The demand for high-/ultra-high-performance concrete has increaseddramatically in recent years, creating demand for high-quality aggregates (Wang et al., Reference Wang, Fang, Kuang, Li, Han and Xing2017; Rashad, Reference Rashad2018; Zhang et al., Reference Zhang, Liu, Tong and Ren2019). This demand cannot be fully met due to the depletion of natural resources, including natural sand and gravel (Ioannidou et al., Reference Ioannidou, Meylan, Sonnemann and Habert2017). Aggregates contaminated by impurities such as clay and silt rather than gravel or sand are typically utilized in this regard.
Various organic chemicals have been applied in clays in efforts to enhance their efficiency, and the interactions between them have been studied (Li et al., Reference Li, Wu, Petit, Gates, Yang, Yu and Zhou2019; Tan et al., Reference Tan, Li, Ren, Deng, Zhang and Nie2019; Guo et al., Reference Guo, Liu, Gates and Zhou2020; Shen et al., Reference Shen, Petit, Li, Li, Khatoon and Zhou2020; Zhang et al., Reference Zhang, Xu, Christidis and Zhou2020). One of the most important chemical admixtures, polycarboxylate superplasticizers (PCEs), is a type of clay-sensitive superplasticizer. However, contamination by clay or silt makes PCE inherently incompatible with aggregates (Wala & Rosiek, Reference Wala and Rosiek2003; Dziekan et al., Reference Dziekan, Laska and Małolepszy2011; Ren et al., Reference Ren, Fang, Ma, Tan, Luo, Liu and Wang2019, Reference Ren, Wang, Xu, Fang, Liu and Luo2021; Tan et al., Reference Tan, Gu, Guo, Ma, Huang and Ren2018a). This negatively impacts the dispersion of PCE and the workability and pumping ability of the concrete (Lei & Plank, Reference Lei and Plank2012b).
There has been extensive research to date conducted on the incompatibility between PCE and clay (Sánchez-Martín et al., Reference Sánchez-Martín, Dorado, Hoyo and Rodríguez-Cruz2008; Ng & Plank, Reference Ng and Plank2012; Zhao et al., Reference Zhao, Wang, Yang, Shu, Yan and Ran2017; Gao et al., Reference Gao, Ren, Liu, Guo and Li2018; Ma et al., Reference Ma, Shi, Lei, Sha, Zhou, Liu and Xiao2020). It is generally accepted that the greater sorption of PCE on clay is due to the larger specific surface of clay grains. Clay minerals have a layered structure with two Si tetrahedra and one Al oxide octahedron in between (2:1 structure), or one Si tetrahedron and one Al oxide octahedron (1:1 structure). Upon contact with water, some 2:1 clay minerals, such as smectite, swell because of expansion of the interlayer space (Tregger et al., Reference Tregger, Pakula and Shah2010; Tan et al., Reference Tan, Gu, Guo, Ma, Huang and Ren2018a). Therefore, the polyethylene oxide (PEO) side-chains can easily become inserted into the interlayer, causing disjunction in the cement particle dispersal with steric repulsion (Suter & Coveney, Reference Suter and Coveney2009; Tan et al., Reference Tan, Gu, Ma, Li, Lin and Li2016a).
Adding small-molecule sacrificial agents such as poly(vinyl alcohol) or cationic surfactants may resolve the incompatibility issue (Nehdi, Reference Nehdi2014; Wang et al., Reference Wang, Deng, Feng, Fu and Zheng2015; Tan et al., Reference Tan, Guo, Ma, Huang, Gu and Zou2018b). Introducing novel PCE structures may also be effective (Chen et al., Reference Chen, Lei, Du, Du and Chen2018; Tang et al., Reference Tang, Zhao, Yang, Dong and Lu2020). The three main approaches to such modifications include increasing the steric hindrance of PCE, introducing a cationic group to produce an amphoteric copolymer and using no PEO side-chains. Xu et al. (Reference Xu, Sun, Yu and Wei2018) synthesized quaternary PCEs grafted with β-cyclodextrin by copolymerizing the monovinyl β-cyclodextrin monomer, sodium methylallyl sulfonate, acrylic acid and methyl allyl polyoxyethylene ether (TPEG) via free-radical polymerization initiated by hydrogen peroxide solution to improve the performance of a kaolin clay. Liu et al. (Reference Liu, Guan, Lai, Zheng, Wang and Cui2017) used star-shaped PCE synthesized by acrylic acid-methyl allyl polyoxyethylene ether (AA-TPEG) copolymer arms on a polyol(pentaerythritol) core to minimize the detrimental effects of clays in cement. These methods are mainly based on increasing the size of PCE. Li et al. (Reference Li, Zheng, Wu and Lu2016) employed the zwitterionic monomer 3-(2-(methacryloyloxy)ethyl)dimethylammonio-propane-1-sulfonate (DMAPS) to prepare a novel amphiphilic polycarboxylate copolymer that may be adsorbed on a clay surface without intercalation.
There have been many other valuable contributions to this literature. Lei & Plank (Reference Lei and Plank2012a, Reference Lei and Plank2014b) prepared a series of PCEs with hydroxy alkyl lateral chains from methacrylic acid and hydroxyl-alkyl methacrylate esters that performed well on dispersing cement with Na-montmorillonite (Na-Mnt; Warr, Reference Warr2020), as they did not intercalate into its layered structure. The same authors also attempted to modify the PCE side-chain by synthesizing a series of vinyl ether-based PCEs containing two monomers: maleic anhydride and 4-hydroxybutyl vinyl ether. The PCE exhibited high cement dispersion ability due to the lack of intercalation with the clay. Xing et al. (Reference Xing, Wang and Xu2016) induced the tertiary amino group from 2-(dimethylamino)ethyl methacrylate as the end group of a side-chain to prepare terpolymer PCEs from acrylic acid and itaconic acid, which showed enhanced sorption on the clay surface by eliminating the intercalation of the PEO side-chain.
The clay-tolerance properties of modified PCEs can be significantly enhanced using the above methods, but the polymerization of chemicals with introduced functional groups requires accurate control over the sophisticated synthesis procedure. Considering its cost, there is demand for novel synthesis techniques for clay-tolerant PCEs (ct-PCEs) based on the conventional PCE. Previous studies have generally involved trial and error rather than precise optimization processes. Compared to traditional experimental methods, response surface methodologies such as Box-Behnken design (BBD) or central composite design (CCD) provide a systematic, efficient strategy to correlate the interactive effects of numerous parameters simultaneously through statistical procedures (Ren et al., Reference Ren, Fang, Ma, Tan, Luo, Liu and Wang2019).
In the present study, a zwitterionic PCE with cationic amide groups and shorter side-chain lengths was synthesized via copolymerization. Because montmorillonite (Na-Mnt) has been identified as the most important mineral composition of the clay (Lei & Plank, Reference Lei and Plank2014a), performance-based optimization of the synthesis conditions of the ct-PCE was conducted by determining the fluidity of the cement paste containing Na-Mnt via BBD. Gel permeation chromatography (GPC), dynamic light scattering (DLS) and Fourier-transform infrared (FTIR) spectroscopy were used to characterize the physicochemical properties of the optimized ct-PCE. The interactions between the sample materials were investigated by total organic carbon (TOC) analysis, X-ray photoelectron spectroscopy (XPS) and X-ray diffraction (XRD) with regards to the sorption amount, sorption thickness and intercalation of the ct-PCE in Na-Mnt.
Materials
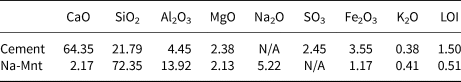
The P.O. 42.5 Cement (PC) (which meets the Chinese standard GB175-2007) used in this study was supplied by China United Cement Co. Ltd. The Na-Mnt with a specific surface area of 24.86 m2 g–1 was supplied by Shenzhen Huanan Xinyang Tech. Co. Ltd, China. The typical chemical compositions of the PC and clay are listed in Table 1.
Table 1. Chemical composition (wt.%) of the PC and clay.
