Introduction
The last 10 years has seen a drive to diagnose diseases causing dementia at the earliest clinical and even preclinical stages. However, in those presenting to memory clinics with mild complaints or mild impairment, biomarker specificity is low and aetiologies heterogeneous.Reference Sierra-Rio , Balasa and Olives1–Reference Mitchell and Shiri-Feshki 5 It is likely that a significant proportion who do not ultimately receive a diagnosis of dementia have functional cognitive disorders: that is, conditions where cognitive symptoms are present and associated with distress and disability, but which are caused by functional disturbances of attention, abnormal metacognitive beliefs, alongside other functional neurological symptoms, or as a result of psychiatric illness.Reference Stone, Pal, Blackburn, Reuber, Thekkumpurath and Carson6, Reference Blackburn, Wakefield, Shanks, Harkness, Reuber and Venneri7
Subjective report of memory impairment generally correlates poorly with performance on cognitive tests, and performance on cognitive screening tests is unpredictable in those with functional disorders: some patients achieve normal scores, but some score very poorly, especially in tests of memory, attention and executive function.Reference Teodoro, Edwards and Isaacs8 Although cognitive screening tests are now heavily used in the diagnosis of dementia and in defining mild cognitive impairment (MCI), the specificity remains unacceptably low. A 2009 review of 41 robust longitudinal cohort studies found that fewer than half of those receiving a description of MCI (described on the basis of memory symptoms and mild impairment on screening tests) progress to dementia even after 10 years of follow up.Reference Mitchell and Shiri-Feshki 5 Over-reliance on cognitive screening tests brings a risk of misdiagnoses and associated iatrogenic harm, and we expect misdiagnoses to become a more pressing problem as preclinical Alzheimer’s disease profiles are increasingly identified in younger people.
It has been traditionally taught that the symptoms of functional neurological disorders are, in part, dependent on the patient’s ideas about the symptoms rather than anatomical and pathophysiological rules.Reference Stone, Mutch, Giannokous, Hoeritzauer and Carson9 Similarities have been observed between simulated and functional paralysis, for example, suggesting to us not that patients with functional disorder are feigning but rather that symptoms in both cases may depend on “top-down” predictions that the brain makes about motor and sensory experience, which in the case of a functional disorder are involuntary.
Experimental simulation—asking a healthy subject to mimic the symptoms of a disease—can give a more detailed insight into that subject’s ideas and beliefs about those symptoms than is possible through interview or questionnaire. Ideas and beliefs about symptoms of dementia may be similar in healthy individuals to in those with functional cognitive disorders; or they may be different. Experimental simulation may be a useful route in which to access these beliefs in order to further investigate how different belief profiles might relate to the experience of cognitive symptoms, and may also suggest avenues for investigation in developing more accurate diagnostic profiles for functional cognitive disorders.
This study therefore aimed to compare performance in easily available cognitive screening tests in young adults simulating mild dementia with normative data, in order to better understand what young adults believe the symptoms of dementia to look like. We hypothesized that simulating individuals would perform as if impaired, but less severely than those with dementia, and that they would present with different patterns of impairment.
Methods
Participants were recruited via peer networks and social media. Inclusion criteria were age over 16 and able to speak and read English. Individuals were excluded if they had a pre-existing neurological disorder or had received any education or training in medicine, healthcare, or clinical neuroscience at college or university level. Educational level and family history of neurological disease was recorded.
Participants were asked to complete a panel of cognitive tests “as if you have mild dementia due to Alzheimer’s disease”; a script was used to standardize examiner suggestion (Appendix A1). The assessment included a brief interview and the Montreal Cognitive Assessment (MoCA), with response times for each item measured using a stopwatch. The Luria 3-step test, interlocking fingers test, and examination of gait and tandem (heel-to-toe) gait were included due to increasing recognition of the diagnostic utility of motor symptoms in dementia.Reference Nasreddine, Phillips and Bedirian10–Reference Ahmed, Baker and Thompson14 The coin-in-hand tests and short digit span trials were included in an attempt to quantify effort or intention to fail.Reference Kapur15 The following procedure was used for the coin-in-hand test: the examiner showed the participant a two-pence coin in the palm of one hand, closed both hands into fists and asked the participant to close their eyes and count aloud backwards from 10; the participant was then asked to open their eyes and indicate which hand the coin was in; 10 trials were completed, the coin appearing in each hand an equal number of times. Any unusual behaviours during testing were noted.
