Introduction
Obsessive–compulsive disorder (OCD) is a chronic debilitating neuropsychiatry disorder that affects 1% to 3% of the population worldwide.Reference Kessler, Berglund and Demler1, Reference Ruscio, Stein, Chiu and Kessler2 Currently, selective serotonin reuptake inhibitors (SSRIs) and cognitive behavior therapy (CBT) are considered the first-line treatment for OCD.Reference Eddy, Dutra, Bradley and Westen3 CBT is at least as effective as pharmacotherapy in children and adults.Reference Franklin and Foa4 However, up to 50% of patients with OCD have failed to respond in SSRI trials.Reference Pittenger and Bloch5 In addition, SSRIs may have side effects that impair patient adherence to these medications (eg, sexual dysfunction or severe nausea).Reference Barth, Kriston and Klostermann6 Therefore, developing better treatment options is a health priority in managing OCD patients.
The most effective strategies in treatment of resistant OCD are the addition of antipsychotics to SSRIs; addition of CBT to current medications, and adding glutamergic agents to the current treatment.Reference Zhou, Zhou and Li7, Reference Albert, Marazziti and Di Salvo8 In a comparative clinical trial on resistant OCD patients, 27.27% of the cases in the aripiprazole group and 54.54% of those in the quetiapine cluster responded moderately to the augmentation.Reference Shoja Shafti and Kaviani9 A study of risperidone augmentation in resistant OCD patients found that 40% of the patients were responsive to the augmentation.Reference Hollander, Baldini Rossi, Sood and Pallanti10 In this line, a response rate of 30 % was reported in resistant OCD patients after augmentation with olanzapine.Reference Koran, Ringold and Elliott11
Pregabalin is S-enantiomer of racemic 3-isobutyl GABA that has structure and actions similar to gabapentin.Reference Zareba12 It has been approved by FDA for partial epilepsy and neuropathic pain. In addition, pregabalin has been extensively studied in relation to generalized anxiety disorders and alcohol and benzodiazepine dependence.Reference Martinotti13, Reference Oulis and Konstantakopoulos14 Pregabalin has multiple mechanisms of action, including inhibition of presynaptic voltage-gated Na+ and Ca2+ channels, thereby inhibiting the release of glutamate.Reference Dooley, Mieske and Borosky15 The rationale for using pregabalin in the treatment of OCD comes from several lines of evidence. First, research has revealed pathologically elevated glutamatergic transmission in cortical–striatal–thalamic circuit of the brain in OCD patients.Reference Chen, Meng and Zhang16, Reference Chakrabarty, Bhattacharyya, Christopher and Khanna17 Following treatment with glutamate modulating agents, a decrease in OCD symptoms severity was detected.Reference Grados, Atkins, Kovacikova and McVicar18–Reference Sahraian, Jahromi, Ghanizadeh and Mowla21 Further evidence for glutamatergic dysfunction in OCD comes from studies that measured levels of glutamate in cerebrospinal fluid (CSF) of OCD patients and matched psychiatrically normal controls.Reference Grados, Atkins, Kovacikova and McVicar18, Reference Bhattacharyya, Khanna and Chakrabarty22 These studies revealed that glutamate concentrations in the CSF of OCD patients were significantly higher than those of normal controls. Second, in a small open study, pregabalin was effective in reducing symptoms of 10 resistant OCD patients.Reference Oulis, Mourikis and Konstantakopoulos23 Third, one clinical trial on the use of gabapentin (a drug with similar mechanism of action to pregabalin) in the treatment of OCD has demonstrated efficacy in reducing obsessions and compulsions,Reference Onder, Tural and Gökbakan24 and fourth, pregabalin has showed to be effective in several other anxiety disorders.Reference Martinotti13 Based on the mentioned rationale, we conducted the first double-blind placebo-controlled clinical trial to examine the efficacy of pregabalin augmentation in treatment of patients with resistant OCD.
Method
Patients
Patients were recruited from Hafez psychiatry clinic affiliated to Shiraz University of Medical Sciences from March 2018 to March 2019. The patients were diagnosed according to Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V), criteria for OCD by a board certified psychiatrist through Structured Clinical Interview for DSM-V, Clinical Version (SCID-I). Exclusion criteria were any other major psychiatry disorder, major medical problems, pregnancy, and substance or alcohol abuse. Thyroid function was especially evaluated as thyroid dysfunction may present with mood disorders.Reference Mowla, Kalantarhormozi and Khazraee25 Our patients had failed to respond to at least 12 weeks of treatment with an adequate and stable dose of sertraline, as reflected by a baseline Yale–Brown Obsessive Compulsive Scale (YBOCS) of 18 or greater before enrollment in our trial. The mean YBOCS baseline score was 26.9. The concurrent medication at baseline was sertraline (mean dosage: 256.5 mg/day; dosage range: 100–300 mg/day). The sertraline dosage had been tittered up until patient’s intolerance.
