Introduction
Stroke is the number one cause of long-term disability in adults worldwide.Reference Mozaffarian, Benjamin and Go1 A particularly disabling syndrome in these patients is hemispatial neglect. This syndrome is defined as the failure to detect, respond, or orient toward stimuli located in the hemi-body and/or hemispace contralateral to the lesioned hemisphere.Reference Heilman, Valenstein and Watson2 Generally, hemispatial neglect is associated with unfavorable post-stroke recovery. Patients that suffer from this syndrome show slower functional progress during rehabilitation and need longer hospitalization times.Reference Cherney, Halper and Kwasnica3 Hemispatial neglect is also an independent predictor of limited post-stroke functional independenceReference Di Monaco, Schintu and Dotta4 and lower likelihood of being discharged home.Reference Wee and Hopman5 Therefore, the development of innovative interventions, supporting the elimination of this syndrome, is urgently needed. Repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS) are innovative techniques that can modulate neural processing within the cortex.Reference Giordano, Bikson and Kappenman6, Reference Siebner and Rothwell7 Both techniques have the potential to antagonize maladaptive neuroplasticity after a stroke and support recovery of the neglect syndrome.Reference Jacquin-Courtois8 This paper reviews the available data of these noninvasive approaches in order to facilitate recovery of hemispatial neglect after stroke.
Hemispatial neglect
The prevalence of hemispatial neglect in acute stroke ranges from 30% to 81%.Reference Arene and Hillis9, Reference Pedersen, Jørgensen and Nakayama10, Reference Stone, Halligan and Greenwood11 The neglect syndrome is most frequently associated with neural damage involving the temporal, parietal, and occipital lobes, and the basal ganglia and thalamus.Reference Ringman, Saver and Woolson12 Generally, hemispatial neglect occurs more frequent, more severe, and more persistent after right than after left hemispheric lesions.Reference Stone, Halligan and Greenwood11, Reference Ringman, Saver and Woolson12 The pathophysiology of the syndrome is linked to a dysfunction of cortical networks within the right hemisphere, involving the posterior parietal cortex, which plays a dominant role in visuospatial attention. Therefore, damage within the right hemisphere often induces neurological disorders characterized by hemispatial neglect.Reference Heilman, Valenstein and Watson2, Reference Vallar, Bottini and Paulesu13
A theory to explain the existence of hemispatial neglect after brain lesion is the interhemispheric rivalry model. It describes reciprocally interactive inhibitory processes exerted by both hemispheres toward one another via the corpus callosum.Reference Kinsbourne14 In the healthy brain, there is an out-weighted balance between both hemispheres regarding reciprocal inhibition, and attention is deployed within the entire extracorporal space with each hemisphere attending the contralateral space. After unilateral brain lesion, this in-between hemispheric balance is disrupted, for example, the contralesional hemisphere is disinhibited and therefore enhanced, what then again may cause stronger inhibition toward the ipsilesional hemisphere. Clinically, a shift of spontaneous spatial attention away from midline toward the ipsilesional side may occur, which leads to an avoidance of the contralesional hemispace and at the same time increased exploration of the ipsilesional hemispace.Reference Hilgetag, Kötter and Young15, Reference Karnath16 This theory received support from several studies. A functional magnetic resonance imaging (fMRI) longitudinal study demonstrated that spatial attention deficits in neglect after right frontal damage correlate with abnormal activation of structurally intact dorsal and ventral parietal regions.Reference Corbetta, Kincade and Lewis17 The recovery from neglect correlates with restoration and rebalance of neural activation within these regions.Reference Corbetta, Kincade and Lewis17 A TMS study demonstrated an increase of functional connectivity between the contralesional posterior parietal cortex and the contralesional primary motor cortex in stroke subjects with hemispatial neglect.Reference Koch, Oliveri and Cheeran18 Amelioration of hemispatial neglect was accompanied by a normalization of enhanced connectivity between the posterior parietal and the primary motor cortices of the unaffected hemisphere.Reference Koch, Oliveri and Cheeran18 Despite its limitations, the theory of interhemispheric imbalance and rivalry provides the theoretical framework for the use of noninvasive brain stimulation techniques in the rehabilitation of hemispatial neglect after stroke.
