FOCUS POINTS
• Treatment-resistant depression (TRD) is a significant problem that may be present in up to 57% of depression cases.
• Monoamine oxidase inhibitors (MAOIs) can be highly effective antidepressants, yet tend to be underused in clinical practice, due in part to misinformation and mythology about their dietary and drug interactions.
• Reversible inhibitors of monoamine oxidase (RIMAs) have shown efficacy in depression, with safety and tolerability comparable to antidepressants such as serotonin-selective reuptake inhibitors (SSRIs).
• With proper consideration of dietary and drug interactions, RIMAs may be safely integrated into clinical practice.
Introduction
Major depressive disorder (MDD) is far more than simply a depressed mood. In fact, “depressed mood” is only one of nine symptoms used to characterize depression on a list that includes apathy, appetite and weight changes, sleep problems, fatigue, and problems thinking. The Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV TR) requires five of the nine symptoms for a diagnosis of depression. Estimates place the prevalence of MDD in the U.S. at 7% in any given year, with a lifetime prevalence of 16%.Reference Kessler, Berglund and Demler1
Considering the range of symptom dimensions in MDD, it is easy to understand why nearly 60% of patients with MDD report severe impairment of work, home, relationship, and social roles.Reference Kessler, Berglund and Demler1 Accordingly, in 2000, the economic burden of MDD totaled $83 billion, of which $26 billion were direct medical costs and $51 million were workplace costs.Reference Greenberg, Kessler and Birnbaum2 Of the medical costs, treatment with antidepressant medications may play a substantial role. In 2006, each of the top 5 antidepressants ranged from $1.1–2.25 billion in annual sales.Reference Ioannidis3
Aside from its direct medical impact, MDD is also associated with increased incidence of conditions such as diabetes,Reference Golden, Williams and Ford4 hypertension,Reference Jonas, Franks and Ingram5 and stroke.Reference Larson, Owens, Ford and Eaton6
Unmet Need in MDD
Considering the burden MDD places on society, the need for effective treatments is self-evident. However, the reality of treatment with antidepressant medication leaves much to be desired. The goal of treatment used to be reduction of symptoms by half (defined as a “response”). However, the current trend is to attempt complete remission of symptoms, and to sustain this remission, because sustained remission leads to better overall outcomes for patients. To attain this goal, multiple rounds of treatment are often necessary.
For example, the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study found that after 1 treatment with an antidepressant, approximately 37% of patients remitted (see Figure 1). Of the non-remitters, the second treatment resulted in 31% remission, or an additional 19% of patients. After the third and fourth treatments, an additional 11% of the patients remitted, leading to an overall 67% remission rate after about a year of treatment. However, taking into account the patients who subsequently relapsed, the number is closer to only 43%.Reference Nelson7, Reference Rush, Trivedi and Wisniewski8 The authors propose two explanations for the substantial numbers of patients who did not achieve remission. First, there could be some types of depression for which the current treatments cannot produce remission. Alternatively, the patients may have developed either comorbid conditions or chronic depression that may have advanced the disease beyond the reach of these treatments. In either case, they suggest that earlier or different treatment may be the solution.

Figure 1 Remission of patients undergoing multiple treatments for depression.
The STAR*D trials also provided evidence in support of the treatment goal of complete remission (Figure 2). Two salient points can be divined from Figure 2. First, patients who attain remission are less likely to relapse. This tendency had been observed for patients attaining remission after a single treatment. However, the STAR*D study demonstrated that attaining remission has benefit, even if it takes multiple treatments. Second, the likelihood of relapse is generally higher as more treatments are required, particularly for those patients not achieving remission.

