The presence of dyes in industrial effluents is a significant environmental problem because of their adverse effects on various life forms (Mills et al., Reference Mills, Hazafy, Parkinson, Tuttle and Hutchings2011; Yaneva & Georgieva, Reference Yaneva and Georgieva2012; Gupta et al., Reference Gupta, Khamparia, Tyagi, Jaspal and Malviya2015). More than 100,000 dyes which are used commercially are estimated to exist and >8 × 105 tons are produced annually (Chen & Zhao, Reference Chen and Zhao2009). The industrial use of these compounds generates a considerable amount of dye wastewater (Mukherjee et al., Reference Mukherjee, Kedia, Rao, Dhir and Paria2015). The presence of dyes in water, even in very small amounts is undesirable (<1 ppm for some dyes, Rafatullah et al., Reference Rafatullah, Othman Sulaiman, Hashim and Ahmad2010): dye in wastewater diminishes the water's transparency, which then reduces the penetration of solar radiation and photosynthetic activity (Mills et al., Reference Mills, Hazafy, Parkinson, Tuttle and Hutchings2011), and affects aquatic life in general.
Methylene blue, also known as methylthioninium chloride, is a basic dye used for leather tinting and cellulose fibres. It is also used as a redox indicator, an antiseptic, a diagnostic agent in renal function tests, as an antidote to cyanide and nitrate poisoning and as a treatment for malaria (Mills et al., Reference Mills, Hazafy, Parkinson, Tuttle and Hutchings2011). It is a cationic dye which is soluble in water. Congo red is a sodium salt of benzidinediazobis-1-naphthylamine-4-sulfonic acid. It is an anionic azo-dye which is very soluble in water. Due to its complex aromatic structure, which makes it physically and chemically stable, it is very resistant to biodegradation and photodegradation (Chen & Zhao, Reference Chen and Zhao2009; Yaneva & Georgieva, Reference Yaneva and Georgieva2012). In the literature, both dyes, but the CR in particular, are considered to be highly toxic to aquatic life, producing carcinogenic and mutagenic effects.
Several decontamination treatments have been explored extensively for removing these organic dyes from wastewater (Chen & Zhao, Reference Chen and Zhao2009; Mills et al., Reference Mills, Hazafy, Parkinson, Tuttle and Hutchings2011; Gupta et al., Reference Gupta, Khamparia, Tyagi, Jaspal and Malviya2015; Mu & Wang, Reference Mu and Wang2016). These treatments are often inefficient, however, due to their cost and/or the generation of secondary contaminants. From a technological point of view, adsorption has been one of the most effective and practical methods, efficient and relatively cheap, for eliminating non-biodegradable contaminants from wastewater. Numerous adsorbents may be used for these purposes, such as activated carbons, alumina, silica, zeolites, clays, chitin and ionic exchange resins (Hajjaji et al., Reference Hajjaji, Andrejkovičová, Pullar, Tobaldi, Lopez-Galindo, Jammousi, Rocha and Labrincha2016; Milošević et al., Reference Milošević, Logar, Dojčinović, Rosić and Erić2016).
Palygorskite is a clay mineral with exciting structural properties: it is a 2:1 layer aluminosilicate, which contains a continuous two-dimensional tetrahedral sheet (SiO44–) and octahedral sheets (AlO3(OH)36–) continuous only in one dimension. The tetrahedral sheets are divided into ribbons, and each ribbon is linked to the next ribbon by inversion of SiO44– tetrahedra along a set of Si–O–Si bonds (Galán, Reference Galán1996; Ismadji et al., Reference Ismadji, Edi, Ayucitra and Sharma2015). These features provide a rigid character to the clay structure, which has large tunnels parallel to the phyllosilicate ribbons which are partially occupied by H2O molecules (called zeolitic water) and exchangeable cations. Partial isomorphous substitution in the tetrahedral and octahedral sheets (Si4+ by Al3+ and, Al3+ and Fe3+ by Mg2+, respectively) results in a negative charge on the surface (Shuchuan et al., Reference Shuchuan, Shisheng, Tianhu, Shaotong and Chuanhu2006; Sattler, Reference Sattler2010; Chen et al., Reference Chen, Zhao, Zhong and Jin2011). As palygorskite has a large specific surface area and contains micropores in its structure, it is used widely as an adsorbent.
