The interplay of genes and environment on children's development is complex and dynamic. Accurately depicting these relations, both conceptually and quantitatively, is essential to elucidating influences on individual differences in development generally and differences in risk for psychopathology more specifically. The complexities of the interplay extend across multiple environmental levels from the cellular to the macroenvironment (Bronfenbrenner & Ceci, Reference Bronfenbrenner and Ceci1994; Wachs, Reference Wachs2010). Here, we simultaneously examine both linear and nonlinear contributions of the family environment to children's temperament in middle childhood. In doing so, we elucidate aspects of the environment that suppress or facilitate the development of temperament. With biometric modeling, we identify passive gene–environment correlation (rGE), such that the relation between the home environment and children's temperament is genetically mediated. We also identify a type of Gene × Environment (G × E) interaction; specifically, we show that the family environment moderates the heritability of temperament.
The Heritability of Temperament
Temperament constitutes early emerging affective and motivational components of behavior that change over time and impact many facets of development. Its associations with later social relationships, academic competence, and psychopathology mark temperament as an important construct. Despite its importance in understanding child development across multiple domains, much remains to be understood about the genetic and environmental underpinnings of temperament. Definitions of temperament, including Rothbart and Bates' (Reference Rothbart, Bates, Damon and Eisenberg1998, p. 109), “constitutionally based individual differences in emotional, motor, and attentional reactivity and self-regulation,” imply that temperament has a strong genetic component. Twin studies that have actually tested the heritability (the proportion of phenotypic variation due to genetic variation) of temperament primarily via parent report have supported a genetic basis to most dimensions (e.g., Gagne & Goldsmith, Reference Gagne and Goldsmith2011; Goldsmith, Buss, & Lemery, Reference Goldsmith, Buss and Lemery1997; Hur, Reference Hur2009; Lemery, Doelge, & Goldsmith, Reference Lemery-Chalfant, Doelger and Goldsmith2008; Rhee et al., Reference Rhee, Cosgrove, Schmitz, Haberstick, Corley and Hewitt2007; Saudino, Reference Saudino2005; Schmitz, Saudino, Plomin, Fulker, & DeFries, Reference Schmitz, Saudino, Plomin, Fulker and DeFries1996).
Classic quantitative biometric modeling of twins is based on the comparison of similarities between monozygotic (MZ) and dizygotic (DZ) co-twins (Neale & Cardon, Reference Neale and Cardon1992). MZ co-twins growing up in the same home share 100% of their genomic DNA and their common environment; any differences between them can be attributed to the nonshared environment. DZ co-twins share 50% of their genomic DNA, so differences between them are due to both genes and nonshared environments (E). Thus, MZ twins should be approximately twice as similar as DZ twins if additive genetic effects (A) contribute to individual differences. That is if the common environment (C) is important. However, MZ twins are less than twice as similar as DZ twins because common environmental influences, by definition, act on both types of twins in similar ways. In this way, the classic ACE model quantifies heritable and environmental contributions to variance in traits.
Heritability estimates resulting from these biometric models vary across dimensions of temperament and perhaps environmental circumstance (Lemery-Chalfant, Reference Lemery-Chalfant, Reich, Zautra and Hall2010; Saudino, Reference Saudino2005). Heritability estimates range broadly from about .20 to about .80 for childhood temperament (Saudino, Reference Saudino2005); Rothbart, Ahadi, Hershey, and Fisher's (Reference Rothbart, Ahadi, Hershey and Fisher2001) higher order factors of temperament are similarly heritable: 0.58 for effortful control, 0.42 for negative affectivity, and 0.41 for extraversion/surgency (Goldsmith et al., Reference Goldsmith, Buss and Lemery1997). These heritability estimates are subject to random sampling error, influences of assessment instruments, population-specific distributions of genotypes and environments, variations in mating patterns, developmental perturbations, and variations in the degree to which other basic assumptions of the underlying quantitative genetic model are met.
The association between temperament and symptoms of mood and behavioral disorders in children are largely accounted for by shared genetic factors (Lemery-Chalfant, Clifford, & Swann, in press). Negative correlations between effortful control at 5 years of age and internalizing and externalizing symptoms at 8 years of age were genetically mediated (Lemery-Chalfant et al., Reference Lemery-Chalfant, Doelger and Goldsmith2008). Thus, the genetic influence on children's symptoms of psychopathology may partially operate by protecting children from disorder. Negative affectivity is positively associated with symptoms, with a common genetic factor accounting for the association between negative affectivity in 7- to 12-year-olds and both attention problems and aggressive behaviors 2 years later (Gjone & Stevenson, Reference Gjone and Stevenson1997). A similar pattern was reported with preadolescents; genetic covariance between negative emotionality and oppositional defiant and conduct disorder symptoms represented 30% and 50% of the variance in symptoms, respectively, although nonshared environment also contributed to the covariances (Singh & Waldman, Reference Singh and Waldman2010). With an adoption design, the association between negative emotionality and internalizing–externalizing symptoms was genetically mediated (Schmitz & Saudino, Reference Schmitz, Saudino, Petrill, Plomin, DeFries and Hewitt2003). Thus, genetic influences on temperament likely also impact child psychopathology.
Contrasting Views of the Importance of the Common Family Environment
Classic biometric modeling of independent ACE contributions to trait variance has generally not supported a significant role of the common environment for temperament and related constructs (e.g., Kendler et al., Reference Kendler, Neale, Prescott, Kessler, Heath and Corey1996; McCall, Reference McCall, Wachs and Plomin1991; Mullineaux, Deater-Deckard, Petrill, Thompson, & DeThorne, Reference Mullineaux, Deater-Deckard, Petrill, Thompson and DeThorne2009; Plomin, Defries, McClearn, & McGuffin, Reference Plomin, DeFries, McClearn and McGuffin2001; Saudino, Reference Saudino2005; Saudino & Cherny, Reference Saudino, Cherny, Emde and Hewitt2001; Slutske et al., Reference Slutske, Heath, Dinwiddie, Madden, Bucholz and Dunne1997), with the reduced AE model often best representing the variance. As a result, several theorists have stressed that family environments within the normal range are not important for child development (Bouchard, Reference Bouchard2004; Harris, Reference Harris1998; Rowe, Reference Rowe1994; Scarr, Reference Scarr1992). Instead, shared genes account for similarities between individuals and account for approximately 50% of the variance, and nonshared environments account for differences between individuals and the remaining variance. Turkheimer (Reference Turkheimer2000) labeled the lack of systematic, common environment effects the “gloomy prospect” and suggested that important environmental contributions to behavior and development are random and unsystematic and thus not conducive to study.