Noninvasive brain stimulation
rTMS and tDCS are noninvasive brain stimulation techniques.Reference Giordano, Bikson and Kappenman6, Reference Siebner and Rothwell7 Both techniques have been used to improve clinical symptoms in neglect syndromes.Reference Song, Du and Xu19–Reference Turgut, Miranda and Kastrup22 The rationale for their application in neglect syndromes after brain damage is the fact that tDCS and rTMS either facilitate or inhibit cortical excitability and thereby neural processing within the stimulated brain areas for time periods outlasting the stimulation period.Reference Giordano, Bikson and Kappenman6, Reference Siebner and Rothwell7 Within the theoretical framework of interhemispheric rivalry, postulating an “overactive” contralesional hemisphere and a “suppressed” ipsilesional hemisphere, the application of noninvasive brain stimulation may outbalance cortical excitability in between both hemispheres by either facilitation of the ipsilesional hemisphere or, alternatively, inhibition of the contralesional hemisphere.Reference Jacquin-Courtois8 TDCS applies a low-intensity current via two electrodes (anode and cathode) placed on the scalp. One of the electrodes is positioned over the target area (active electrode), the other (reference electrode) over another cranial or extracranial position.Reference Nasseri, Nitsche and Ekhtiari23 Anodal stimulation (anode over the target area) induces a depolarization of cortical neurons and thereby an increase of cortical excitability. Cathodal stimulation (cathode over the target area) induces hyperpolarization and decreases cortical excitability.Reference Nitsche and Paulus24
During TMS, a magnetic coil is placed on the scalp overlying the cortical target area. Discharging the electromagnetic coil induces a current flow within the cortex. Depending on frequency, rTMS may increase (representing long-term potentiation) or decrease (representing long-term depression) motor cortical excitability. High-frequency rTMS (≥5 Hz), intermittent theta burst stimulation (iTBS), and paired-pulse stimulation (inter-stimulus interval 1.5 ms) cause an increase of the motor cortex excitability; low-frequency rTMS (1 Hz), continuous theta burst stimulation (cTBS), and paired-pulse stimulation (inter-stimulus interval 3 ms) cause a decrease of the motor cortex excitability.Reference Lang, Siebner, Siebner and Ziemann25 However, recent studies indicate that the responses to excitatory and inhibitory noninvasive brain stimulation protocols are highly variable between individuals.Reference Hamada, Murase and Hasan26, Reference Wiethoff, Hamada and Rothwell27 The factors responsible for the inter-individual variability of the effect of noninvasive brain stimulation on cortical excitability are not completely understood.
Methods
Studies included
We reviewed the PubMed database prior to May 30, 2017, for papers reporting on the use of noninvasive brain stimulation in rehabilitation of hemispatial neglect after stroke. The search terms “transcranial direct current stimulation” and “neglect,” and “repetitive magnetic transcranial stimulation” and “neglect” were used. Studies matching the following criteria were included: (1) human studies, (2) prospective studies, (3) diagnosis of stroke and hemispatial neglect, (4) tDCS or/and rTMS used as intervention for improving hemispatial neglect, (5) assessment of hemispatial neglect before and after the intervention, and (6) placebo-controlled study or study with at least two experimental groups.
Outcomes
The intensity of hemispatial neglect, its change after intervention, the stimulation techniques and stimulation parameters used (rTMS or tDCS), the stimulated hemisphere (ipsilesional/contralesional/bilateral), the stimulated brain regions (P3/P4/P5/P6 of the international electrode positioning system, representing posterior parietal cortex of the left (P3/P5) or right (P4/P6) hemisphere, respectively), stimulation duration, stimulation intensity), characteristics of subjects included (time since stroke, stroke etiology – ischemic/hemorrhagic, stroke location – cortical/subcortical, affected hemisphere – right/left), and study design (crossover/parallel groups, presence/absence of follow-up, presence/absence of additional intervention) were all assessed.