Figure 2 Relapse rates of patients undergoing multiple treatments for MDD.
Treatment-Resistant Depression
The authors also note that the percentages of patients achieving remission after the first and second treatments are approximately the same, yet the percentages drop substantially on the third and fourth treatments. Accordingly, they suggest that the failure of two treatments constitutes a useful definition of treatment-resistant depression (TRD). For the purpose of regulatory approval, the definition has sometimes been expanded to include the failure of 1–3 treatments.
Researchers have found that TRD is associated with twice the hospitalization rate compared to patients with depression that is not resistant to treatment. Of those patients who were hospitalized, medical costs were 6 times those of the comparison group.Reference Crown, Finkelstein and Berndt9
Currently, the U.S. Food and Drug Administration (FDA)-approved medications for TRD, defined as having failed one treatment, include an olanzapine/fluoxetine combination, and two antipsychotic medications (aripiprazole and quetiapine XR) approved for augmentation of SSRIs/SNRIs in patients who have failed one previous treatment with the SSRI/SNRI alone. These medications from the atypical antipsychotic drug class present multiple problems with side effects, such as weight gain, metabolic issues, tardive dyskinesia, and increased incidence of stroke and death in elderly patients with dementia, whereas drugs from the SSRI/SNRI drug class cause sexual dysfunction and weight gain.
Nonpharmacological treatments for TRD include electroconvulsive therapy (ECT), vagus nerve stimulation, and transcranial magnetic stimulation.Reference Shelton, Osuntokun, Heinloth and Corya10 Of these, ECT shows the highest effectiveness, with rapid improvement and response rates of 50–60%. However, relapse rates are high, and clinical data suggest that follow-up with pharmacological therapies or maintenance ECT may be indicated. ECT is most often prescribed as a last resort for patients who have not responded to multiple treatment trials. Cognitive impairment, such as the loss of memory functions, is the most prominent adverse event of ECT. Thus, current treatments for resistant depression represent a substantial economic and side effect burden. Accordingly, there is a significant unmet need for treatments that can bridge the gap between SSRI/SNRI antidepressants and antipsychotics.
This article will review the etiology of MDD, discuss the perceptions and realities of monoamine oxidase inhibitors (MAOIs), and outline opportunities for CX157 in the treatment of MDD.
Etiology of MDD
The substantial burden of MDD accounts for the extensive research that has been undertaken in search of treatment for this condition. Rational strategies for drug treatment have been based on the monoamine hypothesis of depression, which posits that the symptoms of depression are caused by low or dysregulated brain levels of the monoamine neurotransmitters serotonin (5-hydroxytryptamine or 5-HT), norepinephrine (NE), and dopamine (DA), followed by certain adaptive changes in the brain that lead to the symptoms of depression.Reference Stahl11 The source of the monoamine dysregulation has been unclear. However, recent studies have pointed to an enzyme known as monamine oxidase-A (MAO-A) that degrades the neurotransmitters and may be overactive in patients with depression. To understand this more clearly, it is useful to review several critical brain circuits.
There are at least 11 regions of the brain that have been linked to a number of key symptoms associated with MDD (see Figure 3). The information processing required to regulate these behaviors is believed to involve neurons in circuits that are topographically distributed in the brain, with different functions, and thus different symptoms regulated by information processing in unique brain areas. Each of these brain areas can be modulated, and the efficiency of information processes in these brain areas regulated by input from 5-HT, NE, and DA arriving in the various brain areas from monoamine projections coming from the brainstem. Neurotransmission between neurons occurs at synapses between the axon terminal of the sending, or presynaptic, neuron and the dendrite of the receiving, or postsynaptic, neuron (see Figure 4). Neuronal circuits communicate with many neurotransmitters, including glutamate and gamma amino butyric acid (GABA) and are modulated by the neurotransmitters 5-HT, NE, and DA, which are synthesized within their neurons and then stored in synaptic vesicles until ready for use. After release into the synapse, monoamine neurotransmitters can be pumped out of the synaptic cleft by their respective reuptake pumps, where they can be repackaged for future use. All three neurotransmitters can be broken down by the enzyme monamine oxidase (MAO), which is located on the mitochondria of the presynaptic neuron, as well as other neurons and glia. Essentially all known antidepressants block either the reuptake pumps for monoamines or the enzyme MAO.

