Risky decision making is a hallmark phenotypic characteristic of substance use disorders (SUDs). For adaptive decision making, it is essential to determine the positive and negative outcomes rapidly to guide current as well as future actions. Disruption of this process may produce risk-prone behavior, where choice is driven by the positive outcomes, despite possible detrimental consequences (Bechara, Damasio, Damasio, & Anderson, Reference Bechara, Damasio, Damasio and Anderson1994; Fishbein et al., Reference Fishbein, Eldreth, Hyde, Matochik, London and Contoreggi2005). It is well known that adolescents are especially prone to risk-taking behaviors, which makes adolescence a period of heightened vulnerability to substance use. For some adolescents, experimental substance use progresses to substance abuse or even to SUD. The highest risk for developing a SUD exists for adolescents with a family history (FH) of SUD (Kendler, Prescott, Myers, & Neale, Reference Kendler, Prescott, Myers and Neale2003; Sher, Walitzer, Wood, & Brent, Reference Sher, Walitzer, Wood and Brent1991). These adolescents often start using substances at younger ages and display a cluster of behavioral traits described as disinhibited, undercontrolled, or impulsive (Clark et al., Reference Clark, Moss, Kirisci, Mezzich, Miles and Ott1997; Iacono, Carlson, Taylor, Elkins, & McGue, Reference Iacono, Carlson, Taylor, Elkins and McGue1999; Tarter et al., Reference Tarter, Kirisci, Mezzich, Cornelius, Pajer and Vanyukov2003; Tarter, Kirisci, Habeych, Reynolds, & Vanyukov, Reference Tarter, Kirisci, Habeych, Reynolds and Vanyukov2004; Verdejo-García, Lawrence, & Clark, Reference Verdejo-García, Lawrence and Clark2008). Genetic factors play an important role in both the initiation of substance use and the transition to abuse (Bierut et al., Reference Bierut, Dinwiddie, Begleiter, Crowe, Hesselbrock and Nurnberger1998; Rhee et al., Reference Rhee, Hewitt, Young, Corley, Crowley and Stallings2003; Uhl, Reference Uhl2004; Young, Rhee, Stallings, Corley, & Hewitt, Reference Young, Rhee, Stallings, Corley and Hewitt2006). However, the role of genetics is complex, with most behavioral phenotypes reflecting the influence of multiple genes, the environment, and their interplay. Given this complexity, the endophenotype concept has been proposed (Iacono, Carlson, & Malone, Reference Iacono, Carlson and Malone2000).
Endophenotypes are quantitative traits intermediating the putative causal pathway from the underlying genes (i.e., genotype) to the clinical manifestation of the disorder (i.e., phenotype; Gottesman & Gould, Reference Gottesman and Gould2003). Endophenotypes are considered important because they can move us closer to understanding the underlying mechanisms of a disorder and can aid in discovering the disorder's genetic etiology. As defined by Gottesman and Gould (Reference Gottesman and Gould2003), an endophenotype should be heritable, associated with the disorder (i.e., the trait serves as a disease marker), and state independent. One of the most important attributes of an endophenotype is that it should be found in biological relatives of those who have the disorder at a higher rate than in the general population, because family members share on average half their genes with their affected relative (i.e., the trait serves as a vulnerability marker; Frederick & Iacono, Reference Frederick and Iacono2006). Studies of young relatives at high risk, such as offspring of parents with SUD, offer a valuable opportunity to characterize premorbid traits in SUD. The ability to identify vulnerability markers among those adolescents would facilitate prospective studies that could clarify trajectories of developing disorders, delineate etiological mechanisms, identify moderating factors, and facilitate the development of preventive interventions (e.g., Cicchetti & Curtis, Reference Cicchetti and Curtis2007; Gottesman & Gould, Reference Gottesman and Gould2003). Impaired (i.e., risky) decision making might represent one promising endophenotype for SUD, and impaired decision-making processes could be one of the critical mechanisms underlying the transition from casual to compulsive and uncontrollable substance use (Bechara & Damasio, Reference Bechara and Damasio2002; Bechara, Dolan, & Hindes, Reference Bechara, Dolan and Hindes2002).
Impairments in decision making processes have become a principal target in addiction research, particularly owing to the clinical significance given the core feature of SUD that the reinforcing aspects of substance use appreciably outweigh the negative consequences. Employing gambling and other decision making tasks, numerous studies have demonstrated decision making impairments in SUD patients, with a tendency toward riskier choices. Chronic alcoholics recurrently make decisions favoring larger immediate rewards, even in the face of mounting negative long-term consequences (Bickel & Marsch, Reference Bickel and Marsch2001; Cantrell, Finn, Rickert, & Lucas, Reference Cantrell, Finn, Rickert and Lucas2008; Mazas, Finn, & Steinmetz, Reference Mazas, Finn and Steinmetz2000; Miranda, MacKillop, Meyerson, Justus, & Lovallo, Reference Miranda, MacKillop, Meyerson, Justus and Lovallo2009). Similar performance deficits have been reported in illicit substance abusers (Bechara et al., Reference Bechara, Dolan, Denburg, Hindes, Anderson and Nathan2001; Bechara & Martin, Reference Bechara and Martin2004; Ernst et al., Reference Ernst, Grant, London, Contoreggi, Kimes and Spurgeon2003; Ersche et al., Reference Ersche, Roiser, Clark, London, Robbins and Sahakian2005; Grant, Contoreggi, & London, Reference Grant, Contoreggi and London2000; Monterosso, Ehrman, Napier, O'Brien, & Childress, Reference Monterosso, Ehrman, Napier, O'Brien and Childress2001), as well as in long-term abstinent SUD patients (Fein, Klein, & Finn, Reference Fein, Klein and Finn2004; Fishbein et al., Reference Fishbein, Eldreth, Hyde, Matochik, London and Contoreggi2005). It has been suggested that impaired decision making in SUD is associated with altered reactions to rewarding and punishing events (i.e., positive and negative feedback), which makes SUD patients less able to use this feedback to guide and adjust ongoing behavior (Bechara et al., Reference Bechara, Dolan and Hindes2002; Kamarajan et al., Reference Kamarajan, Rangaswamy, Tang, Chorlian, Pandey and Roopesh2010). Risky decision making in SUD individuals therefore may reflect a deficient feedback processing system.
Researchers have recently begun to examine the neural aspects of feedback processing by pairing gambling or decision making tasks with electrophysiological event-related potential (ERP) measurements. ERPs to positive (i.e., gains) and negative feedback stimuli (i.e., losses) may provide useful information on both the timing and the neural substrates of feedback processing. Two major ERP components have been described that are particularly sensitive to feedback: the feedback-related negativity (FRN) and the feedback-related P300 amplitude. The FRN is a negative deflection at frontocentral recording sites that reaches its maximum between 200 and 300 ms postonset of the feedback stimulus. It is generally larger following the presentation of negative feedback associated with unfavorable outcomes than following positive feedback (e.g., Gehring & Willoughby, Reference Gehring and Willoughby2002; Hajcak, Holroyd, Moser, & Simons, Reference Hajcak, Holroyd, Moser and Simons2005; Nieuwenhuis, Slagter, von Geusau, Heslenfeld, & Holroyd, Reference Nieuwenhuis, Slagter, von Geusau, Heslenfeld and Holroyd2005; Nieuwenhuis, Yeung, Holroyd, Schurger, & Cohen, Reference Nieuwenhuis, Yeung, Holroyd, Schurger and Cohen2004). The FRN reflects an early, rapid evaluation of the affective or motivational impact of outcome events, and its amplitude is related to the simple bad versus good appraisal of feedback (Yeung & Sanfey, Reference Yeung and Sanfey2004). The feedback-related P300 amplitude is the most positive peak in the 300–600 ms following feedback and seems to reflect a later, attention-sensitive and more elaborated evaluation of performance outcomes, in which factors that affect the allocation of attentional resources come into play in a top-down controlled manner (e.g., Sato et al., Reference Sato, Yasuda, Ohira, Miyawaki, Nishikawa and Kumano2005; Wu & Zhou, Reference Wu and Zhou2009; Zhou, Yu, & Zhou, Reference Zhou, Yu and Zhou2010). Given that the P300 amplitude is generally believed to be associated with processes of attentional allocation and high-level motivational evaluation (Johnson, Reference Johnson1986; Polich & Criado, Reference Polich and Criado2006), P300 arguably reflects the evaluation of the functional and motivational significance of feedback stimuli.
Accumulating evidence supports the idea that SUD patients display abnormal feedback processing (Kamarajan et al., Reference Kamarajan, Rangaswamy, Tang, Chorlian, Pandey and Roopesh2010; Porjesz, Begleiter, Bihari, & Kissin, Reference Porjesz, Begleiter, Bihari and Kissin1987; Ramsey & Finn, Reference Ramsey and Finn1997). Kamarajan et al. (Reference Kamarajan, Rangaswamy, Tang, Chorlian, Pandey and Roopesh2010), for example, demonstrated that alcoholics show significantly smaller P300 amplitudes during both loss and gain feedback as compared to healthy controls. Although these findings suggest that deficient feedback processing during risky decision making can be considered as a disease marker for SUD, an unanswered question remains whether such deficits reflect only the consequence of chronic substance use on the brain. This view is challenged by studies showing that decision making impairments are even observable after prolonged periods of abstinence (Porjesz et al., Reference Porjesz, Begleiter, Bihari and Kissin1987). Moreover, Fein and Chang (Reference Fein and Chang2008) demonstrated smaller FRN amplitudes in treatment-naive alcoholics with a greater FH density of alcohol problems. These findings raise the possibility that impaired feedback processing during risky decision making might be an antecedent to substance use, thereby reflecting a biological vulnerability marker for SUD, rather than a consequence of prolonged, heavy substance use.