Because montmorillonite is a common component in palygorskite clays, an evaluation of its contribution to the adsorption of organic species onto palygorskite clays is of interest. Based on the elements described above, the palygorskite is suitable for removing pollutants from wastewater. A new deposit of this clay has been discovered in Cuba, but its use as a host for dyes has been explored little. The present study attempts to demonstrate the effectiveness of this natural clay, without modification, at the uptake of dyes (Congo red and methylene blue), potentially toxic to human and aquatic life. The focus here is on the capture of the anionic dye, i.e. Congo red.
MATERIALS
Palygorskite (PAL) from Cuba was used as a raw material. It was supplied by the Research Center for Mining and Metallurgical Industries (CIPIMM). The methylene blue (MB) and Congo red (CR) dyes of analytical reagent grade were provided by Sigma-Aldrich. Natural mixtures of palygorskite and montmorillonite minerals (identified as PAL1 and PAL2) were used in the study. Table 1 lists the quantitative mineralogical compositions and the specific surface areas of the clay samples used in this work.
Table 1. Quantitative mineralogical composition for the raw material (PAL) and the natural mixture of palygorskite and montmorillonite (PAL1 and PAL2), as well as the specific surface areas of the different clay samples.

EXPERIMENTAL
Experiments were performed by mixing 10 mL of aqueous dye solution with 100 mg of PAL in powder form, in a 30 mL conical flask under continuous stirring. After the interaction, the suspensions were centrifuged. In order to guarantee the thermal stability of both composites, the resulting solids were dried at 65°C for 48 h. All the experiments were performed in triplicate and the average values were used in data analysis.
Initially, the clay–dye interaction studies took place at 27°C over a 4 h period and with an initial dye concentration of 1 mg mL–1. In these cases, the pH values at the end of the interaction was 8.5 and 9 for MB and CR, respectively. The dye adsorbed by the PAL was calculated as:
where q e (mg g–1) is the mass of dye adsorbed per unit mass of the adsorbent, C 0 is the initial concentration of the dye solution (mg mL–1), C f is the concentration of the dye solution at equilibrium (mg mL–1), V is the volume of solution (mL) and m is the mass of the adsorbent (g) used in the experiments.