Figure 3 Neurotransmitters that are hypothetically dysregulated in depression. Brain regions are hypothetically associated with symptoms of depression. Each of these brain regions receives neurotransmitters from signals originating in the brainstem (NT). Symptoms of depression are associated with dysregulation in one or more neurotransmitters in each of these brain regions. A = amygdala, BF = basal forebrain, C = cerebellum, H = hypothalamus, NA = nucleus, NT = brainstem neurotransmitter centers, PFC = prefrontal cortex, S = striatum, SC = spinal cord, T = thalamus.
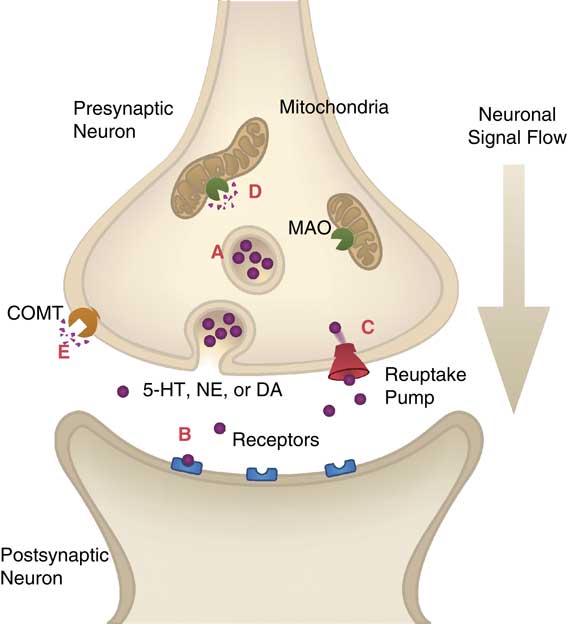
Figure 4 Neurotransmission in the synapse. (A) Neurotransmitters are stored in the synaptic vesicle until released by a neuronal impulse. (B) Neurotransmitters bind to postsynaptic receptors to induce an impulse in the postsynaptic neuron. (C) Reuptake pumps carry neurotransmitters from the synapse back into the presynaptic neuron for repackaging into vesicles. (D) Monoamine oxidase (MAO) on the outer membrane of mitochondria degrades serotonin (5-HT), norepinephrine (NE), and dopamine (DA). (E) Catechol O-methyl transferase (COMT) degrades norepinephrine and dopamine.
Pharmacological Treatment for MDD
The strategy for pharmacological treatment of depression has centered on the strategy of increasing synaptic monoamine levels in an attempt to boost signaling in the associated neurons. Since the symptoms of depression are hypothetically linked to dysfunction of neurotransmitter signaling in various brain circuits, antidepressants hypothetically act to improve symptoms by restoring the efficiency of information processing in these circuits by boosting the availability of 1 or more monoamine neurotransmitters. Although not proven, it seems plausible that some patients respond to 5-HT boosting alone, as with an SSRI; others may require dual action on both 5-HT and NE, as with an SNRI; finally, still other patients may require the unique triple action on serotonin, norepinephrine, and dopamine only possible with an MAO inhibitor. Figure 3 shows the neurotransmitters that are hypothetically dysregulated in regions of the brain in depression. Furthermore, the symptoms of MDD outlined in the DSM-IV TR can also be organized based on their associated neurotransmitters that are hypothetically dysregulated (see Figure 5).
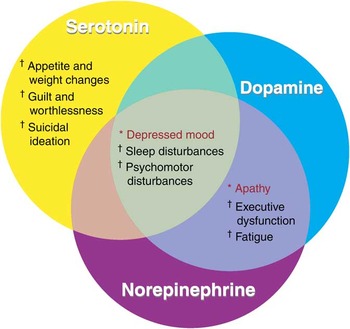
Figure 5 DSM-IV TR symptoms of depression hypothetically associated with dysregulation of neurotransmitters. *Diagnosis requires at least one of these symptoms. †Diagnosis requires at least four of these symptoms. Some symptoms, including depressed mood, are hypothetically linked to dysregulation of all three neurotransmitters.
The Role of MAO-A and the Neurotransmitters 5-HT, NE, and DA in Depression
So far, the discussion of the monoamine hypothesis of depression has explained antidepressant action through its increase of monoamine levels in the synapse. However, it does not explain how monoamine levels may have become low in the first place. Recent work using brain imaging of the MAO-A enzyme has suggested an intriguing possibility. By comparing the MAO-A levels in multiple brain regions of patients with depression, researchers found that the levels of MAO-A were consistently higher than in healthy subjects.Reference Meyer, Ginovart and Boovariwala12 Thus, in the absence of other compelling mechanisms for the depletion of the neurotransmitters 5-HT, NE, and DA, the authors propose MAO-A as the primary monamine-lowering mechanism in depression.
Further studies investigated the MAO-A levels of depressed patients who had been treated with SSRIs.Reference Meyer, Wilson and Sagrati13 These studies revealed that compared to healthy subjects, the MAO-A levels are higher in both depressed patients and patients in recovery (see Figure 6). Furthermore, of patients who had recovered, recurrence of MDD was associated with higher levels of MAO-A compared to those who remained in recovery, suggesting that high levels of MAO-A may be the cause of some types of treatment resistance. Thus, with respect to MAO-A levels, recovery was not the same as in the healthy condition, leading the researchers to propose a model of serotonin dysregulation in which MAO-A plays a role in reducing the overall pool of monoamines (see Figure 7). Therefore during treatment, even though SSRIs may increase the levels of synaptic 5-HT, the underlying issue of low monoamine levels due to high levels of MAO-A activity remains.

Figure 6 Brain levels of MAO-A in depressed patients. Monoamine oxidase A distribution volume (MAO-A VT, a measure of MAO-A levels) in healthy subjects, depressed subjects, and subjects with major depressive disorder (MDD) in recovery. Monoamine oxidase A VT was significantly greater in each patient sample as compared with healthy subjects (*P < 0.01, †P < 0.001). Adapted from Meyer (2009).Reference Meyer, Wilson and Sagrati13

