Introduction
Although first line therapies for obsessive–compulsive disorder (OCD), including serotonin re-uptake inhibitors (SRIs) (selective serotonin re-uptake inhibitors [SSRIs] and clomipramine), and/or psychological interventions (specifically exposure-response prevention; ERP) can elicit clinically meaningful improvements in many patients,Reference Fineberg, Hollander and Pallanti 1 , Reference Skapinakis, Caldwell and Hollingworth 2 a proportion remain “treatment-resistant.”Reference Knopp, Knowles and Bee 3 , Reference Pittenger and Bloch 4 The terms “treatment-resistant” and “treatment refractory” are often used interchangeably in research and clinical practice, but the latter has been described as a greater degree of treatment resistance in which patients have failed to attain adequate improvements with three SRI trials (one of which includes clomipramine), two antipsychotic augmentation strategies as well as cognitive behaviour therapy (CBT) concurrent to pharmacotherapy.Reference Husted and Shapira 5
Although there is a paucity of research into the aetiopathogenesis of treatment-resistant or refractory OCD, positive treatment responses have been demonstrated for glutamatergic modulating pharmacotherapies.Reference Kariuki-Nyuthe, Gomez-Mancilla and Stein 6 Abnormalities of glutamatergic activity may exist in some treatment refractory individuals. In addition, the presence of oxidative stress, which may be greater in magnitude in more chronic and severe cases of OCD warrants further investigation as a treatment target.Reference Kandemir, Abuhandan and Aksoy 7 Thus, strategies which modulate glutamate activity and provide antioxidant and neuroprotective effects may be of benefit in patients with treatment-resistant and refractory OCD. One approach is the use of nutraceutical agents, which have been shown to be potentially effective adjuncts in a range of psychiatric disorders, including depression.Reference Sarris, Murphy and Mischoulon 8 Further the use of “combination” nutraceuticals may potentially exert a greater effect than isolated nutrients via targeting an array of neurobiological pathways underlying the disorder, and potentially via synergism (as nutrients are often more effective in combination and may require certain nutrient co-factors for optimal metabolism).Reference Sarris, Logan and Akbaraly 9
Key nutraceuticals with the potential to modulate implicated pathways involved in OCD include: N-acetyl cysteine (NAC), zinc, L-theanine, selenium, magnesium, and pyridoxal-5′ phosphate (P5P). In brief, NAC has been found to attenuate the synaptic release of glutamate in subcortical brain regions (via glutamate metabotropic autoreceptor 2/3 activation and the involvement of cysteine transporters) and restore extracellular concentrations of glutamate in the nucleus accumbens.Reference Baker, McFarland and Lake 10 - Reference Kupchik, Moussawi and Tang 12 The latter mechanism is postulated to be particularly beneficial for attenuation of compulsive behaviors.Reference Kupchik, Moussawi and Tang 12 Neuroimaging studies highlight NAC’s ability to exert activity in the brain when taken orally. For example, oral administration of NAC in adults can lower elevated levels of glutamate in the dorsal anterior cingulate cortex (dACC), as well as glutamate plus glutamine levels in the anterior cingulate cortex (ACC).Reference Schmaal, Veltman and Nederveen 13 , Reference McQueen, Lally and Collier 14 L-theanine is a nonproteinogenic amino acid derived almost exclusively from tea leaves (Camellia sinensis) and possesses various neuromodulatory and neuroprotective effects, particularly enhancing inhibitory and attenuating excitatory neurotransmission.Reference Lardner 15 Specifically, L-theanine is a glutamate analogue which exerts weak antagonistic effects at ionotropic glutamate receptor subtypes, and has a stronger affinity for inhibiting glutamine transporter activity, which subsequently suppresses exocytotic release of glutamate.Reference Kakuda, Hinoi and Abe 16 , Reference Kakuda, Nozawa and Sugimoto 17
Zinc is an essential trace mineral involved in a diverse range of biochemical processes.Reference Szewczyk, Kubera and Nowak 18 It is a modulator of inhibitory and excitatory neurotransmission with effects on glutamate and GABA, which may in part explain its role in the attenuation of excitotoxicity.Reference Bancila, Nikonenko and Dunant 19 , Reference Peters, Koh and Choi 20 A deficiency of dietary zinc has also been implicated in the excitability of glutamatergic neurons.Reference Takeda, Hirate and Tamano 21 Further, zinc is involved in antioxidant and anti-inflammatory processes, including as a structural component of superoxide dismutase, maintaining metallothionein concentrations in tissues, sequestering the production of reactive oxygen species, inhibiting glutathione depletion and protecting against lipid peroxidation in the brain.Reference Bagchi, Vuchetich and Bagchi 22 , Reference Gropper and Smith 23 One small RCT in OCD revealed that zinc supplementation potentiated the effects of fluoxetine in individuals with moderately severe OCD (minimum score of 21 on the YBOCS at time of entry), which was significant within 2-weeks of administration.Reference Sayyah, Olapour and Saeedabad 24 In addition, serum zinc levels were significantly lower in individuals with OCD compared to healthy controls matched for sex, age, and socioeconomic status.Reference Shohag, Ullah and Qusar 25
Selenium is an essential trace mineral which interacts primarily with a class of enzymes and transporters called “selenoproteins,” many of which are essential antioxidant enzymes. It is an integral part of the key redox enzymes thioredoxin reductase and GSH peroxidases, and also possesses anti-inflammatory activity through suppression of COX-2 enzymes.Reference Rayman 26 Low serum levels of selenium have been detected in some individuals with OCD, and a deficient state of this mineral have been associated with the development of oxidative stress.Reference Ozdemir, Cetinkaya and Ersan 27 Further, in selenium deficient states, the pro-inflammatory COX-2 enzyme becomes upregulated and susceptibility to glutamate induced neurotoxicity increases, which may result in loss of hippocampal neurons and damage to glial cells.Reference Savaskan, Brauer and Kuhbacher 28
Magnesium is an essential dietary mineral and serves as an enzymatic co-factor in over 300 biochemical reactions, most notably in adenosine triphosphate and adenyl cyclase dependent enzymes.Reference Gropper and Smith 23 Its neurobiological activity includes inhibitory effects at NMDA receptors, and thus, attenuation of extracellular glutamate release with subsequent neuroprotective effects, particularly in the hippocampus.Reference Kang, Choi and Park 29 , Reference Furukawa, Kasai and Torimitsu 30 Magnesium supplementation in mice attenuates anxiety-related behaviors and modulates glutamatergic and GABAergic transmission, as well as the activity of the hypothalamic–pituitary axis.Reference Sartori, Whittle and Hetzenauer 31 , Reference Murck 32 P5P, or “activated vitamin B6,” is the phosphorylated form of pyridoxal (the oxidized form of pyridoxine; vitamin B6) and serves as a co-factor in more than 150 enzymatic reactions.Reference Ueland, McCann and Midttun 33 P5P is a rate limiting co-factor in the synthesis of various neurotransmitters, including decarboxylation reactions required in the production of dopamine, serotonin, and GABA as well as noradrenaline and melatonin.Reference Kennedy 34 Even a mild deficiency of this vitamin may result in down-regulation of GABA and serotonin synthesis and subsequent disturbances in sleep, behavior, and cardiovascular function, with a loss of hypothalamus-pituitary control of hormone excretion.Reference Kennedy 34
This 20-week, multicenter open-label clinical trial sought to elucidate the effects of a nutraceutical combination of the aforementioned nutrients, for individuals with treatment-refractory OCD (TR-OCD). This preliminary study was open-label given the severe and chronic nature of TR-OCD and necessity for additional evidence-based treatment approaches for these individuals. 35 , Reference Castle, Bosanac and Rossell 36 Several other clinical trials involving TR-OCD participants have adopted this research design.Reference Pallanti, Marras and Salerno 37 - Reference Bakhla, Verma and Soren 40 Open label designs are arguably more ethical in OCD than in other psychiatric disorders because of very low placebo response rates in OCD.Reference Khan, Kolts and Rapaport 41 , Reference Sugarman, Kirsch and Huppert 42 It was hypothesized that the nutraceutical combination would attenuate symptoms of OCD as measured by a reduction in Yale-Brown Obsessive-Compulsive Scale (YBOCS) score from baseline to week-20 endpoint. Additional benefits were anticipated for alleviating anxiety as well as improving mood, general functioning, and overall quality of life.
