Introduction
Kraepelin conceived mixed states as combinations of two opposite polarities—namely, weakness or excitement of mood, thinking, and volition.Reference Marneros 1 However, his broad concept of mixed states was long neglected.Reference Marneros 1 In the Diagnostic and Statistical Manual of Mental Disorders, 4th ed., text revision (DSM–IV–TR), mixed states corresponded to “mixed episodes,” which were defined quite narrowly as the cooccurrence of full syndromal mania and depression for ≥1 week in the context of bipolar I disorder (BD–I). 2
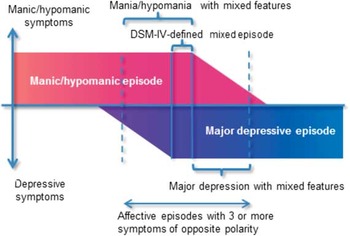
Recently, the broad concept of mixed states has reemerged as a spectrum: mixed (or dysphoric) mania/hypomania (mania/hypomania with subsyndromal depression) → full mixed state corresponding with the DSM–IV-defined mixed episode → mixed depression (depression with subsyndromal mania/hypomania).Reference Swann, Lafer and Perugi 3 Compared to pure manic or depressive episodes, broadly defined mixed states exhibit several specific features: a longer overall course, higher episode frequency, and increased rates of attempted suicide and comorbid substance abuse.Reference Swann, Lafer and Perugi 3 , Reference Vieta and Valenti 4 Based on the prognostic impacts of these additional features, the DSM–5 removed the narrowly defined “mixed episode,” and instead captured ≥3 nonoverlapping symptoms of the opposite polarity using a “with mixed features” specifier to be applied to manic/hypomanic and major depressive episodes (Figure 1). 5 The full mixed state is now included in the category of manic/hypomanic episode with mixed features. 5
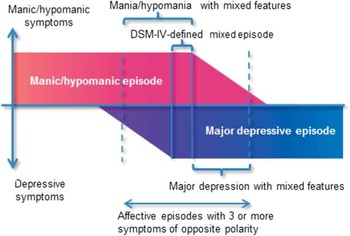
Figure 1 Schematic presentation of mixed features according to the DSM–5.
Pharmacotherapy of mixed states is challenging because the physician is required to treat both manic/hypomanic and depressive symptoms concurrently.Reference Vieta and Valenti 4 Monotherapy with high-potency antipsychotics for manic symptoms can potentially promote depressive symptoms.Reference Vieta and Valenti 4 Conversely, antidepressants can potentially exacerbate agitation and irritability, leading the task force of the International Society for Bipolar Disorders to recommend avoiding antidepressant treatment in mixed states.Reference Pacchiarotti, Bond and Baldessarini 6 Our paper aims to provide a review and synthesis of the available evidence for pharmacotherapy of mixed states, with a focus on mixed mania/hypomania.
Methods
The author searched PubMed for English-language articles published since 1990 on the efficacy of pharmacotherapy in adult (≥19 years old) patients with mixed episode or mania/hypomania with significant depressive symptoms. The search was conducted on 19 December 2015 using the following search terms: (mixed episode* OR mixed mania OR mixed hypomania OR mixed specifier OR mixed feature* OR mixed state* OR dysphoric mania OR bipolar mixed) AND (therapy OR treatment OR pharmacotherapy OR trial). Articles were selected for further evaluation by inspecting abstracts. The bibliographies of the selected articles were also included. The eligible articles were randomized controlled trials (RCTs) of acute-phase and maintenance treatments with a placebo arm and ≥25 cases.
The initial PubMed search identified 919 articles, 81 of which were selected for further evaluation. From those, 34 studies were included in our review (summarized in Tables 1 and 2). Only two RCTs prospectively examined the efficacy of pharmacotherapy for mixed mania/hypomania exclusively; the remaining 32 studies were subgroup or pooled analyses of RCTs for manic and mixed episodes of BD–I.
