Introduction
Hypoactive sexual desire disorder (HSDD) is the most common female sexual dysfunction and is defined by the International Society for the Study of Women’s Sexual Health as a lack of motivation for sexual activity manifested by (i) reduced or absent spontaneous desire (sexual thoughts or fantasies), (ii) reduced or absent responsive desire to erotic cues and stimulation, or inability to maintain desire or interest through sexual activity, or (iii) loss of desire to initiate or participate in sexual activity, including behavioral responses such as avoidance of situations that could lead to sexual activity, that is not secondary to a sexual pain disorder and is associated with clinically significant personal distress.Reference Goldstein, Kim and Clayton 1 , Reference Parish, Goldstein and Goldstein 2 In the United States, HSDD is estimated to affect approximately 10% of women.Reference Rosen, Shifren, Monz, Odom, Russo and Johannes 3 , Reference Shifren, Monz, Russo, Segreti and Johannes 4 Similarly, HSDD has been shown to affect women worldwide. A 2016 systematic review and meta-analysis assessed the prevalence of female sexual dysfunction as 28.2% in 215,740 reproductive-age women worldwide.Reference McCool, Zuelke, Theurich, Knuettel, Ricci and Apfelbacher 5 Prevalence rates of HSDD were comparatively high in gender-equal sexual cultures in Europe and the non-European West. It has been shown that in egalitarian societies, various factors, including women’s employment and childcare/housework imbalance, are predictors of low sexual interest or decreased sexual desire in women.Reference Traeen, Martinussen, Öberg and Kavil 6 , Reference Sanders, Graham and Milhausen 7 Moreover, HSDD is often underdiagnosed and thus undertreated.Reference Goldstein, Kim and Clayton 1
The current treatment approach for HSDD often follows a biopsychosocial model and is guided by medical history and assessment of symptoms.Reference Goldstein, Kim and Clayton 1 Two types of psychotherapy (cognitive behavioral therapy and mindfulness meditation training) appear effective, but adequate randomized controlled trials to support their use in women with HSDD are lacking.Reference Pyke and Clayton 8 Bupropion and buspirone are off-label treatments for HSDD, and efficacy and safety data are limited.Reference Goldstein, Kim and Clayton 1 Testosterone also has been used as an off-label treatment and was demonstrated to be effective in postmenopausal women with HSDD, but potential serious adverse events on fetal development have deterred its use in reproductive-age women.Reference Goldstein, Kim and Clayton 1 , Reference Kingsberg and Rezaee 9
Two drugs are currently approved by the US Food and Drug Administration for the treatment of premenopausal women with HSDD. Flibanserin (Addyi®), a mixed serotonin receptor 1A (5-HT1A) agonist and serotonin 2A (5-HT2A) antagonist, is a pill taken once-daily and was approved in 2015. 10 Bremelanotide (Vyleesi®), a melanocortin receptor (MCR) agonist and an analog of the naturally occurring peptide α-melanocyte-stimulating hormone (α-MSH), is taken as needed using an autoinjector and was approved in 2019. 11 , Reference Kingsberg, Clayton and Portman 12 The mechanism of action of bremelanotide in improving sexual desire and cognitive excitement/arousal in premenopausal women with HSDD is not entirely understood. It has been hypothesized that bremelanotide, as an MCR agonist, stimulates the release of dopamine (DA) in the brain to alter key excitatory pathways involved in sexual response, specifically to increase excitatory signaling components, which differs from the flibanserin mechanism of action that decreases inhibitory sexual signals.
The objective of this article is to review data on the effects of bremelanotide on the physiological and neurobiologic components of female sexual desire and function based on animal studies and imaging studies in humans.