Figure 7 A proposed hypothesis of the role of MAO-A and reuptake pumps in depression. (A) In healthy subjects, MAO-A and the serotonin reuptake transporter (SERT) correctly regulate neurotransmission. (B) In depressed patients, both MAO-A and SERT may be overactive, leading to lower levels of 5-HT and, consequently, the creation of more 5-HT2A receptors to compensate. (C) SSRI treatment blocks the reuptake pumps, leading to higher levels of 5-HT in the synapse, which results in down-regulation of the 5-HT2A receptors and relief from symptoms of depression. However, the underlying problem of high levels of MAO-A still remains.
The plot continues to thicken in the story of MAO-A and its role in depression. Additional research has traced the high MAO-A activity to a protein that controls the genetic expression of MAO-A, known as R1.Reference Johnson, Stockmeier and Meyer14 R1 is a repressor protein, meaning that it represses the genetic machinery that leads to the production of MAO-A. However, researchers found that this R1 repressor was significantly depleted in both treated and untreated depression patients (by 30% and 37.5%, respectively) compared to healthy subjects—and the reduction in R1 was correlated with a 40% increase in MAO-A protein levels. Thus, decreasing repression of MAO-A production provides an explanation for the high levels of MAO-A observed in MDD patients.
MAOIs—Myths and Realities
MAOIs emerged in the early 1960s shortly after the introduction of the tricyclic antidepressants (TCAs). These inhibitors were irreversible and nonselective toward the A and B forms of MAO. Although these drugs were efficacious, within a few years, patient deaths were linked to the drug tranylcypromine, causing its temporary withdrawal from the market. These deaths were due to intracranial bleeding brought on by hypertensive crisis. The drug was reintroduced later that same year, albeit with more limited indications and specific warnings.Reference Atchley15 As a consequence of this potential for adverse effects, the need to monitor diet and to prohibit various concomitant drugs, as well as the development of many effective alternative treatments without these issues, the use of MAOIs has not only fallen off, but numerous myths about the dangers of MAOIs have developed, especially among clinicians with little experience using MAOIs.
MAOI-Induced Hypertension
Dietary interactions—the tyramine reaction
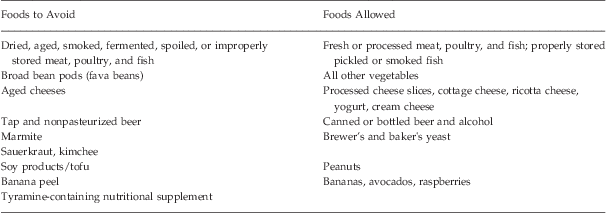
MAO-A and MAO-B are present in both central nervous system (CNS) and peripheral tissues, and specifically in the brain, gut, and liver. Tyramine is a dietary monoamine closely related to DA and is also a potent releaser of NE. Normally, tyramine is easily broken down by MAO in the gut and liver. However, when MAO is inhibited, a tyramine-rich meal can cause a hypertensive crisis by increasing levels of NE in the sympathetic nervous system—a phenomenon known as the “tyramine reaction.” Because virtually all fatalities in patients taking MAOIs resulted from the ingestion of cheese, this reaction has also been termed the “cheese effect.” Table 1Reference Grady and Stahl16 shows a list of foods to be avoided, along with safer alternatives.
Table 1 Dietary considerations while taking MAOIs (adapted from Grady and StahlReference Grady and Stahl16)
The tyramine effect is a consequence of inhibition by these MAOIs that is both nonselective and irreversible. This combination of effects ensures that both forms of MAO are unable to metabolize tyramine in the gut until new MAO is synthesized. However, by developing MAO-A selective inhibitors, MAO-B in the gutReference Billett17 can remain active in the presence of these drugs, and continue to metabolize tyramine. Thus, MAO-A selective inhibitors have a lower risk of tyramine-induced hypertensive crisis.
On the other hand, reversible inhibition means that high concentrations of other MAO substrates, such as tyramine, can displace the inhibitor from the MAO enzyme, essentially “reversing” its inhibition. That is, a high concentration of tyramine would push its way onto the enzyme active sites and facilitate its own metabolism. As an example, it takes 8 times more tyramine to elicit the same 30 mm-Hg increase in blood pressure during treatment with the RIMA moclobemide compared to the irreversible MAOI tranylcypromine (see Figure 8).Reference Lotufo-Neto, Trivedi and Thase18, Reference Bieck and Antonin19 Thus, for inhibitors of MAO-A, reversibility and selectivity both contribute to the safety of these agents with respect to the tyramine reaction. As a result, reversible inhibitors of MAO-A do not require dietary restrictions for tyramine.

Figure 8 The median pressor dose of tyramine during treatment with MAOIs. The pressor dose (PD30) is the dose of tyramine required to increase supine blood pressure by 30 mm-Hg. The FDA currently allows for PD30 ≥ 40 mg of tyramine as the safe threshold for a medication. Three reference compounds that are selective for MAO-A showed a tyramine pressor dose above this threshold.
Drug–drug interactions
Although the MAOI “cheese effect” creates a memorable story because of its catchy name, drug–drug interactions are potentially more common and likely to be clinically significant. Drug–drug interactions are cited by prescribers as the biggest reason they avoid use of MAOIs, usually because of misunderstandings about what is dangerous, what is not, and how to work around the risks that are real. Some drug combinations can in fact be dangerous, and even lethal, because their mechanisms act in concert with MAO inhibition. The two potentially dangerous mechanisms to combine with MAOIs are (1) blocking the reuptake or enhancing the release of norepinephrine and (2) blocking the reuptake of serotonin.
Hypertensive reactions
Drugs that boost adrenergic stimulation through enhancing the actions of norepinephrine can cause dangerous hypertensive reactions (see Figure 9). Drugs that have been implicated in these interactions include some cold preparations, stimulants, anesthetics containing norepinephrine, and antidepressants. However, over-generalizations have led to some misperceptions regarding the use of these drug classes in combination with MAOIs.

