Williams syndrome (WS) is a relatively rare genetic neurodevelopmental disorder that results from the hemizygous microdeletion of approximately 28 genes at band 7q11.23 (Ewart et al., Reference Ewart, Morris, Atkinson, Jin, Sternes and Spallone1993; Schubert, Reference Schubert2009). Brain structure and function are known to be atypical in WS. The volumes of the parietal and occipital lobes are disproportionately reduced, while those of the frontal and temporal lobes, cerebellum, hippocampus, parahippocampal gyrus, and amygdala are relatively preserved (Jernigan, Bellugi, Sowell, Doherty, & Hesselink, Reference Jernigan, Bellugi, Sowell, Doherty and Hesselink1993; Martens, Wilson, Dudgeon, & Reutens, Reference Martens, Wilson, Dudgeon and Reutens2009; Reiss et al., Reference Reiss, Eliez, Schmitt, Straus, Lai and Jones2000, Reference Reiss, Eckert, Rose, Karchemskiy, Kesler and Chang2004). Functional abnormalities of the amygdala have been reported in response to social stimuli and music (Haas et al., Reference Haas, Mills, Yam, Hoeft, Bellugi and Reiss2009; Levitin et al., Reference Levitin, Menon, Schmitt, Eliez, White and Glover2003; Meyer-Lindenberg, Hariri, et al., Reference Meyer-Lindenberg, Hariri, Munoz, Mervis, Mattay and Morris2005). Atypical hippocampal structure and function have also been noted in WS (Meyer-Lindenberg, Mervis, et al., Reference Meyer-Lindenberg, Mervis, Sarpal, Koch, Steele and Kohn2005).
The cognitive profile of individuals with WS is distinctive and is characterized in part by mild to moderate intellectual delay (Martens, Wilson, & Reutens, Reference Martens, Wilson and Reutens2008; Mervis & Klein-Tasman, Reference Mervis and Klein-Tasman2000). Language and face processing skills that were once considered relatively spared are now viewed as atypical (Brock, Reference Brock2007; Mervis & Becerra, Reference Mervis and Becerra2007), and significant deficits have been shown in visuospatial processing and mathematical skills (Farran, Reference Farran2005; Farran & Jarrold, Reference Farran and Jarrold2005; O'Hearn & Landau, Reference O'Hearn and Landau2007; Porter & Coltheart, Reference Porter and Coltheart2006). Individuals with WS are described as hypersociable and highly anxious (Doyle, Bellugi, Korenberg, & Graham, Reference Doyle, Bellugi, Korenberg and Graham2004; Dykens, Reference Dykens2003; Einfeld, Tonge, & Rees, Reference Einfeld, Tonge and Rees2001; Rosner, Hodapp, Fidler, Sagun, & Dykens, Reference Rosner, Hodapp, Fidler, Sagun and Dykens2004). Examining the interrelationships between the distinct cognitive/behavioral profile and the atypical neuroanatomy of WS can yield insights into the links between genes, brain, and behavior.
