Introduction
Functional neurological disorders (FND)/conversion disorders (CD) are highly prevalent neurobehavioral conditions at the interface of neurology and psychiatry. Psychogenic nonepileptic seizures (PNES) are a FND/CD subtype where individuals exhibit paroxysmal convulsive events and/or alterations in behavior and consciousness that resemble epileptic seizures (ES) but are not associated with changes in cortical activity.Reference Devinsky, Gazzola and LaFrance 1 PNES and other somatic symptoms were first introduced in the medical literature as “hysteria” and considered identifiable neurological conditions by Jean-Martin Charcot; early psychological theories were postulated by Sigmund Freud, Pierre Janet, and others.Reference LaFrance and Schachter 2 Modern day conceptualizations of PNES now integrate mind and brain.Reference Perez, Dworetzky and Dickerson 3 , Reference Baslet 4
Over the past decade, notable advances have been made in our ability to diagnose and treat patients with PNES. Despite these achievements, epidemiology and healthcare utilization studies highlight a continued unmet medical need in the comprehensive interdisciplinary care of PNES. Advances in clinical neuroscience and systems-level neurobiological studies have started to elucidate a neurobiology for PNES, which offers promise in clarifying the underlying pathophysiology and identifying biomarkers for guiding prognosis and treatment selection. In this article, we review recently published research in adults with PNES, focusing on semiologic features that distinguish PNES from ES, newly published diagnostic criteria, the intersection of PNES and other psychiatric and neurological comorbidities, neurobiological studies, evidence-based treatment interventions, and outcome studies.
The Scope of the Unmet Medical Need
PNES occur worldwide, and in the United States up to 20% of civilians and up to 25% of Veterans diagnosed as having epilepsy actually have PNES,Reference Salinsky, Evrard, Storzbach and Pugh 5 making PNES as common as multiple sclerosis and Parkinson disease.Reference Hirtz, Thurman, Gwinn-Hardy, Mohamed, Chaudhuri and Zalutsky 6 Comprehensive epidemiological investigations are lacking in PNES; however, the estimated prevalence of PNES is up to 33/100,000 individuals.Reference Asadi-Pooya and Sperling 7 The incidence rate from a prospective study of 367,566 individuals followed over 3 years found that new-onset PNES was observed in 4.9/100,000/year.Reference Duncan, Razvi and Mulhern 8 As is typical for PNES, individuals were 80% women with a mean age of onset of 31 (±15) years. The majority of individuals with PNES were unemployed, and 46% were previously diagnosed with anxiety or depression; 57% of patients also had at least one additional medically unexplained symptom (MUS), highlighting the overlap between PNES and other FNDs. The coincidence of PNES with ES is approximately 10%.Reference Duncan and Oto 9
The economic and healthcare utilization costs incurred in caring for PNES are substantial. As one illustrative example, 15 of 28 subjects with PNES accounted for 34 emergency room visits, and 12 of 28 individuals accounted for 66 inpatient hospital days pre-diagnosis.Reference Razvi, Mulhern and Duncan 10 In a retrospective study from Ireland, the annual estimated cost per patient with undiagnosed PNES was 20,995 euros.Reference Magee, Burke, Delanty, Pender and Fortune 11 While healthcare expenditures decrease substantially post-diagnosis,Reference Razvi, Mulhern and Duncan 10 , Reference Magee, Burke, Delanty, Pender and Fortune 11 multiyear delays in diagnosis following symptom onset, continued PNES events, unemployment, and medical disability perpetuate healthcare and economic costs.
Further compounding the problem, many healthcare providers report frustration when caring for patients with PNES. In a semistructured interview of 75 healthcare clinicians and workers in the Veterans Health Administration system, while some providers endorsed sentiments of hope, many expressed frustration over the complexity of PNES, which included difficulties with patient acceptance of the diagnosis, uncertainties in treatment interventions, and a failure of cross-disciplinary collaborations.Reference McMillan, Pugh and Hamid 12 While neurologists increasingly recognize the scope of the unmet medical need, many remain uncomfortable discussing somatoform symptoms with patientsReference Monzoni, Duncan, Grunewald and Reuber 13 ; psychiatrists and other mental healthcare providers may also potentially feel ill-equipped to treat individuals with PNES.