Figure 9 Hypertension caused by sympathomimetic amines and MAOI inhibition. (A) Decongestants work by constricting nasal blood vessels, but they do not typically elevate blood pressure at the doses used. (B) An MAOI given alone (and without the ingestion of tyramine) increases NE, but does not usually cause vasoconstriction or hypertension. (C) The noradrenergic actions of an MAOI combined with the direct alpha 1 stimulation of a decongestant may be sufficient to cause hypertension or even a hypertensive crisis.
Cold preparations
Most of the hypertensive reactions are reported with over-the-counter decongestants that are either removed from the market or highly restricted: namely, ephedrine, pseudoephedrine, and phenylpropanolamine. In practice, this means that phenylephrine is the main agent that should be avoided or used with caution in patients taking an MAOI. Instead, patients should take an antihistamine (any except chlorpheniramine and brompheniramine, which block serotonin reuptake, as well as acting as antihistamines), cough medicine, or expectorant including codeine. Dextromethorphan is an opiate derivative in cough medicines that should also be avoided, not because it is an opiate, but because it also blocks serotonin reuptake. Table 2 outlines drugs that should be avoided because of their potential to cause hypertension.Reference Grady and Stahl16, Reference Wimbiscus, Kostenko and Malone20, Reference Stahl and Felker21
Table 2 Drugs with the potential to cause hypertensive episodes when taking MAOIs (adapted from Grady and StahlReference Grady and Stahl16)

*Not available in the U.S.
Antidepressants and stimulants
Similarly, other agents that boost norepinephrine should also be avoided or used with caution. Stimulants such as methylphenidate and amphetamine both act on the NE uptake pump, while amphetamine also increases NE release. Many antidepressants block the NE uptake pump as part of their action. This includes many of the tricyclic antidepressants, as well as NE reuptake inhibitors (NRIs), 5-HT/NE reuptake inhibitors (SNRIs), and NE/DA reuptake inhibitors (NDRIs). They are used in conjunction with MAOIs by experienced practitioners in cases where the risk is outweighed by the potential benefit, but are not recommended for routine combination with MAOIs. Thus, the list of medications that should be avoided is short, and there are adequate alternatives.
Anesthetics
Anesthetics often contain epinephrine, norepinephrine, or levonordefrin to modulate the effects of the anesthetic. As a result, it has often been presumed that anesthetics should not be used in conjunction with MAOIs. However, the actual clinical evidence for a hypertensive effect with MAOIs and administered norepinephrine is not compelling,Reference Elis, Laurence, Mattie and Prichard22–Reference Horwitz, Goldberg and Sjoerdsma24 and some have suggested that catechol O-methyl transferase (COMT) plays a role in norepinephrine signal termination, in effect compensating for the MAO inhibition.Reference Yagiela, Duffin and Hunt25 However, these studies were performed on small numbers of healthy subjects, and so care should be taken when using local anesthetics containing epinephrine or norepinephrine. Furthermore, inhaled general anesthetics sensitize the heart to the effect of catecholamines and should also be used with care.
In reality, when taking MAOIs, it is better to use local anesthetics that do not contain vasoconstrictors. For elective surgery, use of the MAOI should be discontinued for the full recommended washout period. For urgent surgery while taking an MAOI, benzodiazepines, mivacurium, rapacuronium, fentanyl, morphine, and codeine may be used cautiously.Reference Wells and Bjorksten26
Serotonin syndrome
Serotonin syndrome, also known as serotonin toxicity, is a complication of drug pharmacotherapy that results from excessive serotonin activity in the brainstem, usually caused by the concurrent use of two or more serotonin-increasing drugs. The syndrome is sometimes life-threatening, and can be difficult to identify because the symptoms are often varied and nonspecific. These symptoms fall into three classes—neuromuscular hyperactivity (e.g., tremor or rigidity), autonomic hyperactivity (e.g., fever or elevated heart rate), and altered mental state (agitation, excitement, or confusion).Reference Sun-Edelstein, Tepper and Shapiro27 MAOIs are the most commonly implicated drugs, most often in combination with SSRIs or TCAs.Reference Hilton, Maradit and Moller28 Furthermore, since the symptoms of serotonin toxicity can present abruptly and progress quickly, these combinations must be avoided.
Antidepressants
Because many of the drugs used for depression have some effect on serotonergic pathways, the perception that MAOIs are incompatible with antidepressants has become common. However, there are many antidepressants that do not significantly affect serotonin levels, including trazodone and some others. The antidepressants that should completely be avoided when taking MAOIs are those that block serotonin reuptake (see Table 3).Reference Grady and Stahl16 The main issue regarding serotonin reuptake inhibitors and MAOIs is how to safely switch from an SSRI to an MAOI, or vice versa. When a patient stops taking an SSRI, the drug is not immediately cleared from the body. There is a time period during which the drug level in the body slowly declines. Taking an MAOI during this period poses an increased risk of serotonin toxicity.
Table 3 Drug with the potential to cause serotonin syndrome when taken with MAOIs (adapted from Grady and StahlReference Grady and Stahl16)

Fortunately, the length of time a drug requires to “wash out” of the system is clearly noted on the Prescribing Information for the various SSRIs. This washout period represents 5 half-lives of the drug, which ranges from 5–7 days for many drugs of this class to 5 weeks for fluoxetine. Thus, the patient simply needs to taper down his or her dose and then observe the washout period before starting an MAOI (see Figure 10).Reference Stahl29 If the patient needs relief from depressive symptoms during this tapering and washout period, there is a wide array of “bridging” medications available. Similarly, when switching from an MAOI to another antidepressant, a washout period of 2 weeks has been required for irreversible MAOIs before starting an SSRI. However, based on the terminal half-life of CX157 of 39 hours, this washout period could potentially be reduced to 8 days.

