Boron is a micronutrient used in plant growth and development (Marschner, Reference Marschner2012). Some of the B used as fertilizer is highly soluble and easily leached into the soil profile. This is particularly important in very rainy areas. While most clayey soils retain for longer periods the B added, this behaviour is also observed in soils with more organic carbon and greater ion exchange capacity (Rosolem & Bíscaro, Reference Rosolem and Bíscaro2007). Thus, increase in the adsorption of this nutrient in the soil, for slow and gradual absorption by the plants, is essential for increasing agricultural productivity.
An alternative way to provide B in a sustainable manner includes storage in layered inorganic materials. The intercalation of borate ions in LDH is of interest as a new form of storage and sustained release of B for plants (Benício et al., Reference Benício, Silva, Lopes, Eulálio, Dos Santos, Aquino, Vergutz, Novais, Costa, Pinto and Tronto2015; Guan et al., Reference Guan, Lv, Bai and Guo2016).
The LDHs, also called hydrotalcite-type compounds, can be described structurally as the stacking of positively charged layers with hydrated anions in the interlamellar domain. To ensure a better understanding of the structure of LDH, it is convenient to start with the brucite structure. In brucite, a Mg(OH)2 mineral, the Mg2+ cations are in the centre of octahedra which have hydroxyl anions at their vertices. These octahedra share their edges, forming flat and neutral layers held together by H bonds. When, in this type of structure, the Mg2+ cations are replaced isomorphically by trivalent cations, the layer has a positive residual charge. To acquire electroneutrality, the system requires the presence of anions between the layers, which, alongside H2O molecules, promote the stacking of double hydroxide layers with an orderly interlamellar domain (Cavani et al., Reference Cavani, Trifirò and Vaccari1991; de Roy et al., Reference De Roy, Forano, El Malki, Besse, Occelli and Robson1992; Evans & Slade, Reference Evans, Slade, Duan and Evans2006; Forano et al., Reference Forano, Hibino, Leroux, Taviot-GuéHo, Bergaya, Theng and Lagaly2006). The general formula ![]() $\left[ {{M}_{{1 - x}}^{{2 +}} {M}_{x}^{{3 +}} \left( {{\rm OH}} \right)_{2}} \right]{A}_{{x}/{m}}^{{m -}} {\cdot} {n \rm H}_{2} {\rm O}$ characterizes these materials, in which M 2+ represents a bivalent cation, M 3+ represents a trivalent cation, and A m- represents an anion with m-charge.
$\left[ {{M}_{{1 - x}}^{{2 +}} {M}_{x}^{{3 +}} \left( {{\rm OH}} \right)_{2}} \right]{A}_{{x}/{m}}^{{m -}} {\cdot} {n \rm H}_{2} {\rm O}$ characterizes these materials, in which M 2+ represents a bivalent cation, M 3+ represents a trivalent cation, and A m- represents an anion with m-charge.
In the agricultural field, LDHs have been used in the intercalation of pesticides, herbicides, plant-growth hormones, as well as the intercalation of nutrients such as nitrate, phosphates (PO43−, HPO42−, H2PO4−), etc., to obtain matrices that have a sustained release of the intercalated anions (Tronto et al., Reference Tronto, Dos Reis, Silverio, Balbo, Marchetti and Valim2004; Cardoso et al., Reference Cardoso, Celis, Cornejo and Valim2006; Ghormade et al., Reference Ghormade, Deshpande and Pakmikar2011; da Silva et al., Reference Da Silva, Kamogawa, Marangoni, Mangrich and Wypych2014; Benício et al., Reference Benício, Constantino, Pinto, Vergutz, Tronto and Costa2016; Everaert et al., Reference Everaert, Warrinnier, Baken, Gustafsson, De Vos and Smolders2016, Reference Everaert, Degryse, McLaughlin, De Vos and Smolders2017; Halajnia et al., Reference Halajnia, Oustan, Najafi, Khataee and Lakzian2016; Yu et al., Reference Yu, Wang, Chen, Wang, Wang, Hayat and Wang2017).
Woo et al. (Reference Woo, Kim, Paek, Ha, Choy and Hwang2011) studied the synthesis, characterization and kinetics of release of phosphate ions intercalated in CaFe-LDH. Those authors suggested the importance of this material as a slow-release phosphate fertilizer and as a soil acidity neutralizer, although they did not conduct any experiments with plants to verify the actual effectiveness of the LDH produced. Everaert et al. (Reference Everaert, Degryse, McLaughlin, De Vos and Smolders2017) noted that, although Mg-Al-LDH has a reduced agronomic potential compared to monoammonium phosphate (MAP) after being incubated with wheat plants, this material has environmental benefits such as P reuse and residue use, thus being attractive for use as a slow-release fertilizer. To compare P-LDH with triple superphosphate in a Neubauer experiment, Benício et al. (Reference Benício, Constantino, Pinto, Vergutz, Tronto and Costa2016) noted an increase in plant mass and height, as well as the total P content in the DM (the weight of the plant when completely dried). The increase of soil pH in P-LDH treatments was the main contributor to the reduction of soil P adsorption.
Previous work has shown that the treatment of LDH to impart slow release of nutrients is of chemical and mineralogical nature, and suggested its applicability as a source of slow-release nutrients without, however, presenting conclusive bioassay results on this application (Berber et al., Reference Berber, Hafez and Minagawa2014; Imran et al., Reference Imran, López-Rayo, Magid and Hansen2016; Moraes et al., Reference Moraes, Tavares, Vaiss and Leitão2016; Ashekuzzaman et al., Reference Ashekuzzaman and Jiang2017). The present work thus has the following objectives: (1) to synthesize and characterize a Mg and Al LDH intercalated with borate ions (Mg2Al-B-LDH); and (2) to evaluate, by bioassay, the potential of the material produced for use as a matrix for storage and sustained release of B for plants.
MATERIALS
All reagents used in this work have a degree of analytical purity. Mg(NO3)2·6H2O (purity > 98%) was purchased from Vetec; Al(NO3)3·9H2O (purity > 98%) was purchased from Sigma-Aldrich; H3BO3 (purity > 99.5%) was purchased from Êxodo Científica, and NaOH (purity = 99%) was purchased from Vetec. The water used in the synthesis reactions was distilled and/or deionized (Milli-Q® system), according to the needs of its use.
LDH synthesis
The preparation of Mg2Al-B-LDH was carried out by the constant pH coprecipitation method (De Roy et al., Reference De Roy, Forano, El Malki, Besse, Occelli and Robson1992). In this method, 250 mL of a solution containing 1.0 mol L−1 of Mg(NO3)2·6H2O and 0.5 mol L−1 of Al(NO3)3·9H2O was added dropwise, under vigorous stirring, to 500 mL of a solution containing 1.25 mol L−1 of H3BO3. The synthesis was carried out under N2 atmosphere to eliminate the influence of atmospheric carbon dioxide. During the synthesis, a 2.0 mol L−1 NaOH solution was added to maintain the pH value at 10.0 ± 0.5. This pH value usually presents highly satisfactory results, obtaining MgAl-LDHs with good structural organization and phase purity (De Roy et al., Reference De Roy, Forano, El Malki, Besse, Occelli and Robson1992). After the synthesis, the solid material was washed with H2O and dried under vacuum in the presence of silica gel.
Characterization of the sample
To characterize Mg2Al-B-LDH, the following analysis techniques were used: X-ray powder diffraction (XRD), attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR), simultaneous thermogravimetric and differential thermal analysis (TGA-DTA), specific surface area (by the Brunauer Emmet Teller method (BET)), scanning electron microscopy (SEM), and ultraviolet-visible molecular absorption spectrophotometry (UV-Vis).
For the XRD analysis, a sample of Mg2Al-B-LDH was macerated to obtain particles of uniform size. A Shimadzu XRD-6000 instrument, with a copper cathode and a graphite monochromator, with a wavelength of 1.5406 Å, was used, at 30 kV and 30 mA. A scanning step of 0.01°, and 10 s per step were used and the scanning range was 4–7°2θ. The same instrument and conditions were used to characterize the soil mineralogy used in the bioassay. The ATR-FTIR spectrum was recorded on a Jasco FTIR 4100 spectrophotometer. The spectrum was obtained with 256 scans, over a wavelength range of 4000 to 400 cm−1. Simultaneous TGA-DTA analyses were performed using an SDT 2960 Simultaneous DTA-TGA thermal analyzer (TA Instruments), at a heating rate of 10°C min−1, with a dry synthetic air flow (80% N2 and 20% O2) of 100 cm3 min−1, in the temperature range of 30–1000°C. The amount of sample mass used was approximately 10 mg. The BET surface area of the Mg2Al-B-LDH was determined with N2 adsorption using a ChemBET Pulsar TPR/TPD device (Quantachrome Instruments©).
