Introduction
For the successful treatment of pediatric diseases, delivering medication via formulations that are acceptable to children is a recommended strategy to improve therapeutic outcomes.Reference Ivanovska, Rademaker, van Dijk and Mantel-Teeuwisse 1 Given that many medications are formulated as capsules and tablets, the development of alternative formulations is important because children may prefer options other than solid oral dosage forms.Reference Patel, Jacobsen, Jhaveri and Bradford 2 , Reference Strickley, Iwata, Wu and Dahl 3 This is particularly relevant to attention-deficit/hyperactivity disorder (ADHD), one of the most common pediatric conditions, which often involves children with a variety of overlapping physical, psychological, and neurodevelopmental disorders.
ADHD is a chronic psychiatric disorder characterized by inattention, hyperactivity, and impulsivity.Reference Pliszka 4 – 6 In the US, ADHD has been reported to affect ∼6–8% of children (in comparison to about a 10% prevalence of asthma in school-aged children 7 ) and 4.4% of adults.Reference Kessler, Adler and Barkley 8 , Reference Kessler, Berglund, Demler, Jin, Merikangas and Walters 9 Approximately 95% of ADHD patients are diagnosed before the age of 12, and up to 60% of them continue to meet the diagnostic criteria for ADHD as adults.Reference Kessler, Berglund, Demler, Jin, Merikangas and Walters 9 Selection of an ADHD treatment approach is dependent on a patient’s age and the severity of the disease. 5 , 6 , 10 Behavioral therapies are the first-line option recommended for preschool-aged children, and pharmacologic agents are often the first-line option for school-aged children and adults, particularly for those with moderate to severe impairment. 10 – Reference Wolraich, Brown and Brown 12 Pharmacotherapy has been proven to be very effective in patients of all ages. Practice guidelines recommend the central nervous system stimulants methylphenidate (MPH) and amphetamine (AMP) as first-line ADHD medications because of their proven efficacy in reducing ADHD core symptoms in both children and adults.Reference Pliszka 4 , 5 , Reference Rabito-Alcon and Correas-Lauffer 13 – Reference Mészáros, Czobor, Bálint, Komlósi, Simon and Bitter 15 While some patients may preferentially respond to or tolerate one better than the other, both stimulant classes are considered appropriate first-line interventions until efficacy and tolerability can be assessed in an individual patient.Reference Pliszka 4 Nonstimulant medications are also indicated for treating ADHD, but they are not as effective as stimulants and, therefore, are recommended as a second-line treatment or adjuvant therapy. 5 , Reference Ramos-Quiroga, Bosch and Castells 16
Most ADHD medications are formulated as solid tablets or capsules that need to be taken daily,Reference Ahmed and Aslani 17 which poses a potential challenge for children who experience difficulties swallowing pills or for individuals with autism or other developmental disabilities who may have tactile issues that preclude the use of tablets, capsules, or even sprinkled preparations.Reference Beck, Cataldo, Slifer, Pulbrook and Guhman 18 – Reference Childress, Sallee and Berry 20 For example, ∼46–89% of children with autism-spectrum disorders have feeding disorders, raising significant concerns for ingesting solid oral formulations.Reference Nadon, Feldman and Gisel 21 , Reference Barnhill, Tami, Schutte, Hewitson and Olive 22 Chewing or crushing pills to ease ingestion may alter the pharmacokinetics of the medications and is inadvisable for most stimulants.Reference Parker 23 Although short-acting liquid formulations of stimulants have long existed, 24 long-acting alternative dosage formulations have only recently become available and include liquid suspensions, chewable tablets, and orally disintegrating tablets (ODTs). 25 – 29 Additionally, the only option for a non-oral route of administration is a transdermal-patch system.Reference Warshaw, Squires, Li, Civil and Paller 30 Despite their proven effectiveness, adherence to ADHD stimulants is poor across patients of all age groups for various reasons,Reference Perwien, Hall, Swensen and Swindle 31 , Reference Winterstein, Gerhard and Shuster 32 and difficulty swallowing or an aversion to oral tablets may be a barrier to ADHD treatment.Reference Beck, Cataldo, Slifer, Pulbrook and Guhman 18 , Reference McCarthy 19 Alternative oral dosage forms, especially those in long-acting formulations with reduced dosing frequencies, may ease medication delivery and improve outcomes for patients. However, incorporating a once-daily long-acting formulation into alternative dosage forms can be challenging.