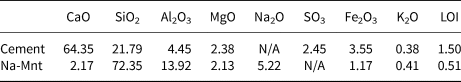
LOI = loss on ignition.
Allyl polyethylene glycol (APEG) (commercial product, purity ≥99.0%) with various molecular weight of 500, 1000, 2000 and 2400 Da was supplied by Nantong Jinlai Chemical Co. Ltd, China, as the side-chain. All chemicals used for polymerization, including acrylic acid (AA), methylacrylamide (AM), diallyldimethylammonium chloride potassium (DMDAAC), 2-acrylamido-2-methyl-1-propanesulfonic acid (AMPS), 2-mercaptoethanol (TGA), potassium persulfate (KPS) and sodium hydroxide (NaOH), were of reagent grade and were supplied by Sinopharm Chemical Reagent Co., Ltd, China. A commercial PCE (c-PCE) from Jiangsu China Railway ARIT New Materials Co., Ltd, was employed for comparison.
Experimental
Polymerization
The ct-PCE used in this study was synthesized via free radical polymerization. APEG and water were added to a 500 mL four-neck round-bottomed flask and magnetically stirred while being heated to the target temperature. The monomer solution was added slowly and evenly as a solution containing the designated KPS dosage and a fixed amount of TGA (0.5 wt.% of the total weight of all monomers) was added. After the addition of all materials in the solution, polymerization proceeded under constant temperature for another 3 h followed by cooling to ambient temperature. The final PCE product was neutralized by NaOH solution to a pH of 7.
Characterization
The molecular weight and molecular distribution, expressed as a polydispersity index (PDI), of PCE were determined by GPC (Wyatt Technology Corporation) with 0.1 M NaNO3 as the eluent and poly(ethylene glycol) (PEG) as the standard. The conversion of the PCE was calculated from GPC results based on a previously reported technique (Sun et al., Reference Sun, Yang, Shui, Liu, Yang and Ji2016). Deionized water was used throughout the experiments. The chemical structure of the PCE was determined by FTIR spectroscopy (Nicolet Instruments, Inc., USA) using the KBr method in the range from 400 to 4000 cm–1. The hydrodynamic radii (Rh) of the PCE were measured by DLS (Malvern Zetasizer Nano ZS) at 25°C.
Test procedure
Sample preparation
To evaluate the dispersion performance of the superplasticizer, a cementitious paste containing 3% Na-Mnt was prepared at a water-to-cementitious material ratio of 0.29. The PCE dosage was controlled to 0.25% by weight of cementitious materials.
Dispersion of cementitious material
The dispersion ability of the PCE was determined via the fluidity of the pastes containing cement and Na-Mnt with a minislump test in accordance with Chinese standard GB/T 8077-2012.
Sorption on Na-Mnt
The sorption on Na-Mnt was measured using the depletion method, in which 0.5 g of Na-Mnt and 26.5 g of the synthetic cement pore solution (prepared by dissolving 1.72 g CaSO4⋅2H2O, 4.76 g K2SO4, 6.96 g Na2SO4 and 7.12 g KOH in 1 L of deionized water; Lei & Plank, Reference Lei and Plank2012a) with various PCE concentrations were poured into a 100 mL container and stirred for 9 min. The sorption of PCE on Na-Mnt was determined by means of TOC analysis (Shimadzu, Japan). A two-stage centrifugation was conducted to separate the solid phase and the aqueous phase. The solution was centrifuged at 3500 rpm for 15 min and then the resulting supernatant was further centrifuged at 12,000 rpm for another 15 min. The supernatant was decanted and diluted with deionized water for TOC analysis. The PCE sorption amount was calculated based on the change in the amount of superplasticizer from before vs after it contacted the binder, which was calculated from the TOC results.
Intercalation with Na-Mnt
X-ray diffraction was employed to examine the d spacing and the interlayer spacing of Na-Mnt (Tan et al., Reference Tan, Gu, Ma, Li, Lin and Li2016a) after hydrating the Na-Mnt specimens for 12 min. Following Xing et al. (Reference Xing, Wang and Xu2016), 0.56 g of clay and 24.56 g of synthetic cement pore solution prepared as described in the previous section with 0.25 wt.% of the tested PCE were added to a 50 mL container. The suspension was stirred for 10 min and centrifuged at 12,000 rpm for another 15 min. The solid was vacuum dried at 45°C for 48 h, followed by selection with a 200-mesh sieve. A Rigaku X-ray diffractometer with a Cu tube (Cu-Kα radiation) was used to characterize the samples. The XRD traces were recorded over the 3–25°2θ range at a speed of 4°2θ min–1 and a step size of 0.02°2θ.
Dried solid powders were prepared for XPS measurements (Thermo Scientific K-Alpha, Thermo Fisher Ltd, USA) to test for Si and Ca elements. Aluminium was used as an anode target and the energy resolution was 0.05 eV. The sorption layer thickness of the PCEs was estimated by calculation of Si2p (Ji et al., Reference Ji, Luo, He, Shi and Gu2012; Tan et al., Reference Tan, Zou, Ma, Liu, Li and Jian2016b).
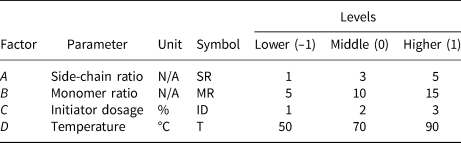
Experimental parameters
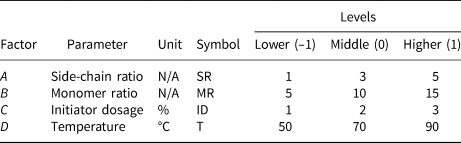
Based on the literature review and preliminary tests, four factors were identified as the most significant in terms of chemical structure. The performance of ct-PCE was analysed according to these factors. The effects of the four factors, namely side-chain ratio (mole ratio between monomer and macromonomer), monomer ratio (mole ratio between AA and AM), initiator dosage (weight percentage of macromonomers and monomers) and synthesis temperature, on the polymerization of ct-PCE were observed and subsequently optimized (Table 2). Three levels were selected for each factor – lower, middle and higher, coded as –1, 0 and 1, respectively. A statistical response model of the initial minislump was quantitatively established for mathematical optimization. The analysis was conducted using the Design Expert software (Stat-Ease, Inc., USA).
Table 2. Parameters for the experimental design.
Results and discussion
Effect of PCE structure on cement with Na-Mnt minislump
The minislump of the Na-Mnt-blended cement incorporated with PCEs composed of various cationic anchor groups was determined and compared with c-PCE (Fig. 1a). A p-value of <0.05 was considered to be statistically significant. Introduction of the cationic group appears to have significantly increased the minislump of the paste, indicating that the ct-PCE performed better at dispersing the cement with the Na-Mnt impurity than c-PCE. The introduced cationic groups promoted electro-attraction between PCE and the negatively charged Mnt surface. Therefore, introducing cationic anchor groups improved the minislump of the sample and AM showed the best performance. The lower value of PCE with AMPS and DMDAAC may be attributed to steric hindrance from the methyl groups.

Fig. 1. Effects of PCEs with various chemical structures on initial minislump of cement paste including 3% Na-Mnt. (a) Effects of cationic anchor group and (b) effects of side-chain length.
The side-chain length (molecular weights of 500, 1000, 2000 and 2400 Da) affects the minislump of the Na-Mnt-containing cement paste because there are statistically significant differences between the various PCEs (Fig. 1b). Compared to c-PCE, the minislump of the paste mixed with PAA–AM–APEG was greater, indicating that PCE copolymerizing AA, AM and APEG has greater Mnt tolerance. The side-chain significantly influenced the performance of PAA–APEG as well. The minislump spread increased when decreasing the side-chain length to give molecular weight of 2400 to 500 g mol–1 and reached its greatest value when the side-chain length gave a molecular weight of 500 Da.
Although it is generally agreed that a greater side-chain length benefits the dispersion ability of PCE, the intercalation of the PEO side-chain into the interlayer of Mnt also causes dysfunction in the steric repulsion of PCE. In this case, a shorter side-chain led to a greater minislump.