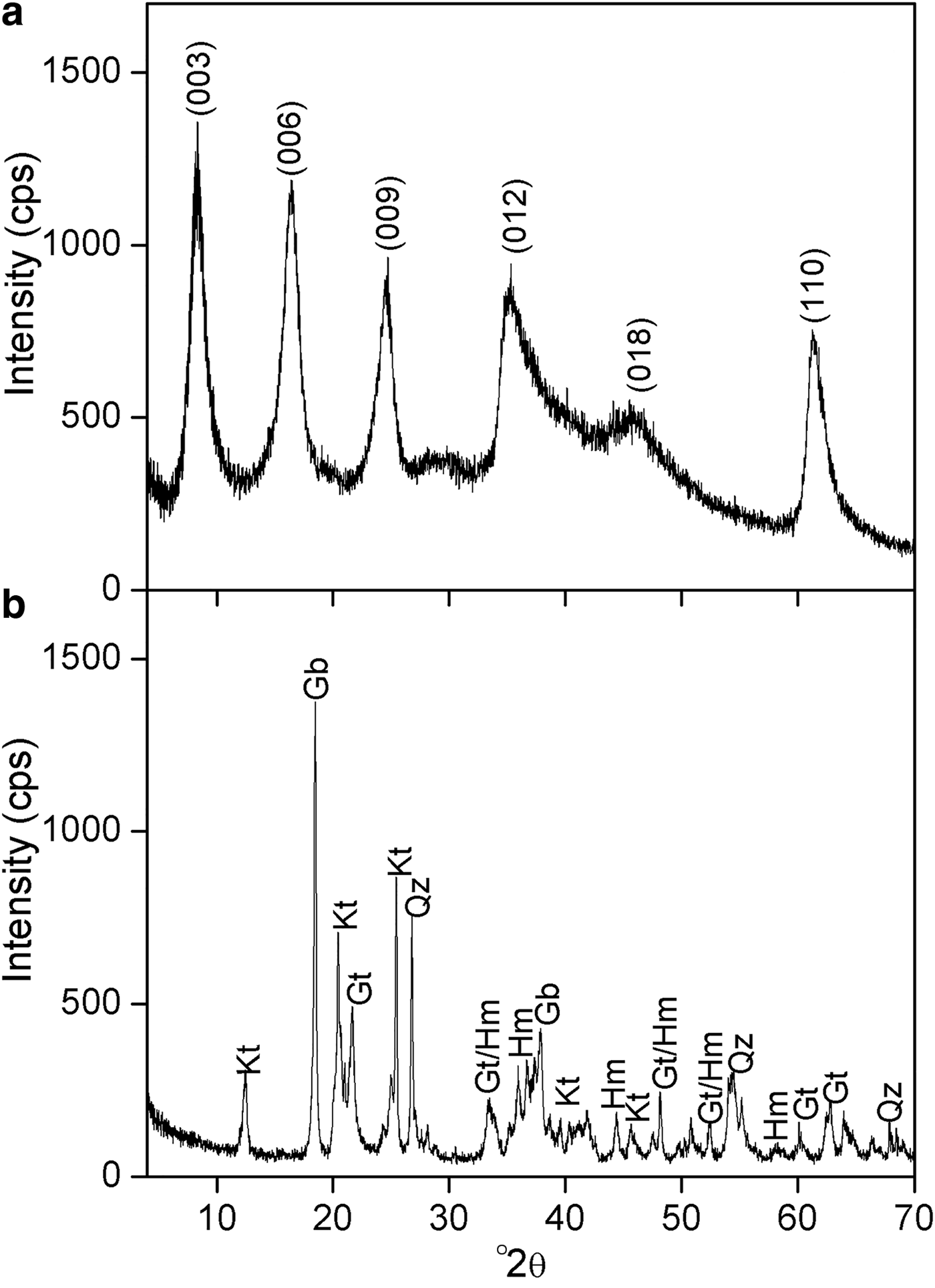
The SEM imaging was carried out on a Carl Zeiss EVO 50 microscope. The sample, in powder form, was supported on the sample holder by dispersion on a conductive double-sided adhesive tape. Gold coating was applied to the samples prior to measurement using a Bal-Tec SCD 050 Sputter Coater spray. The concentration of borate in Mg2Al-B-LDH was determined with Ultraviolet-Visible Molecular Absorption Spectrophotometry (UV-Vis), λ = 420 nm, in a Thermo Scientific Evolution 300 device. The B available in the soil was determined according to López et al. (Reference López, Giménez and Hernández1993). A soil sample for the bioassay was collected at 0–20 cm depth in a Dystrophic Red Latosol (DRL), an Oxisol (USDA, 1999), with a clay-like texture, in the region of the city of Rio Paranaíba (MG). The sample was air-dried, levelled and sieved with a 4-mm mesh sieve. After drying, the sample was homogenized. A sub-sample of this soil was removed and sieved using a 2-mm mesh sieve, obtaining air-dried fine earth (ADFE). The chemical and physical characteristics of the sample are presented in Table 1 and the mineralogical characterization is presented in Fig. 1a.
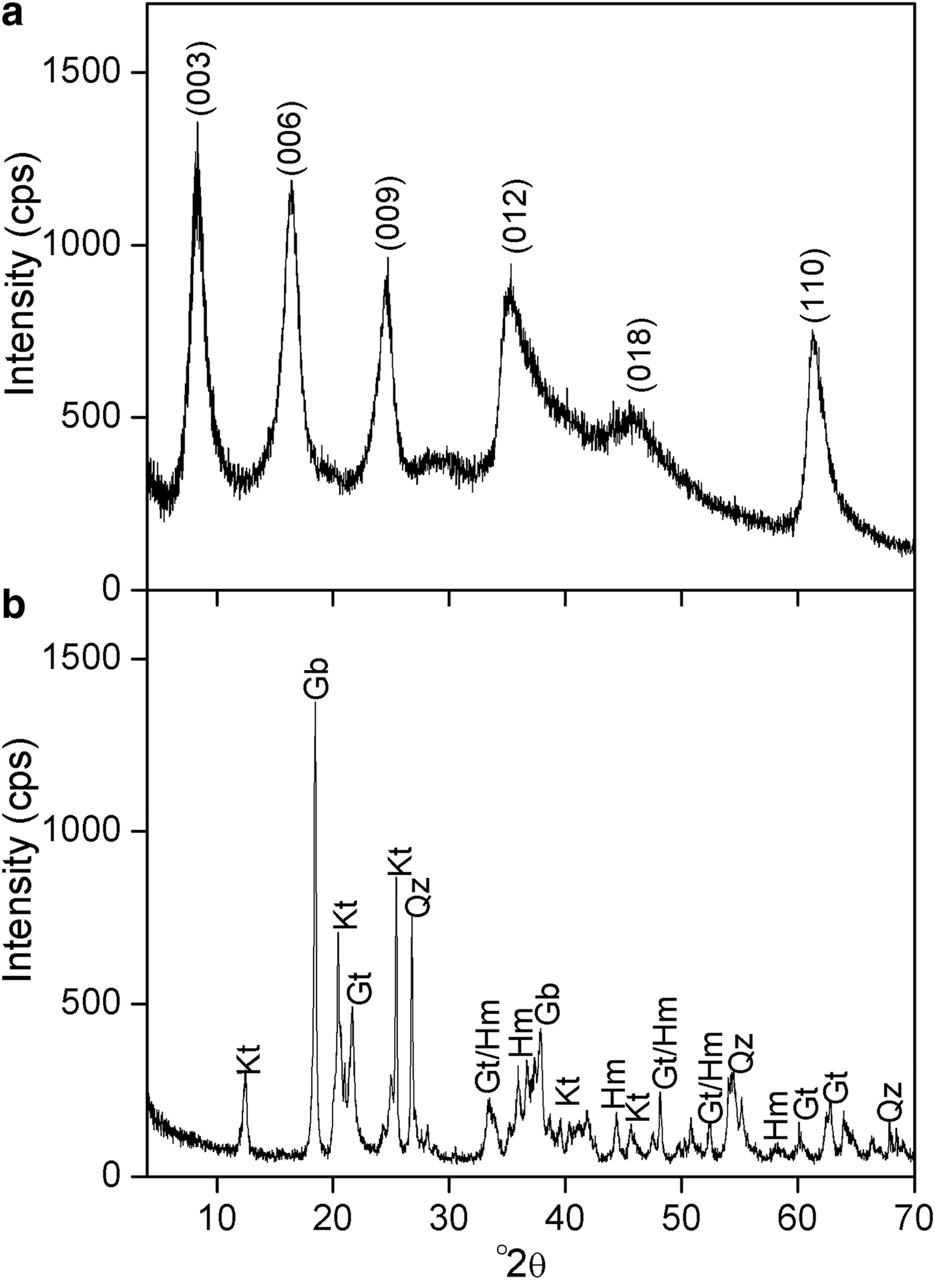
Fig. 1. XRD patterns for (a) Mg2Al-B-LDH and (b) soil sample; Kt = kaolinite; Gb = gibbsite; Qz = quartz; Gt = goethite; Hm = hematite.
