A substantial literature has demonstrated that adverse and traumatic experiences in childhood are linked to differences in the function, volume, and structural integrity of regions and white matter pathways throughout the brain (Nemeroff, Reference Nemeroff2016; Teicher & Samson, Reference Teicher and Samson2016). Studies demonstrating childhood trauma-related differences in brain structure have spanned development (Cicchetti, Reference Cicchetti2016; Teicher & Samson, Reference Teicher and Samson2016) and have included nonpsychiatric (Baker et al., Reference Baker, Williams, Korgaonkar, Cohen, Heaps and Paul2013; Dannlowski et al., Reference Dannlowski, Stuhrmann, Beutelmann, Zwanzger, Lenzen, Grotegerd and Kugel2012; Gorka, Hanson, Radtke, & Hariri, Reference Gorka, Hanson, Radtke and Hariri2014) and psychiatric samples, including those with posttraumatic stress disorder (PTSD) and other comorbidities (Bremner et al., Reference Bremner, Randall, Vermetten, Staib, Bronen, Mazure and Charney1997; Carrion et al., Reference Carrion, Weems, Eliez, Patwardhan, Brown, Ray and Reiss2001; De Bellis et al., Reference De Bellis, Keshavan, Clark, Casey, Giedd, Boring and Ryan1999; Keding & Herringa, Reference Keding and Herringa2015; Stein, Koverola, Hanna, Torchia, & McClarty, Reference Stein, Koverola, Hanna, Torchia and McClarty1997). Studies examining childhood trauma-related differences in white matter integrity among children and adults have generally observed decreases in the structural integrity of limbic white matter pathways, including the cingulum, uncinate fasciculus, and fornix (Choi, Jeong, Rohan, Polcari, & Teicher, Reference Choi, Jeong, Rohan, Polcari and Teicher2009; Eluvathingal et al., Reference Eluvathingal, Chugani, Behen, Juhász, Muzik, Maqbool and Makki2006; Hanson et al., Reference Hanson, Adluru, Chung, Alexander, Davidson and Pollak2013).
Thus, a mounting literature has demonstrated limbic brain differences associated with childhood trauma. However, fewer studies have considered the broader context of early and later life trauma. Some studies of military veterans have considered both childhood trauma and later life trauma, namely, combat exposure. In a prior study with the current sample, we showed that both childhood trauma and combat exposure are independently associated with dorsal anterior cingulate activity in response to emotional faces (Herringa, Phillips, Fournier, Kronhaus, & Germain, Reference Herringa, Phillips, Fournier, Kronhaus and Germain2013). Further, only two studies of military veterans have directly examined and found interaction effects between childhood trauma and combat exposure on gray matter volume. In these studies, combat exposure was associated with decreased amygdala and anterior cingulate cortex volume within individuals with a history of childhood trauma under the age of 13 (Kuo, Kaloupek, & Woodward, Reference Kuo, Kaloupek and Woodward2012; Woodward, Kuo, Schaer, Kaloupek, & Eliez, Reference Woodward, Kuo, Schaer, Kaloupek and Eliez2013). These findings suggest that childhood trauma may sensitize limbic brain regions to later trauma exposure in ways that could contribute to vulnerability to mental illness. However, the impact of interactions between childhood and later life trauma on the structural integrity of limbic white matter pathways has yet to be examined. To our knowledge, this is the first report to examine the moderating effect of childhood maltreatment on the relationship between combat exposure and the structural integrity of limbic white matter pathways in a military sample.
Limbic white matter pathways interconnect cortical and subcortical regions that link visceral states, emotion, cognition, and behavior (for review, see Catani, Dell'Acqua, & Thiebaut de Schotten, Reference Catani, Dell'Acqua and Thiebaut de Schotten2013). For example, the cingulum is a large, limbic white matter bundle involved in executive functions, emotional processing, and memory (Beckmann, Johansen-Berg, & Rushworth, Reference Beckmann, Johansen-Berg and Rushworth2009). The uncinate fasciculus is another large, limbic bundle involved in episodic memory and socioemotional processing (Von Der Heide, Skipper, Klobusicky, & Olson, Reference Von Der Heide, Skipper, Klobusicky and Olson2013). These functions are supported by their anatomical organization; the cingulum and the uncinate fasciculus connect amygdala-related structures with corticofrontal structures, including the cingulate and orbitofrontal cortex, respectively (Catani & De Schotten, Reference Catani and De Schotten2008; Heilbronner & Haber, Reference Heilbronner and Haber2014; Jones, Christiansen, Chapman, & Aggleton, Reference Jones, Christiansen, Chapman and Aggleton2013; Von Der Heide et al., Reference Von Der Heide, Skipper, Klobusicky and Olson2013).
In addition to the cingulum and uncinate fasciculus, the fornix and stria terminalis are limbic white matter pathways that connect amygdalo-hippocampal regions and associated cortices with other subcortical regions, including the hypothalamus and bed nucleus of the stria terminalis (Adelmann, Deller, & Frotscher, Reference Adelmann, Deller and Frotscher1996; De Olmos & Ingram, Reference De Olmos and Ingram1972; Dong, Petrovich, & Swanson, Reference Dong, Petrovich and Swanson2001). As such, these pathways are involved in memory, as well as homeostatic and neuroendocrine functions (Cragg & Hamlyn, Reference Cragg and Hamlyn1959; Feldman & Conforti, Reference Feldman and Conforti1980; Flood, Merbaum, & Morley, Reference Flood, Merbaum and Morley1995; King, Rollins, Grundmann, & Olivier, Reference King, Rollins, Grundmann and Olivier2003; Osborne, Sivakumaran, & Black, Reference Osborne, Sivakumaran and Black1979; Packard, Hirsh, & White, Reference Packard, Hirsh and White1989). Finally, the anterior thalamic projections, running through the anterior limb of the internal capsule, connect thalamic regions carrying visceral/sensory signals to the orbitofrontal cortex and cingulate (Axer & Keyserlingk, Reference Axer and Keyserlingk2000; Catani et al., Reference Catani, Dell'Acqua and Thiebaut de Schotten2013).
These pathways have long-ranging developmental trajectories reaching peak structural integrity between the early 20s and mid-40s (Lebel et al., Reference Lebel, Gee, Camicioli, Wieler, Martin and Beaulieu2012), rendering them potentially malleable to the influences of traumatic events throughout development. Given the functional importance of these pathways, differences in their structural integrity may have important implications for physical and mental health outcomes known to be associated with childhood trauma (Danese et al., Reference Danese, Moffitt, Harrington, Milne, Polanczyk, Pariante and Caspi2009). Thus, the present study of military veterans is focused on examining interactions between childhood maltreatment and combat exposure on the structural integrity of limbic white matter pathways. Structural integrity measures were derived from diffusion tensor imaging (DTI) and extracted from five tracts of interest (TOIs): two components of the cingulum (cingulum, cingulate gyrus [CGC] and cingulum, hippocampus [CGH]), the fornix/stria terminalis (FX/ST), the uncinate fasciculus (UNC), and the anterior limb of the internal capsule (ALIC). We hypothesized that childhood maltreatment may induce vulnerability to further trauma exposure within limbic white matter. This would be demonstrated by childhood maltreatment moderating the relationship between combat exposure and limbic white matter structural integrity within TOIs (i.e., decreased fractional anisotropy and/or increased radial diffusivity), above and beyond the effects of potential confounders, including depressive and posttraumatic stress symptoms (PTSS).
