Introduction
Post-traumatic stress disorder (PTSD), a psychiatric condition consequent to experiencing or witnessing a traumatic event and characterized by symptoms that include distressing dreams or nightmares, avoidance behaviors, and hyperarousal, has a reported prevalence between 10% and 30% in U.S. Veterans, depending on the time or war served. 1 PTSD has been associated with worsening physical function,Reference Hall, Beckham and Bosworth 2 development of chronic conditions,Reference Ryder, Azcarate and Cohen 3 , Reference Hoerster, Campbell and Dolan 4 and increased mortality risk.Reference Forehand, Peltzman and Westgate 5
Patients suffering from PTSD have a number of evidence-based treatment options. These include medications (e.g., serotonin reuptake inhibitors) and psychotherapies (e.g., prolonged exposure and cognitive processing therapy and eye movement desensitization and reprocessing). 1 Unfortunately, these treatments often fall short of treatment goals and many individuals suffer persistent symptoms, including sleep disturbances, that are associated with substantial disability.Reference Steenkamp, Litz, Hoge and Marmar 6 , Reference Berger, Mendlowicz and Marques-Portella 7 As a result, there has been an urgent need for innovation in developing therapies to treat PTSD and its related symptoms.
Prazosin, a centrally acting α1-adrenergic receptor antagonist originally indicated for the treatment of hypertension, was such an innovation as it was found to have benefit in reducing trauma-associated nightmares and improving sleep in PTSD patients. Nearly 20 years ago, Raskind et alReference Raskind, Dobie and Kanter 8 while leading a Vietnam Veteran’s support group, discovered that two members who had begun treatment with prazosin for non-PTSD related medical reasons (to improve urinary flow related to benign prostatic hypertrophy) surprisingly had a reduction in combat related nightmares. This finding ultimately led to a number of placebo-controlled clinical trials that all showed a benefit of prazosin over placebo for reducing patient-reported nightmares and sleep quality, including one study that demonstrated sleep benefits with polysomnography.Reference Taylor, Martin and Thompson 9 Several reviews and meta-analyses have summarized the clinical trial data comparing prazosin vs placebo in alleviating PTSD symptoms finding an overall significant benefit of prazosin.Reference Berger, Mendlowicz and Marques-Portella 7 , Reference De Berardis, Marini and Serroni 10 – 14
Most recently, a large multicenter clinical trial sponsored by the Veterans Health Administration’s Cooperative Studies Program 1 enrolled 304 Veterans at 20 Veterans Affairs (VA) Medical Centers across the country.Reference Raskind, Peskind and Chow 15 Results surprisingly showed that prazosin failed to distinguish itself from placebo on all the primary and secondary outcome measures including nightmares and sleep quality—two features that had consistently shown improvement in previous controlled studies. Upon publication of these study results, commentaries and position papers were published highlighting the negative results of the study and thereby discouraging the use of prazosin in the treatment of PTSD.Reference Morgenthaler, Auerbach and Casey 16 – 18 However, a number of clinician researchers expressed concerns regarding biases and potential errors in the conduct of the trial.Reference Anghelescu and Moschner 19 – 21 What has been lacking in the ensuing discussion is an appreciation of the trial details that could explain the negative findings, and place this one study in the context of the extant literature that presents such a consistent, but contrary story. In consideration of this new study, we performed an updated meta-analysis of the impact of prazosin vs placebo on PTSD symptoms as well as deeper examination of where this new study falls in relation to previously published trial data.
Methods
Search strategies
We performed a wide search to identify trials investigating the effect of prazosin vs placebo on metrics of PTSD, sleep quality, and nightmares. We primarily searched MEDLINE (PubMed) for publications listed through March of 2019 using the following keywords: ([prazosin] AND [posttraumatic stress disorder dream* OR PTSD OR nightmare* OR night terror* OR dyssomnia* OR insomnia* OR parasomnia* OR sleep disorder* OR sleep disruption* OR sleep distress* OR fragmented sleep* AND trial]). The search terms were similar to those listed in a prior published meta-analysis.Reference Khachatryan, Groll, Booji, Sepehry and Shutz 13 To find additional articles that may not have been PubMed indexed, a similar query was used to search Web of Science, EMBASE, Cumulative Index to Nursing and Allied Health Literature (CINAHL), and Cochrane Central Register of Controlled Trials (CENTRAL). To search EMBASE, the above search query was slightly tailored to match the searching keywords to EMTREE (the EMBASE’s indexing thesaurus). Three field experts (B.S., C.R., and E.S.) were consulted to recognize any unidentified relevant study.