Table 1 Summary of the efficacy of pharmacotherapy for acute-phase mixed mania/hypomania

ADRS=Affective Disorder Rating Scale; ARP=aripiprazole; ASE=asenapine; BD–II=bipolar II disorder; CBZ–ERC, extended-release carbamazepine capsule; CGI–BP–D=Clinical Global Impression For Bipolar Disorder–Depression; DSM=Diagnostic and Statistical Manual of Mental Disorders; DVP=divalproex; HAMD–21=Hamilton rating scale for depression, 21-item; LI=lithium; MADRS=Montgomery–Åsberg Depression Rating Scale; NR=not reported; OLA=olanzapine; PAL–ER=extended-release paliperidone; PLA=placebo; QUE=quetiapine; QUE–XR=extended-release quetiapine; RDC=research diagnostic criteria; SADS–C=Schedule for Affective Disorders and Schizophrenia–Change Version; VAL=valproate; YMRS=Young Mania Rating Scale; ZIP=ziprasidone; >PLA=the agent demonstrated a significantly higher efficacy compared to placebo. =PLA=the efficacy of the agent was not significantly different from that of placebo. *=overall severity, Clinical Global Impression for Bipolar Disorder Overall Severity Scale; functioning=Global Assessment of Functioning Scale.
Table 2 Summary of the efficacy of pharmacotherapy for maintenance-phase mixed mania

ARP=aripiprazole; DSM=Diagnostic and Statistical Manual of Mental Disorders; DVP=divalproex; LI=lithium; LTG=lamotrigine; NR=not reported; OLA=olanzapine; PLA=placebo; QUE=quetiapine; SADS–C=Schedule for Affective Disorders and Schizophrenia–Change Version; VAL=valproate; >PLA=the agent demonstrated a significantly higher efficacy compared to placebo. =PLA=the efficacy of the agent was not significantly different from that of placebo. *=mean change in the baseline Young Mania Rating Scale score.
Acute-Phase Treatment
Atypical antipsychotics
Aripiprazole
In a 3-week RCT for acute manic and mixed episodes, aripiprazole demonstrated significantly greater improvements in both manic (by the Young Mania Rating Scale [YMRS]) and depressive (by the Montgomery–Åsberg Depression Rating Scale [MADRS]) symptoms in mixed-episode patients compared to placebo.Reference Sachs, Sanchez and Marcus 7 A pooled analysis of the abovementioned study and another identically designed RCTReference Keck, Marcus and Tourkodimitris 8 examined the efficacy of aripiprazole for manic symptoms in patients with baseline MADRS scores >18 (pure mixed) and 9–18 (intermediate mixed).Reference Suppes, Eudicone, McQuade, Pikalov and Carlson 9 Aripiprazole produced significantly greater endpoint YMRS score improvements than did placebo regardless of baseline MADRS score.
Asenapine
In a 3-week RCTReference McIntyre, Cohen, Zhao, Alphs, Macek and Panagides 10 of asenapine and olanzapine (included as a reference intervention) for acute manic and mixed episodes, asenapine demonstrated a YMRS effect significantly greater compared to placebo and similar to that of olanzapine.Reference McIntyre, Cohen, Zhao, Alphs, Macek and Panagides 10 Further analysis in mixed-episode patients showed that the endpoint YMRS score improvement with asenapine approached statistical significance, while that with olanzapine was highly significant compared to placebo (p=0.05 and 0.006, respectively). However, according to the mixed model for repeated measures analysis, the YMRS effects of asenapine and olanzapine did not reach statistical significance at endpoint compared to placebo.