The corpus callosum is a large white matter structure that connects the two cerebral hemispheres and is integral to interhemispheric exchange of information, including language and visuospatial processing (Giedd et al., Reference Giedd, Rumsey, Castellanos, Rajapakse, Kaysen and Vaituzis1996; Habib, Demonet, & Frackowiak, Reference Habib, Demonet and Frackowiak1996; Hines, Chiu, McAdams, Bentler, & Lipcamon, Reference Hines, Chiu, McAdams, Bentler and Lipcamon1992). Given that both of these cognitive domains are atypical in WS, the corpus callosum has been of increasing interest to WS researchers. The earliest study of callosal morphology in WS suggested that it was similar in shape to controls, whereas in individuals with Down syndrome the rostral section was smaller than in controls (Wang, Doherty, Hesselink, & Bellugi, Reference Wang, Doherty, Hesselink and Bellugi1992). Later studies, however, indicated that callosal morphology was altered in WS, with less curvature and decreased midline length compared to controls (Luders, Di Paola, et al., Reference Luders, Di Paola, Tomaiuolo, Thompson, Toga and Vicari2007; Schmitt, Eliez, Bellugi, & Reiss, Reference Schmitt, Eliez, Bellugi and Reiss2001). In addition, the callosal area has been found to be significantly reduced compared to controls, with disproportionate narrowing posteriorly in the isthmus and splenium (Schmitt, Eliez, Warsofsky, Bellugi, & Reiss, Reference Schmitt, Eliez, Warsofsky, Bellugi and Reiss2001) and in the posterior callosal body and splenium (Tomaiuolo et al., Reference Tomaiuolo, Di Paola, Caravale, Vicari, Petrides and Caltagiorne2002). In 2002, Tomaiuolo and colleagues reported that the midbody and caudal portions of the corpus callosum contained less water compared to controls on the basis of alterations in voxel intensity in T1 weighted magnetic resonance imaging (MRI) scans, suggesting that the cell-packing densities or axonal diameters in these regions may be atypical in WS.
However, previous studies of callosal morphology in WS have not taken handedness, an important confound, into account. In normal controls, the corpus callosum in those who are left-handed has been previously found to be larger than in right-handed individuals (Clarke, Kraftsik, Van Der Loos, & Innocenti, Reference Clarke, Kraftsik, Van Der Loos and Innocenti1989; Driesen & Raz, Reference Driesen and Raz1995). Left-handers show a tendency to process information more bilaterally as compared to right-handers (Bryden, Reference Bryden1982). Given that the corpus callosum plays an integral part in hemispheric integration, it is believed that the enlarged corpus callosum in left-handers supports enhanced bihemispheric processing (Witelson, Reference Witelson1985). Studies of individuals with WS have reported an increased incidence of left-handedness (van Strien et al., Reference van Strien, Lagers-van Haselen, van Hagen, de Coo, Frens and van der Geest2005), or at least inconsistent handedness (Gérard-Desplanches et al., Reference Gérard-Desplanches, Deruelle, Stefanini, Ayoun, Volterra and Vicari2006). In their study of callosal morphology in WS, Tomaiuolo et al. (Reference Tomaiuolo, Di Paola, Caravale, Vicari, Petrides and Caltagiorne2002) were unable to assess the effect of handedness adequately because only two WS and two control participants were left-handed, precluding statistical analysis. The two studies by Schmitt et al. (Schmitt, Eliez, Bellugi, et al., Reference Schmitt, Eliez, Bellugi and Reiss2001; Schmitt, Eliez, Warsofsky, et al., Reference Schmitt, Eliez, Warsofsky, Bellugi and Reiss2001) did not report handedness for their participants.
The aim of the current study was to examine the thickness and area of the corpus callosum in one of the largest studies to date of individuals with WS, compared to chronological age, sex, and handedness-matched healthy controls. As previously noted by Luders, Di Paola, et al. (Reference Luders, Di Paola, Tomaiuolo, Thompson, Toga and Vicari2007), determining callosal thickness in addition to area is important because it evaluates regional changes in callosal morphology without constraining this a priori. It was hypothesized that the thickness of the callosum would be reduced in WS compared to controls, particularly in the splenium and isthmus, and that the area of the corpus callosum would be smaller in individuals with WS. An exploratory analysis was also conducted to determine if there were any age, sex, or handedness effects on callosal area.
