There is now ample evidence that the quality of early attachment experiences shapes expectations of supportive and responsive care and ultimately serves to scaffold adaptation to the salient tasks of development in enduring ways (Cassidy & Shaver, Reference Cassidy and Shaver2008). Nonetheless, only one published report has identified neural mechanisms that may support these links by prospectively examining longitudinal associations between infant attachment insecurity and/or disorganization and adult brain function (Moutsiana et al., Reference Moutsiana, Fearon, Murray, Cooper, Goodyer, Johnstone and Halligan2014). The current study was designed to help fill this notable gap in the literature by focusing on the long-term predictive significance of infant attachment, assessed in the Ainsworth Strange Situation Procedure (SSP; Ainsworth, Blehar, Waters, & Wall, Reference Ainsworth, Blehar, Waters and Wall1978), for patterns of neural activation to reward and loss at age 20 years, assessed during a functional magnetic resonance imaging (fMRI) scan in a moderately large (N = 171), high-risk sample of young men.
Infant attachment behavior during the SSP is most commonly coded using a system designed to inductively sort infant–mother dyads into three primary attachment categories (Ainsworth et al., Reference Ainsworth, Blehar, Waters and Wall1978). Infants with a secure attachment relationship seek out support and comfort from their caregivers when they are reunited after brief separations during the SSP, and this proximity is effective in relieving their emotional distress in that such infants quickly return to engagement with the environment. In contrast, during reunion episodes, insecure–avoidant infants ignore their caregiver and do not seek proximity to resolve their emotional distress following separation, whereas insecure–resistant infants simultaneously seek proximity and resist being soothed by it. In addition to the three “organized” patterns of attachment, a small minority of infants display a mix of patterns and anomalous behaviors suggesting a breakdown of the attachment system. Infants who display these behavioral features are categorized as disorganized (Main & Hesse, Reference Main, Hesse, Greenberg, Cicchetti and Cummings1990).
Meta-analytic reviews of decades of research focused on the developmental origins and consequences of individual differences in infant attachment largely support the core predictions of Bowlby's (Reference Bowlby1980/1969) attachment theory that the quality of early parental care helps organize infant attachment and that in turn the quality of attachments in the early life course has predictive significance for children's social–emotional development and adjustment (Fearon, Bakermans-Kranenburg, van IJzendoorn, Lapsley, & Roisman, Reference Fearon, Bakermans-Kranenburg, van IJzendoorn, Lapsley and Roisman2010; Groh, Roisman, et al., Reference Groh, Fearon, Bakermans-Kranenburg, van IJzendoorn, Steele and Roisman2014; Groh, Roisman, van IJzendoorn, Bakermans-Kranenburg, & Fearon, Reference Groh, Roisman, van IJzendoorn, Bakermans-Kranenburg and Fearon2012; Madigan, Atkinson, Laurin, & Benoit, Reference Madigan, Atkinson, Laurin and Benoit2013). As regards caregiving influences on infant attachment security, maternal sensitivity to infant signals and support for autonomy reliably predicts infant attachment security (Bernier, Matte-Gagne, Belanger, & Whipple, Reference Bernier, Matte-Gagne, Belanger and Whipple2014; Riem, Bakermans-Kranenburg, van IJzendoorn, Out, & Rombouts, Reference Riem, Bakermans-Kranenburg, van IJzendoorn, Out and Rombouts2012). Likewise, infant attachment insecurity has been shown to be a significant predictor of internalizing symptoms (Groh et al., Reference Groh, Roisman, van IJzendoorn, Bakermans-Kranenburg and Fearon2012; Madigan et al., Reference Madigan, Atkinson, Laurin and Benoit2013), externalizing behavior (Fearon et al., Reference Fearon, Bakermans-Kranenburg, van IJzendoorn, Lapsley and Roisman2010), and lower levels of social competence with peers (Groh, Roisman, et al., Reference Groh, Roisman, Haydon, Bost, McElwain, Garcia and Hester2014). Despite this large corpus of research examining the consequences of infant attachment security on multiple behavioral outcomes, there is little knowledge regarding brain-based mechanisms that might account for these associations. Until recently, the methods necessary to examine individual differences in how the brain processes information had not been incorporated into attachment research, particularly in the context of prospective, longitudinal research designs, where attachment security had been assessed during infancy using the SSP.