Substantial nonbehavioral genetic research shows consistent associations between the family environment and children's temperament. Aspects of the home environment, such as level of chaos and quality of the physical environment, are consistently associated with child outcomes when not investigated using genetically informed designs, making nonsignificant findings within the field of behavior genetics suspect (e.g., Leventhal & Brooks-Gunn, Reference Leventhal and Brooks-Gunn2000; Pettit, Bates, & Dodge, Reference Pettit, Bates and Dodge1997). The point of reconciliation would be that “home” environment is not typically experienced as “common” (Goldsmith, Reference Goldsmith1988).
The conceptual framework of facilitative versus determinative environments provides an interpretative context. A facilitative (permissive) environment places few constraints on the child's development. In contrast, a determinative environment either constrains the genetically influenced pathways of development or leverages them beyond their expected expression (Baumrind, Reference Baumrind1993; Bradley, Reference Bradley, Watt, Ayoub, Bradley, Puma and LaBoeuf2006; Novak & Peleaz, Reference Novak and Pelaez2003; Sroufe, Reference Sroufe1997). For example, a chaotic, unstructured home typically does not support development of foundational skills, such as self-regulation and self-efficacy. Similarly, Wachs (Reference Wachs1988) suggested that the physical environment plays a role in the expression of temperament such that the right conditions must be present for the expression of any given dimension of temperament. Thus, environmental affordances affect the expression of heritable traits, which is perhaps an obvious point but one that is worth emphasizing in this context.
Two aspects of the home environment are the focus of our investigation: (a) general levels of home chaos, such as high levels of noise, confusion, and disorganization; and (b) the physical home, including the layout and size of the living environment, safety, and the availability of basic necessities and materials to encourage learning and growth.
Chaotic homes, which provide little structure or routine, are associated with children's poor self-regulatory and cognitive skills, aggression, impulsivity, and internalizing and externalizing problems (e.g., Dumas et al., Reference Dumas, Nissley, Nordstrom, Smith, Prinz and Levine2005; Evans, Wells, & Moch, Reference Evans, Wells and Moch2003; Valiente, Lemery-Chalfant, & Reiser, Reference Valiente, Lemery-Chalfant and Reiser2007; Wachs, Reference Wachs2000). Children raised in highly chaotic homes demonstrate increased behavioral problems over and above the effects of bad parenting and are more likely to engage in externalizing behaviors (Coldwell, Pike, & Dunn, Reference Coldwell, Pike and Dunn2006; Prevatt, Reference Prevatt2003). Home chaos also mediates the relation between socioeconomic status and components of socioemotional development, including self-regulation (Evans, Gonnella, Marcynyszyn, Gentile, & Salpekar, Reference Evans, Gonnella, Marcynyszyn, Gentile and Salpekar2005).
Relations between the physical home environment and temperament have also been reported, although the literature is more limited. Elements in the home contributing to the ambiance have been associated with children's temperament, outcomes in psychopathology, and development in general (Bradley, Reference Bradley1993; Evans, Reference Evans2006; Matheny, Wilson, & Thoben, Reference Matheny, Wilson and Thoben1987; Wachs, Reference Wachs1988). Children's development of fine and gross motor skills is linked with the number of toys in the home (Abbott & Bartlett, Reference Abbott and Bartlett2001). Children in overcrowded homes receive less parental attention (Liddell & Kruger, Reference Liddell and Kruger1989) and score higher on neuroticism (Matheny & Phillips, Reference Matheny, Phillips, Wachs and Kohnstamm2001; Murray, Reference Murray, Canter and Lee1974) and externalizing behaviors (Supplee, Unikel, & Shaw, Reference Supplee, Unikel and Shaw2007).
Integrating Contrasting Literatures by Modeling Gene–Environment Interplay
Many developmental psychologists traditionally adhered to the idea that chaotic, overcrowded, and unsafe environments crucially impact maladaptive child outcomes, given that children are less able to develop optimally in the face of constant disruptions and confusion. In contrast, behavior geneticists have shown that correlations between measured family environments and child traits are often genetically mediated, through the process of rGE; that is, genetic differences are associated with exposure to different environments. Many well-known “environmental” variables demonstrate moderate heritability (Kendler & Baker, Reference Kendler and Baker2007; Plomin et al., Reference Plomin, DeFries, McClearn and McGuffin2001). Passive rGE occurs when heritable traits of the parents influence the family environment, such that biological parents pass on genotypes to their children, as well as an environment that correlates with the genotype. For example, parents with poor self-regulation skills pass on a genotype that does not support the development of adaptive self-regulation in their children, and these same parents likely provide a more chaotic home environment with fewer routines and little predictability. The resulting association between the home environment and children's temperament is ambiguous in terms of whether it is genetically (passive rGE) or environmentally mediated.
These two literatures can be integrated by modeling more complex nonlinear relations between measured aspects of the common environment and genetic influences on temperamental traits. A new generation of quantitative biometric modeling is available that estimates G × E and rGE as well as the main effects of genes and environments that have been studied using the classic ACE models. Specifically, Purcell (Reference Purcell2002) expanded the classic models to incorporate both family-level and individual-level moderation of the ACE pathways, with the estimation of rGE also possible with an individual-level moderator. Price and Jaffee (Reference Price and Jaffee2008) then proposed a model that allows for the estimation of passive rGE with a family-level moderator in the case where significant moderation of heritability is present.