Alternative oral formulations that ease ingestion in children have been successfully established for treating conditions that commonly occur in children, such as bacterial infections, pain, and allergies. Some examples include fruit-flavored liquid amoxicillin, loracarbef, and doxycycline solutions;Reference Gee and Hagemann 33 ibuprofen oral liquid suspension, chewable tablets, and ODTs;Reference Fini, Bergamante, Ceschel, Ronchi and de Moraes 34 , Reference Scott, Retsch-Bogart, Kustra, Graham, Glasscock and Smith 35 and sublingual formulations for allergy and asthma allergen immunotherapy.Reference Cox 36 – Reference Vadlamudi and Shaker 38 Other than ease of ingestion, these formulations may possess additional advantages over their capsule or tablet counterparts, such as a more favorable pharmacokinetic profile or improved safety. Beyond pediatric care, alternative oral dosage forms have also demonstrated benefits in many chronic disease areas—including cardiovascular, dyslipidemia, and chronic pain.Reference Wertheimer, Santella, Finestone and Levy 39 However, most of the alternative dosage forms are short-acting formulations that require multiple daily doses.Reference Strickley, Iwata, Wu and Dahl 3 With advances in technology, long-acting transdermal patches, oral liquid solutions, and ODTs with reduced dosing frequencies are emerging, such as buprenorphine extended-release (ER) transdermal patches, azithromycin oral suspension, and ketoprofen orally disintegrating/sustained-release tablets,Reference Kathpalia, Sule, Patil, Mahadik and Sharma 40 – Reference Pergolizzi, Scholten, Smith, Leighton-Scott, Willis and Henningfield 43 which may further facilitate medication administration.
Alternative Dosage Forms for the Management of Psychiatric Disorders
Alternative oral dosage forms, such as chewable tablets, liquid solutions, sublingual tablets, and ODTs have been developed for the treatment of psychiatric disorders.Reference Keith 44 , Reference Nordstrom and Allen 45 Aside from obviating swallowing difficulties, each oral dosage form provides unique benefits.Reference Strickley, Iwata, Wu and Dahl 3 , Reference Kathpalia, Sule, Patil, Mahadik and Sharma 40 , Reference Keith 44 , Reference Dey and Maiti 46 Liquid agents can be accurately split into a flexible dose, enabling gradual dose adjustment.Reference Kathpalia, Sule, Patil, Mahadik and Sharma 40 The sublingual route of administration avoids first-pass metabolism, resulting in quicker absorption and onset of effect as compared to other oral delivery methods.Reference Nordstrom and Allen 45 The use of ODTs may enhance compliance not only in individuals with swallowing or tactile issues but also in pill-averse patients who willfully refuse medication, as ODTs disintegrate rapidly after administration and prevent surreptitious behaviors, such as cheeking, pouching, or spitting pills out.Reference San, Casillas, Ciudad and Gilaberte 47 The ODT formulation may also reduce misuse of psychotic medications (e.g., stimulants) that can have the potential for abuse, misuse, or diversion.Reference Nordstrom and Allen 45
Different dosage forms may also affect patients’ adherence to their medications. Like many chronic disease areas in which medication discontinuation is common, such as diabetes (adherence=31–87%, depending on the studyReference Johnson 48 ) and hypertension (adherence ∼50%Reference Tajeu, Kent and Kronish 49 ), medication adherence in patients with psychiatric disorders is also suboptimal, ranging from 40 to 60%.Reference Kane, Kishimoto and Correll 50 , Reference Patel and David 51 Psychiatric medications in liquid, sublingual, or ODT forms improve adherence compared with standard tablets.Reference Patel and David 51 In addition, simplifying medication regimens has a positive effect on adherence in patients with psychiatric disorders.Reference Burton 52 , Reference Velligan, Weiden and Sajatovic 53 Long-acting formulations have been shown to enhance medication adherence in the management of depression,Reference Nemeroff 54 Parkinson’s disease,Reference Antonini and Calandrella 55 and dementia.Reference Herrmann, Binder, Dalziel, Smyth and Camacho 56
