Setting the Scene
Pollutant agents are exponentially increasing in modern society since industrialization processes and technology are being developed worldwide. It is well known that there are various environmental pollutants that may have multiple effects on health of living species. In particular, it is suggested that some pollutants may impact on mental health of humans even if psychological and psychopathological consequences are poorly studied and described. In fact, these aspects are still underestimated even if there is so much interest on the physical consequences of the exposure to various environmental pollutants.
From the industrial age, the pollution related to the work of man has been inserted into the balance of our planet’s natural forces. Human industrialization has been also named Anthropocene.Reference Crutzen 1 The negative impact of some global changes and the noxious action of new elements on the ecosystem and human life may be summarized by the term “pollution.”
Introduction
Polluting agents are widespread in a complex way on the planet and it may be difficult to estimate the benefits and costs of pollution for the humans. Pollution and its related outcomes should be compared, in an experimental manner, with those from healthy environments like medically or recreationally structured living conditions.Reference Anakwenze and Zuberi 2 , Reference Khan, Plana-Ripoll and Antonsen 3 Environmental pollution is part of the “flux” of global events which are leading to the transformation of postmodern societies.Reference Cianconi, Tarricone and Ventriglio 4 The polluting elements can also affect the human nervous system with remarkable consequences on population’s mental health.Reference Anakwenze and Zuberi 2 , Reference Khan, Plana-Ripoll and Antonsen 3 Mental health problems may be divided into three main categories according to their causes: psychogenic disorders, endogenous disorders, and exogenous disturbances. 5 In fact, pollution can impact on the expression of genes and the structure of neural tissues (gene × environment interaction), but also generating a social stress related to unhealthy and poor living conditions in degraded environments.Reference Anakwenze and Zuberi 2 , Reference Khan, Plana-Ripoll and Antonsen 3 In this case, pollution can act as a psychogenic agent since psychogenic disorders are the result of stress, shock, or any kind of psychological traumas in childhood, adolescence, or adulthood. 5
Also, Anakwenze and Zuberi, in 2014,Reference Anakwenze and Zuberi 2 pointed out that the impact of pollution on human health can be due to an immediate cause–effect relationship but more significantly to long-term effects, often only hypothesized and poorly described or demonstrated. According to this, the damage of a pollutant can be achieved through “direct” biochemical mechanisms of the polluting agents on the nervous tissue, or indirectly, through the production of a stressful prolonged condition impacting on the mental health status. “Direct” impact can be differentiated into “genetic interference,” “gestational interference,” and “post-gestational interference.”Reference Anakwenze and Zuberi 2 , Reference Khan, Plana-Ripoll and Antonsen 3 “Genetic interference” is related to the biochemical modification of human DNA and the consequent transmission of genetic mutations to the following generations with an increased risk of developing mental illness. In the “gestational interference” model of action, the embryonic or fetal tissues are directly affected, involving the physiological processes of neurodevelopment, triggering the etiopathogenesis of a mental illness, or generating a condition of vulnerability that may favor the onset of it. In the “post-gestational interference” model, additionally, the pollutant interferes with the psychosexual processes occurring from the birth and during the childhood/adolescent stage, or even in the adulthood, favoring a condition of mental illness or distress.Reference Anakwenze and Zuberi 2 , Reference Khan, Plana-Ripoll and Antonsen 3
The so-called “indirect” agents, as stated, may be involved in the etiopathogenesis of a mental disorder by generating a condition of a prolonged stressful condition possibly related to other multiple factors (cultural, psychological, technological, economic and urban, etc.). It is important to highlight that there are several polluting agents that can be defined as “direct” and “indirect” since they may act with two different models of action.Reference Anakwenze and Zuberi 2 , Reference Khan, Plana-Ripoll and Antonsen 3
In this narrative review of literature, we intend to focus on the available evidences about the possible relationships between various environmental pollutants and mental health.
Methods
A literature search on PubMed and EMBASE has been conducted using the following set of keywords: pollution, pollutants, air pollution, mental health, stress, psychological distress, radiations, heavy metals, neurotoxicity, noise, environment, urbanicity, and industrialization. All articles dealing with the issue of pollution and mental health (N = 806) have been considered. Redundant articles were analyzed and authors included those reporting significant evidences. Finally, a total of 134 documents were cited, analyzed in this narrative review, and grouped in specific topics of discussion as following.
