Introduction
Schizophrenia manifests significant clinical heterogeneity concerning its onset, course, outcome, as well as response to available treatments. As a result, the very concept of schizophrenia as a single illness or mental disorder has been criticized,Reference Baruch, Hemsley and Gray 1 and subsequently the search for a unifying dysfunction is therefore seen as misguided. Although a number of subtypes have been identified, these remain problematic due to symptom instability.Reference Pfohl and Winokur 2 More promising are dimensional conceptualizations of the full range of schizophrenic symptomatology,Reference Liddle 3 but again there is no clear consensus of what exactly these dimensions might mean in terms of nosological classification.
This significant clinical heterogeneity in schizophrenia is also reflected in the polythetic way the criteria of both DSM and ICD and could be the result of different causative factors, or alternatively could be due to innate constitutional differences among people including the potential to manifest the symptomatology during different developmental stages. Environmental factors could also play a role in this heterogeneity. Another debate was whether schizophrenia is a purely neurodevelopmental disease, or whether a neuroprogressive component exists.Reference Stein and Broome 4 , Reference Davis, Moylan, Harvey, Maes and Berk 5 Additional issues, theories so far failed to explain, are the age and developmental stage at onset, the frequently episodic nature, and the long-term course and variable outcome.Reference Lieberman, Sheitman and Kinon 6
Recently, a staging method has been proposed.Reference Fountoulakis, Dragioti and Theofilidis 7 Its main advantage was that it was based exclusively on the clinical data of a large multi-national sample. This staging method suggested the presence of four main stages of illness progress (seven when substages are taken into consideration). At each of these stages, a specific aspect of symptomatology is dominant (Figure 1). The factors identified in that publication differ from the original Positive and Negative Syndrome Scale (PANSS) factors in their item composition. Therefore, and in order to avoid confusion, the new factors were named Po (instead of P), Ne (instead of N), EH (for excitation/hostility), DA (reflecting aspects of general psychopathology, but especially depression and anxiety), and Ncog (reflecting neurocognitive impairment).
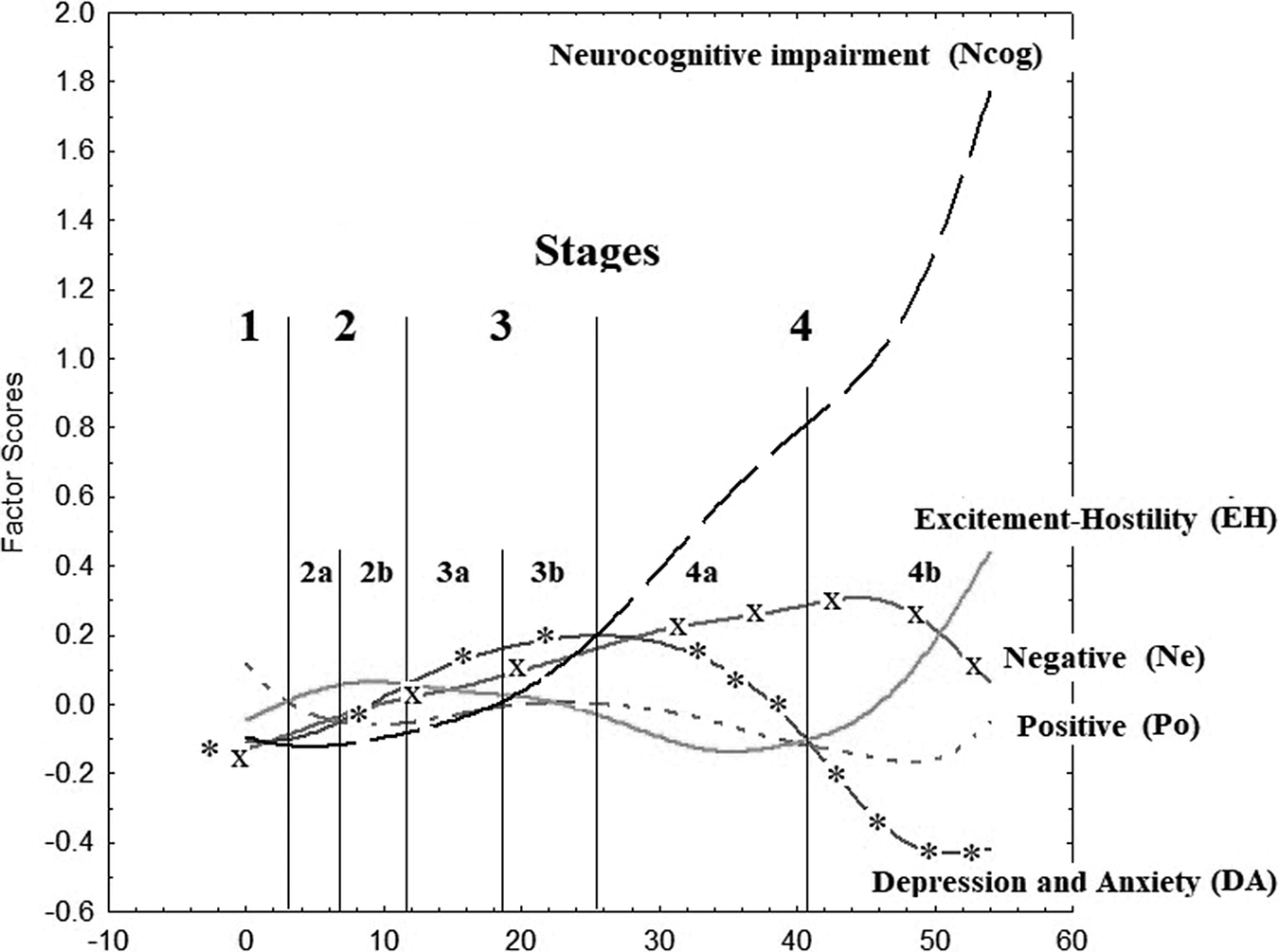
Figure 1. Plot of factor scores (y-axis) vs duration of illness (x-axis) and identification of stages.