Nevertheless, very few studies have examined decision making processing in individuals at high risk (HR) owing to a FH of SUD, and results have been equivocal (Acheson, Robinson, Glahn, Lovallo, & Fox, Reference Acheson, Robinson, Glahn, Lovallo and Fox2009; Herting, Schwartz, Mitchell, & Nagel, Reference Herting, Schwartz, Mitchell and Nagel2010; Lovallo, Yechiam, Sorocco, Vincent, & Collins, Reference Lovallo, Yechiam, Sorocco, Vincent and Collins2006; Petry, Kirby, & Kranzler, Reference Petry, Kirby and Kranzler2002). Petry et al. (Reference Petry, Kirby and Kranzler2002) found that HR women had higher discounting rates (i.e., rates at which individuals discount rewards delayed in time) than did controls without a FH of SUD, suggesting that these women were characterized by impulsive decision making. In contrast, Lovallo et al. (Reference Lovallo, Yechiam, Sorocco, Vincent and Collins2006) initially revealed that there were no overall differences in the proportion of safe versus risky choices during a gambling task, although further analyses demonstrated high attention to gains among HR males, but not in females. More recently, Acheson et al. (Reference Acheson, Robinson, Glahn, Lovallo and Fox2009) also failed to demonstrate performance differences between HR and control groups. However, despite a lack of clear behavioral differences, neurobiological differences have been observed. HR participants show more activation in the anterior cingulate cortex and the caudate nucleus during gambling situations (Acheson et al., Reference Acheson, Robinson, Glahn, Lovallo and Fox2009). Others found blunted activation in the nucleus accumbens (Andrews et al., Reference Andrews, Meda, Thomas, Potenza, Krystal and Worhunsky2010) and white matter microstructure abnormalities in HR youth (Herting et al., Reference Herting, Schwartz, Mitchell and Nagel2010), which likely contributes to less efficient cortical processing. Although these findings lend support to the notion of biases in brain decision making systems underlying elevated risk for SUD, the previous studies cannot draw firm conclusions on whether risky decision making reflects a biological predisposition to SUD, owing to the inconsistent behavioral findings. The lack of clear behavioral differences between HR individuals and controls might also be related to a lack of statistical power, because the majority of previous studies employed relatively small samples of HR subjects.
Therefore, the aims of the current ERP study are (a) to determine whether risky decision making reflects a biological predisposition to SUD, (b) to assess whether risky decision making is driven by blunted feedback processing in the brain, and (c) to further elucidate the neural mechanisms that underlie these feedback processing deficits. A subsidiary aim is to explore whether risky decision making and feedback-related ERPs are associated with generic temperamental or behavioral traits (i.e., impulsiveness, externalizing problem behavior, and frequency of substance use). We also explored gender differences in decision making behavior and feedback-related ERPs, because previous studies found differential effects of gender on decision making skills and P300 amplitude (e.g., Lovallo et al., Reference Lovallo, Yechiam, Sorocco, Vincent and Collins2006).
For these purposes, ERPs elicited by positive and negative feedback were recorded in HR adolescents with at least one parent in treatment for a SUD and in normal-risk controls (NR) while performing a modified version of the Balloon Analogue Risk Task (BART; Euser, Van Meel, Snelleman, & Franken, Reference Euser, Van Meel, Snelleman and Franken2011; Lejuez et al., Reference Lejuez, Read, Kahler, Richards, Ramsey and Stuart2002; Pleskac, Wallsten, Wang, & Lejuez, Reference Pleskac, Wallsten, Wang and Lejuez2008), which has shown to be a sensitive measure of risky decision making. We hypothesized the following: (a) HR adolescents would make more risky decisions during the BART than NR controls as indicated by their choosing to inflate balloons to a greater degree; (b) risky decision making in HR adolescents would be driven by a deficient feedback processing system, characterized by either a hypersensitivity for positive feedback coupled with hyposensitivity to negative feedback or a hyposensitivity to both positive and negative feedback, with reduced FRN and P300 amplitudes indicating decreased sensitivity; and (c) risky decision making during the BART would be related to FRN and P300 amplitudes. We did not have specific hypotheses for the subsidiary aim, because these analyses were explorative.
Methods
Participants
The present study was part of a larger study, the Youth in the Netherlands Study (Huizink et al., in press), which initially included a sample of 65 HR and 110 NR adolescents who were between the ages of 12 and 20 years. For the present analyses, 9 participants were excluded because of EEG measurement errors (i.e., they had fewer than eight artifact-free negative feedback ERP epochs; 3 HR adolescents and 6 NR adolescents). Moreover, because externalizing problem behavior and habitual substance use patterns are essential variables that may confound the effects of having a positive FH of SUD, participants were also excluded from the present analyses when this information was missing. Hence, participants for the present study were only selected from the larger sample when they successfully completed the BART, the Youth Self-Report (YSR), and a substance use questionnaire. As a result, a further 14 adolescents of the initial sample were excluded owing to missing questionnaire data (1 HR and 13 NR adolescents). Consequently, the final sample for the present data analyses consisted of 61 HR and 91 NR adolescents.
HR group
The HR adolescents (n = 61; 29 males; M age = 15.75, SD = 2.54) were included because of at least one biological parent who was undergoing or had undergone treatment for a SUD (i.e., lifetime DSM-IV-TR diagnosis of substance abuse and/or dependence other than nicotine). These HR adolescents were recruited from the outpatient clinics of Bouman GGZ, the primary addiction care provider in the city of Rotterdam and the surrounding area (Zuid-Holland, The Netherlands), where their parents had been diagnosed and treated for a SUD. Diagnosis of SUD in subjects' parent was based on information obtained from the treatment staff and was corroborated during a selection interview, in which the DSM criteria were checked. Eligible patients were informed about the study by the treatment staff. They were given an information package, and parents and children were asked to participate. After permission of both parents and their children, participants were screened by telephone and, if eligible, an appointment for the measurements was made. Two HR participants had parents who were diagnosed with a SUD but were not currently in treatment. These participants were recruited by word of mouth, and SUD was ascertained with the Composite International Diagnostic Interview (Robins et al., Reference Robins, Wing, Wittchen, Helzer, Babor and Burke1989), which was performed by a trained interviewer on the research staff prior to participation of the offspring in the study.
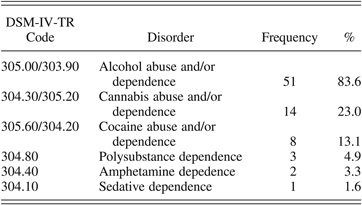
Of the included HR adolescents, 32 (52.5%) had a father with a SUD diagnosis, 28 (45.9%) had a SUD-diagnosed mother, and one adolescent (1.6%) had both a father and mother with a lifetime DSM-IV-TR diagnosis of SUD. The array of parental SUD diagnoses in the final sample was diverse (see Table 1). Thirty-seven parents (60.7%) were diagnosed with an alcohol use disorder only, five used cannabis only (8.2%), one parent used cocaine only (1.6%), and one used sedatives only (1.6%). Seventeen of the parents (27.9%) had more than one SUD diagnosis and used a combination of two or more substances.
Table 1. Percentages of the DSM-IV-TR diagnoses of the parents of the included high-risk adolescents

NR group
Adolescents in the community-based NR group (n = 91; 46 males; M age = 15.20, SD = 2.18) were part of a larger sample that participated in a general population study (n = 2,567) of youth aged 6 to 20 years (Tick, van der Ende, & Verhulst, Reference Tick, van der Ende and Verhulst2007). For this larger study, children and adolescents were randomly drawn from municipal registers of 35 representative municipalities in the Dutch province of South Holland, including urban and rural areas. The NR adolescents included in the present study were matched to HR adolescents by age and gender, and randomly ascertained from the larger sample. To maximize the representativeness of the sample, a psychiatric disorder in the parent, as well as in the adolescent, did not disqualify the adolescent for participation in the study, in order to obtain incidence rates of psychiatric disorders more comparable to those of the general population. Parents and their children were screened by telephone and if eligible, were invited to our laboratory, where relevant data were obtained.
All adolescents included in the study were fluent in Dutch, were physically healthy, and had no history of significant head injury, mental retardation, or neurological disorders. Informed consent was obtained from all parents and adolescents before their participation. The research protocol was approved by the medical ethical committee of the Erasmus Medical Center Rotterdam, The Netherlands. The study was conducted in accordance with the declaration of Helsinki.
Subjective self-report questionnaires and interviews
I7 Questionnaire
The Impulsiveness Scale of the I7 Questionnaire (Eysenck, Pearson, Easting, & Allsopp, Reference Eysenck, Pearson, Easting and Allsopp1985; Lijffijt, Kenemans, & Caci, Reference Lijffijt, Kenemans and Caci2005) was used as a measure of impulsivity. In this questionnaire, impulsiveness is regarded as acting without first considering the possible consequences. The scale has good psychometric properties (Lijffijt et al., Reference Lijffijt, Kenemans and Caci2005).
Brief Sensation Seeking Scale
The Brief Sensation Seeking Scale (Hoyle, Stephenson, Palmgreen, Lorch, & Donohew, Reference Hoyle, Stephenson, Palmgreen, Lorch and Donohew2002) total score was used as a measure of sensation seeking tendencies. The scale has good psychometric properties (Hoyle et al., Reference Hoyle, Stephenson, Palmgreen, Lorch and Donohew2002).
YSR questionnaire
The YSR (Achenbach Reference Achenbach1991) was used to assess self-reported problem behaviors. The externalizing problem behavior scale was used to measure externalizing problems. The good validity and test–retest reliability of the YSR have been established (Achenbach Reference Achenbach1991; Verhulst, Van der Ende, & Koot, Reference Verhulst, Van der Ende and Koot1997).