Data were analysed using R (version 3.5.2), and with group comparisons performed using independent 2-group t-test for continuous and Pearson’s chi-square for dichotomous data; distribution was assessed for normality (Shapiro Wilks). The study received University of Edinburgh ethical approval.
Results
Fifty subjects were recruited: 25 female and 25 male, mean age 22 (range 18–27). Seventy-eight percent were current university students (66% undergraduate and 12% postgraduate), 18% university graduates, and 4% neither students nor graduates.
In response to the question, “Please could you tell me what you think someone with mild or early stage dementia might experience? What symptoms might they have?” ( Table 1): 49/50 participants listed memory problems, of whom 25 specified preferential impairment of “short-term” memory and 12 impairment of both “short-term” and “long-term” memory. Failure to recognize familiar people or faces was the most commonly reported specific memory symptom (20), followed by losing things (7), repetitive conversation (5), forgetting items like keys and shopping lists (5), forgetting tasks while undertaking them (4) (for example, going into a room and forgetting what you went in for), and lack of awareness of current affairs (2). Two described relative preservation of memories with emotional content. Out of the 50 participants 17 listed “confusion,” 14 listed disorientation to place, getting lost, problems with spatial awareness or navigation and 3 disorientation in time. Ten participants listed distress or agitation including “fear,” “irritation and frustration,” “frustration,” “feeling insecure,” and “anxiety.” Eight listed motor impairment including “loss of dexterity,” “slow movement and bad imbalance,” “lacking co-ordination,” “balance problems, falling,” “slower motor skills,” and “slightly restricted mobility”; in contrast, 3 specifically stated they would expect no motor impairment or physical symptoms. Six described changes in behavior: “angry,” “strange behavior,” “short and irritable,” “expressionless,” “slight personality change,” “saying things that are out of character or socially unacceptable,” “reduced social interaction”; 6 listed speech changes, including “difficulty speaking/difficulty forming sentences,” “disorganized speech,” “mixing words up,” “slurred words,” and “slow speech.” Five listed affective symptoms including “sadness,” “negative mood,” “not a full range of emotions,” and another “absence of drive and motivation.” Five listed problems performing simple tasks, one of whom stated that a person with dementia might sustain a greater number of accidental injuries such as burns or cuts from cooking. Three included higher-order problems: “loss of critical reasoning,” “problems with decision making,” “difficulty problem solving.” Two listed “short attention span.” Symptoms mentioned once only included confabulation (“constructing false memories”); “paranoia” and auditory and visual hallucinations (“speaking to self/seeing things”); slow processing speed; lack of insight (“denial of symptoms”); neglect of self, household and pets; “reminiscing”; “removal from reality”; “tiredness”; and “headaches.”
Table 1. Responses of 50 healthy adults questioned about expected symptoms in mild dementia

Mean MOCA score was 16 (±5.5, range 5–26, maximum potential score 30) ( Table 2). The items with most errors were delayed recall of 5 items (100%, with 72% recalling two or fewer items), letter vigilance (86%), digit span (5 digits) (82%), clock-drawing (82%), and sentence repetition (80%). The items with fewest errors were cube drawing (42%) and serial sevens (54%). Sixteen (32%) made at least one perseveration in verbal fluency. We also noted some perseverative responses during delayed recall: “feet” (when previously produced in letter fluency) and “clock”; and some semantic errors such as “rose” for daisy. Median total summed response time for the whole MocA was 7 minutes 57 seconds with an unusually wide range (5 minutes 13 seconds–14 minutes 12 seconds, IQR 2 minutes 4 seconds.)