Method
Participants
The participants were 28 male combat veterans from Operations Enduring and Iraqi Freedom who were between 18 and 35 years old (mean age = 26.4, SD 2.6). This sample has been described previously (Birn, Patriat, Phillips, Germain, & Herringa, Reference Birn, Patriat, Phillips, Germain and Herringa2014; Herringa et al., Reference Herringa, Phillips, Fournier, Kronhaus and Germain2013). Briefly, participants were originally recruited from the Pittsburgh area through public media advertisements from October 2010 to November 2011 to participate in ongoing studies. This sample was 89% White (n = 25); 2 participants were Black or African American, and 1 was multiracial. Of the 25 White participants, one was of Hispanic or Latino descent. Written informed consent was obtained from all participants after procedures were explained in accordance with the University of Pittsburgh Institutional Review Board.
Information regarding the participants’ military service was obtained via Department of Defense Form 214 documentation. Exclusion criteria included left-handedness, magnetic resonance imaging (MRI) contraindications, any medications within the previous 3 weeks (fluoxetine within the past 2 months), active substance abuse within the past month, suicidality, psychotic or bipolar disorder, or neurological disease, including treatment for any sequelae of traumatic brain injury (TBI) or concussive symptoms at the time of the study. TBI information was assessed using the Military Acute Concussion Evaluation (French, McCrea, & Baggett, Reference French, McCrea and Baggett2008) and the Clinician-Administered PTSD Scale (CAPS; Blake et al., Reference Blake, Weathers, Nagy, Kaloupek, Gusman, Charney and Keane1995), and none of the participants reported concussive symptoms at the time of enrollment.
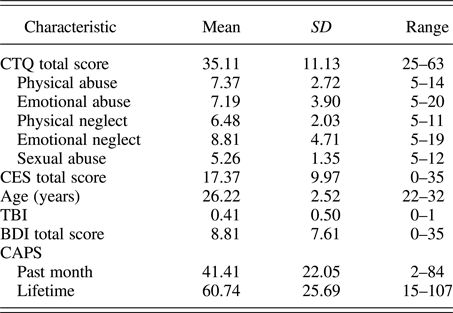
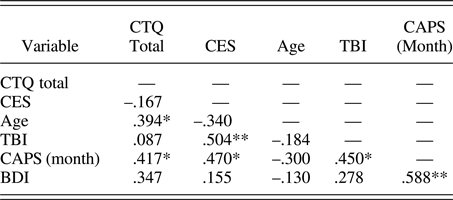
Participants were assessed with the Structured Clinical Interview for DSM-IV Axis I Disorders (First, Spitzer, Gibbon, & Williams, Reference First, Spitzer, Gibbon and Williams2002) and with the CAPS for past month PTSS. Eighteen participants met full criteria for PTSD as identified by the CAPS, using the F1/I2 criteria (Weathers, Keane, & Davidson, Reference Weathers, Keane and Davidson2001). As determined by the Structured Clinical Interview for DSM-IV Axis I Disorders, three participants met criteria for current major depressive disorder and were free of other current comorbid psychiatric disorders. Additional assessments included the Beck Depression Inventory (Beck, Steer, & Carbin, Reference Beck, Steer and Carbin1988) for depressive symptoms, the Combat Exposure Scale (CES; Keane et al., Reference Keane, Fairbank, Caddell, Zimering, Taylor and Mora1989) for the severity of combat exposure, and the Childhood Trauma Questionnaire (CTQ; Bernstein et al., Reference Bernstein, Fink, Handelsman, Foote, Lovejoy, Wenzel and Ruggiero1994) for childhood maltreatment history. Participant characteristics for primary measures of interest are shown in Table 1 and correlations between primary measures of interest are shown in Table 2. All correlations were below r = .7, indicating that multicollinearity was not high among our primary measures of interest.
Table 1. Summary of participant characteristics (n = 27)
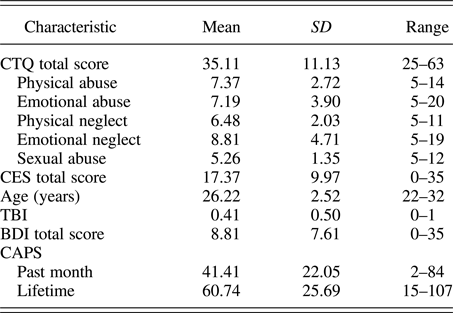
Note: CTQ, Childhood Trauma Questionnaire; CES, Combat Exposure Scale; TBI, Traumatic Brain Injury; BDI, Beck Depression Inventory; CAPS, Clinician Administered PTSD Scale. The CTQ total score corresponds to a minimal level of childhood maltreatment. The CES total score corresponds to a moderate level of combat exposure. The BDI total score corresponds to minimal depressive symptoms. Here, TBI was coded as 0, indicating no TBI, or 1, indicating any past symptoms of TBI.
Table 2. Correlations between primary variables (n = 27)
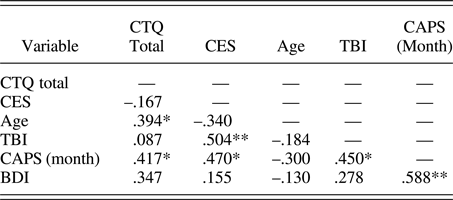
Note: CTQ, Childhood Trauma Questionnaire; CES, Combat Exposure Scale; TBI, Traumatic Brain Injury; CAPS, Clinician Administered PTSD Scale; BDI, Beck Depression Inventory. Spearman rho values are presented. Although some relationships between variables are significant, none represented high multicollinearity.
*p ≤ .05 (two tailed). **p ≤ .01 (two tailed).
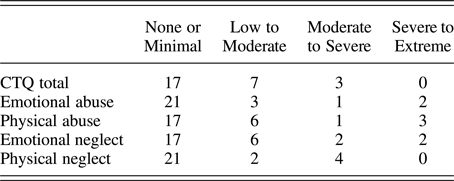
The CTQ total score was used in the present study to examine cumulative adversity from all maltreatment subtypes as it includes physical, emotional, and sexual abuse (PA, EA, and SA) and physical and emotional neglect (PN and EN). Scores range from 25 to 125, with 25 indicating no maltreatment. CTQ total scores were examined first and the subscales were examined post hoc (described below in Data Analyses). Only one participant indicated a history of SA, therefore the SA subscale was not examined. The distribution of CTQ total and subscale scores is presented in Table 3.
Table 3. Proportion of participants in CTQ classification groups (n = 27)
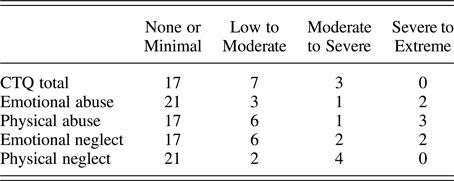
Note: CTQ, Childhood Trauma Questionnaire. The majority of our participants fell in the “none or minimal” range for all of the maltreatment subscales, with decreasing proportions as severity increased. For CTQ total, we considered scores of 25–36 none to minimal, 37–51 low to moderate, 52–68 moderate to severe, and >68 severe to extreme. The CTQ subscale classifications are defined by Bernstein and Fink (Reference Bernstein and Fink1998).