The benefits of ODTs in psychiatric treatment have been documented in studies of olanzapine, an antipsychotic medication indicated for the treatment of schizophrenia and bipolar I disorder.Reference San, Casillas, Ciudad and Gilaberte 47 Clinical studies indicate that olanzapine formulated as an ODT is as effective as the standard tablet formulation,Reference Bitter, Treuer and Dilbaz 57 – Reference Karagianis, Grossman and Landry 59 but 61% of patients opted for ODTs versus 27% for standard tablets in a study, suggesting that ODTs are a patient-preferred formulation.Reference Bitter, Treuer and Dilbaz 57 Furthermore, olanzapine ODT has significantly improved medication adherenceReference Karagianis, Grossman and Landry 59 and reduced caregiver burden because of high patient acceptance.Reference Czekalla, Wagner, Schacht, Kluge and Kinon 58 – Reference Kinon, Hill, Liu and Kollack-Walker 60 Other examples of alternative oral dosage forms in psychiatric treatment include risperidone and aripiprazole ODTs for treating schizophrenia and bipolar disorder,Reference Nordstrom and Allen 45 risperidone oral solution for treating schizophrenia,Reference Kusumi, Honda and Ito 61 mirtazapine ODT for treating depression,Reference Danileviciute, Sveikata, Adomaitiene, Gumbrevicius, Fokas and Sveikatiene 62 asenapine sublingual tablets for treating schizophrenia and bipolar mania, 63 and benzodiazepine (e.g., alprazolam, lorazepam, and clonazepam) ODTs for treating anxiety, depression, and panic disorder.Reference Erdman, Stypinski, Combs, Witt, Stiles and Pollock 64 – Reference Susman and Klee 66 ODTs have consistently demonstrated advantages of easy ingestion and high patient preference across different classes of medications.Reference Danileviciute, Sveikata, Adomaitiene, Gumbrevicius, Fokas and Sveikatiene 62 , Reference Erdman, Stypinski, Combs, Witt, Stiles and Pollock 64 , Reference Susman and Klee 66
Formulations of Stimulants for Treating ADHD
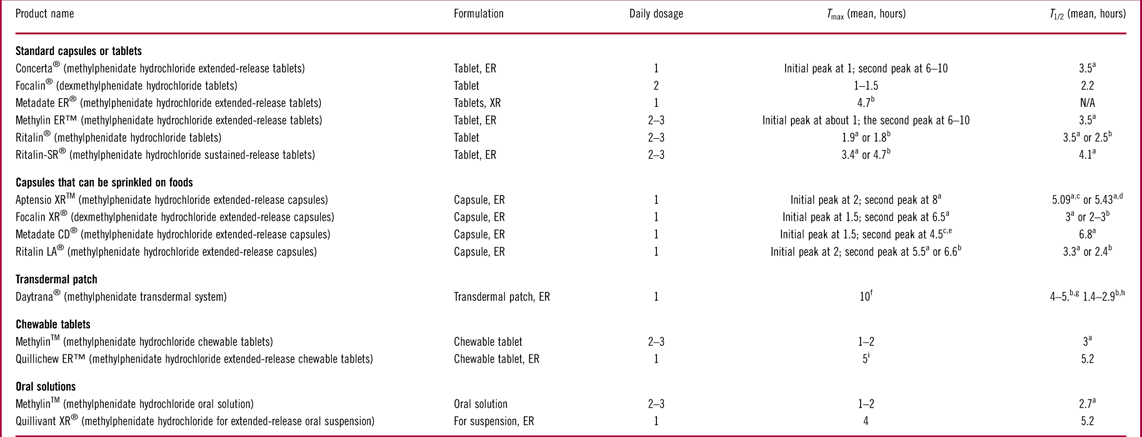
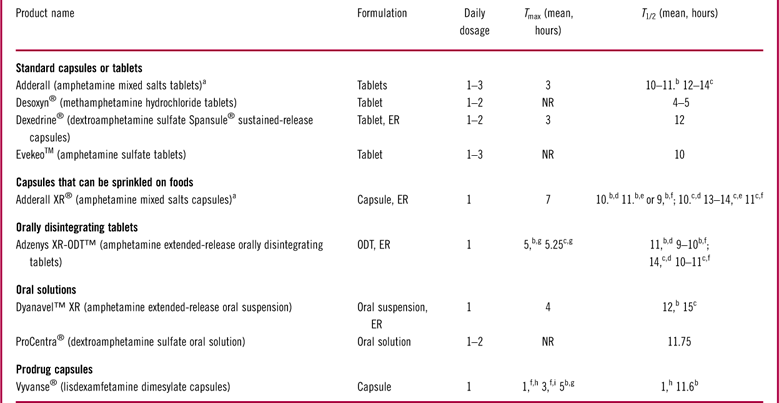
The success of alternative oral formulations has been well-established in psychiatric treatment, but alternative oral formulations for ADHD medications are still limited. The stimulants for ADHD treatment, methylphenidate and amphetamine, are available in a variety of formulationsReference Gajria, Lu and Sikirica 67 (Tables 1 and 2). Although methylphenidate and amphetamine have demonstrated similar efficacies at the population level, individual patients may respond better to one or the other.Reference Pliszka 4 , 5 Thus, the choice as to which stimulant to prescribe depends on factors such as treatment history and patient and prescriber preference.Reference Rabito-Alcon and Correas-Lauffer 13 , Reference Lopez and Leroux 68 Owing to their short halflives, first-generation stimulants were short-acting (or immediate release) medications.Reference Greenhill, Abikoff and Arnold 69 Short-acting methylphenidate and amphetamine typically take effect within 30 minutes after dosing, with durations of action ranging from 3 to 6 hours.Reference Antshel, Hargrave, Simonescu, Kaul, Hendricks and Faraone 70 – Reference Olfson 72 Thus, they require 2 to 4 doses a day to provide 12-hour symptom control.Reference Antshel, Hargrave, Simonescu, Kaul, Hendricks and Faraone 70 , Reference Buitelaar and Medori 71 Despite the proven effectiveness of short-acting stimulants,Reference Greenhill, Abikoff and Arnold 69 , Reference Olfson 72 , Reference Swanson, Wigal and Greenhill 73 the need for multiple daily dosing may lead to fragmented treatment coverage, missed doses, and social stigma for school-aged children, along with liability concerns regarding in-school medication storage due to dosing requirements during school hours.Reference Olfson 72 , Reference Swanson 74 , Reference Wolraich, Greenhill and Pelham 75
Table 1 Summary of short-acting and long-acting methylphenidate 24 - 26 , 29 , Reference Maldonado 76 , 103 , 104 , Reference Focalin 106 , 107 , 109 , 111 , 115 – 119

ER=extended release; N/A=not applicable; T 1/2=halflife; T max=time to peak plasma concentration.