The aim of the current study was to explore the underlying interrelationships among the PANSS-defined clinical dimensions. The study further aims to explore the development of these relationships through the stages of the disorder to assemble a disease model.
Materials and Methods
Study sample
The study sample included patients with a DSM-IV or DSM-5 diagnosis of schizophrenia. 8 , 9 There was much effort to exclude organic mental disorders and, more specifically, dementia of any kind, according to the clinical judgment of the investigators. Participants were either inpatients prior to discharge or outpatients and were collected in a number of clinical settings, including academic units, clinics, and hospitals across different countries.
Eligible patients were stabilized patients, and all were treated with medication based on therapists’ judgment. There were no interventions associated with the current study. Patients were excluded if they had a coexisting diagnosis of substance abuse or dependence or concurrent medical or neurological disorders according to their medical records.
All clinical evaluations were performed by trained psychiatrists before clinic or hospital discharge. The study obtained approval by the Research Ethical Committee of the Aristotle University Medical School, Thessaloniki Greece and the other participating centers. Informed consent was obtained from all patients after a detailed description of the study procedures. Twenty-eight centers from 25 countries around the world participated in the study and contributed a total of 2358 patients (Table 1).
Table 1. Composition of the Study Sample in Terms of Country of Origin, Sex, and First Episode of Schizophrenia (FES) Status.

Abbreviations: F, females; FES-E, early group of no more than 18 months of illness duration (N = 484); FES-L, late group with duration of more than 3 years; FES-M, middle group with duration between 18 months and 3 years; M, males; N, number of subjects; Non-FES, patients who are not during their FES.
Measurements
The study collected socio-demographic information on patients with schizophrenia (age and sex) together with assessment using the PANSS. 10-14 The PANSS is a 30-item rating scale developed by Kay et alReference Kay, Fiszbein and Opler 11 to assess dimensions of schizophrenia symptoms and their severity. Items were initially compacted to resolve three scales: Positive (7 items), Negative (7 items), and General Psychopathology (GP) (16 items). In this study, we used the modified version which includes four dimensions: Positive, Negative, GP, and Excited symptoms.Reference Kay and Sevy 14 Trained interviewers administered the PANSS during structured clinical interviews and scored items on a scale from 1 (asymptomatic) to 7 (extremely symptomatic).
The factor scores for the subscales identified in our previous paper (positive [Po], negative [Ne], excitation/hostility [EH], depression and anxiety [DA], and neurocognitive impairment [Ncog]) have been used.Reference Fountoulakis, Dragioti and Theofilidis 7
Data analyses
The statistical analysis included the creation of tables with descriptive statistics and the Pearson correlation coefficient between total PANSS and illness duration in years.
The plots of total PANSS vs illness duration and stages/substages as well as the plot of factor scores (Po, Ne, EH, DA, and Ncog) vs substages were created.
It also included multiple linear regression analysis (stepwise method) at each stage separately with dependent variable all factor scores (Po, Ne, EH, DA, and Ncog) separately and all the rest factor scores as predictors. The Bonferroni correction was used to correct for multiple testing.
As shown in the previous publication, males did not differ from females in terms of the overall model and its qualitative as well as temporal relationships and therefore no separate analysis was undertaken concerning sexes.
Results
Sociodemographic characteristics
The study population consisted of 2358 patients; 929 females (39.40%) and 1429 males (60.60%), aged 37.21 ± 11.87 years old (range 16-81 years) with the DSM-IV or DSM-5 diagnosis of schizophrenia. 8 , 9 Among these, 602 (25.53%) were in their first episode (mean duration 1.20 ± 2.48 years). Thirty-four patients’ first episode had never resolved and had a duration >5 years. Their age at onset was 26.16 ± 8.07 years and their illness duration was 11.05 ± 10.93 years (range 0-54).
