Introduction
Parkinson’s disease (PD) is a progressive movement disorder that results from the degeneration of dopaminergic neurons in the substantia nigra and other basal ganglia structures. The non-motor features of PD are known to cause significant disability, and their common occurrence qualifies PD as a neuropsychiatric condition. Pharmacological treatments, including levodopa and dopamine agonists, often provide adequate control over the motor symptom for the first few years of therapy. However, their long-term use is associated with decreased effectiveness, including “on/off” fluctuations in the motor state. Bilateral high frequency stimulation of the subthalamic nucleus (STN) is increasingly used as a treatment option for severe disabling presentations that cannot be adequately controlled with medications.
Deep brain stimulation of the STN (STN-DBS) can be associated with adverse cognitive, behavioral, and emotional effects, possibly including apathy. Apathy is often a characteristic of PD and is defined as “a clustering of behavioural and emotional symptoms that manifest as diminished interest and involvement in normal purposeful behaviour, flattened affect, diminished emotional responsivity, lack of initiation of non-routine activity and diminished drive.”Reference Leroi, David and Robert 1 Apathy has a negative effect on prognosis and is associated with an increased rate of cognitive and functional decline.Reference Leroi, David and Robert 1 It can lead to deficits in physical aspects of PD, including poor nutritional status and increased sedentary behavior, which can lead to complications such as deep vein thrombosis.Reference Benoit, Andrieu and Lechowski 2 STN-DBS has been associated with a 14–68% improvement of quality of life (QOL) in patients with PD.Reference Diamond and Jankovic 3 Apathy and other non-motor symptoms of PD have been shown to have a negative effect on QOLReference Barone, Antonini and Colosimo 4 – Reference Antonini, Barone and Marconi 6 and may limit the beneficial effect that STN-DBS has on QOL.
In relation to STN-DBS, a number of studiesReference Thobois, Ardouin and Lhommée 7 – Reference Krack, Batir and Van Blercom 17 have reported an increase in the prevalence and severity of apathy after STN-DBS. However, this is not an established relationship; some studies reported a decrease in apathetic behavior,Reference Campbell, Black and Weaver 18 , Reference Czernecki, Pillon and Houeto 19 and others reported no change.Reference Castelli, Lanotte and Zibetti 20 – Reference Chou, Persad and Patil 22 Whether changes in apathy are a direct effect of STN-DBS or a consequence of the reduction in the daily dose of levodopa is unclear. There are limitations in previous studies, including small sample sizes and the use of assessments that have not been validated. This, combined with the clinically significant effect that apathy has on prognosis, means more research is necessary in this area.
The primary objective of this study was to assess the prevalence and severity of apathy in patients who have undergone STN-DBS compared to patients who are on pharmacotherapy alone. The secondary objective was to assess whether the prevalence and severity of apathy are associated with specific clinico-demographic characteristics. Finally, we aimed to assess whether QOL varies between the groups and whether the severity of apathy is associated with a change in QOL.
Methodology
Participants
A total of 60 patients with PD took part in the study between February 2013 and February 2014. All participants were recruited from the specialist PD clinics at the Queen Elizabeth Hospital, Birmingham, UK. Patients were sent a letter of invitation before their appointment, and those who gave written informed consent after their consultation were included in the study. The study group (DBS group) consisted of 22 patients who had undergone STN-DBS. The control group consisted of 38 patients who were on pharmacotherapy alone. The inclusion criteria were clinical diagnosis of PD according to the UK PD brain brank criteria 23 and stable stimulation settings for at least 3 months for the DBS group. Exclusion criteria included comorbid neurological disorders, dementia, acute psychiatric disorders, and unstable medical status. Clinico-demographic characteristics were collected, including age, gender, disease duration, duration of medication, levodopa equivalent dose (LED), and time since surgery. Medication was not altered to prevent distress to the participants. This study received approval from the National Research Ethics Service.