Methods
Participants
Eligible participants were between the ages of 18 and 75 years with a primary diagnosis of OCD (confirmed with the Structured Clinical Interview for the DSM-5; SCID-5) of moderate or greater severity (YBOCS ≥ 16) who had the desire and capacity to consent to the study and follow its procedures. Participants had TR-OCD, defined as inadequate responses to a minimum of three trials of SRIs, one augmentation strategy (eg, an antipsychotic or mood stabilizer) as well as engagement in adequate CBT therapy specific for OCD (eg, completion of an inpatient OCD program or minimum 15 sessions of outpatient ERP). Treatment refractory criteria for the trial were formulated from the current literature at the time of protocol development and group consensus from the study psychiatrists experienced in OCD treatment. Participants who were already receiving standard treatment for their OCD, for example, psychotropic medication or ERP, were required to be stable prior to entry into the trial (minimum 12 weeks at consistent doses and/or frequency of therapy). Participants were excluded if they had a diagnosis of bipolar disorder, a psychotic disorder, primary diagnosis of an obsessive–compulsive spectrum disorder/s (secondary diagnosis permitted); current alcohol/substance abuse (as per SCID-5); suicidal ideation SIGHD-17 score ≥ 3; were allergic to, or taking medications with known or suspected negative interactions with any of the investigated nutraceuticals (eg, anticoagulants such as warfarin); had a serious or unstable medical condition/s; current gastrointestinal ulcers; pregnancy and lactation. Further, participants were withdrawn from the study if they experienced a severe deterioration in their OCD (medical monitors were notified if there was a ≥25% increase in their YBOCS score from baseline); ceased taking the nutraceuticals for 7+ days; underwent substantial treatment changes (for example, change in primary medication or entry into an inpatient OCD CBT program); emergence of serious side effects/SAEs as (determined by the medical monitors on a case by case basis); or if a participant elected to withdraw from the trial at their own volition.
Intervention
Treatment consisted of the nutraceutical combination taken each day for the 20-week study period in an open-label manner. The nutraceuticals were taken together in combination, and used adjunctively to existing psychotropic medication/s, psychological therapy, or as a monotherapy. Table 1 details the investigated nutraceuticals and dosages used.
Table 1. Nutraceutical Dosage Regime in the TRON Study
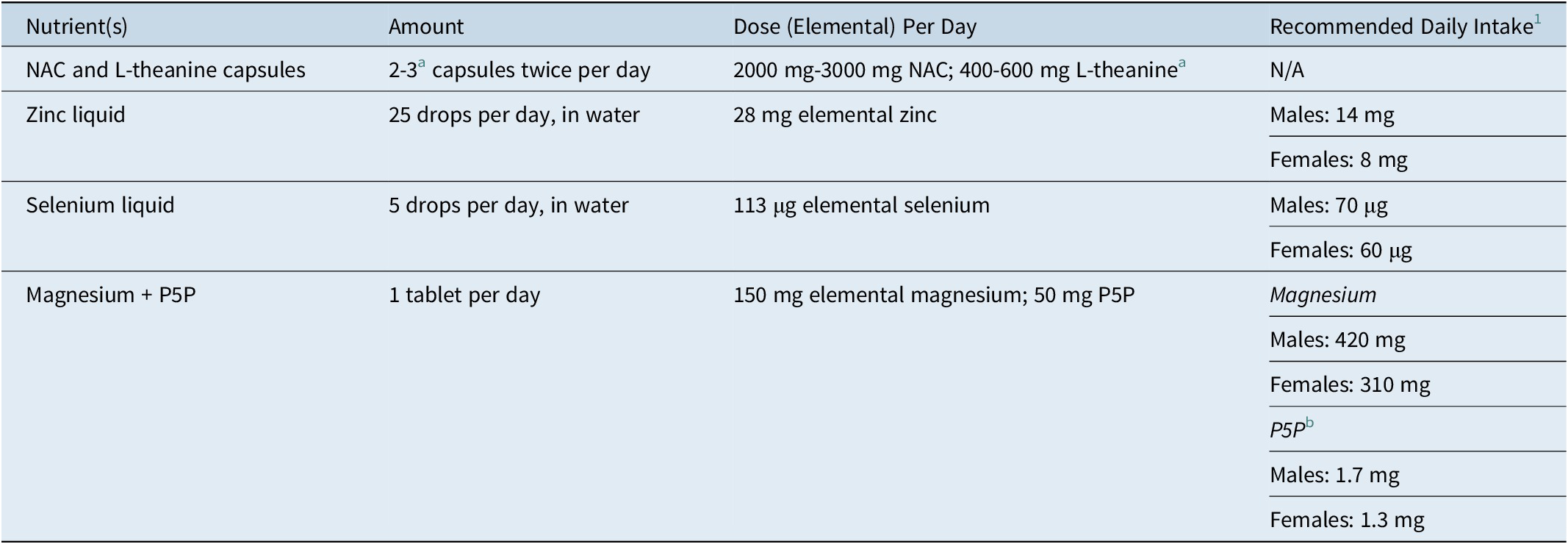
Abbreviations: N/A, not applicable; NAC, N-acetyl cysteine; P5P, Pyridoxal-5′-phosphate.
a At week-8, the NAC and L-theanine capsules were titrated from two to three capsules, twice per day in the event of nonresponse (≤35% reduction from baseline YBOCS score).Reference Fineberg, Hollander and Pallanti 1 Recommended daily intakes are taken from the Nutrient Reference Values for Australian and New Zealand. 43
b Based on B6 RDI levels.