Adult Attachment and Brain Function
Existing research on central nervous system correlates of attachment security has instead focused almost exclusively on concurrent assessments of attachment security in adulthood and brain activity in various fMRI- and EEG-based paradigms (for a broad review, see Swain et al., Reference Swain, Kim, Spicer, Ho, Dayton, Elmadih and Abel2014; see also Gander & Buchheim, Reference Gander and Buchheim2015). Such studies have examined links between maternal sensitivity and/or attachment security (often but not exclusively among parents) with neural responses to a wide range of attachment-relevant tasks, including listening to audio-recordings of infant distress (Riem et al., Reference Riem, Bakermans-Kranenburg, van IJzendoorn, Out and Rombouts2012), passively viewing images of familiar and unfamiliar infants (Strathearn, Fonagy, Amico, & Montague, Reference Strathearn, Fonagy, Amico and Montague2009; Strathearn, Li, Fonagy, & Montague, Reference Strathearn, Li, Fonagy and Montague2008), and engaging in fMRI-compatible paradigms requiring top-down cognitive control in the presence of emotional distractions (Warren et al., Reference Warren, Bost, Roisman, Silton, Spielberg, Engels and Heller2010).
Parental response to infant signals is thought to be a key component of attachment behavior. Research examining neural responses to infant crying in particular has found adult attachment security is associated with differential neural patterns of activation. For example, Riem et al. (Reference Riem, Bakermans-Kranenburg, van IJzendoorn, Out and Rombouts2012) examined associations between adult attachment security and neural activity of 21 women (nonmothers) listening to infant cries. Imaging data revealed heightened amygdala activity among participants with insecure attachments. Similarly, in a sample of 108 mothers, EEG activation in response to infant cries was examined, and mothers with less secure base script knowledge showed a smaller left (vs. right) shift in frontal EEG asymmetry compared to baseline, indicating restricted or inhibited emotional engagement with the stimuli (Groh, Roisman, et al., Reference Groh, Roisman, Haydon, Bost, McElwain, Garcia and Hester2014). These sorts of findings support the argument that individuals’ attachment security is associated with processing of attachment-relevant signals and the corresponding engagement of emotional/motivational states related to caregiving (Lemche et al., Reference Lemche, Giampietro, Surguladze, Amaro, Andrew, Williams and Phillips2006).
Maternal neural responses to infant visual cues, such as smiling, have also been associated with adult attachment security status. For example, Strathearn et al. (Reference Strathearn, Fonagy, Amico and Montague2009) examined associations between adult attachment status and the neural responses of 30 mothers when exposed to images of their infants’ emotional faces during an fMRI scan. Results indicated that mothers with a secure attachment status showed greater activation in reward circuits when viewing images of their smiling infants compared to mothers with an insecure attachment status, suggesting that secure mothers find the positive affect displayed by their infant as more rewarding. Further, insecure mothers showed greater dorsolateral prefrontal cortex (DLPFC) and insula activation when viewing their infants’ sad face compared to securely attached participants. This neural pattern of hyperactivation may be indicative of needing enhanced cognitive control in response to their infants’ negative affect.
Associations between insecure attachment and higher activity of cognitive control and social-processing circuitry have also been found in response to negative affective stimuli more generally. Warren et al. (Reference Warren, Bost, Roisman, Silton, Spielberg, Engels and Heller2010), for example, had adult participants complete an emotional Stroop task during an fMRI scan and found that individuals scoring lower on a measure of secure base script knowledge exhibited more activation to negative words (compared to neutral) in the orbitofrontal cortex and the DLPFC. Similar effects were also found during positive word trials compared to neutral. This suggests that attachment insecurity may result in a general hyperactivation of inhibitory and cognitive control systems in response to emotional stimuli in the environment. Similarly, alterations in basal ganglia and prefrontal cortex (PFC) functioning (key nodes for reward encoding and social–emotional processing) during receipt of reward feedback has been reported in connection to early parenting variables in longitudinal research (Morgan, Shaw, & Forbes, Reference Morgan, Shaw and Forbes2014). Finally, data from Quevedo et al. (Reference Quevedo, Johnson, Loman, Lafavor, Moua and Gunnar2015) suggested that the psychobiological basis of reward processing, supported by the postauricular reflex, a measure of hedonic arousal, would be hypersensitive to any emotionally salient stimuli, pursuant to a history of early attachment disturbances such as those experienced by infants adopted from international institutions. Taken as a whole, the literature suggests that cognitive, social, and reward neurobiological substrates may be hyperactive in response to emotionally charged stimuli after a history of insecure or disturbed attachment experiences.
