Take-Home Points
1. Ketamine administered orally or intravenously as high-dose boluses can induce both anesthesia and psychotomimetic actions, especially when the drug is wearing off, just like phencyclidine (PCP), a related agent.
2. However, given at subanesthetic doses and as a continuous 90-minute infusion, ketamine has few psychotomimetic actions, and instead rapidly relieves depression, suicidal thoughts, and pain in patients who have failed many other treatments.
3. Ketamine's most potent binding affinity is at N-methyl-d-aspartate (NMDA) glutamate receptors. This direct action has downstream consequences, releasing glutamate to stimulate other receptors, notably α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptors, which in turn cause proliferation of dendritic spines.
4. Less potent actions of ketamine are at the sigma 1 receptor, which could also contribute to its antidepressant actions, especially in combination with NMDA antagonist actions.
Ketamine was first characterized as a “dissociative” anesthetic related to phencyclidine (PCP), and later became a “club drug” of abuse, sometimes called Special K, for a hallucinatory and psychotomimetic experience that is a bit milder than that of PCP.Reference Stahl1 Because of theoretical considerations of potentially overactive glutamate systems in depression, this agent was administered intravenously over 1 or 2 hours in subanesthetic and subpsychotomimetic doses, and remarkably, has been shown to reduce depressive symptoms and suicidal symptoms in patients who had failed multiple previous treatments, whether the patients had unipolar or bipolar depression.Reference Cateno-Dell'Ossa, Gagiolini, Rotella, Baroni and Marazziti2–Reference Ibrahim, Diazgranados and Franco-Chaves4 This effect has been replicated by several independent groups. The issue now is to try to extend this incredible clinical result beyond the week or two of relief from a single infusion; however, repeated infusions are not practical and may not even be effective, and “chasing” the initial infusion with other glutamate agents such as memantine, lamotrigine, and riluzole have all been disappointing.Reference Cateno-Dell'Ossa, Gagiolini, Rotella, Baroni and Marazziti2–Reference Ibrahim, Diazgranados and Franco-Chaves4 Thus, an oral agent that targets the same receptors as ketamine is a key way forward. Here we review the potential pharmacologic mechanisms of the action of ketamine.
NMDA receptors
Ketamine has potent actions on NMDA receptors, and less potent actions on sigma 1 receptors (Figure 1).Reference Stahl1 It also binds with even lower affinity to μ opiate receptors as well as to the transporters for norepinephrine and serotonin (Figure 1).Reference Stahl1 Before discussing “which receptors” ketamine blocks, we should mention “how receptors” are blocked. That is, certain receptors seem to react quite differently to pulsatile versus continuous blockade, and that applies to dopamine transporters as well as to NMDA receptors.Reference Stahl1 Thus, rapid but short-acting and high degrees of receptor occupancy of these targets results in increased phasic neurotransmission, subjective “highs,” and abuse potential, whereas slow-onset, long-acting, slow-offset, and moderate degrees of receptor occupancy of these targets result in increased “tonic” neurotransmission. Studies showing antidepressant effects of ketamine all employ subanesthetic doses with slow onset, over 1 or 2 hours of intravenous infusion, slow-offset, and moderate degrees of receptor occupancy, and not intravenous boluses or single large oral doses.Reference Cateno-Dell'Ossa, Gagiolini, Rotella, Baroni and Marazziti2–Reference Ibrahim, Diazgranados and Franco-Chaves4 This manner of blocking NMDA receptors largely avoids both anesthesia and dissociative, psychotomimetic reactions.Reference Cateno-Dell'Ossa, Gagiolini, Rotella, Baroni and Marazziti2–Reference Ibrahim, Diazgranados and Franco-Chaves4 Unfortunately, the antidepressant effects associated with this mode of administration of ketamine are not sustained for more than a few days, leading to a search for an oral ketamine-like agent that could have rapid onset in treatment resistant patients, as well as sustained efficacy.
