The experience of early life stress (ELS) is associated with mental health difficulties such as depression and anxiety (Andersen & Teicher, Reference Andersen and Teicher2008; Heim & Nemeroff, Reference Heim and Nemeroff2001; Lupien, McEwen, Gunnar, & Heim, Reference Lupien, McEwen, Gunnar and Heim2009; Teicher & Samson, Reference Teicher and Samson2016). Exposure to ELS accounts for nearly one-third of all mood and anxiety disorders in the United States, underscoring the critical role of ELS as a risk factor for the onset of internalizing psychopathology (Green et al., Reference Green, Mclaughlin, Berglund, Gruber, Sampson, Zaslavsky and Kessler2010; McLaughlin et al., Reference McLaughlin, Greif Green, Gruber, Sampson, Zaslavsky and Kessler2012). ELS has been theorized to confer risk for the emergence of psychopathology through several stress-related neurobiological pathways. Specifically, ELS may affect functioning of the hypothalamic–pituitary–adrenal axis and consequently influence the development of neural structures with high densities of corticotropin-releasing factor neurons, including portions of the prefrontal cortex (PFC) and amygdala (Heim et al., Reference Heim, Newport, Wagner, Wilcox, Miller and Nemeroff2002; Lupien et al., Reference Lupien, McEwen, Gunnar and Heim2009; Malter Cohen, Tottenham, & Casey, Reference Malter Cohen, Tottenham and Casey2013; Nemeroff, Reference Nemeroff2004). ELS-induced alterations in the development of neural regions involved in this corticolimbic stress regulatory system may contribute to risk for psychopathology (for a review, see Heim & Binder, Reference Heim and Binder2012; Lupien et al., Reference Lupien, McEwen, Gunnar and Heim2009).
For instance, researchers have found that adults with a history of ELS exhibit both heightened amygdala response and positive functional connectivity, or temporal correlation, between right ventrolateral PFC (right vlPFC) and amygdala while labeling threatening (i.e., fearful and angry) faces, compared to adults without histories of adversity (Taylor, Eisenberger, Saxbe, Lehman, & Lieberman, Reference Taylor, Eisenberger, Saxbe, Lehman and Lieberman2006). Negative functional connectivity between PFC and amygdala during the processing of threat-related stimuli is typically found in adults and is posited to reflect adaptive emotion regulation by the PFC (Gee, Humphreys, et al., Reference Gee, Humphreys, Flannery, Goff, Telzer, Shapiro and Tottenham2013). In contrast, the positive functional connectivity of PFC and amygdala in adults with ELS suggests that stress leads to atypical emotion regulation, presumably through ineffective PFC regulation of amygdala responses (Taylor et al., Reference Taylor, Eisenberger, Saxbe, Lehman and Lieberman2006). It is important to note, however, that most of the studies that have found altered corticolimbic function following exposure to ELS have been conducted with adult samples and have used retrospective reports of ELS obtained many years after exposure to stress (Burghy et al., Reference Burghy, Stodola, Ruttle, Molloy, Armstrong, Oler and Birn2012; Dannlowski et al., Reference Dannlowski, Stuhrmann, Beutelmann, Zwanzger, Lenzen, Grotegerd and Kugel2012; Fan et al., Reference Fan, Herrera-Melendez, Pestke, Feeser, Aust, Otte and Grimm2014; Heim & Binder, Reference Heim and Binder2012; Herringa et al., Reference Herringa, Birn, Ruttle, Burghy, Stodola, Davidson and Essex2013, Reference Herringa, Burghy, Stodola, Fox, Davidson and Essex2016; Taylor et al., Reference Taylor, Eisenberger, Saxbe, Lehman and Lieberman2006; van Harmelen et al., Reference van Harmelen, van Tol, Demenescu, van der Wee, Veltman, Aleman and Elzinga2013, Reference van Harmelen, van Tol, Dalgleish, van der Wee, Veltman, Aleman and Elzinga2014). Thus, it is difficult to assess whether these neurobiological changes are a direct consequence of having experienced adversity early in development or, alternatively, are a signature of adult-onset psychopathologies.
Given the significant consequences of exposure to ELS, combined with the growing recognition that many of these effects are not observed until adolescence (Gee & Casey, Reference Gee and Casey2015; Kessler et al., Reference Kessler, Berglund, Demler, Jin, Merikangas and Walters2005; Lee et al., Reference Lee, Heimer, Giedd, Lein, Estan, Weinberger and Casey2014), researchers have begun to focus on elucidating the psychological and neurobiological consequences of ELS during this developmental period (see Tottenham & Galván, Reference Tottenham and Galván2016, for a review). Investigators have posited that exposure to ELS alters the development of neural structures and connections that underlie emotion processing and regulation, including the PFC and amygdala, that, in turn, increases adolescents' risk for developing internalizing disorders (Burghy et al., Reference Burghy, Stodola, Ruttle, Molloy, Armstrong, Oler and Birn2012; Eiland & Romeo, Reference Eiland and Romeo2013; Maughan & Cicchetti, Reference Maughan and Cicchetti2002; Pechtel & Pizzagalli, Reference Pechtel and Pizzagalli2011; Tottenham et al., Reference Tottenham, Hare, Quinn, McCarry, Nurse, Gilhooly and Casey2010). This is particularly salient in adolescence when these neural systems are undergoing dramatic reorganization (Casey, Jones, & Hare, Reference Casey, Jones and Hare2008). Supporting this formulation, researchers have found that children and adolescents who have experienced high levels of ELS exhibit heightened activation to emotionally evocative faces and images in brain regions that are involved in processing salient stimuli (i.e., amygdala, anterior insula, dorsal anterior cingulate cortex; Garrett et al., Reference Garrett, Carrion, Kletter, Karchemskiy, Weems and Reiss2012; Gee, Gabard-Durnam, et al., Reference Gee, Gabard-Durnam, Flannery, Goff, Humphreys, Telzer and Tottenham2013; Marusak, Martin, Etkin, & Thomason, Reference Marusak, Martin, Etkin and Thomason2014; McCrory et al., Reference McCrory, De Brito, Kelly, Bird, Sebastian, Mechelli and Viding2013; McLaughlin, Peverill, Gold, Alves, & Sheridan, Reference McLaughlin, Peverill, Gold, Alves and Sheridan2015; Suzuki et al., Reference Suzuki, Luby, Botteron, Dietrich, McAvoy and Barch2014; Tottenham, Hare, Millner, et al., Reference Tottenham, Hare, Millner, Gilhooly, Zevin and Casey2011). Fewer investigators have examined how ELS may affect PFC regulation of the processing of emotional stimuli.
