Introduction
Huntington’s disease (HD) is an autosomal-dominant neurodegenerative disease caused by the expansion of CAG repeats in exon 1 of the huntingtin (HTT) gene at the short arm of chromosome 4 (4p16.9), responsible for the synthesis of the huntingtin protein. The disease was described in 1872 by Huntington.Reference Huntington1 Since the discovery of the gene locus near the tip of the short arm of chromosome 4 in 1983 and the cloning of the gene 10 years later, there has been an extensive research on the genetics of HD, although the exact role of the abnormal gene product HTT in neurodegeneration is still not well understood.
Genetic confirmation of CAG repeat expansion is the hallmark of current epidemiological measures of HD. Accurate prevalence estimates depend on comprehensive genetic testing coupled with neurological evaluation. Prevalence studies incorporating both genetic and clinical diagnostic standards show that 10.6 to 13.7 individuals per 100 000, are affected in Western populations.2–5 Prevalence studies that include genetic (molecular) diagnostics report higher rates of the disease than those using clinical measures alone.Reference Bates, Dorsey and Gusella6 Longitudinal analyses show an increase in the prevalence of HD over the past several years, probably owing to the wider availability of the genetic testing.Reference Evans, Douglas, Rawlins, Wexler, Tabrizi and Smeeth3, Reference Morrison7 The incidence of HD is estimated to be 4.7 to 6.9 new cases per million people per year in Western populationsReference Ramos-Arroyo, Moreno and Valiente8; it is endemic to all populations but occurs at much higher frequencies among individuals of European ancestry. Populations in Japan, Taiwan, and Hong Kong have a much lower incidence of HD with a prevalence of one to seven cases per million people, approximately one-tenth as frequently as in Europe and North America.Reference Rawlins, Wexler and Wexler5, Reference Squitieri, Andrew and Goldberg9 In South Africa, black people also present with lower rates than white and mixed-ancestry subpopulations.Reference Hayden, MacGregor and Beighton10, Reference Baine, Krause and Greenberg11 These differences are ancestry-specific,Reference Fisher and Hayden4 relating to genetic differences in the HTT gene.
HD is diagnosed on the basis of clinical evaluation, family history, and, in most cases, genetic testing for the presence of the CAG expansion in HTT. The disease is a fully penetrant, autosomal-dominant, inherited disorder; therefore, a carrier of an expansion greater than 39 CAG repeats is genetically diagnosed with HD. The triad of symptoms that characterize the condition are motor dysfunction (most typically chorea), cognitive impairment (eg, problems with executive functions, attention, and emotion recognition), and neuropsychiatric features (such as apathy and blunted affect).Reference Bates, Dorsey and Gusella6 The clinical diagnosis of manifest HD is based on the presence of motor manifestations, which are the best known and most visible symptoms in HD. Among them, involuntary movements are the most obvious. The motor findings are fairly sensitive and specific. However, patients with HD may exhibit a variety of movement disorders, with the most common being chorea, but also parkinsonism (characteristic of juvenile HD), ataxia, dystonia, bruxism, myoclonus, tics, and tourettism. Nonetheless, these signs appear chronologically late compared with other manifestations such as cognitive impairment.
Mild cognitive impairment (MCI) has been reported to be present in approximately 40% of people with premotor (or prodromal, genetically confirmed) HD.Reference Duff, Paulsen and Beglinger12 At the onset of motor symptoms, MCI was found in 84% of patients with HD and dementia in 5% of patients with HD. After 5 years of motor symptoms, 24% of HD patients met the criteria for MCI and 69% met the criteria for dementia.Reference Julayanont, McFarland and Heilman13
Cognitive impairment begins prior to the clinical diagnosis of motor symptoms, in the premotor or premanifest period, and progresses gradually throughout the course of the disease.14–16 The features of cognitive disability in HD are similar to disorders associated with subcortical brain pathology (eg, Parkinson’s disease [PD]) but are dissimilar to Alzheimer disease.Reference Aretouli and Brandt17, Reference Peavy, Jacobson and Goldstein18 HD differentially affects specific domains of cognition throughout the course of the disease. The impaired cognitive domains include executive function, mental flexibility, psychomotor performance, attention, working memory, and emotion recognition. The largest cross-sectional effect sizes between early manifest HD and controls were demonstrated in information processing speed, executive function, attention, memory, visuospatial skills, timing, and emotion processing.Reference Stout, Jones and Labuschagne15, Reference Tabrizi, Scahill and Owen16, 19–21 These cognitive deficits are detectable in the premanifest stages and develop slowly. Among these cognitive deficits, motor planning/speed and sensory perceptual processing are cognitive domains that may be important for predicting progression in the premanifest population.Reference Harrington, Smith and Zhang22 The earliest change and best predictor of disease progression is psychomotor slowing.Reference Tabrizi, Scahill and Owen16, 23–25 Executive difficulties in HD include problems in planning,Reference Watkins, Rogers, Lawrence, Sahakian, Rosser and Robbins26, Reference Unschuld, Liu and Shanahan27 organization and sequencing,Reference Snowden, Craufurd, Griffiths and Thompson23 cognitive flexibility, and set shifting.Reference Watkins, Rogers, Lawrence, Sahakian, Rosser and Robbins26, Reference Paulsen, Salmon, Monsch, Butters, Swanson and Bondi28 A common practical difficulty observed in HD is in multitasking, with evidence of attention problems.Reference Georgiou, Bradshaw, Phillips, Bradshaw and Chiu29
Learning and retrieval of new information are affected, but the impairment differs from Alzheimer disease, with rapid forgetting being less pronounced.Reference Aretouli and Brandt17 Studies investigating memory in HD patients have shown proportionally poorer free recall than recognition memory and cued recall,30–32 more passive learning strategies in HD than controls,Reference Lundervold, Reinvang and Lundervold32 problems in source memoryReference Brandt, Bylsma, Aylward, Rothlind and Gow33 and in prospective memory,Reference Nicoll, Pirogovsky and Woods34 and relatively preserved retention from immediate to delayed recall. The profile of memory disturbances suggests a strong executive contribution to memory failures, in keeping with disrupted striatal–frontal pathways.Reference Snowden35 In addition to the problems in declarative memory (ie, explicit memory for material previously presented), people with HD show procedural memory impairments (ie, skill and habit learning).Reference Gabrieli, Stebbins, Singh, Willingham and Goetz36, Reference Thompson, Poliakoff and Sollom37 Language (eg, syntax) impairments are demonstrated early in the disease course, with progressive difficulties evident in understanding and producing complex sentences. In addition, patients with HD commonly show reduced performance on verbal fluency tasks.Reference Rohrer, Salmon, Wixted and Paulsen38, Reference Henry, Crawford and Phillips39 A reduction in lexical capacity appears later and often might be overlooked.Reference Bachoud-Lévi, Ferreira and Massart40
Disorientation, both in time and space, appears during the progression of HD, and the temporal orientation is altered earlier.41–43 Visuospatial and visual perceptual impairments are present late in the course of the disease through interference with the integration and understanding of visual information.Reference Lawrence, Watkins, Sahakian, Hodges and Robbins41 Several studies reported that patients with HD have difficulties with high-level perceptual discrimination,Reference Brouwers, Cox, Martin, Chase and Fedio44, Reference Lawrence, Sahakian, Hodges, Rosser, Lange and Robbins45 perceptual integration,Reference Gomez Tortosa, del Barrio, Barroso and Garcia Ruiz46 and constructional tasks,Reference Bamford, Caine, Kido, Plassche and Shoulson47 which utilize executive processes. Spatially, people with HD present impairments on tasks involving mental rotation or manipulation of informationReference Mohr, Brouwers, Claus, Mann, Fedio and Chase48, Reference Bylsma, Brandt and Strauss49 and a timed visual search.Reference Labuschagne, Mulick Cassidy and Schahill50
Some cognitive disturbances such as problems with initiation, lack of awareness of deficits, and disinhibition are at the intersection between cognitive and psychiatric domains.Reference Duff, Paulsen and Beglinger12 Patients with HD can have social disengagement, decreased participation in conversation, and slowed mentation, often accompanied by lack of awareness of deficits and by impulsivity.Reference Papoutsi, Labuschagne, Tabrizi and Stout51
The psychiatric and behavioral manifestations of the disease are also very debilitating. These include irritability, depression and suicidal ideation or attempts, anxiety, apathy, obsessions, paranoia, and hallucinations.
