Objective
The prevalence of sexual dysfunction is greater in patients with major depressive disorder (MDD) than in the general population,Reference Kennedy, Dickens and Eisfeld 1 with some reports estimating an incidence as high as 75%.Reference Williams and Reynolds 2 Treatment-induced phase-specific sexual dysfunction (sexual problems in ≥1 phase of the sexual response cycle, ie, desire, arousal, or orgasm) has been reported in patients without clinically significant global sexual dysfunction.Reference Clayton, Keller and McGarvey 3 In addition, treatment-related sexual dysfunction is recognized as a significant factor in treatment non-adherence and discontinuation, with approximately 25% of patients reporting adverse events (AEs) related to sexual dysfunction as one of the most common reasons for non-adherence.Reference Ashton, Jamerson and Weinstein 4
The prevalence of antidepressant-related sexual dysfunction has been shown to be similar in patients with MDD receiving selective serotonin reuptake inhibitors (SSRIs; fluoxetine, 57.7%; sertraline, 62.9%; fluvoxamine, 62.3%; paroxetine, 70.7%; citalopram, 72.7%) and the serotonin norepinephrine reuptake inhibitor (SNRI) venlafaxine (67.3%).Reference Montejo, Llorca and Izquierdo 5 A more recent short-term study estimated the incidence of treatment-emergent sexual dysfunction at 48.7% with the SSRI escitalopram and 33.3% with the SNRI duloxetine.Reference Clayton, Kornstein and Prakash 6 However, both SSRIs and SNRIs are associated with a greater incidence of sexual dysfunction compared with other antidepressant agents.Reference Montejo, Llorca and Izquierdo 5 , Reference Clayton, Pradko and Croft 7 Furthermore, there is evidence of a trend toward dose-related increases in sexual dysfunction for paroxetine, venlafaxine, sertraline, and citalopram.Reference Clayton, Pradko and Croft 7
Once-daily extended release quetiapine fumarate (quetiapine XR) is approved as adjunct therapy in the EU and the U.S. (and as monotherapy in a limited number of countries including Australia and Canada) in patients with MDD and an inadequate response to current antidepressant therapy. 8 , 9 This approval was based on results from a clinical development program in MDD, which found that quetiapine XR improved depressive symptoms in 3 out of 4 acute monotherapy studies in adults,Reference Weisler, Joyce and McGill 10 – Reference Earley, McIntyre and Wang 13 2 acute adjunct therapy studies in adults,Reference El-Khalili, Joyce and Atkinson 14 , Reference Bauer, Pretorius and Constant 15 an acute monotherapy study in elderly patients,Reference Katila, Mezhebovsky and Mulroy 16 and a maintenance therapy study in adults.Reference Liebowitz, Lam and Lepola 17 Across these studies, quetiapine XR was found to be generally well tolerated, with a safety profile consistent with the known pharmacologic profile of quetiapine.
Beyond the aforementioned detrimental impact on adherence and treatment discontinuation, antidepressant-induced sexual dysfunction is of key clinical relevance because the majority of patients with MDD consider sexual function to be important to their overall quality of life.Reference Clayton, Pradko and Croft 7 , Reference Hu, Bull and Hunkeler 18 , Reference Williams, Baldwin and Hogue 19 Therefore, the objective of this study was to investigate the effect of quetiapine XR on sexual functioning, as measured by the Changes in Sexual Functioning Questionnaire (CSFQ), in adults with MDD who participated in 6 acute (short-term) randomized, placebo-controlled studies. We report findings from pre-planned and post hoc analyses of pooled data.
Methods
The study designs and methodology for the 4 acute monotherapy studies (D1448C00001, D1448C00002, D1448C00003, and D1448C00004) and 2 acute adjunct therapy studies (D1448C00006, D1448C00007) included in our pooled analyses have been reported previously.Reference Weisler, Joyce and McGill 10 – Reference Bauer, Pretorius and Constant 15 Studies 1, 2, 3, and 6 were performed in the United States, and Studies 4 and 7 were conducted outside of the United States. Study details described here are intended as a brief summary only.
Study design
Acute monotherapy
Data were pooled from 4 multicenter, randomized, double-blind, parallel-group, placebo-controlled, phase 3 studies. All studies included a 7-to-28-day enrollment/washout period and a 2-week, post-treatment follow-up period. Active treatment periods were 6 weeks in Studies D1448C00001 and D1448C00002 (Studies 1 and 2, fixed-dose quetiapine XR 50, 150, and 300 mg/day) and 8 weeks in Studies D1448C00003 and D1448C00004 (Studies 3 and 4, modified fixed-dose quetiapine XR 150–300 mg/day).
Acute adjunct therapy
Studies D1448C00006 and D1448C00007 (Studies 6 and 7) were 6-week, multicenter, randomized, double-blind, parallel-group, placebo-controlled, phase 3 studies. Both studies consisted of a 2-week enrollment period and a 6-week active treatment period. Study D1448C00006 included a 2-week post-treatment follow-up period. Patients received adjunct quetiapine XR (fixed-dose quetiapine XR 150 and 300 mg/day) or placebo as adjunct to ongoing antidepressant treatment.
Patients
Inclusion criteria
In all 6 studies, eligible patients were male or female outpatients (aged 18–65 years), with a Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) diagnosis of MDD single episode, or MDD recurrent, as confirmed by the Mini-International Neuropsychiatric Interview (MINI) questionnaire.Reference Sheehan, Lecrubier and Sheehan 20
In the monotherapy studies, patients were required to have a Hamilton Rating Scale for Depression (HAM-D)Reference Hamilton 21 total score ≥22 and a HAM-D Item 1 (depressed mood) score ≥2 at enrollment and randomization. In the adjunct therapy studies, patients were required to have a HAM-D total score ≥20 and a HAM-D Item 1 score ≥2 at enrollment and randomization.
In the adjunct studies, patients were required to have a history during the current depressive episode of an inadequate response to ≥1 of the following antidepressants: amitriptyline, bupropion, citalopram, duloxetine, escitalopram, fluoxetine, paroxetine, sertraline, or venlafaxine. An inadequate response was defined as persistent symptoms despite receiving at least the minimum effective dose according to prescribing information for 6 weeks, including at least 1 dose increase when permitted according to the prescribing information at enrollment.
Exclusion criteria
Patients were excluded from the study if they were diagnosed with any DSM-IV Axis I disorder other than MDD within the 6 months prior to enrollment or any Axis II disorder that had a major impact on current psychiatric status, or if the current episode of depression exceeded 12 months’ duration or began <4 weeks prior to enrollment. Other exclusion criteria included a current serious suicidal or homicidal risk, a HAM-D Item 3 (suicide) score ≥3, or a suicide attempt during the 6 months prior to enrollment; or substance or alcohol abuse during the 6 months prior to enrollment.