Findings
Air pollution
Air pollution, based on chemical emissions essentially derived from various urban and industrial activities, can have significant consequences on human neuro-developmental processes and on the Central Nervous System (CNS).Reference Annavarapu and Kathi 6 Heavy metals are the most studied components of air pollution that have been associated with neurotoxicity; they include polycyclic aromatic hydrocarbons, volatile organic compounds, black coal, environmental tobacco smoke, carbon dioxide, ozone, nitrogen dioxide, and sulfur dioxide, particulate matter (PM) including fine PM < 2.5 μm (PM2.5), ultrafine particulates <0.1 μm, and larger particles. It has been demonstrated that air pollutants can have synergistic impact on various human physiological systems such as the neuroendocrine system, the pro-inflammatory immune pathways, and on the redox balance.Reference Wright 7 Several epidemiological and experimental studies describe the association between atmospheric pollutants and chronic inflammation of brain tissues with an activation of microglia and alterations of the brain white matter.Reference Block, Elder and Auten 8 Also, it has been described that children, elderly subjects, those with pre-existing health problems, socially excluded or economically disadvantaged subjects, and racial or cultural minorities report greater vulnerability to air pollution impact (probably due to an additive effect to their pre-existing vulnerability).Reference Harlan and Ruddell 9 , Reference O’Neill and Ebi 10 In particular, exposure to urban pollution is greater if close to major roads, landfills, and factories, it can seriously harm children’s health especially when associated with socio-economic poor conditions.Reference Calderon-Garciduenas and Torres-Jardon 11 Outdoor air pollution may lead to an immunological activation with systemic inflammation, neuroinflammation, oxidative stress, and immunodisregulation. 12 Also, children may present greater vulnerability to environmental pollutants due to differences in absorption, metabolism, and excretion of chemical agents if compared to adults. In fact, environmental contaminants may have longer half-lives in young children due to the immaturity of metabolic enzyme systems. Cell immaturity and brain susceptibility represent a high risk for children exposed to pollutants, particularly PM, so that they can be considered the most vulnerable subjects of the general population. 12 , Reference Heinrich and Slama 13 Recently, a comprehensive review confirmed the association between various fossil fuel combustion pollutants and multiple health effects in children.Reference Perera, Ashrafi, Kinney and Mills 14
Exposure to air pollutants during fetal life may be an important risk factor for neurodevelopmental disorders such as autism and major psychoses like schizophrenia.Reference Genc, Zadeoglulari and Fuss 15 It is generally recognized that the developing brain is much more vulnerable than the mature nervous system.Reference Clifford, Lang and Chen 16 This is due to the phase of synaptogenesis which is considerably susceptible to neurotoxicity.Reference Rizzi, Ori and Jevtovic-Todorovic 17 , Reference Levy 18 A recent review conducted by Annavarapu and KathiReference Annavarapu and Kathi 6 explores recent advances in scientific research about the possible association between the exposure of children to vehicular emissions mainly from 2005 to 2015 and oxidative stress, neurodegeneration, neurodysfunction, attention-deficit/hyperactivity disorder (ADHD), and autism.Reference Annavarapu and Kathi 6 This study also highlights an important influence of some additional association factors such as younger age, gender, proximity to the source of contamination, and socio-economic conditions of children.Reference Annavarapu and Kathi 6 Sunyer et alReference Sunyer, Esnaola and Alvarez-Pedrerol 19 described a correlation between exposure to air pollution and an inflammatory reaction of some specific brain structures such as the prefrontal cortex and the striatum. It has been suggested this may lead to deficits in cognitive development among children. These evidences were confirmed in a study conducted in Mexico City on children reporting neuroinflammation, neurodegeneration, and cognitive deficit after a prolonged exposition to air pollution. 20–24 Authors documented volumetric alterations at magnetic resonance imaging (MRI) involving bilateral temporal and right parietal areas of the brain. According to the same authors, the presence of heavy metals was found in frontal lobes of lifelong exposed children with consequent neuroinflammation and up-regulation of the frontal interleukin-1b and of the cyclooxygenase-2.Reference Calderon-Garciduenas, Serrano-Sierra and Torres-Jardo 25 Recently, de Prado Bert et alReference de Prado Bert, Mercader and Pujol 26 summarized evidences regarding the effect of traffic-related air pollution (TRAP) on the human brain integrating epidemiological data with neuroimaging findings. They pointed out that long-term exposure to air pollution agents may have impacts on brain structures (especially white matter) and brain functioning which are described with different MRI techniques. It is suggestive that brain changes may mediate the impact of TRAP on human cognition and to be causally associated to cognitive disorders.
It has been described that air pollutants, in particular PM, promote oxidative stress. In fact, children exposed to emissions also show mutations of the genes involved in redox pathways.Reference Annavarapu and Kathi 6 , Reference Calderon-Garciduenas, Franco-Lira and Mora-Tiscareno 27 Oxidative stress is a central mechanism through which exposure to PM can lead to pathological conditions.Reference Ghio, Carraway and Madden 28 In particular, some authors described a specific correlation between autism and the exposure to urban TRAP during the pregnancy and the first year of life.Reference Becerra, Wilhelm and Olsen 29 – Reference Volk, Lurmann and Penfold 32 Ehrenstein et alReference Ehrenstein, Ondine and Hilary 33 have shown that exposure to air pollutants from urban traffic and industry emissions during the intrauterine life can be considered an important risk factor for autism. Also, Siddique et alReference Siddique, Banerjee and Ray 34 studied the association between atmospheric pollution and neurobehavioral disorders in children, concluding that the prevalence of ADHD was significantly higher among Indian children living in urban areas than children of the same age and gender living in nonurbanized areas. In 2019, Burkhardt et alReference Burkhardt, Bayham and Wilson 35 found also an association between the increase of air pollution and violent crime rates across the continental United States.