Although these models have not yet been used with temperament, rGEs were found between adolescent attitudes about their relationship with their parents (measured at the individual level) and their negativity and positivity personality factors (Krueger, South, Johnson, & Iacono, Reference Krueger, South, Johnson and Iacono2008), which often are interpreted in hedonic temperamental terms. Simultaneously, parent–child relationships significantly moderated the heritability of personality, such that heritability was higher for positivity when regard for parents was higher, and heritability was higher for negativity when regard and conflict were higher. Similarly, rGEs were reported between income and adult internalizing problems, interpreted as social selection, or active rGE (South & Krueger, Reference South and Krueger2011). Income simultaneously moderated the heritability of internalizing problem behaviors, with heritability higher with higher income, interpreted as social causation.
The Present Study
We aimed to uncover the role of measured aspects of the common family environment on children's temperament using the twin design to integrate the developmental psychology literature that emphasizes the importance of the home environment with the behavior genetic literature that does not support an important role of the common family environment on children's traits and development. Using contemporary biometric modeling, we aimed to elucidate passive rGE, such that the correlations between measured aspects of the home environment and children's temperament as genetically mediated, and G × E, or family environment moderation of the heritability of temperament. Temperament was assessed with both mother and father report of effortful control, negative affectivity, and surgency, and measured aspects of the home environment included mother report of home chaos and experimenter observation of the physical home environment. We hypothesized that higher chaos and poorer quality physical environments would be both correlated with and moderate the heritability of children's temperament, based on the facilitative versus determinative environments conceptual framework.
Methods
Participants
The sample consisted of 807 twin pairs participating in the longitudinal Wisconsin Twin Project, with 51% boys and mean age of 7.93 years (SD = 0.87). There were 301 MZ, 263 same-sex DZ, and 243 opposite-sex DZ twin pairs. Methods of recruitment from state birth records have previously been described in depth (see Lemery-Chalfant, Goldsmith, Schmidt, Arneson, & Van Hulle, Reference Lemery-Chalfant, Goldsmith, Schmidt, Arneson and Van Hulle2006). Ethnicity was 88.5% Caucasian, 4.1% African American, and 5.8% other or mixed. Parent education ranged from no formal education to a graduate degree, with a majority of parents having completed some or all of college (M mothers = 14.91 years, M fathers = 14.45 years). Family income ranged from unemployed to $200,000+ a year, with the average family earning $51,000–$70,000.
Procedure
Mothers completed demographic information and the zygosity questionnaire via a phone interview. Around 3 to 4 months later (M = 3.72, SD = 4.77), mothers participated in another phone interview during which they completed Rothbart's Children's Behavior Questionnaire (CBQ) and the Confusion, Hubbub, and Order Scale (CHAOS). At this time, the father also completed the CBQ over the telephone. In addition, families participated in a 4-hr home visit, which involved a number of assessments. Child testers completed the Home Observation for Measurement of the Environment (HOME) during and immediately after the home visit.
Measures
Parents provided demographic information, and zygosity was determined through agreement between parent and observer ratings. Mothers completed the Zygosity Questionnaire for Young Twins (Goldsmith, Reference Goldsmith1991), which measures physical similarities. The agreement of this questionnaire with genotyping is greater than 95% (Forget-Dubois et al., Reference Forget-Dubois, Pérusse, Turecki, Girard, Billette and Rouleau2003; Price et al., Reference Price, Freeman, Craig, Petrill, Ebersole and Plomin2000). Observers also completed questions concerning zygosity after a home visit. For six of the twin pairs in this sample, parents and observers did not agree, and these pairs were omitted from genetic analyses.
Socioeconomic status
A mean composite was formed from standardized years of mother education, father education, and total family income (rs = .45–.55, p < .0001).
CBQ
The CBQ (Rothbart et al., Reference Rothbart, Ahadi, Hershey and Fisher2001) consisted of 180 questions scored on a 7-point Likert scale, ranging from extremely untrue of your child to extremely true of your child over the past 6 months. Sample questions include “My child is full of energy, even in the evening,” “My child will move from one task to another without completing any of them,” and “My child is afraid of loud noises.” Internal consistency for the CBQ scales ranged from 0.69 (sadness) to 0.89 (shyness), averaging 0.73 across all scales used to form the superordinate factors used in model fitting. Preliminary analyses showed that mother-report and father-report CBQ were moderately correlated (rs = .41–.65, p < .0001), and thus mean scale composites across reporter were formed. Based on Rothbart et al. (Reference Rothbart, Ahadi, Hershey and Fisher2001), scales were further composited into three higher order factors: effortful control, negative affectivity, and extraversion/surgency. Effortful control is a mean composite of inhibitory control and attentional focusing (r = .72, p < .0001); negative affectivity is a mean composite of distress to novelty, distress to limitations, sadness, and lack of soothability (rs = .24–.59, p < .0001); and extraversion/surgency is a mean composite of activity level, lack of shyness, impulsivity, smiling and laughter, and approach (rs = .15–.66, p < .0001).
CHAOS
This mother report questionnaire (Matheny, Wachs, Ludwig, & Phillips, Reference Matheny, Wachs, Ludwig and Phillips1995) consists of 15 true/false statements asking participants to think about how each item described their home. Sample questions include, “It's a real ‘zoo’ at our home,” “Our home is a good place to relax (reverse scored),” “First thing in the day, we have a regular routine at home (reverse scored),” and “We almost always seem to be rushed.” The global mean was computed such that higher scores indicated higher chaos, with an internal consistency of 0.80.
Living Environment Observation Scale (LEOS)
Adapted from Caldwell and Bradley's (Reference Caldwell and Bradley1984) HOME, observers completed this scale immediately after visiting the home, rating nine dimensions of the home on a scale of 1 to 3, where 1 = no/minimal evidence, 2 = moderate evidence, and 3 = substantial evidence. The nine dimensions were structural safety of the home, home décor, child-friendly home, adequate living space for number of individuals in the home, interpersonal space, overall organization, cleanliness, outside play environment, and condition of street where the twins live. A sample item (overall organization) includes “This item reflects the overall physical organization of the house: (a) home is cluttered making it difficult to walk around objects, unable to find a clear space to do assessments activities, (b) home is generally clean though floors may need to be vacuumed or washed; noticeable dust on furniture, and (c) home is clean and appears to have been cleaned recently or on a regular basis.” The global mean was computed such that higher scores indicated a higher quality physical environment, with an internal consistency of 0.84. The LEOS was transformed for negative skewness (–2.079 to –1.260; SE = 0.066) and kurtosis (4.650 to 0.430; SE = 0.132) by taking an inverse transformation (1/x).