Substance use questionnaire
A self-report substance use questionnaire (Evans, Greaves-Lord, Euser, Franken, & Huizink, Reference Evans, Greaves-Lord, Euser, Franken and Huizink2012) was used to assess adolescents' early onset experimentation with alcohol, tobacco, and cannabis use. Analyses first focused on use versus never use. Subsequently, for adolescents who had already experimented with alcohol, tobacco, or cannabis, the age of onset (i.e., first use) was assessed. Frequency of use was examined for the total sample by calculating the number of drinks/uses per week.
Diagnostic Interview Schedule for Children (DISC)
The National Institute for Mental Health DISC (Saffer, Restifo, Lucas, Dulcan, & Schwab-Stone, Reference Shaffer, Restifo, Lucas, Dulcan and Schwab-Stone2000; Shaffer et al., Reference Shaffer, Fisher, Dulcan, Davies, Placentini and Schwab-Stone1996), a highly structured respondent-based interview, was applied to determine adolescents' current risk status and to assess whether symptoms of substance abuse and dependence were already present. The DISC has two parallel forms: the DISC-C is administered directly to the adolescent, and the DISC-P is administered to the parent. In this study, the Dutch translation of the substance use module of the DISC-IV child and parent version were used to obtain the prevalence of current and lifetime DSM-IV diagnoses of substance abuse or dependency. The reliability and validity of the DISC have been supported by previous studies (Fischer, Parra, Wicks, Reyland, & Shaffer, Reference Fisher, Parra, Wicks, Reyland and Shaffer1992; Verhulst, Van der Ende, Ferdinand, & Kasius, Reference Verhulst, Van der Ende, Ferdinand and Kasius1997). The DISC was administered by trained and certified students.
BART
An automatic response mode version of the BART was used as a behavioral measure to assess risky decision making (automatic BART; e.g., Euser et al., Reference Euser, Van Meel, Snelleman and Franken2011; Pleskac et al., Reference Pleskac, Wallsten, Wang and Lejuez2008), which involves inflating a simulated balloon on a computer screen that could either grow larger or explode. Instead of sequentially pumping the balloon (as in the standard BART; Lejuez et al., Reference Lejuez, Read, Kahler, Richards, Ramsey and Stuart2002), this automatic BART requires participants to select the target number of pumps (corresponding to how much risk) they wish to perform at the beginning of each trial. The task was presented on a computer screen, which included a small blue balloon (about 7 × 5 cm), accompanied by a dial of numbers (0–9), a reset button, and three permanent displays listing the current reward/loss magnitude of the balloon (“pumps selected”), the total money earned (“total earned”), and the money earned on the last balloon (“last balloon”).
Participants were told that they had to pump up 60 separate balloons. For each pump, participants could obtain one point (corresponding to 1 cent for each pump). Hence, the higher the target number of pumps, the higher the money that could be earned. At the beginning of each trial, the participant had to determine how many times this specific balloon should be pumped in order to get the best score. This number could be selected by clicking on the number dial on the screen and then selecting “pump.” Each balloon had an inflation time of 4–6 s, in which the balloon was inflated incrementally. After inflation time, there were two possible outcomes: the balloon remained whole and the money (corresponding to the selected number of pumps) was earned (i.e., positive feedback), or the balloon was pumped past its individual explosion point and thus popped, and the money was lost (i.e., negative feedback). After the feedback of each balloon, a new uninflated balloon appeared on the screen until a total of 60 balloons were completed.
The maximum number of pumps possible was set to 128 for each balloon with an explosion a priori equally likely to occur on any given pump subject to the constraint that within each sequence of 10 balloons, the average explosion point was on pump 64. All participants were presented the same balloons in the same order to limit extraneous variability. ERPs were time locked to a 7 × 5 cm green dollar figure superimposed over a whole balloon for positive feedback, and to a 7 × 5 cm red cross superimposed over an exploded balloon for negative feedback (see Figure 1). The two types of feedback were used for analyses of FRN and P300 amplitude.

Figure 1. (Color online) An example of the stimulus–response–feedback timeline for the Balloon Analogue Risk Task. Upon balloon presentation, participants select the target number of pumps they wish to take. Once this value is accepted, participants watch the balloon as it automatically inflates until either the stated number of pumps is reached and the money is earned (i.e., positive feedback, top right) or the balloon explodes and the money is lost (i.e., negative feedback, bottom right).
Procedure
Participants were invited to the Erasmus Behavioral Lab (Erasmus University Rotterdam) and were scheduled for a 3- to 4-hr experimental session, which included two laboratory protocols that were part of a larger study. At arrival, the participants signed informed consent and completed the self-report questionnaires with respect to several temperamental and behavioral traits as well as the current degree of exposure or experimentation with substances. Then, all participants took part in the EEG session, which lasted approximately 75 min. Participants were seated on a comfortable chair in a light- and sound-attenuated room. After the EEG electrodes were attached, participants completed two cognitive tasks (not reported in this paper). Subsequently, the BART was administered (~20 min) to measure feedback processing during risky decision making. Participants were told they were going to pump up 60 balloons on the computer screen. The goal was to obtain as many points as possible. As an additional incentive, participants were informed that the participant who obtained the highest score of all would receive an extra reward of €100. DISC data of the HR adolescents were collected during a consecutive visit. Parents of the HR participants who had completed the experimental sessions were contacted by telephone by a research assistant to make an appointment for interviews with the parents as well as the adolescents. For the NR group, this interview was conducted during a visit before the EEG session. All participants received a financial compensation for participation.
EEG acquisition and analysis
The EEG was recorded with BioSemi Active-Two using 34 scalp sites (10–10 system, and two additional electrodes at FCz and CPz) with Ag/AgCl active electrodes mounted in an elastic cap. Six additional electrodes were attached: two placed to the left and right mastoids as reference electrodes, two placed next to each eye for the horizontal electrooculogram to record ocular movement and to be able to correct for ocular artifact, and two placed above and below the left eye for vertical electrooculogram. Online signals were recorded with a low-pass filter of 134 Hz. All signals were digitized with a sample rate of 512 Hz and 24 bit A/D conversion.
Data were offline referenced to mathematically linked mastoids. Because we were interested in FRN as well as P300 amplitudes, EEG data were filtered offline with different parameters, in line with previous literature (e.g., Donkers, Nieuwenhuis, & van Boxtel, Reference Donkers, Nieuwenhuis and van Boxtel2005; Euser et al., Reference Euser, Van Meel, Snelleman and Franken2011; Luu, Tucker, Derryberry, Reed, & Poulsen, Reference Luu, Tucker, Derryberry, Reed and Poulsen2003; Wu & Zhou, Reference Wu and Zhou2009). For the FRN, data were filtered using a 2–12 Hz bandpass filter, which removes low-frequency waves from the EEG and minimizes overlap between the FRN and other ERP components (Donkers et al., Reference Donkers, Nieuwenhuis and van Boxtel2005; Euser et al., Reference Euser, Van Meel, Snelleman and Franken2011). A conventional wide band filter of 0.10–30 Hz (phase shift-free Butterworth filters; 24 dB/octave slope) was used to investigate the feedback-related P300 amplitude. Data were segmented in feedback-locked epochs of 1000 ms (200 ms prestimulus until 800 ms poststimulus). After ocular correction (Gratton, Coles, & Donchin, Reference Gratton, Coles and Donchin1983), epochs including out of range voltages (±100 µV) were rejected as artifacts and were excluded from further processing. The mean 200 ms preresponse period served as baseline. After baseline correction, epochs locked to positive and negative feedback were averaged separately for artifact-free trials at each scalp site, producing one average waveform per feedback condition per participant. The mean number of included positive feedback trials was 28.93 (SD = 5.95; 90% of all epochs), and the mean number of negative feedback trials was 24.44 (SD = 5.63; 88% of all epochs). The mean number of available feedback-related epochs did not differ between groups, t (150) = 0.77, p = .45, for positive feedback, and t (150) = –0.53, p = .60, for negative feedback.
The FRN component was identified as the most negative amplitude within a 200–300 ms window following feedback onset, based on previous literature. The P300 component was defined as the maximum amplitude within 300–400 ms following the FRN. For the purpose of statistical analyses, we focused on the FRN amplitudes on the frontocentral midline electrode FCz, and the P300 amplitudes on the parietal midline electrode Pz, because the FRN and P300 amplitude effects were the largest on these electrodes.
Statistical analyses
Differences between HR and NR groups with respect to age, scores on the subjective self-report ratings, externalizing problem behavior, and frequency and age of onset of substance use were assessed with independent samples t tests. Differences with respect to gender distribution and use versus nonuse of alcohol, nicotine, and cannabis were examined by using chi-square tests.
As in previous research using the automatic response mode version of the BART, for the behavioral measure of risk taking, we analyzed the mean target number of pumps across balloons during the BART as the primary dependent variable (e.g., Euser et al., Reference Euser, Van Meel, Snelleman and Franken2011; Pleskac et al., Reference Pleskac, Wallsten, Wang and Lejuez2008). A study comparing the automatic and the original manual BART has shown that this target score tends to be a more reliable and unbiased estimator of risk-taking propensity (i.e., yields an unbiased statistic), whereas it maintains the BART's predictive validity for assessing risk-taking behavior (Pleskac et al., Reference Pleskac, Wallsten, Wang and Lejuez2008). In addition, we examined the maximum number of pumps on a balloon, the total number of explosions, the total amount of money earned, and the deliberation time (i.e., the amount of time a participant required to make a decision on a particular trial and to determine how many times the specific balloon should be pumped). The effect of a parental history of SUD on these measures was assessed with univariate 2 × 2 analyses of variance (ANOVAs) with Group (HR vs. NR) and Gender (male vs. female) as between-subject factors. Moreover, we stratified the BART into three different blocks, corresponding to the number of pumps for each group of 20 of the 60 balloons. This measure reveals whether there was a strategy shift during the task and was assessed with a 2 × 2 × 3 repeated measures ANOVA, with Group (HR vs. NR) and Gender (male vs. female) as between-subject factors and Block (pumps 1–20, pumps 21–40, and pumps 41–60) as the within-subject factor.