Carbon monoxide and neurotoxicity
Carbon monoxide (CO) is a common contaminant of both indoor and outdoor environments and it is a product of incomplete combustion of hydrocarbons. CO is a component of vehicle-related pollution, gas stove pollution, and tobacco smoke. CO can cross the placenta and reach the developing brain via the fetal circulation.Reference Levy 18 , 36 , Reference Vrijheid, Martinez and Aguilera 37 LevyReference Levy 18 proposed the theory according to which CO is responsible for alterations of neurodevelopment especially in case of exposure during the perinatal period. A work conducted by Vrijheid et alReference Vrijheid, Martinez and Aguilera 37 showed an association between exposure to CO during fetal life and abnormalities in cognitive development beyond 14 months of age, regardless of socio-economic conditions. A further study showed a reduction of neuropsychological performance among Guatemalan children at 6 to 7 years of age, after being exposed to chronic indoor wood smoke during the third trimester of pregnancy.Reference Dix-Cooper, Eskenazi and Romero 38 From all these studies, a correlation emerges between any exposure to CO in both internal and external environments and abnormalities in neurological development especially if exposure occurs during fetal life. Animal studies have shown that perinatal exposure to CO interferes with the physiological stages of neurodevelopment. For example, Cheng et al, in 2012, identified some important biochemical effects on various elements involved in the programmed cell-death pathways of the brain tissues of mice exposed to CO in the postnatal period.Reference Levy 18 , Reference Cheng, Thomas and Mardini 39
PM2.5 and neurotoxicity
Fine PM with an aerodynamic diameter of less than or equal to 2.5 μm (PM2.5) is now considered an important risk factor for neurodegenerative disorders and neurological development disorders including autism.Reference Wei, Feng and Liang 40 , Reference Wei, Liang and Meng 41 A study conducted by Wei et al demonstrated that PM2.5 involves the redox balance leading to a decrease in GSH/GSSG (glutathione/oxidized glutathione) ratio and abnormal DNA methylation patterns which in turn may cause cognitive deficits or behavioral disorders.Reference Wei, Liang and Meng 41 , Reference Ronan, Wu and Crabtree 42 This work has shown that PM2.5 and its extracts impair the SAMe (S-adenosylmethionine) synthesis, remembering that SAMe is fundamental for DNA methylation. The impaired capacity of methylation leads to a global hypomethylation of DNA but also to hyper- or hypo-methylation of some candidate genes for autism. Authors have further analyzed the influence of PM2.5 on gene-specific DNA methylation and correlative mRNA expression in some autism candidate genes such as NRXN1 and NLGN3 which codify for synaptic-adhesion molecules. NRXN1 and NLGN3 play a fundamental role in the synaptic transmission by connecting presynaptic and postsynaptic neurons. PM2.5 exposition seems also associated with the reduction of expression of adhesion proteins such as Synapsin-1 and PSD-95, which are fundamental for the establishment of synapses, neurotransmission, and axonal growth.Reference Nikolaev and Heggelund 43 , Reference Schaevitz, Berger-Sweeney and Ricceri 44 These findings suggest the hypothesis that PM2.5 is associated with synaptic homeostasis and activities involved in neurodevelopmental disorders.Reference Wei, Liang and Meng 41 Wei et alReference Wei, Feng and Liang 40 have founded that PM2.5 and its extracts promote cell cycle arrest, cell apoptosis and inhibition of cell proliferation in neuronal tissues. Moreover, they highlight that PM2.5 and its extracts increase the hydroxymethylation of the global DNA and in particular of neuronal genes, thus interfering with their expression.
Findings from several studies support a correlation between air pollution and depression. Air pollution exposure has been found to lead to depression-like behaviors in animal studies as well as exposure to PM2.5 is associated with increased depression-like responses in mice.Reference Fonken, Xu and Weil 45 Mokoena et alReference Mokoena, Harvey and Viljoen 46 reported anxiety and depression-like behavior in rats following chronic ozone inhalation.Reference Fonken, Xu and Weil 45 , Reference Mokoena, Harvey and Viljoen 46 Jones and ThomsenReference Jones and Thomsen 47 have reported an association between the increase of pro-inflammatory cytokines and behavioral abnormalities in animals and air pollution has been consistently associated with increases of blood proinflammatory markers and systemic oxidative stress, both involved in the pathogenesis of psychiatric disorders such as depression.Reference Thompson, Zanobetti and Silverman 48 Some other studies have reported an association between air pollution and suicide. 49 – Reference Power, Kioumourtzoglou and Hart 51 In a recent prospective cohort study conducted by Kioumourtzoglou et al,Reference Kioumourtzoglou, Power and Hart 52 authors investigated the association between long-term exposures to PM2.5 and ozone and the onset of depression among a nationwide cohort of mid-life and older women, during a period of 12 years. The findingsReference Kioumourtzoglou, Power and Hart 52 of this study suggest that both PM2.5 and ozone are potential risk factors for depression even if Zijlema et alReference Zijlema, Wolf and Emeny 53 investigated the association between air pollution and depressed mood in four European general population cohorts and they found no consistent evidence of an association between air pollution and depressed mood.