The NAC and L-theanine capsules were supplied by BioCeuticals in line with strict pharmaceutical Good Manufacturing Practices (GMP). The Zinc (Zinc Drops), Selenium (Selenium Drops), and Magnesium + P5P (Ultra Musceleze P5P) tablets were also produced and supplied by BioCeuticals, in line with strict pharmaceutical GMP.
Measures
Baseline measures
After providing written, informed consent, participants’ demographic information was obtained including age, gender, education level, marital and employment status, country of birth, and ancestry. A medical, mental health, and treatment history was also obtained. Height and weight (to calculate body mass index [BMI]) and blood pressure were also recorded at baseline. The NetSCID-5,Reference Brodey, First and Linthicum 44 a computerized version of the SCID-5, was used to confirm the diagnosis of OCD, screen for exclusionary disorders applicable the study (psychosis, bipolar disorder, and substance abuse/dependence) and to determine the presence of comorbidities. A condensed version of the SCID-5 was administered using the following DSM-5 diagnostic modules: mood disorders, psychotic disorders, substance use disorders, anxiety disorders, obsessive–compulsive and related disorders, and gambling disorder.
Outcome measures
The YBOCS—severity scaleReference Goodman, Price and Rasmussen 45 was used as the primary outcome measure. The YBOCS-severity scale is a 10-item, semi-structured, clinician administrated instrument that is regarded as the gold standard for measuring the severity of obsessive and compulsive symptoms.Reference Rapp, Bergman and Piacentini 46 The self-reported Dimensional Obsessive–Compulsive Scale (DOCS)Reference Abramowitz, Deacon and Olatunji 47 was used to capture dimensional or thematic aspects of OCD symptoms as well as their perceived severity. The DOCS assesses the following symptom dimensions: germs and contamination; responsibility for harm, injury, or bad luck; unacceptable obsessional thoughts; symmetry, completeness, and exactness. Depressive symptoms were assessed using the Structured Interview Guide for the Hamilton Depression Rating Scale (SIGHD-17). Physiological and psychological anxiety symptoms were assessed using the 21-item, self-reported Beck Anxiety Inventory (BAI).Reference Beck, Epstein and Brown 48 The Sheehan Disability Scale (SDS)Reference Sheehan, Harnett-Sheehan and Raj 49 was used to measure the extent of which three domains (work; social life or leisure activities; and home life or family responsibilities) were impaired by mental health symptoms as perceived by the participant. The clinician-rated Clinical Global Impression scale (CGI) and patient rated Patient Global Impression scale (PGI) were used to rate OCD severity and change relative to baseline.Reference Guy 50 , Reference Mohebbi, Dodd and Dean 51 Quality of life was assessed using the 25-item, self-reported World Health Organization Quality of Life-BREF (WHOQOL-BREF). 52 Adverse events (AEs) were described qualitatively by the participant at the start of each post-baseline visit. AEs were further assessed using the self-reported Systematic Assessment for Treatment Emergent Events (SAFTEE),Reference Levine and Schooler 53 a 55-item symptom checklist assessing the presence and severity of emerging side effects. Caffeine was measured in milligrams based on standard amounts found in beverages, for example, instant coffee = 60 mg and black tea = 40 mg.
Procedures
The study was a 20-week, open-label, multicentre, pilot study conducted at The Melbourne Clinic (TMC) Professorial Unit in Melbourne, VIC (University of Melbourne); The Royal Brisbane and Women’s Hospital in Brisbane, QLD (University of Queensland) and NICM Heath Research Institute, Westmead, NSW (Western Sydney University). Recruitment occurred between August 2017 and May 2020. The study was registered on the ANZCTR (ACTRN12617001140347) and approved by TMC Research Ethics Committee (290), WSU Human Research Ethics Committee (H12331) and UQ Medical Research Ethics Committee (2018000339) prior to recruitment.
Participants were recruited via invitation letters to past attendees of an OCD inpatient program at TMC, clinician referrals at each recruitment site, as well as radio and Facebook advertising. Those who expressed interest in study participation completed a brief phone screening assessment with a research assistant to review their treatment and medical history to gauge study suitability. Baseline screening appointments were scheduled thereafter. After providing written informed consent, participants were assessed by a research assistant (trained via workshops led by clinicians experienced in OCD assessment) at baseline and every 4 weeks thereafter throughout the 20-week intervention using the measures outlined above. At each follow-up visit participants were asked about any significant life events, notable changes in OCD symptoms, the presence of side effects/new symptoms since last visit, healthcare visits, treatment changes, and compliance (ie, recalled missed doses of the nutraceuticals). Alcohol, drug use, and average daily caffeine intake were also estimated at each study visit, and blood pressure taken. At week-8, participants who had not experienced a ≥35% reduction in their YBOCS score from baseline were titrated from two capsules twice per day to three capsules twice per day (where tolerable), of the NAC and L-theanine capsules (max dose equating to 3000 mg of NAC and 600 mg of L-theanine per day).
Participants were asked to return remaining nutraceuticals at the end of the study to estimate adherence. Further, at the end of the 20-weeks, participants were entitled to an additional supply of the nutraceuticals (three containers of NAC and L-theanine capsules, one bottle of Zinc liquid, one bottle of Selenium liquid, and one bottle of Magnesium with P5P “Ultramuscleze” [BioCeuticals]) accompanied by a letter with recommended dosage instructions as specified by the Therapeutic Good Administration (Australia) and general safety information. The letter also advised the participant to discuss with their treating doctor prior to continuing the nutraceuticals post-study completion.
Data and statistical analysis
The analysis of primary and secondary outcomes was undertaken using linear mixed effects models (LMMs; R package lme4 Reference Bates, Sarkar and Bates 54 ) and included all participants with outcome data available. Models included a random intercept term. Such models account for correlated measurements within individuals and allow for the use of all available data. For each model, the outcome of interest was the fixed effect of “time” which represented the mean change in symptoms between study visits. LMMs included baseline score as a covariate and a baseline score–by time interaction (to allow treatment response to depend on baseline severity). For the primary outcome (total YBOCS), we also investigated an interaction between time and baseline caffeine intake, and time and number of prior failed medication therapies, to determine whether either variable modified treatment response. The likelihood ratio test was used to determine whether treatment response followed a linear trend. In the absence of clear evidence of nonlinearity, time was modeled as linear. A sample size calculation was not performed as we sort to recruit as many eligible people as possible to this pilot study within our 3-year recruitment window.