Neurobiology of Female Sexual Behavior
Female sexual response and desire
A complex interplay of interpersonal, physiological, and psychological factors contributes to the female sexual response.Reference Rosen and Barsky 13 Specifically, a balance between excitatory and inhibitory pathways in the brain regulates sexual desire.Reference Pfaus 14 Separate pathways for excitation and inhibition were first proposed by Sechenov, Sherrington, and Pavlov, and were applied by Gray to study anxiety.Reference Pfaus 14 , Reference Gray 15 The dual-control model of sexual behavior, which was proposed by Bancroft and Janssen with regard to male erectile response, considered excitement or inhibition to be an individual propensity.Reference Pfaus 14 , Reference Bancroft and Janssen 16 In the Sexual Tipping Point® model from Perelman, the sexual response in men and women is balanced in a neutral/resting state.Reference Perelman 17 The shift toward excitation or inhibition is influenced by a range of factors that can be classified as “physiological/organic” or “psychosocial/interpersonal.”Reference Perelman 17 Psychosocial/interpersonal inputs are relationship- or experience-based, whereas neurotransmitters and hormones represent the physiological/organic inputs. Important excitatory neurotransmitters and hormones include DA, melanocortin, oxytocin, estrogen, and testosterone; inhibitory neurotransmitters and hormones include serotonin, endocannabinoids, opioids, and prolactin (Figure 1).Reference Pfaus 14 , Reference Clayton and Hamilton 18 Shifts in the equilibrium of neurotransmitters or hormones that result in excess inhibition or diminished excitation may play an important etiological role in the manifestations of HSDD.Reference Perelman 17
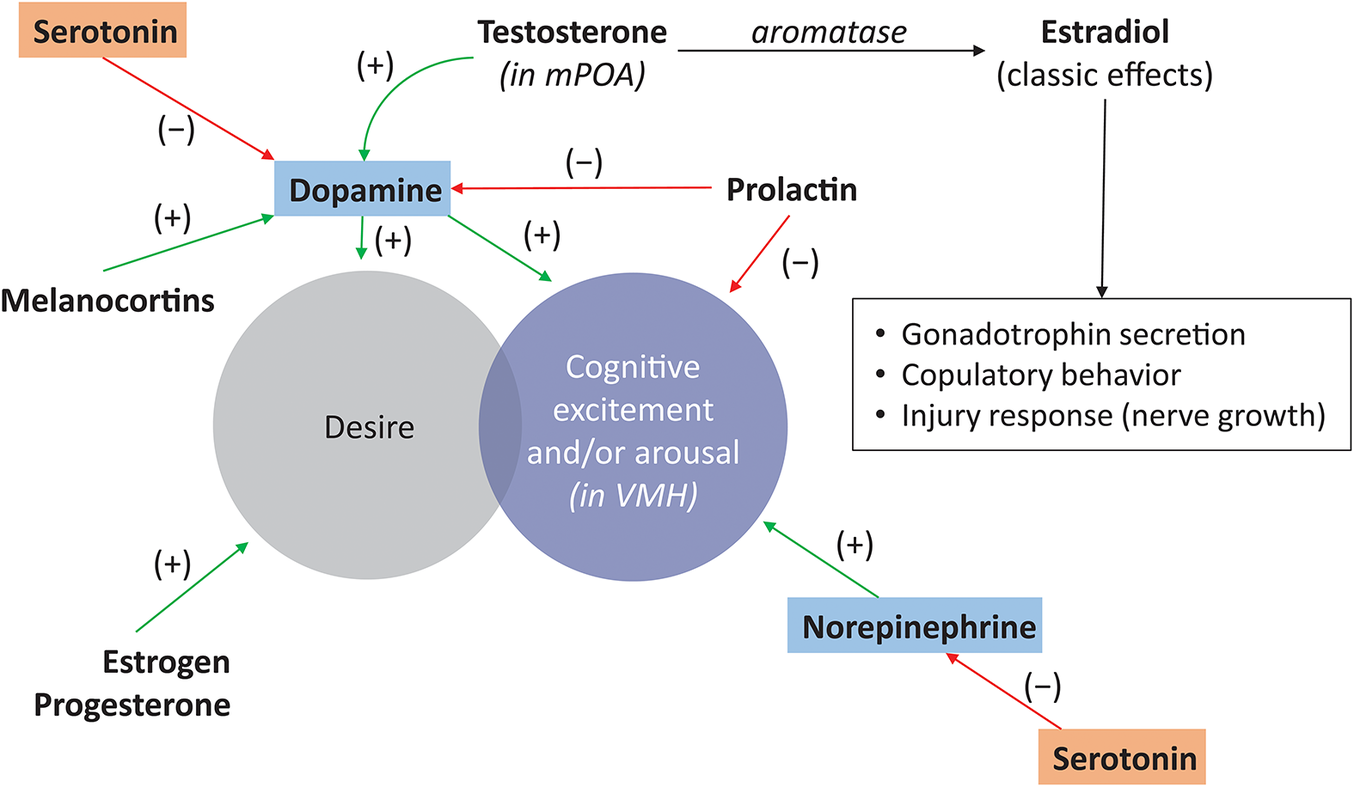
Figure 1. Central effects of neurotransmitters and hormones on sexual functioning. Sexual responsiveness involves interaction between excitatory and inhibitory neuro-modulatory processes; excitatory components include norepinephrine (stimulation of sexual excitement) and dopamine and melanocortins (stimulation of desire); inhibitory components include serotonin (regulation of satiety) and prolactin.Reference Pfaus 14 , Reference Clayton and Hamilton 18 mPOA, medial preoptic area; VMH, ventromedial hypothalamus.