Environmental ultrafine particles and neurotoxicity
Allen et alReference Allen, Oberdorster and Morris-Schaffer 54 showed the effects of exposure to concentrated environmental ultrafine particles (UFPs) on mice during their postnatal day (equivalent to the third human trimester of life) correlating them with the onset of pathological conditions. The morphological and neurobiological changes found in some brain structures suggest that the equivalent of the third human trimester may be a period of potential vulnerability to the toxicity of UFP, particularly in males. Moreover, it has been hypothesized that exposure to UFP during periods of neurogenesis may be a risk factor for schizophrenia, attention deficit disorder and periventricular leukomalacia. A recent study also has shown that short-term exposure to ultrafine particles has been associated with exacerbations of psychiatric conditions in children as suggested by increased access to the Cincinnati Children’s emergency department for psychiatric issues.Reference Brokamp, Strawn, Beck and Ryan 55
Heavy metals
Metals are known to impact on early neurodevelopment, but their effects later in life are not well described. Heavy metals are often generated by the electronic waste. In fact, electronic waste is constituted by plastic components and precious materials such as gold, silver, copper, platinum, and palladium but also iron, aluminium, small amounts of heavy metals (like mercury, lead, cadmium).
According to the World Health Organization (2007), 56 risks for our health may derive from direct contact with harmful materials, inhalation of toxic fumes, and accumulation of chemical substances in the soil, water, and food that constitute also a threat for the biodiversity. Grant et alReference Grant, Goldizen and Sly 57 described change in the thyroid function, worsening in the neonatal health, increases in spontaneous abortions, premature births, reduction of the lung functionality after exposure to e-waste (e: electronic). Even if there are possible associations between heavy metals exposure and psychosis, only few studies have investigated putative linking mechanisms.Reference Modabbernia, Arora and Reichenberg 58
Lead (Pb)
One study investigated the linking mechanism between early exposure to Pb and the gene expression influencing the risk of psychosis. Lead seems to impact dopaminergic and glutamatergic neurotransmission both involved in the pathophysiology of psychosis. In fact, Cory-Slechta et al showed that the exposure to Pb in early life can lead to hyperactivity of the dopaminergic system.Reference Cory-Slechta, Brockel and O’Mara 59 The N-methyl-d-aspartate receptor (NMDAR) for glutamate, which is an excitatory aminoacid playing an important role in regulating dopamine activity, seems to be antagonized by Pb. Since the early ‘90s, Guilarte and MiceliReference Guilarte and Miceli 60 reported evidences that Pb2+ is a noncompetitive antagonist of the NMDAR and disrupts neuronal processes depending on NMDAR activation. Authors added that exposure to Pb during early brain development leads to onset of psychotic symptoms as later confirmed by Opler in 2004.Reference Opler, Brown and Graziano 61
Mercury
Brain pathophysiology of autism seems to show similarities with brain abnormalities found in mercury intoxication.Reference Kern, Geier, Audhya and King 62 It is of note that mercury and other heavy metals can employ the sulfur-dependent detoxification system, and glutathione reserves in particular.Reference Kern, Geier and Sykes 63 Children from the Western Amazon have been studied after being exposed to acute TCV-EtHg (thimerosal-containing vaccines—ethylmercury) and to chronic MeHg (methylmercury) from fish consumption. The Mental Development Indices (MDI) and Psychomotor Development Indices (PDI) were calculated at 6 and 24 months of age. The combined exposure to MeHg and EtHg was not associated with differences in PDI but some differences were found for the MDI. However, it has been observed a significant decrease in both PDI scores and MDI scores at 24 months. Also, the combined exposure to Hg caused an increase in neurodevelopmental delays.Reference Marques, Abreu and Bernardi 64 In 1985, a 16-year-old female, while was working at the thermometer manufacturing factory, developed a mental disorder that was probably provoked by an inorganic mercury poisoning as reported by Jeong et al.Reference Jeong, Cheon and Chang 65
Cadmium
Cadmium impacts on brain and increases reactive oxygen species (ROS) damaging antioxidant defence systems.Reference Vaziri 66 The mechanism of lead- and cadmium-induced neurotoxicity leading to neuropsychiatric disorder is not fully understood. On the other hand, one developmental hypothesis is that cadmium could be another environmental factor inducing early pathological changes in the brain.Reference Orisakwe 67
Ionizing radiation
IRs certainly act through “direct” biological mechanisms on the CNS with consequent alterations. However, it should be emphasized that the awareness of being exposed to radiations and the consequent perception of risk to own health is already an important source of psychological stress for healthy subjects. This condition, in the long run, can promote a cascade of biological and pathophysiological mechanisms possibly leading to the etiopathogenesis of mental distress such as depressive and anxious disorders. In eukaryotic cells, IR induces damage to DNA, proteins, and lipids, directly or indirectly, increasing the production of highly reactive free radicals. IR-related brain injuries do not occur as a unitary and immediate event, but can be considered as dynamic and multiphase processes over time. In vitro findings have shown that IR pathophysiologically can lead to direct neuronal damage and death. The CNS histological alterations produced by IR mainly concern the process of demyelination. In Western countries, radiodiagnostics and nuclear medicine are the main sources of exposure to IR and most of the data concerning exposure to IR derive from the evaluations of patients exposed to radiotherapy. Little information is available on medium to low dose range exposure. More evidence is needed to explore dangerous effects of low-moderate doses of IR on the brain which is one of the main organs limiting the dose in radiotherapy.Reference Marazziti, Baroni and Lombardi 68