The PFC may be particularly sensitive to exposure to ELS given its high density of glucocorticoid receptors and its protracted development into adulthood (Giedd, Reference Giedd2004; McEwen & Morrison, Reference McEwen and Morrison2013; Pechtel & Pizzagalli, Reference Pechtel and Pizzagalli2011; Sanchez, Young, Plotsky, & Insel, Reference Sanchez, Young, Plotsky and Insel2000; Teicher et al., Reference Teicher, Andersen, Polcari, Anderson, Navalta and Kim2003). For example, McLaughlin et al. (Reference McLaughlin, Peverill, Gold, Alves and Sheridan2015) used a cognitive reappraisal task to examine functional magnetic resonance imaging (fMRI) responses of 13- to 19-year-old participants as they attempted to explicitly regulate their affective responses to emotionally salient images. Although previously maltreated adolescents showed the typical increased response in regions involved in processing salient stimuli to viewing negative images, they also showed increased activation in the superior frontal gyrus and frontal pole when downregulating their negative affect to a negative image, relative to nonmaltreated adolescents. Similarly, Marusak et al. (Reference Marusak, Martin, Etkin and Thomason2014) found that adolescents exposed to childhood trauma exhibited elevated dorsolateral PFC (dlPFC) activation when performing an emotional conflict task, suggesting early adverse experiences may have important consequences on the development of the PFC in adolescence.
In addition to the effects of ELS on activation to salient stimuli in the amygdala and the PFC, researchers have documented that ELS affects the functional connectivity between these two structures (Burghy et al., Reference Burghy, Stodola, Ruttle, Molloy, Armstrong, Oler and Birn2012; Gee, Gabard-Durnam, et al., Reference Gee, Gabard-Durnam, Flannery, Goff, Humphreys, Telzer and Tottenham2013; Marusak et al., Reference Marusak, Martin, Etkin and Thomason2014; Wolf & Herringa, Reference Wolf and Herringa2016). Using task-based connectivity analyses, researchers have found that adolescents exposed to ELS show stronger negative amygdala-PFC connectivity, a more mature, adultlike pattern of connectivity, while viewing negative stimuli (Gee, Gabard-Durnam, et al., Reference Gee, Gabard-Durnam, Flannery, Goff, Humphreys, Telzer and Tottenham2013; Wolf & Herringa, Reference Wolf and Herringa2016). These findings may reflect adaptive functioning in the face of adversity: children exposed to ELS may develop more adultlike patterns of connectivity at an earlier age in order to deal more effectively with environmental adversity. Gee, Gabard-Durnam, et al. (Reference Gee, Gabard-Durnam, Flannery, Goff, Humphreys, Telzer and Tottenham2013) showed that among previously institutionalized children and adolescents, having a negative pattern of amygdala–medial PFC connectivity was associated with lower separation anxiety, indicating that this adultlike pattern may be protective following experiences of early adversity. However, this pattern may also represent a premature end to a sensitive period for the development of this circuit. The long-term consequences of this earlier maturation are unclear, as are the developmental trajectories of the effects of ELS on this circuitry from childhood to adulthood.
Although researchers have now demonstrated that ELS affects the development of corticolimbic circuitry in childhood and adolescence, they have not examined possible sex differences in this association. ELS has, among females, been found to be a particularly important risk factor in the development of major depressive disorder (Ge, Conger, & Elder, Reference Ge, Conger and Elder2001; Ge, Lorenz, Conger, Elder, & Simons, Reference Ge, Lorenz, Conger, Elder and Simons1994; Rudolph & Flynn, Reference Rudolph and Flynn2007; Weiss, Longhurt, & Mazure, Reference Weiss, Longhurt and Mazure1999). Females also differ from males in their perceptions of stressful life events (Raffaelli et al., Reference Raffaelli, Strache, Parchetka, Artiges, Banaschewski, Bokde and Gallinat2016), in their biological response to both acute and chronic stressors, and in their neural responses to negative stimuli (Bourke, Harrell, & Neigh, Reference Bourke, Harrell and Neigh2012; Kajantie & Phillips, Reference Kajantie and Phillips2006; for reviews, see Bangasser & Valentino, Reference Bangasser and Valentino2014; Novais, Monteiro, Roque, Correia-Neves, & Sousa, Reference Novais, Monteiro, Roque, Correia-Neves and Sousa2016; Ordaz & Luna, Reference Ordaz and Luna2012; Stevens & Hamann, Reference Stevens and Hamann2012). Given striking sex differences in the incidence of internalizing disorders, including evidence that females are twice as likely as males to develop major depressive disorder in adolescence and adulthood (Hankin & Abramson, Reference Hankin and Abramson1999), there may be sex-specific mechanisms, including sex-specific effects on corticolimbic circuitry, through which ELS contributes to vulnerability for internalizing problems in adolescence (Teicher et al., Reference Teicher, Andersen, Polcari, Anderson, Navalta and Kim2003).
In this context, the age at which sex differences in internalizing disorders become most pronounced corresponds to the complex developmental period of puberty; moreover, there is evidence that pubertal status is a stronger predictor of the onset of depression than is chronological age (Angold, Costello, & Worthman, Reference Angold, Costello and Worthman1998; Hayward, Gotlib, Schraedley, & Litt, Reference Hayward, Gotlib, Schraedley and Litt1999; Oldehinkel, Verhulst, & Ormel, Reference Oldehinkel, Verhulst and Ormel2011). Thus, puberty is a critically important period to study in order to understand neurobiological differences between males and females that may underlie sex differences in internalizing problems. It is important to note, however, that males and females differ significantly in pubertal timing; females typically experience the onset of puberty 1.5 years earlier than do males (Negriff & Susman, Reference Negriff and Susman2011). These sex differences in pubertal timing mean that age-matched samples of adolescent males and females are almost certain to be confounded by sex differences in pubertal stage. Similarly, given the difficulties inherent in recruiting and studying high-risk samples such as adolescents who have been exposed to maltreatment or who have experienced institutional care, many studies are simply not sufficiently powered to detect sex differences in the neurobiological effects of ELS during the narrow developmental window of early puberty during which sex differences in internalizing problems begin to emerge. Significant puberty-related changes occur in corticolimbic circuitry in adolescence (Peters, Jolles, Duijvenvoorde, Crone, & Peper, Reference Peters, Jolles, Duijvenvoorde, Crone and Peper2015; Spielberg, Forbes, et al., Reference Spielberg, Forbes, Ladouceur, Worthman, Olino, Ryan and Dahl2014; Spielberg, Olino, Forbes, & Dahl, Reference Spielberg, Olino, Forbes and Dahl2014). Therefore, it is critical that we examine sex differences in corticolimbic development in carefully characterized samples of adolescent males and females, matched for pubertal development.