Other symptoms besides motor, cognitive, and psychiatric disorders are often present. Among those, weight loss, dysphagia, and sleep disturbance are sometimes the most prominent symptoms.Reference Bachoud-Lévi, Ferreira and Massart40 In addition, patients with HD might present other debilitating symptoms such as urinary incontinence, pain, excessive perspiration, hypersalivation, and reduced lung function and respiratory muscle strength.Reference Bachoud-Lévi, Ferreira and Massart40
Although the clinical diagnosis of HD has traditionally been based on motor signs and symptoms, neuroimaging and other tests can support the diagnosis, primarily by ruling out other conditions. Typically, they are not necessary, especially if there is a characteristic presentation of an individual with a known family history and a positive genetic test. An MRI or computed tomography (CT) scan may reveal symmetrical striatal atrophy (and often, to a lesser degree, atrophy in other subcortical regions, cerebral cortical gray matter, and subcortical white matter). Such changes might be detectible even prior to the development of motor symptoms and are strongly suggestive of a diagnosis of HD.Reference Bates, Dorsey and Gusella6
In HD, in contrast to other neurological disorders (eg, PD, MCI, HIV-associated neurocognitive disorders [HAND]), there is no “gold standard” definition for MCI and dementia.Reference Mestre, van Duijn and Davis52 Therefore, it is challenging to provide a formal definition of MCI or dementia in HD. Adopting the general neurological definition, MCI is delineated as the transition between normal cognition and dementia, in which an individual develops subjective cognitive symptoms with objective evidence of cognitive impairment on a standardized neuropsychological evaluation but is still functionally independent. When cognitive impairment progresses to affect daily functions, dementia is diagnosed. The diagnosis of HD dementia should include demonstrable evidence of impairment in at least two areas of cognition (eg, attention, information processing speed, executive functions, visuospatial abilities, memory) but without the requirement of memory impairment in the context of impaired functional abilities and a deteriorating course.Reference Peavy, Jacobson and Goldstein18 The adoption of these definitions carries the challenge of identifying functional impairment strictly associated with cognitive impairment in a complex disease such as HD, in which other clinical features may contribute to a functional limitation.Reference Mestre, van Duijn and Davis52
However, applying an extensive battery of neuropsychological tests is time consuming, is expensive, necessitates trained personnel, and is generally not feasible in most facilities. Therefore, brief cognitive tests, such as the Montreal Cognitive Assessment (MoCA) or Mini-Mental State Examination (MMSE), could be useful in evaluating patients with HD. Recently, the International Parkinson and Movement Disorder Society (MDS) invited an international group of experts on cognition in HD to review and critique scales evaluating global cognitive performance in HD patients.Reference Mestre, van Duijn and Davis52 The authors retrieved all the manuscripts published before September 2016 and considered a total of 17 cognitive scales for in-depth assessment. None of the scales met the criteria for a “recommended” status. To assess the severity of cognitive dysfunction, the MoCA was “recommended with caveats.” In addition, it was “suggested” as a screening tool for cognitive impairment. Eight scales were classified as “suggested” for the purpose of measuring cognitive dysfunction severity, namely the Unified Huntington’s Disease Rating Scale (UHDRS) Cognitive Assessment, the cognitive section of the UHDRS-For Advanced Patients (FAP), the Alzheimer’s Disease Assessment Scale—Cognitive Subscale, the Frontal Assessment Battery, the Mattis Dementia Rating Scale, the MMSE, and the Repeatable Battery for the Assessment of Neuropsychological Status.Reference Mestre, van Duijn and Davis52
The MoCA was developed in 2005 for detecting MCI and has been shown to be highly sensitive and specific in the older adult population.Reference Nasreddine, Phillips and Bedirian53 It is a brief bedside test; the administration time is approximately 10 minutes. The MoCA evaluates executive functions, memory, and attention, which are commonly affected in patients with HD, and also evaluates visuospatial functions, naming, language, abstraction, and orientation. Scores on the MoCA range from 0 to 30 points; a score of 25 or lower indicates cognitive dysfunction. This cutoff is now widely used as a threshold for detecting cognitive impairment and possible dementia. To minimize practice effects, three versions of the MoCA have been developed in English, which test the same domains, but the contents of the tasks are different. The alternative versions of the MoCA present comparable reliability to the original test.Reference Costa, Fimm and Friesen54 Translations in multiple languages are also available.
Several studies have consistently reported that the MoCA has good overall psychometric properties and good sensitivity in identifying milder forms of cognitive impairment in many clinical conditions. Therefore, the MoCA has widespread international use and is recognized as one of the best screening tests for cognitive impairment.Reference Ismail, Rajji and Shulman55 For example, in MCI, the MoCA demonstrated excellent internal consistency, with a Cronbach’s alpha of 0.83 on the standardized items.Reference Nasreddine, Phillips and Bedirian53 The test–retest reliability was also good, with a mean change in MoCA scores from the first to second evaluation of 0.9 points.Reference Nasreddine, Phillips and Bedirian53 In addition, in studies that applied Rasch analysis techniques, the researchers found that scores on the MoCA can be used to quantify the amount of cognitive ability a person has and can be used to track changes in cognitive functions over time.Reference Koski, Xie and Finch56
Validation studies of the MoCA have been conducted in patients with different types of neurological disorders, such as MCI,Reference Freitas, Simoes, Alves and Santana57 Alzheimer’s disease,Reference Freitas, Simoes, Alves and Santana57 and PD.Reference Hoops, Nazem and Siderowf58 Nonetheless, recent systematic reviews found that cutoff scores lower than 26 on the MoCA were likely to be more useful for optimal diagnostic accuracy in patients with stroke,Reference Lees, Selvarajah and Fenton59 dementia (including Alzheimer’s disease, vascular dementia, Lewy body dementia, and frontotemporal dementia),Reference Davis, Creavin, Yip, Noel-Storr, Brayne and Cullum60 MCI,Reference Carson, Leach and Murphy61 and HAND.Reference Rosca, Albarqouni and Simu62
Early diagnosis and specific care of cognitive impairment in individuals with HD are essential, as it is an important cause of functional disability and related outcomes. Although physicians tend to focus on motor disturbances, rather than cognitive impairments when considering treatment, probably because they are the most visible symptoms, several studies have demonstrated the impact of cognitive disturbances on patients and caretakers.Reference Sampaio, Borowsky and Reichmann21 Cognitive impairment, along with motor disturbances and apathy, was demonstrated to be an independent predictor of disability.Reference Banaszkiewicz, Sitek and Rudzińska63 In addition, cognitive disturbances determined a patient’s quality of life,63–66 while motor disturbances and depression were predictors of caregiver burden.Reference Banaszkiewicz, Sitek and Rudzińska63
There is currently no cure or treatment that can halt, slow, or reverse the progression of the disease. Based on the present knowledge, no pharmacological treatment is recommended for the treatment of cognitive symptoms. However, multiple rehabilitation strategies (speech therapy, occupational therapy, cognitive, and psychomotricity) might improve or stabilize transitorily cognitive functions at some point of time in the course of the disease.Reference Bachoud-Lévi, Ferreira and Massart40 Several clinical trials have investigated means to alleviate or reduce symptoms and slow progression in clinically diagnosed as well as prodromal HD, but most of the clinical trials used a total motor score and a measure of functional capacity as primary and secondary outcomes. However, recent research has suggested that traditional outcomes designed for diagnosed HD may lack sensitivity for individuals with early HD and those with prodromal HD; thus, cognitive, psychiatric, and new functional capacity outcomes should be assessed.Reference Vaccarino, Sills and Anderson66, Reference Beglinger, Adams and Paulson67 Therefore, the validation of new measures for cognition and psychiatric disturbances will be critical to efforts to better treat HD,Reference Paulsen20 as neuropsychological assessment has a crucial role in the identification of cognitive changes in the early phases of the disease, in monitoring progression, and in the evaluations of therapeutic interventions outcomes.