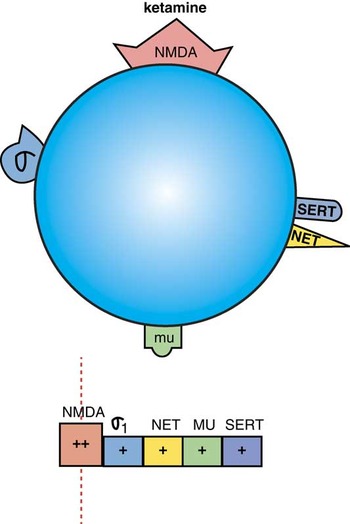
Figure 1. Ketamine is an NMDA (N-methyl- Daspartate) receptor antagonist, with additional actions at σ1 receptors, and weaker actions at the norepinephrine transporter (NET), μ-opioid receptors, and the serotonin transporter (SERT). The horizontal binding strip below the icon shows potency left to right, with highest potency binding to the left with the vertical line indicating the target engaged at effective doses, and with progressively less potent binding shown further and further to the right. Depending upon dose, some of these additional receptors may be important in the actions of ketamine as well as the NMDA receptor which has the highest affinity.
Direct effects on NMDA receptors
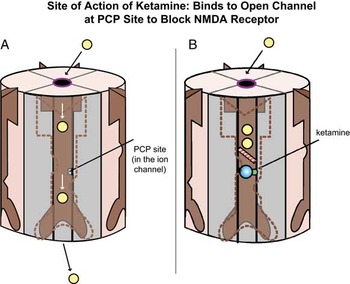
There are several types of glutamate receptors, and in order to understand the potential mechanism of action of ketamine, it is important to understand at least two of them: NMDA receptors and AMPA receptors. Ketamine blocks NMDA receptors by binding to the so-called “PCP site” inside the calcium channel of this receptor (Figures 2A and 2B).Reference Stahl1 NMDA receptors are postsynaptic and activate long-term potentiation and synaptic plasticity.Reference Stahl1

Figure 2. Site of action of ketamine. Ketamine binds to the open channel conformation of the N-methyl-D-aspartate (NMDA) receptor (A). Specifically, it binds to a site within the calcium channel of this receptor, which is often termed the PCP site because it is also where phencyclidine (PCP) binds (B).
Downstream effects on AMPA receptors
Due to blockade of NMDA receptors on inhibitory gamma amino butyric acid (GABA) neurons in the prefrontal cortex, ketamine also results in glutamate release downstream (Figure 3).Reference Stahl1 This glutamate stimulates postsynaptic AMPA receptors (Figure 4) that mediate fast, excitatory neurotransmission by allowing sodium to enter the neuron to depolarize it. A debate is ongoing as to whether it is the direct actions of ketamine at the PCP site on the NMDA receptor that account for its actions, or the downstream stimulation of AMPA receptors. One hypothesis for why ketamine has antidepressant actions proposes that it is actually the stimulation of AMPA receptors and not the blockade of NMDA receptors per se that causes the antidepressant action.Reference Li, Lee and Liu5
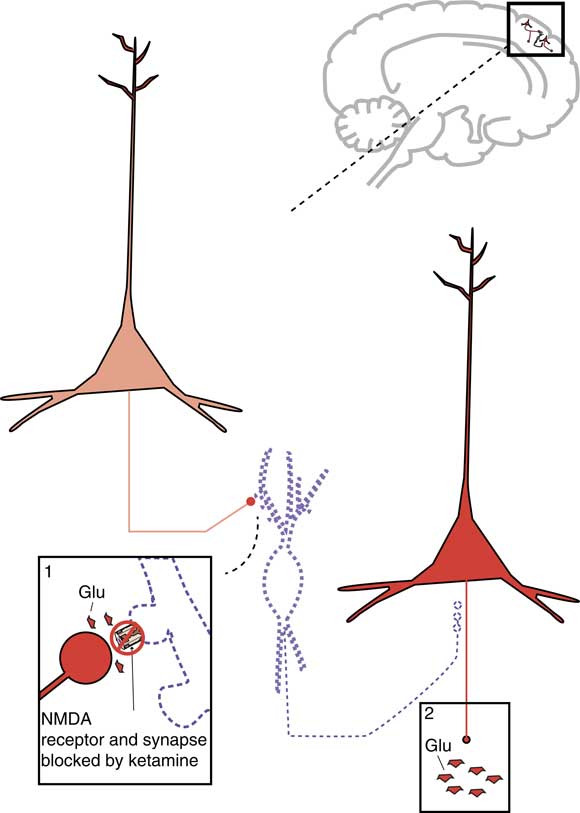
Figure 3. Downstream mechanism of action of ketamine. Shown here are two cortical glutamatergic pyramidal neurons and a GABAergic interneuron between them. If an N-methyl-D-aspartate (NMDA) receptor on a GABAergic interneuron is blocked by ketamine, this prevents the excitatory actions of glutamate (Glu) there. Thus, the GABA neuron is inactivated and does not release GABA (indicated by the dotted outline of the neuron). GABA binding at the second cortical glutamatergic pyramidal neuron normally inhibits glutamate release: thus, the absence of GABA there means that the neuron is disinhibited and glutamate release is increased.