All of this said, to date, there has only been one prospective, longitudinal investigation of the impact of attachment security in the early life course on the neural underpinnings of adult emotional processing. Specifically, Moutsiana et al. (Reference Moutsiana, Fearon, Murray, Cooper, Goodyer, Johnstone and Halligan2014) followed up with 54 adults whose attachment security had been assessed 20 years prior (at age 18 months) as part of a longitudinal study of the impacts of maternal depression (Murray, Reference Murray1992). They found that adults with insecure attachment histories in infancy showed greater neural activation when attempting to upregulate positive emotion (Moutsiana et al., Reference Moutsiana, Fearon, Murray, Cooper, Goodyer, Johnstone and Halligan2014). Specifically, individuals who had been insecurely attached as infants showed greater activation in prefrontal cortical regions associated with cognitive control and social processing, and reduced coactivation of the nucleus accumbens and the PFC (Moutsiana et al., Reference Moutsiana, Fearon, Murray, Cooper, Goodyer, Johnstone and Halligan2014). These results suggest that early attachment security plays a role in shaping the neurological functioning of the emotion regulation and cognitive control system. Unfortunately, the study sample was small and did not allow for the examination of the impact of attachment disorganization, focusing exclusively on secure versus insecure attachment. Further, a significant portion of the participants were selected based on having mothers who met clinical criteria for depression. As a result, replication with a larger sample containing adequate numbers of organized and disorganized attachment cases and with participants who experienced a more representative frequency of maternal mental illness during development is needed to examine the generalizability of these results. In particular, it is important also to evaluate the prediction that insecure and/or disorganized attachment has a strong impact on social–emotional function, emotion regulation, and cognitive control during reward processing, as these are the hypothesized areas of adaptive functioning that would be affected by early rewarding or unrewarding close relationships with caregivers.
The Present Study
Most research studying associations between individual differences in attachment and neural correlates of emotional and cognitive processing has focused on cross-sectional designs with relatively small samples of adults. This is problematic for several reasons. First, these studies have been unable to evaluate the impact of infant attachment on later neurological functioning, which is a central claim on attachment theory. Second, small samples do not allow for the examination of the impact of attachment classifications with relatively low base rates (e.g., disorganization) on later neural processing. Third, there are currently no published data regarding the long-term consequences of infant attachment status for the neural basis of reward processing during the transition into adulthood. Infant attachment insecurity might have particularly strong long-term influence on systems of reward and positive emotion because adaptive social behavior hinges decisively on both deriving and eliciting rewards in the context of social relationships and regulating emotions in the midst of social challenges.
Building on the Moutsiana et al. (Reference Moutsiana, Fearon, Murray, Cooper, Goodyer, Johnstone and Halligan2014) findings suggesting that insecure infant attachment would result in both basal ganglia and PFC hyperactivity during the processing of positive (e.g., rewards) and negative (e.g., loss) outcomes, we hypothesized that young adults with a history of insecure infant attachment would evidence hyperactive function in both basal ganglia and PFC during the anticipation and receipt of reward-related feedback compared to infants with a history of secure attachment. We also expected that young adults with a history of disorganized infant attachment in particular would show limbic hyperactivity compared to participants with organized attachment during both anticipation and receipt of losses and rewards, specifically the amygdala and insula, which are substrates for fear, intensely salient emotions (including intense positive ones), and awareness of interoceptive negative information and pain, respectively, all of which are central to putative explanations of the origins of disorganization in infancy (Main & Hesse, Reference Main, Hesse, Greenberg, Cicchetti and Cummings1990).
Methods
Participants
Participants are a subset of individuals enrolled in the Pitt Mother and Child Project, an ongoing longitudinal study of child vulnerability and resiliency in low-income families (Shaw, Gilliom, Ingoldsby, & Nagin, Reference Shaw, Gilliom, Ingoldsby and Nagin2003). From 1991 to 1992, 310 infant boys and their mothers were recruited from Women, Infants, and Children nutrition supplement clinics in Allegheny County, Pennsylvania, when the boys were between 6 and 17 months old. At the time of recruitment, 53% of the target children in the sample were European American, 36% were African American, 5% were biracial, and 6% were of other races (e.g., Hispanic American or Asian American). Two-thirds of mothers in the sample had 12 years of education or less. The mean per capita income was $241 per month ($2,892 per year), and the mean Hollingshead socioeconomic status score was 24.5, indicative of a working-class sample. Thus, many boys in this study were considered at elevated risk for maladaptive outcomes because of their socioeconomic standing. The present study included 171 young adults (Table 1) who had both assessments of attachment classifications when they were 18 month of age using the only SSP assessment performed at that age and imaging data free of movement artifact when they were young adults (20 years old).