Materials and Methods
Participants
Twenty-five individuals with WS participated in the study (mean age = 18.3 years, range = 8–41 years; full-scale IQ = 53.3, SD = 9.6). They were recruited through the Victoria Williams Syndrome Association via advertisements placed in their monthly newsletter and through attendance at events organized by the association. In 23 of these participants, the diagnosis was confirmed genetically using fluorescence in situ hybridization, and the clinical diagnosis was confirmed by geneticists or physicians. The remaining 2 WS participants declined genetic testing and were diagnosed on the basis of their clinical and medical phenotype. This cohort was described by Martens et al. (Reference Martens, Wilson, Dudgeon and Reutens2009) and Martens, Reutens, and Wilson (Reference Martens, Reutens and Wilson2010), but it has not been previously investigated in terms of callosal morphology. Twenty-five healthy control participants (mean age = 18.3 years, range = 8–41 years; full-scale IQ = 110, S = 11.4) were recruited through community advertisements placed in local newspapers and on noticeboards and via associates of the researchers. A specific request was made for both right- and left-handed participants, who were screened for developmental, neurological, and psychiatric abnormalities. IQ was measured using the age-appropriate Wechsler Intelligence Scale. The control participants were matched on chronological age (within 1 year), sex, and handedness (18 right-handed and 7 left-handed in each group). Ethics approval was obtained from the relevant Human Research Ethics committees. All participants were fully informed, and written consent was given by all participants or their guardians.
Determination of handedness
Handedness was determined by the Edinburgh Handedness Inventory (Oldfield, Reference Oldfield1971). The standard scoring procedure described by Oldfield was used, in which a laterality quotient was determined by subtracting the sum for the left hand from the sum for the right hand, dividing by 10, and then multiplying by 100. A negative laterality quotient was the criterion for left-handedness.
Image acquisition
MRI brain scans were acquired for each participant, using a 1.5 Signa Echospeed Superconducting Imaging System (General Electric Medical Systems, Milwaukee, WI) at Austin Health, Melbourne, Australia. A three-dimensional T1-weighted radiofrequency spoiled gradient echo sequence was used (echo time = 2.2 ms, repetition time = 10.5 ms, inversion time = 350 ms, flip angle = 20 degrees, matrix size = 256 × 256, NEX 1, field of view = 25 cm, voxel size = 1.5 × 0.9 × 0.9 × mm [1.2 mm3]).
Image analysis
The MRI images were registered into stereotaxic coordinate space using an automated procedure based on the 152 subject T1-weighted average template from the Montreal Neurological Institute, using a nine-parameter linear transformation (rotation, translation, and rescaling along the principal axes), and Automatic Image Registration 3.0 (Woods, Grafton, Watson, Sicotte, & Mazziotta, Reference Woods, Grafton, Watson, Sicotte and Mazziotta1998). Intersubject registration has been found to be necessary in studies examining morphological differences in brain structures between groups to control for differences in overall brain volume (Woods, Reference Woods, Toga and Mazziotta1996). The midsagittal slice of the registered images was automatically identified using a reproducible method that does not require manual definition of a partitioning plane. Recent evidence indicates that automated methods of callosal morphology are robust and reproducible in studies of individuals with other neurological disorders (Adamson et al., Reference Adamson, Wood, Chen, Barton, Reutens and Pantelis2011; Walterfang et al., Reference Walterfang, Wood, Reutens, Wood, Chen and Velakoulis2008). White matter voxels were then identified based on a histogram segmentation procedure (Otsu, Reference Otsu1979), which requires minimal manual correction to remove noncallosal pixels. This correction was made by one of the coauthors (J.C.), who was blind to subject group, using interactive mouse-driven software (Display) made available from the Montreal Neurological Institute. A measure of overall callosal area in square millimeters was then generated. The regional callosal area (genu, anterior body, midbody, isthmus, and splenium) was determined using Witelson partitioning, which divides the corpus callosum along its first principal axis, which corresponds to the longest axis of the outline (Ryberg et al., Reference Ryberg, Stegmann, Sjostrand, Rostrup, Barkhof and Fazekas2006). Four division points (1/3, 1/2, 2/3, and 4/5) were referenced along the first principal axis, resulting in a mean area for each of the five regions.