Development of total burden of psychopathology with the progression of the stages
While there is a gradual increase of total PANSS score as years being ill increase (Figure 2A) and this is also reflected by a significant correlation between them and illness duration (R = 0.15, P < .05), in sharp contrast when the total PANSS is considered against the stages and substages (Figure 2B) it manifests a dramatic drop during the middle stages and eventually returns to baseline levels. This cannot be considered to be a mathematical artifact of the staging method, but instead reflects the conceptual difficulties when attempting to interpret the data and the differences between the observed raw picture vs the refined one.
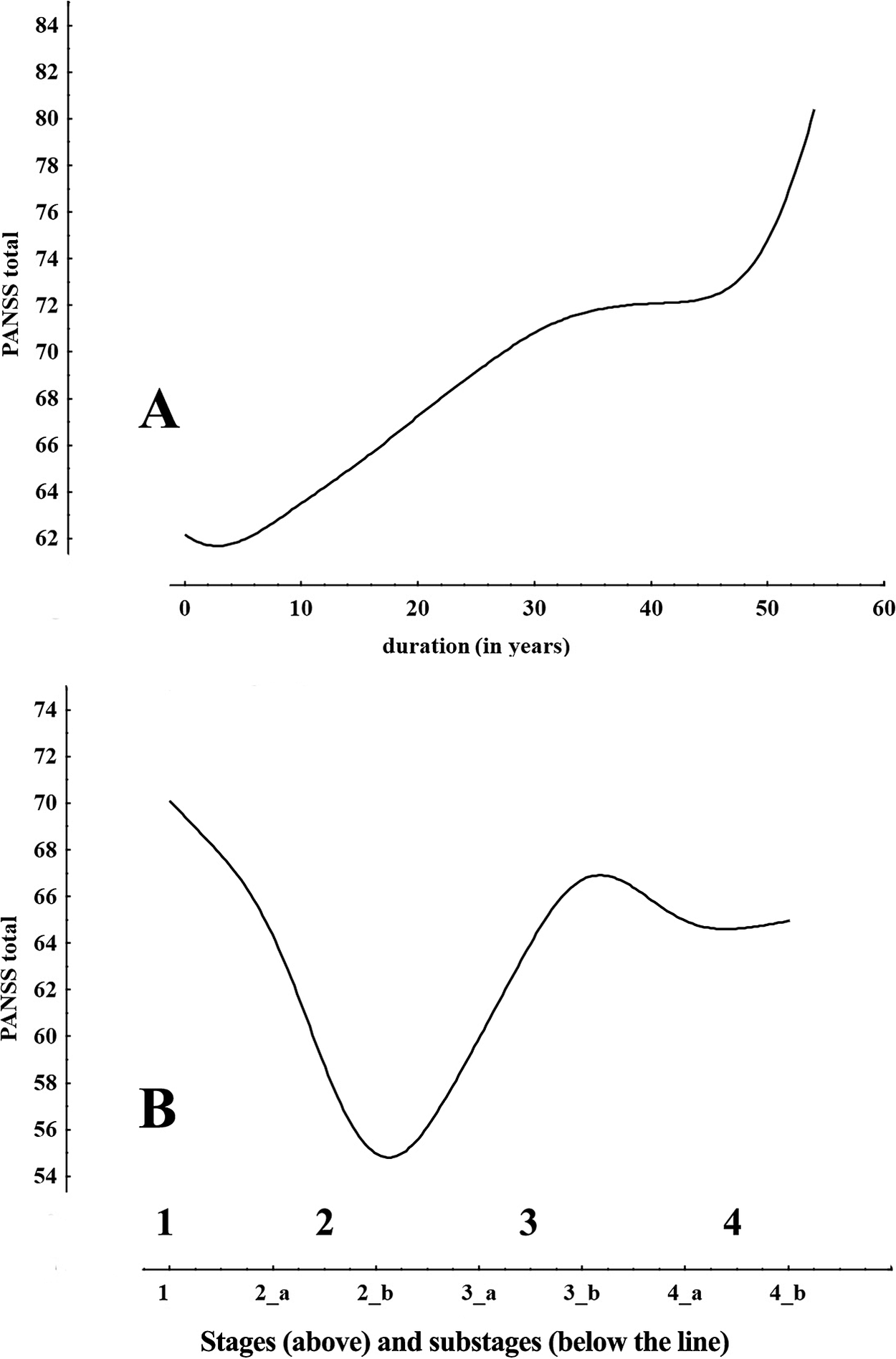
Figure 2. Plot of PANSS total score vs duration of illness and stages and substages. The differences in the two plots make evident how the hidden clinical structure emerges with the staging method.
The multiple linear regression analysis results suggest that there are changing correlations among PANSS factors at each stage (Table 2). The first major observation is that each factor correlates with all the others in that particular stage in which this factor is dominant. There is one exception, Po and stage 1. One the other hand, of all PANSS factors, only Po not only correlates with all the others during three of the four stages, but it also manifests the strongest correlations. All the rest correlate less among each other with EH having the least correlations.