Table 1. Percentages and frequencies of sociodemographic variables

Note: No significant differences were found between attachment groups. SES, Socioeceonomic status.
a Statistics represent comparisons between the secure vs all insecure attachment histories.
fMRI paradigm
Participants completed a slow event-related fMRI reward paradigm (t = 8 ms) adapted from a task originally designed by Delgado, Nystrom, Fissell, Noll, and Fiez (Reference Delgado, Nystrom, Fissell, Noll and Fiez2000). On each trial, participants guessed whether a number would be greater or smaller than 5, and were led to believe that their performance would determine the amount of money to be received after the scan. Losing or winning would diminish (by $0.50) or increase (by $1) their earnings. Some trials included neutral outcomes, when guesses resulted in no change in earnings. During anticipation the subjects believed they had a high likelihood of winning or losing money based on their guesses. During the outcome phase, the actual number was displayed along either an arrow or a dot (win = up arrow, loss = down arrow, or neutral/no change = dot). Outcomes could be classified as “win” (guessing correctly after an anticipated monetary reward); “loss” (guessing incorrectly after an anticipated loss); “disappointment” (not receiving money in a neutral outcome after an anticipated monetary reward); or “relief” (being spared an anticipated monetary loss on a neutral outcome). Baseline between trials required no task-related efforts aside from watching a fixation cross. Trials were presented in a single run, with 24 trials total and a balanced number of trial types within runs.
fMRI acquisition, processing, and analysis
Each participant underwent scanning using a Siemens 3T Trio scanner. Blood oxygen level dependent (BOLD) functional images were acquired with a gradient echo planar imaging sequence and covered 39 axial slices (3.1 mm thick) beginning at the cerebral vertex and encompassing the entire cerebrum and the majority of the cerebellum (repetition time/time to echo = 2000/25 ms, field of view = 20 cm, matrix = 64 × 64). All scanning parameters were selected to optimize the quality of the BOLD signal while maintaining a sufficient number of slices to acquire whole-brain data. Before the collection of fMRI data for each participant, we acquired a reference echo planar imaging scan that was visually inspected for artifacts (e.g., ghosting) and for good signal across the entire volume of acquisition. The fMRI data from all included participants were cleared of such problems. Preprocessing and whole-brain image analyses were completed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm). For each scan, structural images for each participant were segmented, and functional images were realigned to correct for head motion, coregistered to the segmented structural data, spatially normalized into standard stereotaxic space (Montreal Neurological Institute template) using a 12-parameter affine model, and smoothed with a 6-mm full width at half-maximum Gaussian filter. Participants’ data were inspected for adequate coverage of the ventral striatum (>80%). All included participants had movement of <2 mm in each plane on average across all frames. Attachment groups did not differ in movement.
Data analysis
In all analyses, variables at age 18 month (family income, mother employment, mother age at birth of participant, mother marital status, mother ethnicity, mother education, father/partner education, and occupation) and for the participant at age 20 (income, education, and relationship status) and nicotine use were used as covariates. These control variables were included to rule out socioeconomic factors, including maternal education and family income, before attributing brain function differences to attachment security. Differences could be attributable to growing up in different levels of poverty versus having an insecure and/or disorganized attachment.
A first-level fixed-effect model was constructed for each participant producing a statistical image for two contrasts reflecting each possible anticipation condition relative to baseline: “reward anticipation > baseline = anticipation reward” and “loss anticipation > baseline = anticipation loss,” and four contrasts reflecting each possible outcomes category relative to baseline: “win,” “disappointment,” “loss,” and “relief.” Disappointment was a thwarted anticipation of winning, and relief avoiding a possible loss announced during the anticipation condition. The first-level activation maps were submitted to second-level random effects analyses (generalized linear modeling) to investigate infant attachment classification effects on BOLD activity during the anticipation (of reward or loss) and the receipt (win, loss, disappointment, or relief) stages. Participants were divided in the two security classifications (secure vs. insecure) and in organization subclassifications (secure vs. insecure versus disorganized) and within group conditions with either two anticipation (loss and reward) or four outcome (win, disappointment, loss, or reward) conditions. Both whole-brain and region of interest (ROIs) analyses were conducted. ROIs reflected significant prior findings with this task in adult and adolescent populations (Forbes et al., Reference Forbes, Hariri, Martin, Silk, Moyles, Fisher and Dahl2009; Forbes, Miller, Cohn, Fox, & Kovacs, Reference Forbes, Miller, Cohn, Fox and Kovacs2005; Forbes, Shaw, & Dahl, Reference Forbes, Shaw and Dahl2007).