In order to establish the optimal conditions to achieve maximum uptake of dye by the clay, various physical-chemical parameters – initial dye concentration, time, temperature and pH – were evaluated. Taking into account the residual concentrations of these organic contaminants, the initial concentrations tested were 0.1‒5 mg mL–1 for MB and 0.1‒2 mg mL–1 for CR. The reaction time was 1‒8 h and the temperature ranged between 27 and 65°C. In addition, the pH assayed for the MB varied from 2 to 8.5 to avoid chemical modifications of MB at higher pH (Mills et al., Reference Mills, Hazafy, Parkinson, Tuttle and Hutchings2011). For the CR, the pH range was 5.5‒9, considering that at these pH values the dye molecule appears in wastewater (Yaneva & Georgieva, Reference Yaneva and Georgieva2012). The chemical structure and dimensions of the CR and MB molecules are shown in Fig. 1.
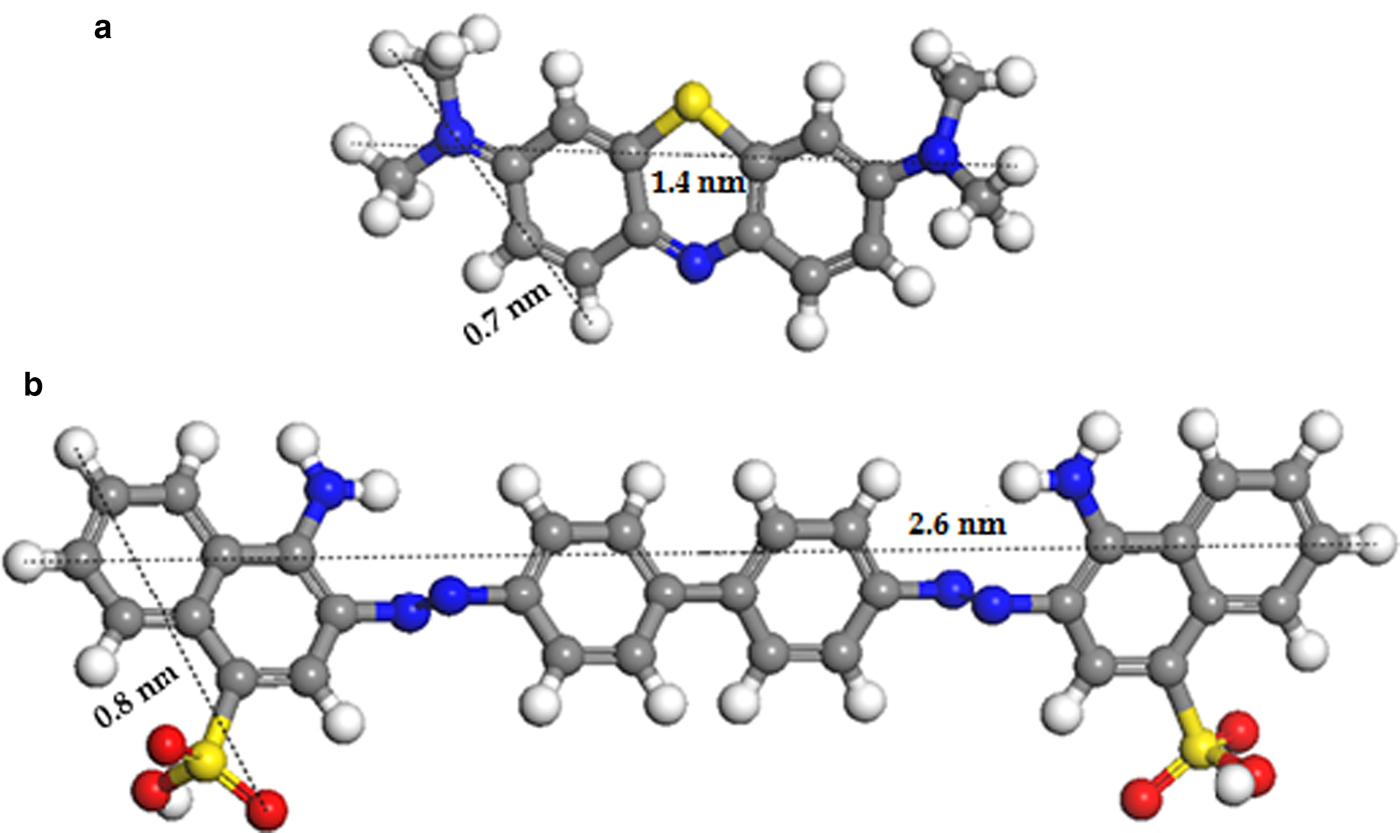
Fig. 1. Chemical structure and dimensions of the dyes molecules MB (a) and CR (b). White, grey, blue, red and yellow balls correspond to hydrogen, carbon, nitrogen, oxygen and sulfur atoms, respectively.
Various palygorskite samples, containing different amounts of montmorillonite (natural mixtures of the two minerals), were evaluated considering the experimental conditions for maximum adsorption of dye onto PAL.
The dye solutions, before and after the interactions with the clays, were analysed by UV-vis spectrometry (Rayleigh UV-2601 spectrophotometer) in the visible region: at 668 nm for MB+ and 746 nm for MBH2+ in its protonated form (Mills et al., Reference Mills, Hazafy, Parkinson, Tuttle and Hutchings2011), and at 499 nm for CR with an error of ± 0.5 nm (Yaneva & Georgieva, Reference Yaneva and Georgieva2012). The X-ray diffraction (XRD) patterns for the various solid samples were recorded on a Philips Xpert diffractometer, using Cu-Kα radiation (λ = 1.54 Å) at room temperature. The experiments were performed at a scan rate of 1° min–1 for a range of 3–60°2θ.
The surface charge of the samples was evaluated using a zeta potential analyzer (Malvern Nano Zetasizer instrument). Before measurements, 1 mg of sample was dispersed fully in 2 mL of a 0.001 mol L–1 KCl solution under ultrasonic stirring for 1 min. The pH was adjusted in the range 2.0–10.0 pH units using small amounts of HCl (10.0 mol L−1) or NaOH (4.0 mol L−1) solutions. The measurements were made in triplicate and the averages were reported.