For FRN and P300 amplitudes, two sets of 2 × 2 × 2 repeated measures ANOVAs were performed with Group (HR vs. NR) and Gender (male vs. female) as between-subject factors and Feedback (positive vs. negative) as the within-subject factor. Greenhouse–Geisser corrections were adopted where appropriate. All significant ANOVA effects were further analyzed using Bonferroni-corrected post hoc t tests.
Furthermore, bivariate correlation analyses using Pearson correlation coefficients were computed to examine associations between the behavioral measures of risk taking during the BART (i.e., mean number of pumps, maximum number of pumps on a balloon, and total number of explosions), feedback-related ERPs, and self-reported temperamental and behavioral traits across groups. Finally, linear regression analyses and scatterplots were used to investigate changes between HR and NR groups with respect to developmental trends of risky decision making and the brain's feedback processing mechanisms over different ages. For all analyses, a .05 level of significance was employed.
Results
Sample characteristics
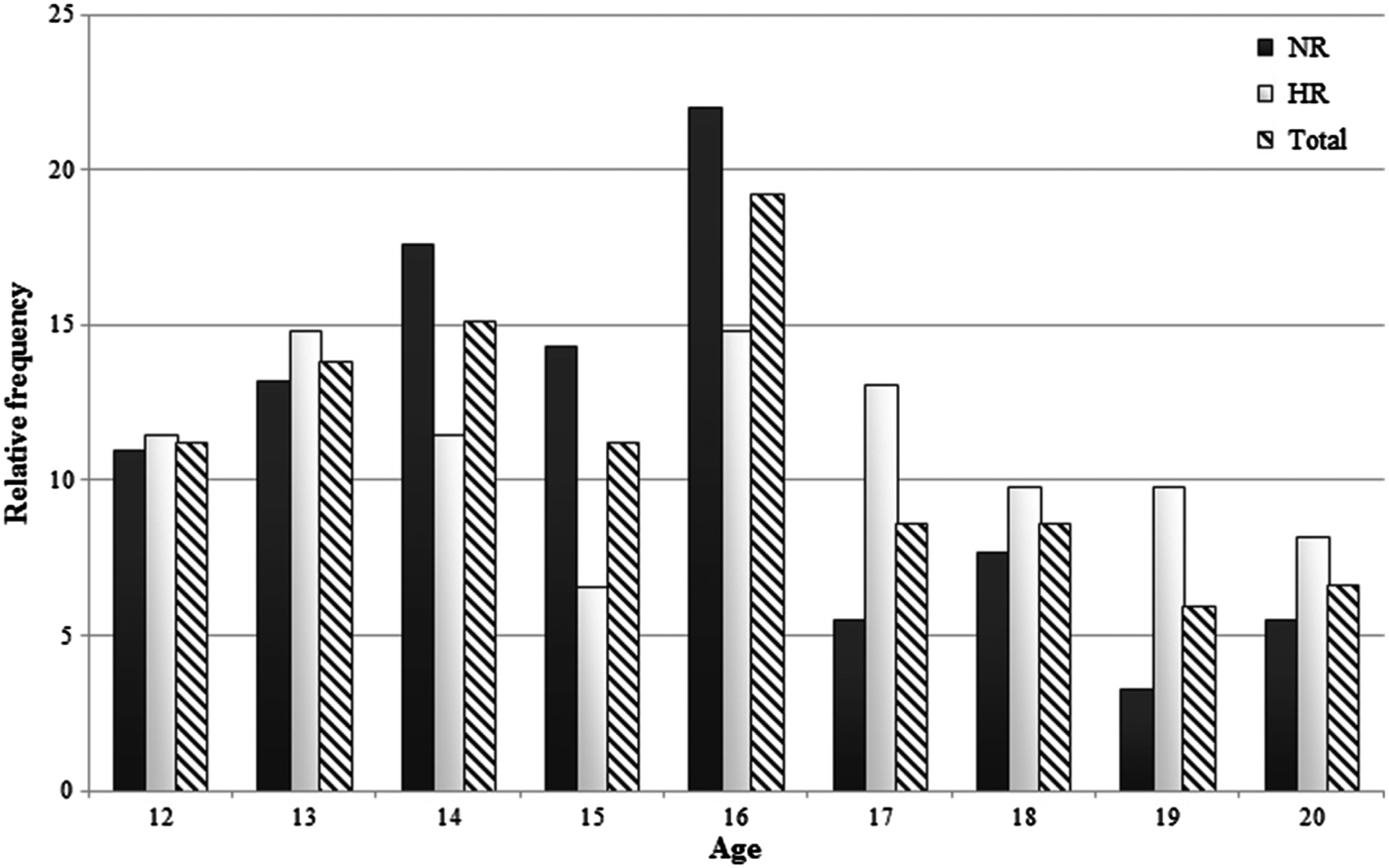
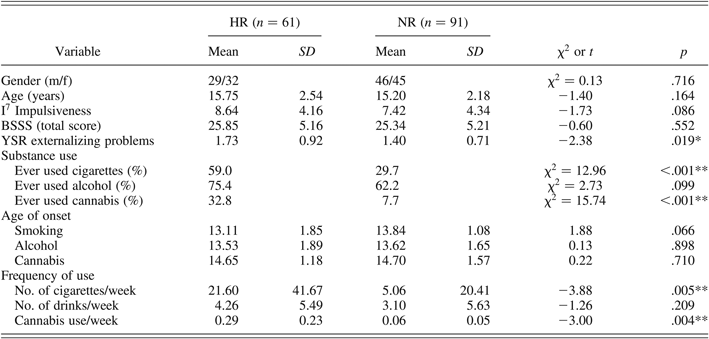
Table 2 shows the descriptive information, including temperamental and behavioral characteristics of the HR and NR adolescents. Figure 2 displays the age distribution of the NR and HR adolescents in a relative frequency histogram, in which the relative frequency (%) of each age across the continuum from age 12 to age 20 appears on the vertical axis. Adolescents in both groups were comparable in age (p = .16) and gender (p = .72). As expected, HR adolescents scored higher on externalizing problem behavior (p < .05, partial η2 = 0.04) and tended to score higher on impulsiveness (p = .09) than did adolescents without a parental history of SUD. None of the other temperamental traits differed between HR and NR adolescents. With respect to self-reported substance use, groups did not differ significantly in alcohol intake. However, significantly more HR adolescents had ever smoked cigarettes or ever experimented with cannabis compared to matched NR controls (both ps < .001). There was no significant group difference in the age of onset, though the HR group tended to display a younger age of onset of nicotine use (p = .07). Regarding frequency of substance use, HR adolescents smoked more cigarettes (p < .01, partial η2 = 0.07) and used significantly more cannabis per week than did NR controls (p < .01, partial η2 = 0.08), yet the mean number of times cannabis was used was small (less than once per 3 weeks).

Figure 2. The age distribution of the included participants. A relative frequency histogram is provided for each age across the continuum from age 12 to age 20.
Table 2. Descriptive information, including temperamental and behavioral characteristics of the HR and NR groups

Note: Age of onset of substance use represents the mean age of first drink/use for only those adolescents who already experimented with substances (ever used); frequency of use represents the mean number of drinks/use per week for the total sample. HR, high-risk group; NR, normal-risk group; I7 Impulsiveness, impulsiveness scale of the I7 Questionnaire; BSSS, Brief Sensation Seeking Scale; YSR, Youth Self-Report.
Nevertheless, these findings indicate that HR participants are at high risk, because of their risky substance use behaviors. This high-risk status was further evidenced by the DISC data, showing that although there was no evidence of DSM-IV diagnoses of SUD in the NR group,Footnote 1 4 HR adolescents (6.6%) had a current DSM-IV diagnosis of nicotine dependency and 3 HR adolescents (4.9%) obtained a lifetime DSM-IV diagnosis of alcohol abuse. Moreover, 6 HR adolescents (9.8%) had already developed cannabis-related problems and had been treated for a DSM-IV diagnosis of cannabis abuse and/or dependence at an outpatient youth clinic of Bouman GGZ.Footnote 2 It is important that HR individuals with a SUD were not excluded at the screening stage, because this sampling approach procedure would result in the selection of low-risk individuals from HR families (assuming that there is variability of risk among the offspring of SUD-diagnosed parents). Instead, analyses in the total sample were controlled for preexisting differences, and an additional analysis was performed without all participants characterized by excessive substance use.
Behavioral results
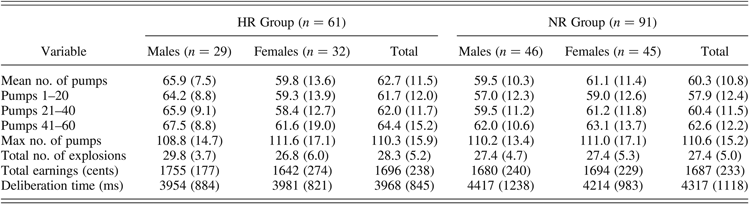
Table 3 shows the descriptive statistics for all behavioral measures of the BART. Regarding the primary measure of risky decision making, univariate ANOVA revealed no significant main effect of group, F (1, 148) = 1.97, p = .16, or gender, F (1, 148) = 1.55, p = .22, on mean number of pumps. However, a significant Group × Gender interaction effect could be observed, F (1, 148) = 4.45, p = .04, partial η2 = 0.03). Post hoc analyses revealed that the mean target number of pumps during the entire task was significantly higher for HR males than for NR controls (p = .015), whereas this difference was absent in females (p = .61), indicating that male HR adolescents made more risky decisions during the BART. When stratifying the task in three blocks of 20 trials, repeated measures ANOVA further revealed a significant main effect of block, F (2, 296) = 10.62, p < .001, partial η2 = 0.07. All participants slowly increased the number of pumps throughout the task, showing a significant difference between the last versus the first and second blocks (both ps < .01). No Block × Group, Block × Gender, or Block × Group × Gender interaction effects were observed (all ps > .38).