DTI acquisition
MRI was performed on a 3-Tesla Trio TIM whole-body MRI scanner (Siemens), equipped with a 12-channel phased-array head coil. DTI was performed using a pair of pulsed-gradient, spin-echo sequences with a single-shot echo-planar imaging readout. A parallel imaging algorithm (generalized autocalibrating partial-parallel acquisition) was applied during diffusion imaging to reduce echo-planar distortion. DTI parameters were 8400-ms time to repetition, 91-ms time to echo, 180° flip angle, 2 × 2 mm pixel size, 128 × 128 resolution (256 × 256 mm field of view), 64 2-mm thick slices with no gap, and 9 min 56 s total imaging time. Diffusion-sensitizing gradient encoding was applied in 68 uniform angular directions with a diffusion weighting of b = 1000 s/mm2. A reference image with no diffusion gradient (b = 0) was also acquired.
DTI processing and analysis
DTI data were first processed using the FSL Diffusion Toolbox (Version 2.0; http://www.fmrib.ox.ac.uk/fsl/fdt/index.html), which comprised correction for motion and eddy current distortions by affine registration to the reference image, removal of skull and nonbrain tissue, and calculation of diffusion parameters by fitting the diffusion images to a diffusion tensor model. The voxelwise eigenvalues λ1, λ2, and λ3 and the eigenvectors of the diffusion tensor were computed from each participant's image. Fractional anisotropy (FA), the variance of diffusion rate, was computed from the eigenvalues by the following equation:
 $${\rm FA} = {\mkern 1mu} \sqrt {\displaystyle{3 \over 2}} \sqrt {\displaystyle{{{(\lambda _1 - \hat \lambda )}^2 - {(\lambda _2 - \hat \lambda )}^2 - {(\lambda _3 - {\rm }\hat \lambda )}^2} \over {\sqrt {(\lambda _1^2 + \lambda _2^2 + \lambda _3^2 )} }}} ,$$
$${\rm FA} = {\mkern 1mu} \sqrt {\displaystyle{3 \over 2}} \sqrt {\displaystyle{{{(\lambda _1 - \hat \lambda )}^2 - {(\lambda _2 - \hat \lambda )}^2 - {(\lambda _3 - {\rm }\hat \lambda )}^2} \over {\sqrt {(\lambda _1^2 + \lambda _2^2 + \lambda _3^2 )} }}} ,$$
where axial diffusivity equals λ1, which is the largest eigenvalue reflecting water diffusivity parallel to the principle fiber direction, and radial diffusivity (RD) is the average of λ2 and λ3 corresponding to perpendicular water diffusivity (Hagmann et al., Reference Hagmann, Jonasson, Maeder, Thiran, Wedeen and Meuli2006). FA is the primary DTI measure to assess structural integrity, with larger FA values reflecting greater “structural integrity.”
The FSL tract-based spatial statistics toolbox (Version 1.2, http://www.fmrib.ox.ac.uk/fsl/tbss/index.html) was used to compute a skeleton-projected FA map for each participant following prior guidelines (Smith et al., Reference Smith, Johansen-Berg, Jenkinson, Rueckert, Nichols, Miller and Bartsch2007). For these analyses, FA images were eroded to remove likely outliers from the diffusion tensor-fitting step. FA images were then normalized to the 1 × 1 × 1 mm MNI152 stereotaxic space via the FSL FA template (FMRIB58_FA). This was done by combining two transformations: (a) a nonlinear registration of each participant's FA image to the FMRIB59_FA template, and (b) an affine transformation of the template to MNI152 space. Next, a mean FA image of all participants was computed and “thinned” to create a skeleton, or a single surface line corresponding to the center of the tracts common to all participants. A threshold was then applied to the mean skeleton according to convention (Smith et al., Reference Smith, Johansen-Berg, Jenkinson, Rueckert, Nichols, Miller and Bartsch2007) to retain FA values above 0.2. Finally, each participant's FA map was projected onto the mean FA skeleton by searching for the local center of the relevant white matter tract (i.e., the location of the local maximum of FA values). This was done to account for residual misalignments uncorrected by the nonlinear registration described above. After processing, each image was smoothed with a 2-mm3 Gaussian smoothing kernel.
Region of interest analyses
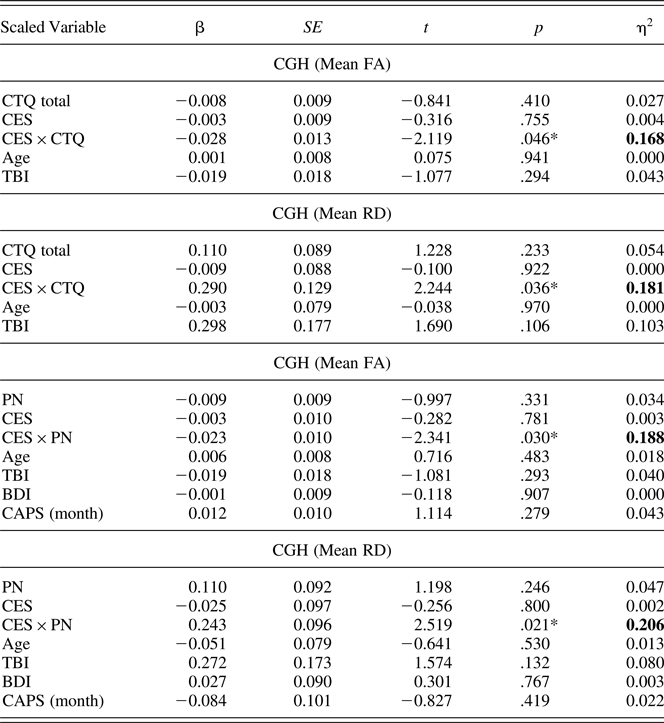
In order to examine whether childhood trauma and/or combat exposure predict DTI measures within specific TOIs, we used a 48-tract white-matter atlas (Mori, Wakana, Van Zijl, & Nagae-Poetscher, Reference Mori, Wakana, Van Zijl and Nagae-Poetscher2005; http://cmrm.med.jhmi.edu/). Of these 48 tracts, we selected five limbic TOIs: CGC, CGH, UNC, FX/ST, and ALIC (Figure 1). For each TOI, the mean of the FA and RD values across all voxels were extracted. We had no a priori hypotheses regarding laterality; thus, DTI values were averaged across hemispheres.

Figure 1. (Color online) Three-dimensional representation of tracts of interest. The cingulum, cingulate gyrus (CGC; maroon online), cingulum, hippocampus (CGH; red online), fornix/stria terminalis (FX/ST; blue online), uncinate fasciculus (UNC; green online), and anterior limb of the internal capsule (ALIC; yellow online) tracts of interest are displayed.