Ovarian steroids are known to prime excitatory sexual systems in the brain and periphery through epigenetic alterations in gene transcription related to activating excitatory neurochemical transmission and reducing inhibitory neurochemical transmission.Reference Goldstein, Kim and Clayton 1 , Reference Pfaus 14 Preclinical studies have demonstrated that estradiol, testosterone, and progesterone affect subtypes of the DA receptor and DA release in the medial preoptic area (mPOA) of female rats.Reference Pfaus 14 , Reference Matuszewich, Lorrain and Hull 19
Although ovarian steroids modulate women’s sexual desire, the exact role of these hormones in HSDD is unclear.Reference Goldstein, Kim and Clayton 1 Lower testosterone levels have been associated with reduced sexual desire; however, there is no level of testosterone predictive for HSDD.Reference Goldstein, Kim and Clayton 1 Lower estradiol levels have also been associated with reduced sexual desire, but estradiol levels are not necessarily low in premenopausal women with HSDD.Reference Goldstein, Kim and Clayton 1 It has been hypothesized that HSDD is associated with neuroendocrine dysregulation/imbalance leading to functional and structural neuroadaptations in these systems, resulting in decreased excitation, increased inhibition, or a combination of both.Reference Goldstein, Kim and Clayton 1
Animal models of sexual desire and responses
Because it is difficult to study the neurobiology of human sexual behavior directly, animal models provide the ability to investigate the neurochemical and neuroanatomical processes underlying behaviors that are homologous and analogous to the human female sexual response. Animals engage in appetitive and consummatory sexual behaviors like those of humans, which are controlled by similar or identical neurochemical and hormonal systems.Reference Pfaus, Kippin and Coria-Avila 20 Although the outward expression of appetitive behaviors or copulation may be species specific, animal models have predictive validity, and can be used to understand the human sexual response if the process and endpoints of sexual response are equivalent.Reference Pfaus, Kippin and Coria-Avila 20 In order to facilitate comparisons between species, sexual behavior can be described as having three phases: wanting, liking, and inhibition, which are aligned with the motivation, consummation, and satiety phases related to other reward cycles.Reference Georgiadis, Kringelbach and Pfaus 21
Rats have been used to study sexual behavior and response because female rats display both appetitive (proceptive) and receptive sexual behaviors during their periovulatory period (or if they are ovariectomized, with appropriate replacement of estradiol and progesterone, or estradiol and testosterone).Reference Pfaus, Kippin and Coria-Avila 20 In particular, proceptive behaviors in female rats, such as solicitation, pacing, and hops and darts, are considered analogous to desire in human females.Reference Pfaus 14 , Reference Pfaus, Kippin and Coria-Avila 20 , 22-31 Female rats solicit sexual contact from males by means of a headwise orientation to the male followed by running away.Reference Pfaus, Kippin and Coria-Avila 20 This behavior entices males to chase them until the female stops and holds a receptive crouch so that the male can mount and intromit, or penetrate the vagina.Reference Pfaus, Kippin and Coria-Avila 20 Women also display increased female-initiated solicitation and sexual activity during their periovulatory periods, although they can and do have sex throughout their ovulatory cycles (Figure 2).Reference Pfaus, Kippin and Coria-Avila 20

Figure 2. Incentive sequences for rat and human sexual behavior. The behavioral stream moves from left to right, through appetitive, precopulatory, and consummatory phases of behavior. This conforms to the movement of animals and humans from distal to proximal to interactive with respect to a sex partner. Understanding which behaviors are homologous and analogous in the three phases allows researchers to choose appropriate behaviors in animals that are homologous or analogous to those in humans.Reference Pfaus, Kippin and Coria-Avila 20 PEI, postejaculatory interval. From Pfaus JG, Kippin TE, Coria-Avila G. What can animal models tell us about human sexual response? Annu Rev Sex Res. 2003;14:1–63, with permission. Copyright © 2012 Taylor & Francis, www.tandfonline.com.