Schizophrenia and IRs
According to the diathesis-stressor model of schizophrenia, environmental stressors can influence the genetically determined neurobiological vulnerability to schizophrenia. There are comparable reports on the increase of Schizophrenia Spectrum Disorders in persons exposed to IRs as a result of Chernobyl accident, atomic bombings, nuclear weapons tests, environmental contamination by radioactive waste, and finally radiotherapy. 69–71 It has been described that exposure to IR causes brain damage with limbic dysfunctions and affects the processing of information at a molecular level, influencing the development of schizophrenia in exposed subjects.Reference Loganovsky, Volovik and Manton 71 Also, exposure to IR during the early years of life leads to problems in neurodevelopment. This evidence has been reported in an epidemiological study carried out on the Norwegian population with the aim of describing the impact of the Chernobyl nuclear accident in 1989. It has been shown a statistically significant relationship between IR exposure and schizophrenia, epilepsy, cerebral palsy, mental retardation, or hearing or vision problems.Reference Lie, Moster, Strand and Wilcox 72
Depression and IR
Depression is one of the most important and long-term effect of nuclear accident (atomic bombings, nuclear testing, and radiation emergences). Chernobyl accident survivors have shown an increased prevalence of depression (18.0% vs 13.1% in controls) and suicide rates.Reference Bolt, Helming and Tintle 73 The clinical manifestation of depression would be characterized above all by symptoms of an asthenic type. The etiopathogenesis of depression in these cases is multifactorial, linked to genetic vulnerability, medical comorbidity, psychological factors, environmental conditions, and finally biochemical processes directly derived from the impact of IR with the CNS tissues.Reference Loganovsky and Vasilenko 70
Alzheimer’s disease and IR
Significant increase in the incidence of cerebrovascular disease has been demonstrated among nuclear workers employed at the Mayak Production Association (Mayak PA).Reference Azizova, Muirhead and Moseeva 74 It has been studied that cerebrovascular dysfunctions are a relevant pathogenetic factor in Alzheimer’s disease. 75–77 There would also be other evidences regarding the relationship between cerebrovascular diseases and cortical neurodegenerative diseases affecting the brain, which all are characteristics of Alzheimer’s disease such as cerebral β-amyloidosis and cerebral amyloidal angiopathy with amyloid-β plaque production.Reference Zlokovic 78
Parkinson’s disease and IR
CNS exposure to IR would result in inflammatory tissue changes. Histological studies on Parkinson’s disease (PD) would confirm the fundamental role of inflammation in the etiopathogenetic neurodegenerative pathway.Reference Mrak and Griffin 79 It is demonstrated that oxidative stress is involved in PD progression and increased levels of ROS can damage the target neuronal cells. The dopaminergic neurons of the basal ganglia are particularly vulnerable to oxidative stress and this would be linked to their low intracellular levels of antioxidants and the high rate of metabolic oxygen consumption.Reference Licker, Kovari and Hochstrasser 80
Cognitive deficits and IR
Pediatric patients with brain tumors treated with radiotherapy cycles show a decrease in brain volume during the neurodevelopment process, especially at the frontal and temporal lobes level, which is directly proportional to the dose of IR administered; this condition is related to deficit in cognitive performances as assessed through neuropsychological tests.Reference Agbahiwe, Rashid and Horska 81 Therefore, despite the significant therapeutic role of radiotherapy in the brain, it would result in organ damage leading to impairment of acquired cognitive abilities. The neurocognitive domains affected by radiation seem to be executive functions, verbal and nonverbal memory, sustained attention and information processing speed as well as brain structures most affected by radiation damage are the prefrontal cortex and hippocampus.Reference Agbahiwe, Rashid and Horska 81 Cognitive processes are mainly determined by a complex network of connections that exists between the frontal cortex and the subcortical level. Radiation injury can affect some or all of these associative pathways through a multifactorial physiopathological mechanism, characterized by inflammation, vascular anomalies, gliosis, demyelination, and necrosis of the white matter in the case of high doses of IR.Reference Makale, McDonald and Hattangadi-Gluth 82
Organophosphate pesticides