a In adults.
b In children.
c Capsule.
d Sprinkle.
e 25–30% of subjects had only one observed peak.
f 8 after repeat patch applications when worn up to 9 hours.
g d-methylphenidate.
h l-methylphenidate.
i Median.

ER=extended release; NR=not reported; ODT=orally disintegrating tablets; T 1/2=halflife; T max=time to peak plasma concentration.
a Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate.
b d-amphetamine.
c l-amphetamine.
d In adults.
e In adolescents.
f In children.
g Median.
h Lisdexamfetamine.
i Dextroamphetamine.
Long-acting (or ER) methylphenidate and amphetamine formulations have been developed to overcome the issues caused by frequent dosing associated with short-acting stimulants. Some long-acting stimulants contain two compartments or different populations of beads that release medication at different speeds, providing both rapid onset of action and long-lasting treatment effects; while others are prepared in a controlled-release formulation that delivers the active ingredient under osmotic pressure at a controlled rate throughout the day.Reference Maldonado 76 , Reference Punja, Zorzela, Hartling, Urichuk and Vohra 77 In addition, one long-acting amphetamine formulation is supplied as an inactive prodrug, lisdexamfetamine, which is converted to active amphetamine after being absorbed from the gastrointestinal tract.Reference Maneeton, Maneeton and Likhitsathian 78 Long-acting stimulants are designed for patients to take once daily in the morning, with symptom control lasting for 8 to 12 hours.Reference Lopez and Leroux 68 , Reference Olfson 72 , Reference Maldonado 76 Clinical studies have demonstrated that once-daily long-acting stimulants are as effective as multiple-dosed short-acting stimulants in reducing ADHD core symptoms and improving cognitive skills in both children and adults.Reference Wolraich, Greenhill and Pelham 75 , Reference Adler, Zimmerman and Starr 79 – Reference Findling, Quinn, Hatch, Cameron, DeCory and McDowell 81 Insurance claim data suggest that ADHD children who received long-acting stimulants were less likely to visit an emergency department or become hospitalized than those receiving short-acting stimulants.Reference Kemner and Lage 82 Compared with short-acting stimulants, the advantages of long-acting stimulants include simpler dosing, reduced stigma related to medication use, reduced burden on schools regarding medication storage, and improved adherence.Reference Buitelaar and Medori 71 , Reference Feldman and Bélanger 83 – Reference Christensen, Sasané, Hodgkins, Harley and Tetali 85 In addition, long-acting stimulants have a smoother pharmacokinetic profile, with reduced fluctuation in plasma medication concentrations compared with their short-acting counterparts, which may prevent euphoria and rebound effects.Reference Buitelaar and Medori 71 Furthermore, stimulants are controlled substances that have the potential for diversion and abuse.Reference Modesto-Lowe, Chaplin, Sinha and Woodard 86 , Reference Wilens, Adler and Adams 87 Long-acting stimulants are associated with lower rates of misuse or abuse than their short-acting counterparts because their use does not correlate as strongly with euphoria and it is more difficult to extract active drug ingredients.Reference Feldman and Bélanger 83 , Reference Wilens, Adler and Adams 87 , Reference Barner, Khoza and Oladapo 88 Several long-acting alternative formulations of stimulants have recently become available, including liquid suspensions, chewable tablets, and ODTs. 25 , 26 , 29 While the extent to which a formulation affects adherence to ADHD medication regimens remains to be investigated, the potential for better treatment individualization through the development of alternative dosage forms may improve outcomes in patients with ADHD.