Inhibition and excitation in sexual response
Studies in animal models of sexual function have identified the interplay between excitatory and inhibitory neurochemical processes in the brain as central to sexual responsiveness.Reference Pfaus 14 These studies have identified certain neurotransmitters (DA and norepinephrine) and neuropeptide hormones (melanocortin and oxytocin) as excitatory components.Reference Pfaus 14 Agents that increase these excitatory signaling components, such as DA agonists, α2 receptor antagonists (which increase norepinephrine signaling by blocking inhibitory feedback), melanocortin agonists, and oxytocin infusion, stimulate sexual desire and/or arousal in male and female rats. Decreased excitatory signaling produced by DA antagonists, α2 receptor agonists, noradrenergic lesions, norepinephrine synthesis inhibitors, or oxytocin receptor antagonists results in decreased sexual function.Reference Pfaus 14 The most well-defined inhibitory neurotransmitters are serotonin, endocannabinoids, and endogenous opioids.Reference Pfaus 14 All of the above-mentioned neurotransmitters act on similar regions of the brain, including the mPOA in the hypothalamus, the attention- and reward-related regions of the limbic system, and the prefrontal cortex (Figure 3A).Reference Pfaus 14 , Reference Kingsberg, Clayton and Pfaus 32 Activation of presynaptic type 4 melanocortin receptors (MC4Rs) through endogenous or exogenous agonists enhances cellular excitability of these neurons and increases DA release into synaptic terminals of the mPOA.Reference Pfaus 14 , Reference Pfaus, Giuliano and Gelez 33 , Reference Ückert, Bannowsky, Albrecht and Kuczyk 34 Notably, DA release in the mPOA serves as a general neural switch that controls sympathetic and parasympathetic blood flow in the presence of sexual cues.Reference Goldstein, Kim and Clayton 1 , Reference Pfaus 14 , Reference Matuszewich, Lorrain and Hull 19 , 35-37 For example, in sexually mature female ovariectomized rats primed with a low dose of estradiol benzoate, an injection of progesterone increased extracellular DA and facilitated copulatory behavior. These results suggest that DA in the mPOA may be important for the facilitation of sexual behavior by progesterone.Reference Matuszewich, Lorrain and Hull 19 Studies in male rats showed that a receptive female results in DA release in the mPOA, and that small mPOA DA increases are associated with parasympathetic-mediated erections, while higher DA levels are associated with sympathetic-mediated ejaculation.Reference Pfaus 14 , Reference Hull, Lorrain and Du 37
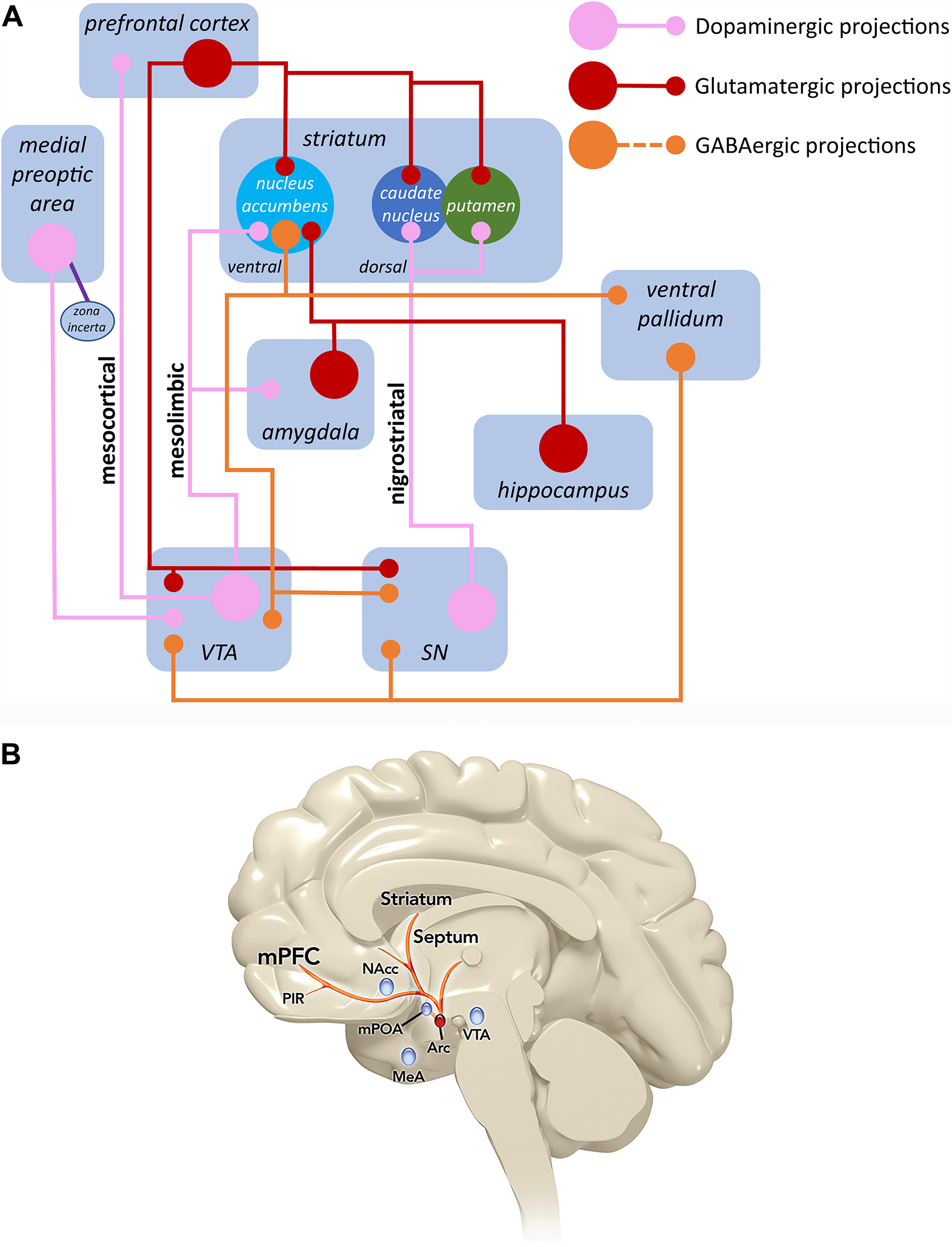
Figure 3. Sexual excitation and melanocortin pathways in the brain. (A) The major brain neurochemical systems involved in sexual desire are shown here. DA projections from the zona incerta to the mPOA of the hypothalamus drive inhibitory GABA neurons that project to the VTA in the midbrain. These neurons make contact with inhibitory GABA interneurons that suppress mesolimbic and mesocortical DA neurons in the VTA. The disinhibition that occurs from this action drives mesolimbic and mesocortical DA neurons, allowing for desire to occur in the presence of sexually stimulating cues in the environment by linking emotional responses with DA’s motor actions in the nigrostriatal system (SN). In turn, the outflow from the prefrontal cortex activates inhibitory circuits in limbic and motor structures, essentially turning off the excitatory system. The activation of inhibitory outflow from the prefrontal cortex is modulated powerfully by serotonin (5-HT).Reference Kingsberg, Clayton and Pfaus 32 (B) The melanocortin system arises in the arcuate nucleus of the hypothalamus (Arc) and projects rostrally to hypothalamic and limbic forebrain regions. This system potentiates sexual desire through an interaction with DA release in the mPOA.Reference Pfaus 14 DA, dopamine; GABA, γ-aminobutyric acid; MeA, medial amygdala; mPFC, medial prefrontal cortex; mPOA, medial preoptic area; NAcc, nucleus accumbens; PIR, piriform cortex; VTA, ventral tegmental area. Top figure (A) from Kingsberg SA, Clayton AH, Pfaus JG. The female sexual response: current models, neurobiological underpinnings and agents currently approved or under investigation for the treatment of hypoactive sexual desire disorder. CNS Drugs. 2015;29(11):915–933, with permission. Copyright © 2015 Springer International Publishing. Bottom figure (B) from Pfaus JG. Pathways of sexual desire. J Sex Med. 2009;6(6):1506–1533, with permission. Copyright © 2009 International Society for Sexual Medicine.