Table 3. Means (standard deviations) of the behavioral measures of risk taking during the BART for the HR and NR groups

Note: BART, Balloon Analogue Risk Task; HR, high-risk group; NR, normal-risk group.
With respect to the additional BART measures, there was no evidence of a main effect of group for the total number of explosions during the BART, F (1, 148) = 1.18, p = .28. However, a marginally significant Group × Gender interaction effect was found, F (1, 148) = 3.27, p = .07. Univariate post hoc tests revealed that male HR adolescents burst more balloons than did NR males (p <.05, η2 = 0.03), whereas there was no significant difference between female HR and NR adolescents (p = .61). Groups did not differ regarding the maximum number of pumps selected on a balloon, F (1, 148) = 0.02, p = .88, or the total amount of earnings, F (1, 148) = 0.09, p = .77. Neither the main effect of gender nor the interaction effects of these measures reached statistical significance (all ps > .11). Although the higher mean target number of pumps could potentially have resulted in HR males earning more money from the task than NR controls did, this was not the case. We presume that this lack of group difference was because the larger number of pumps for HR males was offset by this group tending to burst more balloons than did NR controls. A univariate ANOVA further revealed a significant main effect of group for deliberation time, F (1, 148) = 4.23, p = .04, partial η2 = 0.03, indicating that HR adolescents made their decisions during the task significantly faster (M = 3968 ms, range = 2572–6146 ms) than did NR controls (M = 4315 ms, range = 2391–8998 ms). Neither the main effect of gender nor the interaction effect of Group × Gender reached statistical significance (all ps > .50).
It is more important that all behavioral results remained identical after controlling for the preexisting differences between HR and NR adolescents with respect to externalizing problem behavior and frequency of nicotine and cannabis use. Performing an analysis of covariance with these variables as covariates, the significant group or Group × Gender effects became even stronger, Group × Gender interaction mean number of pumps: F (1, 145) = 4.97, p = .03; Group × Gender effect number of exploding balloons: F (1, 145) = 3.60, p = .06; group main effect deliberation time: F (1, 145) = 5.85, p = .017, respectively.
Electrophysiological results
FRN
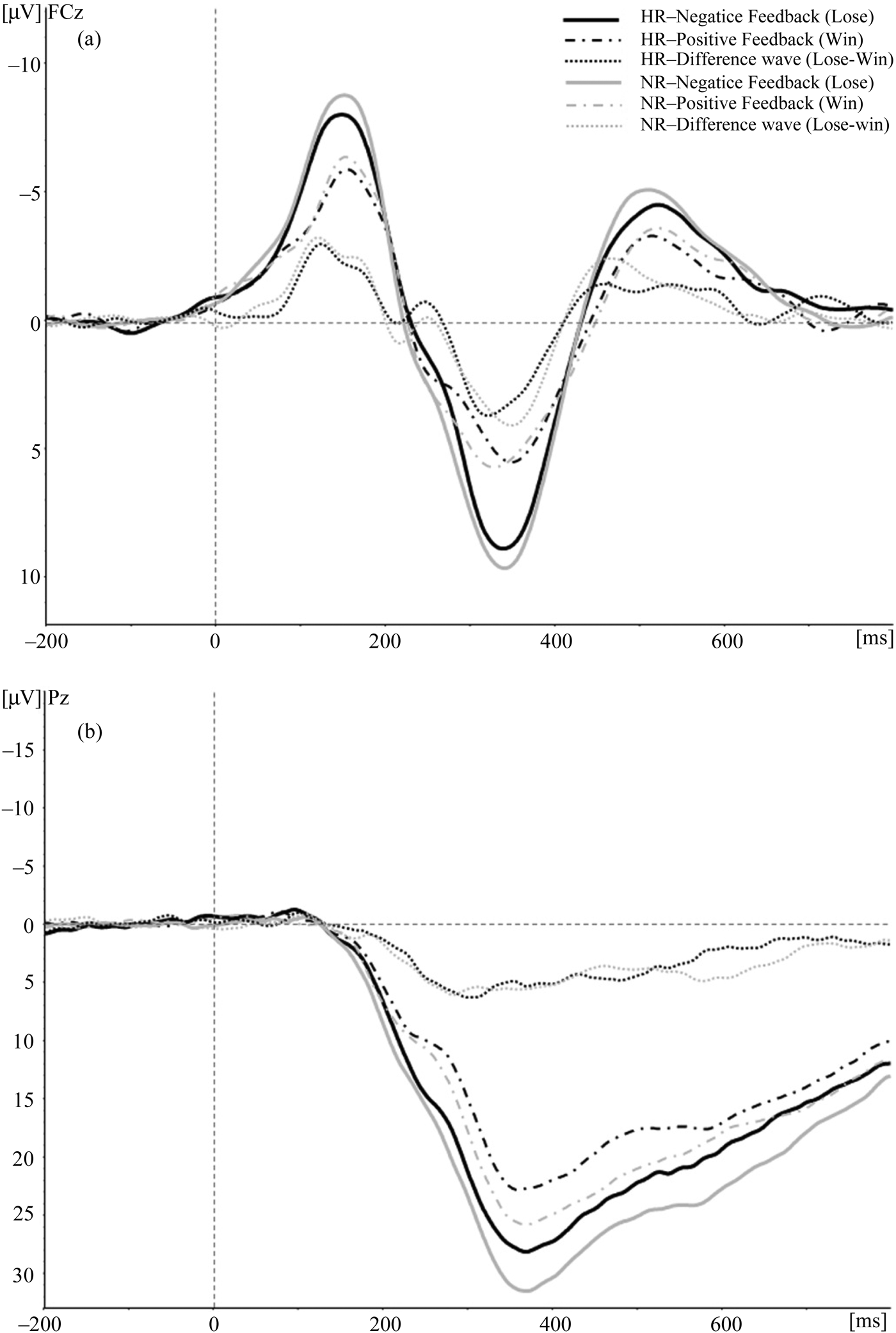
Mean FRN amplitudes for positive and negative feedback for electrode site FCz are presented in Table 4. Figure 3a shows the grand averages for the two types of feedback. Repeated measures ANOVA conducted on peak FRN amplitude did not reveal any significant main effects of feedback valence, F (1, 148) = 0.05, p = .82; group, F (1, 148) = 0.01, p = .94; or gender, F (1, 148) = 0.55, p = .46. Neither the interaction effect of Group × Feedback valence nor the interaction effects of Group × Gender, Gender × Feedback Valence, or Group × Gender × Feedback Valence reached statistical significance (all ps > .74).Footnote 3 The paradigm used in the present study apparently failed to elicit a distinct FRN and, as can be seen in Figure 3, there is no negative component at all. Consequently, any further analyses of the FRN will not provide additional valuable information.

Figure 3. Stimulus-locked grand average waveforms evoked by positive (Win) and negative (Lose) feedback in the Balloon Analogue Risk Task for (a) the feedback-related negativity (upper figure; site FCz, filtered 2–12 Hz) and (b) the feedback-related P300 amplitude (bottom figure; site Pz, filtered 0.1–30 Hz). For illustration purposes, difference waves (Lose–Win) are plotted in the figures.
Table 4. Means (standard deviations) of FRN and feedback-related P300 amplitudes for the HR and NR groups

Note: FRN, feedback-related negativity; HR, high-risk group; NR, normal-risk group.
Feedback-related P300
Mean P300 amplitudes for positive and negative feedback for electrode site Pz are presented in Table 4. Figure 3b shows the grand averages for the two types of feedback. Repeated measures ANOVA conducted on peak P300 amplitude revealed a significant main effect of feedback valence, F (1, 148) = 62.01, p < .001, partial η2 = 0.30, indicating that P300 amplitude was larger in response to negative feedback (31.6 µV) than to positive feedback (26.3 µV). It is more important that a significant main effect of group was observed, F (1, 148) = 4.55, p < .05, partial η2 = 0.03.Footnote 4 Post hoc analyses revealed that, in response to both positive and negative feedback, HR adolescents showed significantly smaller P300 amplitudes than did NR controls (27.4 vs. 30.4 µV, respectively), indicating a reduced sensitivity for feedback stimuli in HR adolescents, regardless of the valence of feedback. The main effect of gender was not statistically significant, F (1, 148) = 0.21, p = .65. Neither the interaction effect of Group × Feedback Valence, nor the interaction effects of Group × Gender, Gender × Feedback Valence, or Group × Gender × Feedback Valence reached statistical significance (all ps > .12).
In addition, because there was evidence of preexisting differences between the HR and the NR adolescents on self-reported externalizing problem behavior and frequency of nicotine and cannabis use per week, and previous work has demonstrated effects of these variables on P300 amplitude (Anokhin et al., Reference Anokhin, Vedeniapin, Sirevaag, Bauer, O'Connor and Kuperman2000; Iacono, Malone, & McGue, Reference Iacono, Malone and McGue2003; Patrick et al., Reference Patrick, Bernat, Malone, Iacono, Krueger and McGue2006), an additional analysis was performed with these variables included as covariates. The main effect of feedback valence remained statistically significant, F (1, 145) = 17.27, p < .001, partial η2 = 0.11. However, it is more important that the group main effect remained significant after controlling for the covariates, F (1, 145) = 3.86, p = .05, partial η2 = 0.03. Neither the main effects of gender and the included covariates nor the interaction effects reached statistical significance (all ps > .12), indicating that feedback-related P300 amplitude was not influenced by externalizing problem behavior or frequency of nicotine and cannabis use.