Table 1. Chemical and physical characteristics of the soil sample.

*soil/water ratio 1:2.5; P, K, Cu, Mn, Fe and Zn – Mehlich-1 extractor; Ca2+, Mg2+ and Al3+ – 1.0 mol/L KCl extractor; S-Extractor Ca (H2PO4)2, 500 mg/L of P in HOAc 2 mol/L; B – “Hot water” extractor; H + Al – Calcium Acetate Extractor 0.5 mol/L (pH 7.0); SB = sum of bases; t = effective cation exchange capacity; T = cation exchange capacity at pH = 7; V = base saturation; m = saturation by Al; OM = organic matter; P-rem = remaining phosphorus; DRL = Dystrophic Red Latosol.
Kinetics of boron adsorption
To evaluate the stability of B adsorption in the soil, an assay was conducted to evaluate the effect of the contact time of increasing doses of this micronutrient on the soil. For this test, 0.00, 10.00 and 20.00 mg dm−3 of total B were used in the soil, in the forms of H3BO3 and Mg2Al-B-LDH. The sources, in their doses, were homogenized thoroughly with the soil. Plastic bags were filled with treatments (soil + B sources) and received distilled H2O in a volume equivalent to soil field capacity (FC). They were then closed to prevent water loss. Every 2 days, checks were made to verify that the plastic bags with the treatments maintained their initial weights; if they had not, they were topped up with distilled H2O. The contact times for each soil sample with the B sources were 1, 2, 3, 5, 10, 20 and 40 days. After this contact time, the treatment samples were duly dried, and the available B content in the soil was determined by the hot-water extraction method in 5.0 mmol L−1 CaCl2 solution, heated in a microwave oven (Raij et al., Reference Raij, Andrade, Cantarella and Quaggi2001). The available B content was analyzed by UV-Vis spectrophotometry according to the method of López et al. (Reference López, Giménez and Hernández1993).
Bioassay
The bioassay to evaluate the use of Mg2Al-B-LDH as a source of B for plants was carried out in a greenhouse from October to November 2016.
The treatments were defined by the combination of 2 × 6 factorial design, using two sources of B (Mg2Al-B-LDH and H3BO3) and six doses of B (0.0, 0.5, 1.0, 2.0, 3.0 and 5.0 mg dm−3 of total B). The experimental design was performed in randomized blocks (RBD) with four replications. The experimental units consisted of a plastic pot with capacity for 2.0 dm3 of soil. Four sunflower plants (Helianthus annuus Helio 250 hybrid) were cultivated in each pot. This plant was chosen because of its high B requirement compared to other species.
The liming requirement (LR) calculations were performed with the aim of correcting the acidity and supplying Ca and Mg. These calculations were determined according to the Basal Saturation method, aiming to raise the saturation to 50% (Alvarez et al., Reference Alvarez, Novais, Barros, Cantarutti, Lopes, Ribeiro, Guimaraes and Alvarez1999; Caires et al., Reference Caires, Garbuio, Churka and Joris2011). The limestone used was composed of a mixture of CaCO3 and MgCO3, with a Ca/Mg ratio of 4:1 and Effective Calcium Carbonate Equivalent (ECCE) = 103%. After the complete homogenization of the limestone with the soil, the samples were packed in plastic bags and the soil moisture was raised to 80% of the field capacity. The incubation period was 25 days, and after 15 days of incubation, humidity was restored to 80% of the FC. After incubation, the soil sample was air-dried again and sieved through a 2-mm mesh sieve.
The sources and respective doses of B were homogenized in the total soil volume of each pot. On the same day N, P, K and S nutrients were applied as a nutrient solution. Fertilization with P and S was defined according to P-rem (Alvarez et al., Reference Alvarez, Novais., Dias. and Oliveira.2000; Côrrea et al., Reference Corrêa, Nascimento, Souza, Freire and Silva2005), and fertilization with N and K as recommended by Novais et al. (Reference Novais, Neves, Barros, Oliveira, Garrido, Araújo and Lourenço1991) and Marcato et al. (Reference Marcato, Reissmann, Marques, Oliveira and Taffarel2005). The nutrient doses applied to the soil were 450 mg dm−3 of P, 140 mg dm−3 of K, 100 mg dm−3 of S and 100 mg dm−3 of N. The sources used were KH2PO4, MAP, CaH2(PO4)2 and (NH4)2SO4.
After 5 days of nutrient application, eight sunflower seeds were sown per pot. The seedlings were thinned seven days after emergence, to leave four plants per pot. The cover fertilizations were divided into two applications during the cultivation, in the form of the nutrient solution, as recommended by Novais et al. (Reference Novais, Neves, Barros, Oliveira, Garrido, Araújo and Lourenço1991) and Marcato et al. (Reference Marcato, Reissmann, Marques, Oliveira and Taffarel2005). For this phase, the nutrient doses and sources corresponded to 200 mg m−3 of N (urea), 1.32 mg dm−3 of Cu (CuSO4·5H2O), 1.55 mg dm−3 of Fe (FeSO4·7H2O), 3.66 mg dm−3 of Mn (MnSO4·H2O), 0.15 mg dm−3 of Mo [(NH4)6Mo7O24] and 4.0 mg dm−3 of Zn (ZnSO4·7H2O).
The plants were cultivated for 45 days. During this period, humidity was maintained at close to 80% of the FC. The irrigations, with distilled water, were made daily in the upper part of the pots; the H2O volume and the frequency of irrigations varied according to the average temperature during the cultivation days. The plant material was dried in a forced circulation oven at 65°C for 96 h. After drying, it was weighed, passed through a Wiley mill using a 20-mesh sieve, and stored in paper bags.
The determination of B in plant tissue was performed according to the method described by Embrapa (2000). After determining the total B content in the aerial part of the plants (Bernardi et al., Reference Bernardi, Monte, Paiva, Werneck, Haim and Barros2010) from the four plants cultivated in each experimental unit, the B content in the aerial part was calculated. A sub-sample of 300 g of soil from each experimental unit was taken before and after planting for the determination of available B and soil pH, according to the method described by Raij et al. (Reference Raij, Andrade, Cantarella and Quaggi2001). The results were submitted to analysis of variance and regression.
RESULTS AND DISCUSSION
Characterizations
The XRD patterns for the soil and Mg2Al-B-LDH are shown in Fig. 1. The soil is a typical Latosol consisting of hematite, gibbsite, goethite and kaolinite (Fig. 1a). The XRD pattern of Mg2Al-B-LDH is presented in Fig. 1b. The pattern is characteristic of hydrotalcite-type compounds, with (003), (006) and (009) basal peaks at 7.26, 15.6 and 23.6 °2θ, respectively. For this material, the basal spacing calculated by the Bragg equation was 12.0 Å. This value coincides with the values reported in the literature for intercalation of tetraborate octahydrate ions [B4O5(OH)42−]·8H2O between the layers of LDH (Ay et al., Reference Ay, Zümreoglu-Karan, Temel and Mafra2011).