Similarly, in humans, the mPOA appears to be a key region of the hypothalamus in the regulation of sexual desire and response.Reference Goldstein, Kim and Clayton 1 Consistent with data from animal models, DA and norepinephrine (excitatory neurotransmitters) and serotonin and endogenous opioids (inhibitory neurotransmitters) in the mPOA have been shown to regulate sexual excitation and inhibition in humans.Reference Goldstein, Kim and Clayton 1 , Reference Pfaus 14 , Reference Pfaus, Kippin and Coria-Avila 20 This proposed mechanism is consistent with differential brain activity patterns and structural differences between women with and without HSDD as shown by several neuroimaging (functional magnetic resonance imaging [fMRI] and positron emission tomography [PET]) studies.Reference Goldstein, Kim and Clayton 1 , Reference Arnow, Millheiser and Garrett 35 , Reference Woodard, Nowak, Balon, Tancer and Diamond 36
Functional Brain Imaging
Functional brain imaging of women with HSDD using various imaging modalities have revealed differences compared to women without HSDD.Reference Arnow, Millheiser and Garrett 35 , Reference Bianchi-Demicheli, Cojan, Waber, Recordon, Vuilleumier and Ortigue 38 In a fMRI study to assess sexual arousal, peripheral sexual response (using a vaginal photoplethysmograph [VPP]), and brain activation, 20 women with no history of sexual dysfunction (NHSD) were compared to 16 women with HSDD. Video stimuli included content containing erotic, sports, and relaxing segments.Reference Arnow, Millheiser and Garrett 35 Subjective arousal to erotic stimuli was significantly greater in women with NHSD compared to those with HSDD. In the erotic/sports contrast scans, women with NHSD showed significantly greater activation in the bilateral entorhinal cortex than women with HSDD, who also demonstrated higher activation in the medial frontal gyrus, right inferior frontal gyrus, and bilateral putamen. There were no differences in VPP-correlated brain activation, nor was peripheral sexual response significantly associated with either subjective sexual response or brain activation patterns. These results suggest differences between women with NHSD and women with HSDD in encoding arousing stimuli and/or retrieval of past erotic experiences.Reference Arnow, Millheiser and Garrett 35
In another fMRI study of 28 premenopausal women, 13 with HSDD, and 15 with NHSD, regional cerebral blood flow responses between these two groups of participants were measured by fMRI while they were looking at erotic vs non-erotic stimuli.Reference Bianchi-Demicheli, Cojan, Waber, Recordon, Vuilleumier and Ortigue 38 Behavioral results indicated that women with NHSD rated erotic stimuli significantly higher than did women with HSDD. Two distinct types of neural changes in women with and without HSDD were shown by functional neuroimaging. In comparison with women with HSDD, those without HSDD demonstrated more activation in brain areas known to be associated with the processing of erotic stimuli, including the intraparietal sulcus, dorsal anterior cingulate gyrus, and ento/perirhinal region. Women with HSDD also showed additional activation in higher order social and cognitive functioning brain areas, including the inferior parietal lobule, inferior frontal gyrus, and posterior medial occipital gyrus.Reference Bianchi-Demicheli, Cojan, Waber, Recordon, Vuilleumier and Ortigue 38
In another imaging study, PET scans were performed in women while they watched videos with erotic or non-erotic physical activity. In women with HSDD, watching erotic videos activated regions in the parietal cortex on the right side to a lesser extent than in women without HSDD (Figure 4).Reference Goldstein, Kim and Clayton 1 , Reference Holstege 39 Additionally, there was less deactivation in the left hemisphere of women with HSDD compared to women without HSDD, indicating differences in how erotic stimuli are processed.