STN-DBS
All patients in the DBS group underwent STN-DBS at the Queen Elizabeth Hospital, Birmingham, UK. A cannula and a recording microelectrode were inserted into the target area using MRI-calculated stereotactic guidance under general anesthesia. The correct positioning of the recording electrode tip in the STN was confirmed by electrophysiological recording. This was followed by removal of the recording electrode and insertion of the permanent one. The electrode was then clamped to the skull. Leads connected to these STN electrodes were tunneled subcutaneously to the upper chest wall where they were connected to a pulse generator. Following the surgery, the patients were sent home with the stimulator turned off. After about 4 weeks, stimulation was commenced and gradually increased, while medication was decreased. Stability was achieved after an average period of 6 months, and patients were recruited at least 3 months after this point.
Assessment
The assessment was completed after the patient’s consultation in the PD clinic and included 4 psychometric instruments. Three rating scales were completed by the patients in the following order: Lille Apathy Rating Scale, Apathy Scale, and 39-item Parkinson’s Disease Questionnaire. Finally, the treating clinician completed the Unified Parkinson’s Disease Rating Scale.
Lille Apathy Rating Scale (LARS)
The LARS is a semistructured interview specifically designed to assess apathy in patients with PD. It consists of 33 items divided into 9 domains ranging from everyday productivity to personal interests. The total score ranges from –36 (least severe apathy) to +36 (most severe apathy).Reference Leentjens, Dujardin and Marsh 25 The LARS is a psychometric tool recommended by the Movement Disorders Society (MDS) 26 based on its good sensitivity and specificity properties.Reference Tomlinson, Stowe, Patel, Rick, Gray and Clarke 24 , Reference Drijgers, Dujardin, Reijnders, Defebvre and Leentjens 27 A score of -16 or greater indicates clinically significant apathy. 26
Apathy Scale (AS)
The AS consists of 14 questions that are read aloud by the examiner and completed by the participant; it was designed specifically for patients with PD. Scores range between 0 (least severe apathy) and 42 (most severe apathy). This scale is also recommended by the MDS based on its good validity, internal consistency, and reliability properties in the PD population.Reference Tomlinson, Stowe, Patel, Rick, Gray and Clarke 24 A score of 14 or greater indicates clinically significant apathy.Reference Zahodne, Young and Kirsch-Darrow 28
Parkinson’s Disease Questionnaire (PDQ-39)
The PDQ-39 is a 39-item self-administrated questionnaire specifically developed to assess QOL in patients with PD. This psychometric instrument covers 8 domains, ranging from mobility to emotional well-being, yielding a score between 0 and 100 (with 100 indicating maximum measurable negative impact of PD on QOL). The PDQ-39 has been shown to have satisfactory internal and test–retest reliability.Reference Peto, Jenkinson, Fitzpatrick and Greenhall 29
Unified Parkinson’s Disease Rating Scale (UPDRS)
The UPDRS is a commonly used assessment tool for the evaluation of clinical status of PD. It has 4 parts: Part I (non-motor aspects of experiences of daily living), Part II (motor aspects of experiences of daily living), Part III (motor examination), and Part IV (motor complications). Items are scored by the clinician on a 5-point Likert scale, providing a score between 0 and 260 (corresponding to maximum level of disability due to PD).
Statistical analysis
Descriptive statistics included mean, standard deviation, 95% confidence intervals for normally distributed data, and median and interquartile range for nonparametric data. The frequency of participants with apathy (LARS score >–16, AS score>14) was compared between patients who underwent STN-DBS (DBS group) and patients on pharmacotherapy alone (control group) using a 2-tailed Fisher’s Exact Test. Continuous variables, including clinico-demographic data, UPDRS, PDQ-39, and both LARS and AS scores, were compared between the 2 groups using a 2-tailed Student’s t test (or a 2-tailed Mann–Whitney U test for nonparametric data). Correlation analyses were carried out using Pearson’s correlation coefficient (or Spearman’s rank correlation coefficient for nonparametric data). Data were analyzed using SPSS Version 21.0.
Results
Participants had a mean age of 66.3 years (SD 8.9) and a mean UPDRS score of 64.1 (SD 27.6). The mean LED was 581.4 (sd 326.2). For the group of patients who underwent STN-DBS, the mean post-operative evaluation time was 48 months (SD 3.3). Most clinico-demographic characteristics, including total UPDRS score, did not differ significantly between the patients who had undergone STN-DBS and those on pharmacological treatment alone. The only differences were reported in duration of disease and medication (which were significantly longer in the DBS group) and age (as a result of the selection process for the DBS procedure) (Table 1).