Patients were excluded from the monotherapy studies if they had a history of inadequate response to ≥6 weeks’ treatment with at least 2 different classes of antidepressants during the current depressive episode.
Study treatments
Acute monotherapy
Patients were randomized to receive quetiapine XR 50 mg/day (Study 1 only), 150 mg/day (all 4 studies), 300 mg/day (Studies 1 and 2), or placebo (all 4 studies). Studies 2 and 4 also included an active control to determine assay sensitivity (duloxetine 60 mg/day [Study 2] and escitalopram 10/20 mg/day [Study 4]; doses as per prescribing information guidelines). In Studies 3 and 4, patients with an inadequate response to quetiapine XR 150 mg/day after 2 weeks (defined as failure to achieve ≥20% reduction in Montgomery–Åsberg Depression Rating Scale [MADRS] total score) received double the randomized dose of the investigational drug. All study treatments were administered orally, once daily in the evening.
Acute adjunct therapy
Patients were randomized to receive quetiapine XR 150 mg/day, 300 mg/day, or placebo as adjunct therapy to their ongoing antidepressant treatment (amitriptyline, bupropion, citalopram, duloxetine, escitalopram, fluoxetine, paroxetine, sertraline, or venlafaxine), which had been ongoing for ≥6 weeks at study entry. Patients were also required to stay on the same dose of antidepressant throughout the study period. All study treatments were administered orally, once daily in the evening.
Concomitant medication
The use of psychoactive medications was prohibited for the duration of the study, with the exceptions of alprazolam (1 mg), chloral hydrate (1 g), estazolam (4 mg), lorazepam (2 mg or its benzodiazepine equivalent), oxazepam (30 mg), temazepam (60 mg), zaleplon (20 mg), zolpidem tartrate (10 mg), or zopiclone (7.5 mg). Medications to treat extrapyramidal symptoms (EPS) were permitted but were not to be taken prophylactically.
Assessment of sexual dysfunction
The CSFQReference Keller, McGarvey and Clayton 22 was completed at randomization, week 4, and at the final visit in all pooled populations. Additional assessments were made at week 6 (Studies 1, 2, 6, and 7) and week 8 (Studies 3 and 4). The CSFQ is a 14-item, gender-specific questionnaire, which can be scored to examine global sexual functioning (total score) and to measure 5 different domains of sexual functioning: pleasure (Item 1), desire/frequency (Items 2 and 3), desire/interest (Items 4, 5, and 6), arousal/excitement (Items 7, 8, and 9), and orgasm/completion (Items 11, 12, and 13). Items 10 and 14 are not assigned to a subdomain. All 14 items in the CSFQ are scored on a 5-point scale, the sum of which provides a CSFQ total score which may range from 14 to 70. CSFQ total scores ≤47 (men) and ≤41 (women) are indicative of sexual dysfunction, with higher CSFQ scores associated with adequate sexual functioning.Reference Keller, McGarvey and Clayton 22
We present a pre-planned, non-inferiority analysis for change from randomization to end of treatment in CSFQ total score for patients receiving quetiapine XR versus placebo in the acute monotherapy studies (pooled Studies 1–4). To evaluate assay sensitivity and to validate the non-inferiority analysis, pre-planned analyses also assessed changes in CFSQ total and domain scores for duloxetine 60 mg/day (Study 2) and escitalopram 10/20 mg/day (Study 4).
In a post hoc analysis, change in CSFQ total score by gender was also analyzed for the acute monotherapy studies. In addition, we present post hoc analyses for change in CSFQ total and domain scores for the fixed-dose monotherapy studies (pooled Studies 1 and 2), modified fixed-dose studies (pooled Studies 3 and 4), and adjunct therapy studies (pooled Studies 6 and 7). CSFQ total and domain scores from the quetiapine XR 50 mg/day arm in Study 1 were also assessed. Other outcomes included change from randomization in individual CSFQ domains, which were analyzed by gender (post hoc). Additional post hoc analyses of change from randomization in CSFQ total score (all patients and by gender) were performed for MADRS responders (≥50% reduction from randomization in MADRS total score) and nonresponders, and for MADRS remitters (MADRS ≤10) and nonremitters. The remission cut-off criterion of MADRS ≤8 was predefined in the study protocol; however, as MADRS ≤10 is more commonly used in the literature, this has been reported here. In addition, post hoc evaluation of the percentage of patients with sexual dysfunction (CSFQ total score ≤47 for men and ≤41 for womenReference Keller, McGarvey and Clayton 22 ) at randomization and end of study was made.
Incidence and severity of AEs (and withdrawals due to AEs) potentially related to sexual dysfunction were recorded throughout the studies.
Statistical analyses
As part of a predefined pooling strategy, which was submitted prior to determination of the primary data analysis, all monotherapy studies (Studies 1, 2, 3, and 4) were pooled for the pre-planned, non-inferiority analysis of change from randomization to end of treatment in CSFQ total score (all patients). Least squares mean (LSM) changes in CSFQ total score were assessed using an analysis of covariance (ANCOVA) model and last observation carried forward (LOCF) approach for imputation of missing data. If post-baseline data were not available, baseline data were not carried forward into the randomized treatment period. Non-inferiority for quetiapine XR versus placebo was to be concluded if the lower limit of the 95% confidence interval (CI) for the estimated difference between quetiapine XR and placebo exceeded −0.75, a change that is considered to not be clinically relevant, where a negative value indicates worse sexual functioning for quetiapine XR versus placebo.
LSM changes from randomization in CSFQ total and domain scores for duloxetine 60 mg/day (Study 2; week 6), and escitalopram 10/20 mg/day (Study 4; week 8) were analyzed using an ANCOVA model and LOCF approach. In this model, CSFQ total score at randomization was used as the covariate, treatment and gender were used as fixed effects, and center was used as a random effect.
For all other post hoc assessments, studies were pooled as follows: fixed-dose monotherapy studies (Studies 1 and 2), modified fixed-dose monotherapy studies (Studies 3 and 4), and adjunct therapy studies (Studies 6 and 7). Quetiapine XR data were analyzed by pooling data from all quetiapine XR arms. All pooled data analyses were performed using the safety populations, defined as patients who had received ≥1 dose of study drug. In addition, data from the quetiapine XR 50 mg/day treatment group of Study 1 were assessed. LSM changes were also analyzed for all post hoc assessments using an ANCOVA model. For analyses of all patients in pooled datasets, the ANCOVA model included treatment, gender, and study as fixed effects, with center as a random effect nested within the study, and score at randomization as a covariate. For gender-specific analyses of pooled datasets, the ANCOVA model included treatment and study as fixed effects, center as a random effect nested within the study, and score at randomization as a covariate.