Performance-based optimization of zwitterionic PCE
Box-Behnken design was employed to establish the optimal levels of four selected factors, namely side chain ratio (A), monomer ratio (B), initiator dosage (C) and synthesis temperature (D), which significantly affect the chemical structure and performance of ct-PCE. Twenty-four tests at factorial points and five repeated tests at central points were run in Design Expert software. The minislump spread was set as the response for determining the above four factors of PCE in dispersing cement with 3% Na-Mnt.
Equation 1 was generated based on the best-fitting model assessed by the software; it is a quadratic equation of the minislump (Y) as the response surface for the results and is visualized as a three-dimensional response surface plot (Fig. 2).


Fig. 2. Three-dimensional response surface plot of minislump of Na-Mnt-incorporated cement with ct-PCE in relation to (a) side-chain ratio and monomer ratio, (b) side-chain ratio and initiator dosage, (c) monomer ratio and synthesis temperature and (d) initiator dosage and synthesis temperature.
The maximal minislump of the Na-Mnt-incorporated cement with PCE was set as the target to optimize the dispersion performance of ct-PCE. The maximal minislump (286 mm) was achieved with a side-chain ratio ((AA + AM):APEG) of 4.9, a monomer ratio (AA:AM) of 9.8, an initiator dosage of 2.9% and a synthesis temperature of 81°C. Experiments were conducted using the optimized levels to check for differences between the experimental and the calculated values. The experimental value was 277 ± 15 mm, which was 96.86 ± 0.05% of the predicted value. Thus, the optimization model was considered accurate.
Chemical properties of PCE
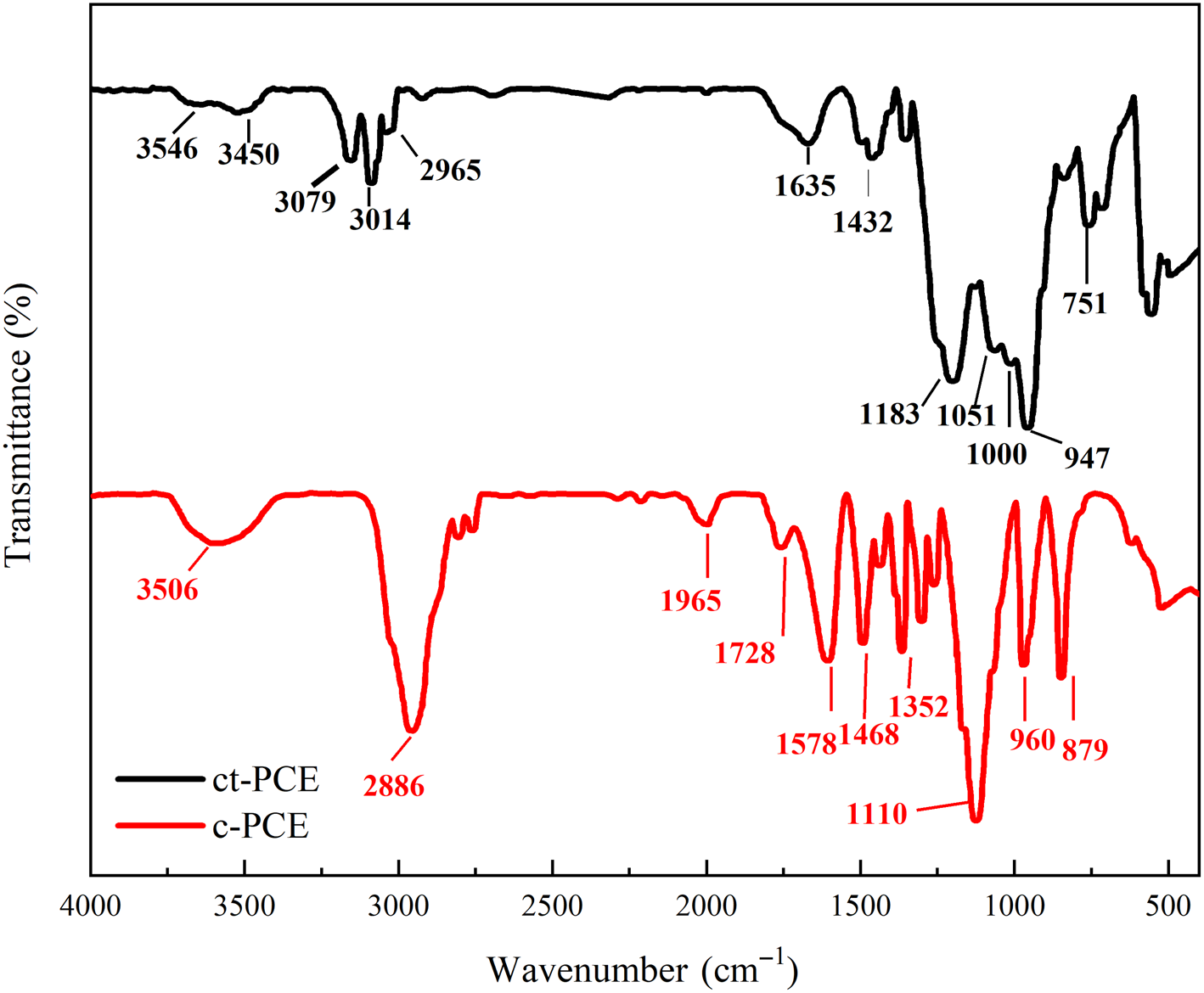
The FTIR spectra of both ct-PCE and c-PCE are shown in Fig. 3. For c-PCE, the bands at ~3506 cm–1 represented the O–H stretching band of carboxylate. Bands at 1965, 1728, 1578 and 1352 cm–1 might be attributed to carbon–oxide bonds in carboxyl groups. Bands at 1110 and 879 cm–1 were attributed to the C–O–C bond in polyether and the PEO side-chain. For ct-PCE, in addition to the bands at 3450, 2965 and 1635 cm–1 for the carboxyl group and that at 1000 cm–1 for the APEG side-chain, the bands at 3546, 1183 and 1051 cm–1 confirmed that the cationic amide group was successfully grafted to ct-PCE.