Completed secondary outcome scales included occasional missed responses for some scale items. Across all visits and participants, a total of nine items were missed on the DOCS, two on the SIGHD, four on the BAI and six on the SDS (<1% missing for each outcome). These missed item responses were imputed using predictive mean matching (R package mice Reference Buuren and Groothuis-Oudshoorn 55 ) prior to calculating total scores. Imputation was only used for completed outcome scales with no more than one-third missed items, and not for scales which were not completed (for instance, due to participant dropout). For outcomes in which treatment response depended on baseline symptom severity, the estimated follow-up scores were calculated with the baseline score at its mean. Treatment response was defined as a ≥35% reduction in YBOCS as well as a CGI-I rating of 1 (“very much improved”) or 2 (“much improved”) at study endpoint.Reference Mataix‐Cols, de la Cruz and Nordsletten 56 We additionally used the criteria of Jacobson and TruaxReference Jacobson and Truax 57 to evaluate the proportion of participants with “reliable” and “clinically significant” change at study endpoint. A change ≥10 points on the YBOCS constituted a reliable change and a final total YBOCS score ≤ 14 constituted a clinically significant change (as YBOCS scores below this threshold are closer to the normal mean than the clinical mean).Reference Fisher and Wells 58 For the primary outcome we performed a sensitivity per-protocol analysis, including only participants with high (>75%) treatment compliance. Additionally, because of the large proportion of study noncompleters, we performed a sensitivity analysis to assess the impact of dropout. This sensitivity analysis was performed using a pattern-mixture model in which intercepts and slopes are estimated separately for study completers and noncompleters and the results are combined in a weighted average.Reference Hedeker and Gibbons 59 A P value of <.05 was considered statistically significant. All statistical analysis was performed using R version 4.0.2 60 and plots were produced using R packages sjPlot Reference Lüdecke 61 and ggplot2. Reference Wickham 62
Results
Participant characteristics
A total of 975 people initially expressed interest in the trial, with only 30 participants being regarded as potentially meeting inclusion criteria (with many not meeting the criteria of TR-OCD). These 30 were assessed for study eligibility in a baseline screening appointment. Two participants were subsequently excluded during baseline assessment, one because of a SIGHD-17 suicide item score ≥ 3, the other for deteriorating mental health symptoms. Both were referred to their treating psychiatrist for ongoing care. Thus, 28 participants met inclusion criteria and provided written informed consent to proceed with the trial. Baseline characteristics of the enrolled participants are shown in Tables 2 and 3.
Table 2. Baseline Participant Characteristics
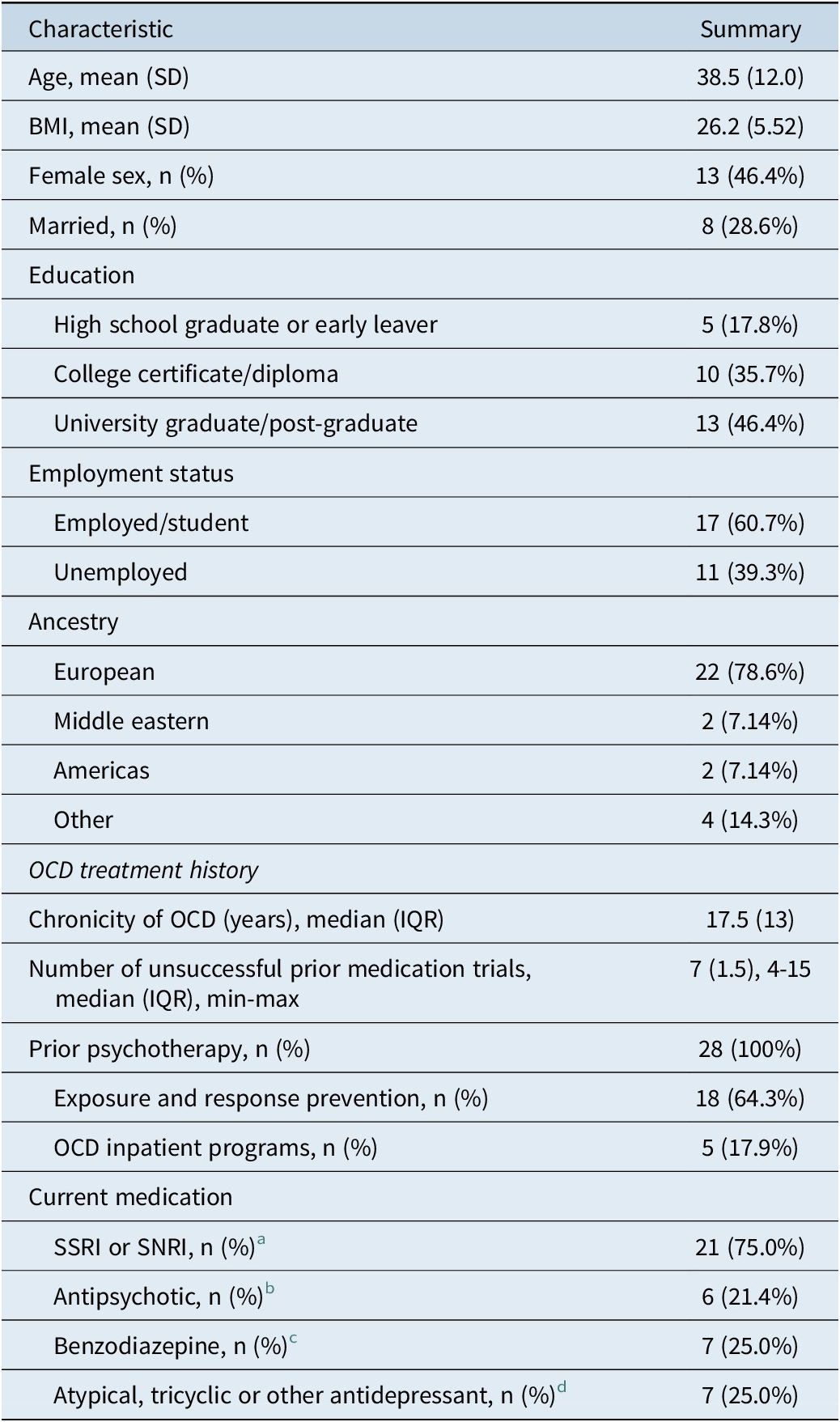
a Citalopram (n = 1), escitalopram (n = 4), fluoxetine (n = 3), fluvoxamine (n = 3), paroxetine (n = 1), sertraline (n = 7), venlafaxine (n = 1), and desvenlafaxine (n = 1).
b Quetiapine (n = 4), risperidone (n = 1), and aripiprazole (n = 1).
c Alprazolam (n = 1) lorazepam (n = 2), diazepam (n = 2), and clonazepam (n = 1).
d Clomipramine (n = 4), amitriptyline (n = 1), mirtazapine (n = 1), agomelatine (n = 1), and lithium (n = 1).
Table 3. Baseline Psychological Features

Abbreviations: BAI, Beck Anxiety Inventory; DOCS, Dimensional Obsessive–Compulsive Scale; SDS, The Sheehan Disability Scale; SIGHD, Structured Interview Guide for the Hamilton Depression Rating Scale; WHOQOL-BREF, World Health Organization Quality of Life-BREF; YBOCS, Yale-Brown Obsessive–Compulsive Scale.
a “How would you rate your quality of life?”