Data analysis
Effect size and the 95% confidence intervals were calculated for each study to evaluate the efficiency of non-invasive brain stimulation for recovery from hemispatial neglect. The effect size was calculated either based on means and SD of repeated measures (pre and post) or based on means and SD of pre–post differences. For studies using more than one hemispatial neglect assessment, effect size and confidence intervals were calculated for each assessment. Finally, means were calculated for each study and a forest plot was constructed. For interpretation, the Cohen definition of effect size was used (d = 0.2 “small,” d = 0.5 “medium”, d = 0.8 “large”). Effect size calculation provides a possibility to compare the effectivities of treatments reported in different trials. However, it should be kept in mind that comparing effect sizes (e.g. between studies evaluating various therapeutic strategies) is useful only in case the method of calculating effect size is comparable between the studies included. Studies that differ substantially regarding design or methodology could taint the comparison of effect size and therefore cause incorrect conclusions.Reference Aarts, van den Akker and Winkens28
Results
Transcanial direct current stimulation
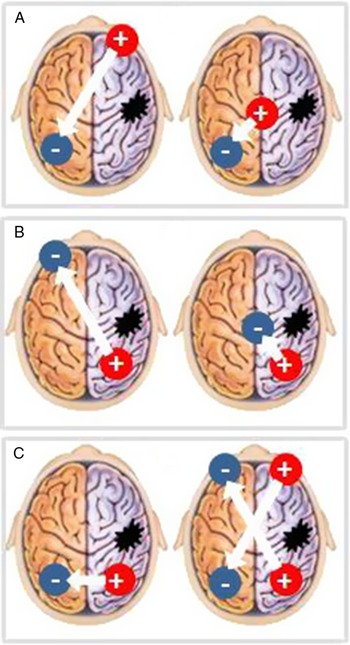
A total of eight studies were detected. All tested the effect of tDCS to improve hemispatial neglect after stroke.Reference Sunwoo, Kim and Chang20, Reference Turgut, Miranda and Kastrup22, Reference Bang and Bong29–Reference Yi, Chun and Do34 A total of 146 stroke subjects were included. Studies were heterogeneous regarding study population included and methods used (Table 1). Serious adverse events were not described. Figure 1 illustrates the electrode positions used in the studies.
TABLE 1. Overview of studies investigating tDCS for recovery of hemispatial neglect.

Notes: mA = milliampere; na = not available, not applicable; and tDCS = transcranial direct current stimulation.

FIGURE 1. Positioning of the anode and of the cathode during (A) inhibitory tDCS over the contralesional hemisphere, (B) facilitatory tDCS over the ipsilesional hemisphere, and (C) bilateral stimulation. + = anode; – = cathode.
Facilitatory tDCS over the ipsilesional hemisphere
Five controlled trials tested the effect of anodal tDCS over the ipsilesional hemisphere. One study revealed a significant improvement of hemispatial neglect with 2 mA anodal tDCS, compared to sham treatment.Reference Làdavas, Giulietti and Avenanti31 The remaining studies showed a significant improvement with 1 or 2 mA anodal tDCS (compared to sham stimulation) but only for some of the performed tests.Reference Sunwoo, Kim and Chang20, Reference Ko, Han and Park30, Reference Sparing, Thimm and Hesse33, Reference Yi, Chun and Do34
Facilitatory tDCS over the contralesional hemisphere
Only one study investigated the efficiency of 1 mA anodal tDCS over the contralesional hemisphere.Reference Sparing, Thimm and Hesse33 The results showed no treatment-related improvement of hemi-spatial neglect, compared to sham tDCS.
Inhibitory tDCS over the contralesional hemisphere
Three studies probed the effect of cathodal tDCS over the contalesional hemisphere in comparison to sham stimulation. Two of them revealed significant treatment-related positive effects of real 2 mAReference Yi, Chun and Do34 and real 1 mAReference Sparing, Thimm and Hesse33 tDCS, again only for a sub-group of hemispatial neglect tests performed. One study detected a deteriorating effect of real 2 mA tDCS.Reference Làdavas, Giulietti and Avenanti31
Bilateral tDCS
Four controlled studies tested the efficiency of bilateral tDCS. Two studies revealed a significant improvement of hemispatial neglect with 1 mA bilateral tDCS, compared to sham stimulation.Reference Sunwoo, Kim and Chang20, Reference Bang and Bong29 One study showed a significant improvement with 1.5–2 mA bilateral tDCS combined with optokinetic treatment in subacute stroke, but only in a sub-set of tests performedReference Turgut, Miranda and Kastrup22 One study found no treatment-related effect of 2 mA bilateral tDCS on improvement of chronic hemispatial neglect.Reference Smit, Schutter and Nijboer32
Comparison of different protocols
Four trials compared different tDCS protocols to improve hemispatial neglect in stroke. Three of them compared anodal tDCS over the ipsilesional hemisphere with cathodal tDCS over the contralesional hemisphere. One of them found a better recovery with anodal tDCS.Reference Làdavas, Giulietti and Avenanti31 Two found no significant treatment-related effects on hemispatial neglect.Reference Sparing, Thimm and Hesse33, Reference Yi, Chun and Do34 One study compared anodal tDCS over the ipsilesional hemisphere with bilateral tDCS and found a significant greater improvement with bilateral tDCS.Reference Sunwoo, Kim and Chang20
Summary
Despite the very limited amount of data available today, the use of tDCS to improve hemispatial neglect after stroke is a promising approach. Anodal and bilateral tDCS application appears to be more effective than cathodal tDCS (Figure 2). Future studies should differentially probe different tDCS protocols in rehabilitation of hemispatial neglect. Larger patient cohorts and long-term effects should be evaluated.

