly accounted for the remaining variation. Of note, this pattern was evident already at 1.5 years of age. Furthermore, our analyses showed that both stable as well as developmentally emerging genetic effects were important for explaining trajectories of early ADHD symptoms, whereas unique environmental effects were mainly specific to each stage of development. Genetic effects present at 1.5 years of age accounted for a nontrivial proportion of variation also at 5 years of age. Consequentially, these findings suggest that the stability over time in early ADHD appears to be driven mainly by genetic influences. Finally, these developmental patterns could be considered equivalent for males and females.
Age-specific contributions
In the current study, heritability estimates of ADHD symptoms at each of the three different ages ranged from 54% to 70%, and the highest estimate was found at 1.5 years of age. Two twin studies based on a sample from England and Wales found slightly higher genetic influences for ADHD symptoms, ranging from 76% to 86% for children aged 2 to 8 years of age (Kuntsi et al., Reference Kuntsi, Rijsdijk, Ronald, Asherson and Plomin2005; Price et al., Reference Price, Simonoff, Asherson, Curran, Kuntsi, Waldman and Plomin2005). Comparable estimates were found in a study of ADHD symptoms in children aged 3 to 12 years of age in a Dutch twin sample (Rietveld et al., Reference Rietveld, Hudziak, Bartels, van Beijsterveldt and Boomsma2004). Broad heritability, including both additive and dominance genetic effects, was estimated between 70% and 74% at all ages. Neither of these studies found that shared environmental influences play an important role in explaining ADHD symptoms.
Overall, our findings are consistent with prior studies based on samples from other populations, showing that individual differences in ADHD in early childhood is largely attributable to genetic variation, whereas environmental effects are mainly accounted for by nonshared environmental influences. Shared environmental effects are typically not found in investigations of ADHD (Burt, Reference Burt2009; Polderman et al., Reference Polderman, Benyamin, De Leeuw, Sullivan, Van Bochoven, Visscher and Posthuma2015; Wesseldijk et al., Reference Wesseldijk, Fedko, Bartels, Nivard, van Beijsterveldt, Boomsma and Middeldorp2017). The convergence of results across studies and samples strengthens the external validity of these findings, and our analysis further showed that the results are generalizable to children as early as 1.5 years of age. Our study corroborates these overall findings in two further ways. First, we did not rely on a targeted twin sample, but utilized a population-based sample that also included regular siblings (Zyphur, Zhang, Barsky, & Li, Reference Zyphur, Zhang, Barsky and Li2013). Second, we found negligible influences of twin-specific effects, suggesting that inferences based on the classic twin design may generalize outside twin populations. However, the twin-specific effects were not stable across time, and therefore more likely to reflect factors in childhood (e.g., social environment) rather than factors during pregnancy. Given that twins share pregnancy, and the twin-specific effects should index additive effects of exposures during pregnancy (e.g., maternal smoking, infections, nutrition, and medication), our findings do not indicate that such factors have a prominent role in the development of symptoms of ADHD in the general population.
Innovation
Across-age correlations index the degree of stability in terms of the relative position of individuals across the assessments, and are informative with regard to the consistency of individual differences on the ADHD symptom construct through development. We found levels of ADHD to be moderately stable across the investigated ages, with correlations ranging from .44 to .65. Successive occasions (1.5–3 years and 3–5 years) were more highly correlated than between the first and last assessments (1.5–5 years).
Cross-time genetic correlations ranged from .58 to .89, suggesting some developmentally dynamic genetic influences. These estimates are higher than, but comparable to, findings from the Dutch twin sample where genetic correlations ranged from .4 to .7 (Rietveld et al., Reference Rietveld, Hudziak, Bartels, van Beijsterveldt and Boomsma2004), and findings from England and Wales where genetic correlations ranged from .3 to .6 (Kuntsi et al., Reference Kuntsi, Rijsdijk, Ronald, Asherson and Plomin2005). Taken together, these results suggest that genetic influences appear to be mainly responsible for the longitudinal stability that characterizes early ADHD symptoms. Because we explicitly modeled latent ADHD levels in the current study, our estimates are less impacted by assessment errors than studies that use sum scores of observed items as proxies for the ADHD construct, which could explain why we find higher genetic correlations than other studies. Overall, our findings indicate that genome-wide association studies could have relatively homogenous findings across ages from age 3 and onward. However, taken together with estimates from the Netherlands and Britain, our results suggest that genome-wide association studies on childhood ADHD should take age into account whenever possible.