Figure 4. Imaging of neural activity in women with or without HSDD. Changes in neural activity in response to viewing an erotic video in women with or without HSDD were assessed by PET. Decreased activation is shown in red in the left hemisphere, and increased activation is shown in green in the right hemisphere.Reference Goldstein, Kim and Clayton 1 From Goldstein I, Kim NN, Clayton AH, et al. Hypoactive sexual desire disorder: International Society for the Study of Women’s Sexual Health (ISSWSH) Expert Consensus Panel Review. Mayo Clin Proc. 2017;92(1):114–128, with permission. Copyright © 2016 Mayo Foundation for Medical Education and Research. Published by Elsevier Inc.
Melanocortin system
Melanocortins are a family of endogenous neuropeptides that are derived from posttranslational processing of the parent molecule proopiomelanocortin (POMC).Reference Eves and Haycock 40 POMC is generated mainly in the pituitary gland and the hypothalamus, and is cleaved by prohormone convertases, with differential processing based on cell and tissue type.Reference Cawley, Li and Loh 41 There is also evidence that POMC may be expressed and processed in other tissues, such as the skin.Reference Eves and Haycock 40 , Reference Cawley, Li and Loh 41 Cleavage of POMC results in the formation of adrenocorticotrophic hormone (ACTH), α-, β-, and γ-MSH, which are MCR agonists, as well as β-endorphin and β-lipoprotein.Reference Eves and Haycock 40 , Reference Ericson, Lensing, Fleming, Schlasner, Doering and Haskell-Luevano 42
Melanocortins bind to and activate MCRs found throughout the body, including the central nervous system (CNS). Five subtypes of MCRs (MC1R through MC5R) have been identified and show tissue-specific expression patterns with different binding affinities.Reference Gantz and Fong 43 MC1R through MC5R are seven transmembrane G-protein coupled receptors with recognition sites for protein kinase A and protein kinase C.Reference Eves and Haycock 40 They are implicated in a diverse set of effects, such as sexual function, pigmentation, inflammation, energy homeostasis, steroidogenesis, and exocrine function.Reference Kingsberg, Clayton and Pfaus 32 , Reference Eves and Haycock 40 , Reference Ericson, Lensing, Fleming, Schlasner, Doering and Haskell-Luevano 42 In the CNS, melanocortin projections originate in the arcuate nucleus of the hypothalamus and the nucleus of the solitary tract, with axons innervating other hypothalamic areas, limbic regions, midbrain, brainstem, and spinal cord (Figure 3B).Reference Pfaus 14 , Reference Kingsberg, Clayton and Pfaus 32 , Reference Tao 44 MCRs are found in these CNS regions as well as in peripheral organs.Reference Tao 44 The most important MCRs for sexual desire are MC3R and MC4R.Reference Kingsberg, Clayton and Pfaus 32 MC4R is expressed in the CNS, including the spinal cord, brainstem, hypothalamus, and cortex, and has been shown to be important for sexual function.Reference Eves and Haycock 40 , Reference Ericson, Lensing, Fleming, Schlasner, Doering and Haskell-Luevano 42 , Reference Gelez, Poirier and Facchinetti 45 MC4R binds with high affinity to ACTH and α-MSH, which cause activation of adenyl cyclase, resulting in elevated cAMP levels in the cell and downstream signaling related to the neuroendocrine and autonomic systems.Reference Eves and Haycock 40 As described above, activation of presynaptic MC4Rs through endogenous or exogenous agonists enhances neuronal excitability and increases DA release into synaptic terminals of the mPOA.Reference Pfaus 14 , Reference Pfaus, Giuliano and Gelez 33 , Reference Ückert, Bannowsky, Albrecht and Kuczyk 34 MC3R, which is expressed in the CNS and other sites in the body, binds γ-MSH with a comparable affinity to ACTH, resulting in elevated cAMP levels; in some conditions, there also may be an inositol Ca2+-phospholipid system present.Reference Eves and Haycock 40 Studies in both rats and humans have demonstrated that MCR agonists stimulate sexual desire, and infusion of a melanocortin agonist into the mPOA of female rats resulted in increased solicitations and DA release.Reference Kingsberg, Clayton and Pfaus 32 , Reference Pfaus, Giuliano and Gelez 33 , 46-51 Preclinical studies with the MCR agonist bremelanotide are discussed in greater detail below.