Study selection and quality assessment
Search results were imported into EndNote software and duplicate records were removed. Inclusion criteria were the following: (a) clinical trial study design in which participants were randomly assigned to either a prazosin treatment group or to a control group; (b) study participants ≥18 years of age that met criteria for PTSD according to the Diagnostic and Statistical Manual of Mental Disorders, Third Edition (DSM-III), Third Edition, Revised (DSM-III-R), Fourth Edition (DSM-IV), or Fifth Edition (DSM-V); and (c) reporting of baseline (pre-treatment) and follow-up (post-treatment) measures of PTSD-related symptoms. Two investigators (B.S. and E.S.) independently screened and selected studies that met inclusion criteria. The Cochrane Collaboration Risk of Bias ToolReference Higgins 22 was used to evaluate each study’s quality. For each published trial, allocation concealment, study blinding, selective reporting, and other biases were evaluated. Any discrepancies between the two reviewers on study eligibility were resolved by discussion and consensus with a third author (C.R.).
Data extraction
Two authors (B.S. and C.T.) independently extracted relevant study data from the identified studies, including study characteristics (author, year, country, study design, cohort size, demographics of participants, baseline blood pressures), follow-up time, dosage, and baseline and follow-up outcome measures. Overall PTSD score was assessed using Clinician-Administered PTSD Scale (CAPS)Reference Blake, Weathers and Nagy 23 or PTSD checklist-Civilian (PCL-C).Reference Blanchard, Jones-Alexander and Buckley 24 Sleep quality was assessed using the Pittsburgh Sleep Quality Index (PSQI)Reference Buysse, Reynolds and Monk 25 scales and CAPS item 13, while nightmares were assessed using questions from CAPS and PSQI and/or sleep diary information on nightmare frequency. In cases of missing data, we sought to obtain additional information by contacting study authors.
Statistical analyses
We calculated a Hedges standardized mean difference (SMD)Reference Higgins and Green 26 , Reference Hedges and Olkin 27 with a 95% confidence interval (CI) for each study, comparing outcomes relating to overall PTSD severity, nightmares, and sleep quality in prazosin vs placebo treated study arms. SMD is used as a summary statistic in meta-analysis to standardize the estimates when studies use different measures to assess outcomes. In primary analysis, the mean and standard deviation at follow-up for each outcome were compared across treatment arms. Standard deviations were back-calculated from 95% CI in studies where only the latter were reported.Reference Higgins and Green 26 In sensitivity analysis, we used differences in means from baseline to follow-up, back-calculating the standard deviations for differences in both study arms from between-group P values, mean differences, and sample sizes according to calculators provided by the Cochrane Handbook.Reference Higgins and Green 26
We also conducted a placebo-effect meta-analysis for all three outcomes with and without the inclusion of the 2018 Raskind trialReference Raskind, Peskind and Chow 15 (n = 271; patients who completed 10 weeks of the study). In this analysis, we calculated SMD values at baseline and follow-up for only the placebo arm of each study. All meta-analyses estimates were calculated using random-effects models due to varying study characteristics. For sensitivity analysis, we additionally examined effect estimates including the four clinical trial design studies and excluding the two crossover design studies. We further assessed study heterogeneity by running a univariate, random-effects meta-regression for each outcome. In addition, we ran Egger’s and Begg’s tests to assess potential publication bias across published and included studies. A 95% CI with no overlap of the null effect parameter value was considered significant. For all statistical procedures, we used StataMP 13 (StataCorp, College Station, TX).