RESULTS AND DISCUSSION
Dye adsorption on the clay
The preliminary results, without parameter selection, allowed us to verify the affinity of the PAL clay for the dyes. The amounts adsorbed were ~100 and 35 mg g–1 ± 1 mg g–1 for MB and CR, respectively. Based on their chemical structures, these results were expected. For example, the MB is in cationic form, with a resonance structure (positive, decentralized charge), which favours electrostatic interactions with the negative surface of the clay. The presence of weaker interactions (H-bonds) between the protons of the MB and the silanol groups of the PAL surface also contribute to the adsorption of MB on the clay. For the CR2–, which is in divalent anionic form, strong electrostatic repulsions with the negative surface of the clay might take place. However, the small amount of CR adsorbed might be explained based on the formation of H-bonds between electronegative atoms (N, S and O) of the dye and the protonated silanol groups at the PAL surface.
Effect of time, temperature and initial concentration of MB and CR on the adsorption process on the PAL sample
The effect of the initial dye concentration on the adsorption capacity of the clay is illustrated in Fig. 2.
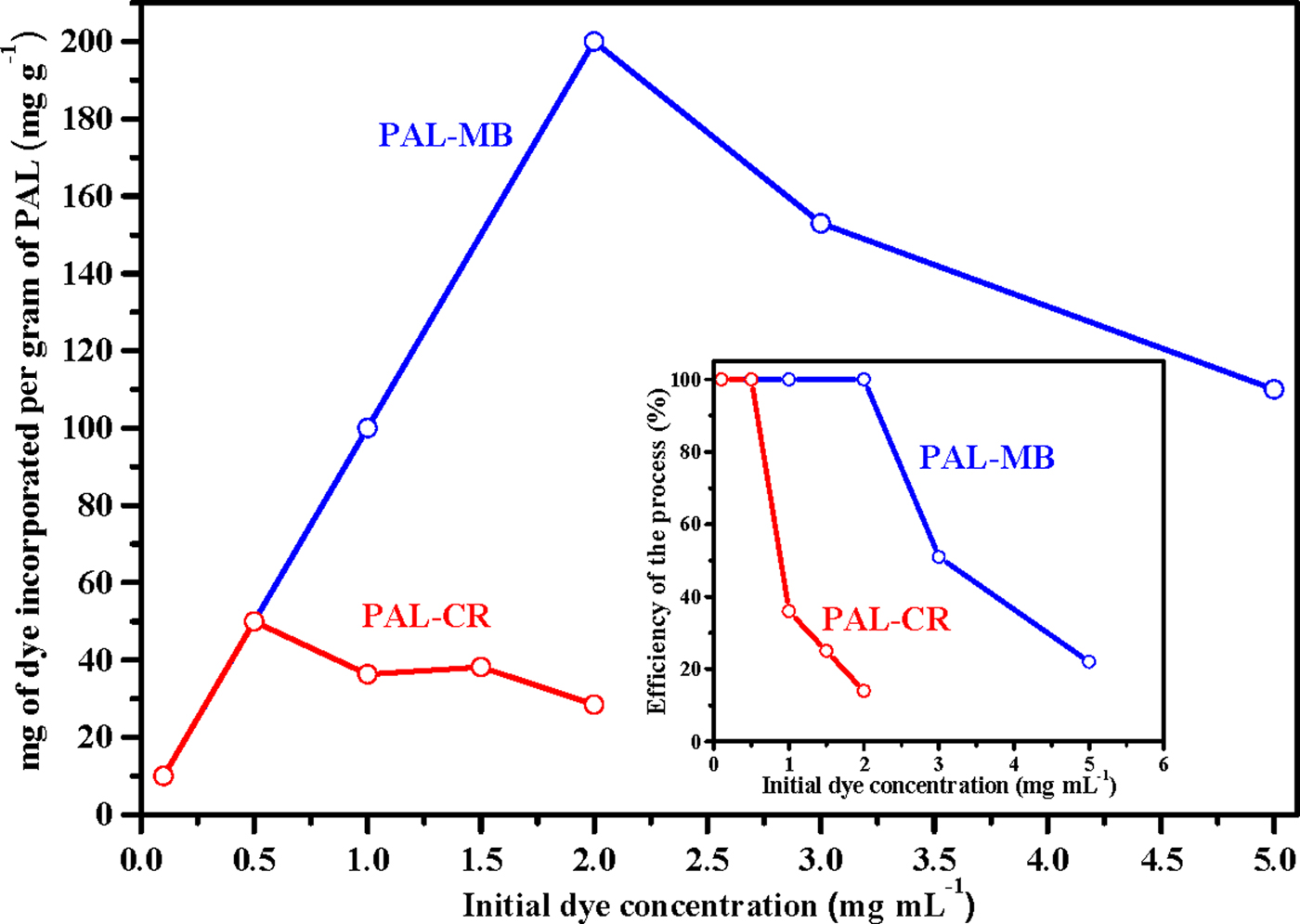
Fig. 2. Dependence of the amount of dye incorporated on the PAL sample as a function of the initial dye concentration. The inset shows the efficiency of the incorporation process with the initial dye concentration in solution. The efficiency was calculated as the percentage of dye incorporated on the clay relative to the initial amount of dye in the solution.