Poor medication adherence in ADHD treatment
Analyses of insurance claims have revealed that medication adherence rates among ADHD patients are about 50% in the first year, but the rates decrease considerably over time (32.8% at 2 years; 17.2% at 5 years).Reference Winterstein, Gerhard and Shuster 32 On average, children and adults only strictly adhere to their initially prescribed dosing regimens for 34.2 and 49.5 days, respectively, although they may continue with their medications on compromised schedules or doses for approximately 200 days.Reference Perwien, Hall, Swensen and Swindle 31 These studies were based on claim data from 2008 or earlier, when most ADHD medications were likely to be short-acting agents. Later analyses of data, including both short- and long-acting ADHD medications, demonstrated an approximate 24–30% increase in adherence for long-acting medications compared with their short-acting counterparts.Reference Lachaine, Beauchemin, Sasane and Hodgkins 84 , Reference Christensen, Sasané, Hodgkins, Harley and Tetali 85 The reasons for poor medication adherence in ADHD include side effects, ineffectiveness, lack of insight into the disease, dosing inconvenience, social stigma, and costs.Reference Gajria, Lu and Sikirica 67 Specific reasons for medication nonadherence vary among patients of different age groups. Children often discontinue medications under their parents’ or caregivers’ discretion because of side effects, high medication costs, denial of illness, and difficulty swallowing.Reference Ahmed and Aslani 17 , Reference Gajria, Lu and Sikirica 67 , Reference Brinkman, Sherman and Zmitrovich 89 Together, the need for school-aged children, especially those taking short-acting stimulants, to go to the nurse’s office for dosing during school hours, which can lead to unwanted social stigma, and liability issues related to school storage of controlled medications may discourage medication adherence.Reference Buitelaar and Medori 71 , Reference Swanson 74 , Reference Wolraich, Greenhill and Pelham 75 Parents and caregivers play a critical role in medication adherence.Reference Ahmed and Aslani 17 A family history of ADHD (especially parental) is associated with decreased adherence,Reference Ahmed and Aslani 17 possibly because of ADHD symptoms in the adult, such as forgetfulness and difficulty managing the child’s medication regimen. Fears about the long-term risks or drug abuse associated with stimulant use may be another reason that affects adherence,Reference Ahmed and Aslani 17 , Reference Swanson 74 , Reference Brinkman, Sherman and Zmitrovich 89 although these risks are low when medications are used appropriately. Disagreement between parents about their children’s need for medication could also impact medication use.Reference Brinkman, Sherman and Zmitrovich 89 This may be particularly relevant for children with ADHD who split time with their separated or divorced parents, only one of whom supports use of the medication. Additionally, perceiving ADHD as a parenting problem rather than as a disease with biological origins may lead to resistance to medication therapy and inadequate parental supervision of medication adherence.Reference Gau, Chen and Chou 90 , Reference Peters and Jackson 91
Adolescents are generally less willing to use ADHD medications than children and adults because of their growing autonomy and rebelliousness.Reference Ahmed and Aslani 17 , Reference McCarthy 19 , Reference Bussing, Koro-Ljungberg, Noguchi, Mason, Mayerson and Garvan 92 , Reference Charach and Fernandez 93 According to interviews with ADHD patients between ages 11 and 16 years, some patients felt that medications deprived them of their self-identity and “gained control over them.”Reference McCarthy 19 As adolescents become older and more involved in treatment decision making, they may deliberately stop taking their medications.Reference McCarthy 19 Other common reasons for medication discontinuation in this population include high medication costs, denial of illness, lack of insight, and forgetfulness.Reference McCarthy 19 , Reference Gajria, Lu and Sikirica 67 , Reference Bussing, Koro-Ljungberg, Noguchi, Mason, Mayerson and Garvan 92 , Reference Taddeo, Egedy and Frappier 94
Among adults with ADHD, side effects, high medication costs, and forgetfulness are the most commonly reported reasons for medication nonadherence.Reference McCarthy 19 , Reference Gajria, Lu and Sikirica 67 Additionally, lack of insight—not viewing ADHD as a disease with a biological basis—and negative attitudes toward pharmacotherapy, such as fear of side effects and loss of authentic self, may also prevent patients from adhering to their medication regimens.Reference McCarthy 19 , Reference Gajria, Lu and Sikirica 67 , Reference Charach and Fernandez 93