Figure 10 Appropriate dosing schedule when switching from an SSRI to an MAOI. In order to avoid the risk of serotonin syndrome, as well as side effects from withdrawal, the dose of the SSRI should be gradually stepped down. Then a washout period of 4–5 half-lives should be observed to allow the SSRI to adequately clear from the body before starting an MAOI. The titration schedule may vary, depending on the individual MAOI agent. When switching in the other direction, the washout period must be observed, but MAOIs generally do not require stepping down the dose.
Tricyclic antidepressants
Tricyclic antidepressants are perceived as having a dangerous set of side effects because of their multiple pharmacological effects. However, as we have discussed previously, the only drugs that should be completely avoided involve those with actions on serotonin, which includes clomipramine. Other TCAs may be used with caution, including cyclobenzaprine, carbamazepine, and oxcarbazepine, which do not block serotonin or norepinephrine uptake.
Opioids and painkillers
MAOIs do not interact with opioid mechanisms, but some opioids are serotonin reuptake inhibitors and these must be avoided. Thus, the association of opiates with MAOI drug interactions stems from their actions on serotonin pumps, not their opiate actions. Accordingly, meperidine is a potent serotonin reuptake inhibitor, and methadone and tramadol are weak serotonin reuptake inhibitors and should be avoided. Dextromethorphan, which we mentioned earlier, is an opiate that also has potent SSRI action. In reality, if a patient needs pain relief, many alternatives are available for those using MAOIs. Morphine, fentanyl, codeine, oxycodone, hydrocodone, suboxone, and NSAIDs can all be used.
Navigating the Myths and Realities of MAOI Drug Interactions
Many of the concerns about drug interactions with MAOIs arose from real risks that were generalized for the wrong reasons. Armed with a clear understanding of the mechanism behind hypertensive reactions and serotonin syndrome, medical practitioners will find that they have a wide array of options available to them to treat a similarly wide variety of patients and circumstances. Table 4 summarizes the alternatives that physicians may consider for patients taking MAOIs.
Table 4 Medications that may be used in conjunction with an MAOI without substantial risk of serotonin syndrome or hypertensive crisis
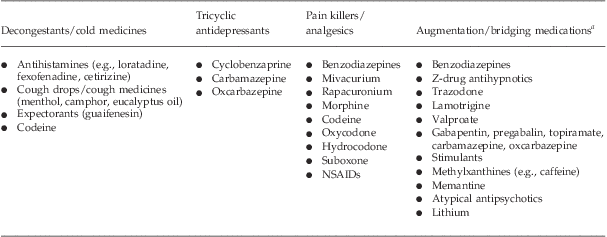
aMay be used with caution by experienced practitioners as augmentation to an MAOI, when switching from an MAOI to an SSRI, or from an SSRI to an MAOI.
Reversible Inhibitors of Monoamine Oxidase-A (RIMAs)
RIMAs represent a new class of drug in the treatment of depression. While most currently used antidepressant drugs have targeted their effect on neurotransmitter reuptake mechanisms, RIMAs act through modulation of the metabolism of neurotransmitters, and specifically on MAO-A. Recent studies have suggested that overactive MAO-A may be at the root of many depressive disorders.Reference Meyer, Ginovart and Boovariwala12 A further investigation of MAO-A levels in SSRI-treated patients showed that these enzyme levels continue to be elevated even when symptoms remit, suggesting that MAO-A depletion of neurotransmitters is upstream of the reuptake mechanism.Reference Meyer, Wilson and Sagrati13
By way of analogy, SSRIs and SNRIs may act by increasing the level of neurotransmitters within the synapse (Figure 11B). However, because MAO-A is still overactive in this circumstance, the overall neurotransmitter levels in the neuron may still be depleted. MAOIs, on the other hand, may reduce the effect of elevated MAO-A activity and increase levels of neurotransmitters in the neuron, as well as in the synapse (Figure 11C). Irreversible MAOIs such as tranylcypromine and phenelzine are powerful antidepressants, and are also effective for certain anxiety disorders such as panic disorder and social phobia. However, their use in treating depression is usually avoided in favor of SSRIs, for the safety and tolerability reasons previously discussed. As a result, MAOIs may account for less than 0.1% of antidepressant prescriptions.Reference Raymond, Morgan and Caetano30–Reference Coupland, Dhiman and Barton33 However, SSRIs and SNRIs have a different set of side effects, including weight gain and sexual dysfunction.