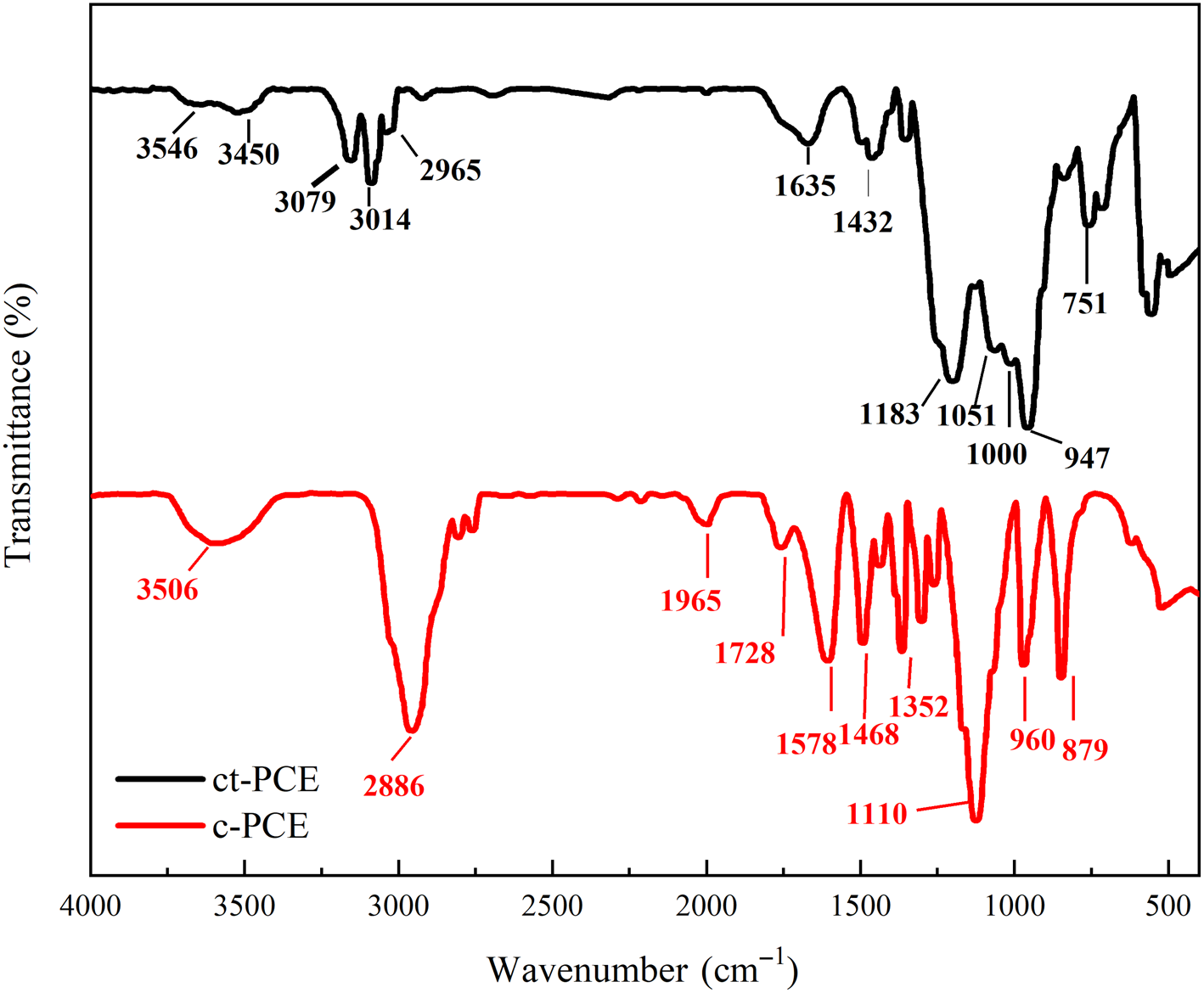
Fig. 3. FTIR spectra of ct-PCE and c-PCE.
GPC was used to characterize the molecular weight and weight distribution of ct-PCE and c-PCE. For comparison, the molecular information is presented in Table 3. The ct-PCE showed a smaller molecular weight than c-PCE, potentially due to the introduced shorter side-chain by APEG 500. However, for c-PCE, the materials often employed are macromonomers with molecular weights of ~2400 Da. The smaller PDI of ct-PCE further suggests that the synthesized terpolymer has a uniform molecular weight distribution. The ct-PCE sample also shows greater conversion than c-PCE. The configuration of PCE expressed by the hydrodynamic radius from the DLS test is shown in Table 3, where ct-PCE had a smaller hydrodynamic radius, probably due to the small side-chain.
Table 3. Molecular information for the ct-PCE and c-PCE samples.

Mn = number-average molecular weight; Mw = mass-average molecular weight; Rh = hydrodynamic radii.
PCE dosage requirement
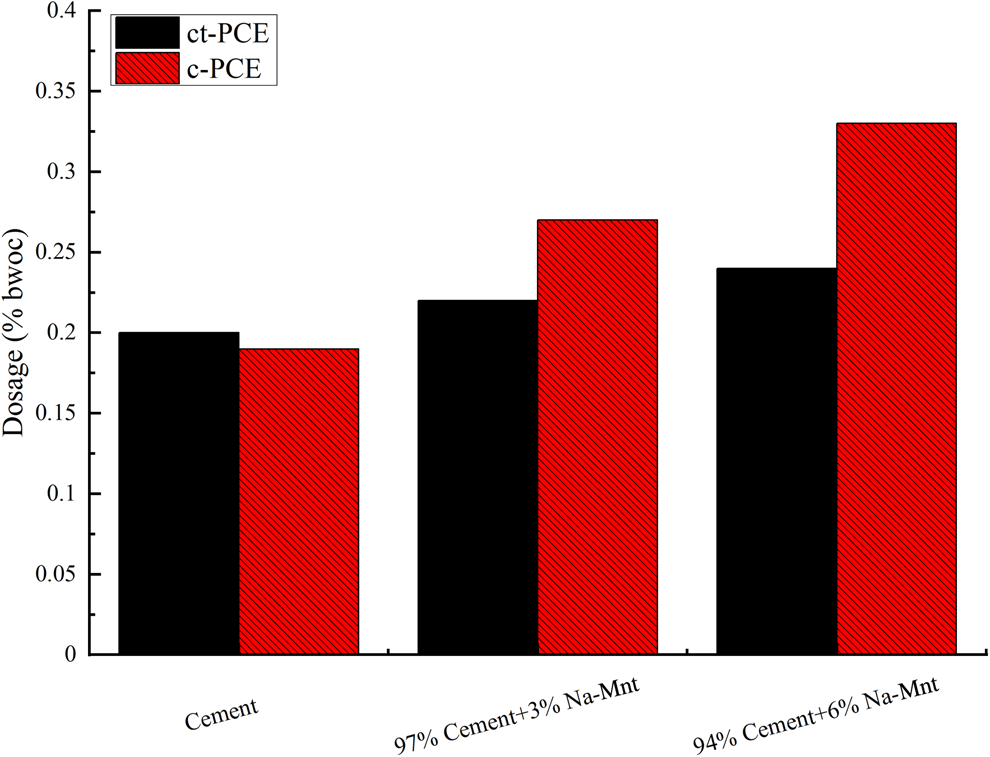
The effect of ct-PCE on the workability of cement containing Na-Mnt was investigated by determining the dosage requirement. The dosages of PCEs to achieve a minislump spread of 260 ± 5 mm were determined accordingly. The results that statistically differed among different samples (p < 0.05) are shown in Fig. 4. In the absence of Na-Mnt, the required dosage of ct-PCE was greater than that of c-PCE because the reduced side-chain hindered the steric repulsion of the PCE (Ran et al., Reference Ran, Somasundaran, Miao, Liu, Wu and Shen2009). The required dosage of both PCEs increased with increasing Na-Mnt dosage.
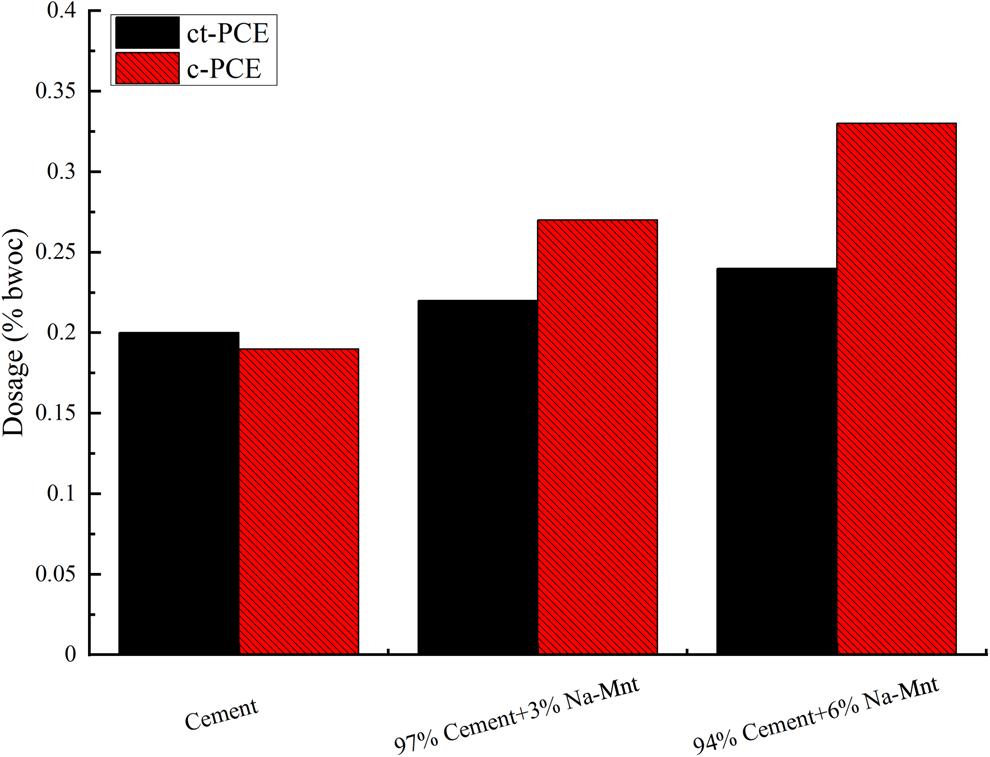
Fig. 4. Dosages of PCE required to achieve a minislump of 260 ± 5 mm (water-to-cementitious material ratio = 0.29). bwoc = by weight of cementitious materials.