Table 2. Multiple Linear Regression Analysis (Forward Stepwise) by Stage.
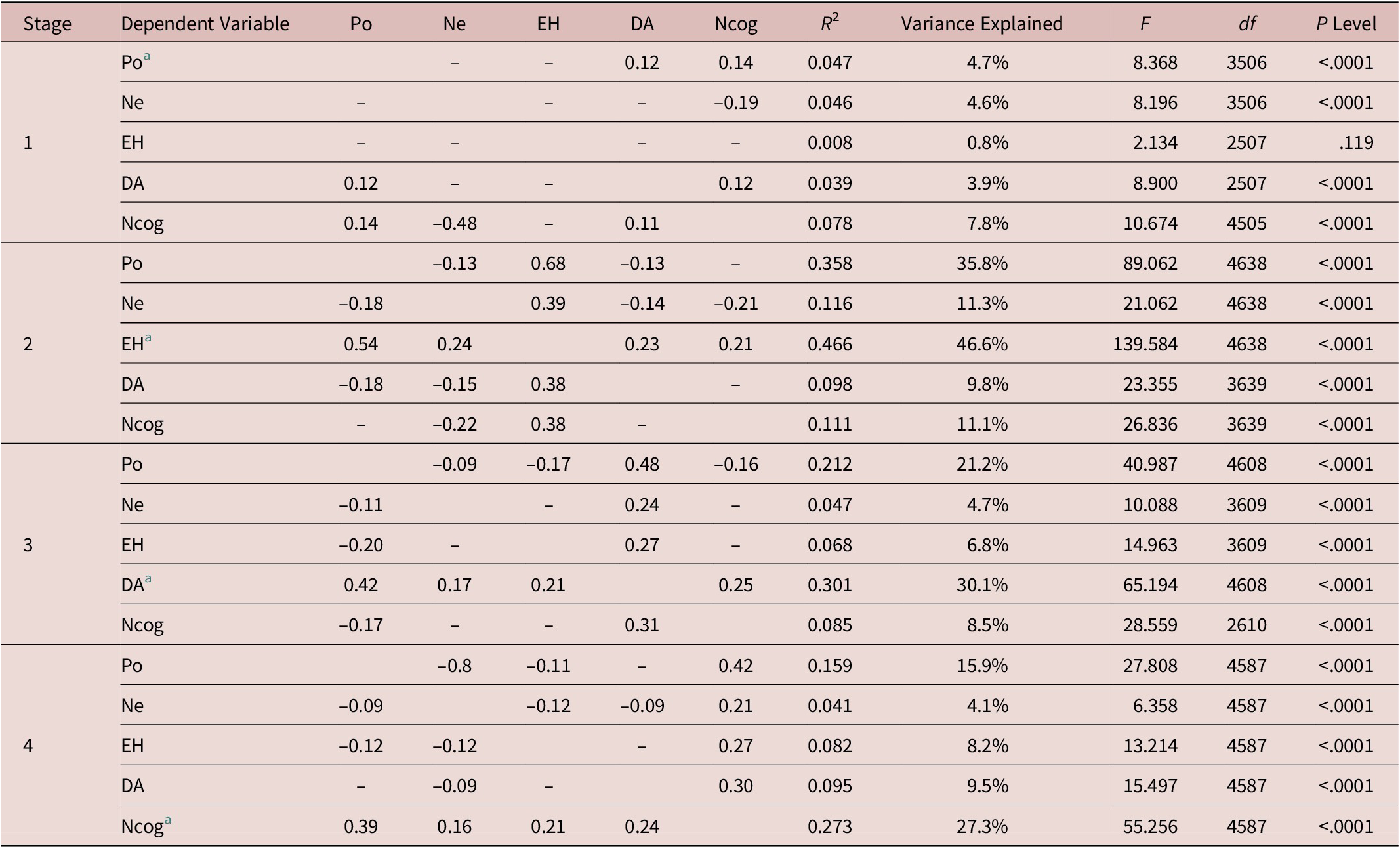
Abbreviations: DA, depression and anxiety; EH, excitement/hostility; Ne, negative; Ncog, neurocognitive decline; Po, positive.
a Dominant factor for this particular stage.
A graphical representation of how the factor scores evolve as the illness progresses through stages is shown in Figure 3. Ne remains unchanged throughout the stages, while Ncog sharply rises in stage 4. The graph will be interpreted in the discussion.