Data analyses
Our primary goal was to determine whether cumulative childhood maltreatment (the CTQ total score) moderated the effect of combat exposure (the CES score) on limbic white matter structural integrity, including DTI variables FA and RD within our five limbic TOIs (CGC, CGH, UNC, FX/ST, and ALIC). TOI axial diffusivity was also examined for exploratory purposes (not reported here). Age and neurological insults, such as TBI, are important determinants of white matter structural integrity (Kraus et al., Reference Kraus, Susmaras, Caughlin, Walker, Sweeney and Little2007; Lebel et al., Reference Lebel, Gee, Camicioli, Wieler, Martin and Beaulieu2012; Shenton et al., Reference Shenton, Hamoda, Schneiderman, Bouix, Pasternak, Rathi and Koerte2012). Thus, all models adjusted for age and history of TBI. However, it is also important to consider the impact of childhood maltreatment and combat exposure above and beyond mood and anxiety symptoms (Korgaonkar et al., Reference Korgaonkar, Grieve, Koslow, Gabrieli, Gordon and Williams2011; Lebel et al., Reference Lebel, Gee, Camicioli, Wieler, Martin and Beaulieu2012; Long et al., Reference Long, Duan, Xie, Du, Li, Xu and Chen2013; Shenton et al., Reference Shenton, Hamoda, Schneiderman, Bouix, Pasternak, Rathi and Koerte2012). Therefore, we also evaluated whether our findings remained after adjusting for depressive (assessed with the Beck Depression Inventory) and PTSS (assessed with the CAPS, past month), in addition to age and history of TBI.
In accordance with the American Statistical Association's recent statement, this report focuses on effect sizes, which is the appropriate method to examine a small sample in order to emphasize the clinical (rather than statistical) significance of our findings (Wasserstein & Lazar, Reference Wasserstein and Lazar2016). Our strategy was to first determine whether there was a clinically meaningful interactive effect of CTQ total and CES on each outcome after controlling for age and TBI. We defined a clinically meaningful interactive effect as one with at least a moderate η2 effect size (i.e., η2 ≥ 0.13; Levine & Hullett, Reference Levine and Hullett2002), with values of 0.02, 0.13, and 0.26 proposed as estimates of small, moderate, and large effect sizes, respectively (Cohen, Cohen, West, & Aiken, Reference Cohen, Cohen, West and Aiken2003). This effect size measure is equivalent to R 2 change related to the interaction term in a multiple regression model including the interaction term, the main effects of CTQ and CES, and our a priori selected covariates of interest. If no clinically meaningful interactive effect was found, we removed the interaction term and evaluated whether the main effects of CTQ and/or CES were associated with at least moderate η2 effect sizes. For outcomes with clinically meaningful interaction or main effects, we explored which CTQ subscale may have been driving the effects (i.e., PA, EA, PN, or EN) and also whether the finding remained after adjusting for depressive symptoms and PTSS. For clinically meaningful moderator or main effects, we used multiple regression methods to further examine the relative contributions of each of the variables in the model. Finally, when we identified that CTQ (total or subscale) moderated the effects of CES on a DTI outcome within a TOI, we used simple slopes analysis (Aiken, West, & Reno, Reference Aiken, West and Reno1991) to further investigate the interactive relationship. For all simple slopes analyses, CTQ total or subscale values that indicate either “none” or “moderate” levels of maltreatment, based on defined severity classifications (Bernstein & Fink, Reference Bernstein and Fink1998), were used in order to provide estimates of predicted structural integrity at that value (i.e., CTQ total: none = 25, moderate = 51; physical neglect: none = 5, moderate = 9).
We then evaluated whether childhood maltreatment moderated the effects of combat exposure on PTSS. We determined whether there was a clinically meaningful interactive effect of CTQ total and CES on PTSS after adjusting for age and past TBI. If no clinically meaningful interactive effect was found, we removed the interaction term and evaluated whether the main effects of CTQ and/or CES were associated with at least moderate η2 effect sizes. For outcomes with clinically meaningful main effects, we explored which CTQ subscale might be driving the effects and also investigated whether the finding remained after adjusting for depressive symptoms.
All continuous variables were standardized, and TBI history was coded as 0.5 (yes) versus –0.5 (no) for interpretability. One participant with an extreme CTQ total score was considered a statistical outlier (>3 SD from the mean) and was excluded from the analyses. However, for completeness and transparency, we also fit models including this outlier and summarize the results. Because of their small size, CGH RD values were multiplied by 10,000 in order to obtain values that would not be rounded to 0 using the statistical package, R.R Version 3.3.0 (used for all analyses).
Results
DTI outcomes
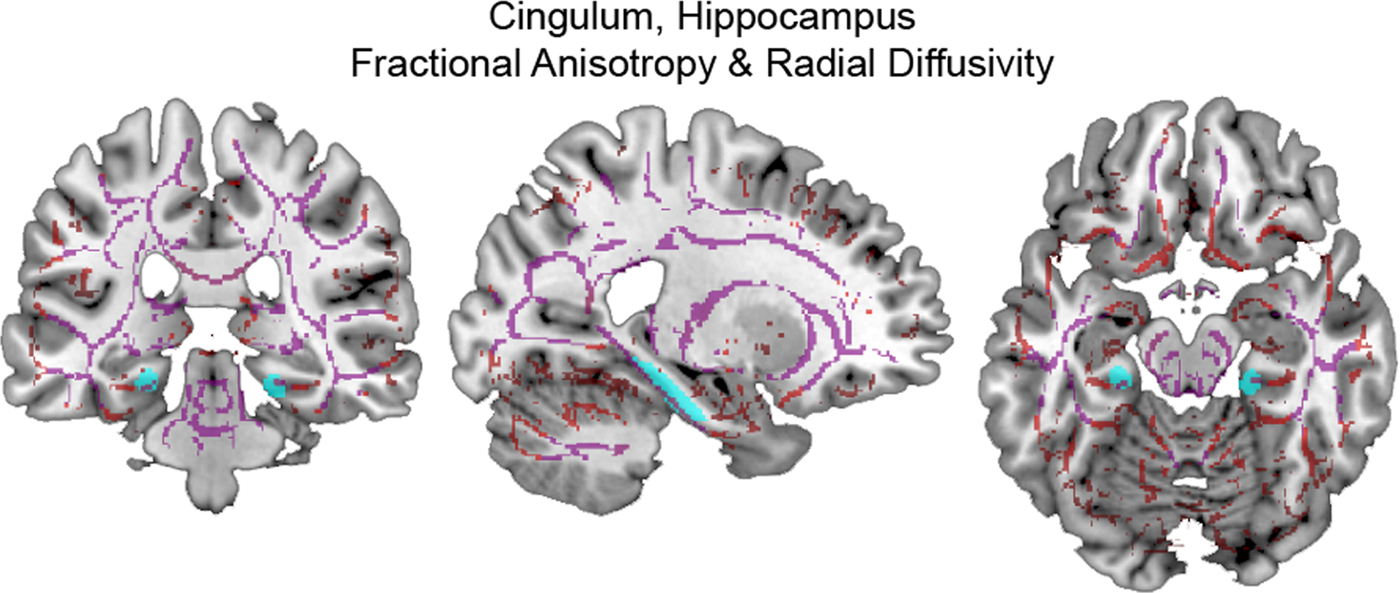
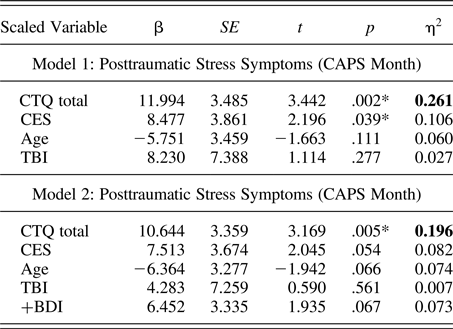
Using CTQ total and adjusting for age and past TBI, we found significant, clinically meaningful moderator effects on CGH FA (η2 = 0.168, p = .046) and RD (η2 = 0.181, p = .036). These effects, their consistency within CTQ subscales, and after adjusting for depressive symptoms and PTSS, are described in further detail below. (See Figure 2 for images of DTI measures within the CGH.)