The MoCA fulfils important feasibility criteria for use in clinical practice: it has a short administration time and with multiple translations. Furthermore, online training and certification are available on the MoCA website. The test has been proven to have good psychometric properties in other populations and to assess a broad range of cognitive domains. Therefore, the MoCA may help identify individuals with cognitive impairments that might require further assessments and specific care, facilitating access to appropriate services. Nonetheless, incorrectly evaluated as having cognitive impairment implies significant costs due to further unnecessary investigations. Furthermore, cognitive assessments have exceptional potential to determine excellent potential for the early detection of HD in persons with genetic risk and have exceptional potential to determine sensitive outcomes in clinical trials, where reliable cognitive tools are needed in order to detect changes secondary to interventions in HD.
To date, no assessment scale has been sufficiently investigated to be classified as “recommended” for evaluating cognitive impairment in individuals with HD.Reference Mestre, van Duijn and Davis52 Among the scales “suggested” by the MDS, there are two scales that were specifically designed for HD: the UHDRS Cognitive Assessment and the UHDRS-FAP cognitive section. However, data regarding their sensibility and specificity and test–retest reliability are lacking. In addition, the UHDRS Cognitive Assessment has only a reduced number of tests, and, therefore, it is not considered to be sufficient for evaluating all relevant cognitive domains in HD.Reference Mestre, van Duijn and Davis52 The UHDRS-FAP is “suggested” for assessing severity of cognitive impairment only in late stages of HD, and further research is needed regarding its ability to discriminate across stages of cognitive dysfunction.Reference Mestre, van Duijn and Davis52 The other scales “suggested” by the MDS were not developed specifically for HD. Among them, the MoCA is the only scale that is also “recommended with caveats” for assessing cognitive impairment in HD individuals.Reference Mestre, van Duijn and Davis52
However, in the literature, there is conflicting evidence regarding the optimal cutoff of the MoCA score,59–62 and the MDS reviewed the articles published before September 2016. Therefore, there is considerable value in determining the strength of the empirical evidence that supports the use of MoCA as a screening test for cognitive impairment in patients with HD.
We aim to collate evidence from different studies, integrating the existing information and providing data for rational decision-making, highlighting possible answers that are easily accessible to clinicians, health care providers, and policy makers. The objective of this systematic review is to evaluate research regarding the accuracy of the MoCA for diagnosing cognitive impairment in individuals with HD and to highlight the methodological quality (in terms of risk of bias) and quantity of evidence available in this regard. In addition, we aim to identify the gaps in the literature concerning this screening test.
Methods
The present systematic review was performed following the recommendations described in the Cochrane Handbook for Diagnostic Test Accuracy Reviews68 and the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA).Reference Moher, Liberati, Tetzlaff and Altman69
Search strategy and selection criteria
Figure 1 shows the search strategy used in the systematic review.

Figure 1. Study selection flow chart.
A computerized bibliographic search was performed from the inception of the database to April 2020 for the following databases: MEDLINE/PubMed, Scopus, Latin American and Caribbean Health Sciences Literature (LILACS), and PsychINFO. In addition, a complementary manual search was performed on the MoCA website, and the reference lists of all relevant research papers were checked to identify possible additional studies.
The following key words were used: “Montreal Cognitive Assessment” or the acronym “MoCA” and “Huntington’s disease” (MeSH). These search terms were used with PubMed database, the primary source of citations. Searches in other data sources used similar versions of these terms, appropriate for each database. We did not utilize search filters (the collection of terms aimed at reducing the number of results that needed to be screened) because our aim was to generate a comprehensive list of studies that would be suitable for answering the research question. Even the most sensitive filters have been found to miss relevant studies and perform inconsistently across subject areas and study designs, while at the same time, they have not significantly reduced the number of studies that need to be assessed for inclusion.68, Reference Davis, Creavin and Noel-Storr70 In addition, we did not apply any language restrictions to our search.
Two authors reviewed the title, abstract, and full text (when needed) of all retrieved research papers and assessed whether the study met the inclusion criteria. Disagreements were solved through discussion, and the participation of a third rater was not needed to address discrepancies.
To perform a systematic review of MoCA use in the context of HD, we selected all the studies in which the MoCA was used to assess the cognitive abilities in HD individuals. The main types of eligible studies, using the MoCA as an index test, were (i) cross-sectional studies in which participants received the index test (MoCA) and a reference standard diagnostic assessment; (ii) case–control studies comparing the MoCA to a battery of tests; (iii) studies comparing the MoCA to the MMSE, the most widely used screening instrument to detect cognitive impairment; (iv) studies estimating the prevalence of cognitive impairment in individuals with HD; and (v) studies or clinical trials in which the MoCA was used for the cognitive assessment of patients with HD.
We included studies reporting adults (over 18 years old) with confirmed HD in which the association between the MoCA score and cognitive impairment was assessed, with the MoCA being used as an index test. The index test was any full version of the MoCA. Although we expected to find the recommended cutoff score of 26 or below to differentiate normal cognition (scores of 26 and above) from impaired cognition (scores less than 26), we also included studies using other thresholds. The target condition was cognitive impairment, including MCI and dementia. As a reference standard for cognitive impairment, we used a complex neuropsychological assessment that evaluated at least five neurocognitive domains (including verbal and language, attention and working memory, abstraction, and executive function, learning and recall, speed of information processing, and motor skills), with consensual recommendations on appropriate tests. We excluded studies with fewer than 10 participants. In addition, we excluded studies with patients with confounding factors such as neurological disorders (eg, recent traumatic brain injury, CNS infections, stroke, other neurodegenerative disorders, and brain tumors), drug or alcohol addiction, and active infections.