Of the 28 participants, 13 (46%) completed the 20-week study intervention, and 15 (54%) were withdrawn at various stages for the following reasons (note, two participants had dual reasons for withdrawing): AEs (n = 6), occurrence of an SAE warranting withdrawal as per study protocol/medical investigators (n = 2), participant decision due to lack of improvement in OCD (n = 3), unable to attend follow-up visits (n = 3), lost to follow-up (n = 1), noncompliance with the investigational product (n = 1), and approaching expiry of the nutraceuticals (n = 1). Three of the 15 withdrawn participants did not attend a study visit after baseline. There were 13 (46.4%) participants whose treatment (NAC and L-theanine capsules) was titrated to a higher dose due to nonresponse at week 8.
Primary outcome
The primary LMM revealed that OCD symptom severity improved from baseline (mean = 25.9, SD = 5.05) to week-20 with a mean change in total YBOCS symptoms of −7.13 (95% CI = −9.24, −5.01, P < .001), for those with the mean severity at baseline. This was equivalent to a 28% drop in total YBOCS score for the overall group. The mean reduction in symptoms between post-baseline visits was −1.21 points (95% CI = −1.66, −0.764; P < .001). Estimated follow-up means are displayed in Table 4. Notably, there was a significant baseline YBOCS score by time interaction (P = .005), with the slope of treatment response greatest in those with lower symptom levels at baseline (see Figure 1). The estimated mean treatment response in those with mild baseline symptoms (baseline YBOCS = 20.6; 20th percentile) was −9.45 points (95% CI = −12.4, −6.48), while in those with more severe symptoms (baseline YBOCS = 28.6; 80th percentile) the mean change was −5.49 points (95% CI = −8.05, −2.94). After accounting for the interaction between baseline YBOCS score and time, treatment response was further modified by baseline caffeine intake. Higher baseline caffeine intake was associated with greater treatment response, with an increase of one interquartile range in daily caffeine intake (eg, 50 mg to 232 mg/day; 25-75th percentile) associated with a 4.04-point greater reduction in YBOCS score over the trial (P < .001; see Supplementary Figure S1). There was no evidence to suggest that treatment response was influenced by the number of prior unsuccessful medication trials (P interaction = .71), after accounting for the interaction between baseline YBOCS severity and time.
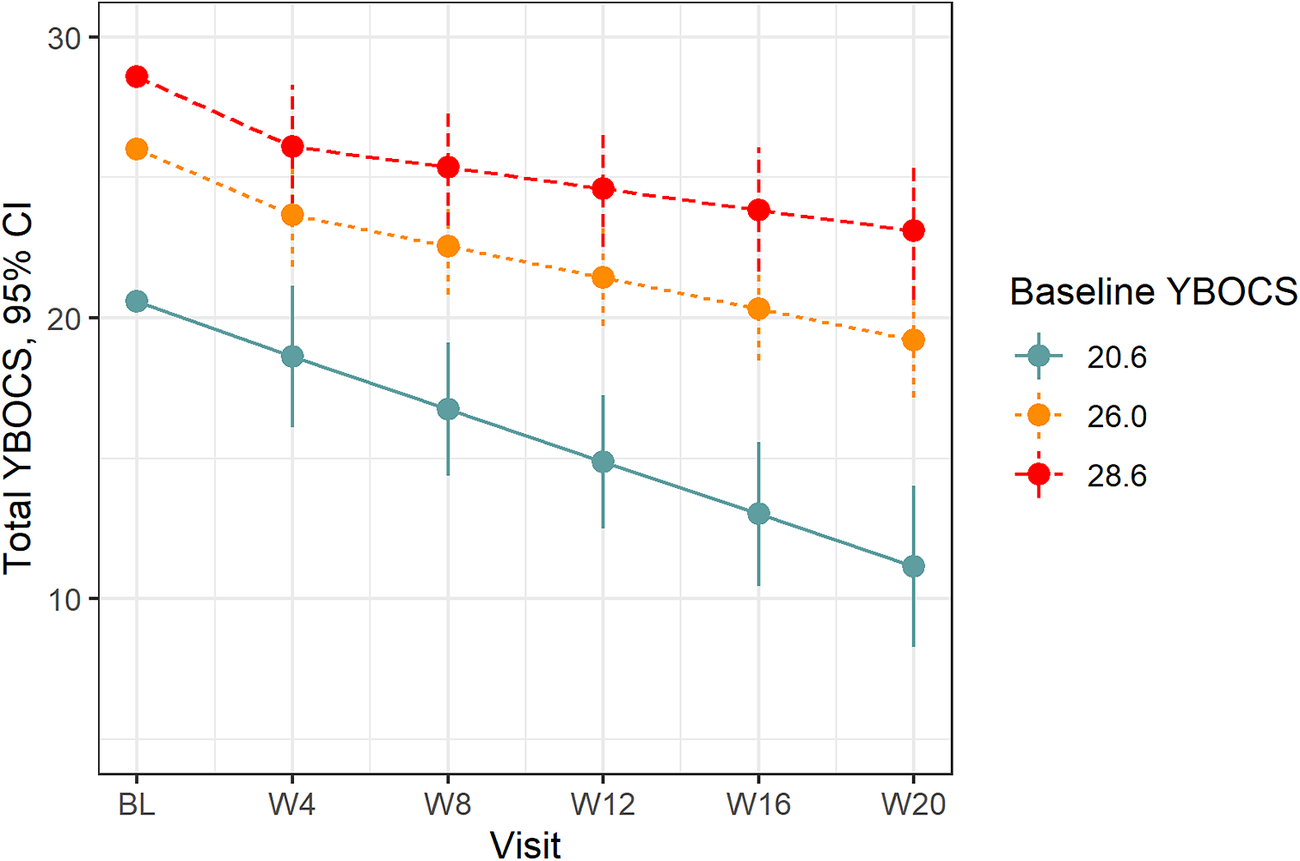
Figure 1. Treatment response on total YBOCS by baseline severity. Treatment response on the YBOCS at the 20th (20.6), 50th (26.0), and 80th percentiles (28.6) of baseline total YBOCS severity. YBOCS, Yale-Brown Obsessive–Compulsive Scale.