Using long-acting stimulants improves medication adherence. Clinical studies based on patient self-reports, pill counts, and claims analyses have shown that long-acting stimulants significantly improve adherence in both children and adult patients with ADHD compared to short-acting stimulants.Reference Ahmed and Aslani 17 , Reference Gau, Chen and Chou 90 , Reference Adler, Lynch and Shaw 95 – Reference Tzang, Wang and Yeh 97 For nonadherent ADHD patients who have been on short-acting medications, switching to long-acting formulations enhances medication adherence.Reference Ramos-Quiroga, Bosch and Castells 16 , Reference Chou, Chou and Tzang 98 The once-daily long-acting formulations simplify dosing regimens and avoid the social stigma caused by administration in public or at school. The convenience and improved patient acceptance offered by long-acting stimulants may account for the increased adherence.Reference Palli, Kamble, Chen and Aparasu 96
Difficulty swallowing
Difficulty swallowing represents a significant barrier to pharmacotherapy.Reference Marquis, Schneider and Payot 99 , Reference Schiele, Quinzler, Klimm, Pruszydlo and Haefeli 100 A wide variety of medical conditions, such as stroke, cancer, neurological disorders, connective tissue diseases, and gastroesophageal reflux disease, can lead to swallowing difficulties.Reference Palmer, Drennan and Baba 101 In addition, many adults without conditions affecting their ability to swallow have difficulty swallowing and express a preference for formulations that eliminate swallowing pills or capsules.Reference Patel, Jacobsen, Jhaveri and Bradford 2 , Reference Schiele, Quinzler, Klimm, Pruszydlo and Haefeli 100
Pediatric patients are more likely to encounter difficulties in swallowing solid dosage forms than adults.Reference Patel, Jacobsen, Jhaveri and Bradford 2 , Reference Schiele, Quinzler, Klimm, Pruszydlo and Haefeli 100 A survey of 304 parents with children aged 0 to 26 years (less than 1% over age 21) reported that more than 50% of the children were unable to swallow a standard-sized pill or a small capsule.Reference Patel, Jacobsen, Jhaveri and Bradford 2 , Reference Polaha, Dalton and Lancaster 102 In addition to physical inabilities, children may refuse to ingest pills because of conditioned anxiety associated with repeated negative experiences, such as aversive medication taste and fear of gagging.Reference Beck, Cataldo, Slifer, Pulbrook and Guhman 18 Parents may resort to cajoling, threatening, or crushing and mixing medications in food to improve adherence, potentially altering medication pharmacokinetics and negatively affecting efficacy and safety and causing distress in children.Reference Beck, Cataldo, Slifer, Pulbrook and Guhman 18 Adults who experience difficulties swallowing often complain about large pill size, sticky pill texture, and bad taste or smell of medications.Reference Marquis, Schneider and Payot 99 , Reference Schiele, Quinzler, Klimm, Pruszydlo and Haefeli 100 Common approaches used to address this issue include drinking more water, opening capsules, splitting or crushing pills, mixing contents with food, tilting the head backward when swallowing, and switching to a different dosage form.Reference Marquis, Schneider and Payot 99 , Reference Schiele, Quinzler, Klimm, Pruszydlo and Haefeli 100 However, crushing tablets and capsules may result in inaccurate dosing.Reference Schiele, Quinzler, Klimm, Pruszydlo and Haefeli 100
Difficulty swallowing is a common issue in treatment and can affect medication adherence in ADHD patients of all ages;Reference Swanson 74 therefore, several techniques have been developed to ease pill ingestion. Some stimulant dosage forms allow patients to open the capsule and sprinkle the contents on applesauce (Metadate CD®, Ritalin LA®, Adderall XR®, Aptensio XRTM, Focalin XR®) or in yogurt, water, or orange juice (Vyvanse®). 27 , 103 – 107 However, to ensure effectiveness and dosage accuracy, the entire contents of the capsule need to be ingested without being chewed. 103 – 105 Among children with ADHD or autism, swallowing training by encouraging the swallowing of mock pills of increasing size has resulted in successful pill ingestion.Reference Beck, Cataldo, Slifer, Pulbrook and Guhman 18 In other pediatric disease areas, such swallowing exercises as modeling-based behavioral intervention, swallowing technique instructions, and coating the back of the mouth and tongue with flavored spray have demonstrated effectiveness.Reference Patel, Jacobsen, Jhaveri and Bradford 2 These strategies may also reduce barriers to ADHD treatment.