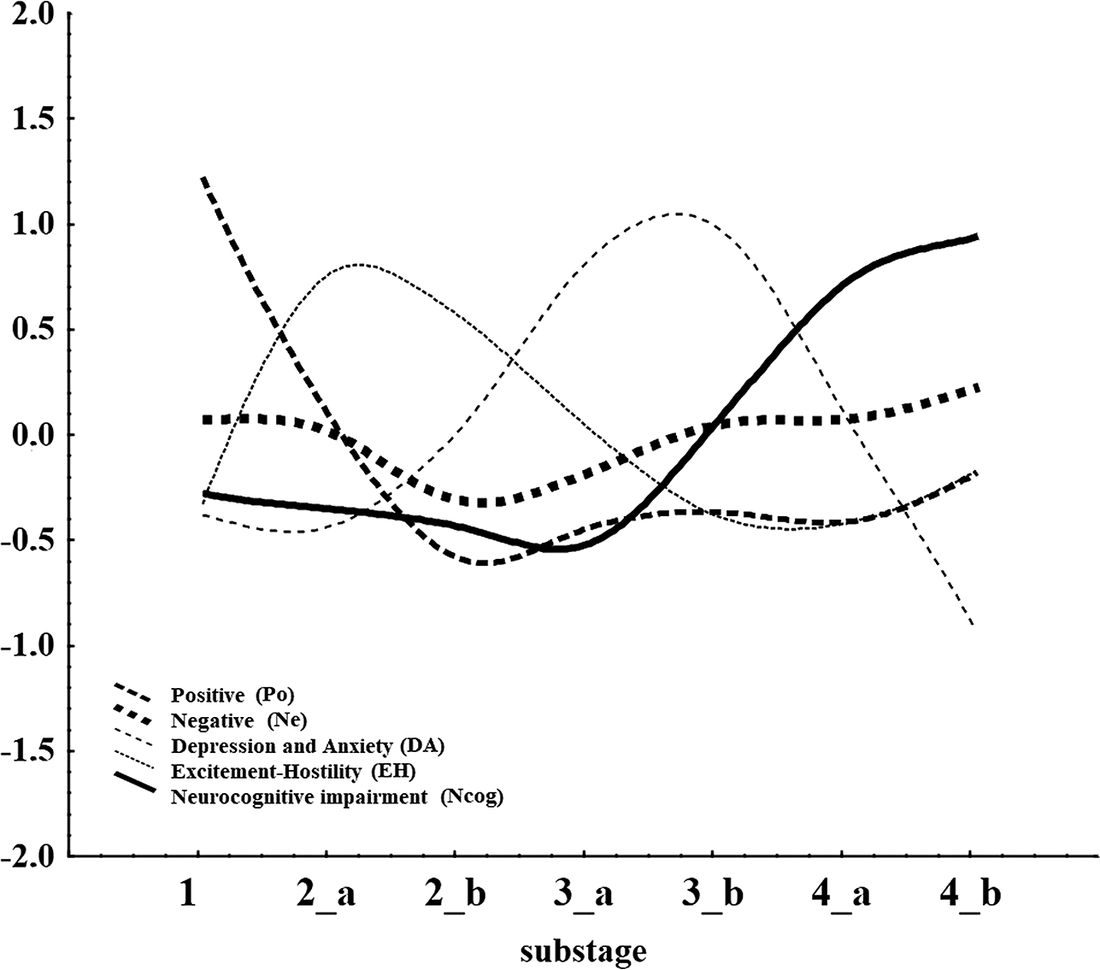
Figure 3. Plot of factor scores (y-axis) vs stages and substages of illness progression.
Discussion
Summary of findings
The results of the current study suggest that with progressing of the stages, there are changing intercorrelations among PANSS factors (Po, Ne, EH, DA, and Ncog) at each stage and each factor correlates with all the others in that particular stage in which this factor is dominant. One the other hand, of all PANSS factors, only Po not only correlates with all the others during three of the four stages, but it also manifests the strongest correlations. Overall, a four-stage model could be proposed with Positive symptoms (Po) dominating the first stage, EH the second, DA the third, and Ncog the last stage (Figure 3).
Interpretation of findings
The overall correlational structure suggests that psychotic symptoms (Po) are relatively independent from the rest of symptomatology especially during stage 1, when they emerge and dominate the clinical picture. On the contrary, all other factors, when dominant, are strongly correlated with the others and much of their variance could be explained by the other factors (eg, 46.6% of EH variance during stage 2). In this frame, stage 1 seems to be “qualitatively distinct” from the other stages in terms of mental functioning and could suggest that the cause of positive symptoms may pre-exist.
One possible explanation could be that stage 1 (and positive symptoms) constitutes the end product of a neurodevelopmental process while during stages 2 to 4, the accumulated burden or neurotoxicity of psychotic symptoms fuels the development of the other aspects of symptomatology and a possible neurodegenerative process. This explains why Po is independent during stage 1 and correlates with all other factors during stages 2 to 4.