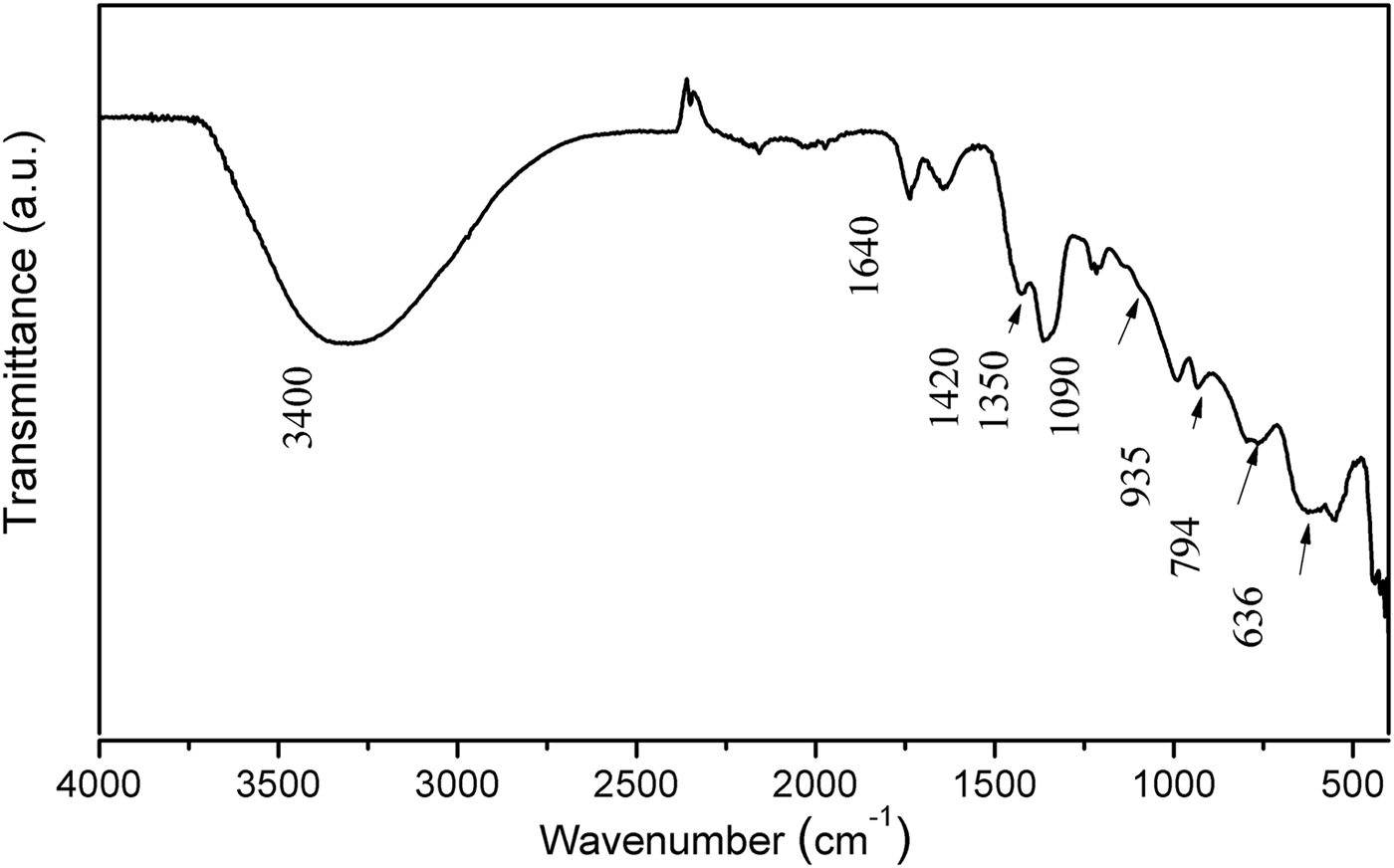
The FTIR/ATR spectrum of Mg2Al-B-LDH is shown in Fig. 2. The presence of the broad band centred at 3400 cm−1 is attributed to the stretching of the O-H bonds of the hydroxyl groups and/or H2O molecules. Bands in the region between 1600 and 500 cm−1 are attributed to the various modes of vibration related to the presence of borate anions. More specifically, the bands at 1420 and 1350 cm−1, 1090 cm−1, 935 cm−1, 794 cm−1, 636 cm−1 are attributed to the vibrational modes υas(B3-O), υas(B4-O), υs(B3-O), υs(B4-O), υs(B3-O), respectively (Ay et al., Reference Ay, Zümreoglu-Karan, Temel and Mafra2011).
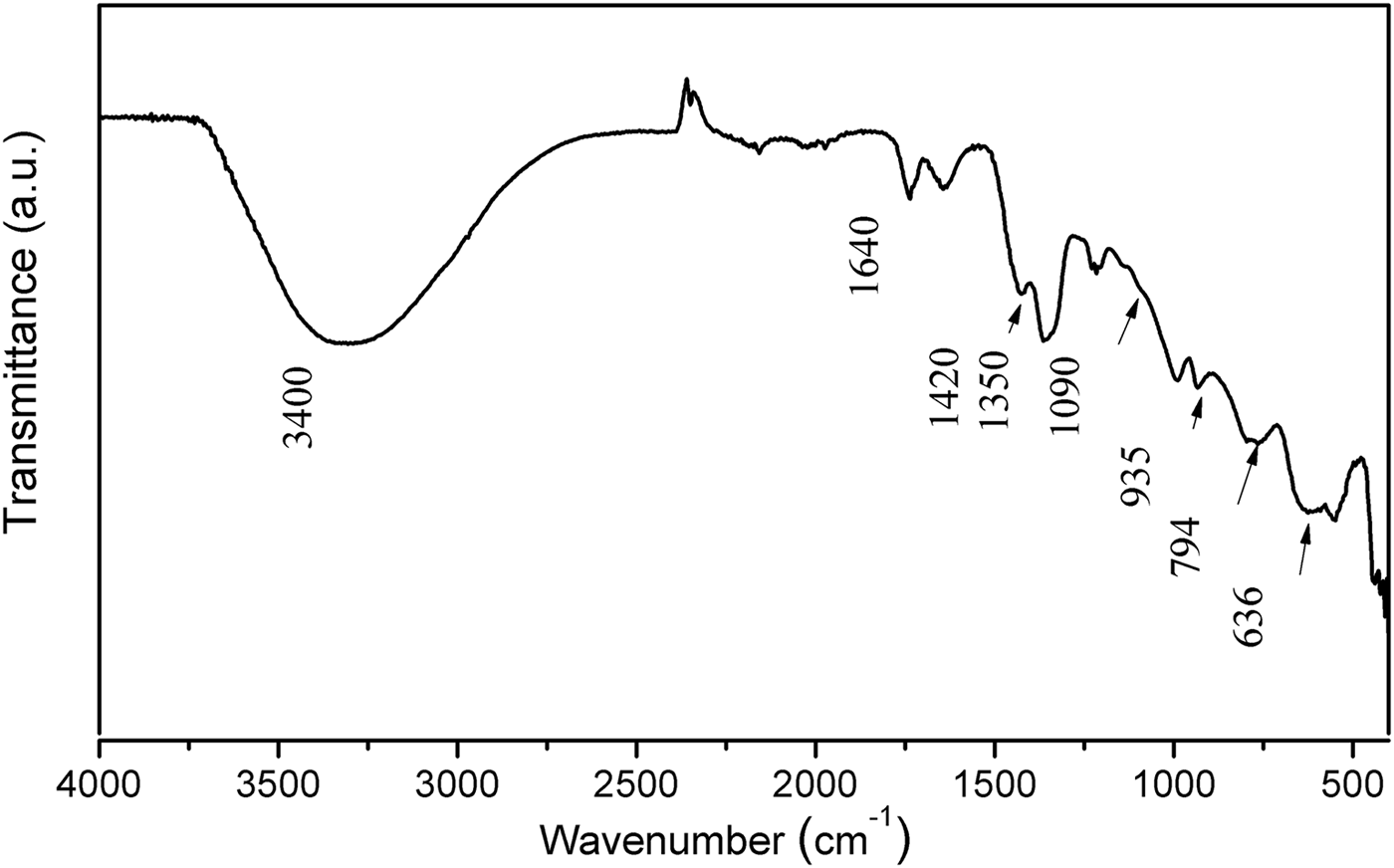
Fig. 2. FTIR/ATR spectrum of Mg2Al-B-LDH.