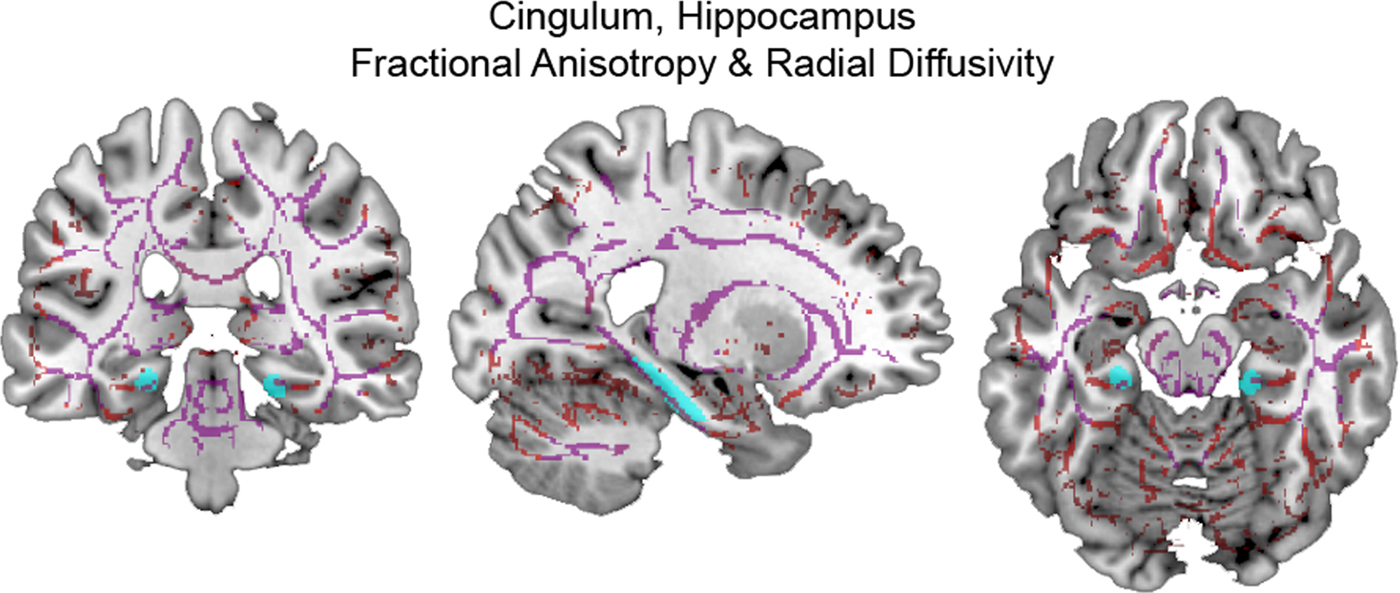
Figure 2. (Color online) Skeletonized diffusion tensor imaging measures within cingulum, hippocampus. (Left) coronal, (middle) sagittal, and (right) axial sections display the cingulum, hippocampus (cyan online) tract of interest overlaid on skeletonized fractional anisotropy (violet online) and radial diffusivity (red online) maps.
Moderator effects within the CGH
CTQ total moderated the effect of combat exposure on mean FA within the CGH after adjusting for age and past TBI (η2 = 0.168, p = .046; Figure 3a). This moderation was also observed with PN (η2 = 0.257, p = .011), EN (η2 = 0.145, p = .066), and EA (η2 = 0.163, p = .051) subscales adjusting for age and past TBI. Simple slopes analysis indicated that at the “none” level of the CTQ total (CTQ = 25), the estimated simple slope of CES was β = 0.002 (SE = 0.002; t = 1.398, p = .178), corresponding to a correlation of r = .269. Conversely, at the “moderate” level of the CTQ total (CTQ = 51) the estimated simple slope of CES was β = –0.004 (SE = 0.002; t = –2.200, p = .040), corresponding to a correlation of r = –.402.

Figure 3. (Color online) Cumulative childhood maltreatment moderated the effect of combat exposure on cingulum, hippocampus structural integrity. Plots display (a) mean fractional anisotropy (FA) and (b) mean radial diffusivity (RD) extracted from the cingulum, hippocampus (CGH) tract of interest. Lines represent predicted FA and RD values at “none” (Childhood Trauma Questionnaire [CTQ] = 25, solid red line online) and “moderate” (CTQ = 51, dashed blue line online) CTQ total scores, adjusting for age and past traumatic brain injury. Thus, higher levels of combat exposure accompanied by greater childhood maltreatment was associated with decreased CGH FA and increased CGH RD. Shaded regions depict 95% confidence intervals.
After including depressive symptoms and PTSS in the model (i.e., adjusting for age, past TBI, depressive symptoms, and PTSS), this moderation effect was observed only in the PN subscale (η2 = 0.188, p = .030; Figure 4a). At the none level of PN (PN = 5), the estimated simple slope of CES on CGH FA was β = 0.001 (SE = 0.001; t = 0.983, p = .339), corresponding to a correlation of r = .193. At moderate PN (PN = 9), the estimated simple slope of CES on CGH FA was β = –0.003 (SE = 0.001; t = –2.504, p = .022), corresponding to a correlation of r = –.448.

Figure 4. (Color online) Physical neglect moderated the effect of combat exposure on cingulum, hippocampus structural integrity. Plots display (a) mean fractional anisotropy (FA) and (b) mean radial diffusivity (RD) extracted from the cingulum, hippocampus (CGH) tract of interest. Lines represent predicted FA and RD values at “none” (physical neglect; PN = 5, solid red line online) and “moderate” (PN = 9, dashed blue line online) PN scores, adjusting for age, past traumatic brain injury, and depressive and posttraumatic stress symptoms. Thus, higher levels of combat accompanied by greater childhood PN was associated with decreased CGH FA and increased CGH RD. Shaded regions depict 95% confidence intervals.
CTQ total also moderated the effect of combat exposure on mean CGH RD after adjusting for age and past TBI (η2 = 0.181, p = .036; Figure 3b). This moderation was also observed in PN (η2 = 0.284, p = .006), EN (η2 = 0.144, p = .065), and EA (η2 = 0.139, p = .069) subscales when adjusting for age and past TBI. Simple slopes analysis indicated that at the none level of the CTQ total (CTQ = 25), the estimated simple slope of CES was β = –0.027 (SE = 0.016; t = –1.722, p = .101), corresponding to a correlation of r = –.326. Conversely, at the moderate level of the CTQ total (CTQ = 51), the estimated simple slope of CES was β = 0.041 (SE = 0.019; t = 2.125, p = .046), corresponding to a correlation of r = .391.