Organophosphorus pesticides (OPs) are commonly used as insecticides and are particularly toxic to humans.Reference Grube, Donaldson and Timothy Kiely 83 , Reference Terry 84 The OPs are rapidly absorbed through the respiration, through the skin, the mucous membranes, and the gastrointestinal tract. Usually, acute intoxication occurs after inhalation and causes the rapid onset of multiple symptoms caused by inhibition of AChE (acetylcholinesterase), the enzyme that inactivates acetylcholine; this process is responsible for the accumulation of acetylcholine in cholinergic synapses and then for a peculiar syndromic picture.Reference Vale 85 , Reference Stallones and Beseler 86 Unlike acute exposure to high concentrations of OPs, chronic exposure is associated with sometimes debilitating neuropsychiatric conditions such as anxiety, depression, and suicide. 87–96 A review by Voorhees et alReference Voorhees, Rohlman and Lein 96 shows the correlation between chronic occupational exposure to OPs and neurotoxicity. Concerning the correlation between OPs exposure and psychiatric disorders, controversial data emerge from scientific studies with some epidemiological studies supporting the close correlation as well as a meta-analysis conducted in 2013 showing results as limited and not very conclusive.Reference Parrón, Hernández and Pla 88 , Reference Lee, Lewis and Ippolito 91 – Reference Zaganas, Kapetanaki and Mastorodemos 94 Some other evidences have described alterations in cytoarchitecture or in the morphology of nerve cells among OPs exposure effectsReference Voorhees, Rohlman and Lein 96 : these alterations mainly consist in variation in length, number, or ramifications of axons or dendrites and all have been associated with psychiatric manifestations, including depression, anxiety, Alzheimer Disease (AD), and PD. 96–99 A substantial part of the scientific literature indicates that chronic exposure to OPs is associated with deficits in cognition, impairment of learning, memory, and behavior on the base of neuroinflammation.Reference Voorhees, Rohlman and Lein 96 Paul et alReference Paul, Ling and Lee 100 recently published a study that strongly supports a relationship between OP pesticides, cognitive decline and mortality among older Mexican Americans. In fact, exposure to pesticides has been associated with the pathogenesis of various mental disorders, especially in individuals exposed for professional reasons, such as farmers. In a recent Brazilian study conducted on a sample of a rural population resident in a place where tobacco farming is the main economic activity, researchers found an association between pesticides exposure and common mental disorders, including self-reported depression.Reference Campos, dos Santos Pinto da Silva and Sarpa Campos de Mello 101 Harrison and MackenzieReference Harrison and Mackenzie 102 in a study published in 2016, analyzed the relationship between low-level cumulative exposure of OPs in sheep farmers and their mental health. In this study, the researchers used both self-evaluation measures and structured clinical interviews. The exposed cohort reported significantly higher rates of anxiety and depression than the control subjects when self-report questionnaires were used taking into account stressful life events and other factors.
Light pollution
Life on Earth is adapted to the 24-hours solar day allowing the synchronization of behavioral and biological processes to the external environment.Reference Bedrosian and Nelson 103 The invention of electric light has led to “round-the-clock” societies: all persons living in the United States and Europe are exposed to unnatural light and about 20% of the population has a shift work.Reference Navara and Nelson 104 These changes obviously have biological implications. In mammals, circadian photoentrainment is mediated by intrinsically photosensitive retinal ganglion cells (ipRGCs) that project these information to various brain regions such as the suprachiasmatic nucleus regulating the circadian rhythm. The same projections reach other areas involved in mood regulation, such as the prefrontal cortex, hippocampus, and amygdale, suggesting that artificial light could have a role in influencing mood.Reference Karatsoreos and McEwen 105
Deregulation of circadian rhythm and mood disorders
Many studies identified a link between the induced deregulation of circadian rhythm and the onset of mood disorders. Any unnatural timing of light exposure can cause a desynchronization between internal biological processes and the external environment, leading to mood alterations. There are in fact several instances associating depression with environmental lighting cues. Seasonal depression is in fact a well-known phenomenon afflicting nearly 10% of the population.Reference Rosen, Targum and Terman 106 In such cases, morning bright light therapy, particularly blue wavelengths, may have a therapeutic role.Reference Glickman, Byrne and Pineda 107 Another study considered shift workers population. In this population, the prevalence of major depression disorder is higher than in general population.Reference Scott, Monk and Brink 108 Young student nurses performing night shift work developed depressive symptoms after only 3 months of night work.Reference Healy, Minors and Waterhouse 109 The involvement of psychiatric disorders is due to the consequences that sleep deprivation has on the monoaminergic system.Reference Wirz-Justice 110 For example, monoamine oxidase A transcription is regulated by some clock components.Reference Hampp, Ripperger and Houben 111 Environmental circadian disruption alternating clock-genes expression could modulate neurotransmitters system, potentially leading to depressed mood. Moreover, exposure to unnatural light at night alter neurotrophin and neurotransmitter systems as demonstrated among mice exposed to 4 weeks of dim light at night which showed depressive symptoms and lower brain-derived neurotrophic factor mRNA in the hippocampus than mice exposed to a typical light–dark cycle.Reference Fonken and Nelson 112 Moreover, the disruption of circadian rhythms plays an important role even in generating organic health problems. For example, the WHO recently referred to shift work as a probable carcinogen.Reference Stevens, Hansen and Costa 113 In Denmark, for example, it is recognized a reward for those women who developed breast cancer after working night shifts.Reference Noone 114 In conclusion, further studies are needed to better know the artificial light effects on human health.