Using the data from the abovementioned and an identically designed 3-week RCT,Reference McIntyre, Cohen, Zhao, Alphs, Macek and Panagides 11 three pooled studies examined the efficacies of asenapine and olanzapine for variously defined mixed mania.Reference Szegedi, Zhao, van Willigenburg, Nations, Mackle and Panagides 12 – Reference McIntyre, Tohen, Berk, Zhao and Weiller 14 Szegedi et al.Reference Szegedi, Zhao, van Willigenburg, Nations, Mackle and Panagides 12 analyzed the efficacy of asenapine for depressive symptoms (MADRS and Clinical Global Impression for Bipolar Disorder-Depression [CGI–BP–D] scale) in patients with mixed mania as defined by the following criteria: population 1, MADRS scores ≥20; population 2, CGI–BP–D scale severity scores ≥4; population 3, a diagnosis of mixed episode.Reference McIntyre, Tohen, Berk, Zhao and Weiller 14 The baseline severity of depressive symptoms was significantly reduced by asenapine compared with placebo: the MADRS scores were improved at days 7 and 21 in all populations, and the CGI–BP–D scores were improved at day 7 in all populations and at day 21 in population 1. The CGI–BP–D severity score was significantly reduced by olanzapine compared to placebo at day 7 in populations 2 and 3, and at day 21 in population 1. Olanzapine did not affect the MADRS scores significantly. Azorin et al.Reference Azorin, Sapin and Weiller 13 , Reference Tohen, Jacobs and Grundy 15 extracted the data of the mixed-episode patients.Reference Tohen, Jacobs and Grundy 15 The improvements in YMRS and MADRS baseline scores at 3 weeks were significantly greater with asenapine than placebo; olanzapine had no statistically significant effects. McIntyre et al.Reference McIntyre, Tohen, Berk, Zhao and Weiller 14 examined the efficacy of asenapine for both manic and depressive symptoms in patients with ≥2 or ≥3 baseline depressive features defined by DSM–5 criteria for a manic episode with mixed features.Reference Tohen, Sanger and McElroy 16 The change in baseline YMRS scores in patients with ≥3 depressive features was significantly greater with asenapine than placebo at day 2 across all depression levels and continued to decrease to endpoint; olanzapine was significantly more efficacious than placebo only in patients with lower baseline depression severities. The differences between the asenapine and placebo groups increased with increasing depression severity. The remission rate of depressive symptoms (MADRS scores ≤12) was significantly higher with asenapine than placebo across most severity levels in patients with ≥3 depressive features. With olanzapine, it was significantly higher only in patients with ≥2 depressive features and mild to moderate depression severity.
Olanzapine
In addition to the aforementioned asenapine trials, other studies support the efficacy of olanzapine for the treatment of mixed mania.Reference Tohen, Jacobs and Grundy 15 – Reference Tohen, McIntyre, Kanba, Fujikoshi and Katagiri 19 In 3- and 4-week RCTs for acute manic and mixed episodes, olanzapine demonstrated significantly greater YMRS effects compared to placebo.Reference Tohen, Jacobs and Grundy 15 , Reference Tohen, Sanger and McElroy 16 The results were not affected by the baseline diagnosis (i.e., manic vs. mixed). One of these RCTs revealed a significantly greater endpoint improvement in depressive symptoms (Hamilton Rating Scale for Depression, 21-item [HAMD–21]) with olanzapine compared to placebo in patients with baseline HAMD–21 total scores ≥20.Reference Tohen, Jacobs and Grundy 15 Pooling the data from these two RCTs, Baker et al.Reference Baker, Tohen and Fawcett 17 , Reference McIntyre, Cohen, Zhao, Alphs, Macek and Panagides 10 examined the efficacy of olanzapine for both manic and depressive symptoms in patients with baseline HAMD–21 total scores ≥20.Reference Tohen, McIntyre, Kanba, Fujikoshi and Katagiri 19 Olanzapine demonstrated significantly greater improvements in YMRS and HAMD–21 scores than did placebo at 3 weeks. With the same pooled data, Baldessarini et al.Reference Baldessarini, Hennen and Wilson 18 analyzed the antimanic efficacy of olanzapine in patients with mixed episodes.Reference Katagiri, Takita, Tohen, Higuchi, Kanba and Takahashi 20 Olanzapine produced a significantly higher response rate for manic symptoms (≥50% YMRS score reduction) than did placebo.
Tohen et al.Reference Tohen, McIntyre, Kanba, Fujikoshi and Katagiri 19 applied the DSM–5 criteria for manic episodes with mixed features to the pooled sample of three RCTs for acute manic and mixed episodes. The RCTs analyzed included the two olanzapine studies described aboveReference Tohen, Jacobs and Grundy 15 , Reference Tohen, Sanger and McElroy 16 and a trial of olanzapine and haloperidol.Reference Katagiri, Takita, Tohen, Higuchi, Kanba and Takahashi 20 In patients with mixed features, olanzapine demonstrated significantly greater YMRS score improvements compared to placebo at 3 weeks. The decrease in depressive symptoms (17-item Hamilton Rating Scale for Depression [HAMD–17]) was greater with olanzapine compared to placebo. However, the difference did not reach statistical significance, which the authors attributed to low statistical power.
Extended-release paliperidone (paliperidone–ER)
In two RCTs for acute manic and mixed episodes, paliperidone–ER demonstrated significantly greater YMRS score improvements compared to placebo.Reference Vieta, Nuamah and Lim 21 , Reference Berwaerts, Xu, Nuamah, Lim and Hough 22 The results were not affected by the baseline diagnosis.
