Background
Central nervous system (CNS) infections are uncommon events characterized by a significant mortality and morbidity. Clinical conditions may differ on the localization, etiology, and patients’ age and comorbidities. Even with an intensive diagnostic workout, a significant proportion of cases remain without causative agent: this percentage is higher in subjects with encephalitis where 30–50% remain undiagnosed. CNS-affecting pathogens and opportunistic agents use different pathways to enter the CNS and they may have a preference for certain cell types. The identification of specific plasma or cerebrospinal fluid (CSF) biomarkers may be relevant for diagnosing certain CNS disease and, potentially, to predict patients’ outcomes even in the absence of a known causative agent.Reference Reiber and Peter1, Reference Reiber and Reiber2, Reference Reiber3 Different studies have already shown an involvement of some neuromarkers in certain CNS infections: an increase in CSF Total tau (T-tau) protein in HHV-6 encephalitis (Tanuma et al. 2014),Reference Tanuma, Miyata and Nakajima4 an elevation of IL-6 and neopterin during herpetic encephalitis (Bociąga-Jasic et al. 2011),Reference Bociąga-Jasik, Cieśla, Kalinowska-Nowak, Skwara, Garlicki and Mach5 a reduction of S-100β levels in VZV CNS infections (Grahn et al. 2013),Reference Grahn, Hagberg, Nilsson, Blennow, Zetterberg and Studahl6 and increased levels of 14.3.3 protein in bacterial meningitis have been reported. The time course of CSF 14.3.3 protein was reported to be a useful prognostic marker in patients with bacterial meningitis: its clearance provided real-time information about the ongoing infection and represented an early favorable prognostic marker (Bonora et al. 2003).Reference Bonora, Zanusso and Raiteri7 Aim of this analysis was to study the CSF concentrations of neuronal damage, β-amyloid deposition, astrocytosis, and immune activation in several CNS-affecting conditions.
Methods
We collected CSF samples from patients among hospitals of Turin and Ivrea between 2000 and 2016, undergoing lumbar puncture (LP) for clinical reasons. Patients signed a written informed consent for CSF withdrawal, storage, and analysis; the protocol received ethics approval (Comitato Etico Interaziendale Città di Torino).
These patients were firstly sorted by clinical features (meningitis, meningoencephalitis, meningomyelitis, and abscess), then by etiology (enterovirus, herpesvirus, Neisseria meningitidis, Streptococcus pneumoniae, other etiological agents, and unknown etiology), and then compared with two control groups; the first one is made up of 15 subjects, undergone a LP for diagnostic evaluation, and proved negative for infective involvement of CNS and the other comprising 70 HIV-positive individuals taking no antiretroviral therapy, without CNS infections or neoplasms, whose CSF was analyzed to monitor CNS involvement due to HIV infection. Samples were analyzed for blood–brain barrier (BBB) indexes of permeability (CSF to serum albumin ratio or CSAR), inflammation (CSF to serum IgG ratio, neopterin), amyloid deposition (1–42 β-amyloid), neuronal damage [T-tau, phosphorylated tau (P-tau), 14.3.3], and astrocyte damage (S-100β). Quantitative determination of albumin in serum and CSF was measured by Immunoturbidimetric methods (AU 5800. Beckman Coulter, Brea, CA. USA), 14.3.3 protein was measured by immunoenzymatic methods (ELISA) (Santa Cruz Biotechnology), CSF-tau, P-tau, and 1–42 β-amyloid were measured by immunoenzymatic methods (Fujirebio diagnostics, Malvern, PA, USA). Neopterin and S-100β were measured through validated ELISA methods [DRG Diagnostics (Marburg, Germany) and DIAMETRA Srl (Spello, Italy), respectively]. Reference values were as follows: CSAR [<6.5 (up to 35 years)], IgG ratio (<0.7), 14.3.3 protein (normally absent), T-tau [<300 pg/ml (in patients aged 21–50), <450 pg/ml (in patients aged 51–70), or <500 pg/ml (in older patients)], P-tau (<61 pg/ml), 1–42 β-amyloid (>500 pg/ml), neopterin (<1.5 ng/ml), and S-100β (<380 pg/ml). Data were analyzed using standard statistical methods: variables were described with medians [interquartile ranges (IQR) or ranges (minimum–maximum)], and they were compared using non-parametric tests (Mann–Whitney, Chi-square and Spearman’ s tests as specified in the text). Data analysis was performed using SPSS software for Mac (version 22.0. IBM Corp).