In addition, the methodological quality of the studies was assessed by two authors independently, using the unmodified Quality Assessment of Diagnostic Accuracy Studies 2 tool.68 Disagreements were solved through discussion.
Results
From a total of 33 unique studies identified using the search strategy and assessed in the full-text, we included 26 studies in the present review: (i) two case–control studies comparing the MoCA to a battery of tests; (ii) three studies comparing the MoCA to the MMSE; (iii) two studies estimating the prevalence of cognitive impairment in individuals with HD; and (iv) 19 studies or clinical trials in which the MoCA was used for the cognitive assessment of participants with HD. We found no cross-sectional studies in which participants received the index test (MoCA) and a reference standard diagnostic assessment composed of an extensive neuropsychological battery.
The characteristics of the included studies are summarized in Table 1. The PRISMA diagram describing the selection process of studies is detailed in Figure 1.
Table 1. Characteristics of Included Studies
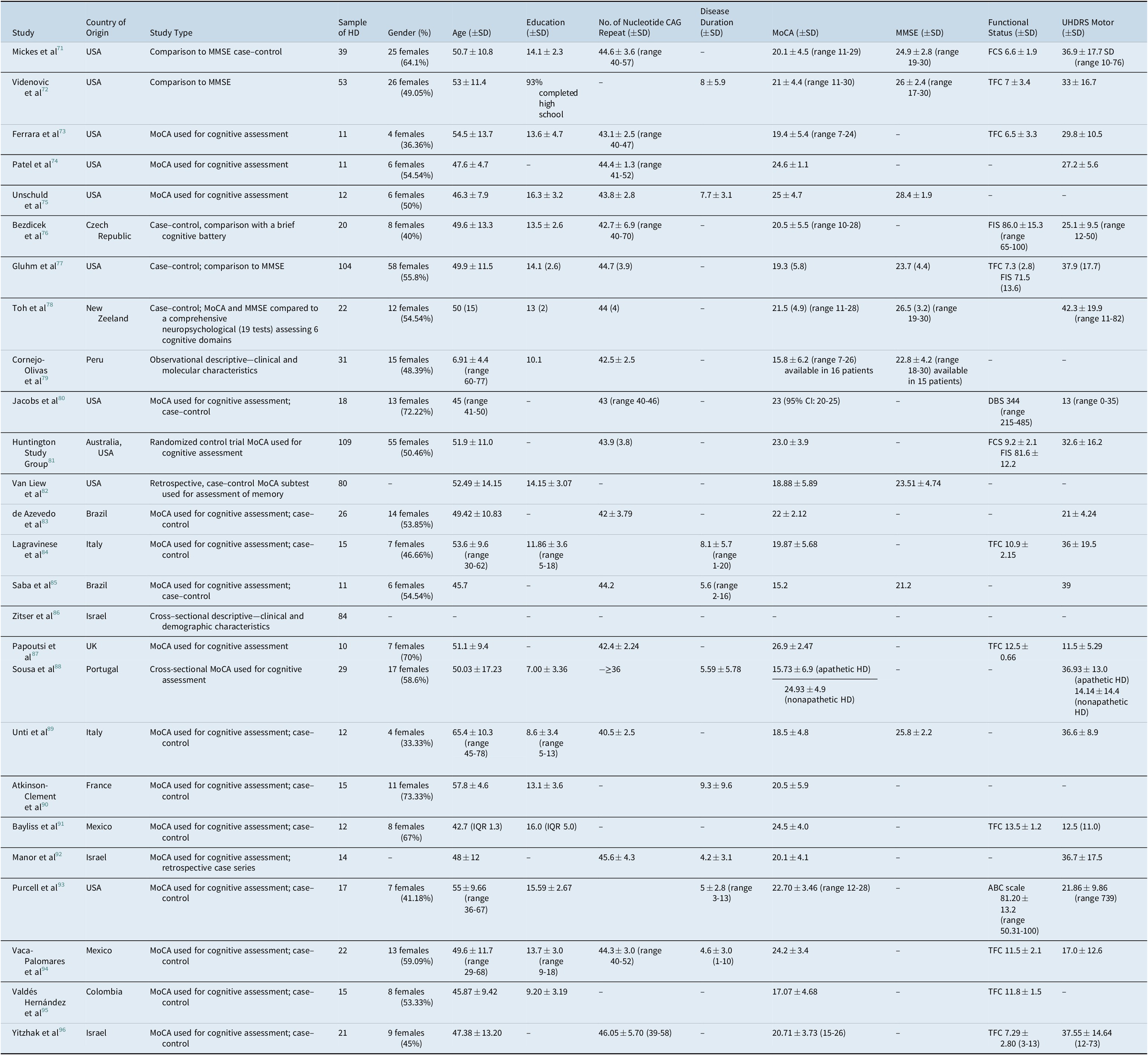
Abbreviations: ABC, activities-specific balance confidence; FCS, functional capacity score; HD, Huntington’s disease; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; DBS, deep brain stimulation; FIS, Functional Independence Scal; IQR, interquartile range.
Table 2. Characteristics of Studies Using MoCA as an Instrument for the Cognitive Assessment of Participants with Huntington’s disease

Abbreviations: ABC, activities-specific balance confidence; AD, Alzheimer’s disease; BDI-II, Beck Depression Inventory-II; BSE, bed side swallowing evaluation; CERAD, consortium to establish a registry for Alzheimer’s disease; DIP, dysarthria impact profile; DT, dual task; DTI, diffusion tensor imaging; FAB, frontal assessment battery; FCS, functional capacity score; FEES, fiberoptic endoscopic evaluation of swallowing; GM, gray matter; HD, Huntington’s disease; IFS, INECO frontal screening; JLO, judgment of line orientation; JTHFT, Jebsen-Taylor Hand Function Test; KDEF, Karolinska Directed Emotional Faces; MRS, magnetic resonance spectroscopy’ NAA, N-acetylaspartate; OT, oxytocin; PD, Parkinson’s disease; PVS, perivascular spaces; RMET, reading mind in the eyes test; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; SMA, supplementary motor area; SDMT, Symbol Digit Modalities Test; SDQ, swallowing disturbances questionnaire; SWAL-QOL, swallowing related quality of life; ToM, theory of mind; TMS, total motor score; UHDRS, Unified Huntington’s Disease Rating Scale; VHI, Voice Handicap Index. APDM, APDM®; WAIS-IV, Wechsler Adult Intelligence Scale.
Seven studies were excluded for the following reasons: the index test was not the MoCA (3), the study population consisted of less than 10 patients (3), or the research paper was in a language other than English or Spanish (1).
Twenty-six studies were included. The year of publication ranged from 2010 to 2020. The study samples were selected from 13 different countries (Australia, Brazil, Colombia, Czech Republic, France, Italy, Israel, Mexico, New Zeeland, Peru, Portugal, the UK, and the USA). Samples varied in size (10-109 participants), sex ratio (33.33%-72.22% females), median age (45-69.1 years), number of CAG repeats (42-46.05), MoCA scores, UHDRS motor scores, functional status, and educational level. The characteristics of the included studies are presented in Tables 1-3.