The need for treatment individualization
Evidence-based ADHD guidelines recommend that medication regimens should be tailored to the individual, as each patient may differ in their response to or tolerability of distinctive medications.Reference Lopez and Leroux 68 In efforts to optimize patient outcomes, clinicians should consider both medication class (stimulants vs. nonstimulants and types of stimulants/nonstimulants) and formulation.Reference Lopez and Leroux 68 The selected formulation should not only provide effective pharmacokinetic and pharmacodynamic profiles but also satisfy the patient’s/family’s preferences. For example, patients who frequently travel may opt for a dosage form that is easy to carry and can be taken without water.Reference San, Casillas, Ciudad and Gilaberte 47 In addition, practice guidelines recommend medication dose titration for individual ADHD patients to achieve an optimal dose with minimal side effects while retaining therapeutic effectiveness. 5
Overview of New Stimulant Formulations
In ADHD management, multiple medication and formulation options better enable treatment individualization, leading to more favorable responses and increased patient satisfaction. Because the majority of treated ADHD patients are children who may be more sensitive to medication texture and tastes and dislike certain flavors,Reference Strickley, Iwata, Wu and Dahl 3 , Reference Squires, Lombardi, Sjostedt and Thompson 108 selection of a specific favored dosage form may enhance their adherence to medications.Reference Strickley, Iwata, Wu and Dahl 3 To satisfy the need, several alternative stimulant dosage forms are under development or have become available, including a transdermal system,Reference Warshaw, Squires, Li, Civil and Paller 30 a long-acting chewable tablet, 25 liquid suspensions, 26 and ODT formulations. 29
Daytrana® (Noven Therapeutics LLC, Miami, FL) is a long-acting methylphenidate transdermal patch that received FDA (U.S. Food and Drug Administration) approval in 2006. 109 This patch is applied to the hip area 2 hours before the treatment effect is needed and can be worn for up to 9 hours.Reference Warshaw, Squires, Li, Civil and Paller 30 , 109 In three randomized, double-blind, placebo-controlled studies conducted in children or adolescents, daily 9-hour usage of Daytrana provided significantly better ADHD symptom control than placebo. Most side effects associated with Daytrana are consistent with those of oral methylphenidate, except for application site reactions, which are specifically related to this formulation. 109 Transdermal methylphenidate not only precludes the need for swallowing pills but also provides flexibility in adjusting the duration of drug exposure, as the treatment effect can be discontinued simply by removing the patch.Reference Patrick, Straughn, Perkins and Gonzalez 110 Because transdermal methylphenidate avoids first-pass metabolism and releases the active ingredient continuously, it provides a smoother pharmacokinetic profile than oral dosage forms, including some ER oral formulations that have a biphasic drug release.Reference Patrick, Straughn, Perkins and Gonzalez 110
Two liquid dosage forms of long-acting stimulants have recently become available, including a methylphenidate oral suspension, Quillivant XR® (Pfizer Inc., New York), approved in 2012, and an amphetamine oral suspension, Dyanavel™ XR (Tris Pharma Inc., Monmouth Junction, NJ), approved in 2015. 26 , 28 Quillivant XR is supplied as a powder that needs to be reconstituted with water prior to dispensing. This agent is dosed once daily in the morning, with the treatment effect taking place within 45 minutes and lasting for 12 hours. In a laboratory classroom study, Quillivant XR demonstrated superior efficacy in controlling ADHD symptoms compared with placebo. 26 Dyanavel XR is supplied as a ready-for-use oral suspension dosed once daily in the morning. 28 The efficacy of Dyanavel XR has been demonstrated in a laboratory classroom study in which Dyanavel XR provided a significant improvement in ADHD symptoms compared with placebo. 28 Liquid dosage forms of long-acting stimulants may represent an appealing option for patients who experience difficulties swallowing pills and who require gradual dose titration or precise doses in between the doses available in solid formulations to achieve optimal treatment effect and tolerability.
Although a short-acting methylphenidate chewable tablet (Methylin® chewable tablet, Shionogi Inc., Florham Park, NJ) has been on the market for years, 111 long-acting methylphenidate chewable tablets were unavailable until very recently. 25 Quillichew ER™ (Pfizer Inc., New York) is an ER chewable tablet that is dosed once daily in the morning. 25 While the prescribing information recommends an initial dose for Quillichew ER, patients receiving this medication may undergo dose titration based on their treatment requirements or responses. To satisfy this need, Quillichew ER tablets are functionally scored and supplied in multiple strengths, which facilitates splitting tablets with accurate doses. In a laboratory classroom study, a single dose of Quillichew ER in the morning significantly improved ADHD symptoms over placebo, with the treatment effect lasting for over 8 hours. 25 This formulation not only circumvents the need for swallowing intact pills but also appeals to patients who prefer to chew their medications.