Results
Two hundred and eighty-one patients were included; their demographic features are shown in Table 1.
TABLE 1. Clinical and demographic features of study participants
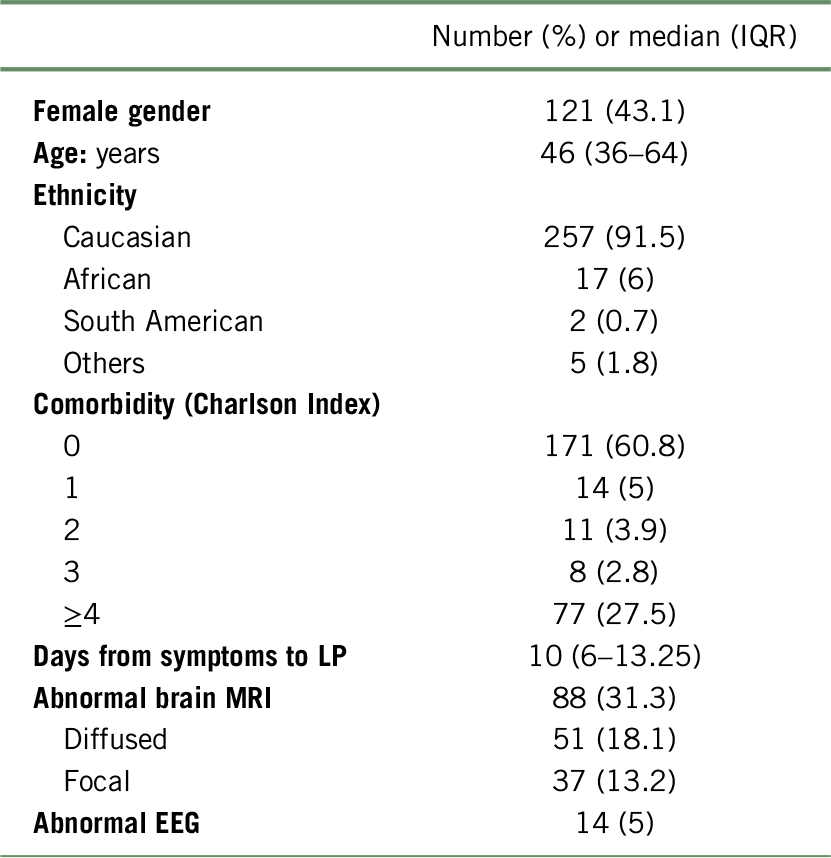
IQR, interquartile range; MRI, magnetic resonance imaging; EEG, electroencephalography
TABLE 2. Clinical, demographic, and laboratory features of study participants

MRI, magnetic resonance imaging; EEG, electroencephalography; IQR, interquartile range; CSAR, CSF to serum albumin ratio.
According to clinical features, patients’ presentations were divided into meningitis (n = 112), meningoencephalitis (n = 78), meningomyelitis (n = 4), abscess (n = 2), a control group (n = 15), and HIV+ patients (n = 70). The most commonly isolated pathogens were herpesvirus (n = 62) enterovirus (n = 32), S. pneumoniae (n = 1), N. meningitidis (n = 5), and others [n = 16 (abscess n = 2, Haemophilus n = 3, Salmonella n = 1, Listeria n = 5, Actinobacillus ureae n = 1, Toscana virus n = 2, and Cryptococcus n = 1] (Their features listed in Table 2). In 60 patients no causative agent was identified, maybe due to empiric treatments, poor sensitivity of the tests, the limited amount of pathogens identified by non-metagenomic approaches, as well as the presence of CNS auto-immune disease (not available for all patients). Considering the clinical features, median age appears slightly lower in meningomyelitis group (40.5 y.o.) and higher in meningoencephalitis group (61.5 y.o.); regarding the etiology, median age is far below the range in enterovirus group (28 y.o.) instead herpesvirus group has the highest value (64 y.o.). Both CSAR and IgG ratios have been assessed in 139 patients and their median values are higher in the other etiologies’ group (13 and 12.8). Interestingly despite ongoing inflammation, we were not able to observe significant BBB damage in herpesvirus infections group. Protein 14.3.3 has been evaluated in 103 subjects based on three variables: negative, slightly positive, and positive. A higher prevalence of positive results was observed in samples from patients affected by bacterial and HSV encephalitis (Figure 1). A linear correlation between IgG ratio and 14.3.3 protein has been found, suggesting possible false positivity results due to cross-reaction of the anti-14.3.3 antibody to immunoglobulins’ structure.