After including depressive symptoms and PTSS in the model, the moderation effect on CGH RD was observed only in the PN subscale (η2 = 0.206, p = .021; Figure 4b). At the none level of PN (PN = 5), the estimated simple slope of CES on CGH RD was β = –0.020 (SE = 0.014; t = –1.448, p = .165), corresponding to a correlation of r = –.278. At moderate PN (PN = 9), the estimated simple slope of CES on CGH RD was β = 0.028 (SE = 0.012; t = 2.251, p = .037), corresponding to a correlation of r = .411.
Examination of the full set of effects from multiple regression (Table 4) indicated that the interaction between CTQ total and CES consistently contributed to the largest increase in R 2 (equivalent to η2) relative to the other covariates in the models. Finally, when the outlier was retained in the CTQ total CGH model after adjusting for age and past TBI, the moderator effects on FA and RD were stronger (CGH FA, η2 = 0.225, p = .011; CGH RD, η2 = 0.280, p = .003).
Table 4. Multiple regression results for childhood maltreatment and combat exposure interaction models
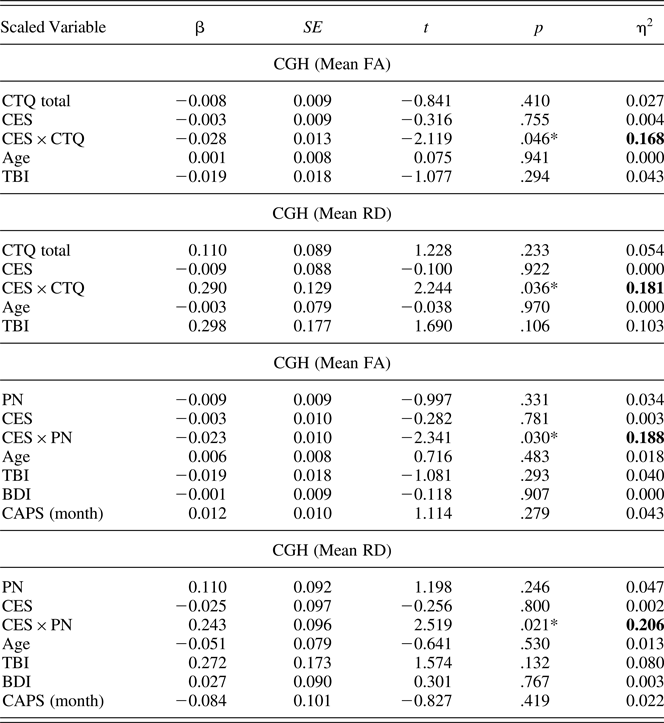
Note: CGH, cingulum, hippocampus; FA, fractional anisotropy; CTQ, Childhood Trauma Questionnaire; CES, Combat Exposure Scale; TBI, traumatic brain injury; RD, radial diffusivity; PN, physical neglect; BDI, Beck Depression Inventory; CAPS, Clinician Administered PTSD Scale. The full set of effects from multiple regression indicated that the interaction between CTQ total and CES on CGH FA and RD contributed to the largest increase in R 2 (equivalent to η2) relative to the other covariates in the model. After including depressive and posttraumatic stress symptoms in the model, the moderation effect on CGH FA and RD was observed only in the PN subscale. *p ≤ .05 (two tailed).
PTSS outcomes
CTQ total did not moderate the effect of CES on PTSS after controlling for age and past TBI. However, there was a large main effect of CTQ total on PTSS after controlling for CES, age, and past TBI (η2 = 0.261, p = .002). This main effect was also observed with the PN (η2 = 0.236, p = .004), EN (η2 = 0.268, p = .002), and PA (η2 = 0.161, p = .022) subscales after controlling for CES, age, and past TBI. When depressive symptoms were added to the model, the main effect of CTQ total was still observed (η2 = 0.196, p = .005), as well as with the PN (η2 = 0.139, p = .021) and EN (η2 = 0.210, p = .003) subscales. (The full set of effects from multiple regression for both models are presented in Table 5.) Finally, when the outlier was included, the main CTQ total effect was still observed after adjusting for CES, age, and past TBI (η2 = 0.217, p = .005), as well as after adding depressive symptoms to the model (η2 = 0.139, p = .018).
Table 5. Multiple regression results for posttraumatic stress symptom outcomes
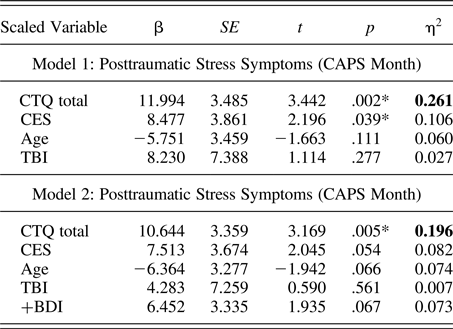
Note: CAPS, Clinician Administered PTSD Scale; CTQ, Childhood Trauma Questionnaire; CES, Combat Exposure Scale; TBI, traumatic brain injury; BDI, Beck Depression Inventory. A large effect of CTQ total on posttraumatic stress symptoms was observed after controlling for CES, age and past TBI, which remained at a moderate effect size after depressive symptoms were added to the model.
*p ≤ .05 (two tailed).
Discussion
The present study extends the current literature by providing preliminary evidence that childhood and later life trauma interact to impact brain structural integrity, as measured by DTI. As limbic white matter pathways have a long developmental trajectory progressing into adulthood, the structural integrity of these pathways may be particularly malleable to the influence of traumatic experiences throughout the life course. Thus, among young adult, male combat veterans, we hypothesized that childhood maltreatment may increase vulnerability to later combat exposure and that this would be revealed by a moderating influence of childhood maltreatment on the relationship between combat exposure and the structural integrity of limbic white matter pathways, above and beyond the influences of potential confounders, including age and TBI. Demonstrated for the first time to our knowledge, cumulative childhood maltreatment (CTQ total, which includes physical, emotional, and sexual abuse and physical and emotional neglect) moderated the effect of combat exposure on the structural integrity of the hippocampal cingulum, such that, among those with high childhood maltreatment, greater combat exposure was associated with decreased FA and increased RD. The moderating effect of childhood maltreatment was unique to the CGH, suggesting specificity to the cingulum bundle. This moderating effect was even more robust when considering the physical neglect subscale, which remained after adjusting for depressive symptoms and PTSS.
Childhood trauma, combat exposure, and the structural integrity of the cingulum
The cingulum is a large bundle containing both association and projection fibers, the longest of which extend from the uncus and parahippocampal gyrus to subgenual areas of the cingulate gyrus (Jones et al., Reference Jones, Christiansen, Chapman and Aggleton2013; Nieuwenhuys, Voogd, & van Huijzen, Reference Nieuwenhuys, Voogd and van Huijzen2008). Among other roles, the cingulum is implicated in autonomic functions, emotional processing and memory, as well as mood and anxiety disorders (Catani et al., Reference Catani, Dell'Acqua and Thiebaut de Schotten2013). The present study demonstrated that childhood trauma moderated the effect of combat exposure on the structural integrity of the CGH, such that those with high levels of childhood trauma and greater combat exposure display decreased FA and increased RD. RD has been shown to increase with demyelination and is canonically interpreted as such (Mori & Zhang, Reference Mori and Zhang2006; Song et al., Reference Song, Sun, Ramsbottom, Chang, Russell and Cross2002). Further, a more disorganized configuration of the axons within the CGH may increase the RD. Taken together, these findings suggest an overall decrease in structural integrity that may be driven by demyelination or disorganization of CGH axons (Johansen-Berg & Behrens, Reference Johansen-Berg and Behrens2009). These differences in CGH structural integrity may have implications for vulnerability to mental illness, specific to its functions.