Correlational analyses
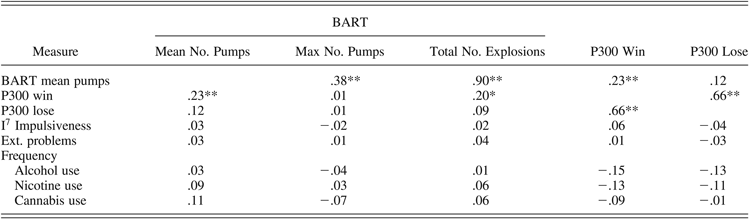
Correlations among the behavioral measures of risk taking during the BART, feedback-related P300 amplitudes,Footnote 5 and temperamental and behavioral traits for the total sample are presented in Table 5. Across groups, risk-taking behavior during the BART was positively associated with P300 amplitude but only when elicited by positive feedback stimuli (r = .23, p = .005 for mean number of pumps, and r = .20, p = .012 for total explosions). None of the generic temperamental or behavioral traits were significantly associated with risk-taking behavior or feedback-related ERPs.
Table 5. Correlations between the behavioral measures of risk taking during the BART, feedback-related P300 amplitudes, and self-reported temperamental and behavioral traits across groups

Note: Correlations are across groups. For all correlations, n = 152. BART, balloon analogue risk task; mean no. pumps, mean number of pumps during the BART as the primary measure of risky decision making; max no. pumps, maximum number of pumps selected on a balloon during the BART; total no. explosions, the total number of explosions during the BART; P300 win, P300 amplitude elicited by positive feedback; P300 lose, P300 amplitude elicited by negative feedback; I7 Impulsiveness, impulsiveness scale of the I7 Questionnaire; Ext. problems, total score of the externalizing problem behavior scale of the Youth Self-Report.
*p < .05. **p < .01.
Developmental trends in risky decision making and feedback-related P300 amplitude
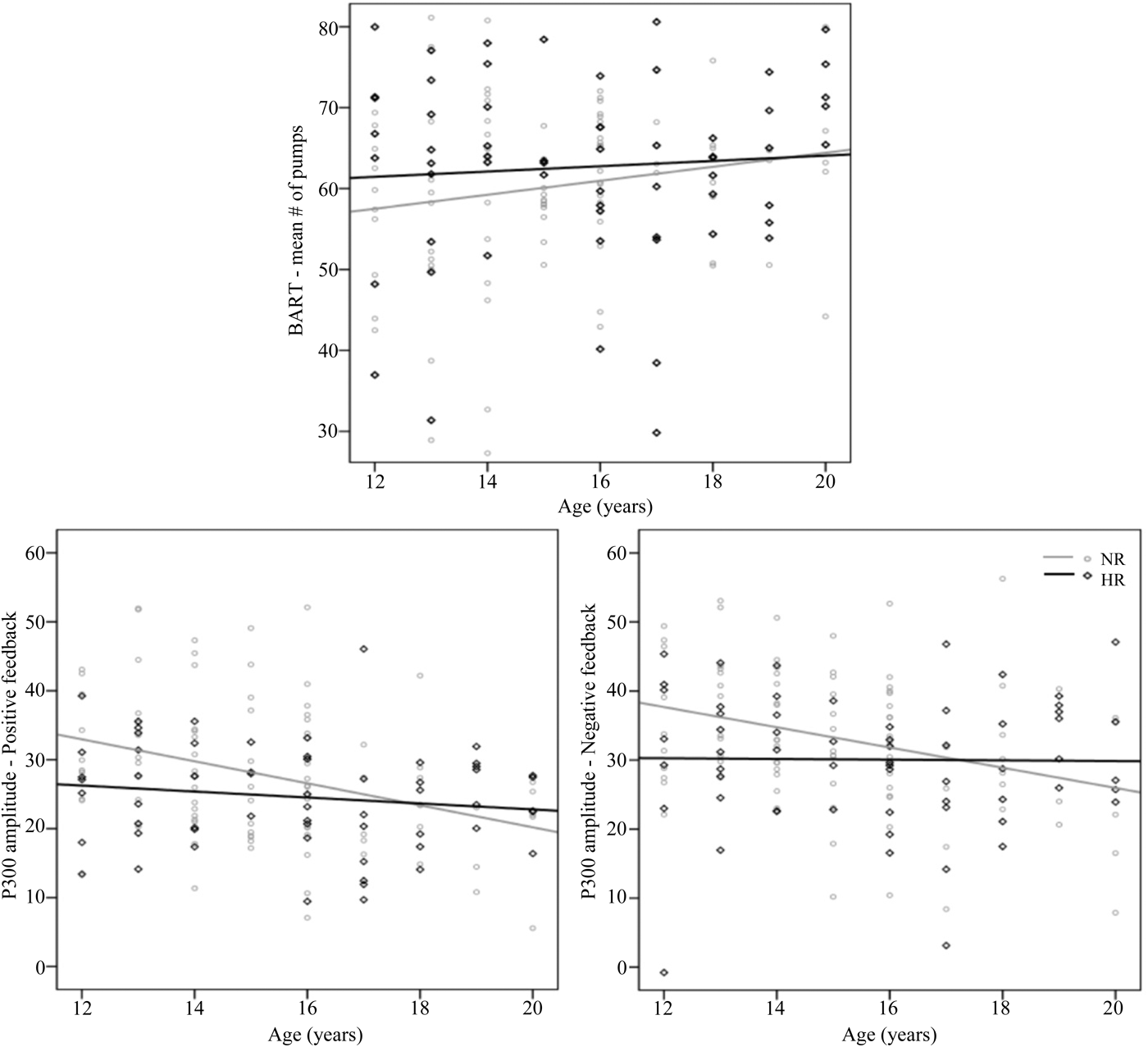
Although the HR and NR adolescents did not differ with respect to mean age, it should be noted that the age range of the participants was quite wide (i.e., 12–20 years). Hence, one would expect that in this broad age range, risky decision making could vary substantially. To test for age-related differences in risky decision making and the brain's underlying feedback mechanisms, we regressed risk-taking behavior during the BART (i.e., mean number of pumps) and the P300 amplitudes elicited by positive as well as negative feedback on age for both groups separately. We observed a trend regarding a linear, age-associated increase in overall risk-taking behavior during the BART in NR adolescents (R 2 = .03, β = 0.17), F (1, 90) = 2.78, p = .10. In contrast, as can be seen in Figure 4, the risk-taking “set” of HR adolescents remained consistent across age (p = .58).

Figure 4. Scatterplots for risky decision making during the Balloon Analogue Risk Task and feedback-related P300 amplitudes in the (grey) normal-risk and (black) high-risk groups over various ages during adolescence.
Furthermore, scatterplots were also created with the feedback-related P300 amplitudes of HR adolescents and NR controls on the y axis and ages of the adolescents on the x axis. The charts were plotted separately for positive and negative feedback. Subsequently, the best-fit line for the scatter for HR and NR adolescents was developed (see Figure 4). Both graphs show that, in NR adolescents, the P300 amplitude is high in the younger age groups and significantly decreases with age for P300 elicited by negative feedback (R 2 = .10, β = –0.32), F (1, 90) = 10.02, p = .002, and for P300 elicited by positive feedback (R 2 = .11, β = –0.34), F (1, 90) = 11.29, p = .001. In the HR group, in contrast, this age-related maturation effect of the P300 amplitude appeared to be absent (both ps > .26). Apparently, the P300 amplitude in HR adolescents is significantly smaller than in NR controls in the younger age groups, but it seems to converge around the age of 18 years old.
Frequent substance use
Some HR and NR adolescents reported excessive alcohol and nicotine use, or more than incidental use of cannabis, which led to an abnormal distribution of these variables. Furthermore, heavy current substance use complicates the conclusion about whether the present findings are due to a positive FH of SUD or rather may be confounded by habitual use. Therefore, we reran the analyses by omitting all excessive-using participants. HR participants with a known current or lifetime DSM-IV diagnosis of alcohol or cannabis abuse and/or dependence (based on the DISC and information from the staff of the Bouman Youth clinic) were omitted from the analyses (n = 9). Furthermore, we also excluded all nonclinical participants who reported heavy substance use, based on self-reported information of the substance use questionnaire (cutoff scores: alcohol use = using more than 14 units of alcohol weeklyFootnote 6; nicotine = using more than 20 cigarettes weekly; cannabis = using more than 0.05 times weekly, i.e., >2.6 times per year). As a result, 21 nondiagnosed participants were omitted (a further 11 from the HR and 10 from the NR group). Hence, the final subsample for this additional analysis consisted of 41 HR and 81 NR adolescents. Mean frequency of substance use in this sample was reduced to 1.7 alcoholic drinks a week, 0.6 cigarettes a week, and no cannabis use, and thus we consider these adolescents to be a substance use-naive sample. Groups did not differ with respect to age, gender, impulsiveness, and frequency of substance use (all ps > .48). Furthermore, groups no longer differed with respect to externalizing problem behavior (p = .10).
Regarding the behavioral data, the above reported results of the primary outcome measure (i.e., mean number of pumps) did not change owing to the exclusion of the substance using participants, Group × Gender interaction mean number of pumps: F (1, 118) = 4.49, p = .04; partial η2 = 0.04, indicating that substance use-naive HR male participants took more risk than did NR controls (mean number of pumps: HR males = 65.7, HR females = 58.5, NR males = 59.5, NR females = 61.3, respectively). Reevaluation of the feedback-related P300 amplitudes again revealed a significant main effect of feedback valence, F (1, 118) = 41.12, p < .001. Most important, however, as in the larger sample, significantly reduced P300 amplitudes in response to both positive and negative feedback were found for substance use-naive HR adolescents as compared to NR controls, as instantiated by a significant main effect of group, F (1, 118) = 4.16, p = .04, partial η2 = 0.03; HR mean = 27.4 µV, NR mean = 30.7 µV. Again, neither the main effect of gender, F (1, 118) = 4.16, p = .04, nor the interaction effects of Group × Feedback Valence, Group × Gender, Gender × Feedback Valence, or Group × Gender × Feedback Valence reached statistical significance (all ps > .10). Hence, these findings provide further evidence that the risk-taking behavior during the BART as well as the reduced P300 amplitudes in response to feedback in our HR sample were not due to the effects of frequent substance use.