A nominal value of p < 0.05 was used as evidence of a difference given the post hoc nature of the analyses. Where appropriate, 95% CIs and nominal p-values are reported.
Results
Patient population
Acute monotherapy
In total, 1797 patients were included in the safety population (all quetiapine XR [50, 150, 300 mg/day], n = 1149; placebo, n = 648) across all 4 monotherapy studies. The pooled safety population for Studies 1 and 2 was 1178 (all quetiapine XR, n = 840; placebo, n = 338), and for Studies 3 and 4 it was 619 (all quetiapine XR, n = 309; placebo, n = 310). The demographic and clinical characteristics of the treatment groups were generally well matched and have been reported previously.Reference Weisler, Joyce and McGill 10 – Reference Earley, McIntyre and Wang 13
Acute adjunct therapy
The pooled safety population for Studies 6 and 7 included 936 patients (all quetiapine XR [150, 300 mg/day], n = 627; placebo, n = 309). The demographic and clinical characteristics of the treatment groups were generally well balanced and have been reported previously.Reference El-Khalili, Joyce and Atkinson 14 , Reference Bauer, Pretorius and Constant 15
Pre-planned, non-inferiority analysis
CSFQ total score: acute monotherapy studies (Studies 1–4)
In the acute monotherapy studies (pooled analysis of Studies 1, 2, 3, and 4), LSM change from randomization to end of treatment in CSFQ total score was 1.90 with quetiapine XR and 1.73 with placebo. The LSM difference (95% CI) in change from randomization in CSFQ total score with quetiapine XR versus placebo was 0.16 (95% CI: −0.59, 0.92), and non-inferiority was demonstrated.
Post hoc analyses and pre-planned analysis of active comparators
CSFQ total score: fixed-dose acute monotherapy studies (Studies 1 and 2)
In the pooled analysis of Studies 1 and 2, LSM changes from randomization to end of treatment in CSFQ total score with quetiapine XR 150, 300 mg/day, and placebo were 1.35, 1.81, and 2.08, respectively. Improvements from randomization were greatest in the placebo group, and LSM differences (95% CI) versus placebo were comparable for quetiapine XR 150 mg/day (−0.74 [−1.84, 0.36]; p = 0.189) and quetiapine XR 300 mg/day (−0.27 [−1.38, 0.84]; p = 0.634). Table 1 shows the LSM difference (95% CI) in change from randomization in CSFQ total score versus placebo for both genders.
Table 1 LSM difference versus placebo (95% CI) in change from randomization to end of treatment in CSFQ total score

a Modified fixed-dose (150/300 mg/day).
DUL: duloxetine 60 mg/day (Study 2 only); ESC: escitalopram 10/20 mg/day (Study 4 only).
Additionally in Study 1, which also included a quetiapine XR 50 mg/day arm, LSM change in CSFQ total score from randomization to end of treatment was 1.66 (n = 160) for quetiapine XR 50 mg/day compared with 2.58 for placebo (n = 155); LSM difference (95% CI) for quetiapine XR 50 mg/day versus placebo was similar at −0.92 (−2.48, 0.64) (p = 0.247).
In Study 2, LSM change in CSFQ total score from randomization to end of treatment for the active comparator duloxetine was 1.74 (n = 127) compared with 1.56 for placebo (n = 137); LSM difference (95% CI) for duloxetine versus placebo was comparable at 0.18 (−1.40, 1.75) (p = 0.823).
The LSM differences (95% CI) in change from randomization in CSFQ total score and domain scores for quetiapine XR versus placebo are shown by gender for the pooled analysis of Studies 1 and 2 in Figure 1A; no difference versus placebo was seen with quetiapine XR for any of the domain scores for either gender.

Figure 1 LSM difference (95% CI) in change from randomization to treatment end in CSFQ total score and domain scores versus placebo (LOCF, safety population); pooled analysis of (A) Studies 1 and 2; (B) Studies 3 and 4; and (C) Studies 6 and 7.
CSFQ total score: modified fixed-dose acute monotherapy studies (Studies 3 and 4)
In the pooled analysis of Studies 3 and 4, LSM change from randomization in CSFQ total score to the end of treatment was 2.96 with quetiapine XR (modified fixed-dose: 150/300 mg/day) and 1.77 with placebo. Improvements in sexual functioning were comparable for quetiapine XR–treated patients compared with placebo; LSM difference between the groups (95% CI) was similar at 1.18 (−0.11, 2.48) (p = 0.074) (Table 1).
In Study 4, which contained the active comparator escitalopram, LSM change in CSFQ total score from randomization to end of treatment was 2.00 with escitalopram (n = 145) and 1.84 with placebo (n = 143); LSM difference (95% CI) for escitalopram versus placebo was comparable at 0.16 (−1.77, 2.10) (p = 0.870).
Figure 1B shows LSM difference in change in CSFQ total score and domain scores by gender for quetiapine XR versus placebo from the pooled analysis of Studies 3 and 4. Males achieved a greater improvement in the CSFQ “pleasure” domain (p < 0.05) with quetiapine XR, and in the “desire/interest” and “arousal/excitement” domains with escitalopram (Study 4 only).
CSFQ total score: acute adjunct therapy studies (Studies 6 and 7)
In the pooled analysis of Studies 6 and 7, LSM changes from randomization to end of treatment in CSFQ total score for quetiapine XR 150, 300 mg/day, and placebo were 2.15, 1.86, and 1.63, respectively. LSM differences (95% CI) versus placebo were of a similar magnitude for quetiapine XR 150 mg/day (0.52 [−0.54, 1.58]; p = 0.338) and 300 mg/day (0.22 [−0.84, 1.28]; p = 0.679) (Table 1).
LSM differences (95% CI) in change in CSFQ total scores and domain scores versus placebo by gender are shown in Figure 1C. Females treated with quetiapine XR 150 mg/day showed improvement versus placebo in 2 out of 5 CSFQ domains (“pleasure” and “desire/interest”; p < 0.05, each).