The parahippocampal retrosplenial network encompasses the CGH TOI used in the present study, which includes both the dorsal and the ventral posterior cingulate cortex (Catani et al., Reference Catani, Dell'Acqua and Thiebaut de Schotten2013; Vogt, Reference Vogt2005). The dorsal posterior cingulate cortex (PCC) plays a role in visuospatial orientation, potentially in orienting toward or away from innocuous or noxious stimuli (Vogt, Reference Vogt2005). Vogt describes the ventral PCC as the preprocessor of information regarding the self-relevance of emotional stimuli. The ventral PCC conveys this information to the subgenual anterior cingulate cortex, involved in autonomic regulation and emotional valence. A meta-analysis of functional studies demonstrates that posterior, retrosplenial clusters are also activated by memory tasks, with these same clusters having a high probability of hippocampal connections (Beckmann et al., Reference Beckmann, Johansen-Berg and Rushworth2009). A small proportion of cingulum fibers course through the entire tract (Jones et al., Reference Jones, Christiansen, Chapman and Aggleton2013); thus, decreased CGH structural integrity and/or demyelination could suggest alterations in the described posterior functions, as well as more anterior functions, such as autonomic and emotion regulation (Vogt, Reference Vogt2005), all of which have potentially maladaptive implications. Among children with a history of institutionalized care, who were likely neglected and/or deprived, decreased CGH FA was associated with worse neurocognitive performance in a test of spatial planning (Hanson et al., Reference Hanson, Adluru, Chung, Alexander, Davidson and Pollak2013). Our results also indicate that physical neglect (e.g., not being attended to with regard to food, clean clothes, medical attention, and/or safety) is the primary type of maltreatment driving the moderator effects of CTQ total on CGH FA and RD. When compared to physical abuse, physical neglect is associated with more severe cognitive deficits, as well as more internalizing and emotional problems (Hildyard & Wolfe, Reference Hildyard and Wolfe2002), which may be due in part to trauma-related alterations in cingulum structural integrity. Further, previous research in both children and adults demonstrates that childhood trauma-related decreases in cingulum FA are associated with depressive symptoms or the development of psychiatric disorders later in life (Choi et al., Reference Choi, Jeong, Rohan, Polcari and Teicher2009; Huang, Gundapuneedi, & Rao, Reference Huang, Gundapuneedi and Rao2012).
No main effects of childhood trauma on FA or RD within any other TOIs were revealed in the present study, which is inconsistent with previous reports that have shown childhood trauma-related decreases in cingulum FA among children and adults. Studies in children/adolescents who have experienced severe neglect or physical or sexual abuse have shown decreased FA in the cingulum compared to controls (Hanson et al., Reference Hanson, Adluru, Chung, Alexander, Davidson and Pollak2013; Huang et al., Reference Huang, Gundapuneedi and Rao2012). Young, healthy adults with a history of parental verbal abuse also show decreased cingulum FA (Choi et al., Reference Choi, Jeong, Rohan, Polcari and Teicher2009). However, individuals with a history of childhood trauma display increased cingulum FA in a study that spanned a large age range and included individuals with major depressive disorder, potentially accounting for the difference in directionality (Ugwu, Amico, Carballedo, Fagan, & Frodl, 2014). The cingulum has the longest developmental trajectory among other major white matter bundles, reaching peak FA in the early 40s (Lebel et al., Reference Lebel, Gee, Camicioli, Wieler, Martin and Beaulieu2012). Thus, it may have the longest window within which both childhood and later life traumas, like combat exposure, could influence its structural integrity, as demonstrated in the present report. Our results support the hypothesis that childhood maltreatment may sensitize the CGH adversely to later combat exposure.
Childhood trauma, combat exposure, and PTSS
While there was no moderating effect of cumulative childhood maltreatment on the relationship between combat exposure and PTSS in our sample, there were statistically significant contributions of both childhood maltreatment and combat exposure to PTSS. However, only the effect of childhood maltreatment was above a moderate effect size and remained so after adjusting for depressive symptoms. Our findings are consistent with previous literature showing that greater childhood adversity increases risk for PTSD (Koenen, Moffitt, Poulton, Martin, & Caspi, Reference Koenen, Moffitt, Poulton, Martin and Caspi2007). Further, adults with higher levels of childhood adversity and recent major stressors show greater prevalence of PTSD, perhaps indicating a stress sensitization process (McLaughlin, Conron, Koenen, & Gilman, Reference McLaughlin, Conron, Koenen and Gilman2010). Further, in military samples, childhood adversity predicts nonresilient postdeployment trajectories of PTSS (Berntstein et al., Reference Berntsein, Johannessen, Thomsen, Bertelsen, Hoyle and Rubin2012) and contributes to PTSS to a greater extent than combat exposure (Cabrera, Hoge, Bliese, Castro, & Messer, Reference Cabrera, Hoge, Bliese, Castro and Messer2007). Thus, our findings support the hypothesis that childhood adversity shapes neural developmental trajectories, particularly cingulum structural integrity, in ways that may sensitize this and potentially other limbic pathways to later life stress or trauma.
Complex trauma intervention implications throughout the life course
Complex trauma is defined as trauma occurring throughout the life course with often prolonged traumas, such as combat exposure, accompanied by a developmental history of childhood maltreatment or adversity. Complex trauma often yields symptom complexity, which can include affect dysregulation, maladaptive coping strategies, impulsivity, self-injurious behavior, substance abuse, and/or comorbidity with other mood or anxiety disorders. This symptom complexity contributes to complex PTSD, developmental trauma disorder, or related conditions, which are more difficult to treat as the therapeutic response is often more modest and/or requires more prolonged, intensive treatment (for a review, see Briere & Scott, Reference Briere and Scott2015).
Our finding that the interaction between childhood maltreatment and combat exposure is associated with decreased CGH structural integrity may implicate the cingulum as a target for prevention and/or intervention. Reduced CGH structural integrity may also represent a neural marker of complex trauma, potential vulnerability to subsequent trauma, and/or a neural predictor of intervention effectiveness. With the cingulum's long developmental trajectory, our findings suggest a potential target of biological or psychosocial intervention that may be responsive even into adulthood. However, the extent to which treatment response to first-line pharmacological and cognitive behavioral treatments of PTSD is mediated by CGH integrity or treatment-induced changes in CGH integrity remains to be determined. Of particular relevance to our findings are interventions that may target cingulum-related functions, including “self-relevance” processing of emotional stimuli and emotional regulation (Vogt, Reference Vogt2005). For example, multitarget interventions including dialectical behavioral therapy and skill training in affect regulation, in which emotional coping skills are developed and emotional processing occurs (Briere & Scott, Reference Briere and Scott2015), may act on cingulum structural integrity to enhance these capabilities and potentially augment first-line treatments.