Discussion
This is the first study we know of to use ERP measurements to investigate the brain dynamics of feedback processing during risky decision making in HR adolescents with a parental history of SUD. We undertook this study to investigate whether blunted feedback processing during risky decision making may represent an endophenotypic vulnerability marker for SUD. Our results show that male adolescents who are at presumed high risk for developing a SUD are characterized by decision making impairments, with a tendency toward riskier choices. Moreover, these impairments were accompanied by aberrations in the later stages of feedback processing and outcome evaluation.
On the behavioral level, it is interesting that all participants increased the number of pumps throughout the task. It may be that participants at the beginning of the BART were more conservative, because in the first block there is still much uncertainty about the chance of explosion occurrences. Likewise, exposure to and experience with the task structure may have led to routinization in the later trials. Moreover, on every pump opportunity trial, participants may adopt some sort of distance to target calculation. If participants know that they have only a few balloons left (i.e., have only a few chances to collect money), they may be more likely to increase the number of pumps in order to get the highest score, which was especially salient because participants were informed that the participant who obtained the highest score of all would receive an additional reward.
Of more importance and in line with our first hypothesis, significant differences in choice behavior were found between HR and NR adolescents, but only in males. Although both groups slowly increased the number of pumps throughout the BART, HR males made significantly riskier choices across all blocks than NR controls, which appeared to be a fairly constant risk-taking set across age during adolescence. This effect was absent in HR females. However, both male and female HR adolescents made their responses significantly faster, indicating more impulsive decision making. It is remarkable that HR and NR adolescents earned a similar amount of money, suggesting that the different response/choice strategies of HR males did not confer an advantage or disadvantage and that the risk-taking tendency of HR males was not maladaptive under BART's conditions. However, it should be borne in mind that under real-life conditions, greater risk taking may have considerably more adverse consequences. The finding that HR males, but not females, were significantly different in choice behavior is consistent with the results of Lovallo et al. (Reference Lovallo, Yechiam, Sorocco, Vincent and Collins2006) and may suggest that HR males have a more enhanced risk-taking propensity than female offspring of parents with a SUD. It may be that HR males are more easily engaged in risk-taking activities, acts from which most others are deterred by the potential loss/reward ratio. We speculate that, by more risky responses in BART and in real life, our HR male adolescents expose themselves to greater loss probabilities and loss/reward ratios than HR females and NR healthy controls. This greater risk-taking propensity in HR males as compared to females might be related to the finding that sons of alcoholic parents have a greater liability for alcoholism than daughters (e.g., Cloninger, Bohman, & Sigvardsson, Reference Cloninger, Bohman and Sigvardsson1981); however, future studies will have to investigate this.
With respect to the effects of NR versus HR status on the neural mechanisms underlying feedback processing during decision making, a differentiation in early versus later feedback processing was revealed: FRN and P300 amplitudes did not show a similar sensitivity to the effect of a parental history of SUD. In contrast to our hypothesis, no differences between HR and NR adolescents were found on the FRN amplitude, indicating that the neural system supporting the rapid evaluation of unfavorable outcomes was not modulated by risk status. However, it should be mentioned that the FRN in the present study was also not modulated by feedback valence, although it has been well established that FRN amplitudes tend to be larger in response to negative feedback than to positive feedback. It is possible that our task was less suitable to elicit robust FRN amplitudes. The feedback delay in the present task varied from 4 to 6 s. Nieuwenhuis et al. (Reference Nieuwenhuis, Slagter, von Geusau, Heslenfeld and Holroyd2005) suggested that delaying feedback might decrease its motivational significance. In the study of Crowley et al. (Reference Crowley, Wu, Crutcher, Bailey, Lejuez and Mayes2009), it was also found that feedback delay was associated with FRN variability. In their study, the 1-s delay produced a more robust feedback response than the 2-s delay, which is consistent with this explanation. Increasing the time between response selection and feedback may lead to reduced expectation of feedback, thereby diminishing the FRN. Future studies should focus on the application of different gambling paradigms with varying feedback delay in order to clarify our FRN results.
In line with our hypothesis, the feedback-related P300 amplitude was found to be modulated by the valence of feedback and was larger in response to negative feedback than to positive feedback. Although some researchers have found increased P300 amplitudes in response to positive feedback, the presence of a large P300 amplitude following negative feedback in our study replicates previous research using a comparable task design (e.g., Crowley et al., Reference Crowley, Wu, Crutcher, Bailey, Lejuez and Mayes2009; Fein & Chang, Reference Fein and Chang2008) and is to be expected because a balloon explosion is an extremely salient and often unexpected task event that grabs attention. It is of more importance that the P300 amplitude was significantly modulated by NR versus HR status: feedback-related P300 amplitudes in response to both positive and negative feedback were reduced in HR adolescents as compared to NR controls, whereas no gender difference could be observed. The P300 is traditionally associated with the mental processes underlying the deployment of attentional resources to an incoming stimulus, the evaluation of that stimulus, and the subsequent memory mechanisms engaged for that stimulus (Donchin, Reference Donchin1981; Johnson, Reference Johnson1986; Polich & Criado, Reference Polich and Criado2006; Polich & Kok, Reference Polich and Kok1995; Pontifex, Hillman, & Polich, Reference Pontifex, Hillman and Polich2009). Here, blunted P300 amplitudes in response to both positive and negative feedback may reflect less effective integration of past occurrences of outcomes over the course of the task, which may suggest a hyposensitivity to future consequences. Our ERP findings thus suggest that it is not the ability to rapidly evaluate feedback valence that is influenced by a parental history of SUD but rather the ability to subsequently assign sufficient attention to further process motivationally salient events. Hence, HR adolescents seem limited in building a reinforcement history essential to guide future behavior.
Note that the differences in feedback-related P300 amplitude between groups appeared to be most prominent in the youngest adolescents, with trajectories in HR and NR adolescents that converge in late adolescence, around the age of 18 years old. Although it must be acknowledged that the best paradigm for tracking developmental trends would be to perform longitudinal evaluations rather than a cross-sectional design, our results are in line with previous (longitudinal) research concerning the developmental course of P300 amplitude (e.g., Hill et al., Reference Hill, Shen, Locke, Steinhauer, Konicky and Lowers1999; Silva, Benega, Devi, & Mukundan, Reference Silva, Benegal, Devi and Mukundan2007). We presume that HR adolescents have age-inappropriate levels of P300 amplitude, and this may be indicative of a developmental delay or deficit in cognitive development in adolescents at high risk because of a positive FH of SUD (Hill et al., Reference Hill, Shen, Locke, Steinhauer, Konicky and Lowers1999). When presuming that reductions in P300 amplitude reflect a genetic predisposition to SUD that is carried by HR offspring, these results suggest that the differentiating capacity of this marker will decline steadily with age and normalize by adulthood. Whether reduced feedback-related P300 amplitudes will specifically predict later development of maladaptive risk-taking behavior or SUD, or is a marker for adult psychopathology, remains to be elucidated. Nevertheless, our results provide important insight into the role of P300 amplitude in childhood and adolescence for predicting problems with adult adjustment.
It is well established that offspring of SUD patients show deficits in P300 amplitude in a variety of cognitive stimulus discrimination tasks (e.g., Euser et al., in press; Hill, Steinhauer, Park, & Zubin, Reference Hill, Steinhauer, Park and Zubin1990; O'Connor, Hesselbrock, Tasman, & DePalma, Reference O'Connor, Hesselbrock, Tasman and DePalma1987; Polich, Pollock, & Bloom, Reference Polich, Pollock and Bloom1994). Our results corroborate findings from previous HR studies and support the notion that reduced P300 amplitudes in HR adolescents represent a bias in attentional and information processing that are related to increased vulnerability to SUD. An intriguing question remains whether the deficits observed in feedback-related P300 in our HR sample are reflective of a specific deficit in feedback processing and outcome evaluation, rather than reflecting a generic cognitive dysfunction.
Although the feedback-related P300 amplitude may share common features of signal processing as indexed by the generic P300 (Kamarajan et al., Reference Kamarajan, Rangaswamy, Tang, Chorlian, Pandey and Roopesh2010), accumulating evidence suggests that the P300 effects that have been repeatedly found in decision making paradigms likely reflect the evaluation of the functional, emotional, or motivational significance of outcomes and feedback stimuli (e.g., Sato et al., Reference Sato, Yasuda, Ohira, Miyawaki, Nishikawa and Kumano2005; Wu & Zhou, Reference Wu and Zhou2009; Yeung & Sanfey, Reference Yeung and Sanfey2004) and is specifically related to outcome evaluation and feedback processing. Furthermore, feedback-related P300 in the present study was found to be sensitive to positive versus negative feedback, which is very distinct from the generic P300 component observed in oddball paradigms. We also found significant associations between risk-taking behavior and feedback-related P300 amplitudes. Although these associations became nonsignificant after a stringent correction for multiple comparisons, it should be noted that the benefit of adjustment for multiple comparisons is controversial because the probability of a Type II error is markedly increased (Rothman, Reference Rothman1990). Our findings may suggest that the present P300 amplitude is specifically associated with risk-taking behavior and may propose a specific role for reward salience. Hence, HR adolescents' hyposensitivity to feedback during risky decision making, both positive and negative, might imply that these adolescents are unable to use this ongoing feedback to guide and adjust their behavior appropriately.