For the MB, the amount incorporated on the PAL sample increased with the increase in the initial dye concentration, reaching a maximum at 2.0 mg mL–1, decreasing at higher concentrations. In the case of CR, an increase in the dye adsorption on clay was detected for an initial dye concentration of up to 0.5 mg mL–1 decreasing at higher concentrations. For relatively low initial concentrations, a diffusive process of mass transfer between aqueous and solid phases (i.e. from the phase with the highest concentration of dye to the lowest concentration) might take place (Valdés et al., Reference Valdés, Martín, Hernandez, Lazo, de Ménorval and Rivera2017). However, at higher concentrations, the interactions between the dye molecules, instead of clay–dye interactions, might be favoured. In addition, the efficiency of the process (% dye adsorbed on the clay/dye mass in the initial solution before interaction with the clay, see inset in Fig. 2) allowed us to select 0.1 mg mL–1 as the initial concentration for both MB and CR.
According to the kinetic study, the maximum efficiency of MB adsorption on PAL occurred in the first contact hour. The maximum uptake of CR took place after 4 h of interaction with the clay. These results are consistent with those discussed above in terms of the interactions involved in the clay–dye system.
The influence of the temperature indicated a slight decrease (~5%) in the amount of CR taken up by the clay, with increase in the temperature from 27 to 65°C. The size and rigidity of the molecules (Fig. 1b) might obstruct their free movement as the kinetic energy of the system increases, affecting the efficiency of the process. For the MB, the temperature did not seem to affect the adsorption equilibrium. The maximum MB incorporation (100% efficiency) is constant throughout the range of temperatures studied. Based on these results, the temperature of 27°C was selected for the rest of the experiments.
Influence of pH on the adsorption process
The experiments demonstrated that the incorporation of MB on PAL did not depend on the pH being constant throughout the pH range studied (Fig. 3) Based on that, the pH value reached spontaneously by the clay-dye suspension (i.e. 8.5) was selected for the study. As mentioned above, the MB is protonated and it can polymerize in acid aqueous solution. In addition, at acidic pH there are also protons (H+) in solution, which will compete with the MB cations for the active sites of the clay. The extent of uptake suggests a strong affinity between PAL and MB cations.

Fig. 3. Incorporation of MB and CR in PAL as a function of pH.
The amount of CR dye adsorbed on the PAL decreased as the pH increased (Fig. 3), which might be related to the pHpzc of the clay (Ismadji et al., Reference Ismadji, Edi, Ayucitra and Sharma2015). The pH values were higher than the pHpzc of the palygorskite, which is ~2.0. So, at the clay surface, for the entire pH range under study, the Si–O‒ groups should dominate, forming a repulsion barrier for the anionic species such as CR2–. For CR, pH 7 was chosen, due to the amount of dye adsorbed on the clay and because at this pH, structural changes to the CR molecule are less likely (i.e. the CR does not change from red to dark blue).
For both dyes, the best composite was obtained at 27°C and an initial dye concentration of 0.1 mg mL–1. The time of interaction was 4 h at pH = 7 for CR and 1 h at pH = 8.5 for MB.
The amount of MB adsorbed by the natural unmodified palygorskite from Cuba is greater than that reported in the literature. Thus, for an initial concentration of 1 mg mL–1, the MB taken up by a natural palygorskite was ~50 mg g–1 with an efficiency of ~80% (Al-Futaisi et al., Reference Al-Futaisi, Jamrah and Al-Hanai2007). Additionally, in some studies where the playgorskite was submitted to chemical modification before interaction with the dye, the MB load per gram of material was comparable, or smaller (Sarkar et al., Reference Sarkar, Erming Liua, McClure, Sundaramurthy, Srinivasan and Naidua2015; Moreira et al., Reference Moreira, Ciuffi, Rives, Vicente, Trujillano, Gil, Korili and de Faria2017), than that reported in the present work. It the case of the anionic dye, CR, the load per gram of natural palygorskite reported in the literature is negligible (Chen & Zhao, Reference Chen and Zhao2009) or at least less than that described here (Taha & Samaka, Reference Taha and Samaka2012).