Callosal thickness was determined using a measure previously described in detail by Walterfang et al. (Reference Walterfang, Wood, Reutens, Wood, Chen and Velakoulis2008). In brief, this method comprised the following steps. Voxels defining the callosal boundary contour were identified first. A user-initialized iterative search was performed for optimum end points that maximized the length of the centerline of the callosum that divided the boundary contour into superior and inferior edges. The centerline was initially defined by dividing the superior and inferior edges into 40 equidistant segments by 39 nodes. The end points and successive corresponding midpoints on the superior and inferior edges were then joined by line segments. Once end points maximizing the length of this center line had been identified by iterative search, cubic spline interpolation between end points and successive midpoints was used to generate a smooth centerline. This curve was divided into 40 segments of equal lengths by 39 nodes. At each node, the distance of the line extending orthogonally to each boundary of the callosum was used as a measure of callosal thickness. This procedure utilizes one-to-one mapping of nodes, which indicates when neighboring nodes show group differences. A measure of overall callosal thickness was obtained by computing the average of the 39 nodes. Regional callosal thickness was determined by computing a mean thickness for each of the five regions, with each comprising eight contiguous segments (see Figure 1).
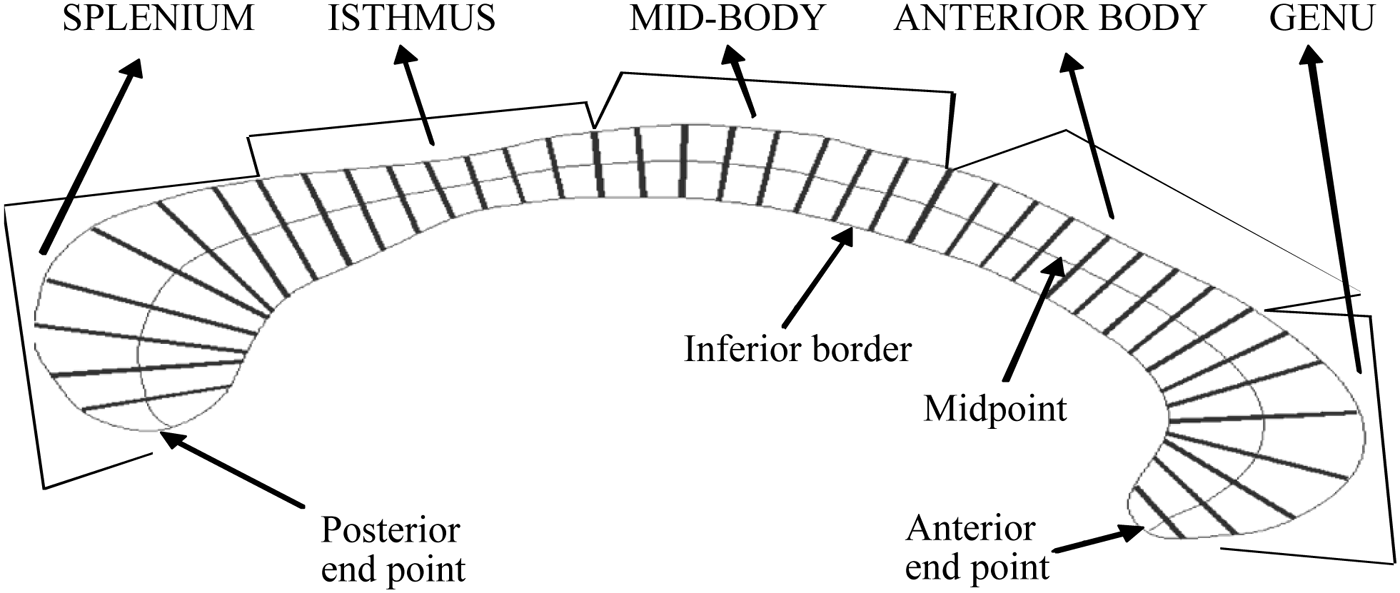
Figure 1. A representation of the corpus callosum with 39 node lengths.