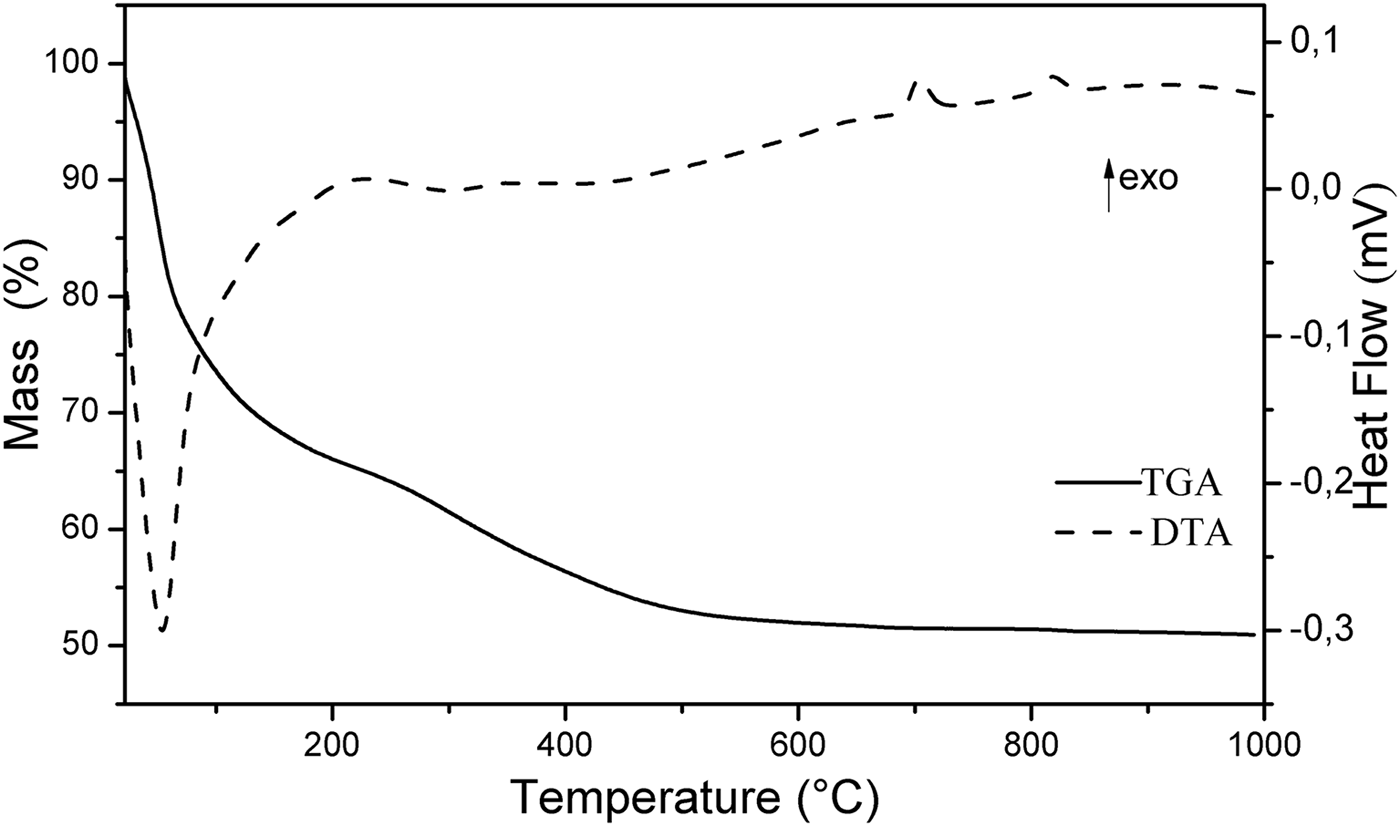
The TGA-DTA curves of Mg2Al-B-LDH are shown in Fig. 3. The TGA curve presents two events of weight loss. The first event occurs in the temperature range of 22–230°C (mass loss of 35%). This thermal decomposition is accompanied by an endothermic process, which can be verified by the inflection of the DTA curve at the same temperature range. In this event, the loss of intercalated H2O and that adsorbed on the surface of the crystallites occurs. The second event of thermal decomposition occurs in the temperature range 230–670°C (mass loss of 13%) and is attributed to the dehydroxylation of inorganic layers and the thermal decomposition of the borate. The TGA curve shows only a very small mass loss above 670°C. At 705°C and 820°C, the DTA curve presented two exothermic events which are produced by recrystallization of Al and Mg borates (Jun et al., Reference Jun, Peng-Sheng and Bai1994; Zhihong et al., Reference Zhihong, Mancheng and Shiyang2004; Douy, Reference Douy2005).
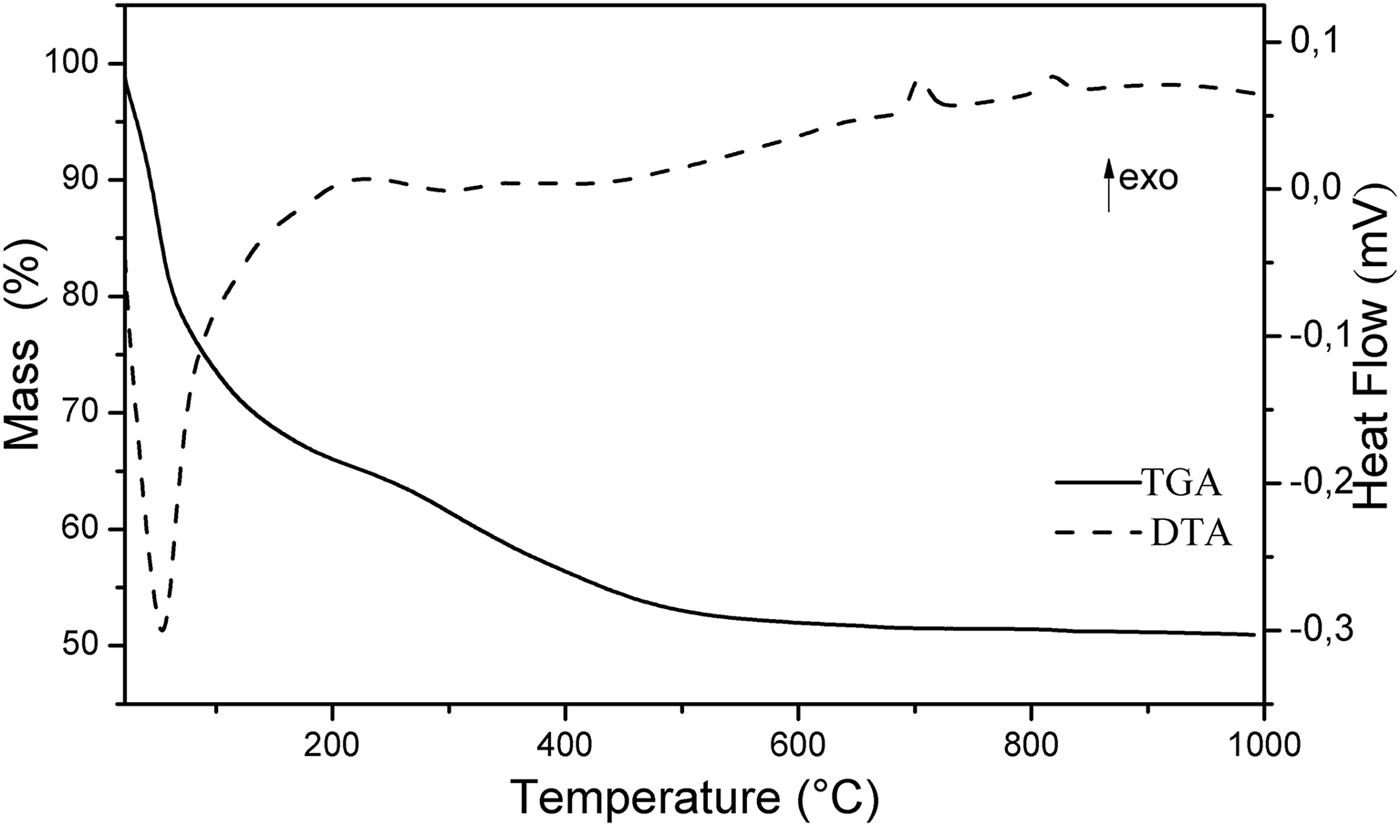
Fig. 3. TGA-DTA of Mg2Al-B-LDH.