Table 3. Characteristics of Studies Comparing MoCA to MMSE
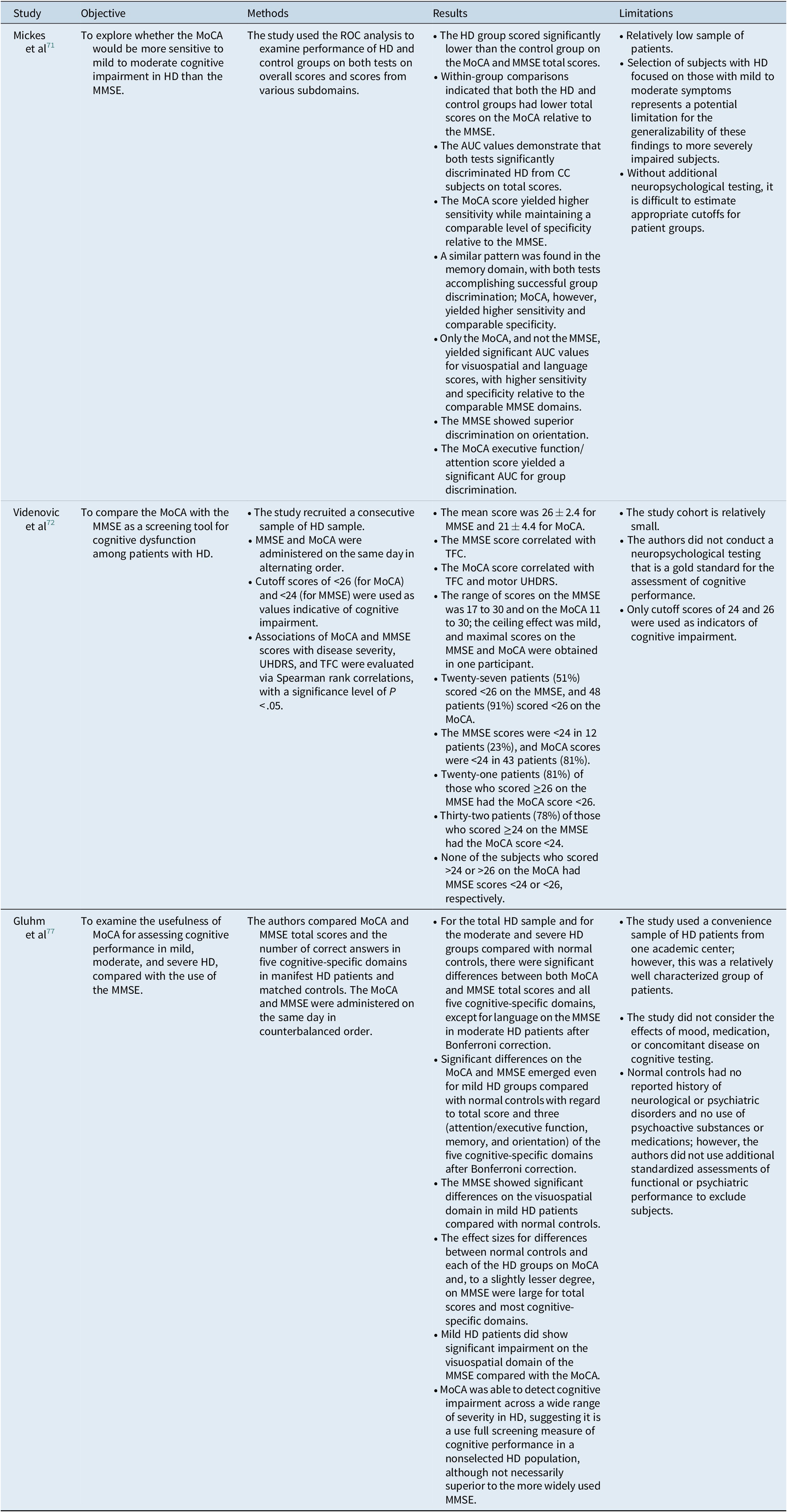
Abbreviations: AUC, area under the curve; HD, Huntington’s disease; ROC, receiver operating characteristic; MoCA, Montreal cognitive assessment; MMSE, mini-mental state examination; TFC, total functional capacity; UHDRS, unified Huntington’s disease rating scale.
Case–control studies comparing the MoCA to a battery of tests (Tables 1 and 2)
To date, only two studies have assessed the validity of the MoCA as a screening tool for cognitive dysfunction in HD, using a cognitive battery as a reference standard.
The case–control study of Bezdicek and his colleaguesReference Bezdicek, Majerova, Novak, Nikolai, Ruzicka and Roth76 analyzed the results of the MoCA in correlation with a brief cognitive battery composite score. The neuropsychological functions of both the HD patients and the normal controls were assessed with the MoCA, and a short battery investigating five cognitive domains (memory, executive functions and set maintenance, set activation, psychomotor speed, and visuoconstructive functions).
The MoCA presented adequate internal consistency, with a Cronbach’s alpha of 0.82 in HD patients. The concurrent validity of the MoCA total score and the composite score of the brief cognitive battery was r = 0.81 (P < .001) using the Spearman rank correlation coefficient. The HD patients scored significantly worse compared with normal controls on six of seven MoCA subtests, specifically the visuospatial/executive, attention, language, abstraction, delayed recall, and orientation subtests. HD patients were comparable to controls only in the naming subtest. The area under the curve (AUC; 95% CI) for the MoCA was 0.90 (0.809-0.997), and the optimal cutoff point was 25/26 (sensitivity = 0.94, specificity = 0.84, positive predictive value [PPV] = 0.81, negative predictive value [NPV] = 0.95) for all three measures; the point of maximum combined sensitivity and specificity, the optimal screening cutoff, and the optimal diagnostic cutoff.
The case–control study of Toh and his collaborators evaluated the utility of MoCA, MMSE, and UHDRS measures and a comprehensive neuropsychological battery of tests in monitoring short-term disease progression in HD patients.Reference Toh, MacAskill and Dalrymple-Alford78 The comprehensive assessment of cognitive function used 19 neuropsychological tests to assess six cognitive domains (executive function, working memory and attention, learning and memory, processing speed, language, and visuospatial functions). The number of tests administered was evenly distributed over two separate sessions that were 1 week apart; the tests were presented in the same order for all participants. Each session began with the MMSE in the first session and the MoCA in the second session. All returning participants were reassessed in an identical manner 12 months later. At baseline, 27.3% of the HD patients had normal cognition, 45.5% met the criteria for MCI, and 27.3% presented with dementia. All the controls had normal cognition. Compared to the controls, the HD group showed significantly reduced scores for overall global cognition and in brief cognitive tests both at baseline and at the 12-month follow-up.
In terms of domain-specific scores, the HD group had significantly lower scores than the controls across all cognitive domains and the mean effect sizes for baseline and 12-month follow-up combined ranged from the smallest (d = 1.5) in the language domain to the largest (d = 2.8) in the executive function domain. Relative to the control group, which showed an increase in the overall global cognitive z-score and the learning and memory domain score over a 12-month period, there was significantly less change in domain-specific scores in the HD group over that period. The MMSE and MoCA were less effective than the UHDRS cognitive assessment for monitoring cognitive changes in manifest HD patients over 12 months. The MoCA, MMSE, and UHDRS cognitive component scores correlated well with overall global cognition, as determined through the comprehensive neuropsychological test battery, in the HD group, supporting the utility of the three brief cognitive assessment tools in the cross-sectional detection of cognitive deficits in manifest HD patients. Furthermore, the results of the study indicated that there were no significant differences between the three brief cognitive tests reflecting overall global cognition in HD patients, thus providing no evidence that one test is better than the other in this respect. However, the UHDRS cognitive component, which focuses on testing executive function and had low variance over time, proved to be a more reliable brief substitute for comprehensive neuropsychological testing than the MMSE and MoCA in monitoring cognitive changes in HD patients after 12 months. With regard to the sensitivity and specificity of the MoCA, no data were presented.