FIGURE 1. CSF 14.3.3 positivity according to etiologies.

FIGURE 2. CSF to serum albumin ratio (left) and S-100β levels according to the etiology groups; the small box represents S-100β concentrations in patients with either HSV or VZV encephalitis. The dotted line represents conservative estimates of “normal” ranges (CSAR<8 and S-100β < 380 pg/ml).
T-tau protein has been evaluated in 147 CSF samples and the highest value can be found in patients with herpesvirus infections (755.7 pg/ml): higher levels were observed in those who received an LP within 7 days from symptoms appearance (in patients with HSV encephalitis, a median value of 1200 pg/ml in the early group versus 311.4 pg/ml, p < 0.001 can be found). P-tau protein has been assessed in 124 subjects and its higher values can be found in enterovirus group (108.6 pg/ml). 1–42 β-amyloid (Aβ1–42), measured in 111 patients, results lower, but above reference value, in pneumococcus group (566 pg/ml). Neopterin, evaluated in 154 subjects presents higher values in herpesvirus (9 ng/ml) groups. Within the herpesvirus group, CSF S-100β levels were compared between subjects with infection due to HSV and VZV: results show higher values in HSV group (median of 548 pg/ml) compared to the other one (280.5 pg/ml) p < 0.05.
An interesting group is represented by subjects without a clear etiologic diagnosis (n=60). This group shows uniform sex distribution (45% females) and median age of 45 years (36–65.5). This group also shows impaired neuroimaging in 28.3% and impaired EEG in 18.3%. Of these subjects 18.3% show BBB alteration. Meningitis is the most represented clinical presentation (n=38, 63.3%). Always in the unknown etiology group, protein 14.3.3 has been analyzed in 42 samples and in 17 of these (40.5%) show clear positive results. Median T-tau value (183 pg/ml) fell within the normality range, as well as P-tau protein (37 pg/ml), Aβ1–42 (855 pg/ml), and S-100β (212 pg/ml), while neopterin median values were 2.17 ng/ml, which are above the normality range.
Thirty-days mortality was 2.7%, and it was higher in patients with unknown etiology (8.3%). CSF T-tau (median value of 431 pg/ml in the deceased group versus 148 pg/ml in the other one, p = 0.1) and Aβ1–42 (527.3 pg/ml in the first group versus 784.1 pg/ml, p < 0.04) showed different levels in patients who died within 30 days of diagnosis (Figure 3).

FIGURE 3. CSF total tau (left) and 1-42 β-amyloid (right) levels in survivors and in those patients who died within 30 days of diagnosis. The dotted line in the graph on the right represents the threshold for Alzheimer’s dementia diagnosis in older subjects (<500 pg/ml).
Discussion
In this study, carried out on 281 subjects, we measured several CSF biomarkers of neuronal damage and inflammation, the heterogeneous etiologies allowed us to explore potential associations with pathogens that warrant further investigations and to compare the biomarkers in patients without CNS involvement or with a chronic low-level viral infection (such as in HIV-positive individuals).