FIGURE 2. Overview of effect size and 95% confidence interval for neglect outcome measures. CL = contralesional; cTBS = continuous theta burst stimulation; Hz = Hertz; IL = ipsilesional; mA = milliampere; rTMS = repetitive transcranial magnetic stimulation; tDCS = transcranial direct current stimulation; ▪,♦ = repetitive transcranial magnetic stimulation; and □,◊ = transcranial direct current stimulation.
Repetitive transcranial magnetic stimulation
Twelve controlled studies investigated the effect of rTMS on recovery of hemispatial neglect.Reference Koch, Bonnì and Giacobbe35, Reference Song, Du and Xu19, Reference Cazzoli, Müri and Schumacher36–Reference Yang, Fong and Li-Tsang45 These studies included a total of 297 stroke subjects. There is a large variability of methods and of subjects included among these trials (Table 2). No study describes serious adverse events.
TABLE 2. Overview of studies investigating rTMS for recovery of hemispatial neglect.

Notes: aMT = active motor threshold; cTBS = continuous theta burst stimulation; na = not available, not applicable; PPC = posterior parietal cortex; rMT = resting motor threshold; and rTMS = repetitive transcranial magnetic stimulation.
Facilitatory rTMS over the ipsilesional hemisphere
Two placebo-controlled studies investigated the efficiency of facilitatory rTMS applied over the ipsilesional hemisphere.Reference Kim, Chun and Kim40, Reference Yang, Liu and Song44 Both found a significant improvement with 10 Hz rTMS (compared to sham), but one of them only in a sub-set of tests.Reference Kim, Chun and Kim40
Inhibitory rTMS over the contralesional hemisphere
Five controlled studies tested the effect of (cTBS) over the contralesional hemisphere on improvement of hemispatial neglect.Reference Koch, Bonnì and Giacobbe35, Reference Cha and Kim37, Reference Fu, Song and Zhang38, Reference Nyffeler, Cazzoli and Hess42, Reference Yang, Liu and Song44 Four studies found a significant greater improvement with real rTMS (compared to control) in all tests performed, one study in only a sub-set of tests.Reference Cazzoli, Müri and Schumacher36 Eight studies investigated inhibitory 1 Hz rTMSReference Cha and Kim37, Reference Kim, Jung and Shin39, Reference Kim, Chun and Kim40, Reference Lim, Kang and Paik41, Reference Oliveri, Bisiach and Brighina43–Reference Yang, Fong and Li-Tsang45 and 0.5 Hz rTMSReference Song, Du and Xu19 over the contralesional hemisphere. Five of them found a significant greater improvement with real rTMS in each assessment performed.Reference Song, Du and Xu19, Reference Cha and Kim37, Reference Kim, Jung and Shin39, Reference Oliveri, Bisiach and Brighina43, Reference Yang, Liu and Song44 Two studies found a greater improvement with rTMS in only a subset of tests performed.Reference Kim, Chun and Kim40, Reference Yang, Fong and Li-Tsang45 One study showed no supportive effect of rTMS.Reference Lim, Kang and Paik41
Comparison of different protocols
Only two studies compared the efficiency of different rTMS protocols in the rehabilitation of hemispatial neglect. 10 Hz rTMS over the ipsilesional hemisphere was compared to 1 Hz rTMSReference Kim, Chun and Kim40 or cTBSReference Yang, Liu and Song44 over the contralesional hemisphere. The results indicate the best improvement with cTBS and the smallest improvement with 1 Hz rTMS.
Summary
The major part of available data implies a positive influence of rTMS on recovery from hemispatial neglect after stroke. Based on current data, cTBS is the most effective stimulation technique. In contrast, conventional rTMS over the contralesional hemisphere appears to be less efficient (Figure 2). Future studies should compare facilitatory and inhibitory rTMS protocols within larger study cohorts and include long-term follow-up investigations.