Table 2. MoCA and additional digit span results.

* Additional digit span trials not part of MoCA.
Data from digit span testing was particularly interesting in relation to what might be expected in mild dementia. Fifty-eight percent of subjects failed a forward digit span of 4 digits, and 26% a forward digit span of three digits.
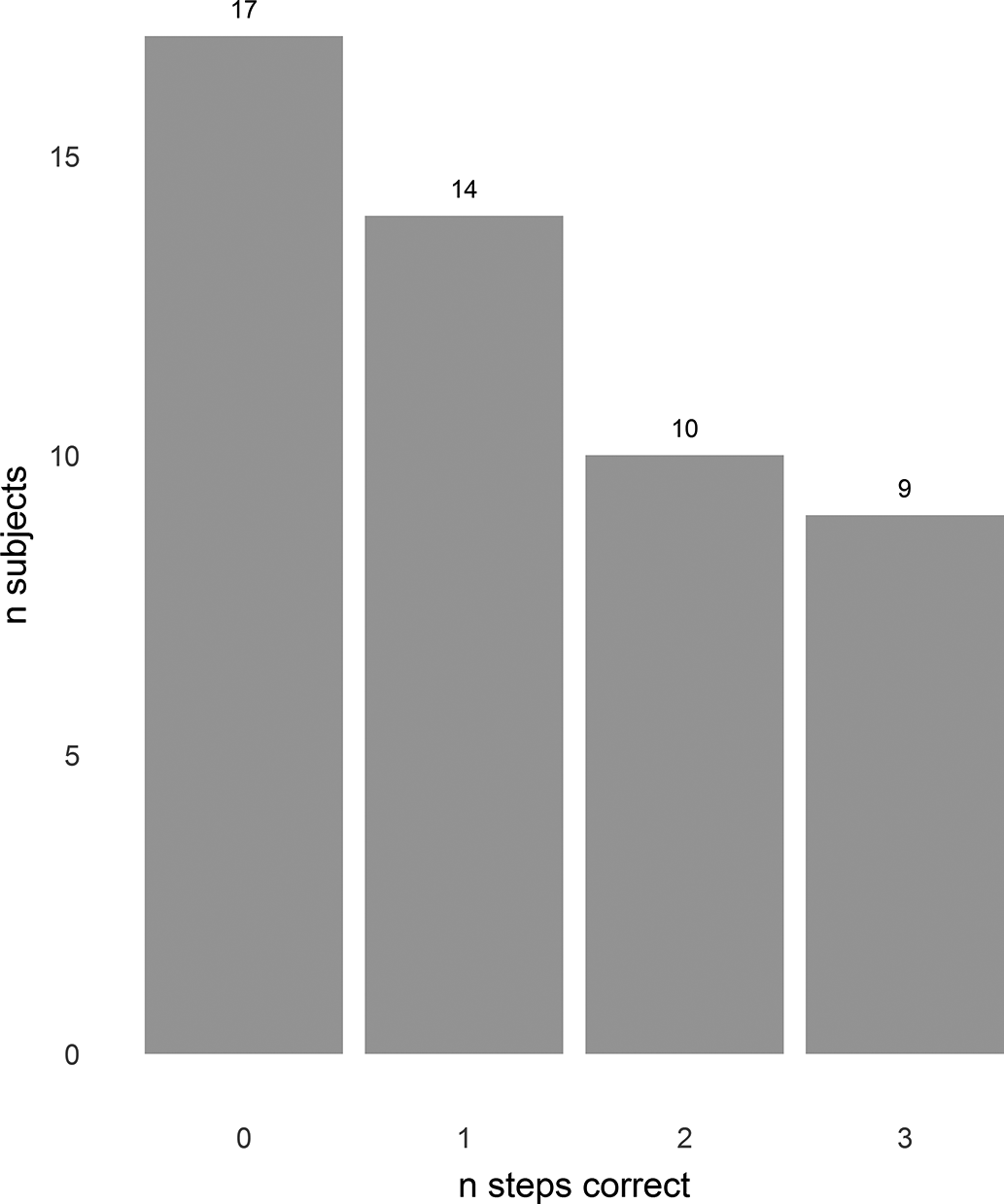
On the coin-in-hand test, 12 (24%) scored at and 4 (8%) below, the level expected by chance ( Figure 1). Three subjects (6%) including 2 of those scoring 10/10 were observed to circumvent the requirement to recall the coin’s location during the test by pointing to the correct hand while they had their eyes closed. Four struggled or failed to count backwards from 10.