The present study was designed to address these issues by examining how ELS differentially affects male and female corticolimbic activation and connectivity during early puberty, a period in which sex differences in rates of internalizing problems begin to emerge. We also examined how corticolimbic circuitry is associated with internalizing disorders in adolescence. Given significant differences between males and females in pubertal timing (Negriff & Susman, Reference Negriff and Susman2011) and the documented impact of pubertal stage on corticolimbic circuitry (Peters et al., Reference Peters, Jolles, Duijvenvoorde, Crone and Peper2015; Spielberg, Forbes, et al., Reference Spielberg, Forbes, Ladouceur, Worthman, Olino, Ryan and Dahl2014; Spielberg, Olino, et al., Reference Spielberg, Olino, Forbes and Dahl2014), in this study we used a puberty-matched sample of early adolescent boys and girls. We examined fMRI activation as boys and girls labeled emotion faces compared to matching emotion faces. Previous findings using this task suggest that labeling versus matching emotional face stimuli recruits implicit emotion regulation processes and elicits strong activation in the PFC, particularly in the vlPFC (Gee et al., Reference Gee, Karlsgodt, van Erp, Bearden, Lieberman, Belger and Cannon2012; Lieberman et al., Reference Lieberman, Eisenberger, Crockett, Tom, Pfeifer and Way2007; Taylor et al., Reference Taylor, Eisenberger, Saxbe, Lehman and Lieberman2006). This design allows us to examine emerging differences in the effects of ELS on corticolimbic circuitry during a period of development in which sex differences in internalizing problems are beginning to emerge. Based on evidence of sex differences in rates of internalizing symptoms in this age group, we hypothesized that early pubertal females will show a stronger association between exposure to ELS and internalizing problems than will their male counterparts. We predicted further that the association between ELS and internalizing problems in females would be mediated by increased amygdala activation and PFC activation during implicit emotion regulation. Finally, we expected to find greater negative connectivity between the amygdala and PFC in individuals who were exposed to more severe ELS and, further, that this negative connectivity would mediate the association between ELS and internalizing problems. We also explored sex differences in patterns of PFC-amygdala connectivity, but made no directional hypotheses.
Method
Participants and procedure
A total of 137 participants met criteria for inclusion in this study and analyses: 59 early pubertal males and 78 early pubertal females, ages 9–13 years (M = 11.42, SD = 1.08). An additional 39 participants were excluded from the analyses due to excessive motion (n = 35) or incomplete scans (n = 4). The participants were part of a larger study examining the effects of ELS on brain structure and function across early adolescence. After completing a brief phone interview to determine eligibility, participants were invited to the laboratory to complete parental consent and child assent forms. In this session, parents and children also completed interviews and questionnaires assessing their experience of ELS and measures of their cognitive and emotional functioning. Males and females were matched on pubertal development based on self-report Tanner stage and exposure to ELS (see Table 1 for descriptive statistics by child sex). Participants were recruited from the San Francisco Bay area through a combination of print and online advertisements. Exclusion criteria included: (a) self-reported Tanner pubertal stage >3 and the experience of menarche in female participants, (b) nonfluent English speakers, (c) contraindications to scan (e.g., metal implants, braces), (d) history of major neurological disorder or illness, and (e) intellectual delay or learning difficulties. Upon confirming eligibility, participants were invited to return within 1 month to complete the fMRI portion of the study. This study was approved by the Stanford University Institutional Review Board and all participants were compensated for their participation in the study.
Table 1. Descriptive statistics for males and females
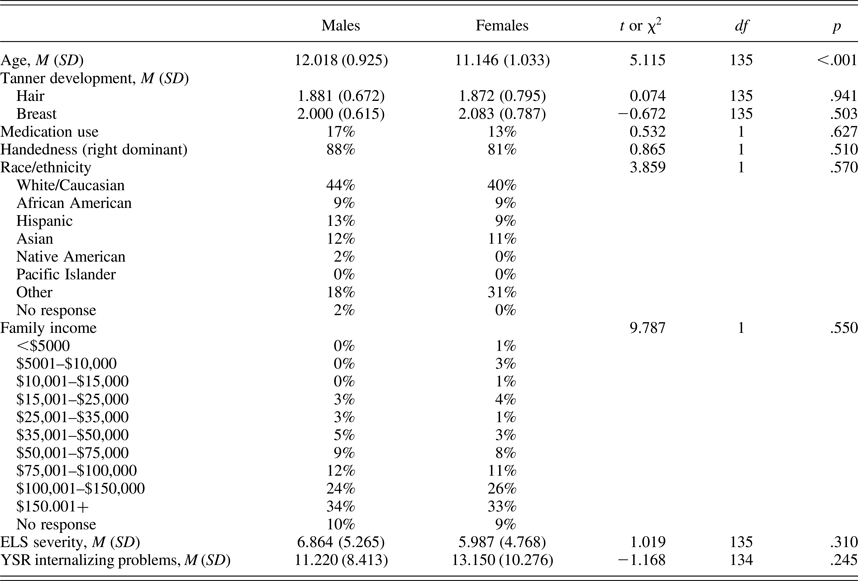
Note: ELS, early life stress; YSR, Youth Self-Report.
Measures
ELS
We assessed levels of ELS severity and the impact of early life stressors using a modified version of Traumatic Events Screening Inventory for Children (Ribbe, Reference Ribbe and Stamm1996). In this interview, we assessed 30+ types of stressful life experiences. For each type of ELS endorsed, interviewers followed up with general and specific probes in order to gather detailed information about the severity of the experience and the child's perceived severity of the stressor (e.g., relationship of persons involved, duration of experience, and consequences of experience). A panel of three coders, blind to the child's subjective severity ratings and reactions and behaviors during the interview, rated the objective severity of each type of stressor based on a modified version of the UCLA Life Stress Interview coding system (Rudolph & Hammen, Reference Rudolph and Hammen1999; Rudolph et al., Reference Rudolph, Hammen, Burge, Lindberg, Herzberg and Daley2000). Coders made objective severity ratings on a 5-point scale (0 = nonevent or no impact, e.g., witnessed debris from car crash; 4 = extremely severe impact, e.g., experienced sexual abuse), interclass correlation = 0.99. For each child, objective ratings for each type of ELS endorsed were summed to create an index of the cumulative severity of ELS.
Pubertal status
Pubertal stage was determined using self-reported Tanner staging (Marshall & Tanner, Reference Marshall and Tanner1969, Reference Marshall and Tanner1970). Using schematic drawings of two secondary sex characteristics (pubic hair and breast/testes development), each participant reported on his/her developmental stage on a scale of 1 to 5. A Tanner staging of 1 signifies that no pubertal development has begun, and a staging of 5 signifies that adult levels of pubertal maturation have been achieved. To be included in this study, all participants rated themselves at Stage 3 or below on measures of both pubic hair and breast/testes development. Tanner staging scores have been found to correlate with physicians' physical examinations of pubertal development (Coleman & Coleman, Reference Coleman and Coleman2002; Slora et al., Reference Slora, Bocian, Herman-Giddens, Harris, Pedlow, Dowshen and Wasserman2009; see Shirtcliff, Dahl, & Pollak, Reference Shirtcliff, Dahl and Pollak2009).
Internalizing problems
Participants completed the Youth Self-Report (YSR; Achenbach, Reference Achenbach1991; Achenbach & Rescorla, Reference Achenbach and Rescorla2001) measure, which assesses a broad array of behavioral problems in children and adolescents. For this study we examined total scores on the internalizing problems subscale of the YSR. One male participant did not complete this questionnaire.