Sample characterization
The XRD patterns for samples with different palygorskite and montmorillonite contents (PAL1 and PAL2) in the natural mixtures and for the resulting composites are shown in Fig. 4. In the PAL sample, the main phases present in the natural clay were montmorillonite, palygorskite and quartz (Mineralogy Database, 2012). These phases were also identified in the XRD patterns for the PAL-MB and PAL-CR composites.

Fig. 4. XRD patterns for PAL, PAL-MB and PAL-CR, including the hkl values of the characteristic planes of each of the phases. The inset shows the patterns for PAL1, PAL2, PAL1-CR and PAL2-CR (i.e. palygorskite samples with different percentages of montmorillonite before and after the interaction with the CR dye).
The characteristic diffraction maxima of palygorskite and montmorillonite did not change after adsorption of the dye, suggesting that the MB and CR were taken up on the clay minerals’ surface. Based on the molecular dimensions of the dyes (see Fig. 1) and on the dimensions of the palygorskite channel (~0.40 nm × 0.60 nm) (Galán, Reference Galán1996; Bergaya & Lagaly, Reference Bergaya, Lagaly, Bergaya, Theng and Lagaly2006; Ismadji et al., Reference Ismadji, Edi, Ayucitra and Sharma2015), the dye molecules in solution are not expected to penetrate into the channels. Therefore, the clay–dye interactions seem to have been governed fundamentally by the functional groups in the clay surface as was reported in previous studies (Shuchuan et al., Reference Shuchuan, Shisheng, Tianhu, Shaotong and Chuanhu2006; Chen et al., Reference Chen, Zhao, Zhong and Jin2011, Reference Chen, Zhong, Wu, Zhao and Yan2012; Yang et al., Reference Yang, Li, Li and Yang2018). From a structural point of view, the isomorphous substitutions and vacant sites in the octahedral sheets generated an ‘excess’ of negative charge. The coordinating OH groups, broken bonds and crystal defects may generate active sites for adsorption. Therefore, the adsorption of positively charged molecules onto this clay will depend to a great extent on the amount and distribution of these active sites. In fact, an important amount is adsorbed on the external surface and on crystal defects rather than within the channels. Thus, at basic pH, the clay surface is more negative due to the ionization of Si–OH to Si–O‒ (i.e. more active adsorption sites), which leads to greater adsorption of cationic dyes such as MB.
Few studies have been conducted on the adsorption of anionic dyes by palygorskite, i.e. without prior chemical modification; where the treatments described are under strong acid conditions; and where electrostatic attractions between protonated Si–OH (Si–OH2+) on the mineral surface and the negatively charged sites of dye molecules seem to govern the process (Chen et al., Reference Chen, Zhong, Wu, Zhao and Yan2012; da Silva et al., Reference da Silva, Ferreira-Santos, Chaves-Santos, Santos Júnior, da Fonseca and da Silva Filho2016). However, in the present study, CR2– uptake takes place at pH = 7, where the clay surface charge is partially negative, in accordance with previous studies on the adsorption of anionic species (dyes and drugs) on palygorskite (Chang et al., Reference Chang, Li, Yu, Munkhbayer, Kuo, Hung, Jean and Lin2009; Chen & Zhao, Reference Chen and Zhao2009; Taha & Samaka, Reference Taha and Samaka2012; Yang et al., Reference Yang, Li, Li and Yang2018). Thus, the CR2– adsorption might be attributed to electrostatic interactions with the edges of the sheets positively charged due to the broken bonds (terminal hydroxyls), and to the presence of metal ions (especially Mg2+, isomorphically substituted) acting as bridges between the anionic dye and clay surface. The anionic dye might interact by ligand exchange with water molecules around compensating cations onto the clay surface involving the inter-particle space. A more detailed study would be necessary to establish the different mechanisms involved in the CR anionic dye adsorption.