Semiologic Differentiation of PNES from ES and Diagnostic Criteria
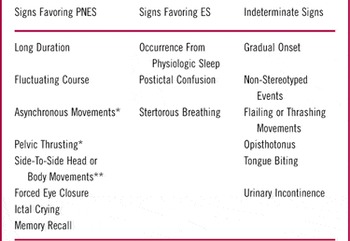
Differentiating PNES from ES can be clinically challenging, and several studies have investigated clinical signs that aid the diagnosis of PNES.Reference Devinsky, Gazzola and LaFrance 1 , Reference Avbersek and Sisodiya 14 A retrospective review detailing signs that reliably distinguished PNES from ES suggested that a diagnosis of PNES was favored for events showing a long duration, a fluctuating course, asynchronous or side-to-side movements, ictal eye closure at onset, ictal crying, and post-ictal recall of information when presented ictally (see Table 1). In addition, urinary incontinence and tongue biting do not reliably distinguish between ES and PNES.Reference Brigo, Nardone and Ausserer 15 A prospective study of 120 seizures in 35 consecutive subjects showed that video-documented preserved awareness, eye fluttering, and the modulation of event intensity by bystanders reliably predicted PNES; abrupt onset, ictal eye-opening, and post-ictal confusion/sleep reliably predicted ES.Reference Syed, LaFrance and Kahriman 16 It is also worth noting that apart from differentiating PNES from ES, additional diagnostic considerations should also be evaluated and ruled out, including paroxysmal movement disorders, panic attacks, and physiologic forms of nonepileptic events including cardiac arrhythmias among other conditions.
Table 1 Semiologic features that support the diagnosis of psychogenic nonepileptic seizures (PNES) vs. epileptic seizures (ES).

* Indicates that sign may not reliably differentiate between PNES and frontal lobe partial epileptic seizures.
** Indicates that sign may only be helpful in distinguishing convulsive PNES and ES.
Adopted from Avbersek and SisodiyaReference Avbersek and Sisodiya 14 with permission.
While semiological features may suggest a diagnosis of PNES over ES, the gold standard for diagnosis is the recording of typical event(s) on video electroencephalography (vEEG) and noting a lack of epileptiform activity in the peri-ictal period, with semiologic and historical consistency with PNES. While this is the diagnostic work-up of choice, access to vEEG may not be available, and some patients may have sufficiently low event frequencies that hospital admission for long-term monitoring is impractical. Recently, the International League Against Epilepsy (ILAE) Commission on Neuropsychobiology Nonepileptic Seizures Task Force published consensus guidelines on the minimum requirements to diagnosis PNES (see Table 2).Reference LaFrance, Baker, Duncan, Goldstein and Reuber 17 Levels of diagnostic certainty are based on the clinical history, ictal semiology of an event, and EEG findings. To meet criteria for documented PNES, the clinical history should be consistent with PNES and habitual events must be recorded on vEEG demonstrating the absence of epileptiform activity. Lower levels of certainty (clinically established, probable, possible) are based on availability of diagnostic components.
Table 2 Diagnostic levels of certainty for psychogenic nonepileptic seizures

+, history characteristics consistent with PNES; PNES, psychogenic nonepileptic seizures; ES, epileptic seizures; EEG, electroencephalogram.
* Captured ictus should not resemble types of ES which thatmay not show ictal epileptiform correlate on EEG (e.g., simple partial epileptic seizures).
Modified from Ref. Reference LaFrance, Baker, Duncan, Goldstein and Reuber17 [LaFrance WC Jr, Baker GA, Duncan R, et al. Minimum requirements for the diagnosis of psychogenic nonepileptic seizures: a staged approach: a report from the International League Against Epilepsy Nonepileptic Seizures Task Force. Epilepsia 2013;54(11):2005—2018]. With permission.