Table 1 Characteristics of patients who underwent STN-DBS (DBS group) and patients on pharmacotherapy alone (control group)

a Student’s t test.
b Chi-Square test.
c Mann–Whitney U test.
UPDRS=Unified Parkinson’s Disease Rating Scale.
There was no significant difference in the proportion of participants with apathy (scores above the cut-off values) between the 2 groups for both LARS (Fisher’s Exact Test P value=0.744) and AS (Chi-Square test: 0.64, p=0.801) (Table 2).
Table 2 Prevalence of apathy according to the Lille Apathy Rating Scale (LARS) and Apathy Scale (AS) in patients who underwent STN-DBS (DBS group) compared to patients on pharmacotherapy alone (control group)

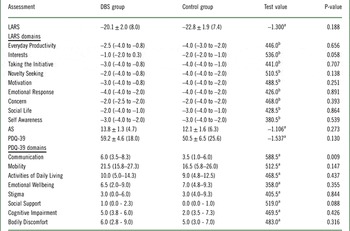
In terms of apathy ratings, the mean LARS and AS scores for the overall sample were –21.8 (SD 7.7) and 12.7 (SD 5.8), respectively. There were no differences between the DBS group and control group in the LARS total score and subscores or total AS score (Table 3). The mean PDQ-39 score for the overall sample was 53.7 (SD 23.3). There were no differences in total PDQ-39 scores between the groups, with the exception of the “Communication” domain, for which the DBS group reported a significantly higher score (Table 3).
Table 3 Lille Apathy Rating Scale (LARS), Apathy Scale (AS), and Parkinson’s Disease Questionnaire (PDQ-39) scores in patients who underwent STN-DBS (DBS group) compared to patients on pharmacotherapy alone (control group)

a Student’s t test.
b Mann–Whitney U test.
The relationship between apathy and clinico-demographic variables was explored through correlation analysis. Although LARS and AS scores were not significantly correlated with years since surgery in the DBS group, both apathy ratings were associated with higher PDQ-39 scores (Pearson correlation coefficients 0.502, p<0.001 and 0.298, p=0.021, respectively). Both LARS and AS scores were also associated with UPDRS scores (Pearson correlation coefficients 0.528, p<0.001 and 0.308, p=0.017, respectively). There was a positive correlation between UPDRS and PDQ-39 scores (Pearson correlation coefficient 0.668, p<0.001). Finally, the correlation between the 2 apathy measures was confirmed by our analysis (Pearson correlation coefficient 0.538, p<0.001).
Discussion
This study did not find any significant difference in the prevalence or severity of apathy between patients with PD who had undergone STN-DBS and those on pharmacotherapy alone. More severe apathy was associated with a higher level of disability due to PD and worse QOL in PD, but no other clinico-demographic characteristics.
Previous studies that have investigated changes in apathy after DBS vary considerably in their methodology and results, and direct comparisons with the present study can be difficult. The majority of research (70% of the total sample size) suggests that apathy increases after STN-DBS. Four studies focused on prevalence of apathy, using either cut-off values from scales or a clinical diagnosis. Thobois et al Reference Thobois, Ardouin and Lhommée 7 reported that 54% of their participants developed apathy after an average of 4.7 months; however, half of these cases resolved by 12 months. Funkiewiez et al Reference Funkiewiez, Ardouin and Caputo 8 used UPDRS item 1.4 to assess apathy and showed that prevalence of apathy doubled between pre-surgery, 1-year, and 3-year assessments. However, this method of assessment consisted of a single question, giving a crude assessment of apathy with a sensitivity of only 52%Reference Kirsch-Darrow, Zahodne and Hass 30 ; furthermore no control group was used. Castelli et al Reference Castelli, Lanotte and Zibetti 20 assessed apathy at 17 months using the AS and found no difference in prevalence of apathy, with a prevalence of 42% in participants before and after surgery. In general, post-implant stimulation and pharmacotherapy practices vary widely across centers where DBS is performed. The pharmacological management of patients with PD who underwent STN-DBS involves different combinations of medications, such as levodopa and dopamine agonists, which can have a significant impact on neuropsychiatric profiles, including apathy symptoms. Therefore, although most previous research indicates that STN-DBS is associated with an increase in prevalence of apathy, there is the possibility that these results are biased by methodological confounders.