Extended-release quetiapine (quetiapine–XR)
In a 3-week RCT of quetiapine–XR for acute manic and mixed episodes, the YMRS and MADRS changes with quetiapine–XR were not significantly different compared to placebo in mixed-episode patients.Reference Cutler, Datto, Nordenhem, Minkwitz, Acevedo and Darko 23
Ziprasidone
In a 3-week RCT for acute manic and mixed episodes, ziprasidone improved manic symptoms significantly compared to placebo (MRS).Reference Keck, Versiani, Potkin, West, Giller and Ice 24 The result was not affected by the baseline diagnosis. In a pooled studyReference Weisler, Kalali and Ketter 27 using the data from the abovementioned and a similarly designed 3-week RCTs,Reference Potkin, Keck, Segal, Ice and English 25 ziprasidone produced significantly greater HAMD and MRS score improvements than did placebo in patients with dysphoric mania, as defined by a manic episode with a score of ≥2 on at least two of the eight selected HAMD items.Reference Stahl, Lombardo, Loebel and Mandel 26
Mood stabilizers
Extended-release carbamazepine capsule (carbamazepine–ERC)
In two identically designed 3-week RCTs for acute manic and mixed episodes, carbamazepine–ERC demonstrated significantly greater YMRS score improvements compared to placebo.Reference Weisler, Kalali and Ketter 27 , Reference Weisler, Keck, Swann, Cutler, Ketter and Kalali 28 The results were not affected by the baseline diagnosis. One of these studies also demonstrated significantly greater HAMD score improvements with carbamazepine–ERC compared to placebo in mixed-episode patients continuing treatment.Reference Weisler, Kalali and Ketter 27 In a pooled analysis of these RCTs, carbamazepine–ERC showed significantly greater YMRS and HAMD changes than did placebo in mixed-episode patients.Reference Weisler, Hirschfeld and Cutler 29
Divalproex and lithium
In a 3-week RCT for acute mania as defined by research diagnostic criteria, both divalproex and lithium demonstrated significantly greater improvements in manic symptoms compared to placebo (Manic Syndrome Scale, Behavior–Ideation Scale, and MRS).Reference Bowden, Brugger and Swann 30 A post-hoc analysis of this RCT revealed that patients with significant depressive symptoms responded similarly to lithium and placebo, whereas those without depressive symptoms responded significantly better to lithium.Reference Swann, Bowden and Morris 31 The response to divalproex was not significantly different between the two patient groups. Depressive symptoms were associated with a better response to divalproex than lithium. In another 3-week RCT for acute manic and mixed episodes, extended-release divalproex demonstrated significantly greater MRS improvements compared to placebo.Reference Bowden, Swann and Calabrese 32 The result was not affected by the baseline diagnosis.
Combination therapy
Olanzapine plus valproate or lithium
A 6-week RCT in patients with acute manic and mixed episodes who were partially nonresponsive to 2-week valproate or lithium monotherapy found adjunctive olanzapine to produce significantly greater YMRS and HAMD–21 score improvements compared to placebo in mixed-episode patients with a baseline HAMD–21 score of ≥20.Reference Tohen, Chengappa and Suppes 33 A post-hoc analysis revealed the efficacy of adjunctive olanzapine in all patients with such HAMD–21 scores.Reference Baker, Brown and Akiskal 34 Houston et al.Reference Houston, Tohen, Degenhardt, Jamal, Liu and Ketter 35 conducted a 6-week RCT of adjunctive olanzapine in patients with acute mixed episodes who showed inadequate response to 2-week valproate monotherapy, the only prospective RCT specifically for acute mixed episodes. Adjunctive olanzapine produced significantly greater endpoint YMRS and HAMD–21 score improvements compared to adjunctive placebo.
Maintenance Treatment
Atypical antipsychotics
Olanzapine
A 48-week RCT in patients achieving remission after open-label acute treatment with olanzapine for manic and mixed-index episodes showed that olanzapine significantly increased the time to relapse into any mood event in patients with mixed-index episodes compared to placebo.Reference Tohen, Calabrese and Sachs 36 Moreover, a post-hoc study found a significantly increased time to depressive and manic relapses in patients with mixed-index episodes treated with olanzapine.Reference Tohen, Sutton, Calabrese, Sachs and Bowden 37
Quetiapine
In a 104-week RCT, patients achieving stabilization after open-label treatment with quetiapine for current or recent manic, mixed, or depressive episodes were randomized to continue quetiapine or to switch to placebo or lithium (included as a reference intervention).Reference Weisler, Nolen, Neijber, Hellqvist and Paulsson 38 In patients with mixed-index episodes, quetiapine significantly increased the time to relapse into any mood, manic, or depressive event compared to placebo.