However, bear in mind that we cannot rule out alternative explanations. Because P300 attenuation in our HR group was nonspecific with respect to feedback valence and in numerous studies HR subjects showed P300 reductions in other paradigms not involving feedback evaluation, one possibility that merits consideration is that the effect might be general and nonspecific. Alternatively, given that P300 amplitude can also be predicted by resting EEG amplitude, the effect may even be nonspecific to ERPs and simply reflect the overall reduction of the amplitude of brain oscillations (e.g., Basar, Basar-Eroglu, Karakas, & Schürmann, Reference Basar, Basar-Eroglu, Karakas and Schürmann2001; Demiralp, Ademoglu, Schürmann, Basar-Eroglu, & Basar, Reference Demiralp, Ademoglu, Schürmann, Basar-Eroglu and Basar1999; Ergen, Marbach, Brand, Basar-Eroglu, & Schürmann, Reference Ergen, Marbach, Brand, Basar-Eroglu and Demiralp2008). Furthermore, deficits in feedback processing during risky decision making, as evidenced by reduced feedback-related P300 amplitudes in adolescents with a parental history of SUD, could also be due to an amalgamation of general signal processing deficits. It is not possible to separate these alternatives with the present data. Nevertheless, we did find evidence that HR male adolescents took more risk during the BART (i.e., displayed a higher mean number of pumps throughout the task, which was the main outcome measure) as compared to NR adolescents, and this risky decision making style was accompanied by blunted P300 amplitudes in response to both positive and negative feedback. Similar P300 amplitude reductions have been reported in an alcoholic patient sample in a study by Kamarajan et al. (Reference Kamarajan, Rangaswamy, Tang, Chorlian, Pandey and Roopesh2010). In light of the current paradigm, we thus suggest that the reduced P300 amplitudes are likely a specific dysfunction in the later, more elaborative, and higher order stages of feedback processing and outcome evaluation (Kamarajan et al., Reference Kamarajan, Rangaswamy, Tang, Chorlian, Pandey and Roopesh2010), in which factors that affect the allocation of attentional resources come into play in a top-down controlled manner (Wu & Zhou, Reference Wu and Zhou2009). However, it remaind rather puzzling in this view that HR females in our study were characterized by the same P300 amplitude reductions and seem to have similar intrinsic neurobiological alterations in feedback-processing as HR males, whereas they did not display the same pattern of risky decision making as HR males and did not differ from NR controls. Although there is some evidence that males generally displayed even lower P300 amplitudes than females (i.e., as evidenced by the significant main effect of gender when conducting a P300 area measure), this effect was absent in the P300 peak analysis. One explanation for the lack of the behavioral expression of the risk-taking phenotype in HR females despite neurobiological alterations may be that environmental factors, such as social relationships, have protected and prevented them from engaging in risk-taking activities and that the environmental interplay may have acted as a resilience factor. Unfortunately, however, we were unable to address this in the present study; hence, firm conclusions about how blunted P300 amplitudes may relate to the FH+ behavioral risk-taking phenotype must await further research.
The present study again showed that adolescents with a parental history of SUD display higher levels of disinhibited and undercontrolled behavioral traits, indexed by more externalizing problem behavior and a tendency toward higher levels of impulsiveness as compared to NR controls, as has been shown repeatedly in previous work (Pihl, Peterson, & Finn, Reference Pihl, Peterson and Finn1990; Verdejo-Garcia et al., Reference Verdejo-García, Lawrence and Clark2008). These findings offer validity to the neurocognitive models of SUD that implicate disinhibited behavior as a major component and suggest the vulnerability account of these behaviors in SUD (e.g., Carlson, McLarnon, & Iacono, Reference Carlson, McLarnon and Iacono2007; Iacono et al., Reference Iacono, Malone and McGue2003; Iacono, Malone, & McGue, Reference Iacono, Malone and McGue2008; Tarter et al., Reference Tarter, Kirisci, Mezzich, Cornelius, Pajer and Vanyukov2003, Reference Tarter, Kirisci, Habeych, Reynolds and Vanyukov2004). However, it is noteworthy that no apparent relationships were found between disinhibited behavioral traits and risk-taking behavior and feedback-related P300 amplitudes. This suggests that the link between being at high risk for SUD and blunted feedback processing during risky decision making in our sample was not modulated by disinhibited and undercontrolled behavioral traits. The high-risk status of our HR sample was further corroborated by their risky substance use behaviors. HR offspring used nicotine and cannabis more frequently than did NR controls, and some of them had already developed substance-related problems and were treated for a SUD themselves. Nevertheless, when taking the preexisting differences in externalizing behavior and frequency of substance use between the HR and the NR adolescents into account, results remained stable. The present study allows us to draw tentative conclusions on causality. After omitting all participants using frequent and heavy alcohol and other drugs from the analysis, enhanced risk-taking behavior during the BART in HR males, as well as reduced feedback-related P300 amplitudes in both male and female HR adolescents, was still evident in a substance use-naive subsample of HR adolescents, who were free of prolonged and excessive substance use. The present findings are thus not secondary to the effects of heavy substance use exposure on the brain, but instead they reflect intrinsic risk-related familial characteristics of the individual that may predate substance use and are present in HR offspring prior to SUD.
A final important issue that needs to be delineated concerns the origins of adolescents' risky decision making and feedback-related brain mechanisms. There are indications that the propensity for risky decision making as measured by the BART as well as the P300 amplitude are highly heritable (Anokhin, Golosheykin, Grant, & Heath, Reference Anokhin, Golosheykin, Grant and Heath2009; van Beijsterveldt & van Baal, Reference van Beijsterveldt and van Baal2002). Our results may suggest that the impaired feedback processing during risky decision making observed in our HR male adolescents reflects a genetic predisposition inherited from a parent with SUD. However, nongenetic influences, such as parenting, the social environment, and peer interactions, cannot be ruled out. Individual behavior carries influences from many past and current interactions within and across individuals and environmental conditions (Masten, Faden, Zucker, & Spear, Reference Masten, Faden, Zucker and Spear2008). To understand behavior, a developmentally framed approach is thus essential, particularly during adolescence, a period that is characterized by rapid transformation, biological changes, and enhanced social pressure. It seems, for example, very reasonable that substance abuse or dependency by adults who play a key role in child development (e.g., parents or teachers) can undermine the achievement of developmental tasks by the children in their care. In our HR adolescents, it is likely that substance use by their parents interfered with parenting, increased the risk of exposure to deviant peers, and in other ways could have increased the general level of adversity and risk faced by the child. Hence, it may be that our HR children were not being provided with the level of support that they needed to promote successful transitions into adolescence (e.g., Masten, Reference Masten2004; Masten et al., Reference Masten, Faden, Zucker and Spear2008; Steinberg et al., Reference Steinberg, Dahl, Keating, Kupfer, Masten, Pine, Cicchetti and Cohen2006). Furthermore, given that adolescence represents a critical period of intense brain maturation (e.g., Casey & Jones, Reference Casey and Jones2010; Crews, He, & Hodge, Reference Crews, He and Hodge2007), it could be possible that early negative environmental experiences, such as negative parenting, also impact the brain's feedback processing system (i.e., reward and punishment sensitivity). Taken together, impaired feedback processing during risky decision making may be influenced by genes and negative environmental experiences in the course of development and eventually moderate the likelihood of a teenager to engage in risky behavior. As development arises from complex interactions among genes, environmental contexts, brain development, and family and peer processes (Gottesman & Hanson, Reference Gottesman and Hanson2005; Masten, Reference Masten2004), integrating good theory and science across multiple levels may provide a better insight into the nature of risky decision making.
There are a number of issues that merit consideration when interpreting the results of the current study. First, although we suggest that the P300 potentials elicited in the present study are feedback specific, rather than represent a general cognitive deficit, we cannot draw firm conclusions yet. Our results are in agreement with previous studies showing reduced P300 amplitude in individuals at high familial risk for SUD; however, the extent to which this P300 amplitude reduction is specific to feedback processing needs to be confirmed in future studies by demonstrating that feedback P300 and oddball P300 show distinct relationships with risk measures. Second, our study had a cross-sectional design, and it should be borne in mind that conclusions based on such evidence must be regarded only as plausible hypotheses until they are confirmed in prospective studies. It is important to note that although HR adolescents are at increased risk for developing a SUD, they may not all develop substance-related problems over time. A longitudinal design would shed more light on the role of risky decision making and blunted feedback processing mechanisms in substance abuse onset and continuation. Further research is needed to examine the longitudinal trajectories of these characteristics and their ability to predict the subsequent onset of SUD. Third, there is the possible influence of feedback delay in our paradigm on the FRN results, which makes the influence of a parental history of SUD on FRN amplitudes still an open question. Fourth, the present study did not examine whether blunted feedback processing during risky decision making mediates the influence of genes on the phenotype of SUD. Identifying the specific brain systems and genes that are involved is a key challenge for future research. Fifth, environmental factors and experiences should not be ruled out, because these may also influence the endophenotype as well as the phenotype and may act as risk or resilience factors. Social factors such as close bonds with family or friends, for example, may have accounted for the lack of behavioral risk-taking propensity in HR females, whereas they do display blunted feedback-related P300 amplitudes. We deem this issue worthy of in-depth consideration in future research.
Despite the acknowledged limitations outlined above, the current results represent a significant contribution to the ongoing development of etiological models of SUD. We presented evidence that male adolescents with a greater than normal genetic risk for substance-related problems owing to a positive parental history of SUD are characterized by decision making deviations, with a tendency toward riskier choices. Moreover, risky decision making in these adolescents was accompanied by aberrations in the later stages of feedback processing, which was, however, also evident in HR females. It is important that the differences between risk groups do not appear to be secondary to prolonged heavy exposure to alcohol or other drugs. We document a prominent role of the feedback-related P300 amplitude as an index of blunted feedback processing and outcome evaluation. HR adolescents seem to be hyposensitive to feedback, both positive and negative. It might be that these adolescents are unable to use this ongoing feedback to guide current as well as future behavior, and, therefore, their behavior is guided by immediate contingency. This could contribute to the onset of substance use and an increased propensity toward substance abuse. Keeping the limitations in mind, it seems that blunted feedback processing during risky decision making may represent a promising endophenotypic vulnerability marker for SUD, at least in males.