Given the clinical need to clarify a diagnosis of PNES vs. ES, whenever possible, seizure induction protocols have drawn increasing interest. While ethical concerns have been raised regarding provocation techniques, given that they may be perceived as misleading, studies suggest that use of conventional EEG activation procedures, including hyperventilation and photic stimulation, may be effective adjunctive techniques in the diagnosis of PNES,Reference Hoepner, Labudda, Schoendienst, May, Bien and Brandt 18 making provocation unnecessary. Providing correct and explicit information about the potential utility of standard induction protocols may alleviate ethical concerns and aid diagnostic evaluations. Surface electromyography recordings may also potentially aid differentiation of convulsive ES and PNES events.Reference Beniczky, Conradsen and Moldovan 19 Approximately twice normal serum prolactin levels drawn 10–20 minutes following an ictal event can also help differentiate convulsive ES from PNES.Reference LaFrance, Baker, Duncan, Goldstein and Reuber 17
Medical, Neurological, and Psychiatric Comorbidities
Apart from their seizures, adult patients with PNES often have comorbid medical, neurologic, and psychiatric conditions that contribute to their overall symptom complex, prognosis, and treatment responses. For example, in a 2-year retrospective review of PNES (N=158) and ES (N=122), individuals with PNES were more likely to report a history of other medical somatic syndromes (eg, fibromyalgia, chronic fatigue syndrome, chronic pain, irritable bowel syndrome) and more frequently endorsed chronic, intermittent medical conditions (eg, migraines, asthma).Reference Dixit, Popescu, Bagić, Ghearing and Hendrickson 20 Compared to ES, patients with PNES also more commonly endorsed complaints on a review-of-systems questionnaire.Reference Robles, Chiang and Haneef 21 VeteranReference Salinsky, Spencer, Boudreau and Ferguson 22 and civilianReference LaFrance, Deluca, Machan and Fava 23 populations with PNES frequently report a history of traumatic brain injury (TBI), and a distinct subset of patients has comorbid intellectual disabilities.Reference Duncan and Oto 9 Categorical psychiatric diagnoses and symptom-specific increases in anxiety, depression, and dissociation are linked to PNES. Patients with PNES often have mood, anxiety, dissociative, and other somatic symptom disorders,Reference Perez, Dworetzky and Dickerson 3 along with personality disorders, including clusters B and C personality disorders.Reference Direk, Kulaksizoglu, Alpay and Gurses 24
Posttraumatic stress disorder (PTSD) is also associated with PNES. Estimates suggest that three-fourths of adults with PNES report prior traumatic experiences, including sexual abuse (~30%) and physical abuse (~25%).Reference Duncan and Oto 9 , Reference Myers, Perrine, Lancman, Fleming and Lancman 25 Individuals with PNES and prior sexual abuse exhibit an earlier event onset, greater diagnostic delay, more severe convulsions, emotional triggers, and traumatic recollections among other symptoms.Reference Selkirk, Duncan, Oto and Pelosi 26 In a study comparing individuals with PNES with and without trauma, traumatized individuals were more likely to have comorbid psychiatric conditions and dissociative symptoms.Reference Hingray, Maillard and Hubsch 27 Antecedent trauma has also been linked to other MUS in PNES.Reference Duncan and Oto 9 Particular associations between PTSD and PNES have been identified in combat Veterans. In a retrospective review of 50 Veterans with PNES and 37 Veterans with ES, PTSD symptoms preceded the diagnosis of PNES in 58%, and PTSD comorbidity differentiated PNES and ES.Reference Salinsky, Evrard, Storzbach and Pugh 5 Veterans with PNES and combat-related PTSD may exhibit more hypomotor or nonmotor PNES events.Reference Chen and Izadyar 28