Preclinical Studies of Bremelanotide
Evidence from animal studies suggests that bremelanotide affects sexual arousal and desire by binding to MC4R in presynaptic neurons of the hypothalamus, activating the release of DA in the mPOA.Reference Pfaus 14 , Reference Pfaus, Giuliano and Gelez 33 , Reference Ückert, Bannowsky, Albrecht and Kuczyk 34 Subcutaneous injection of bremelanotide or infusion directly into the lateral ventricles or POA increased appetitive or consummatory sexual behaviors in ovariectomized female rats primed with estrogen alone or estrogen plus progesterone.Reference Pfaus, Giuliano and Gelez 33 , Reference Pfaus, Shadiack, Van Soest, Tse and Molinoff 50 Treatment with bremelanotide dramatically and selectively increased measures of solicitation in a dose-dependent manner without altering other aspects of copulation, such as pacing or lordosis (Supplementary Figures 1A and 1B).Reference Pfaus, Giuliano and Gelez 33 , Reference Pfaus, Shadiack, Van Soest, Tse and Molinoff 50 As mentioned previously, solicitations in female rats reflect the desire to engage in sexual activity and are indicated by headwise orientation to the male, followed by running away.Reference Pfaus, Kippin and Coria-Avila 20 , Reference Pfaus, Giuliano and Gelez 33 , Reference Pfaus, Shadiack, Van Soest, Tse and Molinoff 50 Bremelanotide stimulated solicitations regardless of hormone priming or testing context.Reference Pfaus, Shadiack, Van Soest, Tse and Molinoff 50 The increase in solicitations was shown in both bilevel and unilevel pacing chambers, suggesting that the effect was not attributed to a general increase in locomotion. Studies in unilevel chambers also showed significant increases in hops and darts, as well as significant increases in female mounting of males and returns to the male following ejaculation.Reference Pfaus, Giuliano and Gelez 33 , Reference Pfaus, Shadiack, Van Soest, Tse and Molinoff 50
Importantly, bremelanotide did not alter the display of an established conditioned partner preference of the female for her preferred male, indicating that it does not disrupt bonding or result in hypersexuality. In a test in which conditioned partner preference was assessed using a preferred (scented) male and an unpreferred (unscented) male, subcutaneous bremelanotide injection increased solicitations only for the preferred partner (unpublished data). Moreover, bremelanotide alone did not induce a conditioned place preference, nor did it increase locomotion, indicating that it has no abuse liability in animal models.Reference Pfaus, Giuliano and Gelez 33
Sexual solicitations induced by bremelanotide were reversed by treatment with a selective MCR antagonist or with a DA D1 antagonist.Reference Pfaus, Giuliano and Gelez 33 Notably, bremelanotide has been shown to stimulate an increase in DA release in the mPOA 30 to 60 min post-dosing, but not in other brain regions such as the nucleus accumbens (NAcc) or ventromedial hypothalamus (VMH) stimulated by drugs of abuse or feeding, respectively (Figure 5).Reference Pfaus, Giuliano and Gelez 33 Several preclinical assays have been used to investigate the effect of bremelanotide on neural pathways. Immunohistochemistry showed that subcutaneous doses of bremelanotide activated solicitations in female rats, and induced Fos protein, a marker of neuronal activation, in brain regions associated with sexual excitation, including the mPOA, NAcc, ventral tegmental area (VTA), and basolateral amygdala.Reference Pfaus, Giuliano and Gelez 33 , Reference Molinoff, Shadiack, Earle, Diamond and Quon 52 Given that these regions are activated identically by sexual incentive cues, these data indicate that bremelanotide acts as a neurochemical priming cue to stimulate activity in important excitatory regions of the brain.Reference Pfaus, Giuliano and Gelez 33

Figure 5. DA release in brain regions following peripheral administration of bremelanotide. DA release in the NAcc, mPOA, and VMH are shown following peripheral (subcutaneous) administration of 200 μg/kg bremelanotide. Data are mean percentages from baseline + standard error of the mean.Reference Pfaus, Giuliano and Gelez 33 *P < .05 from baseline (analysis of variance followed by Tukey post hoc comparisons of individual means). From Pfaus J, Giuliano F, Gelez H. Bremelanotide: an overview of preclinical CNS effects on female sexual function. J Sex Med. 2007;4(Suppl 4):269–279, with permission. Copyright © 2007 International Society for Sexual Medicine.