All the patients provided informed consent, and the study protocol was approved by the ethics committee of Shiraz University of Medical Sciences that adheres to the Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects. This clinical trial was registered on the Iranian Registry of Clinical Trial (IRCT) database with IRCT number: IRCT 20181225042113N1.
Procedure
This study was designed as a double-blind controlled clinical trial. The patients were placed in one of the treatment groups randomly: pregabalin or placebo. We used a standard randomization procedure generated by a computer to have a random sample set. The patients and the examiner were blind about the medication consumed, as the same number of similar pills were provided to all the patients. Rating was done by an examiner who was a trained psychologist. The examiner-psychologist was blind about the treatment groups. The treatment duration was 12 weeks. Subjects were interviewed at screening, baseline, and biweekly (once in 2 weeks) until the end of the study. Safety and tolerability were assessed using spontaneously reported adverse event data and rates of premature termination for side effects. During the trial, no other psychological or medical intervention was allowed.
Intervention
Pregabalin was added to the subject’s OCD regimen initially at 75 mg/day, increased in 75-mg increments weekly. No dose escalation was administered in the case of patient's intolerance or clinical response. The mean dosage of pregabalin was 185.9 mg/day (range 75–225 mg/day). In the placebo group, placebo pills were added to the current medication.
Measurements
Assessments of efficacy of the 2 drug regimens were performed at baseline, and weeks 4, 8, and 12. To assess obsessive compulsive symptoms, we used Y-BOCS.Reference Goodman, Price and Rasmussen26 There is no standard cutoff point for Y-BOCS. A decrease of more than 35% in Y-BOCS was considered a significant response rate in our study based on analysis of data in previous similar studies,Reference Sahraian, Bigdeli, Ghanizadeh and Akhondzadeh27, Reference Sahraian, Ehsaei and Mowla28 although in one study on resistant OCD patients, more than 25% decrease in Y-BOCS was chosen as the cutoff point for treatment response.Reference Mowla and Sahraian20 Adverse effects of the medications were also registered.
Statistical analysis
The obtained data were statistically analyzed with IBM SPSS Statistics 21.0 for Windows. Categorical variables were compared with Chi-square test results, and T-test was applied to compare Y-BOCS score between 2 groups. p-value of less than .05 was considered statistically significant.
Results
Among the 67 patients, 56 met the inclusion criteria and were included in the trial. Finally, 42 subjects completed the study. There were 22 patients in the pregabalin group and 20 in the placebo group. Figure 1 shows the flow chart of the patients in the 2 groups.

Figure 1. Flow chart of the patients in the 2 groups.
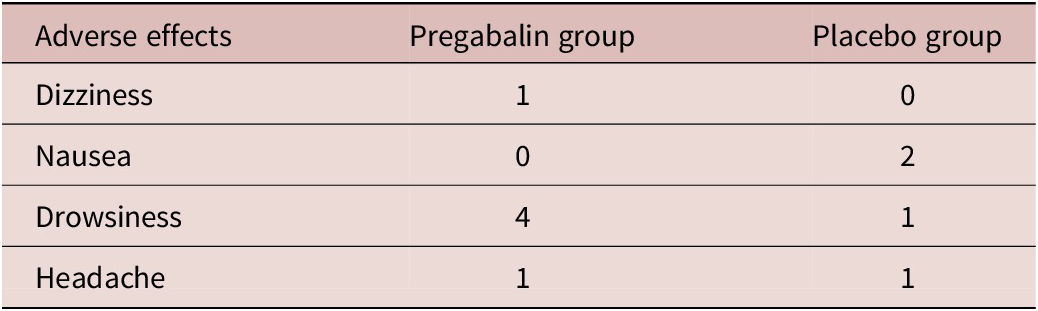
The demographic and clinical data of the patients involved in the trial are depicted in Table 1. Our findings revealed a significant difference between the 2 groups regarding the number of patients with a decline of over 35% in the total Y-BOCS score. At the end of the study, in the pregabalin group, 16 (57.14%) patients showed more than 35% decrease in mean Y-BOCS score compared with only 2 (7.14%) patients in the placebo group. This ratio of the improvement was statistically different between the 2 groups (χReference Ruscio, Stein, Chiu and Kessler2 = 5.0, df = 1, p = 0). The adverse effects reported by the patients of both groups are demonstrated in Table 2. The pregabalin group showed good tolerability and safety.
Table 1. The characteristics of the patients of the 2 groups.