From a dimensional, symptom-specific perspective, patients with PNES exhibit abnormal neuropsychiatric and personality profiles. Associations between PNES and depression have been well established, although depression scores on psychometric measures do not reliably differentiate between PNES and ES.Reference Asmussen, Kirlin, Gale and Chung 29 Patients with PNES report dissociative symptoms [fragmentation of one’s internal experience of the outside world (derealization) and/or fragmentation and compartmentalization of one’s self-perception and body-schema (depersonalization)], and dissociation in PNES has been linked to depression, somatic symptoms, and an external locus of control.Reference Cohen, Testa, Pritchard, Zhu and Hopp 30 Alexithymia, the reduced ability to recognize and express emotions, has also been observed in PNES. It remains unclear, however, if alexithymia scores differentiate PNES and ES.Reference Kaplan, Dwivedi, Privitera, Isaacs, Hughes and Bowman 31 Beliefs about emotions are impaired in PNES compared to healthy subjects, with one study reporting that individuals with PNES described emotions as overwhelming, uncontrollable, shameful, irrational, and damaging.Reference Urbanek, Harvey, McGowan and Agrawal 32 Cluster analyses suggest possible psychopathologic PNES subtypes; in a cohort of 43 PNES individuals, 11 were categorized as having high levels of psychopathology, somatization, alexithymia, and impaired emotion regulation, while 32 subjects reported high somatization and depression scores but relatively normal emotion expression and regulation abilities.Reference Brown, Bouska and Frow 33
Personality measures, including the Personality Assessment Inventory (PAI) and the Minnesota Multiphasic Personality Inventory 2 (MMPI-2), have been widely used in PNES. In a large cohort of PNES (N=75) and ES (N=109) subjects, PNES patients endorsed significantly higher somatic, conversion, depressed, anxious, and suicidal symptoms on the PAI.Reference Thompson, Hantke, Phatak and Chaytor 34 Elevated conversion subscale (SOM-C) scores predict PNES vs. ES.Reference Hill and Gale 35 On the MMPI-2, PNES compared to ES shows a “conversion V” pattern on personality testing, which consists of elevations in hypochondriasis and hysteria subscales relative to the depression subscale.Reference Willment, Hill, Baslet and Loring 36 Predictive regression analyses suggest that dual use of the conversion and health concern subscales of the PAI and the hysteria subscale of the MMPI-2 may also aid PNES vs. ES distinctions.Reference Gale and Hill 37
Neuropsychological studies have started to quantify cognitive profiles in PNES; however, they do not differentiate individuals with PNES from those with ES. Individuals with PNES frequently endorse cognitive symptoms, including memory and concentration difficulties. It has been reported that PNES patients potentially overestimate their cognitive deficits; one study of PNES vs. ES showed that PNES patients endorsed greater word finding difficulties but performed better than ES on the Boston Naming Test.Reference Prigatano and Kirlin 38 Despite this finding, when controlling for effort and IQ, patients with PNES show some impairments in spatial working memory, attention, and executive functions.Reference O’Brien, Fortune and Dicker 39 Several particularly informative studies have probed the intersection of cognitive and emotional functions in PNES.Reference Bakvis, Spinhoven, Putman, Zitman and Roelofs 40 – Reference Gul and Ahmad 42 In a study of 20 subjects with PNES vs. healthy controls, those with PNES exhibited working memory deficits while performing an N-back task paired with facial distractors; following stress induction via the Cold Pressor Test, working memory performance exhibited more generalized impairments.Reference Bakvis, Spinhoven, Putman, Zitman and Roelofs 40 In this cohort, increases in salivary cortisol were associated with larger stress-induced working memory impairments. In a study using an affectively valenced facial viewing task to probe aspects of set shifting, PNES compared to healthy subjects showed greater difficulty switching attention from emotion-related task demands.Reference Gul and Ahmad 42 One study comparing PNES individuals with PTSD, individuals with PNES and prior trauma without PTSD, and PNES subjects without previously experienced trauma showed that subjects with PNES with PTSD exhibited lower episodic verbal memory performance than comparison groups.Reference Myers, Zeng, Perrine, Lancman and Lancman 41 Additional research is necessary to clarify the relationship between neurocognitive deficits and mood and anxiety symptoms in PNES, particularly whether neurocognitive deficits occur independent of mood symptoms.