The evolution of symptoms, as shown in Figure 3 in relation to stages, seem to provide support for the combined presence of a neurodevelopmental and a neurodegeneration component, since neurocognitive impairment is considered as reflecting the later. Notably, the slope of the Ncog curve slopes sharply upwards between stages 3 and 4, suggesting a neuroprogressive element to this domain, evident in Figures 1 and 3. Another interesting thing is the latency time between those two neurodevelopmental and neurodegeneration components, which covers substages 2b and 3a and thus several years. This period is covered by two distinct features, EH and DA which seem to be highly distinct and contained. The presence of this latency time between the periods dominated by Po and Ncog is against the theory that the toxicity of acute psychoses leads directly to degeneration and points either to the presence of a distinct mechanism for the development of neurocognitive decline or to the presence of a mediating processes involving EH and DA. The role of normal aging, which is present also in patients, cannot be assessed with the utilization of the current dataset.
Additionally, Po and EH seem to follow contrasting trajectories. A possible interpretation could be that the psychotic episodes have a toxic effect on those neural circuits responsible of behavioral inhibition and excitement and hostility constitute some kind of residual symptomatology after the attenuation of psychoses (stage 3). Ne seem to be more or less unchanged through stages and independent from the rest of symptomatology, except the final stage.
Overall, this conceptual model for schizophrenia suggests the presence of two distinct “cores” of schizophrenia, the “Positive” and the “Negative.” While the “Positive” shapes directly or indirectly the clinical picture of all stages, the “Negative” core seems to be more pervasive and independent, and determines the final stage by also contributing to the emergence and exponential increase of the neurocognitive deficit during stage 4. The disinhibition symptoms (EH and DA) could serve as the mediating factor between Po and Ncog since they add further burden to the already overstretched brain resources.
The above could be in accord with the presence of five distinct apparatus/circuits that produce the five factors/clusters of symptoms. The Po and the Ne are the two major circuits that give the pace to the disorder, relatively independently of each other. The succession of EH and DA could happen either because the reduction of Po below a certain threshold leads to the decline of EH also and thus the disinhibition of internalizing emotions (DA) prevail, or, alternatively that the EH circuit has low sensitivity (to stimulation by the Po) but high intensity output and this is why it precedes (and masks) the activation of the DA circuit which might have high sensitivity but low intensity output. In other words, it is easier for the brain to elicit aggression and hostility, but since depression is triggered it is stronger as an effect on behavior. A third explanation could be that agitation/hostility are residuals of psychotic symptoms, while depression is the prodromal of an emerging cognitive decline (Figure 3).
Relevance to existing theories of schizophrenia
In spite of the extended literature and the existence of a bulk of data, our understanding of the mechanisms underlying schizophrenia is poor and limited to the early phases of schizophrenia.Reference Millan, Andrieux and Bartzokis 15
The classic theoretical conceptualizations for schizophreniaReference Andreasen and Olsen 16 , Reference Crow 17 are mainly based on the ideas on the organization of the brain into layers, originally suggested by John Hughlings Jackson (1835-1911) and generally they ignore the presence of the neurocognitive deficit. Even the pyramidal modelReference Kay, Fiszbein and Opler 11 considers it to be a negligible aspect of symptomatology. However, more recently, a number of psychological models have been proposed, having as a common denominator a core neurocognitive dysfunction to which, is believed positive symptoms are secondary. According to these theories, positive symptoms constitute an attempt to organize a chaotic input from the environment and its dysfunctional process, while in this frame, negative symptoms are considered to be the result of the eventual exhaustion of mental resources.Reference Andreasen, Paradiso and O’Leary 18 , Reference Hemsley 19 Furthermore, there were some attempts to theoretically bridge dopamine dysregulation, neurocognitive deficit, and positive symptoms, Reference de Lafuente and Romo 20 , Reference de Lafuente and Romo 21 but the data are unconvincing. More refined models proposed that stress acts as the mediator between impaired neurocognition and psychosis.Reference Hemsley 19 , Reference Beck and Rector 22 , Reference Howes and Murray 23 The developmental trajectory of schizophrenia is thought to be driven by a complex process whereby genetic risk interacts with multiple risk and vulnerability factors that operate at serial yet crucial neurodevelopmental periods that cumulatively lead to the expression of disorder.Reference Davis, Eyre and Jacka 24 All these models consider a very specific neurocognitive deficit to be part of the initial mechanism of disease onset and they make no mentioning on a possible late-stage neurodegenerative process.