Table 4. Estimated Mean Follow-up Scores for Primary and Secondary Outcomes
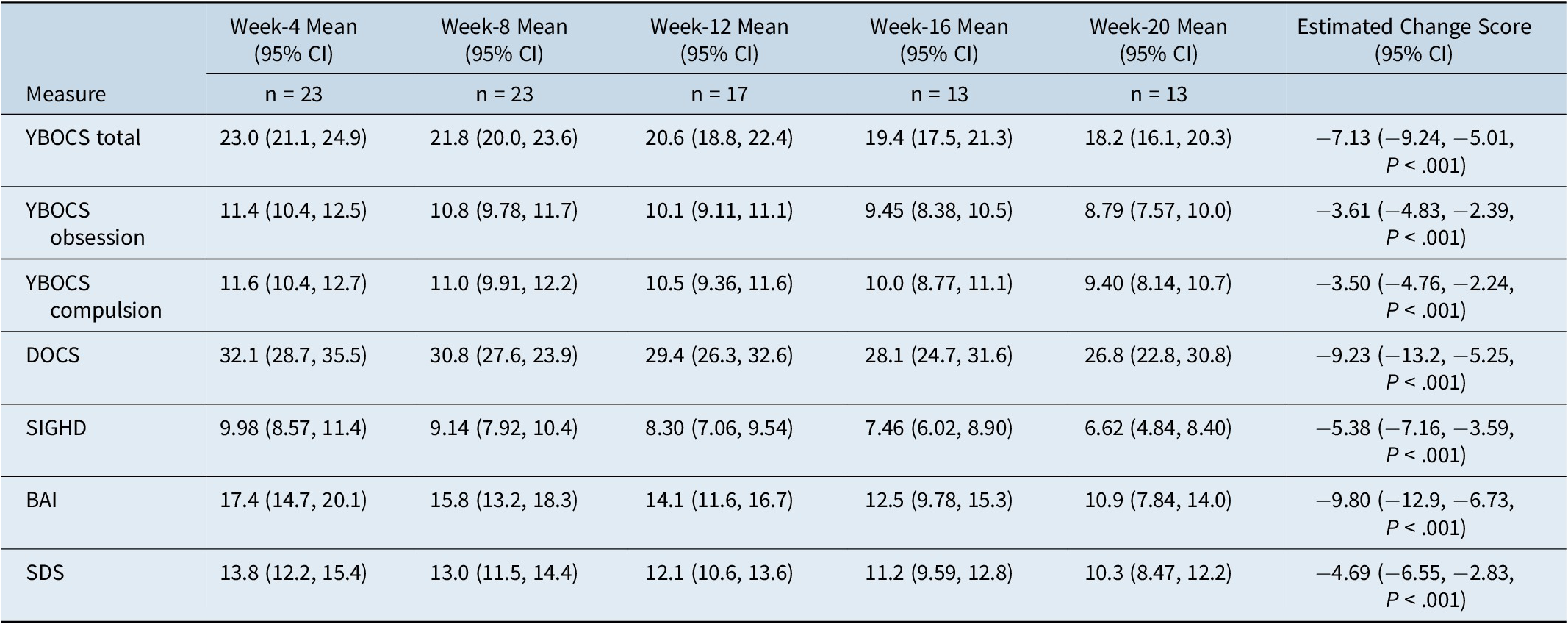
Note: Follow-up means and 95% confidence intervals are estimated from linear mixed effects models adjusted for baseline score. All follow-up means and change scores are calculated with baseline score at its mean value.
Abbreviations: BAI, Beck Anxiety Inventory; DOCS, Dimensional Obsessive–Compulsive Scale; SDS, The Sheehan Disability Scale; SIGHD, Structured Interview Guide for the Hamilton Depression Rating Scale; WHOQOL-BREF, World Health Organization Quality of Life-BREF; YBOCS, Yale-Brown Obsessive–Compulsive Scale.
A total of three (23%) participants who completed the trial met criteria for response (≥35% reduction in total YBOCS scores and “improved” or “very much improved” on the CGI-I at the final visit) and an additional two (15%) met criteria for partial response (>25% and <35% reduction in total YBOCS scores). Finally, three (23%) were estimated to meet the Jacobson and Truax criteria for reliable and clinically significant change at endpoint.
In a per-protocol analysis, including only participants with good compliance (>75%), the estimated mean change in total YBOCS was not substantively changed (mean change = −7.26, 95% CI = −9.53, −4.99, P < .001). Finally, in a pattern-mixture model assessing the influence of participant dropout, the mean change in total YBOCS score was reduced (mean change = −5.04, 95% CI = −8.45, −1.62, P = .004).
Secondary outcomes
Estimated follow-up visit means and mean treatment response for all secondary outcomes are displayed in Table 4. Considering the YBOCS obsession and compulsion subscales, treatment response was greatest in those with lower symptom levels at baseline (P interaction = .085 and P interaction = .020, for obsession and compulsion subscales, respectively). Symptoms improved across the trial for both the obsession (mean change = −3.61; 95% CI = −4.83, −2.39; P < .001) and compulsion subscales (mean change = −3.50; 95% CI = −4.76, −2.24; P < .001), for those with mean baseline severity for each measure. For the DOCS, the slope of treatment response was similarly greatest in those with lower symptom levels at baseline (P interaction = .022; see Figure 2). The estimated mean change in symptoms for those with the mean baseline DOCS score was −9.23 (95% CI = −13.2, −5.25; P < .001). For the SIGHD and BAI, there was little evidence that treatment response depended on baseline severity (P interaction = .54 and P interaction = .18, respectively). For the SIGHD, depressive symptoms improved across the trial with a mean change across the trial of −5.38 (−7.16, −3.59, P < .001) points. For the BAI, the mean change across the trial was −9.80 (95% CI = −12.9, −6.73, P < .001) points. Finally, for the SDS, there was no clear evidence that treatment response depended on baseline severity (P interaction = .17). Self-reported disability improved over the trial with a mean change of −4.69 points (95% CI = −6.55, −2.83, P < .001).