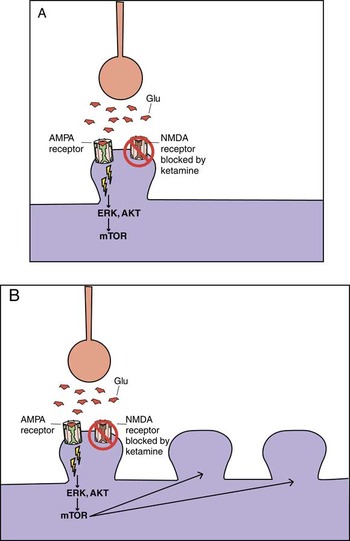
Figure 4. Ketamine, AMPA receptors, and mTOR. Glutamate activity heavily modulates synaptic potentiation; this is specifically modulated through NMDA (N-methyl-D-aspartate) and AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid) receptors. Ketamine is an NMDA receptor antagonist; however, its rapid antidepressant effects may also be related to indirect effects on AMPA receptor signaling and the mammalian target of rapamycin (mTOR) pathway. (A) It may be that blockade of the NMDA receptor leads to rapid activation of AMPA and mTOR signaling pathways. (B) This in turn would lead to rapid AMPA-mediated synaptic potentiation, and rapid induction of dendritic spines. Traditional antidepressants also cause synaptic potentiation; however, they do so via downstream changes in intracellular signaling. This may therefore explain in part the difference in onset of antidepressant action between ketamine and traditional antidepressants.
That is, when AMPA receptors are activated by glutamate release, this first activates the ERK, AKT signal transduction cascade (Figure 4), which next triggers the mammalian target of rapamycin (mTOR) pathway, resulting in the expression of synaptic proteins leading to an increased density of dendritic spines (Figure 4).Reference Stahl1, Reference Li, Lee and Liu5 Increase in synaptic spines can be seen in experimental animals very soon after ketamine administration.Reference Li, Lee and Liu5 One hypothesis is that it is actually this increase in dendritic spines that causes the rapid onset antidepressant effect, and not just the blockade of NMDA receptors or the stimulation of AMPA receptors.Reference Li, Lee and Liu5
Sigma 1 receptors
Evidence currently suggests that sigma 1 receptors both mediate important functional and behavioral actions in the central nervous system, and also serve as novel targets for treatment of depression, cognitive disorders, cocaine abuse, and stroke.Reference Stahl6 Unlike neurotransmitter receptors, which are located within pre- and postsynaptic neuronal membranes, sigma receptors are located at the endoplasmic reticulum where they play a key role in regulating intracellular calcium signaling. Naturally occurring neurosteroids as well as many psychotropic drugs bind to sigma receptors; sigma receptors may thus mediate some actions of fluvoxamine, amantadine, memantine, dextromethorphan, cocaine, ketamine, and PCP. Several selective sigma 1 agents hold promise as novel antidepressants, as drugs for cognition, and possibly for drug abuse, for neuroprotection and for other conditions. Dextromethorphan, a weak NMDA antagonist with sigma 1 properties,Reference Stahl1 is reported to be effective specifically in the treatment of the labile affect associated with pseudobulbar states, sometimes also called involuntary emotional expression disorder,Reference Stahl1 and as such meets some of the binding requirements of an orally active ketamine-like agent. The mechanism of action of dextromethorphan combined with quinidine is discussed in the next issue of Brainstorms, where further details on the functions of sigma 1 receptors will be given.Reference Stahl7
Conclusion
Ketamine is perhaps the most rapid in onset of known antidepressants, and acts in patients who have failed multiple other therapies generally targeting monoamines. To exploit this information to develop a rapid acting oral antidepressant with sustained actions in treatment resistant and suicidal unipolar and bipolar patients, it will require sorting out whether the target should be blockade of NMDA receptors or one of their subtypes, stimulation of AMPA receptors, or additional actions as agonists of sigma 1 receptors.