There was a dramatic increase in PCE dosage observed with c-PCE compared to ct-PCE, which indicated that the compatibility of c-PCE with clay was poor due to intercalation with the PEO side-chain (Tan et al., Reference Tan, Gu, Ma, Li, Lin and Li2016a). A greater dosage was required for the cement without Na-Mnt compared to the cement with c-PCE, implying poor dispersion ability. However, there was no significant increase in the required amount of ct-PCE in the presence of Na-Mnt, suggesting enhanced clay tolerance in the ct-PCE.
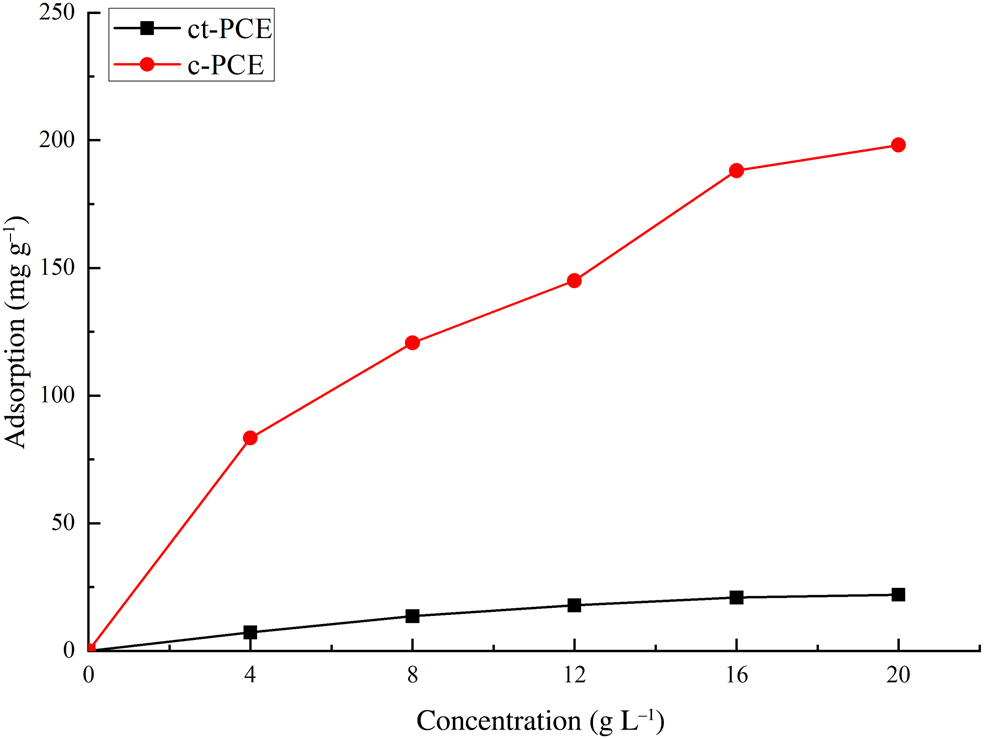
Sorption of PCE on Na-Mnt
The interaction between PCE and Mnt was studied by sorption measurements (Fig. 5). The PCE samples, and specifically ct-PCE, displayed Langmuir-type adsorption, which significantly increased at lower dosages, reaching equilibrium plateau at higher dosages. In addition, an extremely high sorption of commercial PCE with the short PEO side-chains on Na-Mnt (up to 200 mg g–1 Na-Mnt) was observed, while only a small amount of the ct-PCE was sorbed (<25 mg) on Na-Mnt. Less adsorption was observed in ct-PCE, but a smaller required amount of ct-PCE was also observed (see the ‘PCE dosage requirement' section), indicating the improved dispersion ability in ct-PCE. Both the c-PCE and Mnt have negative ζ-potentials (Zadaka et al., Reference Zadaka, Radian and Mishael2010), so the high observed sorption of c-PCE may be attributed to the PEO side-chain intercalating in the interlayer space (Ng & Plank, Reference Ng and Plank2012; Tan et al., Reference Tan, Gu, Ma, Li, Lin and Li2016a). Considering that PCE intercalation does not improve the dispersion of cement particles (Flatt & Houst, Reference Flatt and Houst2001), it was not surprising that greater sorption led to poor dispersion.
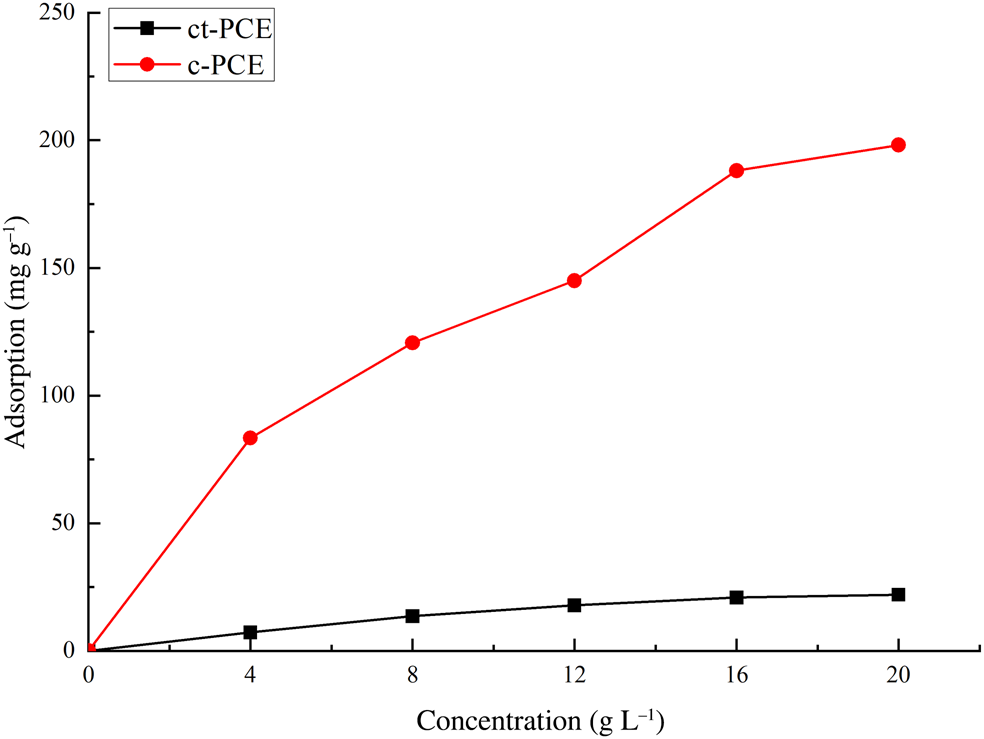
Fig. 5. Amount of ct-PCE and c-PCE sorbed on Na-Mnt.