Mood stabilizers
Lithium and divalproex/valproate
In the abovementioned RCT of quetiapine, lithium significantly increased the time to relapse into any mood event compared to placebo in patients with mixed-index episodes.Reference Weisler, Nolen, Neijber, Hellqvist and Paulsson 38 The time to relapse into manic, but not depressive, events was significantly longer with lithium than with placebo. Bowden et al.Reference Bowden, Collins and McElroy 39 conducted a post-hoc study of the relationship between initial manic symptomatology (euphoric or dysphoric) and the response to maintenance treatment (divalproex, lithium, or placebo) using the data from a 52-week RCTReference Bowden, Calabrese and McElroy 40 for patients who had recovered from manic-index episodes as defined by the DSM–III–R. The time to relapse into any mood, manic, or depressive event was not significantly different between the divalproex, lithium, and placebo groups among initially dysphoric patients.
Combination therapy
Aripiprazole plus lamotrigine
In a 52-week RCT, patients achieving and maintaining stabilization after single-blind treatment with aripiprazole and open-label lamotrigine for recent manic and mixed episodes were randomized to double-blind treatment with lamotrigine plus aripiprazole or placebo.Reference Carlson, Ketter and Sun 41 In patients with mixed-index episodes, the aripiprazole combination significantly increased the time to relapse into a depressive event compared to the placebo combination.
Aripiprazole plus lithium or valproate
In a 52-week RCT, patients achieving stabilization after treatment with single-blind aripiprazole plus open-label lithium or valproate for acute manic and mixed episodes were randomized to double-blind lithium/valproate plus aripiprazole or placebo.Reference Marcus, Khan and Rollin 42 Yatham et al.Reference Yatham, Fountoulakis and Rahman 43 conducted a post-hoc analysis of this RCT to explore the efficacy of aripiprazole plus lithium/valproate stratified by manic or mixed-index episodes. In patients with mixed-index episodes, the aripiprazole combination demonstrated significantly greater YMRS score improvements than did the placebo combination; however, the time to relapse into any mood episode and the MADRS score change with the aripiprazole combination did not differ significantly compared to the placebo combination.
Quetiapine plus lithium or divalproex
In two identically designed 104-week RCTs, patients achieving stabilization after open-label treatment with quetiapine plus lithium or divalproex for current or most recent manic, mixed, or depressive episodes were randomized to double-blind quetiapine or placebo plus lithium/valproate.Reference Suppes, Vieta, Liu, Brecher and Paulsson 44 , Reference Vieta, Suppes, Eggens, Persson, Paulsson and Brecher 45 Each of these studies and their pooled analysis found that the quetiapine combination significantly increased the time to relapse into any mood, manic, or depressive event compared to the placebo combination in patients with mixed-index episodes.Reference Suppes, Vieta, Liu, Brecher and Paulsson 44 – Reference Vieta, Suppes, Ekholm, Udd and Gustafsson 46 Moreover, the pooled study revealed that the quetiapine combination significantly increased the time to relapse into a mixed event compared to the placebo combination.Reference Vieta, Suppes, Ekholm, Udd and Gustafsson 46
Mixed Hypomania
Suppes et al.Reference Suppes, Ketter and Gwizdowski 47 conducted an RCT of adjunctive quetiapine for patients with hypomania with depressive symptoms (YMRS scores ≥12 and MADRS scores ≥15) of BD–II under a stable medication regimen for ≥2 weeks. Adjunctive quetiapine demonstrated significantly greater improvements in the CGI–BP severity and MADRS scores compared to placebo; the YMRS scores did not change significantly.
Synthesis of Evidence
Most evidence for the treatment of mixed mania comes from post-hoc subgroup and pooled analyses of RCTs for patients with manic and mixed episodes of BD–I. Because such analyses often produce false positive and false negative results, they should be interpreted with caution.