Data analysis
To test our first hypothesis of overall reduced callosal thickness, group effects were examined using an independent samples t test. In order to examine callosal thickness of the 39 nodes, we used a nonparametric permutation method of 20,000 randomizations. This takes into account statistical dependence between adjacent thickness measurements and multiple comparisons (Holmes, Blair, Watson, & Ford, Reference Holmes, Blair, Watson and Ford1996). Step-down t testing was then used to localize nodes with significant difference in thickness between the groups (Holm, Reference Holm1979). To test our second hypothesis, a two-way between-groups analysis of covariance was performed to examine differences in callosal area as a function of sex, handedness, age, and total brain volume in WS and control participants, with an omnibus analysis used to control for type 1 error. The dependent variable was total callosal area; the independent variables were sex, handedness, and group; and age and total brain volume were included as covariates. Group was entered as an independent variable to allow examination of possible interaction effects. A further multivariate analysis of variance was performed to explore the nature of significant interaction effects, using a Bonferroni corrected p value of .01. Finally, given previous reports of significant positive correlations between callosal morphology and intelligence (Luders, Narr et al., Reference Luders, Narr, Bilder, Thompson, Szeszko and Hamilton2007), we also examined the relationship between full-scale IQ and callosal morphology for each of the five callosal regions using Pearson product-moment correlations. With the exception of the nodal analysis, the analyses were performed using SPSS 17.0, with a probability level of p ≤ .05 used as the criterion of statistical significance unless otherwise stated. Assumptions of normality and homogeneity of variance were satisfied for parametric analyses.
Results
Callosal thickness
The comparison of overall callosal thickness showed no significant group effect (p = .359). An examination of callosal thickness between groups at each of the 39 nodes showed that the corpus callosum was significantly thicker in the control participants at 3 nodes (nodes 33, 38, and 39), all located in the splenium. Figure 2 depicts the 3 nodes within the corpus callosum at which there was a significant group difference. These results support the hypothesis that the thickness of the corpus callosum at the splenium is reduced in individuals with WS compared to controls; a similar reduction was not noted in the isthmus. The differences in thickness at the splenium are illustrated in a control participant (Figure 3a) and in a WS participant (Figure 3b).

Figure 2. Corpus callosum nodes in which the control participants displayed significantly greater cortical thicknesses than individuals with Williams syndrome.

Figure 3. The splenium of the corpus callosum in (a) a control participant and (b) a Williams syndrome participant. [A color version of this figure can be viewed online at http://journals.cambridge.org/dpp]
Callosal area
Table 1 lists the mean callosal area by sex and handedness for each group. In support of our second hypothesis, the mean callosal area for the participants with WS (651.56 ± 91.48 mm2) was significantly smaller than the mean callosal area for the control participants (711.18 ± 87.43 mm2), as evidenced by a significant main effect of group, F (1, 40) = 11.47, p = .002, partial η2 = 0.22. There was a significant three-way interaction between group, sex, and handedness, F (1, 40) = 4.10, p = .049, partial η2 = 0.09, which was largely carried by a significant two-way interaction between handedness and group, F (1, 40) = 7.49, p = .009, partial η2 = 0.16. In other words, the mean absolute corpus callosal area of left-handed participants with WS was smaller than the mean area of right-handed participants with WS. In contrast, the mean corpus callosal area of left-handed control participants was larger than the mean area of right-handed control participants (see Figure 4). The main effects for sex and handedness were not significant, and there was no significant interaction between sex and group, or between sex and handedness (ps > .05 for all comparisons). The covariates of age, F (1, 40) = 1.49, p = .229, and total brain volume, F (1, 40) = 0.09, p = .762, were not significant.

Figure 4. Area of corpus callosum in stereotaxic coordinate space (mm2) of right-handed Williams syndrome (WS; n = 18) and control (n = 18) participants and left-handed WS (n = 7) and control (n = 7) participants. [A color version of this figure can be viewed online at http://journals.cambridge.org/dpp]
Table 1. Mean corpus callosal area by sex and handedness for WS and control participants

Note: WS, Williams syndrome.