The first approved long-acting ODT stimulant formulation is an amphetamine medication, Adzenys XR–ODT™ (Neos Therapeutics Inc., Grand Prairie, TX). 29 Adzenys XR–ODT is dosed once daily in the morning by placing the ODT on the patient’s tongue, where it disintegrates in saliva and can be swallowed without chewing or crushing. In several clinical studies conducted in pediatric or adult patients, Adzenys XR–ODT provided significantly better control of ADHD symptoms than placebo. 29 In addition, a long-acting methylphenidate XR–ODT is under development. In clinical studies, methylphenidate XR–ODT has shown favorable pharmacokinetics and provided effective ADHD symptom relief relative to placebo without causing serious safety concerns.Reference Childress, Newcorn, Stark, McMahen, Tengler and Sikes 112 , Reference Childress, Kollins, Cutler, Marraffino and Sikes 113 Long-acting ODTs not only ease ingestion but also offer the convenience of once-daily-dosing. Stimulants in chewable pills and ODTs are also portable options, as they are more compact and have no risk of leaking or spilling compared with liquid agents and do not require water or other liquids for ingestion.
Conclusions
ADHD is a common psychiatric disorder that typically develops in childhood and usually persists into adulthood.Reference Kessler, Berglund, Demler, Jin, Merikangas and Walters 9 , Reference Adler and Nierenberg 114 For most ADHD patients, the primary treatment is pharmacotherapy using stimulants, which are commonly formulated as tablets or capsules.Reference Pliszka 4 – 6 , Reference Rabito-Alcon and Correas-Lauffer 13 Solid oral formulations may negatively affect medication adherence in ADHD treatment because of dosing inconvenience, patient discomfort, or difficulty swallowing. Therefore, efforts to refine stimulant formulations can potentially enhance adherence.Reference Perwien, Hall, Swensen and Swindle 31 , Reference Winterstein, Gerhard and Shuster 32 Among ADHD patients, long-acting stimulants have shown proven effectiveness in improving medication adherence compared with their short-acting counterparts.Reference Ahmed and Aslani 17 , Reference Gau, Chen and Chou 90 , Reference Adler, Lynch and Shaw 95 – Reference Tzang, Wang and Yeh 97 Alternative oral dosage forms, such as chewable tablets, liquid solutions, and ODTs, may also increase adherence rates, as shown in other disease areas.Reference Wertheimer, Santella, Finestone and Levy 39 Among various oral dosage forms, ODTs represent a particularly portable and convenient option to ease ingestion as they obviate the need for carrying liquids. To fulfill the need for alternative oral dosage forms in ADHD treatment, several new ER formulations of stimulants have recently become available, including a methylphenidate ER liquid suspension, 26 an amphetamine ER liquid suspension, 28 a methylphenidate ER chewable tablet, 25 and an amphetamine ER–ODT. 29 Furthermore, a methylphenidate ER–ODT is under development.Reference Childress, Newcorn, Stark, McMahen, Tengler and Sikes 112 , Reference Childress, Kollins, Cutler, Marraffino and Sikes 113 The long-acting alternative oral dosage forms have combined advantages of dosing convenience and easy ingestion, thereby potentially improving medication adherence in ADHD patients. However, ER formulations of amphetamine chewable tablets and transdermal patches are still lacking. Future development is directed toward creating more long-acting alternative dosage forms for amphetamine and nonstimulant medications. As more medication formulations become available, clinicians will have more tools to individualize treatment and improve the effectiveness of pharmacotherapy for ADHD.
Disclosures
Dr. Cutler reports grants and personal fees from Akili Interactive, grants and personal fees from Arbor Pharmaceuticals, grants and personal fees from Ironshore Pharmaceuticals, grants and personal fees from Janssen, grants and personal fees from Lilly, grants and personal fees from Lundbeck, grants and personal fees from Neos Therapeutics, grants and personal fees from Novartis, grants and personal fees from Noven, grants and personal fees from Pfizer, grants and personal fees from Purdue, grants and personal fees from Rhodes Pharmaceuticals, grants and personal fees from Shire, grants and personal fees from Sunovion, grants and personal fees from Supernus, and grants and personal fees from Takeda, outside the submitted work.
Dr. Mattingly reports grants from Akili, grants from Alcobra, grants and personal fees from Alkermes, grants from Boehringer, grants and personal fees from Forest, grants and personal fees from Forum, personal fees from Lundbeck, grants from Jansen, personal fees from Merck, personal fees from Otsuka, personal fees from Perdue, grants from Reckitt Benckiser and from Rhodes, grants and personal fees from Shire, grants and personal fees from Sunovion, grants and personal fees from Supernus, grants and personal fees from Takeda, and personal fees from Vanda, outside the submitted work.