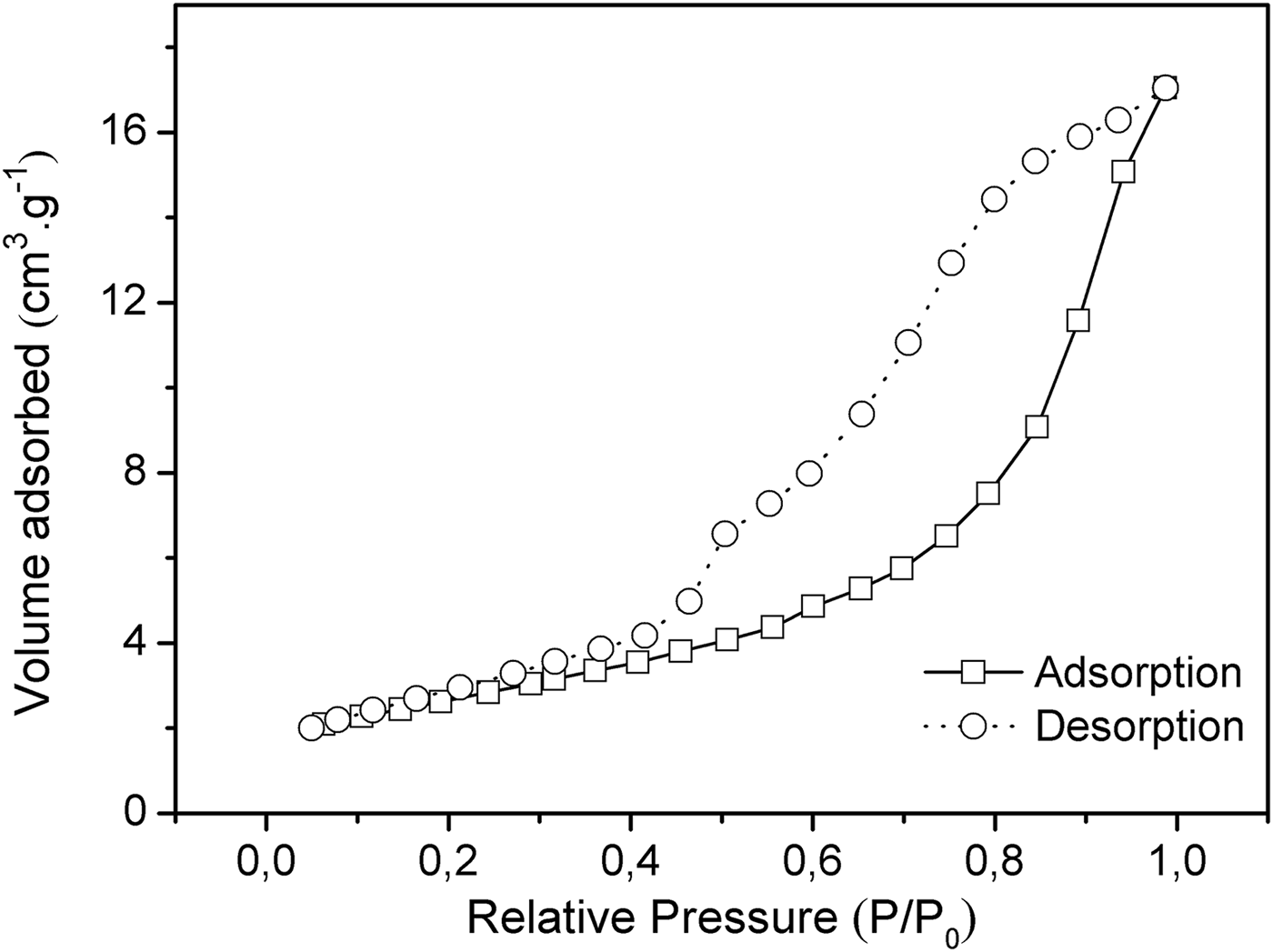
The values of the BET surface area, pore volume and pore size for Mg2Al-B-LDH are shown in Table 2. The N2 adsorption-desorption isotherm of Mg2Al-B-LDH is shown in Fig. 4. According to the IUPAC classification, this isotherm is of the IV type, typical of mesoporous materials with pore diameters of 2–50 nm.
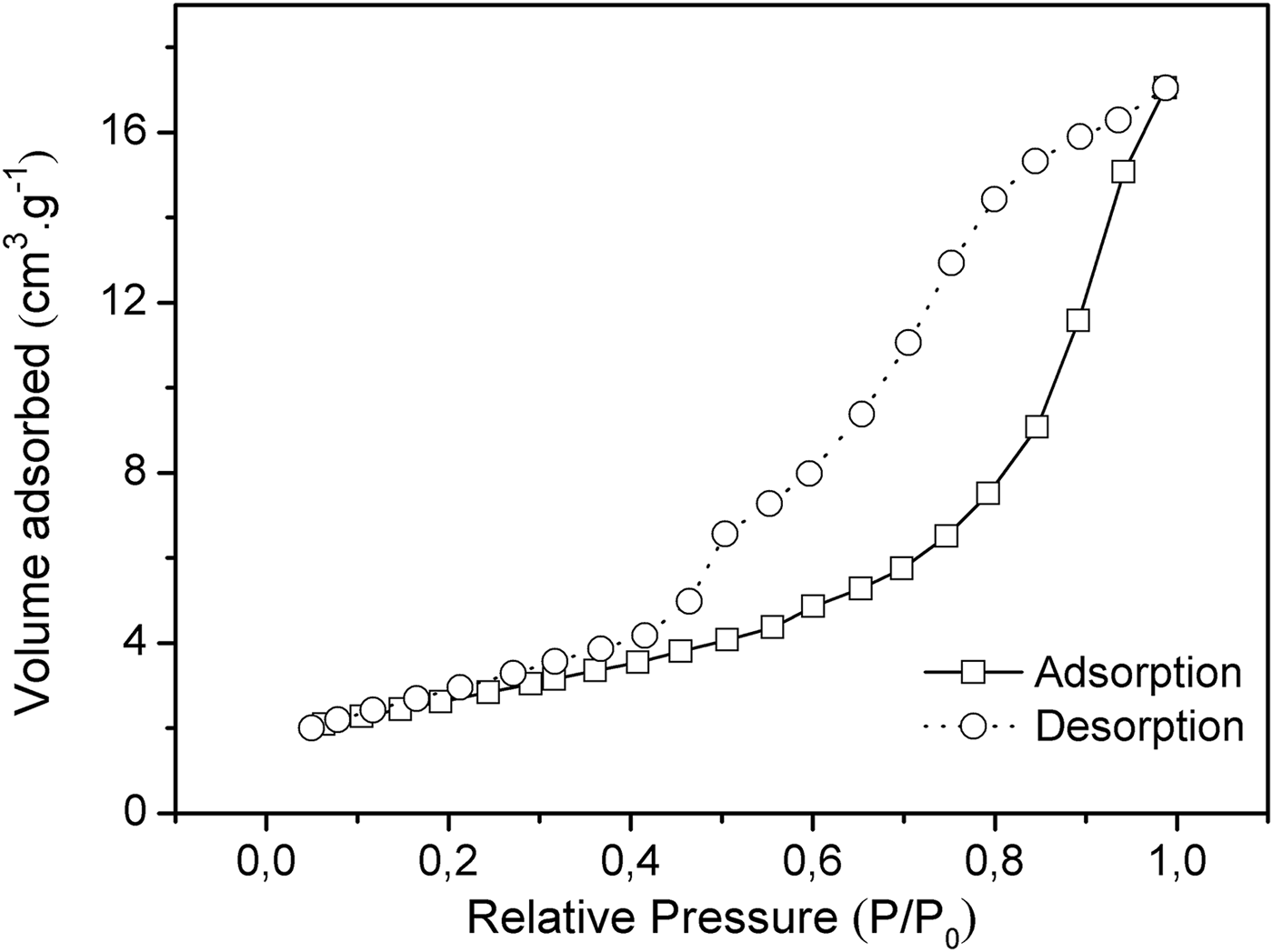
Fig. 4. N2 adsorption-desorption isotherm of Mg2Al-B-LDH.
Table 2. BET specific surface area, pore volume and pore size for the Mg2Al-B-LDH.

*HK method; **Non-local density functional theory (NDFT).
The morphology of the Mg2Al-B-LDH was analysed by means of SEM (Fig. 5). The material consists of spherical aggregates with diameters varying between 1 and 5 µm.

Fig. 5. SEM images of Mg2Al-B-LDH at two different magnifications.
The total B content in Mg2Al-B-LDH as determined by UV-Vis was 45.23 mg g−1 of B per LDH. Previous work reported q max values of 14.0, 37.90 and 25.5 mg g−1 of B for MgAl-CO32−-LDH, MgAl-NO3−LDH, and calcined MgAl-LDH, respectively (Ferreira et al., Reference Ferreira, De Moraes, Duran, Cornejo and Alves2006; Kentjono et al., Reference Kentjono, Liu, Chang and Irawan2010; Isaacs-Paez et al., Reference Isaacs-Paez, Leyva-Ramos, Jacobo-Azuara, Martinez-Rosales and Flores-Cano2014). Therefore, the amount and total B determined for Mg2Al-B-LDH indicates higher incorporation of B compared to the materials cited in the literature. The chemical formula determined for Mg2Al-B-LDH was: Mg2Al(OH)6 [B4O5(OH)42−]0.38(NO3−)0.16(CO32−)0.08·5.4 H2O.