To further examine the nature of the interaction effect between handedness and group, a 2 × 2 (Group × Handedness) multivariate analysis of variance was conducted with the five regional corpus callosal areas, using a Bonferroni corrected p value of .01. The results showed that there was a significant interaction between handedness and group in the anterior body, F (1, 46) = 10.36, p = .002, partial η2 = 0.18, and midbody, F (1, 41) = 12.58, p = .001, partial η2 = 0.22, of the corpus callosum. Specifically, these regions were smaller for left-handed WS participants compared to right-handed WS participants, and they were larger for left-handed controls compared to their right-handed counterparts (see Table 2). The finding that the relationship between handedness and callosal morphology in WS participants was the reverse of that in controls is novel and suggests that corpus callosal morphology may be particularly atypical in left-handed individuals with WS.
Table 2. Mean anterior body and midbody corpus callosal areas by handedness for WS and control participants

Note: WS, Williams syndrome.
We investigated the relationship between corpus callosal thickness and intelligence in each group. Our results showed a positive correlation between full-scale IQ and each of the five corpus callosal areas for the control participants but not for the WS participants, specifically for the genu (control: r = .518, p = .008; WS: r = –.114, p = .597), anterior body (control: r = .397, p = .050; WS: r = –.024, p = .910), midbody (control: r = .524, p = .007; WS: r = .009, p = .968), isthmus (control: r = .428, p = .033; WS: r = –.043, p = .841), and splenium (control: r = .464, p = .019; WS: r = .024, p = .910). There were also no significant correlations between full-scale IQ and the five corpus callosal areas when examined separately for the left- and right-handed WS participants.
Discussion
This study, using one of the largest samples of individuals with WS to date, confirms reduced callosal thickness in the splenium and overall reduced callosal area in WS. In addition, our findings are the first to show that in WS the anterior and midbody callosal areas are smaller in left-handed participants than in right-handed participants, with opposite findings reported in the control group.
The current findings supported our hypothesis that the area of the corpus callosum would be reduced in individuals with WS compared to controls, consistent with previous findings (Schmitt, Eliez, Warsofsky, et al., Reference Schmitt, Eliez, Warsofsky, Bellugi and Reiss2001; Tomaiuolo et al., Reference Tomaiuolo, Di Paola, Caravale, Vicari, Petrides and Caltagiorne2002). The thickness of three nodes in the splenium, which is the most posterior region in the corpus callosum, was significantly reduced in WS compared to healthy participants. No significant differences were found between the groups for the remaining regions, including nodes in the isthmus.
Schmitt, Eliez, Warsofsky, et al. (Reference Schmitt, Eliez, Warsofsky, Bellugi and Reiss2001) reported that the isthmus and splenium are both significantly reduced in WS compared to typical controls and Tomaiuolo et al. (Reference Tomaiuolo, Di Paola, Caravale, Vicari, Petrides and Caltagiorne2002) found that the posterior callosal body and splenium were significantly reduced in WS, while Luders, Di Paola, et al. (Reference Luders, Di Paola, Tomaiuolo, Thompson, Toga and Vicari2007) noted that the posterior callosal regions were significantly thinner than controls. An earlier study found no difference in corpus callosal morphology between WS and control participants (Wang et al., Reference Wang, Doherty, Hesselink and Bellugi1992). There were methodological differences between these studies, including (a) the thickness of MRI acquisition slices, (b) the method used to identify the midsagittal slice, and (c) the method used to define five callosal regions (genu, anterior body, midbody, isthmus, and splenium). In this study, we used a similar method to Tomaiuolo et al. (Reference Tomaiuolo, Di Paola, Caravale, Vicari, Petrides and Caltagiorne2002) to define the five callosal regions, whereas Schmitt, Eliez, Warsofsky, et al. (Reference Schmitt, Eliez, Warsofsky, Bellugi and Reiss2001) used four equidistant lines drawn orthogonal to the centerline. Luders, Di Paola, et al. (Reference Luders, Di Paola, Tomaiuolo, Thompson, Toga and Vicari2007) used mesh-based geometrical modeling to compute 100 points of callosal thickness. This is similar to our method of measuring regional thickness by utilizing multiple subdivisions (39 nodes), which is advantageous because it allows increased detection of subtle regional changes. Furthermore, our statistical methodology takes into account multiple comparisons and dependence on adjacent slice measurements.