Noise pollution
Noise represents the most frequent stressor and is caused by work environment and house hold appliances, planes, and city traffic.Reference Ravindran, Rathinasamy and Samson 115 Acoustic pollution could be defined as an indirect pollutant because essentially its consequences on mental health are mediated by generating stress even if there may be some direct biological mechanisms that involve CNS tissues. Children living in noisy areas reported reduction of problem solving, impaired hearing, poor reading, and frustration.Reference Cohen 116 In fact, noise affects neurotrasmitters level in different parts of the brain, impairs cognition and memory, increases plasma levels of corticosteroids, decreases dendritic count. 117–122
About the relationship between environmental noise and schizophrenia Tregellas et alReference Tregellas, Smucny and Eichman 123 reported that hippocampal hyperactivity, determined by environmental noise, may cause a reduced recruitment of attention networks in schizophrenia. The effects of environmental noise on cognition in schizophrenia have been analyzed by Wright et al in 2016.Reference Wright, Peters and Ettinger 124 Cognitive impairment, especially in executive function domains, memory, and attention are frequently present and linked to low functional outcomes in schizophrenia. In healthy adult population, environmental noise damages many cognitive domains, including those compromised in schizophrenia. It has been demonstrated that noise has negative effects on the working memory and verbal domains in psychotic patients as well as healthy participants. This may add more distress and lead to poorer outcome in schizophrenia patients with pre-existing comprised cognition. The role of noise in clinical environments has been studied by Brown et alReference Brown, Lam and van Kamp 125 with particular interest for the mental health. All clinical environments have a “soundscape.” This includes noises from machines, the rhythms of the day and the activities of other people in the hospital. Distracting sound may have a negative effect especially on those who are more susceptible, yet this does not necessarily mean that silence is better than noise!
Environmental catastrophes
The relationship between environmental catastrophes due to human action and consequences on mental health is a very difficult topic to deal with. Loss of pipelines, damage to oil extraction platforms, contamination of forests and ecosystems may produce enormous psychological distress. Data on these disastrous events are few. The damage is not only due to direct pollution by the polluting agents, but also indirect pollution due to the sense of irreparable catastrophe and injustice. This sense of injustice is due to both the negligence of the control systems and the vastness of the destruction. Mental health assessment after the gulf coast oil spill have been analyzed by Buttke et al.Reference Buttke, Vagi and Bayleyegn 126 On April 20, 2010, 40 miles south of the coast of Louisiana in the United States, the mobile offshore drilling unit Deepwater Horizon exploded. The oil released by Deepwater Horizon has damaged the tourism industries of the Gulf coast, fishing as well as physical and mental health for those affected or exposed to the oil spill. Following an oil spill, however, longitudinal studies indicate that the subsequent psychiatric effects can be more widespread than physical health outcomes.Reference Chung and Kim 127 , Reference Sabucedo, Arce and Senra 128 In fact, these studies have demonstrated higher levels of depression, anxiety, and stress in individuals and communities either exposed to oil, or financially impacted by the spill, than in communities not exposed. People interviewed were divided in three groups reporting, respectively, depressive symptoms, symptoms consistent with an anxiety disorder, and other reporting 14 or more mentally unhealthy days within the past 30 days. A cluster sampling methodology was used to evaluate the mental health status of coastal residents in the Gulf Coast counties in Mississippi at fifth month after the oil spill, in three counties in Alabama 4 months following the 2010 Deepwater Horizon Oil Spill (DWHOS). The proportion of respondents reporting negative mental health parameters in the affected Alabama and Mississippi coastal communities is bigger than the proportion reported in the 2008 and 2009 Behavioral Risk Factor Surveillance System state, indicating that the public health response to the DWHOS should concentrate on mental health services in these communities. Rung et alReference Rung, Gaston and Robinson 129 analyzed domestic conflict, mental distress, and depression among Louisiana Women exposed to the DWHOS in the WATCH Study. A part of them reported symptoms of depression, others severe mental distress, others an increase in the number of fights with their partners, and a small part an increase in the intensity of partner fights. Both economic and physical exposure were significantly associated with depressive symptoms, whereas only physical exposure was related to mental distress. Mental health effects of DWHOS on residents in heavily affected areas have been studied also by Osofsky et al.Reference Osofsky, Osofsky and Hansel 130 The greatest effects on mental health were related to the extent of disruption to participants’ social engagement, family, work, lives with increased symptoms of post-traumatic stress, depression, and anxiety. Given the location of the oil spill hitting communities that had been destroyed by Hurricane Katrina, results also showed that losses from Hurricane Katrina were linked to negative mental health outcomes. The mental health consequences of disasters, including oil spills, have also been analyzed by Rung et al.Reference Rung, Gaston and Oral 131 The social capital and social support were studied and also the effects of exposure to the DWHOS on depression among women. Data for the analysis come from a longitudinal study of the health effects of women exposed to the oil spill in southern Louisiana, United States. Women were interviewed about social support, cognitive social capital (sense of community and informal social control), structural social capital (neighborhood organization participation), depression symptomatology, and their exposure to oil spill. It was demonstrated that structural social capital was linked to increased levels of cognitive social capital, which had as consequence higher levels of social support and then lower levels of depression. Social capital and social support appeared beneficial for depression postoil spill; however, they were themselves negatively damaged by the oil spill. Social capital and social support are coping-resources that are helpful for depression postdisaster. The findings indicate that social resources can be changeable and can be harmed by disasters, conditioning a population’s degree of depression. Responses and resilience of people and communities impacted by the DWHOS have been studied by Glenn Morris.Reference Glenn Morris, Grattan and Mayer 132 Significant social and economic impact in all the four communities, regardless of distance from the site of DWHOS, has been showed. High levels of stress and concern related to the uncertainty of the long-term economic and ecological impacts were commonly noticed by respondents. Mental health problems continue to be present, and in some cases are worsening for residents of northeastern Gulf Coast communities 1 year after the DWHOS. Some of the anxious symptomatology, particularly hyperarousal, may also be linked to media exposure.