Nine out of 14 studies that investigated changes in severity of apathy after DBS (66% of the sample size looking at severity) reported significant increases in the severity of apathy. The results of the present study did not provide confirmatory evidence; however, it is difficult to make direct comparisons because of the different methodological approaches. Moreover, none of the studies that reported an increase in severity of apathy used validated assessments along with a control group, and reported results with 95% confidence intervals that do not overlap. One study used only UPDRS item 1.4,Reference Funkiewiez, Ardouin and Caputo 8 and another used an assessment based on a visual analogue scale,Reference Campbell, Black and Weaver 18 which had not been validated. Several studies that reported significant increases have 95% confidence intervals that overlap, despite significant p-values from analysis. For example, Kirsch-Darrow et al Reference Kirsch-Darrow, Zahodne, Marsiske, Okun, Foote and Bowers 9 reported a significant increase in apathy before surgery (AS score 10.3, SD 1.6) compared to 6 months after surgery (12.6, SD 2.2).
An interesting observation when comparing changes in severity is the time at which assessments were performed. All but 1 of the studiesReference Thobois, Ardouin and Lhommée 7 – Reference Krack, Batir and Van Blercom 17 that reported an increase in severity of apathy assessed participants prior to surgery and between 3 and 6 months post-surgery. In contrast, the 5 studiesReference Campbell, Black and Weaver 18 – Reference Chou, Persad and Patil 22 that reported no change or an improvement in apathy assessed participants between 6 and 17 months post-surgery. In this study, participants were only included if they had been on stable stimulation settings for at least 3 months and the average time since surgery was 53 months. It could be possible that apathy may only be a temporary side effect of STN-DBS or reduction in dopaminergic medication in the post-surgical phase. Further investigation looking at apathy symptoms for longer than 6 months post-surgery would be an important step in order to better understand the temporal trajectory of this presentation.
Correlation analysis showed a strong positive correlation between QOL in PD and disability due to PD, which appears to be an established relationship.Reference Weaver, Follett and Stern 31 – Reference Soha, Morrisa and McGinley 34 In particular, UPDRS Part II (motor aspects of experiences of daily living) is often correlated with QOL assessments, as in this study. The relationship between apathy and disability due to PD is less certain. Some studies have reported correlation between apathy and either total UPDRS or UDPRS Part III.Reference Pederson, Alves, Brønnick, Aarsland, Tysnes and Larsen 35 – Reference Pederson, Larsen, Alves and Aarsland 37 In one study, apathy was reported by 25% of patients at a mild disease stage and by 49% at an advanced disease stage.Reference Robert, Le Jeune and Lozachmeur 38 The study by Czernecki et al Reference Czernecki, Pillon and Houeto 19 did not report any correlation, but showed that patients whose apathy scores improved after STN-DBS had a lower UPDRS score without treatment or stimulation compared with those whose scores worsened or did not change. There are multiple possible mechanisms explaining why apathy occurs in PD and may be affected by DBS. This result supports the view that the mechanisms causing apathy and motor symptoms may be associated. Further research is needed to look at the association between apathy and disease severity, as, if a relationship is established, apathy could be used as a marker for disease severity. Furthermore, dopaminergic replacement therapy may have a similar beneficial effect on apathy as it does on the motor symptoms of PD.
Apathy and QOL were shown to be associated in this study. Other studiesReference Barone, Antonini and Colosimo 4 – Reference Antonini, Barone and Marconi 6 have shown that among the non-motor symptoms of PD, apathy has a large impact on QOL. The significant effect that apathy has on QOL means that it should be considered as an important target for clinical intervention. As in other studies,Reference Barone, Antonini and Colosimo 4 apathy was not correlated with disease duration. This suggests that apathy could be a target for clinical intervention even in the early stages of disease.