Figure 1. Coin in hand test. X axis—number of trials in which side of coin (L or R) correctly identified. Y axis—number of subjects. Sixteen subjects scored at (12) or below (4) the level which would be expected by chance.
Nine (18%) achieved all 3 steps of the Luria three-step test and 17 (34%) did not manage the first step, the remaining 24 (48%) managing one or two steps ( Figure 2). Fifteen (30%) copied all 4 interlocking finger positions, 23 (46%) three, 10 (20%) two; one subject copied only one and another failed to copy any of the hand positions.
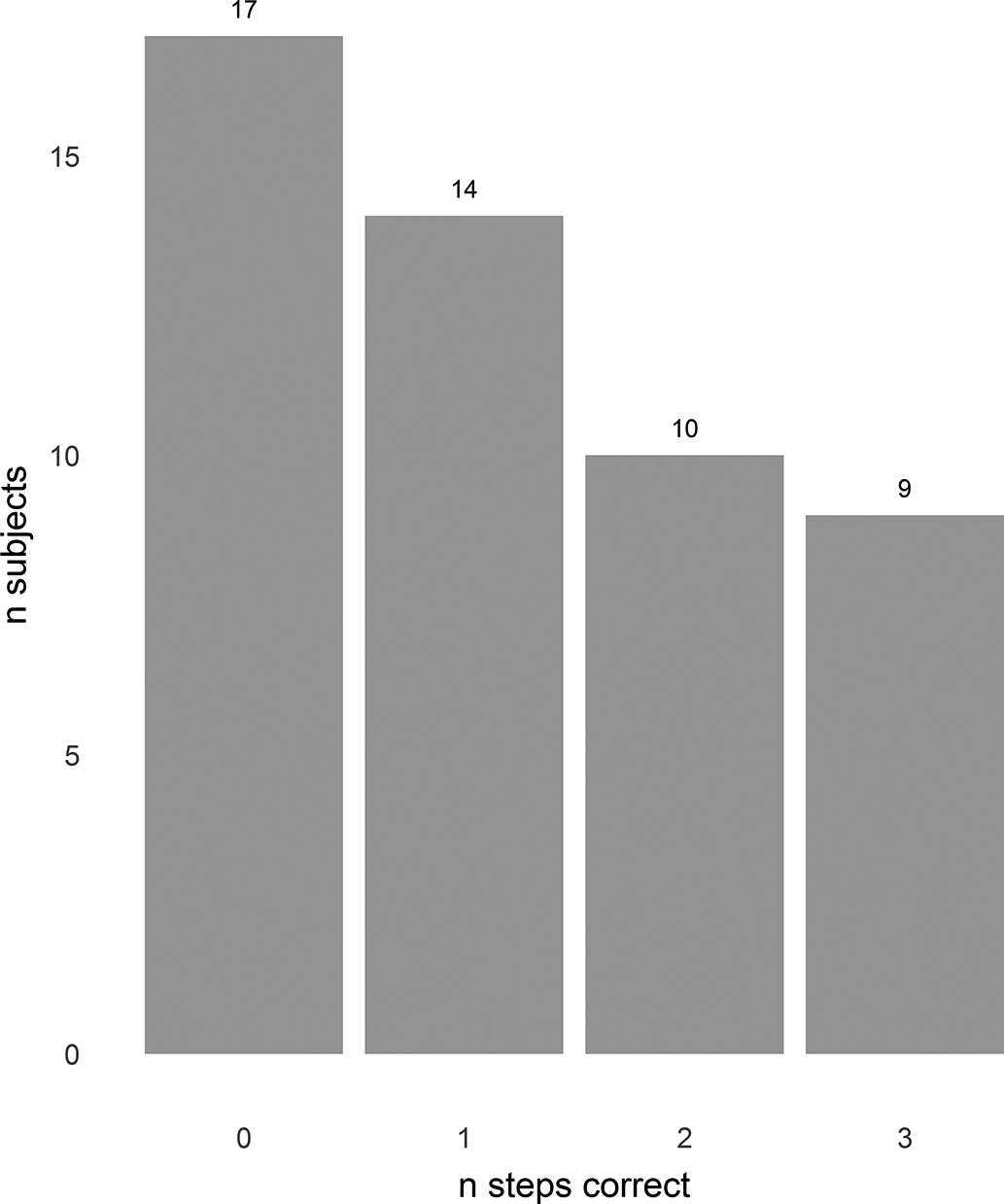
Figure 2. Luria 3-step test. X axis—number of steps correctly completed (1—series copied alongside examiner, 2—series repeated independently, 3—new series successfully copied independently.) Y axis—number of subjects.