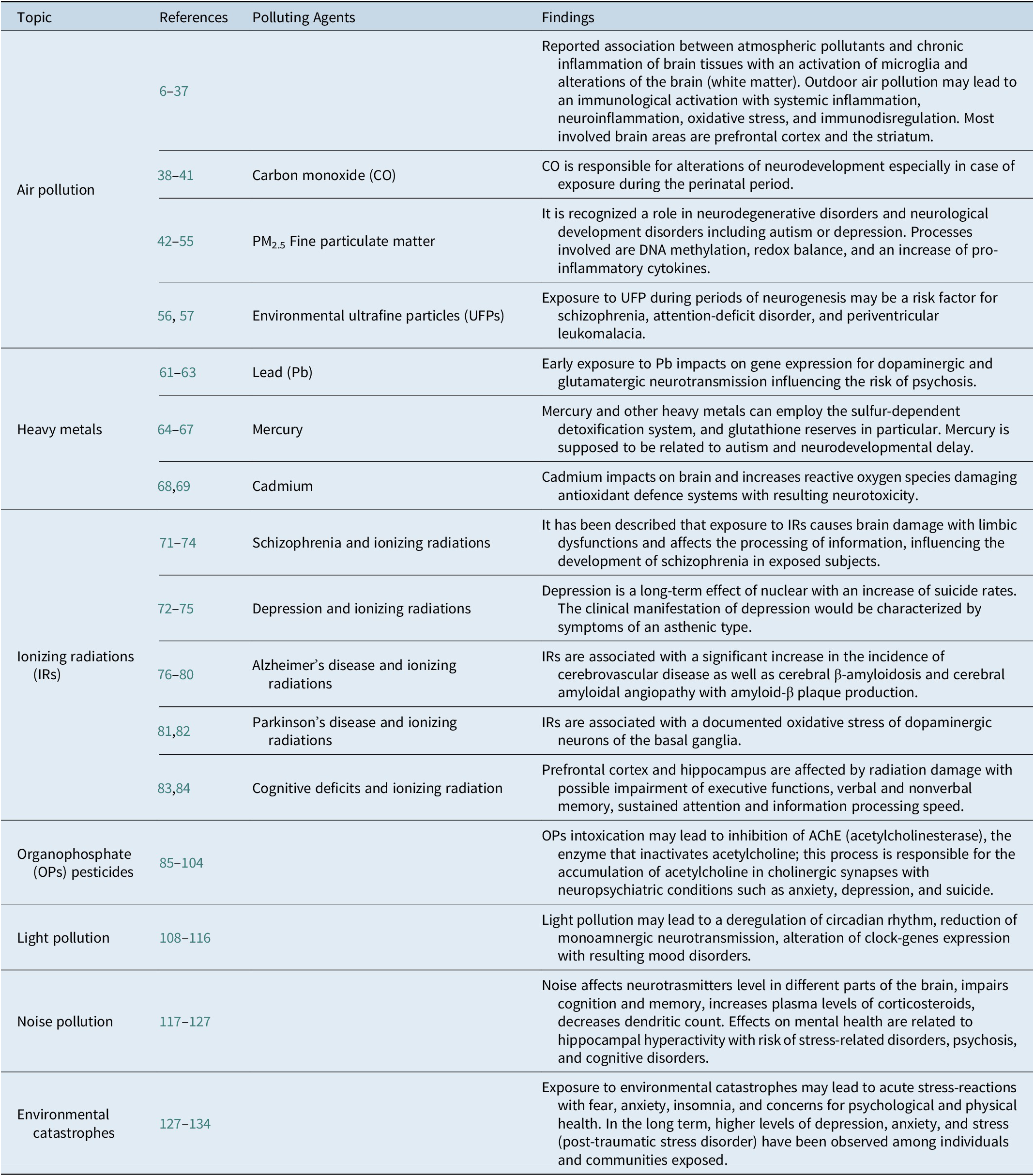
All findings of this narrative review are reported and summarized in the following Table 1.
Table 1. Polluting Agents and their Putative Effects on Human Central Nervous System and Mental Health.
Conclusions
Pollution and mental health is a relevant but poorly described topic. This narrative review aimed to summarize available evidences on sources of pollution and correlation with psychopathology. Polluting elements considered were air pollutants, heavy metals, IR, organophosphate pesticides, light pollution, noise pollution, and environmental catastrophes. It has been highlighted how the effects on the individuals and on the collectives can be direct on the tissues (eg, on the CNS tissue) or indirect, mediated by stress or stigma following the exposure to the known pollutant. This is a relatively new field of studies and more research is needed to understand how direct and indirect effects of pollutants may damage groups and can lead to mental health disorders. For most of described pollutants, data are nonconclusive. Limitations of this review may include the fact that most of evidences come from retrospective studies with a lack of longitudinal studies, and there may be a possible role of socioeconomic status as a mediator of some described effects of pollution on mental health in different layers of population. Further studies should clarify how industrial production, the exploitation of certain resources, the proximity to waste and energy residues, noise and the change in lifestyles are connected with psychological distress and mental health problems for the affected populations.
Disclosure
The authors have nothing to disclose.