The position of the 001 basal reflection of montmorillonite did not change after the incorporation of the dyes, which suggests surface adsorption (Fig. 4), although a decrease in the intensity of this reflection was detected after the interactions with both dyes. This might be explained by the possible dissolution of the montmorillonite phase due to the pH during the interaction process with the MB and CR. The dissolution is minimal at neutral pH but might be significant in more acidic and alkaline conditions (Rozalén et al., Reference Rozalén, Huertas, Brady, Cama, García-Palma and Linares2008, Reference Rozalén, Huertas and Brady2009; Myllykylá et al., Reference Myllykylá, Tanhua-Tyrkko, Bouchet and Tiljander2013). So, for MB where the interaction pH is >7, the dissolution was greater than in the case of CR, when comparing PAL-CR and PAL-MB.
The influence on dye absorption of different percentages of palygorskite and montmorillonite in the natural samples is illustrated in Table 2. For the CR, the decrease in the percentage of palygorskite (see inset in Fig. 4) produced a significant decrease in the amount of CR adsorbed by the clay mixtures. This might be explained by the specific surface area, cation exchange capacity (CEC) and surface charge of the palygorskite and montmorillonite. The CEC of montmorillonite is greater than that of palygorskite (Chen & Zhao, Reference Chen and Zhao2009; Chemeda et al., Reference Chemeda, Christidis, Tauhid-Khan, Koutsopoulou, Hatzistamou and Kelessidis2014; Ismadji et al., Reference Ismadji, Edi, Ayucitra and Sharma2015; Tsai et al., Reference Tsai, Chang, Gao, Xu, Chen, Wang, Chen, Yang, Jean and Lia2016), due to the greater layer charge. Indeed, the zeta-potential results indicated that the surface charge of the PAL sample is smaller (~–20 mV) than a montmorillonite sample (~–30 mV). Thus, it is expected that a decrease in the palygorskite content in the natural mixture would lead to a decrease in the number of CR anions adsorbed onto clay due to the electrostatic repulsion. In addition, a decrease in this phase of the samples, would cause a decrease in the number of active sites available in the mixture due to a smaller specific surface area (see Table 1).
Table 2. Efficiency of CR and MB incorporation (0.1 mg mL–1 of initial dye concentration) in natural samples with different percentages of palygorskite (P) and montmorillonite (M). P/M corresponds to the mass percentage of the two phases relative to the total mass of the sample.

On the other hand, the adsorption efficiency for MB is the same for all the mixtures assayed, confirming the high affinity of the natural mixtures for this cationic dye (Table 2). For the experimental conditions described here, therefore, the average surface negative charge density seems to control the adsorption process beyond the surface area (see Table 1), and the CEC of each individual phase.
In general, the results in Table 2 and the lack of variation in the position of the 001 basal reflection in the XRD patterns, suggest that the dye-incorporation process was controlled mainly by the palygorskite.
CONCLUSIONS
The potential use of a Cuban palygorskite clay, as an adsorbent to remove the Congo red and methylene blue dyes from aqueous solution, was examined. The effects of various physical-chemical parameters on the incorporation of dyes on the palygorskite (PAL), i.e. initial dye concentration, pH, contact time and temperature, were investigated. The adsorption efficiency of the PAL clay for CR and MB was optimal at pH 7 (4 h of interaction) and 8.5 (1 h of interaction), respectively, at 27°C and initial dye concentration of 0.1 mg mL–1. No significant variations were observed when the temperature was varied. The XRD analysis confirmed the adsorption of the dyes on the clay surface. The electrostatic interactions and H-bonding seemed to control the adsorption process. The decrease in the percentage of palygorskite in the natural palygorskite-montmorillonite mixtures diminished the adsorption capacity of CR on the clay, suggesting that the process is essentially controlled by that phase. Finally, it was demonstrated that the use of the Cuban palygorkite offers promise in terms of environmental remediation.
ACKNOWLEDGEMENTS
The authors thank the Organizing Committee of the 16th International Clay Conference and the University of Havana for financial support. Professor E. Alsthuler is acknowledged for a critical review of the manuscript. The help of D. Hernández with the Zeta potential measurements is also acknowledged. A. Rivera thanks the Academy of Sciences for the Developing World (TWAS) for research Grants No. 00-360 RG/CHE/LA and No. 07-016 RG/CHE/LA. Two anonymous referees are thanked for their valuable comments and suggestions.