MADRS responder and remitter analysis: fixed-dose acute monotherapy studies (Studies 1 and 2)
In the pooled analysis of Studies 1 and 2, no differences in LSM changes from randomization in CSFQ total score versus placebo were observed in either MADRS responders (≥50% reduction in MADRS total score: quetiapine XR 150 mg/day, −1.72 [p = 0.051]; 300 mg/day, −1.33 [p = 0.137]) or nonresponders (quetiapine XR 150 mg/day, −1.28 [p = 0.063]; 300 mg/day, −0.57 [p = 0.406]), remitters (MADRS total score ≤10: quetiapine XR 150 mg/day, −1.51 [p = 0.146]; 300 mg/day, −1.48 [p = 0.151]) or non-remitters (quetiapine XR 150 mg/day, −0.91 [p = 0.141]; 300 mg/day, −0.38 [p = 0.553]). No additional post hoc analyses were performed to directly compare responders versus nonresponders and remitters versus nonremitters.
In Study 1, LSM differences in change from randomization to end of treatment in CSFQ total score for quetiapine XR 50 mg/day versus placebo were −1.48 (p = 0.277) for responders and −1.00 (p = 0.253) for nonresponders, and −0.81 (p = 0.605) for remitters and −1.26 (p = 0.139) for nonremitters. In Study 2, LSM differences versus placebo for duloxetine were −0.08 (p = 0.949) for responders versus −0.60 (p = 0.560) for nonresponders, and −0.60 (p = 0.668) for remitters versus −0.10 (p = 0.915) for nonremitters, respectively.
Pooled analysis LSM differences (95% CI) in change from randomization in CSFQ total score for quetiapine XR versus placebo in MADRS-defined responders and nonresponders are shown by gender in Figure 2A and for remitters and nonremitters in Figure 3A.

Figure 2 MADRS responders and nonresponders, LSM difference (95% CI) in change from randomization to treatment end in CSFQ total score versus placebo (safety population); pooled analysis of (A) Studies 1 and 2; (B) Studies 3 and 4; and (C) Studies 6 and 7.

Figure 3 MADRS remitters and nonremitters, LSM difference (95% CI) in change in CSFQ total score from randomization at treatment end versus placebo (safety population); pooled analysis of (A) Studies 1 and 2; (B) Studies 3 and 4; and (C) Studies 6 and 7.
MADRS responder and remitter analysis: modified fixed-dose acute monotherapy studies (Studies 3 and 4)
In the pooled analysis of Studies 3 and 4, LSM differences versus placebo in change from randomization in CSFQ total score with quetiapine XR 150/300 mg/day were 0.84 (p = 0.364) for responders and 0.61 (p = 0.451) for nonresponders, and 0.73 (p = 0.508) in remitters and 0.94 (p = 0.194) in nonremitters. No additional post hoc analyses were performed to directly compare responders versus nonresponders and remitters versus nonremitters.
In Study 4, LSM differences versus placebo in change from randomization in CSFQ total score with escitalopram were −0.24 (p = 0.865) in responders versus −0.16 (p = 0.890) in nonresponders, and −0.03 (p = 0.985) in remitters versus −0.46 (p = 0.661) in nonremitters.
LSM differences (95% CI) for quetiapine XR versus placebo in change from randomization in CSFQ total score in responders and nonresponders from the pooled analysis of Studies 3 and 4 are shown by gender in Figure 2B. Data for remitters and nonremitters are shown by gender in Figure 3B.
MADRS responder and remitter analysis: acute adjunct therapy studies (Studies 6 and 7)
In the pooled analysis of Studies 6 and 7, no differences in LSM change from randomization in CSFQ total score versus placebo were observed with quetiapine XR 150 and 300 mg/day in MADRS-defined responders (quetiapine XR 150 mg/day, −0.08 [p = 0.915]; 300 mg/day, −0.48 [p = 0.533]) or nonresponders (quetiapine XR 150 mg/day, 0.68 [p = 0.327]; 300 mg/day, 0.16 [p = 0.818]). LSM change from randomization in CSFQ total score with quetiapine XR 150 and 300 mg/day versus placebo was 3.46 and 3.32 versus 3.86 for remitters and 1.21 and 0.42 versus 0.58 for nonremitters. LSM difference in change from randomization in CSFQ total score versus placebo was similar for quetiapine XR 150 mg/day (remitters, −0.40 [p = 0.674]; nonremitters, 0.63 [p = 0.314]) and 300 mg/day (remitters, −0.54 [p = 0.559]; nonremitters, −0.17 [p = 0.792]). Although no formal statistical testing was applied, numerically greater improvements in CSFQ total scores appeared to be achieved in MADRS responders versus nonresponders and in remitters versus nonremitters.
LSM difference (95% CI) in change from randomization in CSFQ total score for quetiapine XR versus placebo in responders and nonresponders are shown by gender in Figure 2C. Male responders experienced worsening of overall sexual functioning in the quetiapine XR 150 mg/day group versus placebo (LSM difference in change from randomization in CSFQ total score: −2.62, p = 0.045). Data for remitters and nonremitters are shown by gender in Figure 3C.
CSFQ-defined sexual dysfunction
The proportions of patients with sexual dysfunction (CSFQ total score ≤47 in males and ≤41 in females) at randomization and at the end of treatment were similar in the quetiapine XR and placebo cohorts: 54.5%, 51.0%, and 53.8%, with quetiapine XR 150, 300 mg/day, and placebo, respectively, in the pooled analysis of Studies 1 and 2. In the pooled analysis of Studies 3 and 4, the percentages of patients with sexual dysfunction at randomization and at the end of treatment were 57.5% with quetiapine XR 150/300 mg/day and 62.9% with placebo. In the pooled adjunct therapy dataset (Studies 6 and 7), 60.1%, 65.4%, and 63.1% of patients had sexual dysfunction in the quetiapine XR 150, 300 mg/day, and placebo cohorts, respectively.
In Study 1, the proportion of patients with sexual dysfunction at randomization and at the end of treatment was 56.3% with quetiapine XR 50 mg/day versus 51.0% with placebo.
The percentage of patients with sexual dysfunction at randomization and at the end of treatment was 58.3% with duloxetine (Study 2) versus 56.9% with placebo, and 73.1% with escitalopram (Study 4) versus 67.1% with placebo.
The percentages of patients who had sexual dysfunction at the end of treatment are shown by gender in Table 2.
Table 2 Percentage of patients with sexual dysfunction (CSFQ total score ≤47 in males and ≤41 in females) at randomization and at the end of treatment
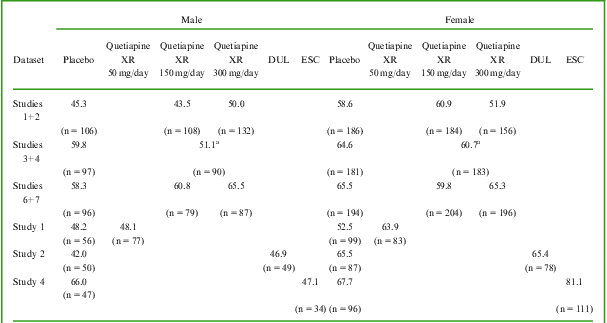
a Modified fixed-dose (150/300 mg/day).