MRI
Implicit emotion regulation fMRI task
We used a modified emotion label task to examine the neural correlates of implicit emotion regulation (see Hariri, Bookheimer, & Mazziotta, Reference Hariri, Bookheimer and Mazziotta2000; Lieberman et al., Reference Lieberman, Eisenberger, Crockett, Tom, Pfeifer and Way2007). This task was designed based on the formulation that linguistic processing of an emotional expression (e.g., labeling an emotional face) requires greater downregulation of regions responsible for processing salient information than does perceptual processing of the same emotional expression (e.g., matching emotional faces), potentially serving as a marker of the neural correlates of implicit emotion regulation (Lieberman et al., Reference Lieberman, Eisenberger, Crockett, Tom, Pfeifer and Way2007). This task requires participants to identify, or label, a target emotional expression (emotion label) or to match a target emotional expression to two other emotional expressions (emotion match). We also included a sensorimotor control condition in which participants were required to match a target shape to two other shapes (shape match). For the label conditions, participants were instructed to make a button press to indicate whether the appropriate label for the target figure was located on the bottom left or the bottom right of the screen. For all match conditions, participants were instructed to make a button press to indicate whether the target figure at the top of the screen was more similar to the figure below it on the left or on the right. Each face/label set was presented on the screen for 5000 ms, and each block of stimuli consisted of 10 trials. All task blocks were interspersed with rest blocks (lasting 15000 ms) in which participants were instructed to focus on a fixation cross in the middle of the screen. Each run consisted of one block of each task condition (positive label, positive match, negative label, negative match, ans shape match) as well as interspersed rest blocks. Presentation of conditions was randomized across participants, and all participants completed two complete runs of the task.
The task was presented using E-Prime software Version 2.0. A total of 50 trials were divided across two emotion conditions (positive and negative) and two response conditions (match and label) in addition to the control shape match condition. The positive and negative emotional expressions were facial displays from the NimStim picture set (Tottenham et al., Reference Tottenham, Tanaka, Leon, McCarry, Nurse, Hare and Nelson2009). Ten actors (5 females, 5 males) were displayed showing positive or negative emotional expressions. Positive emotional expression blocks included both high and low arousal positive displays including happy, excited, surprised, and calm facial expressions. Negative emotional expression blocks included both high and low arousal negative displays including sad, angry, and fearful facial expressions. To optimize power in testing our hypotheses, our analyses focused on the contrast of all emotion label relative to all emotion match conditions.
MRI acquisition
Participants who met eligibility criteria completed an fMRI scan. All scans were conducted on a 3 Tesla GE whole-body scanner (GE Healthcare Systems, Milwaukee, Wisconsin). Foam padding was used to minimize head movement. Two T2*-sensitive gradient echo-planar pulse sequences were used for functional imaging (repetition time = 2000 ms, echo time = 30 ms, flip angle = 77°, matrix size = 70 × 70, 43 axial slices, field of view = 22.4 cm, 3 mm thick), each run lasting 5 min and 54 s. An automated high-order shimming procedure was used to reduce B0 inhomogeneity. Additional high-resolution structural images were acquired with an axial 3D FSPGR sequence with T1 contrast (repetition time = 6.0 ms, echo time = 2 ms, flip angle = 12°, matrix size = 256 × 256, 186 axial slices, field of view = 23 cm, 0.9 mm) for spatial registration.
fMRI data analysis
Analyses were conducted in FSL Version 6.0.0 (FMRIB's Software Library, http://www.fmrib.ox.ac.uk/fsl), using FEAT, the fMRI expert analysis tool. The first four volumes of each participant's functional scan were discarded to allow for stabilization of longitudinal magnetization. The remaining images were preprocessed using standardized procedures, including motion correction to the mean image using motion correction FMRIB's linear image registration tool MCFLIRT (Jenkinson, Bannister, Brady, & Smith, Reference Jenkinson, Bannister, Brady and Smith2002), slice-timing correction using Fourier space–time series phase shifting, spatial smoothing using a Gaussian kernel of full width half maximum 5 mm, grand-mean intensity normalization of the entire four-dimensional data set by a single multiplicative factor, and high-pass temporal filtering (Gaussian-weighted least squares straight line fitting, with σ = 50.0 s). Functional data were linearly registered to a common stereotaxic space by first registering the in-plane T2 image to the T1-weighted structural image (6 degrees of freedom), transforming the T1-weighted structural image to the MNI152 T1 brain, and then applying that deformation matrix and resampling to 2-mm resolution onto the functional images using FMRIB's linear image registration tool FLIRT (12 df; Jenkinson et al., Reference Jenkinson, Bannister, Brady and Smith2002; Jenkinson & Smith, Reference Jenkinson and Smith2001). All fMRI analyses were therefore performed in MNI space at 2 mm3 resolution.
Time series statistical analysis was conducted using full information at maximum likelihood with local autocorrelation correction (Woolrich, Ripley, Brady, & Smith, Reference Woolrich, Ripley, Brady and Smith2001). The voxelwise general linear model included regressors for each block condition (positive/match, positive/label, negative/match, negative/label, and shape match) as well as their temporal derivatives. Twelve motion correction parameters, as well as an indicator function to identify volumes as having excessive motion according to framewise displacement of 0.9 mm, were included as covariates of noninterest. Participants with absolute motion >3 mm or >20% of volumes with framewise displacement >0.9 mm were excluded from the analyses (n = 35). Both within-subject runs were combined in a fixed-effects model for each participant, which averaged the contrast estimates over runs within participant by setting the random effects variance to zero in FMRIB's local analysis of mixed effects (FLAME; Beckmann, Jenkinson, & Smith, Reference Beckmann, Jenkinson and Smith2003; Woolrich, Reference Woolrich2008; Woolrich, Behrens, Beckmann, Jenkinson, & Smith, Reference Woolrich, Behrens, Beckmann, Jenkinson and Smith2004). All participants were then combined in a higher level mixed-effects model to investigate within- and between-group differences. Prior to thresholding, we used a binarized gray matter mask (obtained from https://canlabweb.colorado.edu/wiki/doku.php/help/core/brain_masks). Higher level group analyses were conducted using FLAME Stage 1 (Beckmann et al., Reference Beckmann, Jenkinson and Smith2003; Woolrich, Reference Woolrich2008; Woolrich et al., Reference Woolrich, Behrens, Beckmann, Jenkinson and Smith2004). Given significant age differences between males and females, we included age as a covariate in all analyses.
Whole-brain analyses: Effects of sex and ELS
We examined the effects of sex and ELS severity on blood oxygen level dependent response during contrasts of interest in whole-brain analyses. Statistical images were thresholded using clusters determined by Z > 2.3 and a corrected cluster significance threshold of α = 0.05 (Worsley, Reference Worsley, Jezzard, Matthews and Smith2001).
Region of interest (ROI) analysis of amygdala
We also examined amygdala activation during implicit emotion regulation using a ROI analysis. A bilateral amygdala ROI was created using the Harvard–Oxford subcortical Atlas in FSL (25% threshold).