We noted several inconsistent patterns of response. Fifteen successfully achieved 5/5 in subtraction of serial sevens from 100 but failed to repeat a digit span of five, of whom 6 also failed a reverse digit span of two digits. Of the 29 subjects who failed forward digit span of four digits, Sixteen (55%) passed cube drawing, 10 (34%) passed serial sevens and 4 (14%) achieved full marks for orientation. Of the 15 scoring 4/4 on interlocking fingers, 11 (73%) did not successfully complete the clock-drawing task. Similarly, of the 9 participants scoring 3/3 on the clock-drawing task, 5 (56%) were unable to copy all 4 finger positions.
Gait appeared normal in 26 (52%) and abnormal in 24 (48%): 5 were unusually slow and 1 very quick, 16 appeared unsteady, and 17 failed to manage or lost their balance during tandem gait. Ten in whom gait otherwise appeared normal appeared to have difficulty remembering or following instructions during examination of gait.
There were no significant differences in total or individual item scores between the 24 participants who reported a family history of dementia (all grandparents) and in the 26 who did not. On the clock-drawing task, individuals with a family history were faster than those without (42.3 seconds vs. 54.7 seconds, p = 0.02 [95% CI 1.48,23.3]); there were no other significant differences in response time.
Discussion
Attention and beliefs are widely recognized as important elements in the aetiology of functional neurological disorders.Reference Edwards, Adams, Brown, Parees and Friston16, Reference Bergh O, Witthöft, Petersen and Brown17 In the Bayesian paradigm described by Edwards et al, prior beliefs about movement, typically not held in awareness, exert a top-down influence on sensorimotor processing to produce and maintain symptoms of functional motor disorder.Reference Edwards, Adams, Brown, Parees and Friston16 Experimental simulation has previously demonstrated similarity between simulated and functional paralysis, both groups also demonstrating sensory loss, in patterns (for example, circumferential) that are less common in structural lesions.Reference Stone, Mutch, Giannokous, Hoeritzauer and Carson9, Reference Hurst18 It might therefore be similarly expected that individuals simulating dementia might demonstrate prior beliefs about dementia, which are more characteristic of functional cognitive disorders than of dementia. This study aimed to access prior beliefs about dementia in healthy individuals by asking them to simulate symptoms of mild dementia, with the purpose of clarifying those ideas and identifying behaviors with potential utility in the diagnosis of functional cognitive disorders.