The Emerging Neurobiology of PNES
Systems-level functional and structural neuroimaging studies have started to elucidate the neurobiology of PNES.Reference Perez, Dworetzky and Dickerson 3 . Several functional magnetic resonance imaging (fMRI)Reference van der Kruijs, Bodde and Vaessen 43 – Reference Li, Liu and Ma 47 and one positron emission tomography (PET) study used resting state techniques to investigate neural circuit disturbances in PNES. In a region-of-interest “seed”-based analysis, increased functional connectivity was observed between motor regions (precentral sulcus) and regions involved in emotional processing [anterior cingulate cortex (ACC), insula] and executive functions (inferior frontal gyrus, parietal cortex); among other findings, trait dissociation scores positively correlated with the functional connectivity strength between the precentral sulcus and the posterior insula.Reference van der Kruijs, Bodde and Vaessen 43 Several studies used data-driven, multivariate functional connectivity analyses to demonstrate widespread functional connectivity alterations in emotion control, executive, fronto-parietal (attentional), sensorimotor, and default mode networks.Reference van der Kruijs, Jagannathan and Bodde 44 – Reference Ding, An and Liao 46 Another study specifically evaluated functional connectivity patterns across insular subregions, observing that PNES compared to healthy subjects showed increased functional connectivity between the left ventral anterior insula and the left post-central gyrus and bilateral supplementary motor area (SMA); functional connectivity strength within the bilateral SMA in relation to the left ventral anterior insula positively correlated with PNES event frequency.Reference Li, Liu and Ma 47 Functional connectivity strength between the SMA and ACC has also been shown to positively correlate with PNES frequency.Reference Li, Li, An, Gong, Zhou and Chen 48 In comparison to healthy subjects, a 2-deoxy-2-[fluorine-18]fluoro-D-glucose PET study showed that PNES patients exhibited bilateral ACC and right inferior parietal lobule hypometabolism.Reference Arthuis, Micoulaud-Franchi, Bartolomei, McGonigal and Guedj 49
Quantitative structural MRI comparisons have also been conducted in PNES.Reference Labate, Cerasa and Mula 50 – Reference Ristić, Daković, Kerr, Kovačević, Parojčić and Sokić 52 Compared to matched controls, patients with PNES showed ACC, SMA, and pre- and post-central gyrus atrophy on cortical thickness and voxel-based morphometry analysesReference Labate, Cerasa and Mula 50 ; bilateral precentral gyrus cortical thickness reductions were independently replicated, along with observed increases in insular and orbitofrontal cortical thickness in PNES.Reference Ristić, Daković, Kerr, Kovačević, Parojčić and Sokić 52 A diffusion tensor tractography study showed a right-ward asymmetry of the uncinate fasciculus (a tract connecting medial prefrontal and medial temporal regions) in PNES compared to healthy controls.Reference Hernando, Szaflarski, Ver Hoef, Lee and Allendorfer 51 While functional and structural neuroimaging studies require further replication and control of potential confounding variables, these early results suggest that PNES may manifest in the context of alterations within and abnormal interactions across sensorimotor, emotion regulation/processing, cognitive control, and multimodal integration neural circuits (see Figure 1 for plots of peak coordinates in the above discussed functional and structural neuroimaging studies). Research is needed to evaluate the specificity of PNES-related neural circuit alternations in relation to neurologic (ie, epilepsy, TBI) and psychiatric (ie, PTSD, depression, other functional neurological disorders) control groups. Additional research efforts are also needed to investigate if semiologic differences in PNES (ie, convulsive vs. atonic events) are linked to distinct patterns of neural circuit alterations.