Psychophysiological interaction (PPI) analyses to assess PFC-amygdala functional connectivity
We used PPI analyses to examine functional connectivity during implicit emotion regulation between the bilateral amygdala ROI and the two PFC regions that showed a significant interaction of ELS and sex (Friston et al., Reference Friston, Buechel, Fink, Morris, Rolls and Dolan1997; O'Reilly, Woolrich, Behrens, Smith, & Johansen-Berg, Reference O'Reilly, Woolrich, Behrens, Smith and Johansen-Berg2012). This analysis identifies regions in the brain that are correlated most strongly with the significant prefrontal ROIs during emotion label relative to emotion match conditions. For each PFC ROI, the general linear model analysis was conducted in FSL and included regressors for each task condition, the ROI time series, and the interaction of the task contrast (emotion label vs. emotion match) and the ROI time series. Again, both within-subject runs were combined in a fixed-effects model for each participant, which averaged the contrast estimates over runs within participant by focusing the random effects variance to zero in FLAME (Beckmann et al., Reference Beckmann, Jenkinson and Smith2003; Woolrich, Reference Woolrich2008; Woolrich et al., Reference Woolrich, Behrens, Beckmann, Jenkinson and Smith2004). All participants were then combined in a higher level mixed effects model to investigate connectivity patterns across both groups. Higher level group analyses were conducted using FLAME Stage 1 (Beckmann et al., Reference Beckmann, Jenkinson and Smith2003; Woolrich, Reference Woolrich2008; Woolrich et al., Reference Woolrich, Behrens, Beckmann, Jenkinson and Smith2004). Statistic images were thresholded using clusters determined by Z > 2.3 and a corrected cluster significance threshold of α = 0.05 (Worsley, Reference Worsley, Jezzard, Matthews and Smith2001). We then extracted parameter estimates from the bilateral amygdala ROI (as defined above) and used linear regression in SPSS (Version 23) to examine the effect of sex, ELS, and the Sex × ELS interaction on connectivity patterns between bilateral amygdala and the two PFC regions that showed a significant interaction effect with sex and ELS severity, after covarying for chronological age. We used simple slope analyses to probe significant interactions (Aiken & West, Reference Aiken and West1991).
Demographics and behavioral analyses
All statistical analyses reported below were conducted with SPSS (Version 23) using two-tailed tests (α = 0.05). Independent samples t tests or χ2 tests were used to compare males and females on demographic variables, exposure to ELS severity, and scores on the YSR internalizing problems subscale. We conducted analyses of covariance to compare task performance between males and females, after covarying for chronological age. Finally, we conducted correlational analyses to test whether ELS severity was associated with task performance.
Mediation analyses
Based on our findings (described below), we tested whether blood oxygen level dependent responses to emotion label relative to match in regions that showed a significant interaction between ELS severity and sex mediated the association between ELS severity and YSR internalizing scores in females. To do this, we used a single-step nonparametric resampling procedure (1,000 samples with replacement) for testing indirect effects (Hayes, Reference Hayes2013). Mediation is supported when the indirect effect is statistically significant. To assess the indirect effect, we calculated 95% confidence intervals (CIs) for coefficients; if the CI does not include zero, the indirect effect is considered to be statistically significant.
Results
Demographic and clinical characteristics
Participant demographic and clinical characteristics are presented in Table 1. As shown in this table, boys and girls did not differ significantly in Tanner stage, medication use, handedness, race/ethnicity, or family income. As expected, however, given that we matched boys and girls on pubertal status, the two sexes did differ in chronological age, t (135) = 5.115, p < .001.
Sex differences in the effects of ELS on internalizing problems
There were no significant sex differences in ELS severity or in internalizing problems in our sample (see Table 1). In examining the contributions of sex and ELS severity to self-reported internalizing problems after controlling for chronological age, a linear regression indicated that sex moderated the association between ELS severity and internalizing problems, B = 0.903, SE = 0.306, t (131) = 2.953, p = .004. Post hoc simple slopes analyses within each sex controlling for age indicated that ELS was significantly associated with internalizing problems in females, r (75) = .476, p < .001, but not in males, r (55) = .079, p = .559.
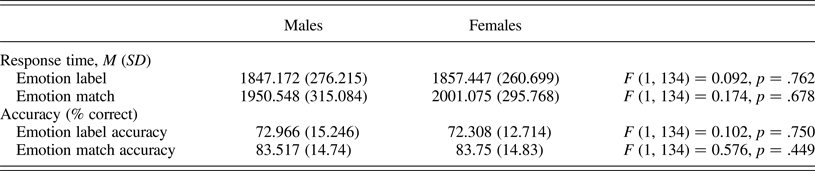
fMRI task behavioral performance
We conducted analyses of covariance to compare task performance between males and females, controlling for age. Males and females did not differ on task accuracy or response time (RT; Table 2). ELS severity was associated with longer RT to match emotions, r (134) = .174, p = .042. ELS severity was not associated with RT to label emotions, r (134) = .069, p = .425, or with task accuracy (|rs| < .048, ps > .576).
Table 2. Task performance
Whole-brain analysis: Main effect of ELS
We conducted whole-brain correlational analyses to examine the effect of ELS severity, controlling for the effects of chronological age, on brain regions involved in implicit emotion regulation (i.e., labeling emotion faces vs. matching emotion faces). These analyses yielded a significant cluster that was negatively correlated with ELS severity: right lateral occipital cortex/precuneus cortex (k = 1,088; peak voxel: x = 18, y = –72, z = 42; Z = 3.95).
We also conducted a linear regression to examine the effect of ELS severity, controlling for the effects of chronological age, on activation in a bilateral amygdala ROI during implicit emotion regulation. There was no association between ELS severity and bilateral amygdala activation, r (134) = –.049, p = .575.
Whole-brain analysis: Main effect of sex
We conducted whole-brain t tests to examine sex differences, controlling for the effects of chronological age, in regions involved in labeling emotion faces versus matching emotion faces. Direct comparisons between males and females yielded one significant cluster in the left lingual gyrus in which males showed significantly greater activation than did females (k = 580; peak voxel: x = –12, y = –70, z = –6; Z = 3.38). There were no clusters in which females showed greater activation than did males.
We also conducted an analysis of covariance to examine sex differences in activation in a bilateral amygdala ROI, controlling for the effects of chronological age. There were no significant differences between males and females in amygdala activation when labeling emotion faces relative to matching emotion faces, F (1, 134) = 0.034, p = .855.
Whole-brain analysis: Interaction of ELS and sex
Finally, we conducted a whole-brain interaction model to examine whether the linear association between ELS severity and brain regions involved in labeling emotion faces versus matching emotion faces differed by sex, controlling for the effects of chronological age. This interaction model yielded three significant clusters (Figure 1). Compared with males, females showed a significantly greater association between ELS and activation in left vlPFC (k = 606; peak voxel: x = –36, y = 48, z = –4; Z = 3.8), right dlPFC/vlPFC (k = 784; peak voxel: x = 44, y = 44, z = 26; Z = 4.1) and bilateral intracalcarine cortex (k = 1,999; peak voxel: x = –18, y = –72, z = 12; Z = 4.18).