Interventions specific to military populations that employ cognitive and emotion regulation strategies may also influence cingulum structural integrity. For example, the Battlemind program, implemented during or after deployment, includes psychological debriefings that facilitate cognitive and emotional processing of traumatic events proximal to adult trauma exposure. This program also incorporates training to cognitively reframe common postdeployment symptoms by addressing how necessary combat-related skills (e.g., maintaining tactical awareness) can naturally become problematic in civilian environments (e.g., hypervigilance; Adler, Bliese, McGurk, Hoge, & Castro, Reference Adler, Bliese, McGurk, Hoge and Castro2009; Adler, Castro, & McGurk, Reference Adler, Bliese, McGurk, Hoge and Castro2009; Castro, Adler, McGurk, & Bliese, Reference Castro, Adler, McGurk and Bliese2012). Veterans with high combat exposure who engaged in the Battlemind program report fewer postdeployment posttraumatic stress and/or depressive symptoms compared to those who received control interventions (Adler, Bliese, et al., Reference Adler, Castro and McGurk2009; Castro et al., Reference Castro, Adler, McGurk and Bliese2012). Thus, we speculate that the cingulum may be a contributing neural mechanism by which these interventions yield effective treatment responses. Such interventions may be particularly useful for individuals with a history of childhood maltreatment, who are presumably more biologically vulnerable to the effects of trauma exposure during combat. However, further study would be needed to examine this possibility.
There is evidence that interventions specifically targeting the cingulum may be effective. For example, therapeutically effective deep brain stimulation used to treat depression includes electrode placements involving various segments of the cingulum bundle (see Heilbronner & Haber, Reference Heilbronner and Haber2014, for review). Further, there is evidence that interventions effective in reducing symptoms enhance cingulum structural integrity. One study showed that electroconvulsive therapy used to treat depression increases FA within dorsal frontolimbic circuits that include the anterior cingulum (Lyden et al., Reference Lyden, Espinoza, Pirnia, Clark, Joshi, Leaver and Narr2014). Lower cingulum FA contributes to a prediction of nonremission with antidepressant medication treatment (Korgaonkar et al., Reference Korgaonkar, Rekshan, Gordon, Rush, Williams, Blasey and Grieve2015). Thus, cingulum structural integrity could also potentially be used as a personalized neural marker to predict treatment response.
Complementary or alternative approaches, such as meditation, may also be useful methods to support or enhance cingulum structural integrity. Luders, Clark, Narr, and Toga (Reference Luders, Clark, Narr and Toga2011) demonstrated that compared to age- and sex-matched controls, long-term meditation practitioners display greater structural integrity of major white matter bundles, including the CGH. Long-term meditation practitioners also display less age-related decline in CGC structural integrity (Luders et al., Reference Luders, Clark, Narr and Toga2011). Further, brief mindfulness training that improves emotion regulation is associated with structural changes within the anterior cingulate cortex (Tang, Holzel, & Posner, Reference Tang, Holzel and Posner2015; Tang, Tang, & Posner, Reference Tang, Tang and Posner2016). Thus, meditation could be used as a noninvasive intervention throughout the life course, particularly among individuals with a history of childhood maltreatment or adversity, to promote cingulum structural integrity and potentially ameliorate neural and psychological effects of subsequent trauma exposure. Further research into how the cingulum could be used as a neural marker of complex trauma and treatment efficacy, and a target for prevention/intervention, is necessary. In addition, research into how interventions may alter the cingulum's developmental trajectory in potentially adaptive ways within maltreated, trauma-exposed, and complex trauma populations is of great interest.
Limitations of the present research
We note several limitations of the present report including a small sample, a cross-sectional study design, and the retrospective recall of childhood maltreatment and combat exposure. Further, as the sample is composed of all male military veterans, the results of the study may not be generalizable to females and other forms of later life trauma, aside from combat exposure.
The present findings are based on a small sample of 27 young adult combat veterans. Given this small sample size, we emphasized clinically meaningful effect sizes that reached at least moderate levels. In the American Statistical Society's recent statement on p values, the authors note that p values do not measure the importance of a result and are highly influenced by sample size: a small p value can be achieved with a small effect if the sample size is large enough and a large effect may produce large p values if the sample size is small (Wasserstein & Lazar, Reference Wasserstein and Lazar2016). The primary moderation effects described in the present report (i.e., CTQ total moderating the effect of CES on CGH FA and RD adjusting for age and TBI, and PN moderating the effect of CES on CGH FA and RD, adjusting for all covariates) were statistically significant, in addition to having moderate effect sizes; however, multiple tests were run to adequately examine these data. Thus, these findings are preliminary, not conclusive, and warrant further replication; however, the effect sizes suggest increased statistical significance with larger samples, and these findings provide an interesting and novel direction for future research, namely, examining interactions between multiple levels of trauma throughout the life span on neural outcomes, including limbic white matter structural integrity.
Although retrospective reporting of childhood maltreatment can be associated with recall bias, congruence between sibling retrospective reports of abuse, and prospectively collected court, clinic, and research records suggests reliability of such reports (Bifulco, Brown, & Harris, Reference Bifulco, Brown and Harris1994; Bifulco, Brown, Lillie, & Jarvis, Reference Bifulco, Brown, Lillie and Jarvis1997; Hardt & Rutter, Reference Hardt and Rutter2004). It is possible that individuals reporting more severe depressive symptoms and PTSS may report more childhood maltreatment. In our sample, CTQ total scores were moderately correlated with depressive (p = .076) and posttraumatic stress symptoms (p = .030, see Table 2). However, Scott, McLaughlin, Smith, and Ellis (Reference Scott, McLaughlin, Smith and Ellis2012) found no differences between prospective and retrospective reports of maltreatment history in the strength of the relationships between maltreatment history and mood or anxiety disorders). Ultimately, longitudinal research is necessary to distinguish the influences of genetic predispositions, prenatal exposures, childhood maltreatment, and later life traumas, as well as the importance of the developmental timing of traumatic experiences. Further analyses examining how the structural integrity of limbic tracts may mediate relationships between childhood maltreatment and/or combat exposure and mood and anxiety symptoms were not feasible in the current study, but are important to investigate in future research.
Summary
In summary, this report provides preliminary evidence of the moderating effect of childhood maltreatment on the relationship between combat exposure and CGH FA and RD, such that having high childhood maltreatment and high combat exposure was associated with decreased FA and increased RD, suggesting decreased myelination contributing to an overall decrease in structural integrity. These findings could potentially have maladaptive implications, particularly for the perception of emotional stimuli, emotional memory, as well as autonomic and emotional regulation. Overall, these differences in CGH structural integrity may contribute to increased risk for psychopathology, particularly with greater cumulative exposure to traumatic experiences across development. Further research is needed to discern changes within more neuroanatomically specific components of these limbic white matter pathways and how these changes may contribute differentially to neural, physiological, and psychological outcomes. Further, our findings suggest the cingulum as a potential target of interventions that may be effective even into adulthood, particularly for individuals with exposure to multiple levels of trauma throughout development.