While it has been solidly proven that people who develop schizophrenia tend to show subtle neurocognitive, social, and motor impairments already since childhood,Reference Ciompi 25 –Reference Miller 27 there are several major problems with all these models. First, the initial neurocognitive deficit is at the level of statistical significance rather than clinical impairment. That is, most patients still manifest neurocognition within the normal range. Furthermore, low cognitive function by itself does not predispose to psychosis. Thus, if an initial neurocognitive deficit is considered to be at the core of the disorder, this should be very finely delineated and very specific, since the degree of neurocognitive impairment does not correlate with psychotic,Reference Goldberg, Gold and Greenberg 28 but on the contrary, their closest association is reported to be with the negative syndrome.Reference Andreasen and Olsen 16 , Reference McKenna, Lund and Mortimer 29 Additionally, similar deficits are found in healthy relatives and in the wider nonschizophrenic psychotic spectrum.Reference Goldberg, Gold and Greenberg 28 , Reference Goldberg, Ragland, Torrey, Gold, Bigelow and Weinberger 30 , Reference Goldberg, Torrey and Gold 31 Second, the worsening of neurocognition with the progression of the illness does not lead to more severe positive symptoms. Third, those with very well organized and resilient delusions and hallucinations (paranoid patients) manifest better neurocognitive function in comparison with the rest. It could be argued that even paranoid schizophrenic patients manifest a significant cognitive disorder, but this is true only concerning their expected level of functioning since it is subclinical in magnitude, and becomes obvious only with twin studies and special testing.Reference Goldberg, Ragland, Torrey, Gold, Bigelow and Weinberger 30 –Reference Suddath, Christison, Torrey, Casanova and Weinberger 32
The fourth and most important objection is that treatment with antipsychotics can lead to a full remission of psychotic symptoms without much change in neurocognition; if even a subtle and very specific neurocognitive deficit leading to a chaotic information process was the cause and psychosis is a dysfunctional attempt to organize a chaotic internal experience, then the suppression of positive symptoms should lead to a more pronounced disorganization by further revealing the underlying cognitive disorder.Reference Kaprinis, Fountoulakis and Kaprinis 33
These theories also fail to explain the often episodic rather than continuous course of the illness and opinions are conflicting concerning whether the neurocognitive deficit is characterized by a gradual deterioration leading to social dysfunctionReference Davidson, Harvey and Haroutunian 26 , Reference Miller 27 or remains stable as neuroimaging findings remain in many patients.Reference Goldberg, Hyde, Kleinman and Weinberger 34 , Reference Hoff, Riordan, O’Donnell, Morris and DeLisi 35 The latter is consistent with the notion that schizophrenia is a static encephalopathy of neurodevelopmental origin.Reference Weinberger 36 The current paper suggests the presence of a gradual deterioration indicative of a neurodegeneration process in addition to the neurodevelopmental.
As discussed above, some authors suggest that negative symptoms are the consequence of resource exhaustion because of the burden of positive symptoms, or some of them even constitute some kind of psychological adjustment or response to the psychotic experience.Reference Andreasen, Paradiso and O’Leary 18 , Reference Hemsley 19 , Reference Beck and Rector 22 Again this is not in accord with the findings of the current study, since our data suggest that Ne is more or less stable through the stages and does not seem to have any temporal relationship with the changes in Po. Additionally, the current model does not support the idea that hostility and violence are a consequence of neurocognitive impairmentReference Picchioni, Harris, Reichenberg, Fahy and Murphy 37 since the increase in EH precedes the increase in Ncog and the two dimensions are generally inversely correlated. Essentially the current model suggests that schizophrenia could be conceptualized as the phasic progression of multiple psychopathologies on a stable background of negative symptoms. It also provides support to the concept of schizophrenia as a single disorder with a single underlying mechanism, producing different psychopathological manifestations.
The current model is in general accord and provides support to the model proposed by Lieberman et al.Reference Lieberman, Sheitman and Kinon 6 , Reference Lieberman, Perkins and Belger 38 This is a more pragmatic model, and is based on clinical as well as neurobiological data, to a larger extend in comparison to the previously discussed models. It suggests that the neurodevelopment phase precedes the onset of psychotic symptoms and this corresponds to the phase of “neuroplasticity.” According to that model, there is an excitatory/inhibitory dysfunction mainly involving the system of excitatory aminoacids, resulting in excitoxicity. In turn, excitoxicity leads to the manifestation of a neurodegenerative phase. Our model adds a latency phase between “neuroplasticity” and “degeneration” and suggests that the excitatory/inhibition imbalance triggers different brain circuits and systems in the row, giving rise to different clusters of symptomatology and subsequent stages of the illness. Alternatively, instead of the presence of a latency period, one could argue that both stages 2 and 3 belong to the “neuroplasticity” period. EH and DA can be explained by disinhibition mechanisms which are also important for memory acquisition and expression.Reference Mohler and Rudolph 39 The neurochemical pathways underlying the process of neuroprogression are complex and are thought to be a cumulative effect of inflammation, oxidative stress, and mitochondrial dysfunction as well as a dysregulated stress response systems.
Strengths and limitations of the current study
The strengths of the current study include the large study sample, which is the largest so far in the literature investigating the underlying mental functioning in patients with schizophrenia with the use of the PANSS and in combination with a staging method. An additional strength is the multi-center and multinational characteristic of the sample.
The most important limitation of the study is that it utilized a cross-sectional design with the utilization of limited demographic and clinical info or treatment resistance status of the patients and these were combined with lack of long-term follow up of patients and in the absence of an external “golden standard.” This absence was intentional and intrinsic to a design that aimed to study the patients on the basis of their current clinical picture alone. This was chosen as an approach because anamnestic data are not reliable in contrast to the assessment of the present state. For a similar reason, only stabilized patients were included.