DUL: duloxetine 60 mg/day (Study 2 only); ESC: escitalopram 10/20 mg/day (Study 4 only).
AEs potentially associated with sexual dysfunction
The incidence of AEs potentially related to sexual dysfunction (Medical Dictionary for Regulatory Activities [MedDRA] preferred terms: anorgasmia, dyspareunia, ejaculation delayed, erectile dysfunction, libido decreased, loss of libido, orgasm abnormal, vulvovaginal dryness, libido increased, sexual dysfunction, retrograde ejaculation, ejaculation failure) is shown for the pooled safety population for the acute monotherapy studies and acute adjunct therapy studies in Table 3. All AEs potentially associated with sexual dysfunction were mild to moderate in intensity, with the majority being mild.
Table 3 Number (%) of AEs potentially related to sexual dysfunction in the acute monotherapy studies and acute adjunct therapy studies

a Study 1: AEs (%) with placebo (n = 181): libido decreased (0.6%).
b Study 2: AEs (%) with placebo (n = 157): erectile dysfunction (0.6%), libido decreased (0.6%).
c Study 4: AEs (%) with placebo (n = 155): erectile dysfunction (0.6%), libido decreased (0.6%), loss of libido (0.6%).
DUL: duloxetine 60 mg/day (Study 2 only); ESC: escitalopram 10/20 mg/day (Study 4 only).
There were no deaths or serious AEs potentially related to sexual dysfunction in any of the 6 studies. In the pooled acute monotherapy studies, there was 1 discontinuation in the quetiapine XR 150 mg/day group due to an AE of erectile dysfunction. In the pooled acute adjunct therapy studies, no discontinuations were reported due to AEs potentially associated with sexual dysfunction.
Discussion
Patients with MDD are underserved by treatment options that focus on improvement in core symptoms of mood and interest but that fail to improve or may even exacerbate sexual dysfunction.Reference Kennedy 23 Sexual dysfunction is particularly problematic for patients with MDD, as it can have a detrimental effect on quality of life and likelihood of adherence to treatment. Therefore, treatments that offer improvement, or no worsening, in sexual functioning, together with at least equivalent efficacy and overall tolerability, may represent a valuable treatment option for some patients. Indeed, it has been reported that avoidance of side effects influences psychiatrists’ choice of therapy on almost 50% of occasions, and sexual dysfunction has been rated as the side effect that has the greatest impact on antidepressant selection.Reference Zimmerman, Posternak and Friedman 24 Reliance on spontaneous reporting of AEs potentially related to sexual dysfunction underestimates the incidence of sexual problems compared with assessments that actively solicit specific information from patients.Reference Clayton, Pradko and Croft 7 , Reference Montejo-Gonzalez, Llorca and Izquierdo 25 In this study, completion of a validated instrument assessing sexual function (CSFQ) by all study subjects may have impacted on the spontaneous reports of sexual dysfunction, leading to the low rates described here. Nonetheless, the CSFQReference Keller, McGarvey and Clayton 22 provides a robust patient-reported method of measuring sexual functioning and is a useful tool to assess sexual dysfunction in MDD.
The pre-planned analysis of data from the acute monotherapy studies demonstrated the non-inferiority of quetiapine XR compared with placebo in terms of CSFQ-measured sexual functioning. Quetiapine XR is approved as adjunct therapy, but not as monotherapy, in the EU and the U.S. in patients with MDD and an inadequate response to current antidepressant therapy. 8 , 9
Previous studies have reported an increase from baseline in overall sexual dysfunction associated with MDD with duloxetine (33%) and escitalopram (44%) compared with placebo (25%) when using CSFQ total score.Reference Clayton, Kornstein and Prakash 6 However, in this study, no significant overall difference in CSFQ total score versus placebo was shown with duloxetine or escitalopram. Overall, comparable differences in CSFQ data versus placebo were shown with the active comparators duloxetine (Study 2) and escitalopram (Study 4), although predefined non-inferiority margins were not determined for these agents.
The uneven distribution of patients limited the ability of this analysis to discern differences between the 2 smallest arms versus placebo: duloxetine (n = 149) and escitalopram (n = 156). However, the number of quetiapine XR patients (n = 1020) was sufficient to show non-inferiority versus placebo (n = 576).
Increased prolactin through suppression of dopamine neurotransmission is a known factor associated with sexual dysfunction. Typical antipsychotics and risperidone are known to increase serum prolactin levels, while treatment with olanzapine, clozapine, quetiapine, aripiprazole, and ziprasidone does not increase serum prolactin, potentially benefiting sexual functioning.Reference Maguire 26 In a study evaluating sexual functioning in schizophrenia, quetiapine was shown to normalize prolactin levels at endpoint whereas risperidone and fluphenazine did not, and quetiapine demonstrated the greatest benefits in terms of sexual functioning.Reference Kelly and Conley 27 Aripiprazole, which has also been shown not to increase prolactin levels, has previously been reported to have some beneficial effects on sexual functioning in patients with MDD.Reference Fava, Dording and Baker 28
It has been reported that SSRI-related sexual dysfunction confined to one or more phases of the sexual response cycle is very common, even if patients do not experience a clinically relevant decrease in sexual function (as measured by the CSFQ total score).Reference Clayton, Keller and McGarvey 3
Furthermore, gender-related differences in the likelihood of experiencing dysfunction in the different phases/CSFQ domains have been reported in patients with MDD.Reference Clayton, Keller and McGarvey 3 In the post hoc analysis reported here with quetiapine XR adjunct therapy, there was a tendency toward a greater improvement in sexual functioning in females compared with males. Moreover, post hoc analyses of changes in individual CSFQ domain scores identified modest differences between male and female patients. Improvements, compared with placebo, were recorded with quetiapine XR in the majority of domains, but for many, the magnitude of score change varied considerably between males and females. In patients who received quetiapine XR, there were improvements compared with placebo in the CSFQ domains “pleasure” (male, monotherapy), and “pleasure” and “desire/interest” (female, adjunct therapy). These results are relatively surprising, as it can be more difficult to demonstrate differences between groups with single-item domains. One potential explanation may be that through the 6- to 8-week active treatment period, quetiapine XR had few negative effects on sexual functioning, so as the depressive symptoms improved, pleasure and desire/interest in general (and specifically sexual pleasure) improved, accounting for the improvement observed in both genders in this domain and in “desire/interest” in women. This appears to remain true in the long-term in other studies; in a 52-week double-blind study of duloxetine, the presence of sexual dysfunction was seen to be more related to the status of patients’ depression than to the treatment received.Reference Montejo, Perahia and Spann 29
The burden of sexual dysfunction among patients receiving SSRI or SNRI antidepressant medication has been well reported.Reference Clayton, Pradko and Croft 7 , Reference Kennedy, Eisfeld and Dickens 30 – Reference Segraves 32 Previously, orgasmic dysfunction has been shown with SSRIs in several studiesReference Clayton, Keller and McGarvey 3 , Reference Clayton, Croft and Horrigan 33 and has recently been reported with escitalopram in female patients with MDD.Reference Weisler, Joyce and McGill 10 Here, the post hoc analyses suggest a trend toward a negative impact of escitalopram on the CSFQ domain “orgasm/completion” in females; this is not shown with quetiapine XR as adjunct therapy to ongoing antidepressants. In contrast, in Study 2 and Study 4, duloxetine and escitalopram, respectively, appear to have had negligible effects on most CSFQ domain scores, with the exception of an improvement in “desire/interest” with escitalopram versus placebo in male patients. It is possible that the analyses were underpowered to show any differences for escitalopram and duloxetine versus placebo, as evidenced by the small patient numbers and wide CIs for escitalopram in male patients with MDD. Furthermore, although LOCF is an approach commonly used in pharmaceutical trials and its use here was pre-specified before the study results were known, it is possible that it may have introduced a potential bias toward the null hypothesis.