Other important methodological differences in the current study include the large sample size of WS participants and the identification of the midsagittal slice after registration into a standardized coordinate space, thus avoiding the variability inherent in relying on visual inspection for slice selection. Despite these differences, however, studies to date are generally consistent in reporting significantly reduced corpus callosal area and reduced splenial thickness in individuals with WS.
This is the first known study to systematically examine the effects of age, sex, and hand preference on corpus callosum morphology in WS. We found that age did not have an effect on callosal area. Driesen and Raz (Reference Driesen and Raz1995) reported that the area of the corpus callosum decreased slightly with age, although the current sample did not include enough older participants to fully examine this effect. Our results indicated that the corpus callosal area was similar in size between males and females, which is inconsistent with previous studies (Driesen & Raz, Reference Driesen and Raz1995; Witelson, Reference Witelson1989). The current findings may be attributed to methodological differences associated with the use of stereotaxic coordinate space, because we did find that callosal area was larger for males than females when measured in absolute space.
The results from our control group support previous research, which indicates that there is a positive association between corpus callosal thickness and IQ in typical individuals (Luders, Narr, et al., Reference Luders, Narr, Bilder, Thompson, Szeszko and Hamilton2007). Luders and colleagues suggest that a larger corpus callosum may enhance interhemispheric communication, which may be associated with increased intellectual functioning. In contrast, there was no relationship between callosal thickness and intelligence for the participants in the WS group. One possibility for this finding is that the reduced variability of IQ in the WS participants affected the statistical outcome. Another possibility is that the overall reduced size of the corpus callosum in WS hinders interhemispheric transfer of information. In either case, this issue clearly warrants further investigation.
We demonstrate the first evidence of a handedness effect on callosal morphology in WS, with callosal area being smaller in left- than right-handed individuals. In contrast, and in keeping with previous research (Clarke et al., Reference Clarke, Kraftsik, Van Der Loos and Innocenti1989; Driesen & Raz, Reference Driesen and Raz1995), our left-handed controls showed a larger callosal area than their right-handed counterparts, particularly in the anterior and midbody of the callosum. Witelson (Reference Witelson1985) found that the midsagittal area of the corpus callosum was increased by 11% in left-handers compared to right-handers. It has been theorized that the larger callosum in left-handers supports increased communication between the hemispheres (Witelson & Pallie, Reference Witelson and Pallie1973), particularly for cognitive functions that tend to be strongly lateralized in right-handers, such as language (Doron & Gazzaniga, Reference Doron and Gazzaniga2008). Consistent with this, a positive association has been reported between left-handedness and atypical language representation (Bishop, Reference Bishop1990). It is reasonable to assume that the significantly smaller corpus callosum in left-handed individuals with WS may represent altered hemispheric dominance, which may partly account for the cognitive and visuospatial heterogeneity that has been reported in the WS phenotype (Porter & Coltheart, Reference Porter and Coltheart2006).
Relatively few studies have evaluated handedness in WS. Using a direct assessment procedure, van Strien et al. (Reference van Strien, Lagers-van Haselen, van Hagen, de Coo, Frens and van der Geest2005) determined that 26% of their WS participants demonstrated strong or weak left-handedness, or mixed handedness, compared to 11% of their control group. Gérald-Desplanches and colleagues (Reference Gérard-Desplanches, Deruelle, Stefanini, Ayoun, Volterra and Vicari2006) used a direct assessment procedure as well as a card-reaching task to determine that individuals with WS display more mixed-handedness than either typical controls (who display more right-handedness) or individuals with Down syndrome (who display more left-handedness).