From a methodological point of view,68, Reference Whiting, Rutjes, Reitsma, Glas, Bossuyt and Kleijnen97 both studies had a case–control design and were considered to present a high risk of bias, as there is consistent evidence that when using a case–control design in diagnostic accuracy studies, both sensitivity and specificity are increased.68 In terms of the patients’ spectrum risk of bias, the sensitivity of a test will often vary according to the severity of disease. The patient groups of both studiesReference Bezdicek, Majerova, Novak, Nikolai, Ruzicka and Roth76, Reference Toh, MacAskill and Dalrymple-Alford78 were not composed of any presymptomatic HD subjects, and therefore, the conclusions cannot be generalized. In addition, the methods used to sample patients may lead to the inclusion of patients different from the spectrum in which the test will be used in practice; the ideal diagnostic accuracy study would prospectively include a consecutive series of patients fulfilling all selection criteria. In the aforementioned studies, it is unclear how the samples were recruited. Regarding the reference standard, the study of Bezdicek et alReference Bezdicek, Majerova, Novak, Nikolai, Ruzicka and Roth76 used only a brief cognitive battery, and its incremental validity in relation to MoCA subscales is limited. Although Toh et alReference Toh, MacAskill and Dalrymple-Alford78 used an extensive battery with 19 neuropsychological tests, this can also cause bias because the probability of an abnormal score increases as the number of tests performed per domain and the number of assessed domains increase.98–100 Furthermore, in both studies,Reference Bezdicek, Majerova, Novak, Nikolai, Ruzicka and Roth76, Reference Toh, MacAskill and Dalrymple-Alford78 it is unclear if the interpretation of the index test was done without knowledge of the results of the reference standard and vice versa (known as test review bias and diagnostic review bias, respectively) or if any patient withdrawals occurred. Empirical evidence shows that a lack of blinding procedures may increase sensitivity, but no systematic effect on specificity was noted.Reference Whiting, Rutjes, Reitsma, Glas, Bossuyt and Kleijnen97 Additionally, incomplete reporting of any withdrawals from the study that might have occurred hinders the evaluation of this aspect.68
Studies comparing the MoCA to the MMSE (Tables 1-3)
We identified only three studies that directly compared the MoCA with the MMSE, the latter being used as a reference standard.Reference Mickes, Jacobson and Peavy71, Reference Videnovic, Bernard, Fan, Jaglin, Leurgans and Shannon72, Reference Gluhm, Goldstein, Brown, Van Liew, Gilbert and Corey-Bloom77 One study had a cross-sectional design,Reference Videnovic, Bernard, Fan, Jaglin, Leurgans and Shannon72 and the others were case–control studies.Reference Mickes, Jacobson and Peavy71, Reference Gluhm, Goldstein, Brown, Van Liew, Gilbert and Corey-Bloom77 A detailed presentation of the studies is provided in Table 3.
In terms of risk of bias, only one study specified that a consecutive sample of HD patients was recruited.Reference Videnovic, Bernard, Fan, Jaglin, Leurgans and Shannon72 In addition, none of the studies specified whether the reference standard results were interpreted without knowledge of the results of the index test and vice versa or if there were any patients who dropped out from the study. Furthermore, using the MMSE as a reference standard introduced incorporation bias, as both tests have some similar items (eg, serial sevens, time, and orientation). Incorporation of the index test in the reference standard is likely to increase the amount of agreement between the results, thereby leading to an overestimation of diagnostic accuracy.68
Studies estimating the prevalence of cognitive impairment in individuals with HD (Tables 1 and 2)
We identified two studies that used the MoCA to assess the prevalence of cognitive impairment in an HD population.Reference Cornejo-Olivas, Inca-Martinez and Espinoza-Huertas79, Reference Zitser, Thaler and Inbar86 Both studies were descriptive, presenting clinical and demographic characteristics of HD patients.
Studies or clinical trials in which the MoCA was used as an instrument for the cognitive assessment of participants with HD (Tables 1 and 2)
The MoCA was used in 19 studies as a cognitive assessment scale. Only two clinical trials used the MoCA to evaluate the cognitive status of the HD individuals.Reference Ferrara, Mostile, Hunter, Adam and Jankovic73, 81
The scale was used in six neuroimaging studies,Reference Unschuld, Edden and Carass75, Reference de Azevedo PC, Guimarães and Piccinin83, Reference Saba, Yared, Doring, Phys, Borges and Ferraz85, Reference Papoutsi, Weiskopf, Langbehn, Reilmann, Rees and Tabrizi87, Reference Vaca-Palomares, Brien and Coe94, Reference Valdés Hernández, Abu-Hussain and Qiu95 eight cognitive studies,Reference Patel, Jankovic, Hood, Jeter and Sereno74, Reference Van Liew, Santoro, Goldstein, Gluhm, Gilbert and Corey-Bloom82, Reference Lagravinese, Avanzino and Raffo De Ferrari84, Reference Sousa, Moreira and Jesus-Ribeiro88, Reference Unti, Mazzucchi and Frosini89, Reference Bayliss, Galvez and Ochoa-Morales91, Reference Purcell, Goldman, Ouyang, Bernard and O’Keefe93, Reference Yitzhak, Gurevich and Inbar96 and three studies examining other clinical aspects of HD (eg, balance, dysphagia).Reference Jacobs, Boyd, Hogarth and Horak80, Reference Atkinson-Clement, Letanneux and Baille90, Reference Manor, Oestreicher-Kedem and Gad92
Discussion
The present systematic review allowed us to make several key observations.
To date, research on the use of the MoCA in individuals with HD is somewhat limited. There is no high-quality cross-sectional study to assess the accuracy of the MoCA in screening cognitive impairment in this population. Only one case–control study compared the MoCA to a short cognitive battery.Reference Bezdicek, Majerova, Novak, Nikolai, Ruzicka and Roth76 The research provided very useful information, demonstrating that the test has robust psychometric properties: good concurrent validity, high sensitivity and high specificity in the detection of cognitive dysfunction in HD patients, and adequate internal consistency. Interestingly, the optimal screening and diagnostic cutoff was a score of 26, which is concordant with the original study on the MoCA.Reference Nasreddine, Phillips and Bedirian53 However, this information must be interpreted with caution, as the authors used only a brief cognitive battery, and its incremental validity in relation to MoCA subscales is limited. Although this is one of the most informative studies conducted in this domain, it presents certain limitations (eg, the sample of patients was relatively low, and the study group was not composed of any presymptomatic HD subjects).
The second case–control study that investigated the utility of the MoCA in HD samples, compared it to a comprehensive neuropsychological battery, composed of 19 neuropsychological tests to assess six domains of cognitive function.Reference Toh, MacAskill and Dalrymple-Alford78 Although the study did not provide any information on the sensitivity and specificity of the MoCA in the HD population, it provided a new perspective on the utility of two widely used brief cognitive assessment tools (the MMSE and MoCA) in comparison to the UHDRS cognitive assessment and other measures for monitoring cognitive changes in manifest HD patients over a 12-month period. The authors concluded that the MMSE and MoCA are less useful for monitoring longitudinal cognitive changes over short-time intervals and the UHDRS cognitive assessment, which focuses on testing executive function, is more sensitive to short-term cognitive changes, and is a more reliable brief assessment tool than the MMSE and MoCA over a period of 12 months.Reference Toh, MacAskill and Dalrymple-Alford78 Nonetheless, we must interpret these results with caution.