To correct for multiple comparisons, we calculated whole-brain, voxelwise, and cluster extent thresholds via Monte Carlo simulations using the program 3dClustSim in AFNI. For ROI results of attachment security across all anticipation conditions and given a voxelwise threshold of p < .05, a cluster-extent threshold of k = 180.3 voxels for the caudate and putamen ROI, k = 71.2 voxels for the DLPFC ROI, k = 161.2 voxels for the insula ROI, and k = 61.2 voxels for the amygdala ROI corresponded with p < .05, family-wise error corrected. Given a voxelwise threshold of p < .005, a cluster-extent threshold of k = 130.1 voxels for whole-brain results of attachment across all outcome conditions corresponded with p < .05, family-wise error corrected. These joint magnitude-extent thresholds in addition to p significant peak activations of <.05 are used to report our results and in our tables and figures.
Results
Anticipation condition
There was no whole-brain level significant effects of attachment security or organization during either the reward or loss anticipation condition; all results during anticipation were found using ROI analyses presented below.
Attachment security by anticipation conditions
During the anticipation reward condition, young males with a history of insecure attachment exhibited significantly higher activity in regions of interest that included the bilateral striatum (caudate and putamen) and right DLPFC, as well as less deactivation in the bilateral insula (Table 2, Figure 1a) compared to those with a secure infant attachment history. Mother's unemployment when the participant was age 18 months was significantly linked to higher striatum activity, F (1, 116) = 4.42, p < .05, yet all findings remained significant. During the anticipation loss condition, adult males with insecure attachment in infancy again showed significantly higher activation in the left DLPFC ROI than those with a history of secure attachment (Table 2, Figure 1b).

Figure 1. (Color online) (a,b) During anticipations of monetary rewards and losses, young males with a history of insecure attachment evidenced higher activity in reward processing (caudate and putamen) and saliency circuitry (insula) as well as in areas supporting executive and cognitive control (dorsolateral prefrontal cortex) compared to young men with a history of secure attachment. (c) Young men with a history of insecure disorganized infant attachment showed higher amygdala activity during reward anticipation versus young men with a history of both organized secure and insecure attachment.
Table 2. Neural regions of interest with significant activity that distinguish infant attachment history among young adult males during anticipation of monetary rewards and losses.

Attachment organization by anticipation conditions
Effects of organization were present only for the anticipation reward condition (Table 2, Figure 1c), specifically young males with a history of disorganized infant attachment exhibited more amygdala activity while anticipating rewards versus those with a history of organized attachment (participants with a history of organized-insecure and organized-secure attachment in infancy did not differ in amygdala activity). Higher family income was linked to lower amygdala activity, F (1, 115) = 4.65, p < .05, yet all findings remained significant when income was used as a covariate.
Outcome conditions
There was an omnibus whole-brain significant main effect of attachment security in midline cortical structures, namely, in the superior medial frontal gyrus, the precuneus, and the limbic cortex: anterior cingulate cortex (ACC) and posterior cingulate cortex, which are all self- and social-processing regions (Kobayakawa & Kawamura, Reference Kobayakawa and Kawamura2011; van Veluw & Chance, Reference van Veluw and Chance2014). Follow-up analyses showed that the omnibus effects were due to young adults with a history of insecure attachment in infancy showing higher activity than those with history of secure attachment, with significant differences present for the loss, disappointment, and relief outcomes but not the win outcome condition (Table 3). Below we describe how the neural structures were active within the three outcome conditions.
Table 3. Neural areas of whole brain significant activity that distinguish infant attachment history among young adult males during the receipt of monetary rewards and losses

Attachment security by outcome condition
The loss and disappointment outcome conditions elicited higher activity in the ACC and superior medial frontal gyrus (Brodmann area 10 [BA10]) in participants with a history of insecure attachment versus adults with a history of secure attachment in infancy, t (679) = 5.14, p < .01. Adults with an insecure history also showed higher precuneus activity to the loss condition, t (679) = 4.71, p < .05, and less insula deactivation to the disappointment and relief conditions compared to adults with a history of attachment security, t (679) = 4.67–4.73, p < .05. In addition, adults with an insecure attachment in infancy exhibited higher DLPFC activation during the relief condition and higher cuneus and lingual gyrus activity during the disappointment condition, t (679) = 5.16–4.73, p < .05, relative to those with secure attachment in infancy (Table 3, Figure 2a–c).