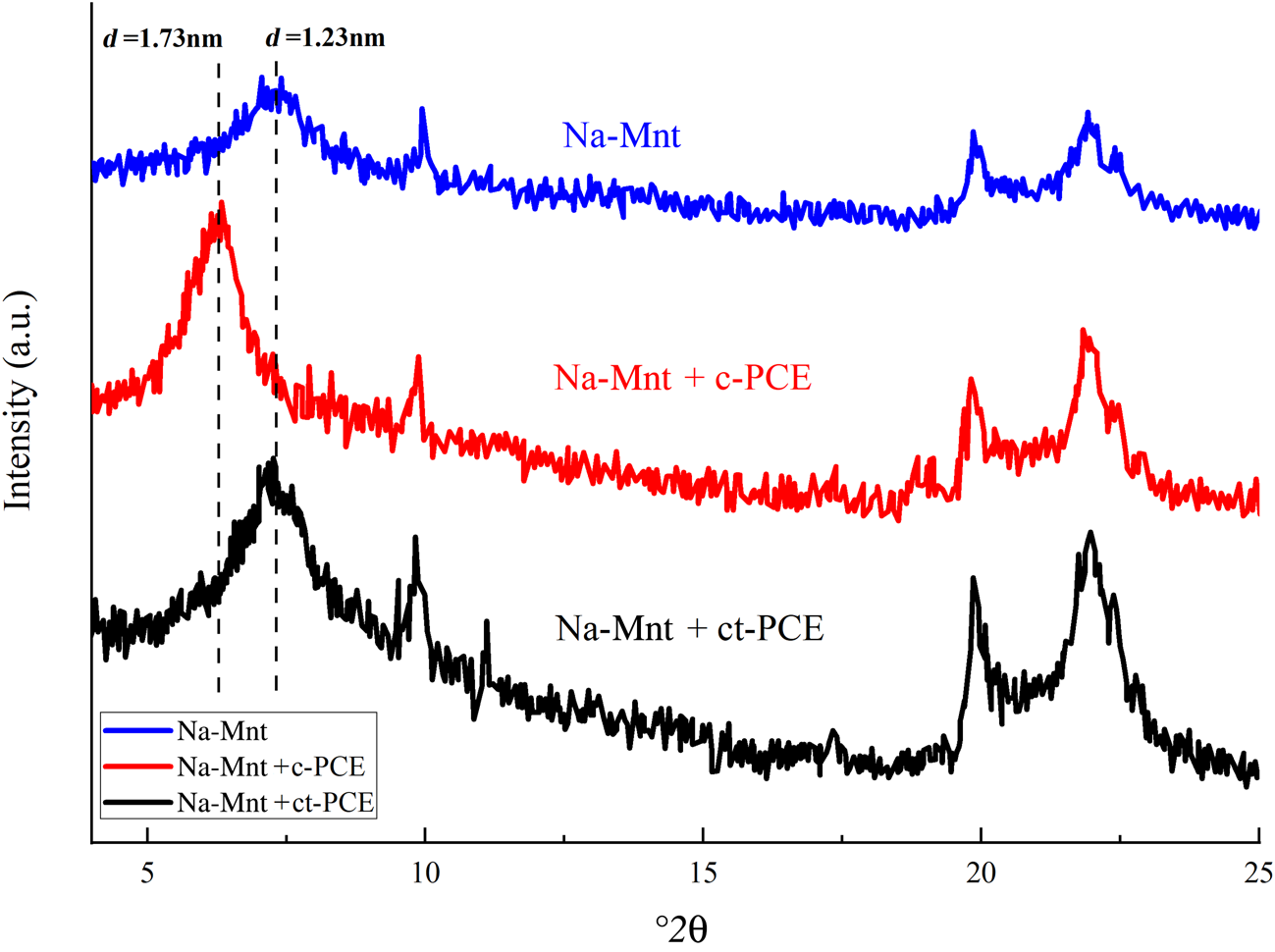
Intercalation behaviour of PCE with Na-Mnt
The XRD traces of Na-Mnt with or without PCE are plotted in Fig. 6. Without adding PCE, the d 001-spacing of Na-Mnt was ~1.23 nm (Tambach et al., Reference Tambach, Hensen and Smit2004). After c-PCE was incorporated with long PEO side-chains, the d 001-spacing increased to 1.73 nm, implying that the PEO side-chains of c-PCE were intercalated into the interlayer space of the clay (Ng & Plank, Reference Ng and Plank2012). However, there was no shift in the d-spacing after the addition of ct-PCE, indicating that there was no intercalation of ct-PCE in Na-Mnt. Therefore, the synthesized ternary ct-PCE did not intercalate in the Na-Mnt.
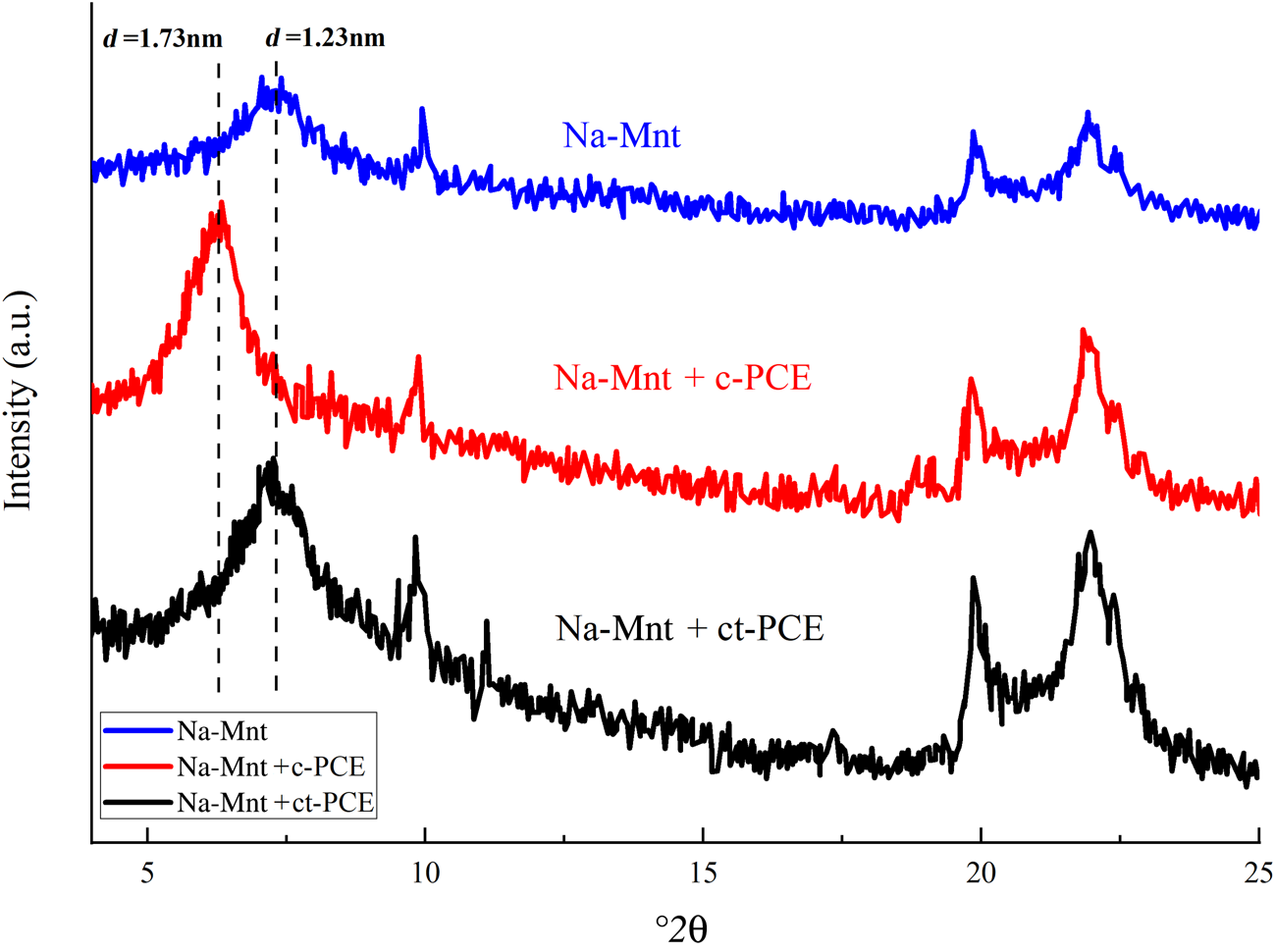
Fig. 6. XRD traces of Na-Mnt with or without PCE.