Figure 1 Display of volumetric and resting-state functional connectivity (FC) findings in psychogenic nonepileptic seizures (PNES) neuroimaging studies. The left panel displays peak coordinates of cortical thickness and voxel-based morphometry findings in PNES vs. healthy controls.Reference Labate, Cerasa and Mula 50 , Reference Ristić, Daković, Kerr, Kovačević, Parojčić and Sokić 52 The right panel shows peak coordinates of abnormal resting-state FC in individuals with PNES vs. healthy controls.Reference van der Kruijs, Bodde and Vaessen 43 , Reference Ding, An and Liao 45 , Reference Li, Liu and Ma 47 , Reference Li, Li, An, Gong, Zhou and Chen 48 Blue circles indicate decrease and red circles indicate increase. Evidence from neuroimaging studies, while requiring replication and further investigations, suggests that PNES may potentially develop in the context of alterations within and across brain networks implicated in sensori-motor (eg, pre- and post-central gyrus, premotor regions), emotion processing/regulation [eg, anterior cingulate cortex (ACC), middle cingulate cortex (MCC), orbitofrontal cortex, insula], cognitive control (eg, lateral prefrontal cortex, dorsal ACC, MCC), and multimodal integration functions (eg, cingulate gyrus, posterior parietal cortex, precuneus). Only main findings are displayed using Caret 5. Cerebellar and basal ganglia foci, and seed region coordinates are not shown.
Electrophysiology and autonomic nervous system studies have also contributed to the understanding of PNES pathophysiology. In a cohort of 18 PNES and 18 healthy subjects, resting state high-density source EEG analyses showed that patients with PNES exhibited decreased functional connectivity between the basal ganglia and cortical regions, along with reduced interhemispheric connectivity across paralimbic regions.Reference Barzegaran, Carmeli, Rossetti, Frackowiak and Knyazeva 53 In a unique case of intracranial recordings during PNES, decreased power in the theta band was observed over the posterior parietal cortex.Reference Arzy, Halje, Schechter, Spinelli, Seeck and Blanke 54 Several investigations have probed autonomic profiles peri-ictally in PNESReference Reinsberger, Sarkis and Papadelis 55 ; some noted increased sympathetic tone ictally in ES vs. PNES,Reference Ponnusamy, Marques and Reuber 56 while others characterized pre-ictal and post-ictal autonomic changes as distinguishing between groups.Reference Reinsberger, Perez, Murphy and Dworetzky 57
A few studies have investigated serologic biomarkers in PNES. Positive correlations were observed between baseline salivary cortisol levels and negative attentional bias during performance of a masked emotional Stroop taskReference Bakvis, Spinhoven and Roelofs 58 ; in this same cohort, negative attentional bias also correlated with past sexual trauma.Reference Bakvis, Roelofs, Kuyk, Edelbroek, Swinkels and Spinhoven 59 Increases in basal diurnal cortisol levels in PNES compared to healthy controls were mediated mainly by patients reporting past sexual trauma.Reference Bakvis, Spinhoven and Giltay 60 Reductions in serum brain derived neurotrophic factor, implicated in synaptic reorganization and neurogenesis, have also been reduced in samples with PNES and with ES, compared to healthy controls.Reference LaFrance, Leaver, Stopa, Papandonatos and Blum 61
Treatment and Outcome Studies
In the past decade, significant progress has been made in the evidence-based management of PNESReference LaFrance, Reuber and Goldstein 62 (see Table 3). In a pilot, randomized, controlled trial of 12-weeks of cognitive-behavioral therapy (CBT) plus standard medical care (SMC) vs. SMC alone, conducted in 66 patients with PNES, intention to treat analyses showed a statistically significant reduction in seizure frequency at the end of the treatment period associated with CBT.Reference Goldstein, Chalder and Chigwedere 63 At a 3-month follow-up, however, mean seizure frequency between the treatment arms did not remain statistically significant. A pilot, double-blind, randomized, placebo-controlled trial of flexible-dose sertraline, conducted over 12 weeks, showed that PNES subjects assigned to the sertraline treatment displayed a 45% reduction in seizure frequency as compared to an 8% seizure frequency increase to placebo.Reference LaFrance, Keitner and Papandonatos 64 Recently a multicenter, randomized clinical trial was conducted in 38 patients with PNES comparing 4 treatment arms: flexible-dose sertraline only, CBT-informed psychotherapy (CBT-ip) only, CBT-ip with flexible-dose sertraline, and SMC.Reference LaFrance, Baird and Barry 65 Within-group analyses for each group showed that the 2 psychotherapy treatment arms exhibited a significant reduction in seizures (51% in the CBT-ip only arm and 59% in the CBT-ip plus sertraline arm), while the sertraline and the SMC arms did not show a significant reduction in seizures. The CBT-ip only group also showed significant improvements in depression, anxiety, quality of life, and global functioning. These findings support the use of a manualized CBT-ip PNES workbook.Reference Reiter, Andrews, Reiter and LaFrance 66 The intervention is being disseminated for treatment by a range of clinicians, from epileptologists/neurologists, psychiatrists, psychologists, and social workers.Reference LaFrance and Wincze 67
Table 3 Summary of controlled clinical trials in the treatment of psychogenic nonepileptic seizures (PNES).