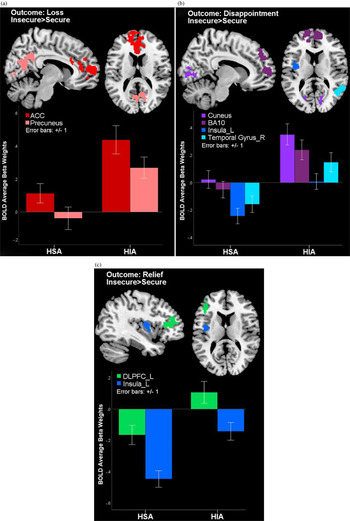
Figure 2. (Color online) (a–c) During receipt of monetary outcomes, young males with a history of insecure attachment showed higher activity in areas supporting self-processing and social cognition (anterior cingulate cortex, precuneus, superior medial frontal gyrus, and temporal gyrus), visual processing (cuneus), and emotional saliency (anterior cingulate cortex and insula), as well as executive control (dorsolateral prefrontal cortex) compared to young males with a history of secure infant attachment.
Effects of attachment organization
Young adults classified as insecure disorganized attachment in infancy showed more activity in the DLPFC during receipt of monetary losses (the loss outcome condition) than those with a history of organized insecure attachment (Table 3), who in turn had more DLPFC activity than those with a history of organized secure attachment, t (675) = 4.85, p < .05.
Discussion
Overall, our results were consistent with previous theory and research suggesting that insecure attachment is associated with altered neural response to emotionally meaningful feedback, specifically demonstrating hyperactivation in the striatal and cortical regions linked to social cognition during an emotion-eliciting reward and losses based task relative to adults with a history of secure attachment. A critical point is that this hyperactivation in striatal, midline cortical regions and regions that support cognitive control was present for both anticipation and receipt of rewards and losses of money. An effect of attachment organization was noted during the anticipation of rewards condition, during which young adult males with a history of disorganized attachment showed higher amygdala activity (a hypothesized ROI for this attachment classification) than young males with a history of both insecure and secure organized infant attachment, yet during the receipt of losses, disorganized infant attachment was associated with more DLFPC activity than insecure attachment in our sample of boys, who in turn had more activity than secure infant attachment history (Table 3). Below, we discuss the details and implications of these findings in detail.
Midline cortical structures, emotion regulation, and the social brain
In the anticipation condition, ROI analyses revealed an effect of attachment insecurity characterized by hyperactivation in bilateral caudate and putamen, right DLPFC, and greater deactivation in the bilateral insula (reward anticipation), greater left DLPFC, and left insula activity (loss anticipation). Cortical structures (i.e., insula and DLPFC) showing heightened activation during the reward anticipation condition suggested that males with a history of insecure attachment were more attentive to interoceptive salient information (insula) and were possibly exercising greater executive and cognitive control in response to the demands of the task and/or internal cues during both reward and loss cues (e.g., Menon & Uddin, Reference Menon and Uddin2010). Greater caudate and putamen activity also suggest greater preparedness and/or reward sensitivity in response to anticipated positive events among young males with a history of insecure attachment (Luking & Barch, Reference Luking and Barch2013). Further, in the anticipation loss condition, participants with a history of insecure attachments in infancy showed heightened activation in the left DLPFC, a region associated with implementation of cognitive control and top-down emotion regulation (e.g., MacDonald, Cohen, Stenger, & Carter, Reference MacDonald, Cohen, Stenger and Carter2000; Miller & Cohen, Reference Miller and Cohen2001). This is consistent with the possible interpretation that individuals with an insecure attachment history may be exerting more cognitive effort to regulate emotions, perform the task, and/or adjust behavior during task conditions that potentially elicit strong positive and negative affect. It is interesting that an area associated with cognitive executive control and planning such as the DLPFC is primarily more active during the anticipation conditions whereas areas associated with social and self-referential processing are active in addition to the neurobiology of cognitive control for young males with a history of disorganized attachment during the outcome condition. This suggests possible greater exertion of cognitive effort during conditions of uncertainty and evaluation of the self-relevance, personal significance, or/and attribution of outcomes to the self upon the receipt of outcomes among young males with a history of insecure attachment.