The main limitation of the present study was its cross-sectional rather than longitudinal design. Our study design did not include analysis of preoperative data on apathy and other clinical features such as dyskinesia, which have been shown to possibly predict postoperative apathy in the acute phase in patients with PD treated with STN-DBS.Reference Higuchi, Tsuboi and Inoue 39 Therefore, our findings need to be validated in further studies where participants are assessed prior to the surgery and at intervals after the surgery and compared to a control group on best pharmacological therapy. These assessments should also address depression: although the relationship between apathy and depression is controversial, with some studies showing that they are discrete constructs,Reference Kirsch-Darrow, Marsiske, Okun, Bauer and Bowers 40 there are shared symptoms that should be accounted for. We did not include a formal neuropsychological assessment, which should be part of the clinical characterization of future study samples: particular attention should be paid to more subtle cognitive symptoms, such as those displayed by patients in the PD–mild cognitive impairment stage. Arguably, both age and disease duration, which significantly differed between our 2 groups, may have interfered in apathy reporting, with the increased possibility of more severe and more frequent apathy in patients with older age and significantly longer duration of disease. These differences should be taken into account when drawing conclusions from our findings. Another limitation of this study is related to the methodology chosen to assess apathy symptoms. The use of 2 psychometric instruments was aimed at reducing social desirability bias: the LARS is a more extensive assessment completed by the assessor, whereas the AS is a briefer assessment completed by the participant, allowing them to answer more freely. Our findings also suggest possible differences in the sensitivity between the 2 instruments. Moreover, given that self-perceived apathy may differ from evaluation from an external rater, the use of self-report measures does not address the role of possible anosognosia. Specifically, it cannot be ruled out that STN-DBS patients who have better communication scores may have reported differently their motivation deficit. A further limitation was that we did not control whether patients were assessed in an “on” or “off” state, in order to reduce stress for the participants. This may have affected both UPDRS scores and apathy assessments, as a study by Czernecki et al Reference Czernecki, Pillon, Houeto, Pochon, Levy and Dubois 41 showed a significant difference in apathy scores between “on” and “off” states. Finally, sample selection bias may have occurred. Participants volunteered to take part in the study, but patients who were more apathetic may have been less likely to take part in the study. This was however unlikely to have a large effect on the study, as only 1 patient declined to participate.
The issue of apathy and STN-DBS is controversial, and this controlled study, which used multiple standardized measures of apathy, did not find an increased prevalence or severity of apathy in patients with PD following STN-DBS compared to patients on pharmacotherapy alone. These findings are in line with the results from a recent study of apathy in patients with PD following internal globus pallidus DBS, which showed absence of deterioration in apathy scores (as well as other psychiatric and cognitive ratings) 3 months and 6 months after pallidal stimulation.Reference Lozachmeur, Drapier and Robert 42 A longitudinal study assessing apathy at regular intervals for longer than 6 months post-surgery would be useful to investigate whether increases in apathy are only temporary features after STN-DBS. Ideally, the study design should allow investigators to disentangle the reciprocal effects of electrical stimulation and dopamine replacement therapy on the development of apathy in patients who underwent STN-DBS. Further research would also be useful to establish whether there is a relationship between apathy and disability due to PD, measured by UPDRS, and to investigate the effect of dopaminergic replacement therapy on apathy. The significant effect that apathy has on QOLReference Martinez-Fernandez, Pelissier and Quesada 43 confirms the importance of the development of effective clinical interventions and further investigation as to how STN-DBS can affect patients’ well-being.
Disclosures
Isabel Hindle Fisher and Jamilla Kausar do not have anything to disclose. Hardev Pall has the following disclosures: Medtronic, researcher, educational grant for meeting attendance; Britannia Pharmaceuticals, consultant neurologist, educational grant for meeting attendance. Rosalind Mitchell has the following disclosure: Medtronic, grant recipient, grant to attend the European Society Stereotactic & Functional Neurosurgery Meeting (September 2014). Andrea Cavanna has the following disclosure: UCB Pharma, speaker’s bureau, speaker’s fee.