Although the LEOS and CHAOS were correlated (r = –.22, p < .001), they were analyzed separately because they measure fundamentally different aspects of the home environment.
Statistical approach
Two independent samples were formed for use with statistical tests that assume independent observations. The Twin 1 subsample was created by randomly selecting one twin from each pair. The Twin 2 subsample included the co-twins of the Twin 1 subsample. Correlations and t tests were tested separately with the Twin 1 and Twin 2 subsamples. Average correlations (using the Fisher Z transformation) across the two samples are reported. We also report twin intraclass correlations.
Age, age-squared, and sex-residualized scores were used for twin modeling to account for any age and sex effects on ACE estimates, as is standard practice to reduce potential biases when it is infeasible to incorporate additional covariates (McGue & Bouchard, Reference McGue and Bouchard1984). Using structural equation modeling, full univariate biometric models decompose the variance into three components: additive genetic (A; the sum of the average effects of individual genes across the genotype), common environment (C; aspects of the environment that make twins growing up in the same home similar to one another), and nonshared environment (E; aspects of the environment that make twins dissimilar to one another as well as measurement error). C is completely shared between co-twins growing up in the same home, and E is completely independent across individuals. The latent A influence is correlated between co-twins and equals 1.00 for MZ twins, because they share 100% of their genomic DNA, and 0.50 for DZ twins, because they share on average 50% of their genomic DNA. Reduced models of the form AE, CE, or E are compared to the full ACE model. E can never be dropped because it also contains measurement error. MZ twins are more similar to each other than DZ twins for heritable traits. If shared environment is significant, however, MZ twins are less than twice as similar as DZ twins because these influences act on both types of twins in similar ways.
Price and Jaffee (Reference Price and Jaffee2008) introduce a classical twin ACE model that estimates passive rGE in the presence of significant family-level moderation of heritability (G × E). The existence of G × E allows the main effect of the measured family environment to be distinguished from passive rGE; thus, this model can only be used when it is first established that there is significant moderation of the latent factor A by the measured family environment. Power to distinguish between a main effect of the family environment and passive rGE increases as the magnitude of A and moderation on A increases.
The variance in the phenotype is partitioned into uncorrelated within- and between-family variables. Between-family differences in the phenotype are then predicted by a measured aspect of the family environment, a measure that differs between, but not within families. The within-family phenotypic variance is not affected by the measured family environment, with passive rGE operating the same way for both MZ and DZ co-twins. The measured environment is modeled as a random effect, and correlations with the latent factor A are estimated to index passive rGE (a linear function of the measured environment), while simultaneously estimating the measured environment's main effect on the phenotype (constant with respect to the measured environment).
A nonsignificant chi-square difference test and a stronger negative Akaike information criterion (AIC; Akaike, Reference Akaike1987) indicate more parsimonious fit of a reduced model over the full model. The statistical program Mx (Neale, Boker, Xie, & Maes, Reference Neale, Boker, Xie and Maes2003) was used to fit the models.
Results
Descriptive statistics and first order associations
The phenotypic correlations, means, and standard deviations for all variables are given in Table 1. Effortful control was moderately negatively correlated with the other two factors, whereas extraversion/surgency and negative affectivity were largely independent. CHAOS and LEOS largely tapped independent aspects of the home environment. The CBQ factors were low to moderately correlated with CHAOS and LEOS in expected directions. Girls were higher on effortful control, t (393) = 5.40, p < .01; M girls = 4.78, M boys = 4.48, and boys were higher on extraversion/surgency, t (389) = –6.70, p < .01; M boys = 4.89, M girls = 4.65. Table 1 also gives twin intraclass correlations. Twin correlations support high heritability of these temperament factors, with MZ co-twins more similar than DZ co-twins. In addition, twin correlations computed separately for male MZ, female MZ, same-sex male DZ, same-sex female DZ, and opposite sex DZ pairs support nonsignificant sex differences.
Table 1. Correlations between study variables, descriptive statistics, and twin intraclass correlations

Note: Correlations are mean correlations of the Twin 1 and Twin 2 subsamples. MZm, monozygotic male; MZf, monozygotic female; DZm, dizygotic male; DZf, dizygotic female; DZos, dizygotic opposite sex twin pairs; CBQ, Children's Behavior Questionnaire; CHAOS, Confusion, Hubbub, and Order Scale; LEOS, Living Environment Observation Scale (higher scores indicate less crowded and more safe conditions).
*p < .01.
We next fit saturated models to the temperament composites to test assumptions of the twin design and provide a more formal test of sex differences.
For effortful control, we fit the saturated model where means, variances, and covariances were estimated separately across the sex and zygosity groups, yielding a fit of –2 log likelihood (–2LL) (1,545) = 3,274.07, AIC = 184.07. Next, a series of nested models were fit. First, we determined that means could not be equated (a) between co-twins within zygosity group, Δχ2 (3) = 13.46, p = .004, AIC = 7.46; (b) between MZ and DZ zygosity groups, Δχ2 (5) = 24.97, p < .001, AIC = 14.97; and (c) between sexes, Δχ2 (1) = 24.38, p < .001, AIC = 22.38. However, variances and covariances could be equated across sex and zygosity groups. We found that variances could be equated (a) between co-twins within zygosity group, Δχ2 (5) = 13.84, p = .02, AIC = 3.84; (b) between MZ and DZ zygosity groups, Δχ2 (6) = 15.33, p = .02, AIC = 3.33; and (c) between sexes, Δχ2 (4) = 9.19, p = .06, AIC = 1.19. Furthermore, covariances could be equated across sexes, Δχ2 (3) = 2.42, p = .49, AIC = –3.58. Because variances and covariances were equivalent across zygosity groups, there is no evidence of sibling interaction effects with the effortful control factor (Neale & Cardon, Reference Neale and Cardon1992). Competition or contrast effects are distinguished from genetic effects because they result in higher total phenotypic variance in DZ than in MZ twins.