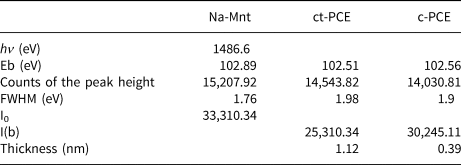
The thicknesses of the adsorption layers of both ct-PCE and c-PCE on Na-Mnt were calculated from Si2p XPS (Table 4). Photoelectron intensity was less in the samples supplied with PCEs than in pure Na-Mnt, indicating that the PCEs were adsorbed on the surface of Na-Mnt. The thickness of the new ct-PCE was calculated as 1.12 nm, while that of the adsorption layer was only 0.39 nm, which can be attributed to the dispersed side-chain in the solution rather than to intercalation between Na-Mnt layers. The increased adsorption layer again indicated that no intercalation of the side-chain of ct-PCE occurred.
Table 4. Si2p data of XPS and thickness of the sorption layer of PCE on Na-Mnt.

Eb = electron binding energy; FWHM = peak width at half-height; hv = photoelectron kinetic energy; I0 = initial photoelectron intensity; I(b) = photoelectron intensity after the photoelectron goes through the adsorption layer.
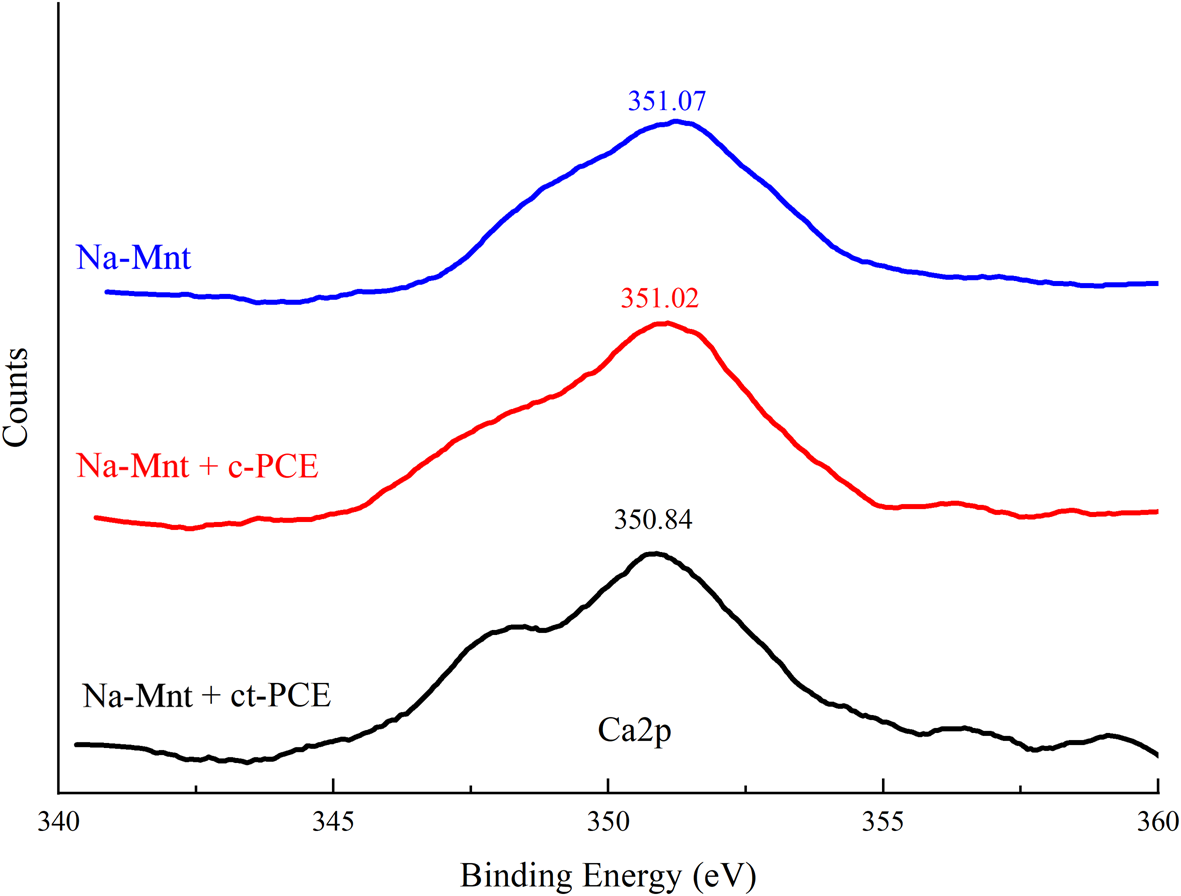
The adsorption of PCEs in a cement system depends on Ca2+ (Tan et al., Reference Tan, Guo, Ma, Huang, Gu and Zou2018b). The bond energy spectra of Ca2+ on the PCE-superplasticized Na-Mnt are shown in Fig. 7. The XPS spectrum revealed that not only does adsorption occur between the cement and PCEs, but there were also some chemical interactions with the dissolved ions. In comparison with pure Na-Mnt (351.07 eV), the binding energies of Ca2p in the presence of ct-PCE and c-PCE were 350.84 and 351.04 eV, respectively, which are close to that of pure Na-Mnt. This result indicated that new types of Ca2+ compounds did not form in the sample.
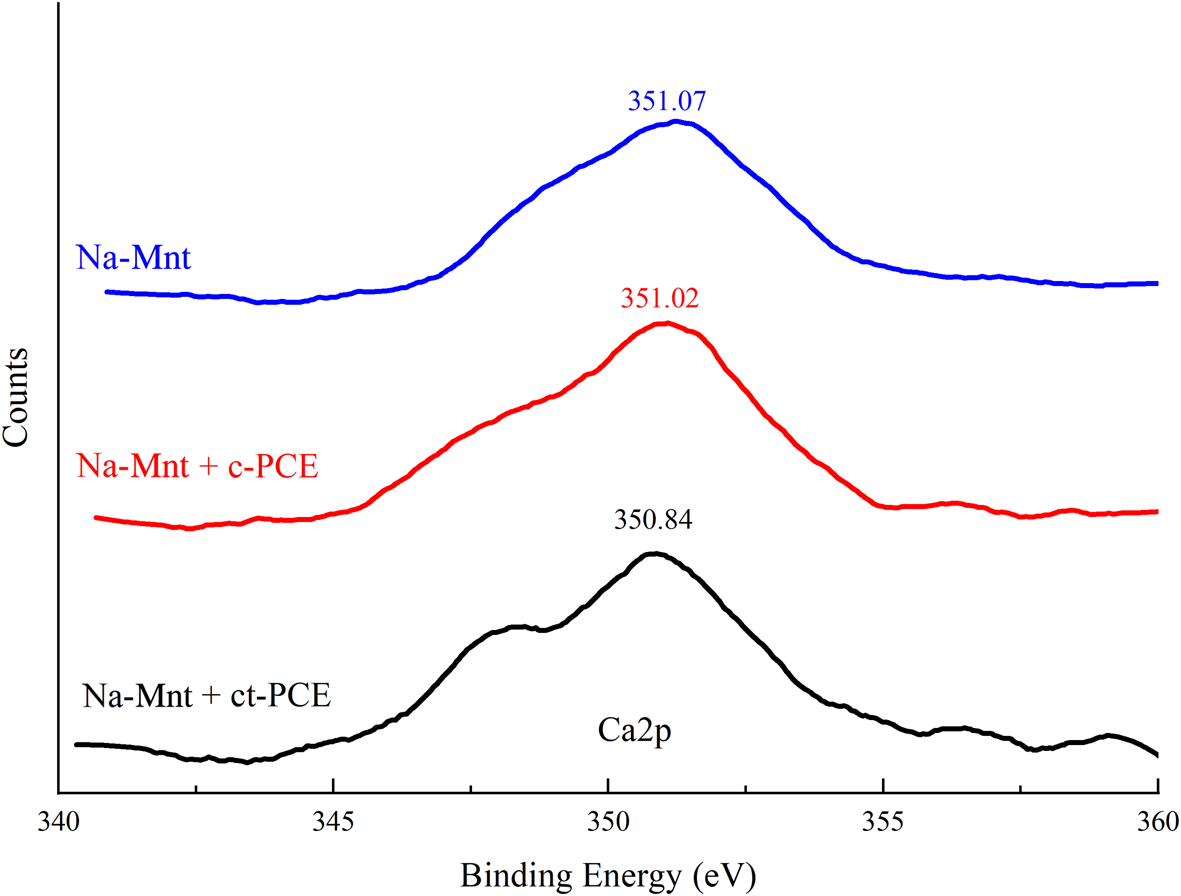
Fig. 7. Binding-energy spectrum of calcium in PCE-Mnt.