Acute-phase treatment
Regarding treatment of manic symptoms, there is evidence of efficacy of aripiprazole, asenapine, carbamazepine–ERC, divalproex/valproate, olanzapine (as monotherapy and co-therapy with lithium or divalproex/valproate), paliperidone–ER, and ziprasidone. Generally, the efficacy for manic symptoms did not differ between mixed and pure mania, which was also shown in a meta-analysis of the efficacies of atypical antipsychotics for acute mixed episodes.Reference Muralidharan, Ali and Silveira 48 However, agents also efficacious for concurrent depressive symptoms are required for the treatment of mixed mania. In this regard, aripiprazole, asenapine, carbamazepine–ERC, olanzapine (as monotherapy and in combination with lithium or valproate), and ziprasidone produced the strongest evidence of efficacy. These results are in line with the three extant reviews of the pharmacotherapy of mixed states.Reference McIntyre and Yoon 49 – Reference Grunze and Azorin 51 Despite having failed to show efficacy in one study, quetiapine is worth considering because of its established efficacy for acute manic and depressive episodes of BD.Reference Bowden, Grunze and Mullen 52 , Reference Calabrese, Keck and Macfadden 53 The efficacy of divalproex/valproate for depressive symptoms was not obvious in the articles included in the present review; however, this agent is also worth considering because of its probable efficacy in bipolar depression.Reference Ghaemi, Gilmer and Goldberg 54 , Reference Muzina, Gao and Kemp 55 Because there is no evidence of differential effectiveness in mixed states among these agents so far, selection should be made based on the profiles of possible adverse effects for individual cases.
For severe cases, combination therapy with these atypical antipsychotics and mood stabilizers, such as olanzapine plus lithium or valproate, is recommended. The poor efficacy of lithium for mixed or dysphoric mania derived from an earlier case series has been widely taken for granted.Reference Swann, Bowden and Morris 31 Surprisingly, only one subgroup analysis has compared the efficacy of lithium with placebo so far. Given the well-known efficacy of lithium for suicide prevention and the high rate of attempted suicide reported in dysphoric mania, lithium may be a valid treatment option.Reference Vieta and Valenti 4 , Reference Cipriani, Hawton, Stockton and Geddes 56 Further studies are needed to determine its value in the treatment of mixed states.
Maintenance treatment
Compared to acute phase, the evidence for maintenance treatment of mixed mania is scarce. Evidence of efficacy in preventing manic relapse was found for aripiprazole plus lithium/divalproex, lithium, olanzapine, and quetiapine (as monotherapy and in combination with lithium/divalproex), whereas evidence of efficacy in preventing depressive relapse was found for aripiprazole plus lamotrigine, olanzapine, and quetiapine (as monotherapy or in combination with lithium/divalproex). Considering the necessity to prevent both manic and depressive relapses, olanzapine and quetiapine (as monotherapy or in combination with lithium/divalproex) have shown the strongest evidence. Although our review did not find any evidence for the efficacy of lithium monotherapy in depressive relapse prevention in addition to its antimanic effects, it may still be worth considering as a maintenance treatment in light of its proven effectiveness in suicide prevention. Patients whose first episode is mixed tend to experience subsequent depressive relapses.Reference Tohen, Zarate and Hennen 57 Therefore, lamotrigine may also be worth considering given its established efficacy in preventing depressive relapses in the entire BD populationReference Goodwin, Bowden and Calabrese 58 and despite its having hardly been studied in the context of mixed states.
Mixed hypomania
In the author’s experience, this state places a substantial burden on outpatients. One prospective RCT suggests that quetiapine and, by analogy, other agents that show efficacy in the treatment of mixed mania could be effective. However, the evidence is too poor to venture any recommendation.
Conclusions
Most evidence for the treatment of mixed mania comes from post-hoc subgroup and pooled analyses. With this limitation, aripiprazole, asenapine, carbamazepine–ERC, olanzapine, and ziprasidone show the strongest evidence of efficacy in acute-phase treatment. Quetiapine and divalproex/valproate are also worth considering. Combination therapies with these atypical antipsychotics and mood stabilizers can be considered in severe cases. Olanzapine and quetiapine (as monotherapy and in combination with lithium/divalproex) show the strongest evidence of efficacy as maintenance treatments. Lithium and lamotrigine may be beneficial given their preventive effects on suicide and depressive relapse. To verify this synthesis, further prospective studies focusing on mixed states are encouraged.
Disclosures
Dr. Takeshima reports personal fees from Astellas, Eli Lilly, GlaxoSmithKline, Meiji Seika Pharma, Otsuka, Sumitomo Dainippon Pharma, and Yoshitomi, outside the present work.