For negative affectivity, we fit the saturated model where means, variances, and covariances were estimated separately across the sex and zygosity groups, yielding a fit of –2LL (1,545) = 2,327.93, AIC = –762.08. We then fit a series of nested models. First, we determined that means could be equated (a) between co-twins within zygosity group, Δχ2 (3) = 1.15, p = .77, AIC = –4.85; (b) between MZ and DZ zygosity groups, Δχ2 (5) = 12.35, p = .03, AIC = 2.35; and (c) between sexes, Δχ2 (1) = 0.29, p = .59, AIC = –1.72. Second, we found that variances could also be equated (a) between co-twins within zygosity group, Δχ2 (5) = 11.67, p = .04, AIC = 1.67; (b) between MZ and DZ zygosity groups, Δχ2 (6) = 7.30, p = .29, AIC = –4.70; and (c) between sexes, Δχ2 (4) = 5.24, p = .26, AIC = –2.76. Furthermore, covariances could be equated across sexes, Δχ2 (3) = 1.73, p = .63, AIC = –4.27.
Similarly, for extraversion/surgency, we fit the saturated model where means, variances, and covariances were estimated separately across the sex and zygosity groups, yielding a fit of –2LL (1,545) = 2,047.93, AIC = –1,042.07. Next, we systematically equated means, variances, and covariances across groups. We determined that means could be equated (a) between co-twins within zygosity group, Δχ2 (3) = 4.14, p = .25, AIC = –1.86, and (b) between MZ and DZ zygosity groups, Δχ2 (5) = 7.09, p = .21, AIC = –2.91. However, means could not be equated between sexes, Δχ2 (1) = 54.42, p < .001, AIC = 52.42. Next, we found that variances could also be equated (a) between co-twins within zygosity group, Δχ2 (5) = 4.22, p = .52, AIC = –5.78; (b) between MZ and DZ zygosity groups, Δχ2 (6) = 9.85, p = .13, AIC = –2.16; and (c) between sexes, Δχ2 (4) = 13.20, p = .01, AIC = 5.20. Furthermore, covariances could be equated across sexes, Δχ2 (3) = 1.76, p = .62, AIC = –4.24.
In summary, we found some mean differences across groups for effortful control and extraversion/surgency, although all variances and covariances were equivalent across groups, indicating that it is not necessary to model competition or contrast effects. Before fitting the following biometric models, we first regressed out the influence of sex, age, and age squared, as is standard practice when multiple covariates cannot be incorporated into the model.
Classic biometric ACE modeling of temperament
We began with the ACE full model (which allows for common environmental influences), then systematically dropped A and C to test the significance of each influence; E is always included because it includes measurement error. Table 2 summarizes the fit statistics for each model, as well as the ACE estimates for the full models and the (bolded) most parsimonious reduced model. For all three temperament factors, the best fitting model was the AE, with 49%–79% of the variance due to heritability. Thus, classic twin modeling focused on independent main effects of genetic and environmental influences indicated that all three components of temperament were moderately to highly heritable, with no significant effects of the common environment.
Table 2. Biometric ACE model fit statistics for effortful control, negative affectivity, and extraversion/surgency

Note: The most parsimonious model is in bold. A, additive genetic; C, shared environment; E, nonshared environment; −2LL, −2 times the log likelihood (fit statistic); AIC, Akaike information criterion (fit index); Δdf, change in the degrees of freedom; Δχ2, change in the chi-square value from the best fitting full model to the reduced model; h 2, additive genetic; c 2, common environment; and e 2, nonshared environment.
Contemporary biometric modeling of gene–environment interplay on temperament
Based on Price and Jaffee (Reference Price and Jaffee2008; see Method), we began by testing whether heritability was moderated by our measured aspects of the family environment (G × E). If G × E was significant, we then modeled rGE. We began with the full moderated ACE model (i.e., the measured family environment is estimated to moderate the A, C, and E parameters), then dropped nonsignificant parameters to reach the most parsimonious model. For each of the three temperament factors, C was nonsignficant and was dropped from the model. Only AE models are presented for simplicity.
Effortful control
A model allowing CHAOS moderation of the A and E paths on effortful control fit significantly better than the AE model with no moderation (see Table 3). Across levels of CHAOS, the estimates of A and E changed such that estimates of A increased from 76% (–1 SD) to 82% (+1 SD), indicating that children's effortful control was modestly more heritable under chaotic home conditions. The overall variance in effortful control was also higher under chaotic home conditions (0.361 at +1 SD; 0.289 at –1 SD for the between-family variable). Passive rGE was also significant (rGE = –.69), supporting genetic mediation of the covariance between CHAOS and children's effortful control. A model allowing LEOS moderation of the A and E paths on effortful control was not more parsimonious than the classic AE model (Table 3); thus, estimates of A (60%) and E (40%) remained constant across levels of LEOS, and the model estimating rGE was not fit.
Table 3. Biometric AE models with moderation and gene–environment correlation
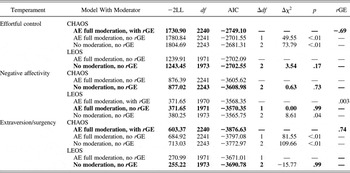
Note: The best fitting model is in bold. CHAOS, Confusion, Hubbub, and Order Scale; LEOS, Living Environment Observation Scale; A, additive genetic; E, nonshared environment; rGE, gene–environment correlation; −2LL, −2 times the log likelihood (fit statistic); AIC, Akaike information criterion (fit index); Δdf, change in the degrees of freedom; Δχ2, change in the chi-square value from the full model to the reduced model.
Negative affectivity
Similarly, a model estimating CHAOS moderation of the A and E paths on negative affectivity was not more parsimonious than the classic AE model (see Table 3); thus, estimates of A (79%) and E (21%) remained constant across levels of home chaos.