The outcome condition revealed strong whole-brain effects. Loss and disappointment outcome conditions resulted in greater activation in midline cortical structures (i.e., ACC and BA10) for participants who had been insecurely attached in infancy. In addition, these participants had greater activation in the left DLPFC (relief) and left insula (relief), and cuneus and lingual gyrus (disappointment). Infant attachment disorganization more specifically was associated with both greater right amygdala activation during anticipation of reward only (discussed below) and greater DLPFC activation during the loss outcome. These results again suggest that males with a history of disorganized attachment might need to expend greater cognitive effort in regulating strong emotions elicited during the anticipation and outcome conditions or to organize behavioral responses during the task.
Across all outcome conditions, individuals with a history of insecure attachment showed hyperactivation in cortical regions associated with task monitoring, self-awareness, and cognitive control. The ACC, BA10, and superior frontal gyrus activation during loss and disappointment conditions suggests such individuals were hypervigilant during negative outcomes and exerting heightened cognitive control (e.g., ACC, Bush et al., Reference Bush, Vogt, Holmes, Dale, Greve, Jenike and Rosen2002; BA10, Koechlin & Hyafil, Reference Koechlin and Hyafil2007; superior frontal gyrus, Petrides, Reference Petrides2005, and Talati & Hirsch, Reference Talati and Hirsch2005). Furthermore, hyperactivity in the precuneus and cuneus suggests enhanced self-referential processing and/or attribution of agency during the loss and disappointment conditions (Cavanna & Trimble, Reference Cavanna and Trimble2006; Farrer & Frith, Reference Farrer and Frith2002) is a finding that is similar to enhanced centrality of regions involved in internal emotional perception, self-referential thinking, and self-awareness linked to more adverse early parenting experiences (Teicher, Anderson, Ohashi, & Polcari, Reference Teicher, Anderson, Ohashi and Polcari2014). Heightened activation in the lingual gyrus during disappointment suggested that participants with a history of insecure attachments were also more sensitive to violation of expectations (Goel & Dolan, Reference Goel and Dolan2001). DLPFC and insula hyperactivation in these participants during the relief condition might have resulted from difficulties in upregulating negative emotion when participants were expecting a negative outcome but instead were surprised with a neutral outcome. Similar processes may have been at play in individuals with a history of disorganized attachment. It is of note that these social cognition areas are engaged in individuals with a history of insecure and insecure disorganized attachment with regards to the loss or disappointing expectation of earning a small monetary reward, suggesting that adverse life events would possibly affect adults with a history of insecure attachment to a greater extent than those with an early secure history.
Limbic systems, striatum, and amygdala, and the emotional experience of loss and gain
The hyperactivation in the basal ganglia during the anticipation of reward condition suggests that individuals who were insecurely attached in infancy develop greater reactivity in regions associated with reward processing and goal-directed action/learning (i.e., caudate and putamen; Grahn, Parkinson, & Owen, Reference Grahn, Parkinson and Owen2008). Prior research suggests that the putamen and caudate are especially important for learning action-outcome contingencies (e.g., Packard & Knowlton, Reference Packard and Knowlton2002; Tricomi, Delgado, & Fiez, Reference Tricomi, Delgado and Fiez2004; Tricomi, Delgado, McCandliss, McClelland, & Fiez, Reference Tricomi, Delgado, McCandliss, McClelland and Fiez2006). Further, the caudate nucleus shows similarly heightened activation independent of the type of reward received: intrinsic versus extrinsic (Tricomi et al., Reference Tricomi, Delgado, McCandliss, McClelland and Fiez2006). Hence, the result obtained here may generalize across these different types of motivated action. Perhaps insecure individuals, who may have more difficulty regulating negative affect or experience more emotional arousal linked to strong positive expectations (e.g., Mikulincer & Shaver, Reference Mikulincer, Shaver, Cassidy and Shaver2008), dedicate greater cognitive resources to learning which actions and contextual factors lead to rewards perhaps to maximize positive affect. Supporting this hypothesis of strong emotionality during anticipation of events is the fact that adult males with a disorganized attachment, in comparison to those with organized attachments during infancy, yielded greater amygdala activation during the anticipation of reward. These results once again suggest that learning and performance in the task was, at least in part, emotionally motivated (Balleine & Killcross, Reference Balleine and Killcross2006; McGaugh, Reference McGaugh2004). The amygdala in particular is strongly implicated in experienced emotion, and it is most reliably activated for negatively valenced emotions (Anders, Eippert, Weiskopf, & Veit, Reference Anders, Eippert, Weiskopf and Veit2008; Kensinger & Schacter, Reference Kensinger and Schacter2006). Although we are unable to differentiate between positive and negative affective experiences here during the anticipation of reward condition, it is possible that the activation observed in the current study reflects a negative appraisal of performance or anticipated outcome influenced by the participants’ developmental histories. However, it is also possible that higher amygdala activity simply represents greater emotional saliency and significance experienced by young men with a history of insecure attachment, and not necessarily a negative emotional experience, given that the amygdala activity also represents emotional saliency regardless of emotional valence (Cunningham & Brosch, Reference Cunningham and Brosch2012).