Kinetics of boron release in soil
The results of soil B levels based on the contact time are shown in Fig. 6. The B values for Mg2Al-B-LDH are lower than those found for H3BO3. This suggests that Mg2Al-B-LDH exhibits greater stability than H3BO3. It was thus noted that B intercalated in Mg2Al-B-LDH is released more slowly than that in H3BO3. It was observed that, in the early days, Mg2Al-B-LDH stabilizes in the soil, following a sustained release profile.
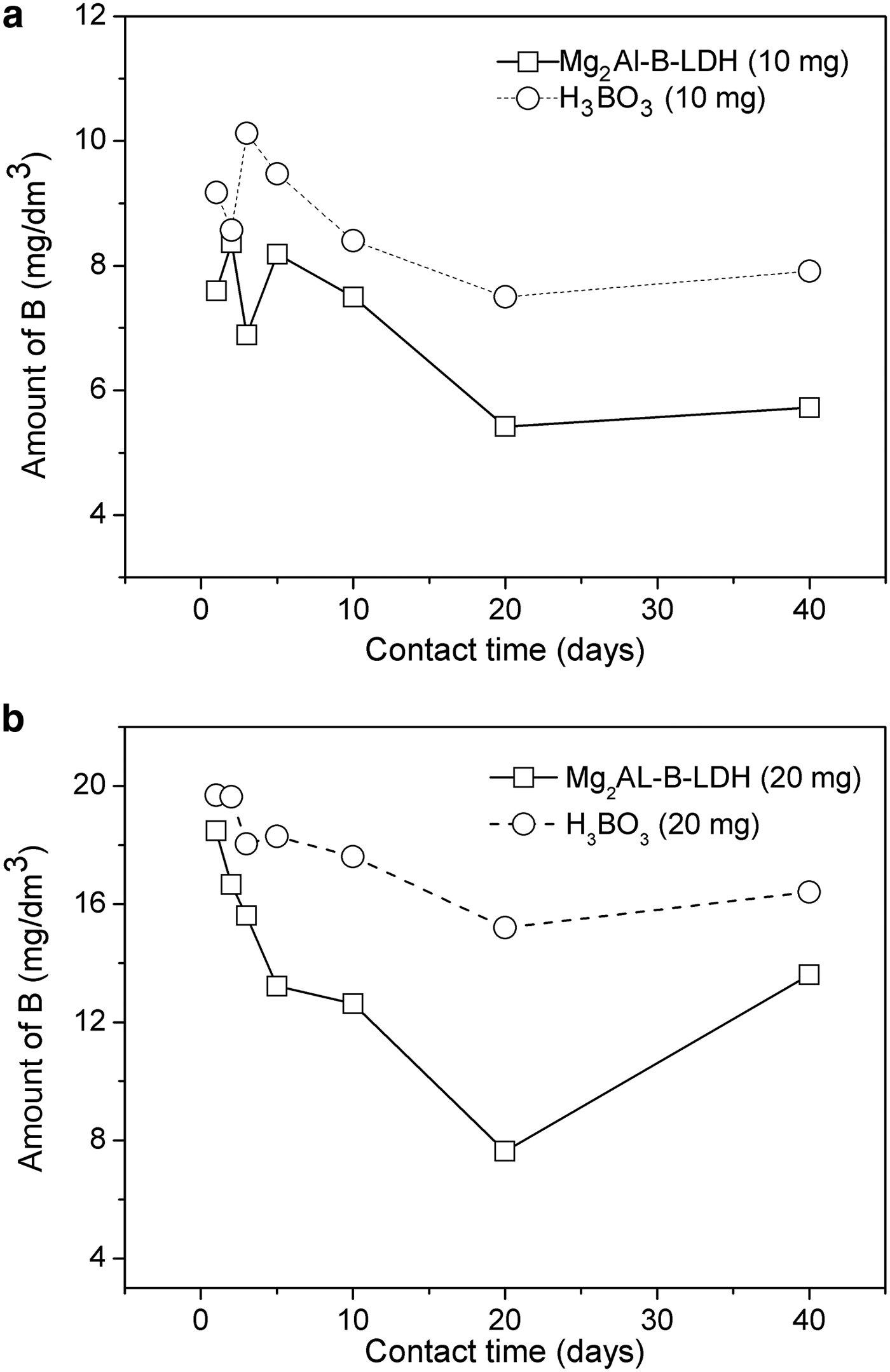
Fig. 6. B available in soil applied in two doses: (a) 10 mg and (b) 20 mg of B, as a function of the equilibrium time for the Mg2Al-B-LDH and H3BO3 sources.
Bioassay
The levels of B available in the soil indicated that, with increase of the applied doses of H3BO3 and Mg2Al-B-LDH, the levels of B increased (Fig. 7). This increase in dose, as demonstrated by the regression analysis, followed a significant linear relationship (p < 0.01), as expected, as the sunflower plants had not been cultivated yet.
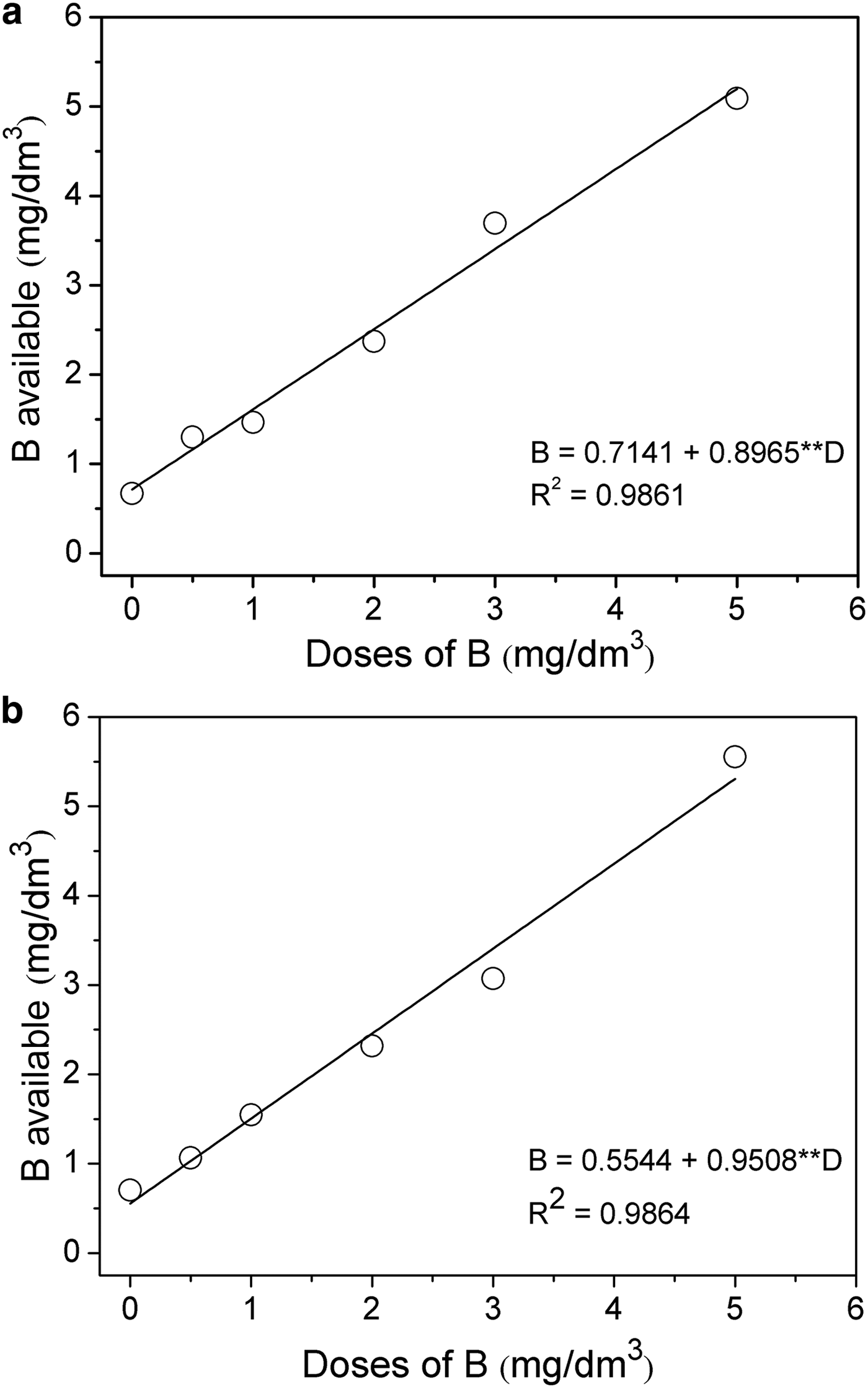
Fig. 7. Simple linear-regression between the available soil B content before the cultivation of sunflower plants as a function of the applied doses of B for: (a) Source of B-H3BO3; (b) Source of B-Mg2Al-B-LDH. ** = significant at 1%; B = boron available; D = doses of boron applied.