However, a model allowing LEOS moderation of the A and E paths on negative affectivity fit significantly better than the AE model without moderation (Table 3). Next, a model that simultaneously estimated rGE was fit, although rGE was estimated at r = .003 and was not significant so was thus dropped. Estimates of A varied from 78% (+1 SD) to 85% (–1 SD), indicating that heritability increased under crowded or unsafe home conditions (i.e., lower LEOS). The overall variance in negative affectivity was also higher under crowded or unsafe home conditions (0.302 at –1 SD; 0.239 at the mean for the between-family variable).
Extraversion/surgency
A model allowing CHAOS moderation of the A and E paths on extraversion/surgency fit significantly better than the AE model without moderation (see Table 3). Across levels of CHAOS, the estimates of A and E changed, such that estimates of A increased modestly from 81% (–1 SD) to 84% (+1 SD), indicating that children's extraversion/surgency was more heritable under chaotic home conditions. Whereas heritability increased, the overall variance in extraversion/surgency minimally changed (0.124 at –1 SD; 0.160 at +1 SD for the between-family variable). Passive rGE was also signficant (rGE = .74), supporting genetic mediation of the covariance between CHAOS and children's extraversion/surgency. A model estimating LEOS moderation of the A and E paths on extraversion/surgency was not more parsimonious than the AE model without moderation (see Table 3); thus, estimates of A (49%) and E (51%) remained constant across levels of the physical home environment.
Discussion
Modeling multiple types of gene–environment interplay uncovered the complex role of genetic factors and the hidden importance of the family environment for children's temperament and development more generally. We considered the genetic and environmental architecture of temperament in middle childhood by simultaneously modeling main effects, G × E, and rGE when possible using a new quantitative genetic model (Price & Jaffee, Reference Price and Jaffee2008; see Method). This new model allows for the simultaneous estimation of G × E and rGE using family-level measures of the environment, such as chaos and the physical environment in the home. Previous models (e.g., Purcell, Reference Purcell2002) allow for the simultaneous estimation of G × E and rGE only with individual-level measures of the environment, such as parent–child conflict and regard measured independently for co-twins. This model has recently been criticized because other, simpler models (i.e., common factor or correlated factor models) may be more parsimonious (Rathouz, Van Hulle, Rodgers, Waldman, & Lahey, Reference Rathouz, Van Hulle, Rodgers, Waldman and Lahey2008).
Given the widespread importance of family-level aspects of the environment for child development, the Price and Jaffee (Reference Price and Jaffee2008) model fills a critical need. It is essential to model G × E and rGE as well as main genetic and environmental effects, given increasing evidence in the literature that all of these processes are relevant for child development.
Passive rGE, children's temperament, and the home
We demonstrated important passive rGE, such that home environments were less chaotic for children with high effortful control, and this association was genetically mediated. Furthermore, children with high extraversion/surgency experienced more chaotic home environments, and this correlation also was genetically mediated. This finding suggests that some of the same genes in parents that contribute to levels of chaos in the home are passed down to children and contribute to their temperament.
Previous literature, largely focused on adolescent adjustment, also documents the important role of genetic mediation of covariation between putative measures of the environment and traits. For example, the correlation between negative relationships of mothers with their adolescents was 0.59, with genetic factors explaining 69% of this covariation (Reiss & Neiderhiser, Reference Reiss and Neiderhiser2000). Shared genetic liability explained part of the co-occurrence of negative life events and depression as well (in adults, Kendler & Karkowski-Shuman, Reference Kendler and Karkowski-Shuman1997; in adolescents, Thapar, Harold & McGuffin, Reference Thapar, Harold and McGuffin1998). Thus, rGE plays a central role in associations between adolescent experiences and adjustment.
Although these findings highlight the importance of rGE, shared genetic variance does not preclude an environmental influence on the trait (Waldman, Reference Waldman2007). Marital stability, corporal punishment, and physical maltreatment have important environmental influences on children's behavioral problems when controlling for genetic associations (D'Onofrio, Turkheimer, Emery, Slutske, & Martin, Reference D'Onofrio, Turkheimer, Emery, Slutske and Martin2005; Jaffee, Caspi, Moffitt, & Taylor, Reference Jaffee, Caspi, Moffitt and Taylor2004).
Our findings bolster the emerging literature showing that “environmental” measures of the home share genetic variance with cognitive abilities, personality, and temperament. In adults, genetic influences on cognitive abilities accounted for a significant portion of the heritability of education and occupational status (Lichtenstein & Pedersen, Reference Lichtenstein and Pedersen1997), and genetic influences on extraversion, openness, and neuroticism accounted for all of the genetic influences on stressful life events (Saudino, Pedersen, Lichtenstein, McClearn, & Plomin, Reference Saudino, Pedersen, Lichtenstein, McClearn and Plomin1997). In toddlers, the heritability of the HOME measure of the home environment was accounted for by cognitive abilities and temperament (Saudino & Plomin, Reference Saudino and Plomin1997).
Unfortunately, we were unable to test for the presence of rGE between the LEOS observational assessment of the physical home context with effortful control and extraversion/surgency because the Price and Jaffee (Reference Price and Jaffee2008) model requires significant moderation of the additive genetic path before rGE can be estimated. The rGE between physical home and negative affectivity was nonsignificant, suggesting that genes that influence children's negative affectivity do not influence the physical home environment.
Moderation of the heritability of temperament by the home environment
We found evidence that the heritability of children's temperament was moderated by home environments, such that effortful control and extraversion/surgency were more heritable in chaotic homes, and negative affectivity was more heritable under crowded or unsafe home conditions. Note that these modest increases in heritability were not due to overall decreases in the phenotypic variance; the between-family variance in effortful control and negative affectivity were higher in adverse home conditions, whereas the variance did not change across the distribution of home environments for extraversion/surgency.