To further characterize the roles of melanocortins and DA in sexual solicitation in female rats, neuropharmacological analyses using melanocortin and DA receptor antagonists were performed. The increase in solicitations with systemic bremelanotide were reversed by intracerebroventricular infusion of an MCR antagonist (unpublished data). Similarly, the increase in solicitations after intra-POA infusion of bremelanotide were reversed by intra-POA infusion of an MCR antagonist (Figure 6A).Reference Pfaus, Giuliano and Gelez 33 Intra-VMH infusion of bremelanotide did not increase solicitations (Figure 6B).

Figure 6. Effect of infusion of bremelanotide to the mPOA and VMH. Bremelanotide (800 ng/2 μL) was infused into the mPOA (A) or VMH (B) of ovariectomized estrogen-primed rats. Data shown are the effects on the mean number of solicitations, hops and darts, pacing, and the lordosis quotient (lordosis to mount ratio). There was an increase in the number of solicitations with intra-mPOA infusion but not with intra-VMH infusion; the increase in solicitations with intra-mPOA infusion was reversed with HS014, a selective MC4R antagonist. Data are means + standard error of the mean.Reference Pfaus, Giuliano and Gelez 33 *P < .05 from control (Student’s t-test between the means). Top figure (A) from Pfaus J, Giuliano F, Gelez H. Bremelanotide: an overview of preclinical CNS effects on female sexual function. J Sex Med. 2007;4(Suppl 4):269–279, with permission. Copyright © 2007 International Society for Sexual Medicine. Bottom figure (B) has been provided by James G. Pfaus, PhD (unpublished data).
Based on preclinical data, bremelanotide is thought to bind to presynaptic MC4R on DA terminals in the mPOA, facilitating release and postsynaptic actions on D1 receptors.Reference Pfaus, Giuliano and Gelez 33 D1 receptors are located on γ-aminobutyric acid (GABA) neurons that project from the mPOA to the VTA, where they likely make contact with GABA interneurons that hold associated DA neurons in tonic inhibition.Reference Micevych and Meisel 53 , Reference Tobiansky, Roma, Hattori, Will, Nutsch and Dominguez 54 Because GABA is inhibitory at a membrane level, a GABA-GABA connection is disinhibitory.Reference Roberts 55 This effect likely produces enough disinhibition of DA neurons in the mesolimbic system such that a competent sexual incentive stimulates appropriate appetitive responses indicative of sexual desire. Bremelanotide and sex-related cues share a common pattern of neuronal activation: downstream consequences of DA release in the mPOA include the activation of neurons in the NAcc, VTA, and basolateral amygdala, which also represent neurons activated by sexual cues.Reference Pfaus, Giuliano and Gelez 33
Conclusions
The female sexual response involves a complex interplay of physiological, neurobiological, hormonal, and psychological factors. The proposed pathophysiology underlying HSDD involves central neuroendocrine dysregulation/imbalance in the brain, resulting in decreased excitation, increased inhibition, or both. Targeting melanocortins is a novel approach to treatment of women who suffer from HSDD by enhancing the activity of excitatory pathways in the brain. Bremelanotide, an analog of α-MSH, primarily acts on excitatory pathways involved in sexual response to enhance sexual desire and arousal by stimulating DA release in the mPOA, as shown in preclinical studies in female rats. Bremelanotide, with its unique mechanism of action, has the potential to fill an unmet medical need for premenopausal women suffering from HSDD who require increased excitation. 56 , 57
Acknowledgments
The authors were responsible for all content and editorial decisions, and received no honoraria related to the development of this manuscript. All authors contributed to the research, writing, and reviewing of all drafts of this manuscript and approved the final version. Editorial support in the preparation of this manuscript was provided by Phase Five Communications, funded by AMAG Pharmaceuticals, Inc., and Palatin Technologies, Inc., the licensee of bremelanotide.
Funding
Editorial support in the preparation of this manuscript was provided by Phase Five Communications, funded by AMAG Pharmaceuticals, Inc. and Palatin Technologies, Inc.
Disclosures
James G. Pfaus has served on advisory boards for Acadia Pharmaceuticals Inc., AMAG Pharmaceuticals, Inc., Emotional Brain Institute, IVIX LLC, Palatin Technologies, Inc., and Viscuris Pharmaceuticals.
Anita H. Clayton has served on advisory boards or has been a consultant for Acadia, Alkermes, Allergan, AMAG Pharmaceuticals, Inc., Daré Bioscience, Fabre Kramer, IVIX, Lundbeck, Palatin Technologies, Inc., S1 Biopharma, Sage Therapeutics, Sprout, and Takeda. She has received grants from Endoceutics, Janssen, Sage Therapeutics, and Takeda. She has shares or restricted stock units in Euthymics and S1 Biopharma.
Amama Sadiq was an employee/shareholder of AMAG Pharmaceuticals, Inc. during the study and during the development of this manuscript.
Carl Spana is an employee/shareholder of Palatin Technologies, Inc.
Supplementary Materials
To view supplementary material for this article, please visit http://doi.org/10.1017/S109285292100002X.