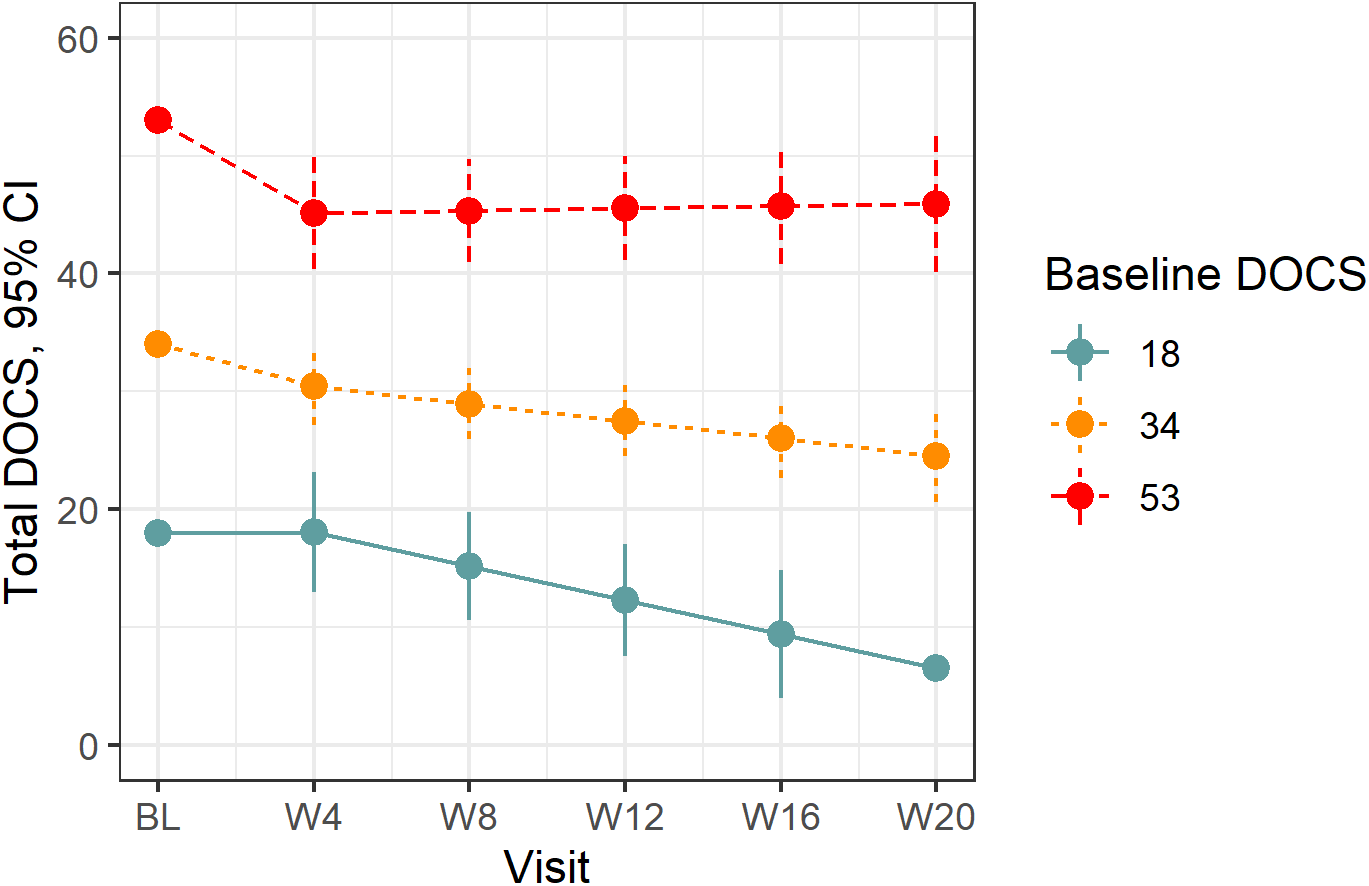
Figure 2. Treatment response on total DOCS by baseline severity. Treatment response on the DOCS at the 20th (18.0), 50th (33.5), and 80th percentiles (49.4) of baseline DOCS severity. DOCS, Dimensional Obsessive–Compulsive Scale.
On the clinician-rated CGI-I, three (25%) were rated as “very much improved,” with seven (58%) rated as “minimally improved” and two (17%) rated as “no change” at the final study visit (week-20). On the participant-rated PGI-I, one participant (8%) reported being “very much better,” five (42%) reported being “much better,” four (33%) reported being “a little better” and the remaining two (17%) reported “no change” at study completion. Finally, for item 1 of the WHOQOL-BREF (“How would you rate your quality of life?”), eight (67%) study completers reported a quality-of-life score higher than their initial baseline score, while quality of life for the remaining four (33%) of participants was unchanged.
Safety data
Eight participants were withdrawn from the study due to AEs. Three of these withdrawals were the participant’s decision. One elected to withdraw due to feelings of agitation, one due to sedation, nausea, and tachycardia, and one due to digestive complaints. Two participants were withdrawn by the study investigators, one for experiences of vivid dreams and fatigue, and a second for worsening physical and mental health and noncompliance with the nutraceuticals. A further participant withdrew based on advice from their general practitioner following an elevated C-reactive protein test. Finally, two participants experienced serious AEs (SAEs) and were withdrawn from the study. The SAEs were both exacerbation in OCD symptoms requiring inpatient admission. Both events were deemed “unlikely” to be related to the study treatment. Across the study, the most reported AEs were nausea (n = 9), which was associated with vomiting in two participants, diarrhea (n = 3), headache (n = 3), constipation (n = 3), reflux (n = 3), and gustatory disturbance (n = 3). Gastric AEs were typically transient and resolved at subsequent visits (however two participants were withdrawn due to this reason). These AEs often improved following advice to consume the nutrients with food.
Discussion
To our knowledge, this pilot study represents the first to explore the use of a nutraceutical combination for OCD, and particularly for a treatment-resistant population. Although our study was open-label, it is noteworthy that this sample had chronic TR-OCD symptoms, and thus any clinical improvement was clinically meaningful. Our results revealed that the combination of NAC, L-theanine, selenium, zinc, magnesium, and P5P had a modest clinical effect in respect to reducing OCD symptoms on both the YBOCS and the DOCS. It was also found to reduce depressed mood and anxiety and improve quality of life.
Of note is that treatment response appeared to depend on baseline severity with regard to OCD symptom outcomes (ie, YBOCS and DOCS). However, there was no clear indication that baseline severity modified treatment response on other outcomes (SIGHD, BAI, and SDS). Those with lower symptom levels may be more likely to benefit, and potentially could be deemed relatively less “treatment-resistant.” These contrasts with the treatment of major depressive disorder, which tends to provide a greater relative symptom reduction with pharmacotherapies in cases of more severe symptomatology (whereas for milder symptom levels medication is equivalent to placebo).Reference Fournier, DeRubeis and Hollon 63 There was also evidence that those with higher baseline caffeine intake were substantially more responsive to the treatment than those with low baseline caffeine intake. Notably, two small double-blind trials, one comparing caffeine to dextroamphetamine and one comparing caffeine with placebo, both found preliminary supportive evidence for the efficacy of caffeine in TR-OCD.Reference Koran, Aboujaoude and Gamel 64 , Reference Shams, Soufi and Zahiroddin 65 These results suggest it is plausible that there may have been a synergistic interaction between caffeine and the treatment formula in this study, particularly given that L-theanine is well known to interact synergistically with caffeine.Reference Bryan 66 Exploring the nature of this potential synergism is a promising avenue for further research.
Our prior systematic review exploring the principal nutraceutical in this formulation (NAC) for OCD and related disorders (OCRD)Reference Oliver, Dean and Camfield 67 found that the balance of evidence supported the use of NAC in presentations of moderate-severity OCD and OCRD, and a more recent meta-analysis supported a small but significant improvement in OCD symptoms from add-on NAC.Reference Gadallah, Ebada and Gadallah 68 Doses of 2.4 to 3 g/day for a minimum of 8 weeks were considered adequate for providing an initial therapeutic effect. However, even within these patient groups, response rates are variable, and some reports have not confirmed efficacy (particularly in treatment-resistant populations). Additionally, our recent 16-week, double-blind RCTReference Sarris, Oliver and Camfield 69 , Reference Sarris, Oliver and Camfield 70 using 3 g/day of NAC (1.5 g twice daily) in 44 participants with DSM-5 diagnosed OCD, while revealing a nonsignificant time × treatment interaction for the YBOCS scale total score, did show a significant time × treatment interaction for the YBOCS “Compulsions” subscale in favor of NAC at week-12 (P = .013). So, the question remained in terms of whether additional nutraceuticals to NAC, with some potentially beneficial mechanistic interaction would further enhance treatment efficacy beyond improvement of compulsive symptoms. This study, while providing benefit in some individuals, needs to be confirmed in a larger RCT in those with treatment-resistance. There is particular potential for its application in those with lower OCD symptom levels.