In the present study, the criteria for MCI followed that described for PD by Dalrymple-Alford et al,Reference Dalrymple-Alford, Livingston and MacAskill101 with a requirement of two measures at—1.5 SD or equivalent within a single domain; the raw score of each component test in the neuropsychological battery was converted to a standard z-score using test-specific norms so that objective comparison could be made across component tests, regardless of individual scale ranges and distributions.Reference Toh, MacAskill and Dalrymple-Alford78 Studies have demonstrated that, assuming a normal distribution of test scores, 7% of people scoring 1.5 SD or more below the mean would be falsely designated as having cognitive decline, even without any change in performance over time (ie, false-positives). The requirement for impaired performance on at least two tests reduces the risk for false-positives but not false-negatives. In PD patients, for example, one study found that the best criterion to minimize the inclusion of cognitively normal patients as having MCI was to require deficits of at least—1.5 SD in two scores within any single domain (resulting in 30% MCI) or deficits of at least—1.5 SD in two scores from different domains (37% PD-MCI).Reference Dalrymple-Alford, Livingston and MacAskill101 Furthermore, studies of complex neuropsychological batteries in healthy controls report that between 15% and 22% of individuals from a normal control group and 20% of a simulated normal population will score below the threshold for cognitive impairment, with false-positive results.Reference Meyer, Boscardin, Kwasa and Price100 These errors are caused by two common practices to increase sensitivity regarding milder neurocognitive abnormalities. First, extensive test batteries will have higher false-positive rates than individual tests because they involve multiple comparisons. The probability of an abnormal score increases as the number of tests performed per domain and the number of assessed domains increase (ie, diagnosing a normal individual as impaired). Second, the high cutoff scores (z-scores with a threshold of 1 SD) will increase the overlap between critical portions of test score distributions in individuals with and without disease.Reference Gisslen, Price and Nilsson98, Reference Meyer, Boscardin, Kwasa and Price100 The result of increased sensitivity is essentially a reduction in specificity. Therefore, false-positive cases will lead to biased prevalence estimates and reductions in power for analytical estimates.Reference Meyer, Boscardin, Kwasa and Price100, Reference Tierney, Sheppard, Kordovski, Faytell, Avci and Woods102
The MoCA was also compared with the MMSE, the most widely used screening instrument for cognitive impairment. The MMSE was used as a reference standard in three studiesReference Mickes, Jacobson and Peavy71, Reference Videnovic, Bernard, Fan, Jaglin, Leurgans and Shannon72, Reference Gluhm, Goldstein, Brown, Van Liew, Gilbert and Corey-Bloom77 with somewhat contradictory results. The study of Mickes and his colleagues,Reference Mickes, Jacobson and Peavy71 using receiver operating characteristic (ROC) analysis, reported that almost all five cognitive-specific domains on the MoCA, but only two on the MMSE, significantly differentiated a sample of mild to moderate HD patients from normal controls. In contrast, a later studyReference Gluhm, Goldstein, Brown, Van Liew, Gilbert and Corey-Bloom77 found that significant differences between both MoCA and MMSE total scores and almost all cognitive-specific domains emerged. Even mild HD subjects showed significant differences with regard to total score and several cognitive domains on both instruments. The authors concluded that the MoCA is a useful instrument for assessing cognitive performance over a broad level of functioning in HD but is not necessarily superior to the MMSE.Reference Gluhm, Goldstein, Brown, Van Liew, Gilbert and Corey-Bloom77 One possible reason for this discrepancy is the difference in the sample size used in the studies; furthermore, the study of Mickes and his colleaguesReference Mickes, Jacobson and Peavy71 excluded serial sevens from analysis. Finally, another study involving moderately impaired HD patients with a mean UHDRS total functional capacity (TFC) score of 7.0 concluded that the MoCA, compared with the MMSE, may be a more sensitive screening instrument for cognitive dysfunction in HD patients on the basis of cutoff points.Reference Videnovic, Bernard, Fan, Jaglin, Leurgans and Shannon72 The different results of the latter study might be due to a different study design: the study of Videnovic and his colleaguesReference Videnovic, Bernard, Fan, Jaglin, Leurgans and Shannon72 was cross-sectional and the other two studiesReference Mickes, Jacobson and Peavy71, Reference Gluhm, Goldstein, Brown, Van Liew, Gilbert and Corey-Bloom77 had a case–control design. The case–control studies are usually considered to present a high risk of bias, compared to cross-sectional studies, as there is consistent evidence that in diagnostic accuracy studies, when using a case–control design, both sensitivity and specificity are increased.68 Nonetheless, we must keep in mind that, for other neurological disorders, the MoCA also presented superior sensitivity for detecting MCI compared with the MMSE, as the MoCA contains more demanding tasks for assessing executive and memory functions.Reference Larner, Julayanont, Phillips, Chertkow and Nasreddine103
When comparing the MoCA directly to the MMSE, all three studies that were conducted involving HD patients presented an incorporation bias, as both tests contain some similar items (eg, serial sevens, time, and orientation). This is likely to increase the amount of agreement between index test results and the reference standard and to overestimate the diagnostic accuracy of the MoCA.68 Finally, there is evidence that the administration of both tests in one session leads to a high level of interference, specifically between the MoCA delayed recall subtest and the MMSE three-word recall subtest, as well as between repeated trials of serial sevens.Reference Bezdıcek, Balabanova, Havrankova, Stochl, Roth and Ruzicka104
The MoCA has also been used as a tool of cognitive assessment in various studies with HD individuals. However, although the scale is “suggested” by the MDS for the screening of the presence of cognitive dysfunction in HD patients and “recommended with caveats” for assessing the severity of cognitive dysfunction,Reference Mestre, van Duijn and Davis52 the results of the MoCA must be interpreted with caution. The data regarding the use of the MoCA in HD patients are quite limited, and the use of this brief cognitive assessment tool requires additional comprehensive testing for complete validation to determine the severity of cognitive dysfunction.Reference Mestre, van Duijn and Davis52
Nonetheless, the studies that used the MoCA as a cognitive assessment tool in participants with HD provided some important information. The MoCA scores correlated with motor function tests, suggesting that factors apart from chorea have an impact on motor tasks (eg, executive functions).Reference Ferrara, Mostile, Hunter, Adam and Jankovic73 In addition, reflexive and voluntary eye motor control,Reference Patel, Jankovic, Hood, Jeter and Sereno74 balance,Reference Jacobs, Boyd, Hogarth and Horak80 and volitional coughReference Manor, Oestreicher-Kedem and Gad92 were correlated with cognitive function as revealed by the MoCA. On the other hand, no correlation was found with the dysarthria scoresReference Atkinson-Clement, Letanneux and Baille90 and postural instability.Reference Purcell, Goldman, Ouyang, Bernard and O’Keefe93 This may be because cognitive and motor abilities do not decline equally together.Reference Vaca-Palomares, Brien and Coe94