Discussion
This review analyzed data from 20 controlled intervention trials including a total of 443 stroke patients with hemispatial neglect. Collectively the data suggest a positive effect of noninvasive brain stimulation to improve hemispatial neglect in patients with stroke, but the current evidence is too small for a routine use in rehabilitation. This is caused by an overall limited number of patients included, heterogeneity of stimulation protocols and assessment regimen, and a very small proportion of studies providing a long-term follow-up.
Stimulation protocols
Inhibitory rTMS over the parietal lobe of the contralesional hemisphere is the most widely used stimulation protocol in rehabilitation of hemispatial neglect after stroke. Its efficiency was tested in 170 patients. Other rTMS protocols were less frequently applied (between 13 and 48 patients). In future, more comparative studies should be designed. In particular, effect size of inhibitory versus facilitatory rTMS and tDCS over the contralesional and ipsilesional hemisphere should be compared.
Bilateral (ipsi- and contralesional) stimulation to improve hemispatial neglect after stroke has been used only when applying tDCS. Future studies should evaluate the potential of bihemispheric rTMS or a combination of rTMS and tDCS. Such stimulation protocols have already been successfully tested in motor rehabilitation after stroke.Reference Turgut, Miranda and Kastrup22, Reference Sung, Wang and Chou46, Reference Takeuchi, Tada and Matsuo47, Reference Wang, Tsai and Yang48
Current data indicate (1) stimulation-method-dependent efficiency and (2) stimulation-protocol-dependent efficiency to improve hemispatial neglect after stroke. Figure 1 illustrates that rTMS appears to be more effective than tDCS in improving hemi-spatial neglect after stroke. In addition, inhibitory stimulation of the contralesional hemisphere appears to be the less effective stimulation protocol. In contrast, bilateral tDCS and cTBS show the best efficiency. However, more data are needed to allow definite conclusions about the efficiency of diverse tDCS/rTMS protocols.
Study design
Current studies investigating rTMS and tDCS to improve hemispatial neglect after stroke differ in many relevant aspects: (1) number of patients included (tDCS:19 participants on average per study, rTMS: 25 patients on average per study), (2) the amount of stimulation sessions (tDCS: eight sessions on average per study and rTMS: 16 sessions on average per study), and (3) long-term follow-up investigation (tDCS: only one study with follow-up over 5 days and rTMS: seven studies with follow-up up to 6 weeks). In consequence, more data are needed to evaluate the long-term effectiveness of the repetitive application of noninvasive brain stimulation for improving hemispatial neglect after stroke.
Only a small part of current studies (four studies evaluating tDCS and four studies evaluating rTMS) mentioned a double-blinded study design. However, blinding of both patients and investigators is critical for the interpretation of meta-analyses. In future, more double-blinded studies are needed to exclude confounders and allow reliable evaluation of the effects of noninvasive brain stimulation on recovery from hemispatial neglect after stroke. Another limitation of the pertinent literature is a lack of elaborate neuro-navigation techniques to position and maintain accurate coil positioning during the rTMS intervention. The majority of studies used coil positioning based on the international 10–20 system.
Patient characteristics
At present, noninvasive brain stimulation techniques have mostly been applied in subacute and chronic stroke survivors with hemispatial neglect. Future trials should investigate the efficiency of rTMS and tDCS in larger study cohorts of acute stroke subjects.
In about one half of all trials, no information about lesion location within the affected hemisphere had been provided. This is a flaw and should change in future studies to allow a proper judgment about lesion location and distribution and its relationship with the effectiveness of brain stimulation techniques. Until today, about three times more stroke subjects with a cortical involvement, as compared to pure subcortical tissue damage, underwent tDCS or rTMS to enhance recovery from neglect.
About a quarter of trials did not provide information regarding stroke etiology. The remaining trials enrolled nearly two times more patients with ischemic stroke than patients with hemorrhagic stroke. Stroke etiology, however, may be relevant as the effectiveness of rTMS and tDCS to overcome hemispatial neglect may differ in ischemic and hemorrhagic stroke, as it may do in different lesion locations and distributions within the brain.
Limitations
The studies included in our review show a large variability of study population (time from stroke, stroke etiology, and stroke location), stimulation protocols used (tDCS/rTMS intensity, duration, number of sessions, stimulated hemisphere, and stimulated area), assessment methods, study design (parallel groups/crossover, and with/without an additional intervention), and methodological quality (blinding, and sham condition technique). All these in-between-study inconsistencies taint the comparison of effect sizes.
Disclosures
Jitka Veldema, Kathrin Bösl, Günter Neumann, Geert Verheyden, and Dennis Alexander Nowak have nothing to disclose.