This cognitively healthy and highly educated group of young adults, asked to simulate mild dementia, scored similarly to individuals with mild Alzheimer’s disease in the original normative data, with a mean score of 16 and 90% falling below the 23/30 cut-off.Reference Nasreddine, Phillips and Bedirian10, Reference Rossetti, Lacritz, Hynan, Cullum, Van Wright and Weiner19–Reference Wilson, Sytsma, Barnes and Boyle22 We were surprised by the severity of apparent impairment displayed, expecting more subtle deficits. In previous studies of simulated ‘mental disorder’ and of cognitive impairment due to brain injury, simulators (particularly student simulators) have produced milder impairments than disease controls.Reference Anderson, Trethowan and Kenna23, Reference Haines and Norris24 However, the overall level and scope of impairment demonstrated by this cohort of simulating subjects reflected the overall impression suggested by their verbal descriptions of mild dementia. Although most identified memory impairment as a key symptom, more often dementia was described as a syndrome of global impairments even at an early stage; motor, speech, emotional, and behavioral symptoms were relatively over-represented in our subjects’ reports of expected symptoms, whereas lack of insight—prevalent in dementia—was only included in the report of 1 subject, suggesting either that lack of insight is not recognized to be important or that it is assumed to be so common as to not be worthy of mention.Reference Wilson, Sytsma, Barnes and Boyle25 In those with functional cognitive disorder, the former seems more likely, given the frequent observation of memory catastrophisation, in which the patient is acutely aware of their deficits whereas others are not. Our first observation, therefore, is that healthy young adults perceive even mild dementia as a condition of significant rather than subtle impairment.
Although total scores were similar, patterns of apparent impairment differed in several important ways from norms for both dementia and cognitively healthy populations. In normative data, individuals with Alzheimer’s Dementia and MCI have been reported to perform worst on trail-making, clock-drawing, naming, delayed recall, phonemic fluency, abstraction, and orientation.Reference Nasreddine, Phillips and Bedirian10 This group of adults simulating dementia performed worst on delayed recall (100%), letter vigilance (86%), clock-drawing (82%), forward digit span (82%), repetition (80%), fluency (78%) and trail-making (72%); letter vigilance (a task involving sustained attention and response inhibition) therefore being more impaired than might be expected in mild dementia. A cognitively healthy cohort of 73 year olds lost most points for delayed recall (mean score 3.1±1.3), fluency (0.7±0.5), and abstraction (1.7±0.6).Reference Borland, Nägga, Nilsson, Minthon, Nilsson and Palmqvist21 Our simulating subjects scored worse than healthy controls in all of these measures: delayed recall (2, IQR 2), fluency (0.22±0.41) and abstraction (1.24±0.71). In another population-based sample, the items with the most frequent errors were cube drawing (59%) and delayed recall (56%).Reference Rossetti, Lacritz, Cullum and Weiner26 Cube drawing was comparatively preserved in our simulating group, 44% making errors, whereas 100% failed delayed recall. It seems that these simulating adults do not perform with an exaggerated pattern of normal failures, nor do they perform similarly to those of individuals with mild dementia.
The degree of effort applied during cognitive testing significantly influences performance but is notoriously difficult to define and measure. Some validity tests are so straightforward that they should be completed without difficulty even in the presence of significant impairment. Others use a “forced choice” paradigm, on the basis that scoring less than chance indicates intention to fail: arguably a completely different concept to that of effort by degree of intention to perform to capacity. The coin-in-hand test meets both criteria, and is easy to perform in clinic without special equipment.Reference Kapur15 In this study, subjects simulating dementia performed poorly on the coin-in-hand test, 84% scoring 7/10 or less, although only 8% scored below the level expected by chance. We have reservations about the utility of these tests in the memory clinic. Critically, individuals with dementia also sometimes fail effort tests. In a study of 6 validity tests 5/22 individuals with moderate to severe dementia failed (scoring 7/10 or less) the coin-in-hand test (although the number scoring at or less than chance is not reported); 16/22 (7/20 with mild dementia) failed the Medical Symptom Validity Test and 16/22 failed the Rey 15 item test.Reference Rudman, Oyebode, Jones and Bentham27 So, although a cut-off score of 7/10 on the coin-in-hand test would correctly identify 84% of our simulating patients, this could not be relied on in a clinical context to exclude other causes of cognitive impairment. Moreover, interpretation of validity test performance in nonsimulating, nonlitigating patients with functional neurological disorders is complex: in some individuals, the interference from pathologically excessive effort, and anxiety, might disrupt normally automatic cognitive processes in order to produce results suggesting, paradoxically, a lack of effort.