The post hoc all-patient analyses of each pooled dataset appeared to identify a greater magnitude of improvement in CSFQ total score among MADRS-defined responders compared with nonresponders. Similar observations were made between MADRS-defined remitters and nonremitters, although no additional post hoc analyses were performed. These findings suggest that improvement in sexual functioning in patients with MDD is influenced by achieving improvement in depressive symptoms. The only gender-specific difference identified by the post hoc analysis was in male responders receiving quetiapine XR 150 mg/day as adjunct therapy who showed an improvement in CSFQ total score versus placebo (p = 0.045). Clearly, not all patients achieving improvement in their depressive symptoms achieved an improvement in their sexual functioning. Improvement in depressive symptoms alone may be insufficient to improve sexual functioning in some patients whose sexual problems arise from a cause other than the effects of major depression, such as primary sexual disorder, or a medical or psychiatric condition that impacts sexual function. Improvement in sexual functioning may also not occur if residual symptoms of depression (including sexual dysfunction) remain/remission of depressive symptoms is not achieved. Furthermore, patients may also possess specific genetic risk factors for antidepressant-associated sexual dysfunction.Reference Perlis, Laje and Smoller 34 , Reference Bishop, Moline and Ellingrod 35 Evaluating the temporal relationship between onset and resolution of depressive symptoms compared with onset and reduction in sexual dysfunction may delineate which factor(s) are impacting sexual function in a specific patient. Only by identifying the underlying cause of a sexual problem can physicians start to consider an appropriate and effective treatment strategy.
Although similar for quetiapine XR and placebo with no gender-specific differences identified, the proportion of patients with sexual dysfunction both at randomization and at the end of treatment was approximately 50% across all quetiapine XR treatment groups. Nonetheless, the findings suggest that acute quetiapine XR (50–300 mg/day) treatment (as monotherapy or adjunct therapy) is not associated with negative effects on sexual functioning. Moreover, our findings compare quetiapine XR favorably with previous studies with alternative agents (e.g., venlafaxine) that report a relatively high incidence of treatment-emergent sexual dysfunction (43%) with these agents.Reference Clayton, Kornstein and Prakash 6
As the sample analyzed here included only patients with MDD, a disease state known to impair sexual function, these data should not be extrapolated to the use of quetiapine XR in other populations. Furthermore, the short duration of the studies included in these analyses (active treatment period ≤8 weeks) also limits the interpretation of our findings to acute therapy. However, the AE profiles reported here are consistent with the known safety profile of quetiapine. 8 A long-term study of quetiapine XR monotherapy as maintenance treatment for MDD was not included in these analyses, as CSFQ total score was not assessed; AEs related to sexual dysfunction during the randomized phase were slightly higher for the quetiapine XR group (1.5%) compared with the placebo group (0.5%).Reference Liebowitz, Lam and Lepola 17
As reported previously for the individual studies, discontinuation rates were higher for quetiapine XR compared with placebo, and this could be attributed to the greater rates of withdrawal due to AEs in the quetiapine XR groups across each of the studies.Reference Weisler, Joyce and McGill 10 – Reference Bauer, Pretorius and Constant 15 Therefore, one possible limitation of the non-inferiority analysis reported here is the potential for bias towards a finding of no difference through the use of the LOCF method to account for missing data.
In considering the findings reported here, it should be noted that only the following analyses were pre-planned: the non-inferiority analysis of quetiapine XR versus placebo for change from randomization to end of treatment in CSFQ total score for Studies 1–4, and the active comparator analyses used to demonstrate assay specificity. All other analyses were conducted post hoc, and should therefore be interpreted with caution and considered as a limitation of this study.
Treatment acceptability (dropout rate) was recently used as a primary outcome in a meta-analysis to compare and rank antidepressant medications.Reference Cipriani, Furukawa and Salanti 36 Sexual dysfunction is a component of treatment acceptability, and future analyses that assess the balance between efficacy and acceptability of quetiapine XR in relation to other antidepressant agents could prove useful for prescribing physicians making decisions regarding the treatment of MDD.
Conclusion
These pooled analyses show that short-term treatment with quetiapine XR monotherapy was non-inferior to placebo in terms of impact on sexual functioning in patients with MDD. Furthermore, quetiapine XR as adjunct therapy to antidepressants resulted in numerical improvements in sexual function compared with placebo.