Strengths and limitations
The current study has several strengths. First, the large sample size allowed us to estimate parameters of interest with high precision. Second, prospective data collection strengthens the validity of our assessments of ADHD symptoms. Third, the population-based sample, including relatives other than twins, gives our findings added generalizability. Fourth, all sibling pairs were assessed at the same biological age, rendering the sibling intraclass correlations comparable to the twin intraclass correlations. Fifth and finally, we explicitly modeled measurements errors, which is an essential aspect of most assessments of psychopathology.
Our findings should be interpreted with respect to some possible limitations. The most important pertains to our assessment of the ADHD symptoms. Two of the scale items were not available at the 1.5-year assessments; consequently, the ADHD symptoms scale is by definition not invariant over time. Because of this, changes in genetic and environmental contributions over time could in part be confounded by changes in the measurement model. We could have formally investigated measurement invariance over time, but given the large sample size, such an approach would be highly sensitive to only minor changes in item performance and would likely be of limited utility. It is however worth noting that the relative ranking of the estimated factor loadings was generally consistent across age and sex, suggesting at least partial equivalence in measurements (see online-only Supplementary Material).
Our conceptualization of ADHD as a continuous construct measured by several indicators can be seen as a disadvantage when viewed from a clinical perspective. In addition to being more easily interpretable, clinical diagnoses are typically formed by multiple sources of information, including patient history and expert judgments, and might therefore encompass a more valid measure. However, requiring clinical diagnosis would render our research question difficult to investigate empirically as most ADHD diagnosis is first given between 4 and 11 years of age, with a median around 7–9 years (Kessler et al., Reference Kessler, Amminger, Aguilar-Gaxiola, Alonso, Lee and Ustun2007). Even if children at this age have not yet reached the threshold for a clinical diagnosis, they are still likely to exhibit different levels of symptomatology, of which a continuous representation is more sensitive to pick up on (Willoughby, Reference Willoughby2003). Empirical investigations have also emphasized the benefits of a continuous approach to measurement of psychopathology, in terms of both reliability and validity (Markon, Chmielewski, & Miller, Reference Markon, Chmielewski and Miller2011).
Finally, item responses were based on maternal reports of their children's behavior. Especially for ADHD, maternal reports have been shown to be highly reliable (Faraone, Biederman, & Milberger, Reference Faraone, Biederman and Milberger1995). However, negative contrast effects (Carey, Reference Carey1986) have previously been reported in twin studies of ADHD symptoms in young children utilizing parental ratings (Kuntsi et al., Reference Kuntsi, Rijsdijk, Ronald, Asherson and Plomin2005; Rietveld et al., Reference Rietveld, Hudziak, Bartels, van Beijsterveldt and Boomsma2004). Such effects could arise if parents rated the behavior of one sibling by making comparisons to the other, resulting in an overestimation of such differences (Rietveld, Posthuma, Dolan, & Boomsma, Reference Rietveld, Posthuma, Dolan and Boomsma2003). Our findings of shared environmental influences, although small, is not consistent with such contrast effects because relative differences in intraclass correlations is expected to increase between groups (Simonoff et al., Reference Simonoff, Pickles, Hervas, Silberg, Rutter and Eaves1998). If contrast effects induced through parental reports are more prominent among parents of twins than among parents of siblings of different ages, our inclusion of nontwin siblings might have mitigated the influence of such effects.
Conclusion
The results from this study add support to the general finding of substantial genetic influences underlying early ADHD symptom expressions, and extends these findings to 1.5 years of age. We found that genetic influences make up the most important contributions to the developmental stability in ADHD symptoms, but we also found evidence that new genetic influences may play a role in this developmental process. These findings are in agreement with a view that the etiology of early ADHD should be understood as a complex dynamic process. The robustness of our findings is strengthened by utilizing a population-based sample including not only twins but also regular siblings. From a molecular genetics perspective, our results indicate that partially different genetic variants are likely to be important in explaining individual differences in ADHD symptoms at different stages of development.
Funding statement
The work was supported by the Medicine, Health Sciences and Biology Program at the Norwegian Research Council (Grant 231105). The Norwegian Mother and Child Cohort Study are supported by the Norwegian Ministry of Health and Care Services and the Ministry of Education and Research, NIH/NIEHS (contract no N01-ES-75558), and NIH/NINDS (Grant UO1 NS 047537-01 and Grant UO1 NS 047537-06A1). We are grateful to all the participating families in Norway who take part in this ongoing cohort study.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0954579418000731