Figure 1. (Color online) A whole-brain interaction model examining whether the linear association between early life stress severity and brain regions involved in labeling emotion faces versus matching emotion faces differed by sex yielded three significant clusters in the left vlPFC, right dlPFC/vlPFC, and bilateral intracalcarine cortex. Activation maps are thresholded at Z > 2.3 and corrected for multiple comparisons using a cluster-based p <. 05. MNI coordinates are indicated for slice distance (mm). Parameter estimates (showing the amount of signal change measured in arbitrary units) of blood oxygen level dependent signal response were extracted from each significant cluster and plotted in the bar graph. Parameter estimates were also related to internalizing problems in males and females separately. vlPFC, ventrolateral prefrontal cortex; dlPFC, dorsolateral prefrontal cortex.
ROI analysis of amygdala: Interaction of ELS and sex
We also conducted a linear regression to examine the interaction between ELS severity and sex in predicting bilateral amygdala ROI response to labeling emotion faces relative to matching emotion faces. This interaction was not significant, B = 0.488, SE = 1.147, t (132) = 0.426, p = .671.
Associations between brain regions and internalizing symptoms
We next examined the association between self-reported internalizing problems and parameter estimates extracted from the ROIs defined by the interaction of ELS and sex, including left vlPFC, right dlPFC/vlPFC, and bilateral intracalcarine cortex, separately for males and females (Figure 2). Females showed a positive association between all three ROIs and YSR internalizing problems total score, such that greater activation in left vlPFC, right dlPFC/vlPFC, and intracalcarine cortex was associated with greater internalizing problems, left vlPFC: r (78) = .237, p = .037; right dlPFC/vlPFC: r (78) = .232, p = .041; intracalcarine cortex: r (78) = .260, p = .021. In contrast, males showed a positive association between internalizing problems and activation only in intracalcarine cortex, intracalcarine cortex: r (58) = .320, p = .014; there was no significant relation in males between internalizing problems and activation in left vlPFC or in right dlPFC/vlPFC, left vlPFC: r (58) = –.070, p = .601; right dlPFC/vlPFC: r (58) = .141, p = .293.
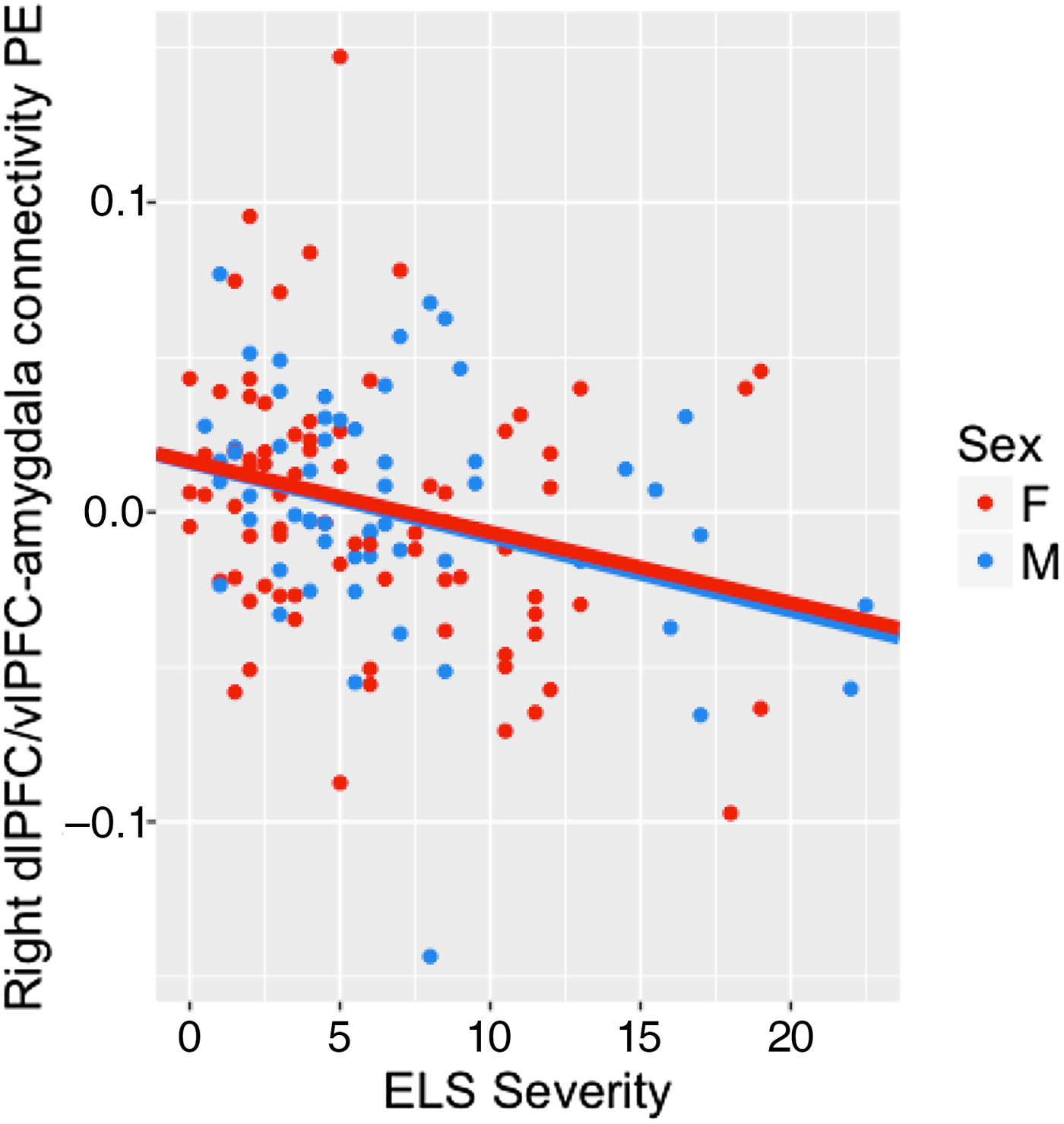
Figure 2. (Color online) Strength of connectivity between right the dlPFC/vlPFC and bilateral amygdala was negatively associated with early life stress severity across males and females. vlPFC, ventrolateral prefrontal cortex; dlPFC, dorsolateral prefrontal cortex.
Mediation analysis: Testing whether brain activation mediates the association between ELS severity and internalizing symptoms in females
Because there was a significant positive association between activation during implicit emotion regulation in left vlPFC, right dlPFC/vlPFC, and intracalcarine cortex and internalizing symptoms in females only, we tested whether activation in these regions mediated ELS severity and internalizing symptoms. Activation during implicit emotion regulation in these three regions did not mediate the association between ELS and internalizing problems in females; that is, the indirect effect did not differ significantly from zero, left vlPFC: point estimate = 0.010 (0.018), 95% CI [–0.011, 0.070]; right dlPFC/vlPFC: point estimate = 0.025 (0.034), 95% CI [–0.023, 0.117]; intracalcarine cortex: point estimate = 0.076 (0.081), 95% CI [–0.057, 0.270].