BBB impairment and IgG synthesis were observed in several infections; CSAR and IgG ratios were higher in the other etiologies’ group (Figure 2). In addition, a linear correlation exists between these markers and tau and neopterin. Clear 14.3.3 positivity was found in bacterial and HSV infections but not in controls nor in HIV-positive patients; this protein accumulates in CSF after neuronal damage especially during bacterial involvement of CNS, and it is cleared from the CFS after some period.Reference Shimada, Fournier and Yamagata8 Bonora et al. found a relationship between bacterial meningitis, 14.3.3 trend, and prognosis, proving that its clearance may be the earliest parameter to provide a favorable prognostic sign.Reference Grahn, Hagberg, Nilsson, Blennow, Zetterberg and Studahl6 The evaluation of this neuromarker could also be important to distinguish between a bacterial and a non-bacterial encephalopathy when the etiology is uncertain.Reference Lu, Chang and Chang9 Nonetheless, antibodies anti-14.3.3 may non-specifically recognize an epitope in CSF IgG, indicating active production of intrathecal antibodies, but a falsely positive 14.3.3 detection. Protein T-tau results higher in herpesvirus group suggesting significant neuronal damage in herpetic encephalitis. In addition, arranging results based on time interval between the beginning of symptoms and the execution of the LP, a sharp increase of this protein can be seen in the first 7 days; for this reason, it is possible to assume an important role of this neuromarker in the early stage of acute herpetic encephalopathy. These data confirm Tanuma’s results which notice an increased level of tau protein and an important role of oxidative stress in HHV-6 encephalitis, suggesting an essential contribution of the neuronal oxidative damage in viral encephalopathy.Reference Reiber3, Reference Mietelska-Porowska, Wasik, Goras, Filipek and Niewiadomska10 In his article, Tanuma also finds no correlation between T-tau levels and prognosis; instead, in this work, T-tau level seems to be higher in patients deceased, but there is no statistical difference, probably due to a lack of samples. Protein P-tau, instead, is more elevated in enterovirus infections, suggesting marked tau phosphorylation activity in enteroviral meningitis. Therefore, an increased level of these two indexes of neuronal damage (T-tau and P-tau) could provide a valid tool to distinguish between viral and bacterial involvement of CNS. These CSF data differ from the laboratory findings in Alzheimer’s disease, where, besides elevated T-tau and P-Tau, a reduced amount of Aβ1–42 can be found.Reference Bloom11, Reference Kumar, Choi and Washicosky12 CNS levels of neopterin denote intrathecal production by microglia, as the BBB has a low permeability for peripheral neopterin, it represents a relevant index of local inflammation.Reference Tanuma, Miyata and Nakajima4, Reference Eisenhut13, Reference Molero-Luis, Fernández-Ureña and Jordán14 This marker shows higher values in herpesvirus group, suggesting an important role in the diagnosis of herpetic encephalitis, as assessed by Bociaga and Eisenhut in their works, and helping clinician to distinguish from other viral involvement of CNS, like enteroviral meningitis in which neopterin presents lower values, maybe for the less cytotoxic damage.Reference Tanuma, Miyata and Nakajima4, Reference Eisenhut13 S100-β is a cytoplasmic calcium-binding protein, abundant in astroglial cells, represents an important marker of astrocytic damage.Reference Eisenhut13, Reference Berdowska and Zwirska-Korczala15 Different articles demonstrate a higher level of this protein in HSV infection rather than in VZV ones, like Grahn’s study.Reference Bociąga-Jasik, Cieśla, Kalinowska-Nowak, Skwara, Garlicki and Mach5 In this article too, it is possible to confirm the result; therefore, S100-β could be a useful item to distinguish between these two viral infections. The group of patients with unexplained etiology is noteworthy: it includes 60 subjects whose CSF sample analysis did not show any pathogen. This could be explained by different reasons, including empiric treatments, poor sensitivity of the tests, and the amount of pathogen identified by non-metagenomic approaches, besides the autoimmune disease of CNS (unavailable for most of the patients). In this case clinical investigation, neuroimaging, antibodies, and specific neuromarkers research can lead diagnosis to set an appropriate immunomodulatory therapy.
The prognostic usefulness of CSF biomarkers might be relevant: our sample size does not allow for definitive conclusions. Nevertheless, the finding of higher T-tau and lower Aβ1–42 concentrations warrant further analysis in controlled settings.
In conclusion, we can state that routine CSF analysis provides the majority of information in CNS infections; nevertheless, new biomarkers could support clinician when a diagnosis is difficult to achieve (long time for microbiological culture, possibility of false-positive and negative results in PCR amplification) or when prognosis is severe and patient’s stratification may allow for prompt intensive treatment. In this heterogeneous cohort of patients with CNS-affecting disorders, we differentiate concentrations of several CSF biomarkers that warrant further analysis for both diagnostic and prognostic evaluations.
Disclosures
The authors declare that they have nothing to disclose.