We propose that left-handedness in WS may have a different genetic basis than left-handedness in typical individuals. Hemizygosity for LIM-kinase 1 is proposed to underlie the reduced corpus callosum volume in WS (Schmitt, Eliez, Warsofsky, et al., Reference Schmitt, Eliez, Warsofsky, Bellugi and Reiss2001). LIM-kinase 1 regulates actin polymerization, which has been shown to influence the development of handedness in some animal systems (Shibazaki, Shimizu, & Kuroda, Reference Shibazaki, Shimizu and Kuroda2004). It may be that left-handed individuals with WS have an even greater reduction in LIM-kinase 1 expression, which brings about a disproportionately reduced corpus callosum and impeded development of increased white matter tracts that are found in typical left-handed individuals. The creation of mouse models may help clarify genetic markers for left-handedness in WS, as has been done in Down syndrome (Roubertoux et al., Reference Roubertoux, Bichler, Pinoteau, Seregaza, Fortes and Jamon2005).
Left-handedness has also been associated with delayed puberty and reduced height, suggesting that developmental hormones may be involved in brain laterality (Mulligan, Stratford, Bailey, McCaughey, & Betts, Reference Mulligan, Stratford, Bailey, McCaughey and Betts2001). Given that some, but not all, individuals with WS display early puberty (Pober, Reference Pober2010), its relationship to handedness is worthy of further investigation. It is also the case that most studies of cognition in WS have not accounted for handedness, even though it has been suggested that the reduced posterior corpus callosum may negatively impact communication between the hemispheres (Paul, Reference Paul2010). It would be of interest to examine a range of cognitive domains that may elucidate the relationships between handedness, lateralization, and corpus callosal morphology in WS, such as visual–spatial cognition, auditory processing of emotion (see Järvinen-Pasley et al., Reference Järvinen-Pasley, Pollak, Yam, Hill, Grichanik and Mills2010), and language-based tasks.
The limitations of our study should be acknowledged. We utilized one measure of laterality, handedness, using the Edinburgh Handedness Inventory (Oldfield, Reference Oldfield1971). It has been demonstrated that some individuals with WS may display an increased incidence of leftward or mixed laterality, depending upon whether laterality is measured using the hand, foot, ear, or eye (Gérard-Desplanches et al., Reference Gérard-Desplanches, Deruelle, Stefanini, Ayoun, Volterra and Vicari2006). Future cognitive, behavioral, and neuroanatomical studies should examine larger groups of left-handed individuals with WS using a variety of laterality measures. We believe that the precision of using automated measures to identify the midsagittal slice of the corpus callosum is superior to manual delineation and is reproducible. However, it is possible that nonlinear variations in brain morphology in WS introduce systematic bias when linear transformation is used to normalize for overall differences in brain volume. We also recognize, as noted by Luders, Di Paola et al. (Reference Luders, Di Paola, Tomaiuolo, Thompson, Toga and Vicari2007), that group effects may be less pronounced in standard than in native space. We acknowledge the limitation of performing multiple statistical tests with small samples. To address this issue, we used a permutation method of step-down testing to assess callosal thickness effects and utilized omnibus analysis of variance with a Bonferroni correction to examine interactions between variables of interest. Finally, our findings should be viewed as preliminary, given the small number of left-handed participants in each group.
In conclusion, we show a smaller callosal area in left-handed individuals with WS than their right-handed counterparts, which is opposite to what is observed in the typical population, suggesting possible aberrant interhemispheric communication in this subgroup. Examining the effects of handedness in WS is critical, given that research is beginning to investigate the relationships between aberrant cerebral shape, morphology of the corpus callosum, and cognitive abilities now considered atypical in WS (Gothelf et al., Reference Gothelf, Searcy, Reilly, Lai, Lanre-Amos and Mills2008). Future studies that incorporate both left- and right-handed individuals with WS will advance our understanding of the morphological and cognitive heterogeneity associated with this disorder and may clarify the genetic basis of left-handedness in WS.