PPI analysis to assess PFC-amygdala functional connectivity
We also conducted PPI analyses to examine task-dependent functional connectivity between the two prefrontal regions that showed a significant interaction of ELS severity and sex and the bilateral amygdala ROI. We tested the main effects of ELS severity and sex and their interaction on the strength of these connections during emotion label relative to emotion match conditions. The main effects of ELS and sex, and the ELS × Sex interaction, on the strength of connectivity between the left vlPFC and bilateral amygdala were not significant. There was a significant main effect of ELS severity on the strength of connectivity between the right dlPFC/vlPFC and bilateral amygdala, B = –0.002, SE = 0.001, t (132) = –2.352, p = .020, such that greater ELS severity is associated with more negative amygdala-PFC connectivity for emotion label relative to emotion match conditions (Figure 2).
Associations between PFC-amygdala functional connectivity and internalizing symptoms
Functional connectivity between the bilateral amygdala and left vlPFC and right dlPFC/vlPFC was not associated with internalizing symptoms across all participants, left vlPFC: r (133) = –.138, p = .110; right dlPFC/vlPFC: r (133) = –.084, p = .334, or in each sex separately, males: left vlPFC: r (58) = –.038, p = .776, right dlPFC/vlPFC: r (58) = .162, p = .225; females: left vlPFC: r (78) = –.181, p = .112, right dlPFC/vlPFC: r (78) = –.210, p = .065. Given null associations between functional connectivity and internalizing symptoms, we did not conduct follow-up mediation analyses.
Discussion
In a sample of puberty-matched early adolescent males and females, we documented differential associations between ELS severity and internalizing problems. Among girls, there was a significant association between ELS severity and internalizing problems, whereas among boys there was no such association. In attempting to understand the neurobiological bases of emerging sex differences in the effects of ELS on internalizing problems, we conducted whole-brain and a priori ROI analyses of brain activation while participants were performing an emotion label task designed to measure implicit emotion regulation. We found a significant interaction of ELS severity and sex in the left vlPFC, right dlPFC/vlPFC, and bilateral intracalcarine cortex. Among girls, there was a positive association between ELS severity and activation in these regions during implicit emotion regulation, and as above, among boys there was no association between ELS severity and activation in these regions. Moreover, greater activation in these three regions was associated with higher levels of internalizing problems in girls. Nonetheless, this activation did not statistically mediate the association between ELS severity and internalizing problems. We also found a significant effect of ELS severity on the right lateral occipital/precuneus cortex and a significant main effect of sex in the left lingual gyrus during emotion label relative to emotion match conditions. Contrary to our hypothesis, ROI analyses yielded no significant main effects or interactions of ELS severity and sex on amygdala response during emotion label relative to emotion match conditions. When we examined patterns of PFC-amygdala functional connectivity, we found greater negative association between ELS severity and connectivity in both sexes combined between the right dlPFC/vlPFC and bilateral amygdala during implicit emotion regulation; this did not mediate the relation between ELS and internalizing symptoms.
The vlPFC is largely involved in cognitive responses to negative emotions, including cognitive reappraisal and emotion regulation (Ochsner, Silvers, & Buhle, Reference Ochsner, Silvers and Buhle2012) and thus plays a significant role in modulating negative affect (Forbes, Phillips, Silk, Ryan, & Dahl, Reference Forbes, Phillips, Silk, Ryan and Dahl2011; Phan et al., Reference Phan, Fitzgerald, Nathan, Moore, Uhde and Tancer2005). Although there are no known direct anatomical connections between the vlPFC and the amygdala, the vlPFC is posited to modulate amygdala response through activation in the medial PFC (Pessoa, Reference Pessoa2010; Pessoa, Kastner, & Ungerleider, Reference Pessoa, Kastner and Ungerleider2002; Silvers et al., Reference Silvers, Insel, Powers, Franz, Helion, Martin and Ochsner2016). Cognitive reappraisal has been associated with a negative correlation between vlPFC and amygdala activation (Silvers et al., Reference Silvers, Insel, Powers, Franz, Helion, Martin and Ochsner2016). The vlPFC does, however, share direct anatomical connections with the dlPFC; research suggests that both of these regions support executive function and inhibitory processes (Pessoa, Reference Pessoa2010; Pessoa et al., Reference Pessoa, Kastner and Ungerleider2002). Given our finding that greater vlPFC activation is associated with higher levels of ELS severity in early pubertal females, several possible interpretations can be generated. For example, heightened PFC activation in response to affective stimuli has been interpreted as reflecting immaturities in regulating an affective response (see Pfeifer & Blakemore, Reference Pfeifer and Blakemore2012, for a review of this literature in adolescence). In this context, it is plausible that early pubertal females require additional neural engagement to label the facial emotion and, in turn, to downregulate amygdala response. Given the nonsignificant results of our mediation analyses, however, it is not clear how increased activation in these brain regions during this implicit emotion regulation task gives rise to heightened internalizing symptoms in early pubertal females. It is possible that increased neural recruitment at this point in early puberty contributes to maladaptive cognitive processes and risk for disorder in the future (i.e., late puberty when first depressive episodes commonly occur and sex differences in depressive symptoms peak); it is important that this possibility be examined longitudinally in future research.
One additional theoretical explanation for our finding that greater exposure to ELS is associated with greater recruitment of PFC in early pubertal females is the stress acceleration hypothesis. Integrating evidence from both animal and human studies of exposure to early adversity, the stress acceleration hypothesis posits that experiencing ELS leads to a faster maturation of neural circuits involved in emotional functioning, primarily the corticolimbic circuit. Callaghan and Tottenham (Reference Callaghan and Tottenham2016) argue that in the face of high levels of stress, accelerated development of neural circuitry involved in emotion functioning is adaptive in the short term, as it facilitates earlier independence from a potentially unstable or harmful environment. Gee, Humphreys, et al. (Reference Gee, Humphreys, Flannery, Goff, Telzer, Shapiro and Tottenham2013) found a more mature pattern of amygdala-mPFC functional connectivity (i.e., more negative connectivity) in previously institutionalized children and adolescents relative to never-institutionalized children and adolescents, which was in turn associated with reduced separation anxiety in the previously institutionalized group only. However, this accelerated development could have later consequences, and researchers have yet to explore this possibility longitudinally. Increasing age in adolescence has been associated with greater vlPFC activation both in an emotion regulation context and during passive viewing of fearful faces (McRae et al., Reference McRae, Gross, Weber, Robertson, Sokol-Hessner, Ray and Ochsner2012; Yurgelun-Todd & Killgore, Reference Yurgelun-Todd and Killgore2006; see Forbes et al., Reference Forbes, Phillips, Silk, Ryan and Dahl2011). Given typical developmental increases in the recruitment of the dlPFC and vlPFC (Cohen-Gilbert & Thomas, Reference Cohen-Gilbert and Thomas2013; Somerville, Hare, & Casey, Reference Somerville, Hare and Casey2011; Tottenham, Hare, & Casey, Reference Tottenham, Hare and Casey2011) in support of emotion regulation, the increased recruitment of left vlPFC and right dlPFC/vlPFC that we found in our sample of early adolescent females who were exposed to high levels of ELS may represent a more developmentally mature pattern of neural function. Gee, Gabard-Durnam, et al. (Reference Gee, Gabard-Durnam, Flannery, Goff, Humphreys, Telzer and Tottenham2013) found heightened activation in the amygdala and in prefrontal and superior temporal gyrus when viewing fear faces in a sample of previously institutionalized children and adolescents, but no difference in mPFC activation between children with and without a history of adversity. When we examined functional connectivity of prefrontal ROIs that exhibited significant interactions of sex and ELS severity, we also found that ELS severity was associated with greater negative connectivity of the right dlPFC/vlPFC and bilateral amygdala for emotion label relative to emotion match conditions. Although sex did not moderate this association, we believe that this is nonetheless an important demonstration of the stress acceleration hypothesis.