Also, it is true that the use of simple neuropsychological methods may be misleadingReference Goldberg, Weinberger, Berman, Pliskin and Podd 40 and therefore it is not peculiar that in the pyramidal model of Kay the neurocognitive factor is not included, because it explained only 5.21% of the total variance of symptomatology. In this model, neurocognitive disorder is considered to be equivalent with disorganization and is placed in the middle between the positive and the negative symptoms. In this way, it is in fact suggested that neurocognitive symptoms are the result of the co-existence of positive and negative symptoms (more or less identical with disorganization).
Another limitation was that neurocognition was assessed on the basis of the therapist’s clinical impression and PANSS scoring rather than the use of detailed neuropsychological testing. Also, in elderly patients, the presence of a comorbid underlying vascular or Alzheimer’s pathology cannot be ruled out.
It is important to note that our results come from “stabilized” patients, that is, patients already treated with antipsychotics and in partial remission. The effect of current as well as past treatment of antipsychotics is unknown and the possibility of the decline in positive symptoms with time is due to their effect cannot be ruled out. Whether this constitutes a true illness progression or reflects the results of treatment with antipsychotics which have a primary beneficial effect on positive symptoms is unclear. However, a possible such effect of antipsychotics is not in accord with the emergence of EH in parallel with the decline of positive symptoms. Our model, however, is in partial accordance with the suggestion that after 3 years, there is attenuation in the relapse rateReference Wunderink, Nieboer, Wiersma, Sytema and Nienhuis 41 or possibly a change in their pattern with more frequent and shorter relapses during the early stages making way for less frequent but more chronic episodes.Reference Andreasen, Liu, Ziebell, Vora and Ho 42 It is also known that antipsychotics are efficacious as well against manic-like symptoms, EH, and therefore it could be suggested that more patients with schizophrenia might manifest a more severe form of this kind of symptomatology especially during the acute psychotic episode. Probably as a result of the psychometric tools used (which in most studies are restricted to “classic” schizophrenia scales such as the PANSS and SAPS/SANS but not YMRS), manic-like symptoms have been identified only in a minority of reports that have studied the factor structure of clinical symptoms of schizophrenia in samples similar to ours,Reference Lorr, Mc, Klett and Lasky 43 –Reference Fountoulakis, Popovic, Mosheva, Siamouli, Moutou and Gonda 47 or in recent-onset cases,Reference van Os, Fahy and Jones 48 , Reference McGorry, Bell, Dudgeon and Jackson 49 but rarely in follow-up studies.Reference Willem Van der Does, Dingemans, Linszen, Nugter and Scholte 50 , Reference Salokangas 51 In a recent 14-years follow-up study from rural China, partial and complete remission in treated patients (57.3%) was significantly higher than that in the never-treated group (29.8%), but such results neither support not dispute our model (mainly concerning the decline of positive symptoms with time).Reference Ran, Weng and Chan 52
A further limitation is that the study sample was not epidemiologically selected and therefore may not represent the general population of patients with schizophrenia. Instead, it represents those patients with at least less than ideal remission who remained in contact with mental health services for several years. It is unclear whether the differences observed among countries were because of this selection method, however, such a nonsystematic heterogeneity among countries is expected and does not seem to determine the overall outcome and results of the study. In the previous publication, the issue of heterogeneity of the data among centers has been investigated. The results suggested that such a heterogeneity did existed, however, it was not systematic and it did not seem to push the results toward a specific direction. The conclusion was that between centers, heterogeneity though present, had no impact on the results of that or the current study.Reference Fountoulakis, Dragioti and Theofilidis 7
Conclusion
The current study investigated the mental organization and functioning in patients with schizophrenia in relation to different stages of illness progression. The proposed organization suggests the presence of two distinct “cores” of schizophrenia, the “Positive” and the “Negative.” While the “Positive” shapes directly or indirectly the clinical picture of three out of the four stages, the “Negative” core seems to be more pervasive and independent and determines the final stage by also determining the emergence of the Ncog. Finally, the Ncog curve escalates during the later stages. Different sensitivity and intensity of output of the various neuronal circuits involved could explain the sequential succession of clusters of symptoms and the emergence of stages. Future research should focus on the therapeutic implications of such a model, since the stabilization of the illness could demand to stop the succession of stages not only by both halting the triggering effect of positive and negative symptoms, but also by stopping the sensitization effect on hostility, excitement, anxiety, and depression neural pathways as well as the deleterious effect on neural networks responsible for neurocognition.
Disclosures
M.B. is supported by a NHMRC Senior Principal Research Fellowship (APP1059660 and APP1156072). Several of the coauthors have received grants and support from the Pharmaceutical industry but this had no influence on the results and interpretation and the writing of the current study. None of the coauthors have any disclosures that pertain to the current study.