In normative Wechsler Adult Intelligence Scale (WAIS) digit span data, individuals with memory impairment, including due to Alzheimer’s dementia or vascular dementia, traumatic brain injury, Korsakoff’s syndrome, and temporal lobectomy, generally scored between 5 and 8 on forward digit span; 4.1%, of all clinical groups and 10.5% with Alzheimer’s disease scored a maximum of 4 and 2.6% of the Alzheimer’s disease group scored a maximum of 3; young adults aged 20–24 scored a mean of 6.8±1.3, reducing gradually over age to a mean of 5.7 ± 1.0 in those aged 85–89.Reference Iverson and Tulsky28 In a study of 18 individuals with Alzheimer’s dementia, 18 with vascular dementia and 26 controls, neither Alzheimer’s nor vascular dementia were associated with impaired performance on forward digit span (mean scores around 5.5 in both groups), although both dementia groups were impaired on backward digit span compared with controls.Reference Carlesimo, Fadd, Lorusso and Caltagirone29 Reliable digit span (summed maximum forward and backwards span measured using the WAIS) has been used in attempts to measure effort.Reference Butters and Cermak30–Reference Mathias, Greve, Bianchini, Houston and Crouch32 Although Reliable Digit Span could not be calculated here due to the simple method used to test digit span, addition of short digit span trials to those included in the MoCA enriched the examination by demonstrating exceedingly poor performance in some individuals; 26% failed a digit span of three and 36% failed reverse span of two digits, suggesting that a substantial minority of those tested believe working memory to be significantly impaired in individuals with mild dementia.
Internal inconsistency is a key feature of functional neurological disorders; for example, in Hoover’s test, hip extension is weak during active movement but returns to normal with contralateral flexion against resistance. Inconsistency was also a prominent feature in these simulating young adults. Atypical performance patterns in less widely used neuropsychological tests have been described as a marker of malingering.Reference Larrabee33 In our study, discrepant patterns such as poor performance in digit span relative to serial sevens (examining overlapping functions of sustained attention and working memory), and poor performance in construction tasks relative to performance in imitation of hand gestures were potential indicators of functional cognitive disorders which merit testing in larger cohorts of individuals with both neurodegenerative and functional disorders.
Family history of dementia did not influence performance in this study. However, our subjects were young (mean age 22), and family history of dementia related to a grandparent in all cases. They were significantly younger than the reported mean age (54.6 ± 13.0 years) of people with functional cognitive disorders in a series of memory clinic patients, in whom family history was associated with increased likelihood of functional cognitive disorder.Reference Bharambe and Larner34 We predict that older individuals are more likely to have experience of dementia in a first degree relative, and that this might impact on beliefs about symptoms, although how this might manifest is an interesting topic for further investigation
Although there may be similarities in beliefs about dementia between those with functional cognitive disorders and healthy adults, there are also likely to be differences, and these differences may be important in determining why functional cognitive symptoms develop in some people and not others. At a group level, people who develop functional neurological disorders have a greater experience of ill health and psychiatric comorbidity, and may also have different background experiences. These factors, together with general factors such as gender, educational background, and ethnicity, are likely to influence beliefs about illness. Further research using an experimental simulation paradigm in those with experiences of chronic pain or ill health, or who have experienced adverse events, might be used to further explore the relationship between beliefs and cognitive symptoms in those with functional disorders.