Table 2. Adverse effects in pregabalin and placebo group.
Discussion
In this double-blind placebo-controlled study, we evaluated the efficacy of pregabalin in treatment of OC symptoms in patients with resistant OCD. Our findings showed that pregabalin augmentation decreased the Y-BOCS scores significantly more than placebo. The response rate in pregabalin group was 57.14% compared to 7.14% in the placebo group. Moreover, pregabalin was well tolerated, and there was also no reported serious adverse effect in the pregabalin group.
Several lines of evidence from basic neurobiological studies have indicated the role of glutamatergic abnormalities in the pathogenesis of OCD.Reference Chen, Meng and Zhang16–Reference Grados, Atkins, Kovacikova and McVicar18, Reference Parent and Hazrati29, Reference Ting and Feng30 These studies demonstrated pathologically elevated glutamatergic transmission in the cortical–striatal–thalamic circuitry of the brain in the OCD patients. Following treatment, the decrease in caudate glutamatergic concentration is associated with the decrease in OCD severity. Glutamatergic modulating agents that were successful in the treatment of OCD have shown the net effect of reducing glutamate output or signaling.Reference Wu, Hanna, Rosenberg and Arnold31 Riluzole and memantine are glutamate antagonists that have been found to be effective in the treatment of OCD patients.Reference Grant, Joseph and Farmer19, Reference Sahraian, Jahromi, Ghanizadeh and Mowla21, Reference Stewart, Jenike and Hezel32 Other medication with glutamate modulating properties that have shown anti-OCD efficacy are lamotrigine, topiramate, amantadine, ketamine, glycine, and D-cycloserine.Reference Bruno, Micò and Pandolfo33–Reference Wilhelm, Buhlmann and Tolin38 Consistent with these studies, our trial showed that pregabalin, a glutamate inhibitor, is beneficial to OCD patients.
In a small open study, pregabalin was used for 8 weeks in 10 OCD resistant patients.Reference Oulis, Mourikis and Konstantakopoulos23 Adjunctive pregabalin was administered at 225–675 mg/day, resulting in a significant reduction of OCD YBOCS severity scores from 27.1 (standard deviation [SD] = 6.5) to 12.3 (SD = 7.1). Overall, 35% symptom improvement was seen in 8 of the patients’ YBOCS scores. The results of our double-blind placebo-controlled study were in line with the above reference.
There are different strategies to augment the treatment response in nonresponsive OCD patients. Adding antipsychotics is the most evidence-based strategy.Reference Zhou, Zhou and Li7, Reference Albert, Marazziti and Di Salvo8 Quetiapine augmentation has shown a response rate of 54% in a clinical trial of patients with resistant OCD.Reference Shoja Shafti and Kaviani9 Augmentation of risperidone in a similar study has revealed a response rate of 40%.Reference Hollander, Baldini Rossi, Sood and Pallanti10 In another study of antipsychotic augmentation for resistant OCD patients, olanzapine showed a response rate of 30%.Reference Koran, Ringold and Elliott11 We found a response rate of 57.14% with pregabaline augmentation in our patients. Glutamatergic agents also recently have been shown to be effective in the treatment of resistant OCD patients.Reference Grados, Atkins, Kovacikova and McVicar18 In this regard, topiramate augmentation in a clinical trial has shown a response rate of 50% in the treatment of resistant OCD patients.Reference Mowla and Sahraian20 Adding cognitive–behavioral therapy to baseline pharmacotherapy is another strategy to augment treatment in resistant OCD patients.Reference Boschen, Drummond and Pillay39 In a study of using CBT in OCD patients, 32% of patients treated with CBT presented a complete remission of OCD symptoms (Y-BOCS score ≤ 8).Reference Sousa, Isolan and Oliveira40
Limitations
There are some limitations in our study. Small sample size of patients is a pitfall of this trial. Also, the study was conducted only in a single center. Short duration of the study is another pitfall. Larger and multicenter clinical trials are needed to confirm our results.
Conclusion
Our study revealed that pregabalin augmentation decreased the Y-BOCS scores significantly more than placebo in patients with resistant OCD. Our results add to the literature implicating glutamate in the pathophysiology of OCD and suggest that glutamate-modulating agents may be considered new pharmacotherapy options. However, it needs to be mentioned that our study is preliminary and larger clinical trials are needed to confirm the results.
Acknowledgments.
This study was performed as postgraduate thesis for graduation of Dr. Mehrnoosh Ghaedsharaf in Shiraz University of Medical Sciences and was financially supported by Shiraz University of Medical Sciences with grant number of 97-01-01-17965.
Disclosure.
The authors have no conflicts of interest to disclose.