Figure 11 The effect of SSRI/SNRIs and MAOIs on neurotransmitter levels in the synapse. (A) Neurotransmitter levels in the neuron may be depleted as a result of elevated MAO-A activity. (B) SSRI/SNRI antidepressants hypothetically work by increasing synaptic levels of neurotransmitters, represented here by a watering can. However, because MAO is unaffected, the neurotransmitter levels in the neuron may remain depleted. (C) By reducing the activity of MAO-A with an MAOI, the levels of neurotransmitters may be increased more broadly, in the neuron as well as in the synapse.
Potential advantages of RIMAs
By selectively inhibiting MAO-A and avoiding irreversible inhibition, the new class of RIMAs has addressed the major safety concerns of dietary and drug interactions leading to hypertension. Moclobemide is an example of a RIMA that has demonstrated the potential of this new class of drugs. Although not approved for use in the United States, moclobemide has a long record of use in European markets. During this time, numerous head-to-head studies have compared moclobemide with other antidepressants. Brofaromine has demonstrated its efficacy and tolerability in numerous studies involving active comparators. However, it is no longer under investigation due to a corporate decision for reasons that are apparently unrelated to its efficacy and side effect profile.
A meta-analysis of 8 studies of moclobemide has shown that it produces response rates at least as high as SSRIs such as fluvoxamine and fluoxetine, with a mean response rate of 67.9% (SD = 3.8%) among those that completed an adequate treatment (AT) trial. This response rate was actually 4.8% (SD = 4.1%) higher than for the SSRIs,Reference Lotufo-Neto, Trivedi and Thase18 which is a small but reliable advantage in favor of moclobemide.
The safety and tolerability studies of 2203 patients treated with moclobemide revealed only one incidence of hypertensive crisis (< 0.1%). The most frequently reported adverse events that were more frequent than in placebo were dizziness, nausea, and insomnia. In comparison to patients treated with TCAs, moclobemide was associated with lower incidence of dry mouth, sweating, tremor, somnolence, dizziness, blurred vision, and sexual side effects. However, moclobemide therapy resulted in significantly more complaints of headaches and insomnia than TCAs.
In a meta-analysis of 6 studies, brofaromine showed a response rate of 66.7% (SD = 7.4%) among the AT group, with a slight advantage of 0.2% (SD = 17.6%) in favor over the active comparators, which included imipramine, maprotiline plus lithium, and tranylcypromine. The tolerability of brofaromine was either significantly better tolerated than imipramine (two studies) and tranylcypromine (two studies) or showed no difference (three studies). Brofaromine was generally better tolerated than the maprotiline plus lithium combination.
Furthermore, a meta-analysis of two studies comparing moclobemide with an older MAOI revealed a potential opportunity for improvements in treatment using RIMAs. In comparison with tranylcypromine, the mean response rate for moclobemide in the adequately treated group was 71.6% (SD = 6.8%), a response rate that was 13.3% (SD = 6.8%) less than for tranylcypromine. The higher response rate for this first-generation MAOI suggests the intriguing possibility that, since these agents work on a similar mechanism, the RIMA class may have some room for improvement in reaching for the efficacy of its MAOI predecessors.
Another RIMA, CX157, is currently in phase II clinical trials for TRD. This agent has demonstrated its ability to selectively target MAO-A with high potency in the CNS, as well as demonstrating rapid reversibility. In vitro measurements show that CX157 inhibits MAO-A in human brain tissues with high potency (IC50 = 3.3 nanomolar).Reference Fowler, Logan and Azzaro34 This represents a 100-fold increase in potency over moclobemide (data on file). On the other hand, CX157 shows no inhibition of MAO-B at up to 10 micromolar concentrations.
Brain imaging studies using the MAO-A specific radioligand [11C]clorgyline showed that treatment with a 60 mg dose of CX157 reaches mean plasma levels of 60.6 ± 84.4 ng/mL within 2 hours, and significantly reduces (by almost 50%) the amount of clorgyline binding (see Figure 12). That amount of binding returns to 80% of its original amount within 12 hours after administration of CX157, demonstrating that its inhibition is readily reversible. Furthermore, these studies also showed that plasma levels of drug correlate with the degree of MAO-A inhibition in the brain. This characteristic is unique among the RIMA class and may provide a useful tool to optimize the dose for the maximum therapeutic effect while minimizing side effects.Reference Fowler, Logan and Azzaro34