Acknowledgments
The authors would like to thank Gail Gilmour, PhD, from Complete Medical Communications, who provided medical writing support, funded by AstraZeneca. The following investigators were involved in these studies:
MDD1: Mohammed Alam (Oak Brook, Illinois), Grant Belnap (Eagle, Idaho), Guy Brannon (Shreveport, Louisiana), John Carman (Smyrna, Georgia), Harry Croft (San Antonio, Texas), Himasiri DeSilva (Santa Ana, California), Bradley Diner (Little Rock, Arkansas), Michael Downing (Dallas, Texas), Neil Dubin (Cincinnati, Ohio), Beal Essink (Portland, Oregon), Donald Garcia (Austin, Texas), Steven Glass (Clementon, New Jersey), Susanna Goldstein (New York, New York), Daniel Grosz (Encino, California), Mark Joyce (Jacksonville, Florida), Arifulla Khan (Bellevue, Washington), Irving Kolin (Winter Park, Florida), Jelena Kunovac (Oceanside, California), Sidney Lerfald (Charleston, West Virginia), Michael Levy (Staten Island, New York), Arnold Licht (Brooklyn, New York), Adam Lowy (Washington, DC), Azfar Malik (St Louis, Missouri), Laura McGill (Memphis, Tennessee), Leslie Moldauer (Denver, Colorado), Mahmoud Okasha (Norwich, Connecticut), Nader Oskooilar (Newport Beach, California), Angela Pinhiero (Okemos, Michigan), Robert Riesenberg (Atlanta, Georgia), Leon Rosenberg (Cherry Hill, New Jersey), Dwight St. Clair (Wichita, Kansas), Jeffrey Simon (Brown Deer, Wisconsin), Ward Smith (Portland, Oregon), Jerry Steiert (Seattle, Washington), Richard Weisler (Raleigh, North Carolina), Nicholas Vatakis (New York, New York), Cherian Verghese (Norristown, Pennsylvania), Inna Yuryev-Golger (Brooklyn, New York).
MDD2: Fares Arguello (Salt Lake City, Utah), Jason Baron (Houston, Texas), Louise Beckett (Oklahoma City, Oklahoma), Robert Bielski (Okemos, Missouri), Lynn Cunningham (Edwardsville, Illinois), Andrew Cutler (Bradenton, Florida), Michael Dempsey (Albuquerque, New Mexico), Bernadette D'Souza (Dayton, Ohio), Steven Eisen (Philadelphia, Pennsylvania), Naresh Emmanuel (Columbia, South Carolina), David Feifel (San Diego, California), Thomas Gazda (Scottsdale, Arizona), John Gilliam (Richmond, Virginia), Lawrence Ginsberg (Houston, Texas), Linda Harper (Orlando, Florida), Willis Holloway (Oklahoma City, Oklahoma), Alexander Horwitz (Salem, Oregon), Alan Jones (Baltimore, Maryland), Mark Joyce (Jacksonville, Florida), James Knuston (South Kirkland, Washington), Rolando Larice (St. Louis, Missouri), Robert Levine (New York, New York), Harry Logue (Birmingham, Alabama), Lora McGill (Memphis, Tennessee), Douglas McLaughlin (Mayfield Village, Ohio), Jeffrey Mattes (Princeton, New Jersey), Charles Meredith (San Diego, California), Janice Miller (West Palm Beach, Florida), Eliot Moon (Wildomar, California), Dennis Munjack (Beverly Hills, California), Elizabeth Reeve (St. Paul, Minnesota), Fredrick Reimherr (Salt Lake City, Utah), Marc Shay (Haverhill, Massachusetts), Thomas Shiovitz (Sherman Oaks, California), Mary Stedman (Tampa, Florida), James Stevenson (Morgantown, West Virginia), Abbey Strauss (Boca Raton, Florida), Roger Vogelfanger (Pasadena, Tennessee), Kashinath Yadalam (Lake Charles, Los Angeles).
MDD3: David Adson (Minneapolis, Minnesota), Diab Almhana (Avon Lake, Ohio), Amit Anand (Indianapolis, Indiana), Roy Autry (Atlanta, Georgia), Michael Banov (Roswell, Georgia), Brian Bortnick (Atlanta, Georgia), Edward Cherlin (El Centro, California), Lisa Cohen (New Port Richey, Florida), Nizar El-Khalili (Lafayette, Indiana), John Goethe (Hartford, Connecticut), George Konis (Little Rock, Arkansas), Paul Gross (Allentown, Pennsylvania), William Jeter (DeLand, Florida), Ali Kashfi (Alamonte Springs, Florida), Zerrin Kayatekin (Pittsfield, Massachusetts), Gustavo Kinrys (Cambridge, Massachusetts), Paul Markovitz (Fresno, California), Charles Morin (Braintree, Massachusetts), Amy Mulroy (San Antonio, Texas), Erik Nelson (Cincinnati, Ohio), John Prater (Ft. Myers, Florida), Veronique Sebastian (Oklahoma City, Oklahoma), Andrew Sedillo (Owensboro, Kentucky), Rajinder Shiwach (Desoto, Texas), John Downs (Memphis, Tennessee), Jeffrey Danziger (Maitland, Florida), Andre Burton (Anaheim, California), Carlos Guerra (Houston, Texas), Delquis Mendoza (Decatur, Georgia), Mohammed Bari (National City, California), Robert Dahmes (New Orleans, Lousiana), Jerold Kreisman (St. Louis, Missouri), Barbara Harris (Phoenix, Arizona), Mark DiBuono (Staten Island, New York), David Walling (Garden Grove, California).
MDD4: Richard Bergeron, Paul Latimer, Claire O'Donovan, Paul Lesperance, Sidney Kennedy, Shaila Misri, Alexander McIntyre, Satpal Girgla, Jean-Guy Gagnon, Autar Munshi, Smadar Tourjman, Andree Daigneault, Nizar Ladha, Pierre Blier (Canada); Niufan Gu, Gang Wang, Jian Hu, Shiping Xie, Xiaoping Wang (China); Grigori Joffe, Liisa Lahdelma, Jussi Turtonen, Sanna Blanco Sequeiros, Tarja Ruotsalainen, Olli Piirtola, Marko Sorvaniemi, Markku Timonen (Finland); Won-Myong Bahk, Min-Soo Lee, Se Joo Kim, Young Chul Shin (Korea); Ahmad Sulaiman, Benjamin Chan, Suarn Singh (Malaysia); Vasavan Agambaram, Shlomo Brook, Yao Mfodwo, Karen Vukovic, Marius Steyn (South Africa); Celso Iglesias García, Raúl Vázquez-Noguerol, Manuel Franco Martín (Spain); Efren Reyes, Constantine Della, Jacqueline Sy, Agnes Padilla, Ruby Manalastas (Philippines); Isaac De la Parra, Sergio Villaseñor, Susana Garcia, Rogelio Apiquian, Ricardo Secin, Felipe Ortega, Juan Bautista Corral (Mexico).