Results
Our initial search yielded 11 records, from which six studiesReference Taylor, Martin and Thompson 9 , Reference Raskind, Peskind and Chow 15 , Reference Raskind, Peskind and Kanter28–Reference Raskind, Peterson and Williams31 were included in this meta-analysis (Figure S1). All of the included studies reported scores at baseline and follow-up outcomes. Results from all six studies were included in meta-analysis of each PTSD outcome (Table S1). Table 1 describes the characteristics of each study. Among the six included studies, there were two crossover studies and four randomized controlled trials. Mean age per study varied from 30 to 56 years and mean follow-up time varied from 7 to 20 weeks. Participants in the majority of studies were comprised primarily of male, U.S. Veteran or active-duty military subjects with the prazosin study dosage ranging from 8.9 to 15.6 mg per day. The Taylor study differed from the other studies in that it had a civilian, mostly female sample and achieved a much lower mean prazosin study dose of 3.1 mg per day.Reference Taylor, Martin and Thompson 9
Table 1. Characteristics of Included Studies
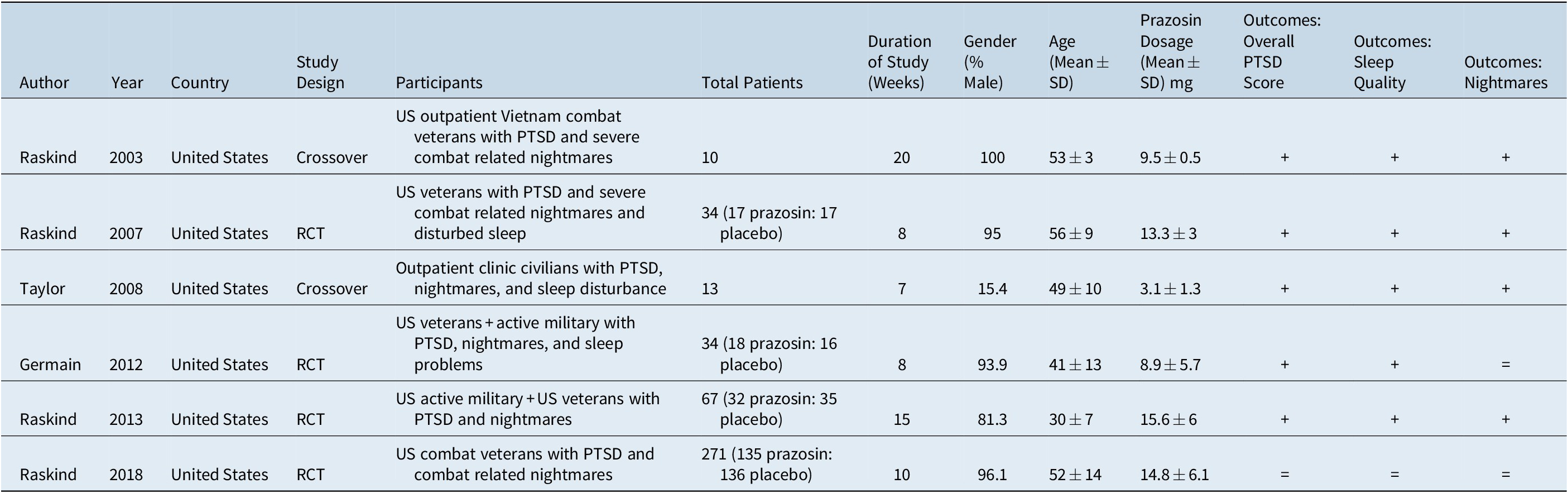
Abbreviations: PTSD, post-traumatic stress disorder; SD, standard deviation; RCT, randomized control trial.
+, if a benefit was demonstrated; =, if no significant change was observed. Among all outcomes, no adverse effect was demonstrated and all data was obtained with respect to that outcome measure.