Overall, our results indicate that young males with a history of insecure attachment evidence neural activity suggestive of greater intensity of emotional experience and cognitive control and executive preparedness during conditions of expectation and uncertainty (i.e., anticipation of rewards or losses) and greater engagement in self-referential cognition, socioemotional processing, and/or self-attributions during the receipt of outcomes, particularly during negative events such as losses and disappointment, given that no differences where found between those with a history of insecure attachment and those with a history of secure attachment for the receipt of rewards. Furthermore, again given the small value of the monetary losses and rewards involved in the present task, it is remarkable the level of emotional and cognitive investment that adults with a history of insecure attachment may be displaying. Greater emotional reactivity and need for effortful emotion regulation or cognitive control might mean fewer resources available to adaptively and efficiently respond to larger emotional and environmental challenges. However, whether these heightened patterns of neural activity represent a disadvantage for adults with a history of insecure attachment compared with those with a history of secure attachment during more emotionally challenging and/or cognitively demanding circumstances would need to be demonstrated. The significance of the current findings for adaptive behavior thus still needs to be determined.
Limitations
Despite the importance of the current novel findings linking infant attachment security prospectively to differences in cognitive and information processing during early adulthood, the study is not without its methodological limitations. First, because of the study's original focus on the developmental precursors of antisocial behavior and a limited budget, only families with male infants were recruited for participation because of the much higher number of females who would have been needed to be recruited to obtain comparable number of youth with clinically meaningful levels of antisocial behavior. As a result, we were unable to examine any potential gender differences in neural processing, and the current findings can only be inferred to apply to low-income males from urban communities. Future studies should be carried out with females from low-income urban contexts and males and females varying in socioeconomic status and urbanicity (e.g., from suburban and rural communities).
Second, infant attachment security was only assessed once. Additional assessments of attachment across the childhood and adolescent period would have allowed for the examination of differential influences of early versus later versus concurrent attachment experience on the functioning of neural systems. Third, future research would also benefit from the inclusion of fMRI tasks that also include attachment-specific stimuli. The paradigm employed here examined more generic stimuli designed to activate the reward, emotion, and cognitive control systems. It is essential to examine neural responses to positive and negative attachment stimuli in a similar way to establish if the present findings are unique to the paradigm and also examine the possibility of additional regions of hyperactivation depending on the attachment relevance of the stimuli. This would allow researchers to disentangle domain-general and domain-specific developmental effects of attachment on the neural system. Fourth, while we found theory-consistent results for amygdala activation among young men with a history of disorganized attachment, a small sample size (n = 15) for these participants underscores the need for replication, including whether this pattern of neural response mediates early attachment and later outcomes such as competence in social and intimate relationships.
Conclusions
Despite these caveats, the results from anticipation and outcome conditions taken together suggest that having an insecure attachment history in infancy may result in hypersensitivity/vigilance to both positive and negative stimuli (anticipated or experienced) and dedication of high-level cognitive resources to processing negative outcomes in the environment in early adulthood in comparison to individuals with a secure attachment history. These results are consistent with the interpretation that individuals with a secure attachment history more easily and flexibly respond to negative and positive experiences (e.g., Cassidy, Reference Cassidy1994; Mikulincer, Shaver, & Pereg, Reference Mikulincer, Shaver and Pereg2003). Further, a disorganized attachment in infancy potentially results in more emotional reactivity to the possibility of positive stimuli and the deployment of greater cognitive resources to regulate affect when negative stimuli are encountered. Such results support the hypothesis that attachment quality early in life impacts later behavior and reactivity in the form of distinctive patterns of neural activation. These effects were observed at both the cortical and the limbic emotional supporting subcortical levels. It remains an open question as to when the particular patterns of hyperactivation observed here manifest and how they contribute to adaptive and maladaptive functioning across development.