Figure 12 Brain imaging of MAO-A inhibition. Binding of MAO-A by [11C ]clorgyline is presented on a rainbow scale with red indicating high binding.Reference Fowler, Logan and Azzaro34 (A) No CX157 treatment. (B) [11C ]clorgyline binding 2 hours after treatment with 60 mg of CX157. (C) [11C ]clorgyline binding returns to 80% of its original levels 12 hours after administration of CX157. Adapted from Fowler (2010).Reference Fowler, Logan and Azzaro34
Additionally, a tyramine challenge study with CX157 at 250 mg/day (125 mg BID) has shown that therapeutic levels of the medication are unlikely to present a risk of dietary-induced hypertension. Studies have shown that the terminal half-life of ingested tyramine ranges from about 30 minutes in the fasted state, to slightly less than an hour when taken with a meal (see Table 5).Reference VanDenBerg, Blob, Kemper and Azzaro35 Hypothetically, with a tmax significantly more than an hour, a drug could potentially mitigate its proximal effect on MAO-A in the gut. A modified release tablet formulation of CX157 administered at 125 mg twice daily has demonstrated a 2 hour lag, followed by rapid absorption with a tmax at 3.4 hours.36 The drug reached steady state within 3 days with a mean steady state concentration of 87.2 ng/mL. The effect of dietary tyramine on blood pressure was measured in subjects before CX157 treatment, and then compared to the tyramine effect in these same subjects after 7 days of treatment with CX157 (see Figure 13A). Out of 10 subjects, none of them experienced a measurable pressor effect up to a tyramine dose of 80 mg.These results are in contrast to a similar study (Figure 13B) performed using tranylcypromine in which all of 9 subjects experienced a severe pressor effect while on the drug after receiving 10 mg of administered tyramine.Reference Azzaro, Vandenberg and Blob37
Table 5 Pharmacokinetic parameters for tyramine and modified release CX157

SD=standard deviation.

Figure 13 Individual tyramine pressor response on medication. (A) After 7 days of twice daily dosing with 125 mg CX157, no pressor effect was observed in any of the subjects upon administration of up to 80 mg of tyramine. Adapted from Burch etal.36 (B) After 10 days dosing subjects with 20 mg tranylcypromine in the morning and 10 mg in the afternoon, all 9 subjects experienced a severe pressor effect upon administration of 10 mg tyramine.Reference Azzaro, Vandenberg and Blob37
Advantages of a RIMA within the antidepressant landscape
RIMAs have a triple action in enhancing DA, as well as 5-HT and NE. This mechanism of action suggests that they may have efficacy for patients who have failed 1–3 previous antidepressants that act only on 5-HT and/or NE reuptake. Moclobemide and brofaromine have been shown to be effective and well-tolerated antidepressants. However, they are not available in the U.S. market. The current treatments for TRD include augmentation of SSRI/SNRI antidepressants with antipsychotics, which introduce an additional side effect burden (see Figure 14). CX157 has the potential to be the first monotherapy for TRD that does not exhibit the side effects associated with antipsychotics and SSRI/SNRI antidepressants, and is the first treatment to work for patients who have failed more than 1 prior antidepressant. Thus, the efficacy, safety, and tolerability profile of CX157 may fill an unmet need within the treatment landscape. Additionally, because of the selectivity of CX157 for MAO-A, as well as its reversible inhibition, the medication will require no dietary restrictions.

Figure 14 The efficacy, safety, and tolerability profile of CX157 may bridge an unmet need in the treatment of TRD. CX157 may be effective in cases where SSRI/SNRIs fail to achieve remission, while its safety and tolerability profile does not have many of the side effects associated with ECT or antipsychotics.
Furthermore, due to their reversible enzyme inhibition, RIMAs would theoretically be safer for drug interactions with agents that inhibit 5-HT or NE reuptake. The increased level of monoamines could potentially reverse the MAO inhibition and allow degradation of the monoamines 5-HT and NE, thereby avoiding a buildup of these neurotransmitters. This is theoretical, however, and needs to be shown.
In cases where there is a need to stop CX157 in order to start another antidepressant, the pharmacokinetics of CX157 suggest that its washout period may be more rapid than for many other antidepressants.
Many of the barriers to prescribing RIMAs are based on misperceptions or lack of information and can be countered by education. The facts support many safe avenues for MAO inhibitor use in the treatment of depression, with multiple practical tips and “work-around” solutions available for the unrestricted use of RIMAs in clinical practice.
Disclosures
Christopher T. Lum has nothing to disclose. Stephen M. Stahl is a consultant for Acadia, AstraZeneca, Avanir, Biomarin, Bristol-Myers Squibb, CeNeRx, Dey, Eli Lilly and Company, Forest, Genomind, GlaxoSmithKline, Johnson and Johnson, Jazz, Lundbeck, Merck, Neuronetics, Novartis, Noven, ONO, Orexigen, Otsuka, Pamlab, Pfizer, PGxHealth, RCT Logic, Rexahn, Roche, Servier, Shire, Solvay, Sunovion, Trius, and Valeant. He is on the speakers bureau for Arbor Scientia, AstraZeneca, Eli Lilly and Company, Forest, Johnson and Johnson, Merck, Neuroscience Education Institute, Pfizer, Servier, and Sunovion. He has received research grants from AstraZeneca, CeNeRx, Eli Lilly and Company, Forest, Genomind, Merck, Neuronetics, Pamlab, Pfizer, Roche, Schering-Plough, Sepracor, Servier, Shire, Sunovion, Torrent, and Trovis.





