Furthermore, the MoCA proved to be a valuable tool that is capable of differentiating participants with AD and HD and able to identify specific memory deficits.Reference Van Liew, Santoro, Goldstein, Gluhm, Gilbert and Corey-Bloom82 The MoCA scores also presented a positive correlation with the TFC scoresReference Bayliss, Galvez and Ochoa-Morales91 and a moderate correlation with apathy.Reference Sousa, Moreira and Jesus-Ribeiro88 With regards to the “theory of mind” or the ability to attribute mental states (to oneself and others), which was found to be impaired in patients with HD, Reading Mind in the Eyes Test performance was correlated with visuospatial abilities as assessed by the MoCA in one study,Reference Lagravinese, Avanzino and Raffo De Ferrari84 but these findings were not replicated by later research.Reference Bayliss, Galvez and Ochoa-Morales91 Some studies, investigating social cognition in HD, found that the MoCA scores were correlated with tasks of social cognition tests,Reference Unti, Mazzucchi and Frosini89 but others reported that emotion recognition was not predicted by the MoCA.Reference Yitzhak, Gurevich and Inbar96
Neuroimaging studies revealed that MoCA scores present significant correlation with N-acetylaspartate (NAA) and glutamate brain levelsReference Unschuld, Edden and Carass75 and with gray matter density in the cerebellumReference de Azevedo PC, Guimarães and Piccinin83 and subcortical structures.Reference Valdés Hernández, Abu-Hussain and Qiu95
To date, the MoCA has rarely been used as a cognitive assessment tool in clinical trials. In a study investigating the safety, tolerability, and efficacy of a metal protein-attenuating compound that might reduce metal-induced aggregation of mutant HTT, although the scores on the Trial Making test part B were improved after treatment, though the MoCA scores did not show significant improvement.81 However, another study, intended to provide pilot data regarding tools that may be used to objectively assess the effects of tetrabenazine on hand function and balance, showed that the performance on the Jebsen-Taylor Hand Function Test (JTHFT) correlated with cognition, specifically the MoCA, but did not correlate with UHDRS maximal chorea scores.Reference Ferrara, Mostile, Hunter, Adam and Jankovic73 In addition, in a neurofeedback training study, MoCA scores revealed improvement in cognition.Reference Papoutsi, Weiskopf, Langbehn, Reilmann, Rees and Tabrizi87
Although the MoCA seems to be a promising screening test for people with HD, our systematic review found that further studies are necessary regarding this issue. Even if the MoCA demonstrated good sensitivity and specificity when used at the recommended threshold score of 26, studies conducted in patients with different types of neurological disorders revealed that lowering the threshold offers a better balance between true-positive and false-positive results.59–62 Therefore, the MoCA deserves closer scrutiny to assess its properties in the HD population. Further studies are necessary to determine whether the MoCA adequately assesses cognitive status in HD individuals. In addition, further cross-sectional studies are required to examine the optimum cutoff score for detecting cognitive impairments in patients with HD.
The present review confirms the main potential benefit of the MoCA as a test promising to significantly decrease the cognitive assessment time and costs, with robust psychometric properties—good concurrent validity and adequate internal consistency. However, the optimal threshold should probably be further investigated. In addition, different thresholds should be tested in individuals with multiple cultural and educational backgrounds and speaking different languages. Researchers should also consider the value of the MoCA in a diagnostic workup so that clinicians can understand how to use this screening test to attain relevant outcomes for patients, such as the benefits of earlier diagnosis. Nonetheless, patients with abnormal screening results should be further assessed with a full neuropsychological assessment. A stepwise protocol including cognitive screening would be easy to implement in routine clinical practice and would show physicians how to address this complex problem.
After publication of the MDS recommendations regarding the cognitive rating scales to be used in HD individuals, the number of research papers reporting the use of MoCA in HD increased. Before 2017, MoCA was used in one prevalence study,Reference Cornejo-Olivas, Inca-Martinez and Espinoza-Huertas79 one neuroimaging study,Reference Unschuld, Edden and Carass75 two cognitive studies,Reference Patel, Jankovic, Hood, Jeter and Sereno74, Reference Van Liew, Santoro, Goldstein, Gluhm, Gilbert and Corey-Bloom82 two clinical trials,Reference Ferrara, Mostile, Hunter, Adam and Jankovic73, 81 and one study regarding clinical aspects of HD.Reference Jacobs, Boyd, Hogarth and Horak80 In addition, all the diagnostic test accuracy studiesReference Mickes, Jacobson and Peavy71, Reference Videnovic, Bernard, Fan, Jaglin, Leurgans and Shannon72, 76–78 were published before 2017. In the last 4 years, 14 studies reported the use of MoCA in HD patients: one prevalence study,Reference Zitser, Thaler and Inbar86 five neuroimaging studies,Reference de Azevedo PC, Guimarães and Piccinin83, Reference Saba, Yared, Doring, Phys, Borges and Ferraz85, Reference Papoutsi, Weiskopf, Langbehn, Reilmann, Rees and Tabrizi87, Reference Vaca-Palomares, Brien and Coe94, Reference Valdés Hernández, Abu-Hussain and Qiu95 six cognitive studies,Reference Lagravinese, Avanzino and Raffo De Ferrari84, Reference Sousa, Moreira and Jesus-Ribeiro88, Reference Unti, Mazzucchi and Frosini89, Reference Bayliss, Galvez and Ochoa-Morales91, Reference Purcell, Goldman, Ouyang, Bernard and O’Keefe93, Reference Yitzhak, Gurevich and Inbar96 and two studies investigating clinical aspects of HD.Reference Atkinson-Clement, Letanneux and Baille90, Reference Manor, Oestreicher-Kedem and Gad92 Nonetheless, the last diagnostic test accuracy study was done in 2013.Reference Gluhm, Goldstein, Brown, Van Liew, Gilbert and Corey-Bloom77
Our systematic review identified several research gaps regarding the use of the MoCA in HD individuals. The most important gap is the need to conduct high-quality, cross-sectional studies in order to obtain data regarding the optimal threshold for detecting cognitive impairment in this population. Moreover, there is a need to investigate the use of the MoCA in different HD stages, including the prodromal stage. Another consideration for future research is the fact that more longitudinal studies are needed to investigate whether MoCA can be a reliable instrument for assessing changes in cognitive function over time. Besides, future clinical trials should use both, the MoCA and an extensive neuropsychological battery, to document whether the MoCA correctly identifies cognitive changes after treatment.
Our study has certain limitations as we did not perform any meta-analyses because the extensive literature search revealed only a low number of studies, with relatively small samples of patients. Also, the included studies had significant heterogeneity among them with regard to study design, patient samples, demographic differences, language and educational background, and reference standard. In any case, the presentation of the studies investigating the use of the MoCA in HD patients provided both an overall picture of the current state of the evidence in the field and identified knowledge gaps in the matter. The results of the present synthesis allowed us to illustrate several research gaps, including the absence of studies, and the lack of knowledge around optimal cutoff, delineating areas of future research initiatives. In addition, the results of quality appraisal were summarized to offer a general impression of the validity of the available evidence.
In conclusion, despite the limitations mentioned before, our study represents the first systematic review of the literature published in this field and describes an accurate state of knowledge on the use of the MoCA in people with HD.
Funding
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Ethical Standards
This manuscript does not contain clinical studies or patient data apart from those identified through literature search.
Disclosure
Rosca Elena Cecilia and Simu Mihaela declare that they have no conflict of interest.