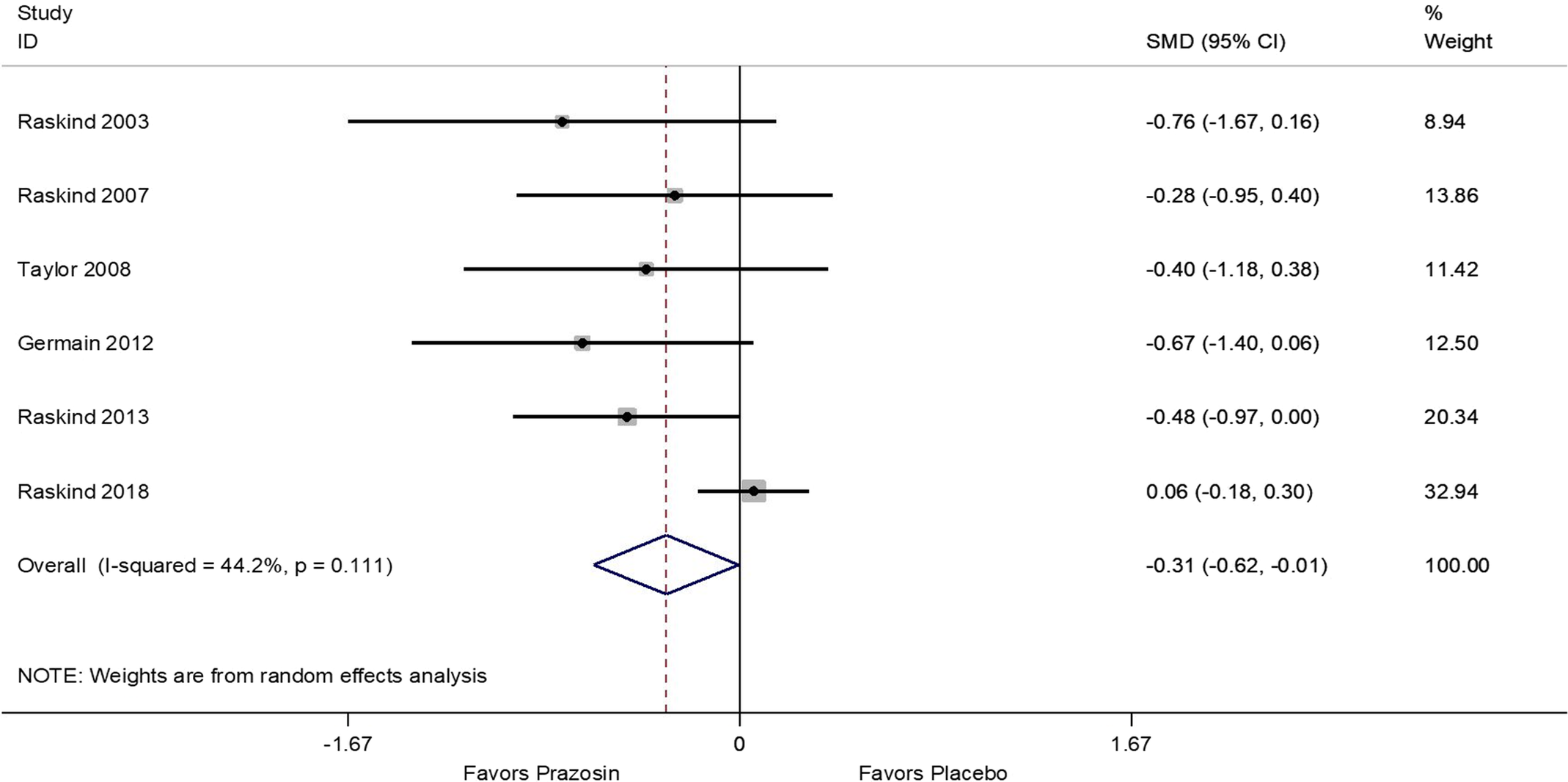
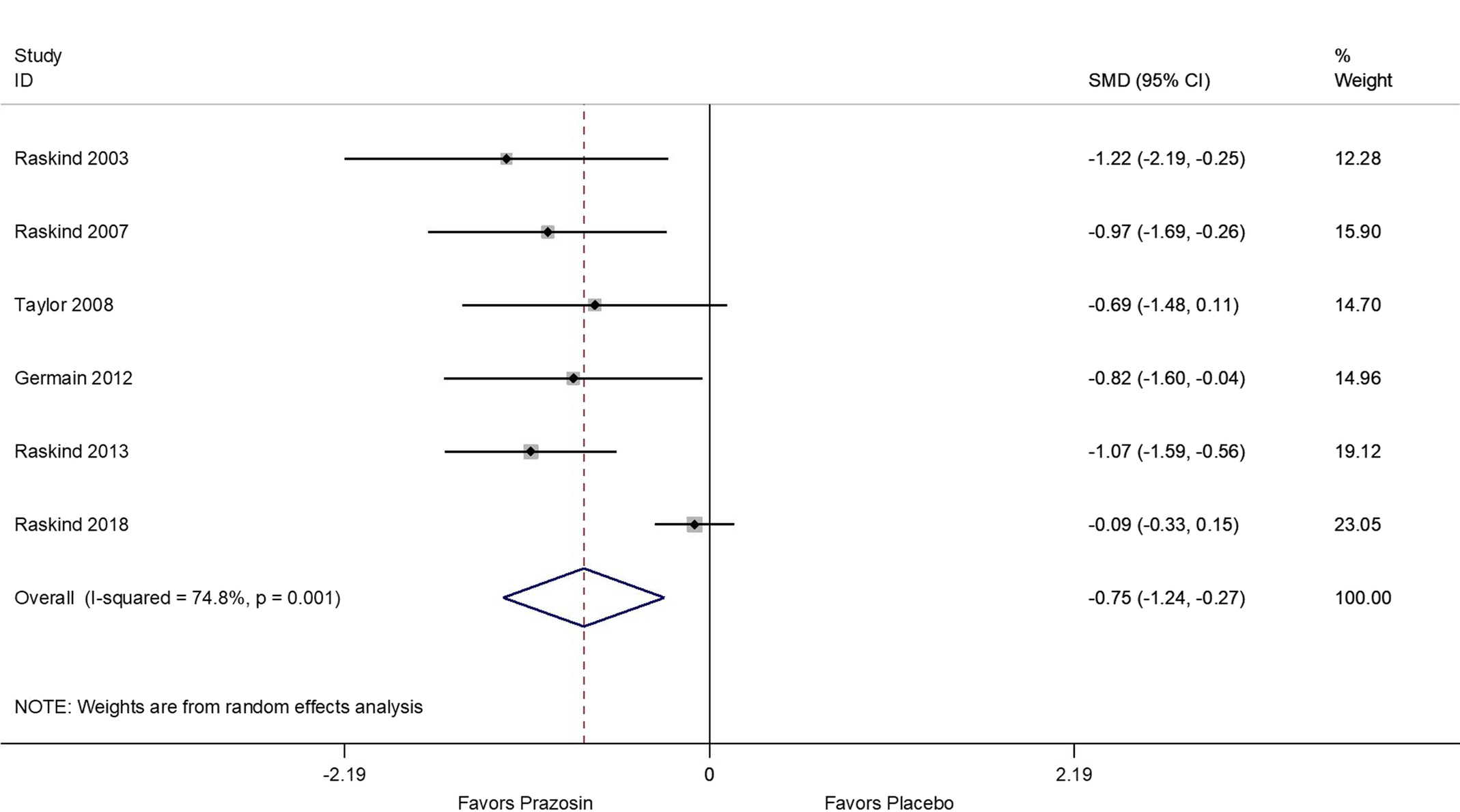
Results of the meta-analyses showed that, compared to the placebo arms, prazosin treatment resulted in a significant decrease of overall PTSD symptoms (SMD = −0.31; 95% CI: −0.62, −0.01; Figure 1), decrease in nightmares (SMD = −0.75; 95% CI: −1.24, −0.27; Figure 2), and improvement of sleep quality (SMD = −0.57; 95% CI: −1.02, −0.13; Figure 3). Sensitivity analyses using differences in baseline and follow-up mean scores to compute estimates showed similar results (Figures S2 and S3). In addition, analysis including only trial studies and exclusion of the two crossover studies yielded similar effect estimates.