Contrary to our hypothesis, we did not find significant effects of stress, sex, or their interaction on amygdala activation during implicit emotion regulation. Activation in the amygdala increases in response to salient (both positive and negative) stimuli in the environment; amygdala activation is posited to serve as a motivating signal to guide learning and memory for emotional material. Our nonsignificant findings for the effect of ELS on amygdala activation during implicit emotion regulation stand in contrast to a large body of work suggesting that individuals exposed to ELS show heightened amygdala reactivity to emotion face stimuli (including angry, happy, fearful, sad, and neutral faces; Garrett et al., Reference Garrett, Carrion, Kletter, Karchemskiy, Weems and Reiss2012; Gee, Gabard-Durnam, et al., Reference Gee, Gabard-Durnam, Flannery, Goff, Humphreys, Telzer and Tottenham2013; Marusak et al., Reference Marusak, Martin, Etkin and Thomason2014; McCrory et al., Reference McCrory, De Brito, Kelly, Bird, Sebastian, Mechelli and Viding2013; Suzuki et al., Reference Suzuki, Luby, Botteron, Dietrich, McAvoy and Barch2014; Tottenham, Hare, Millner, et al., Reference Tottenham, Hare, Millner, Gilhooly, Zevin and Casey2011), as well as to negative images (McLaughlin et al., Reference McLaughlin, Peverill, Gold, Alves and Sheridan2015). In understanding these discrepant findings, it is instructive to note that because of the block design of this task, we combined a range of emotional face stimuli, including happy, surprised, sad, angry, and fearful faces. The block design of the task prevents us from being able to assess reliably amygdala activation to each emotion. It is possible that the effects of ELS on amygdala activation are selective to fearful or angry faces or specific to task demands (i.e., passively attending to facial stimuli or performing a cognitive task involving emotional face stimuli). Future research should investigate the specificity of this effect across a range of emotional faces and task conditions.
Similarly, the majority of existing work examining the effects of ELS on amygdala activation to threat-related stimuli in adolescence uses an extreme-group approach to examine brain function in individuals exposed to severe and homogenous forms of ELS, including early institutional care and severe maltreatment, relative to healthy controls (McLaughlin et al., Reference McLaughlin, Peverill, Gold, Alves and Sheridan2015; Mueller et al., Reference Mueller, Maheu, Dozier, Peloso, Mandell, Leibenluft and Ernst2010; Tottenham, Hare, Millner, et al., Reference Tottenham, Hare, Millner, Gilhooly, Zevin and Casey2011). Less work has focused on the neurobiological consequences of more commonly experienced forms of ELS such as those reported by the young participants in this study, including witnessing an injury or an accident and moving homes. It is possible that heightened amygdala reactivity as a consequence of ELS occurs only after exposure to extreme forms of early adversity or to specific types of ELS, such as exposure to severe threat or harmful input from caregivers early in development (Humphreys & Zeanah, Reference Humphreys and Zeanah2015; McLaughlin, Sheridan, & Lambert, Reference McLaughlin, Sheridan and Lambert2014; Teicher & Samson, Reference Teicher and Samson2016). The measure of ELS severity that we used in this study includes both more severe forms of ELS such as physical and sexual abuse, as well as less severe stressors. Thus, heightened amygdala reactivity may occur only after exposure to specific types of extreme ELS, a possibility that should be examined more explicitly in future research.
In summary, this is the first study to carefully assess sex differences in corticolimbic activation and connectivity during implicit emotion regulation in a unique sample of early pubertal youth as a function of exposure to ELS severity. In addition to the strengths of this investigation, there are three important limitations of this investigation. First, although we used careful coding systems to evaluate the cumulative effects of objectively rated ELS severity on corticolimbic development, we use the term ELS broadly to include multiple forms of adverse childhood experiences, ranging from maltreatment and neglect to residential moves and exposure to marital disagreements. As we noted above, researchers have suggested that different forms of early adverse experiences have different psychobiological consequences (Humphreys & Zeanah, Reference Humphreys and Zeanah2015; McLaughlin & Sheridan, Reference McLaughlin and Sheridan2016; Teicher & Samson, Reference Teicher and Samson2016; Teicher, Samson, Anderson, & Ohashi, Reference Teicher, Samson, Anderson and Ohashi2016). Future research should examine the differential effects of types of stress exposure, including exposure to threat and neglect, on neural functioning. Second, we did not examine effects of the timing of ELS severity or its chronicity in this study. Researchers have developed coding systems to describe more fully experiences of maltreatment; one notable example of this is the Maltreatment Classification System (Barnett, Manly, & Cicchetti, Reference Barnett, Manly, Cicchetti, Cicchetti and Toth1993). Given our use of child-reported stressors and objectively rated severity of stressors (as opposed to obtaining both child and parent reports, or reports from Child Protective Services for more severe stressors), we did not have sufficient details in our stress assessment to explore specific and differential effects of onset, chronicity, or developmental timing. It will be important in future research to elucidate how exposure to ELS at different points in development, as well as chronicity of adverse experiences, differentially affect corticolimbic circuitry, given that this system is still maturing through adulthood. In the present study, we defined ELS as any adverse experience prior to participating in the study, when adolescents were in early pubertal development. There is evidence in both human and rodent models, however, to suggest that the effects of adverse experiences on different neural systems depends on the individual's developmental stage at the time of exposure (Andersen & Teicher, Reference Andersen and Teicher2008; Andersen et al., Reference Andersen, Tomada, Vincow, Valente, Polcari and Teicher2008; Lupien et al., Reference Lupien, McEwen, Gunnar and Heim2009; Pechtel, Lyons-Ruth, Anderson, & Teicher, Reference Pechtel, Lyons-Ruth, Anderson and Teicher2014). Third, it is important to mention the limitations of cross-sectional investigations such as the present study, which precludes our ability to draw causal inferences. It will be important in future research to understand how corticolimbic development across puberty differs in males and females, particularly with respect to its temporal relation to ELS and risk for internalizing problems. Elucidating deviations from normative neural development in individuals who are exposed to ELS will lead to a better understanding of the etiology of internalizing problems and will highlight potential ways to intervene in the association between ELS and internalizing problems.
Despite these limitations, this study documents the effects of a wide range of ELS exposure on corticolimbic function in early puberty, and elucidates how sex moderates the effects of ELS on corticolimbic function. These results are important in refining models describing the impact of ELS on development, and highlight the need to study homogenous samples of well-characterized individuals at specific points in development. Understanding how ELS affects psychobiological development over time will facilitate the generation of a model describing the emergence of internalizing disorders, and will help to identify specific points or sensitive periods at which intervention may be most effective.