Describing performance patterns in simulating adults and, in future, in individuals with functional cognitive disorders is an important step in improving our understanding of functional cognitive disorder phenotypes. However, overall, we suspect that raw cognitive test results will continue to have a limited reach in discriminating between neurodegenerative disease and functional cognitive disorders as preliminary clinical studies have suggested.Reference Bharambe and Larner34 Promising work in this area has concentrated instead on linguistic and behavioral features during the clinical consultation, finding for example that individuals with functional cognitive disorders are more likely to attend clinic alone, more likely to provide detailed accounts of forgetting events, and less likely to ‘head turn’ toward an accompanying adult.35–Reference Jones, Drew and Elsey39
The conclusions we can draw from this study are limited by the lack of functional cognitive disorder controls. In addition, detailed enquiry was not made into the extent to which the instruction to simulate mild (rather than moderate or severe) dementia was understood, and it is possible that some subjects aimed to simulate more severe impairment than we intended. Finally, digit span was measured on the basis of single trials and not according the method used in the WAIS (not available for general clinical use by nonpsychologists), and as a result it was not possible to calculate reliable digit span in order to compare directly with normative data.
In summary, cognitively healthy individuals simulating dementia attain similar overall scores in cognitive screening tests as individuals with mild dementia, but with particularly poor performance on short digit span trials, relative preservation of cube drawing and abstraction, inconsistent patterns of performance and higher rates of effort test failure. Experimental simulation of cognitive impairment is a novel method of accessing beliefs about dementia with potential utility in the development of diagnostic tools for functional cognitive disorders.
Funding.
Laura McWhirter is funded by a University of Edinburgh Clinical Research Fellowship funded philanthropically by Baillie Gifford. Professor Jon Stone is a Chief Scientists Office NHS Research Scotland Career Researcher.
Disclosures.
Laura McWhirter, Brendan Sargent, and Craig Ritchie have nothing to disclose. Alan Carson is a director of a limited personal services company that provides independent medical testimony in Court Cases on a range of neuropsychiatric topics on a 50% pursuer 50% defender basis. Jon Stone provides independent medical testimony in court cases regarding patients with functional disorders.
Ethical Statement.
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Appendix
Script Used During Interview and Cognitive Examination
Introduction
“Hi my name is [researcher’s name], I’m undertaking a study with Dr Jon Stone, a consultant Neurologist, to find out the ideas that healthy people have about certain symptoms and illnesses. This interview will take around 20 to 25 minutes. Is that OK? What I would like to do, is ask you some questions, ask you to do some movements and ask you to fill out a form used to test cognitive (mental) function. During this interview I would like you to imagine that you have mild dementia due to Alzheimer’s disease. Don’t worry if you’re not exactly sure how someone with dementia might respond, this research is interested in your perceptions and results will be anonymous.
Do you have any questions or concerns about this? There is a consent form to show you some options and you can sign (show subject the consent form and answer any questions). If at any point you do not wish to continue with the interview or exam, please let me know.
(Record subject’s basic details on consent form, assign them a volunteer number). Do you have any medical condition that affects your memory, or any condition that affects the movement of your hands or fingers? Do you or your close friends or family have any neurological disorders such as seizures, MS or a tremor? Is there any family history of dementia or other neurological conditions?
Ideas about symptoms and investigations
Please could you tell me what you think someone with mild or early stage dementia might experience? What symptoms might they have? As mentioned previously, I’d like you to imagine you have mild dementia for the rest of this interview.
MoCA
I’m now going to ask you to perform this short test, I’d like you to do it pretending you have mild dementia. (Record performance and time on each individual component, follow MoCA script.)
Gait
If you could now walk from here to the end of the room (about 5 meters?) in a straight line, and back walking heel-to-toe please.
Coin in hand test
I’m now going to show you a coin in one of my hands. I’d then like you to close your eyes, count backwards from 10 and then open your eyes. Then I’d like you to tell me which of my closed hands contains the coin. We’ll repeat this ten times.
Luria
I’d now like you to observe these three movements of my hand in sequence (do cut, fist, slap three times, pausing between each). Now please could you copy this sequence with me (three times)?Now could you complete the sequence on your own please (three times). This time, please could you follow another sequence (show slap, cut, fist once and then ask them to do it)?
Interlocking fingers
Now I’d like you to copy each of these finger movements one by one. (Show each movement, and wait for it to be copied: index and thumbs in pincers, pinkies hooked, fingers laced backwards, two fingers through split fingers.) That’s the end of the interview, thank you very much for your time.