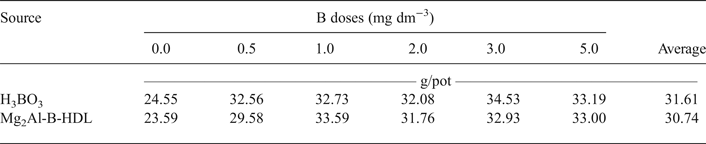
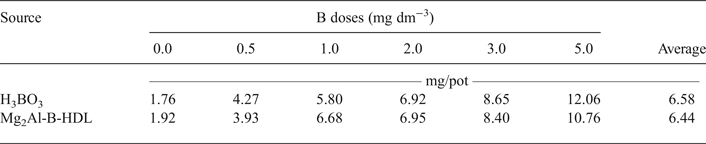
The data for DM production and B content in the aerial parts of the plants are listed in Tables 3 and 4, respectively. For the plants treated with Mg2Al-B-LDH and H3BO3, there was an increase in the DM and B content in the aerial part of the plants, relative to the treatments that did not receive B. The DM production was not statistically different when comparing the two sources of B used. During the bioassay, it was also noted visually that the sunflower plants showed a similar development for the two sources of B studied. Additionally, it was possible to note plants with symptoms of B deficiency in the treatments without B. The images of the cultivated sunflower plants are presented in Appendices 1 and 2.
Table 3. Dry-matter production in the aerial part of the sunflower plants* as a function of the doses and sources of B added to the soil.
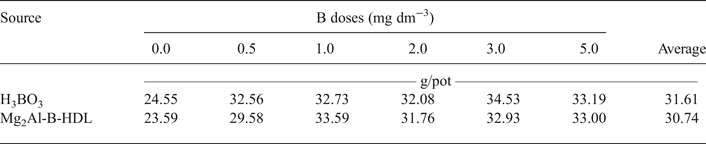
*There was no significant difference (p < 0.05) between the sources of B.
Table 4. B content in the aerial part of the sunflower plants* as a function of the doses added to the soil.
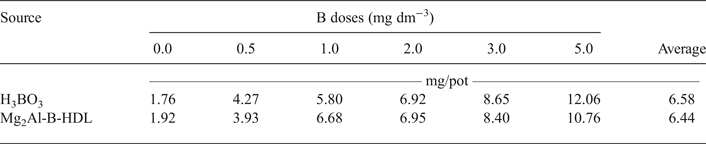
*There was no significant difference (p < 0.05) between the sources of B.
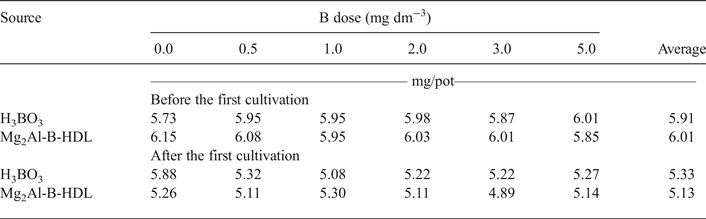
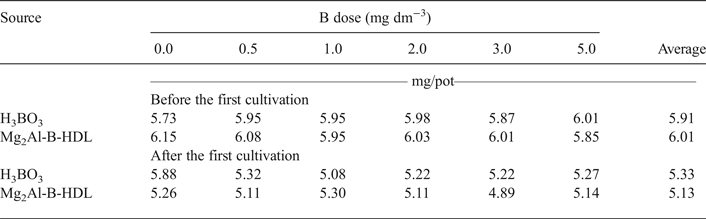
The pH values of the soil before and after the cultivation of sunflower plants are presented in Table 5. Before cultivation, the average pH ranged between 5.73 and 6.15. This pH range, ideal for plant cultivation, chemically classifies the soil as having medium to weak acidity (Alvarez et al., Reference Alvarez, Novais, Barros, Cantarutti, Lopes, Ribeiro, Guimaraes and Alvarez1999; Caires, et al., Reference Caires, Garbuio, Churka and Joris2011). This acidity provided destabilization of the lamellar structure of Mg2Al-B-LDH and the subsequent release of B. Recent work using a MgAl-LDH as a matrix for storage and release of phosphorus presented, in a bioassay carried out with maize (Zea mays), an increase in the soil pH value based on the dose increase of the P matrix applied (Benício et al., Reference Benício, Constantino, Pinto, Vergutz, Tronto and Costa2016). The release of intercalated anions in LDH depends on the pH value of medium. At acid pH value, the LDH structures are unstable and the release of the intercalated anions occurs due to destruction of the layered material by acid attack, as LDHs are unstable in acid media. Another possibility for the release of intercalated anions is through anion-exchange with the anions in the medium (Tronto et al., Reference Tronto, Cardoso and Valim2003, Reference Tronto, Dos Reis, Silverio, Balbo, Marchetti and Valim2004; Benício et al, Reference Benício, Constantino, Pinto, Vergutz, Tronto and Costa2016). In this case, as P is a nutrient required in large quantities for plant growth, the amount of LDH used was greater than the values used for Mg2Al-B-LDH, which favoured the maintenance of the lamellar structure of LDH intercalated with P. Note that, in this work, a small amount of Mg2Al-B-LDH was applied to plant cultivation, as B is a micronutrient and thus required in small amounts only. This small amount was not able to change the pH of the soil (Table 5) to values that might favour the maintenance of the structure of Mg2Al-B-LDH, unlike the result presented by Benício et al. (Reference Benício, Constantino, Pinto, Vergutz, Tronto and Costa2016). In addition, plant cultivation itself may acidify the soils according to post-cultivation pH results (Table 5). Acidification is often associated with the removal of exchangeable bases from the soil (Zinn et al., Reference Zinn, Lal and Resck2005; Caputo et al., Reference Caputo, Beier, Sullivan and Lawrence2016; Zhang et al., Reference Zhang, He, Liang, Zhao, Zhang, Xu and Shi2016), which may favour a lower stability of Mg2Al-B-LDH and greater nutrient release compared to H3BO3.
Table 5. Soil pH as a function of the added B doses before and after the first cultivation.
The expected result for the utilization of Mg2Al-B-LDH as a source of B for plants is that there would be a sustainable release (constant concentration of B over a long period of time) of this micronutrient, more pronounced than that presented by normal H3BO3. However, this work showed that Mg2Al-B-LDH, applied in the soil with acid pH values, cannot be characterized as a sustainable release matrix of this micronutrient, as the DM yield and B content in the aerial part of the plants were statistically the same for both sources.
CONCLUSIONS
The average results of DM yield and B content in the aerial part of the plants between the two sources of B (Mg2Al-B-LDH and H3BO3) were similar, and not significantly different from each other. The results obtained for Mg2Al-B-LDH used as a matrix of sustainable release of B, are probably reflections of the small amount of LDH used in the soil, as B is a micronutrient required in small quantities by the plants. This small amount of Mg2Al-B-LDH was not sufficient to increase soil pH, which would allow the stability of the LDH structure and would promote the sustainable release of B by means of anionic exchange. The two sources used were solubilized similarly in the soil and released B equally to the plants. Thus, the Mg2Al-B-LDH used in agricultural soils with normal acid pH value can be compared to the commercially available, soluble H3BO3, not as a slow-release source of B as expected initially. Additional experiments using M2Al-B-LDH as stores and sources of B for plant growth in soils at alkaline pH values are being performed by the authors.
ACKNOWLEDGMENTS
This study was supported by the Foundation for Research Support of the State of Minas Gerais (FAPEMIG) and the Coordination for the Improvement of Higher Education Personnel (CAPES). This work is a collaborative research project of members of Minas Gerais Chemistry Network (RQ-MG). The authors thank Heliagro for supplying the sunflower seeds used in this research. The authors also thank Vera R.L. Constantino of University of São Paulo for valuable support and useful discussion.

Appendix 1. Response of sunflower plants to increasing doses of B from each of two sources in the soil. From left to right, the pots correspond to doses 0.0, 0.5, 1.0, 2.0, 3.0 and 5.0 mg dm−3 of B, H3BO3 (a) and Mg2A1-B-LDH (b).

Appendix 2. Sunflower plant with B deficiency symptom at the floral apex (a) and without that symptom (b).