At this point in this relatively new literature, it is difficult to form directional hypotheses concerning moderation of heritability. Johnson (Reference Johnson2007) hypothesized that adaptive behaviors are more heritable under positive environments, and risky behaviors are more heritable under adverse environments. However, it is challenging to cleanly distinguish adaptive and risky behaviors, because they vary depending on the broader context and the outcome. Temperamental shyness, for example, increases risk of later anxiety disorders but is protective against later externalizing problems (e.g., Rubin, Coplan, & Bowker, Reference Rubin, Coplan and Bowker2009; Schwartz, Snidman, & Kagan, Reference Schwartz, Snidman and Kagan1996). High empathy, prosociality, and strong inhibitory processes are generally adaptive features of temperament and childhood personality; however, if these processes are overdeveloped, they may contribute to risk for mood disorders in adolescent girls (Zahn-Waxler, Shirtcliff, & Marceau, Reference Zahn-Waxler, Shirtcliff and Marceau2008). The opposite argument also seems likely, where the importance of genetic factors for individual differences is lower in portions of the population exposed to adversities that impact the disorder or trait (e.g., posttraumatic stress disorder in war veterans; Button, Scourfield, Martin, Purcell, & McGuffin, Reference Button, Scourfield, Martin, Purcell and McGuffin2005; Wichers et al., Reference Wichers, Purcell, Danckaerts, Derom, Derom and Vlietinck2002). A broader statement is perhaps more accurate: Social control constrains heritability (Rutter, Reference Rutter2006; Shanahan & Hofer, Reference Shanahan and Hofer2005).
The literature supports the idea that social control constrains heritability, whereas the environment is permissive and heritability is higher under conditions of low social constraint. Individual differences in disinhibition (i.e., partying, drinking, and multiple sex partners) were not heritable for those with a religious upbringing (Boomsma, deGeus, van Baal, & Koopmans, Reference Boomsma, de Geus, van Baal and Koopmans1999). There are also numerous examples of heritability varying by cohort, with lower social controls allowing for the expression of heritable individual differences. The heritability of smoking in females rose over time as it became more socially acceptable, with no change in men (Kendler, Thornton, & Pedersen, Reference Kendler, Thornton and Pederson2000). Similarly, when overall mean height increased in Finland from 1928 to 1957 (attributed to improved nutrition), heritability increased (Silventoinen, Kaprio, Lahelma, & Koskenvuo, Reference Silventoinen, Kaprio, Lahelma and Koskenvuo2000). Perhaps structured, secure, and safe home environments exert social control over children's temperament and thus decrease heritability. It would be informative to determine how stable these effects are across various environments and developmental periods.
Multiple pathways of temperament development and risk for child psychopathology
Developmental processes that contribute to temperament and child psychopathology include direct effect of genes and environments, environmental moderation of genetic effects, and indirect effects of genes through environments and social conditions.
The direct effect of genes, termed the reactive pathway by Reiss and Leve (Reference Reiss and Leve2007), suggests that genes and environments make independent contributions to children's outcomes. This main effects model can be expanded by considering G × E. Multiple types of moderational models have been proposed (Shanahan & Hofer, Reference Shanahan and Hofer2005), including diathesis stress (does an adverse environment act on a vulnerable, often genetic substrate?), social context as enhancement (does a positive environment act on a positive or adaptive genetic substrate?), social context as compensation (does a positive environment suppress the expression of a vulnerable genetic diathesis?), and social context as social control (do social norms or institutions limit choices, preventing or minimizing the expression of the diathesis?). The more recent biological sensitivity to context/differential susceptibility model also falls under this category (Ellis, Boyce, Belsky, Bakermans-Kranenburg, & van IJzendoorn, Reference Ellis, Boyce, Belsky, Bakermans-Kranenburg and van IJzendoorn2011), because it predicts a crossover interaction (are some individuals genetically or temperamentally more sensitive to both adverse and protective environments?). Illustrating this approach, difficult temperament may both impede healthy development in adverse environments and facilitate positive development in supportive contexts (Pluess & Belsky, Reference Pluess and Belsky2010).
These models can be examined using molecular, as well as quantitative genetic approaches (e.g., children carrying the short version of the tandem repeat in the promoter of the serotonin transporter gene paired with mothers with low social support were more likely to be behaviorally inhibited). Moffitt, Caspi, and Rutter (Reference Moffitt, Caspi and Rutter2006) and Rutter, Moffitt, and Caspi (Reference Rutter, Moffitt and Caspi2006) provide thorough reviews of G × E findings involving measured genes interacting with measured environments to predict problem behaviors and psychopathologies.
The literature utilizing these interactive models is expanding, and evidence of G × E is accumulating. One limitation is that these models do not explicitly address rGE, and the extent to which the genetic diathesis and the measured environment are correlated can introduce bias to the estimates (Aiken & West, Reference Aiken and West1991). In terms of quantitative genetic models, parsing out the main effect of the measured environment can affect estimates of moderation of the ACE variance components if the moderator shares genetic variance with the trait of interest (rGE).
The third model, the social mediation pathway model (Reiss & Leve, Reference Reiss and Leve2007), is relatively new to the literature. In this case, poor home environments mediate the genetic influence on child adjustment through rGE. For example, infant behavioral inhibition evokes parental insensitivity, which then potentiates maladaptive parent–child interactions over time, exacerbating the child's fear of novelty toward clinical levels of anxiety. In support of this pathway, previous literature has shown that children's heritable traits evoke parent–child mutuality (Deater-Deckard & O'Connor, Reference Deater-Deckard and O'Connor2000; Deater-Deckard & Petrill, Reference Deater-Deckard and Petrill2004), parental emotional overinvolvement (Moberg, Lichtenstein, Forsman, & Larsson, Reference Moberg, Lichtenstein, Forsman and Larsson2011), hostility (Boivin et al., Reference Boivin, Pérusse, Dionne, Saysset, Zoccolillo and Tarabulsy2005), and discipline (Riggins-Caspers, Cadoret, Knutson, & Langbehn, Reference Riggins-Caspers, Cadoret, Knutson and Langbehn2003).
Current findings of large rGE between children's temperament and chaos in the home support the importance of considering the social mediation pathway model in addition to the more commonly used G × E moderational models. Effortful control, for example, may protect children from internalizing and externalizing disorders (Eisenberg et al., Reference Eisenberg, Valiente, Spinrad, Liew, Zhou and Losoya2009) by decreasing chaos in the home. Testing these competing causal models of the etiology of psychopathology would greatly advance the literature.