Figure 1. Meta-analysis of standardized mean differences between prazosin and placebo for Overall PTSD Score using study follow-up (post-treatment) means and standard deviations.

Figure 2. Meta-analysis of standardized mean differences between prazosin and placebo for Nightmares using study follow-up (post-treatment) means and standard deviations.

Figure 3. Meta-analysis of standardized mean differences between prazosin and placebo forSleep Quality using study follow-up (post-treatment) means and standard deviations.
Significant heterogeneity was present for nightmare (I 2: 74.8%, P value: .001; Figure 2) and sleep quality (I 2: 70.5%, P value = .005; Figure 3) outcomes, but not for overall PTSD (I-squared: 44.2%, P value = .111; Figure 1). In univariate meta-regression analysis (Table S2) neither age, gender, study duration, nor study dosage could explain observed heterogeneity (P > .05 for all estimates per outcome) across study results.
Meta-analysis of the placebo-effect for nightmares showed that placebo-treated patients had significant improvement between baseline and follow-up (pooled SMD of 0.40; 95% CI: 0.10-0.70; Figure 4) even with exclusion of Raskind et al’s study.Reference Raskind, Peskind and Chow 15 The Raskind et al’s studyReference Raskind, Peskind and Chow 15 alone had a large placebo effect SMD of 0.99, 95% CI: 0.75-1.24, which, when included with the other studies, increased the pooled SMD by 20% (pooled SMD with Raskind et alReference Raskind, Peskind and Chow 15: 0.51; 95% CI: 0.13, 0.89; Figure 4). Similar impacts of the Raskind et al’s studyReference Raskind, Peskind and Chow 15 were observed for overall PTSD placebo effects (Figure S5A,B) as well as sleep quality placebo effects (Figure S6A,B).

Figure 4. Forest plot of standardized mean differences between baseline (pre-treatment)and follow-up (post-treatment) for only the placebo arms of nightmares outcome, with andwithout inclusion of Raskind et al. (2018).
As for publication bias, Egger’s tests detected no significant reporting bias for overall PTSD, nightmares, and sleep quality outcomes (P values: .144, .299, and .371, respectively). Begg’s test was also not significant for overall PTSD, nightmares, and sleep quality evaluated (P values: .260, .452, and .133, respectively).
Discussion
The results of our six-study meta-analysis indicate that patients receiving prazosin have significant improvements in overall PTSD scores, nightmares, and sleep quality as compared to placebo, even after inclusion of the large, randomized control trial by Raskind et al,Reference Raskind, Peskind and Chow 15 which failed to show benefit of prazosin for any outcome. Results were robust independent of the use of baseline to follow-up differences or follow up means for effect estimates. Our results build upon meta-analyses published by Khachatryan et alReference Khachatryan, Groll, Booji, Sepehry and Shutz 13 and Singh et alReference Singh, Hughes, Mehta, Erwin and Parsaik 14 that similarly found overall benefit of prazosin vs placebo.