Model of interaction between PCE and Na-Mnt
The Mnt has a 2:1 structure with water and cations occupying the interlayer space (Fig. 8). Upon contact with water, Mnt layers swell due to expansion of the interlayer volume. Water molecules can enter the interlayers and cations within the interlayer space exchange with cations in the solution. The d 001 spacing of the original Mnt varied between 1.20 and 1.90 nm depending on the type of interlayer cations (Ait-Akbour et al., Reference Ait-Akbour, Boustingorry, Leroux, Leising and Taviot-Guého2015). As PCE is larger than the interlayer space, the polymer cannot easily enter the interlayer space and instead is adsorbed on the Mnt surface due to electrostatic attraction (Table 4). The side-chain of the PCE may also intercalate between the layers.
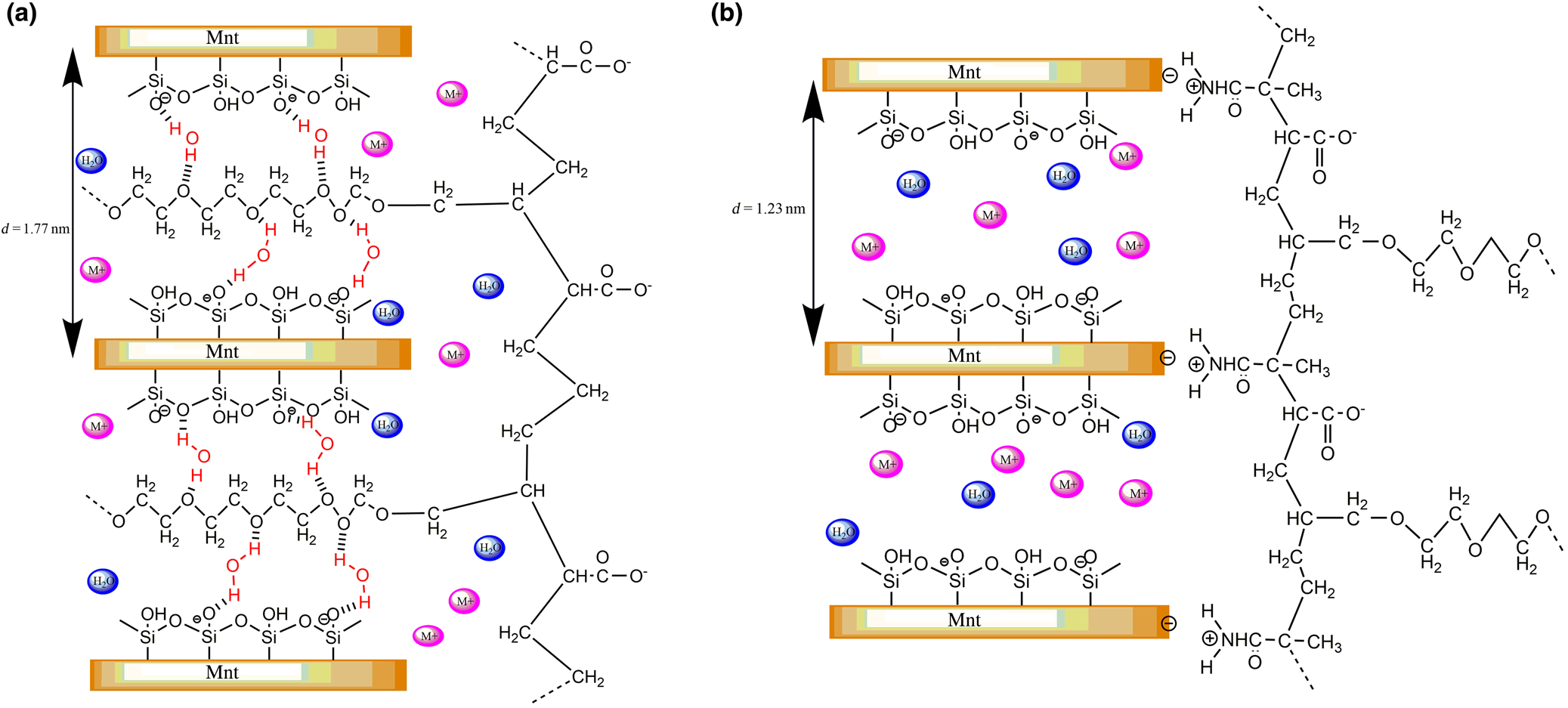
Fig. 8. Schematic diagram of Mnt with PCEs: (a) c-PCE and (b) ct-PCE.
In the presence of c-PCE, the d 001-spacing of Mnt increased from ~1.23 to 1.73 nm. The increased spacing between layers suggests that the PEO side-chain of the c-PCE entered the interlayer of Na-Mnt. The conformation size of the PEO side-chain in solution is ~0.70–1.40 nm (Houst et al., Reference Houst, Bowen, Perche, Kauppi, Borget and Galmiche2008), which is less than the interlayer space; hence, the PEO side-chain may enter the Mnt. As discussed in the ‘Sorption of PCE on Na-Mnt' section, a much greater sorption of c-PCE in Na-Mnt was observed, but the dispersion ability was still poor. This suggests that, compared to sorption, intercalation by the PEO side-chain was the dominant mechanism for c-PCE in Na-Mnt. Compared to ct-PCE, the smaller thickness of the sorbed PCE layer also indicates that intercalation of the PEO side-chain occurred in the presence of c-PCE (Table 4).
The conformation size of the ct-PCE was ~5.25 nm, which was larger than the spacing between the layers (1.23 nm) (Table 3). The 001 peak of Mnt did not shift with the addition of ct-PCE, indicating that no intercalation of ct-PCE occurred in the interlayer space (Fig. 6). This may be because the introduced cationic groups inhibited the expansion of Na-Mnt and consequently reduced the likelihood of intercalation by PEO. Lower sorption appears to enhance ct-PCE dispersion performance, which indicates that the new terpolymer is clay-tolerant.
Conclusions
The results of this work are summarized as follows:
(1) Introducing cationic amide groups with shorter side-chain lengths improved the dispersion performance of PCE in cement containing Na-Mnt by increasing the initial minislump of the cement paste.
(2) A quadratic model for the minislump of the cement pastes mixed with Na-Mnt was developed statistically. The optimal conditions for ct-PCE dispersion performance in Na-Mnt-incorporated cement paste were a side-chain ratio ((AA + AM):APEG) of 4:9, a monomer ratio (AA:AM) of 9:8, an initiator dosage of 2.9% and a synthesis temperature of 81°C. Experimental values confirmed the optimization accuracy of the model.
(3) The dosage requirement of both ct-PCE and c-PCE increased as the Na-Mnt dosage increased. Compared to commercial c-PCE, the modified ct-PCE showed a lower dosage requirement.
(4) Unlike commercial PCE, no intercalation occurred in the newly manufactured ct-PCE with clay, leading to better cement dispersion.
Although the ct-PCE was successfully synthesized and its compatibility with Na-Mnt was investigated in this study, a comprehensive understanding of the mechanism was not achieved. Further research is necessary.
Acknowledgements
Mr Shengye Xu is acknowledged for his contribution to this manuscript.
Financial support
Financial support from the National Natural Science Foundation of China including 51908526 (Jun Ren), 51808196 (Shuqiong Luo), 51772227 (Hongbo Tan) and 52008256 (Min Liu), and from College Student Innovation and Entrepreneurship Training Project of Yunnan University (202105091, Jun Ren), is gratefully acknowledged.