CBT, cognitive behavioral therapy; CBT-ip, cognitive behavioral therapy–informed psychotherapy; SMC, standard medical care; AED, anti-epileptic drug; Sz, seizures; f/up, follow-up; ↓ - reduction in.
A randomized, controlled trial of a post-PNES diagnosis group psychoeducation program (3 successive monthly, 1.5-hour group sessions) also showed that patients in the psychoeducation intervention reported improvements in perceived psychosocial functioning and a trend toward decreased seizure-related emergency room visits or hospitalizations; however, seizure frequency reduction was not observed.Reference Chen, Maheshwari, Franks, Trolley, Robinson and Hrachovy 68 Last, a randomized controlled trial of immediate vs delayed withdrawal of antiepileptic drugs (AED) in PNES showed that immediate AED withdrawal was associated with a reduction in seizures, decreased use of rescue medications, and greater perceived internal locus of control.Reference Oto, Espie and Duncan 69 The long-term benefits of therapy and other treatments can be examined in randomized, controlled, clinical trials along with other potential pharmacologic and psychotherapy treatment interventions.Reference Baslet, Dworetzky, Perez and Oser 70 , Reference Bullock, Mirza, Forte and Trockel 71
Outcome and predictor of outcome studies have been performed in PNES. Given that the initial stages of treatment include accurate diagnosis and effective communication of the diagnosis, it is noteworthy that diagnostic acceptance by patients improves outcomes.Reference Duncan, Graham and Oto 72 Effective communication alone, however, is insufficient to improve outcomes in most patients.Reference Mayor, Brown and Cock 73 In a retrospective study of 260 consecutively evaluated PNES patients measuring outcome at 6 and 12 months post diagnosis, 18% showed an increase in seizures, 38% were event free, but the majority of patients continued to have seizures.Reference McKenzie, Oto, Russell, Pelosi and Duncan 74 Women, individuals with anxiety/depression, and patients receiving social security benefits were less likely to become event-free. Post-diagnosis, many also developed other MUS functional neurological symptoms.Reference Jones, TJ and Adams 75 In addition to psychosocial and psychiatric variables impacting outcome, patients with PNES with a history of TBI exhibit worse outcomes, as exemplified by increased likelihood of having major depression, behavioral impulsivity, PTSD, receiving disability, and lower global functioning.Reference LaFrance, Deluca, Machan and Fava 23
Conclusions
PNES are highly prevalent, complex neuropsychiatric symptoms. Over the past 5–10 years, notable advancements have been made in the diagnosis and management of this previously enigmatic condition. Neurobiological studies using systems-level, clinical neuroscience techniques are elucidating an emergent neurobiology for PNES, which will hopefully help reduce the stigma associated with this condition and facilitate additional research efforts to identify diagnostic biomarkers of prognosis and treatment response. Future investigations will also help clarify gender differences, and similarities and differences between PNES and other somatic symptom subtypes.
Disclosures
David Perez has the following disclosure: National Institute of Neurological Disorders and Stroke, Researcher, Research grant (D.L.P., R25NS065743-05S1). W. Curt LaFrance has the following disclosures: Cambridge University Press, book co-editor, editor’s royalties; Oxford University, book co-author, author’s royalties; Matty Fund, researcher, research support; Veterans Administration, researcher, research support; Brown University, researcher, research support.