As an α1-adrenergic receptor antagonist, prazosin blocks noradrenergic activity in the central nervous systemReference Boehnlein and Kinzie 32 in areas related to hyperarousal of symptoms typical of PTSD, including irritability,Reference Yamamoto, Shinba and Yoshii 33 sleep disturbances,Reference Mallick, Majumdar and Faisal 34 increased cognitive processing and fear response,Reference Birnbaum, Gobeske and Auerbach 35 and related release of cortisol.Reference Vythilingam, Anderson and Owens 36 Successful trials and case reports have reported that prazosin treatment is related to improvement in these specific symptoms.Reference Raskind, Dobie and Kanter 8 , Reference Singh, Hughes, Mehta, Erwin and Parsaik 14 , Reference Raskind, Peskind and Kanter 28 , Reference Raskind, Peskind and Hoff 29 , Reference Raskind, Peterson and Williams 31 , Reference Daly, Doyle and Radkind 37
Does the single, large Raskind et al’s studyReference Raskind, Peskind and Chow 15 completely negate the previous literature? Our random effects meta-analyses indicate that it does not. The estimated average effect sizes across studies remained statistically significant in all three outcome domains, even with inclusion of Raskind et al.Reference Raskind, Peskind and Chow 15 Did the “negative finding” in that study reflect poor outcomes in patients treated with prazosin? On the contrary, patients treated with prazosin had statistically and clinically large positive effects in all outcomes. In fact, the standardized pre-post change in overall PTSD severity for prazosin-treated patients was nearly the equivalent of the average prazosin effect size in the previously conducted studies. The negative finding resulted from large effect sizes for patients who received placebo, particularly for nightmares. The PTSD pre-post effect for placebo-treated patients was the largest of all six studies. A large effect size for placebo-treated patients obviously minimizes differences between prazosin and placebo-treated patients.
The reasons for the large placebo effects are not clear. The trial did not differ significantly in achieved dose, trial duration, or demographics (age, % male) of the study population compared to previous trials that demonstrated benefit of prazosin (Table 1). An unusual feature of the Raskind trial was that the population was likely more stable with more concurrent treatment due to inclusion and exclusion criteria than in previous trials. For example, due to rightful concerns pertaining to clinical risk for patients only receiving placebo, “psychosocial instability” was an exclusion, thus allowing participants to continue ongoing supportive therapy and other possibly effective pharmacotherapy if initiated 4 weeks prior to the trial. Approximately 80% of subjects were receiving antidepressants and 40% psychotherapy at the time of baseline assessments. These clinical and treatment features could negate any real difference between prazosin and placebo. Other multicenter VA studies have produced negative outcomes in trial for medications with established efficacy,Reference Friedman, Marmar and Baker 38 , Reference Rosenheck, Krystal and Lew 39 but several of the trials included in this meta-analyses were also conducted on U.S. Veterans at VA clinics and found a benefit for prazosin.Reference Raskind, Peskind and Kanter 28–Reference Raskind, Peterson and Williams31 Of note, all studies reported demographics and clinical characteristics potentially related to PTSD treatment such as concurrent use of other medications and abuse of alcohol or drugs. However, data on other comorbidities were not reported and therefore we are unable to ascertain if these comorbidities differed across treatment groups and across studies.
Despite commentary and press regarding the negative results of Raskind et al’sReference Raskind, Peskind and Chow 15 large trial, prescription rates for prazosin in patients with PTSD across the VA increased from 2007 (7%) to 2012 (15%) and then 2017 (20%), and did not differ substantially between last year (2018, 19%) and this year to date (2019, 18%). Although these numbers are estimations, this observation likely represents the value (benefit/risk) clinicians associate with this treatment which continues to drive its use.
Although our meta-analysis included studies on prazosin and placebo within our aforementioned criteria, results should be interpreted with caution as the majority of the studies, including the largest and most recent trial, were conducted by or in collaboration with a single researcher. Our assessment of publication bias did not find any significant biases, however, the small number of pooled studies included may limit the accuracy of this result. Additionally, there were large heterogeneity in outcome effect estimates which could not be accounted for by differences in study mean age, gender distribution, mean prazosin dosage, or duration of follow up.
Overall, the addition of the negative results of the Raskind trial does not negate a significant pooled overall effect observed for prazosin compared to placebo across randomized trials for overall PTSD score, nightmares, and sleep quality. The current pooled analysis demonstrates that prazosin has a moderate benefit compared to placebo in improving these symptoms. Additional studies to further identify the populations and contexts that are best suited for prazosin therapy may be beneficial in maximizing the utility of this medication. This remains important as nearly all studies to date have chiefly focused on Veteran populations, despite the extensive use of prazosin for civilians.
Funding
There was no funding for this project.
Disclosure
The authors have no disclosures to report.
Supplementary Materials
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S1092852920001121.