Relevance of gene–environment interplay for preventive interventions
Testing the reactive and the social mediation pathways is important for elucidating proximal processes of child development and risk for psychopathology. Infant difficult temperament, for example, evokes adverse responses from caregivers (Boivin et al., Reference Boivin, Pérusse, Dionne, Saysset, Zoccolillo and Tarabulsy2005); however, a parenting intervention may extinguish these negative genetic influences, such that parents learn to respond to their infant's irritability and unadaptability with patience and consistency. As discussed above, protective environments can exert social control over a heritable risk for depression (Shanahan & Hofer, Reference Shanahan and Hofer2005).
In contrast, the presence of a significant rGE does not always support the social mediation pathway. Chaotic home environments may not have a causal impact on children's adjustment; rather, the association may simply reflect genetic risk that parents transmit to their children. In this case, interventions targeting the home environment may be ineffective in reducing risk for psychopathology. Testing these competing process models would be informative for designing effective interventions.
Limitations of the study
The study faced several limitations. First, generalizability to other populations is limited since the sample was predominantly composed of Caucasian families. Home environments vary by ethnic and cultural groups, which may lead to different patterns of genetic and environmental influence on children's temperament. For example, ethnic differences in children's IQ scores were significantly associated with the HOME (Brooks-Gunn, Klebanov, & Duncan, Reference Brooks-Gunn, Klebanov and Duncan1996). Different cultural values and beliefs lead to different levels and types of affordances available in the home, such as parental responsiveness and the availability of learning materials (e.g., Bradley, Reference Bradley, Watt, Ayoub, Bradley, Puma and LaBoeuf2006; Bradley & Corwyn, Reference Bradley and Corwyn2005).
Second, participants in this study were twins, so there is a question as to whether findings from a study of temperament in twin children can be generalized to single-born children. Moilanen et al. (Reference Moilanen, Linna, Ebeling, Kumpulainen, Tamminen and Piha1999) found nonsignificant trends suggesting lower rates of emotional and behavioral problems in twins, whereas others have reported no differences in depression scores for twins and singletons (Angold, Erkanli, Silberg, Eaves, & Costello, Reference Angold, Erkanli, Silberg, Eaves and Costello2002). Studies with larger, population-based samples usually find no differences between twins and singletons in internalizing and externalizing problems (Lytton & Gallagher, Reference Lytton, Gallagher and Bornstein2002). Specific to temperament, there were no differences between twin and singleton infants' parent-reported temperament (Goldsmith, Lemery, Buss, & Campos, Reference Goldsmith, Lemery, Buss and Campos1999). Thus, most literature supports the generalizability of twin data to singletons for temperament and symptoms of psychopathology.
Third, the equal environments assumption (EEA), or the assumption that the environment influencing the behavior being studied is no more similar for MZ twins than DZ twins, is central to the classic twin study. If MZ twins have more similar trait relevant environments than DZ twins, then estimates of heritability would be too large. This assumption has been tested with large samples of twins. Comparing models of mother report of child and adolescent mood and behavioral disorders controlling for environmental similarity in twin pairs to models that did not control for environmental similarity, Cronk and colleagues (Reference Cronk, Slutske, Madden, Bucholz, Reich and Heath2002) reported that estimates were not greatly affected, supporting the EEA. Twin children's similarity in personality was unrelated to their similarity of childhood experience, which also supports the EEA (Borkenau, Riemann, Angleitner, & Spinath, Reference Borkenau, Riemann, Angleitner and Spinath2002). Thus, investigators continue to test the EEA, and the validity of this assumption is supported for personality and mood and behavior problems.
Fourth, independent mother and father reports were composited to assess children's temperament, and although the physical home environment was assessed through trained observation after a home visit, mothers were relied upon to report on levels of chaos in the home. The question, of course, is the extent to which parent report assesses temperament with optimal validity and the extent to which shared rater variance accounts for associations between temperament and home chaos (Saudino, Reference Saudino2005). Parent-report measures are inexpensive, easy to administer, and parents are more knowledgeable and familiar with their children in multiple contexts than other caregivers, such as teachers or other relatives (Rothbart & Bates, Reference Rothbart, Bates, Damon and Eisenberg2006). The CBQ has been used in conjunction with the Laboratory Temperament Assessment Battery (Goldsmith, Reilly, Lemery, Longley, & Prescott, Reference Goldsmith, Reilly, Lemery, Longley and Prescott1993), demonstrating its validity (Gagne, Van Hulle, Aksan, Essex, & Goldsmith, Reference Gagne, Van Hulle, Aksan, Essex and Goldsmith2011).
Fifth, the Price and Jaffee model (Reference Price and Jaffee2008) is limited in that it cannot estimate rGE in the absence of G × E. The existence of G × E allows the main effect of the measured family environment to be distinguished from passive rGE, with the measured environment's main effect on the phenotype constant with respect to the measured environment and rGE modeled as a linear function of the measured environment. Thus, there are likely additional rGEs that could not be detected with this model.
Future directions
In addition to testing the reactive and social mediation process models described above, other lines of inquiry would be useful. First, our study focused on the three main temperament factors found in childhood. Future studies should consider temperament at the lower order scale level, because the genetic architecture of temperament, using the classic ACE model, has sometimes been found to vary by scale (Goldsmith et al., Reference Goldsmith, Lemery, Buss and Campos1999). Second, it will be important to consider age differences in the genetic and environmental underpinnings of temperament, preferably with a longitudinal design. Genes and environments probably have age-dependent influences on temperament, and documenting these differences will inform understanding of the course of development.
Conclusion
We considered the genetic and environmental architecture of temperament in middle childhood, simultaneously modeling main effects, G × E, and rGE using a new quantitative genetic model (Price & Jaffee, Reference Price and Jaffee2008). Children with high effortful control had less chaotic home environments, and children with high extraversion/surgency had more chaotic home environments; these associations were mediated genetically through the process of passive rGE. At the same time, effortful control and extraversion/surgency were modestly more heritable in chaotic homes, and negative affectivity was modestly more heritable under crowded or unsafe home conditions. It may be that structured, secure, and safe home environments exert social control over children's temperament and thus decrease heritability. Gene–environment interplay on children's temperament should continue to be examined to uncover permissive and deterministic influences across a spectrum of environments.