MDD6: Amit Anand (Indianapolis, Indiana), Sarah Atkinson (Rochester, New York), Michael Banov (Roswell, Georgia), Benny Barnhart (Wichita Falls, Texas), Brian Bortnick (Atlanta, Georgia), Ronald Brenner (Cedarhurst, New York), Edward Cherlin (El Centro, California), Adnan Dahdul (Springfield, Massachusetts), Nizar El-Khalili (Lafayette, Indiana), Prakash Ettigi (Richmond, Virginia), Miguel Flores (Hialeah, Florida), Richard Jackson (Royal Oaks, Michigan), Elias Sarkis (Gainsville, Florida), Anita Kablinger (Shreveport, Louisiana), James Kocsis (New York, New York), Jelena Kunovac (Oceanside, California), Charles Morin (Braintree, Massachusetts), Amy Mulroy (San Antonio, Texas), Veronique Sebastian (Oklahoma City, Oklahoma), Ismail Sendi (Clinton, Michigan), Phebe Tucker (Oklahoma City, Oklahoma), Robert Buynak (Valparaiso, Indiana), Carlos Danger (Miami, Florida), Kettlie Daniels (Toledo, Ohio), Robert Earle (Friendswood, Texas), Sanjay Gupta (Olean, New York), Gregory Mattingly (St. Charles, Missouri), Haydn Thomas (Prairie Village, Kansas), Ethan Kass (Coral Springs, Florida), James Whalen (Lincoln, Rhode Island), Jeffrey Ross (Hoffman Estates, Illinois), Jerold Kreisman (St. Louis, Missouri), Barbara Harris (Phoenix, Arizona), Joseph Ripperger (Norman, Oklahoma), Michael Levy (Staten Island, New York), Lora McGill (Memphis, Tennessee), Mark Joyce (Jacksonville, Florida), Charles Bailey (Orlando, Florida), Charles Meredith (San Diego, California), Ramanath Gopalan (Arlington, Virginia), Riaz Baber (Naperville, Illinois), Bijan Bastani (Beechwood, Ohio), Elly Lee (Irvine, California), Robert Hudrick (Cherry Hill, New Jersey), Deborah Bergen (Wichita, Kansas), Susanna Goldstein (New York, New York), Robert Riesenberg (Atlanta, Georgia), Fares Arguello (Salt Lake City, Utah), David Krefetz (Clementon, New Jersey), Steven Eisen (Philadelphia, Pennsylvania), Mohammed Alam (Oak Brook, Illinois), Arifulla Khan (Bellevue, Washington).
MDD7: Kurt Audenaert, William Pitchot, Joseph Lejeune, Serge Seghers, Stefaan Geerts, Antonio Gazziano, Bart Leroy, Firmin Janssen (Belgium); Dennis O'Keefe, Brian Ramjattan, John Li, Jeannette Janzen, Allan Kelly, Howard Conter, Lew Pliamm, Thomas George, Michael Theodoros, Nevin Taylor, Jayashri Kulkarni, Peter Farnbach, Isaac Schweitzer, Richard Newton, Bernhard Baune (Canada); Marketa Zemanova, Tibor Miklos, Yvona Hendrychova, Alena Railova, Michaela Klabusayova, Ivan Drabek, Libor Chvila, Marek Perez, Silvia Musilova, Marcela Bolkova Janikova, Zdenek Solle (Czech Republic); Antti Ahokas, Anna Savela, Raili Kansanen, Riitta Jokinen, Jukka Penttinen, Juhani Aer (Finland); Jean-Pierre Olie, Joël Gailledreau, Eric Neuman, Marcel Zins-Ritter, Frédéric Chapelle, Christian Gaussares, Mocrane Abbar, Christian Geraud, Francis Gheysen, Paule Khalifa, Nicole Parant, Jean Audet, Bertrand Baranovsky, Bernard Vanier (France); Matthias Rothermundt, Albert Diefenbacher, Hans Gutzmann, Volker Schumann, Niels Bergemann, Thomas Messer, Franz-Markus Leweke, Günther Schumann, Eugen Schlegel (Germany); Espen Anker, Andrea Berz, Dag Oulie, Neh Geok Lim, Magne Hompland, Kjetil Høye, Erik Shetelig, Jan Wimpelmann, Shaheen Asghar, Torbjørn Tvedten, Sverre Tønseth (Norway); Mieczyslaw Janiszewski, Maciej Jachymiak, Jolanta Ferszka, Jerzy Samochowiec, Andrzej Kokoszka, Wlodzimierz Chrzanowski, Dariusz Juszczak, Krzysztof Walczewski, Krzysztof Klinke, Zbigniew Wawrzyniak (Poland); Mirela Manea, Elena Gherman, Maria Ladea, Cristian Marinescu, Jolanta Rabe-Jablońska (Romania); Lynette Nel, Christiaan Verster, John Joska, Mahomed Salduker, Reino Verster (South Africa); Curt Rolleri, Per Ekdahl, Konrad Rosman, Kurt Wahlstedt, Göran Björling, Eija Maahr, Anna Loewenstein, Hans Ågren, Lars Häggström (Sweden).
Conflicts of Interest and Source of Funding
These studies (D1448C00001, D1448C00002, D1448C00003, D1448C00004, D1448C00006, and D1448C00007) were funded by AstraZeneca Pharmaceuticals; Clinical Trials Registry numbers were as follows: NCT00320268, NCT00321490, NCT00326144, NCT00351169, NCT00326105, NCT00351910.
Professor Clayton has received research grants and provided speaker/advisor services or acted as a consultant in the last 3 years to Astellas Pharma US, Bayer, BioSante Pharmaceuticals, Boehringer-Ingelheim, Brain Resource Limited, Bristol-Myers Squibb, Eli Lilly, Euthymics, Forest Research Institute, Labopharm, Lundbeck, New England Research Institutes, Palatin Technologies, Pfizer, PGxHealth, Repligen Corporation, sanofi-aventis, Takeda, and Trimel Pharmaceuticals. In addition, Dr. Clayton has received royalties from Ballantine Books/Random House and holds shares/restricted stock units in Euthymics and S1 Biopharmaceuticals. Dr. Locklear was an employee of AstraZeneca at the time these analyses were conducted. Dr. Svedsäter was an employee of AstraZeneca at the time these analyses were conducted. Professor McIntyre has received research grants, provided speaker/advisor services to, participated in CME activities with, or received travel funding from: AstraZeneca, Biovail, Bristol-Myers Squibb, CME Outfitters, Eli Lilly, France Foundation, GlaxoSmithKline, I3CME, Janssen-Ortho, Lundbeck, Merck, Optum Health, Organon, Physician's Postgraduate Press, Pfizer, Schering-Plough, Shire, and Solvay/Wyeth.