Clinically, it is worth noting that several participants had gastrointestinal problems when consuming the nutrient combination. While the 2 to 3 g of NAC used in the study appeared to be well-tolerated, it did potentially cause mild to moderate gastrointestinal effects. This was noted to be the most common symptom in our previous NAC studies (potentially due to the irritant effect of the sulfur-based compound). This was largely offset by the recommendation of co-consumption with or after food (this irritation could also be due to the zinc). Further, some symptoms, such as dizziness, agitation, and insomnia, were present in some participants, and were likely unrelated to study medications as such symptoms are common in people with anxiety or may be due to either a nocebo reaction or a psychosomatic experience in this population. However, an interaction with medications such as SRIs (potentially increasing serotonergic activation) are also possible. A future placebo-controlled study could elucidate this.
The current study has several limitations. Firstly, it was an open-label design (though as noted previously placebo response rates may be lower in OCD than comparable disorders, and such pilot studies are crucial to determine feasibility for future larger RCT designs).Reference Sugarman, Kirsch and Huppert 42 , Reference Leon, Davis and Kraemer 71 Another limitation was that while the study included people with OCD who were on “treatment as usual,” potential effect-modifying factors were present with participants taking different types of medications, or psychological interventions. The study was not adequately powered to assess differences in subgroups. Overall sample size was modest, and there was a high withdrawal rate, which is common in the more severe presentations of psychiatric disorders. This high withdrawal rate is a recognized limitation and may have led to estimated treatment effects being inflated, as participant withdrawal may have coincided with worsening mental health symptoms. Indeed, there was a 30% smaller estimated treatment effect in the pattern-mixture model, which accounted for different treatment response between completers and noncompleters. We also did not measure blood levels of relevant nutrients, as this would help to test the pharmacokinetics and serum levels in relation to efficacy, in addition to compliance. Regardless, our study had a 20-week interventional period, and thus should have duration long enough for any potential benefits to be apparent. Additionally, it is recognized that the pharmacokinetic and pharmacodynamic interaction between the nutrients (and potential synergy) is at present unknown and needs to be teased out in preclinical and clinical models. Other nutrients which have antioxidant effects potentially dampening glutaminergic neurotoxicity, such as vitamin C and B12, may also be of benefit. Finally, the treatment effects reported in this study are likely to be overstated as open-label trials are subject to bias by placebo effects and other nonspecific effects (such as regression to the mean, Hawthorne effects, and disease natural course variation).Reference Ernst and Resch 72 OCD patients may have slow and steady improvements on SSRI treatments for periods longer than 12 weeks, and so a longer waiting period for those who recently commenced new treatments may have been required.Reference Diniz, Shavitt and Fossaluza 73 , Reference Jakubovski, Diniz and Valerio 74 Strengths of the study include the use of a titration mechanism for those who were initially nonresponsive, and that the use of a multinutrient combination provided the potential for synergistic neurobiological activity beyond the usual monotherapy approach. Further research to experiment with formulation adjustments and use in concert with ERP may also be beneficial.
In conclusion, this pilot study tentatively supports the benefits of nutraceuticals for the treatment of OCD and encourages further research into their effectiveness and their place within current treatment guidelines for OCD.
Acknowledgments
Thanks are extended to the much-appreciated participants in this study. Jerome Sarris is supported by an NHMRC Clinical Research Fellowship (APP1125000). Michael Berk is supported by a NHMRC Senior Principal Research Fellowship (1156072).
Funding
BioCeuticals and Blackmores Institute unconditionally provided the study’s interventional product free of charge. They were not involved in the study’s conception, design, conduct, data-analysis, or publication of the results.
Conflict of Interest
The authors declare that there are no conflicts of interest.
Disclosures
Jerome Sarris has received either presentation honoraria, travel support, clinical trial grants, book royalties, or independent consultancy payments from: Integria Healthcare & MediHerb, Pfizer, Scius Health, Key Pharmaceuticals, Taki Mai, Fiji Kava, FIT-BioCeuticals, Blackmores, Soho-Flordis, Healthworld, HealthEd, HealthMasters, Kantar Consulting, Grunbiotics, Australian Natural Therapeutics Group, Polistudium (Italy), Research Reviews, Elsevier, Chaminade University, International Society for Affective Disorders, Complementary Medicines Australia, SPRIM, Terry White Chemists, ANS, Society for Medicinal Plant and Natural Product Research, Sanofi-Aventis, Omega-3 Centre, the National Health and Medical Research Council, and CR Roper Fellowship.
Michael Berk has received Grant/Research Support from the NIH, Cooperative Research Centre, Simons Autism Foundation, Cancer Council of Victoria, Stanley Medical Research Foundation, Medical Benefits Fund, National Health and Medical Research Council, Medical Research Futures Fund, Beyond Blue, Rotary Health, A2 milk company, Meat and Livestock Board, Woolworths, and Avant and the Harry Windsor Foundation, has been a speaker for Abbot, Astra Zeneca, Janssen and Janssen, Lundbeck, and Merck, and served as a consultant to Allergan, Astra Zeneca, Bioadvantex, Bionomics, Collaborative Medicinal Development, Janssen and Janssen, Lundbeck, Merck, Pfizer, and Servier—all unrelated to this work.
Chee H. Ng had served as a consultant for Lundbeck, Grunbiotics, Servier, Janssen-Cilag, Wyeth, and Eli Lilly, received research grant support from Wyeth and Lundbeck, and speaker honoraria from Servier, Lundbeck, Bristol-Myers Squibb, Organon, Eli Lilly, GlaxoSmithKline, Janssen-Cilag, Astra-Zeneca, Wyeth, and Pfizer, all outside and unrelated to this work.
Gerard J. Byrne has received, through his employer, grant income from Eli Lilly, Janssen-Cilag, Biogen, the Australian National Health and Medical Research Council, and the Royal Brisbane and Women’s Hospital. He has received royalties from book sales and licensing fees for the use of psychometric scales.
As a medical research institute, NICM Health Research Institute receives research grants and donations from foundations, universities, government agencies, individuals, and industry. Sponsors and donors also provide untied funding for work to advance the vision and mission of the Institute.
Georgina Oliver, Lachlan Cribb, Scott Blair-West, David Castle, Olivia M. Dean, David A. Camfield, Vlasios Brakoulias, Chad Bousman, Nathan Dowling, Carolyn Ee, Jenifer Murphy, Ranjit Menon, Suneel Chamoli, and Mark Boschen do not have any disclosures.
Authorship Contributions
Jerome Sarris, Lachlan Cribb, and Georgina Oliver were primarily involved in the first iteration of the manuscript. Lachlan Cribb analyzed the data. All other co-authors participated in the editing and final approval of the manuscript.
Supplementary Materials
To view supplementary material for this article, please visit http://doi.org/10.1017/S1092852921000638.








