Our modern civilization is built on developments in technology and industry. However, from the last quarter of the twentieth century onwards, we have had to deal with the challenges of industrial and agricultural wastes, among other types of waste. Clays and clay minerals have been proven to be good candidates for pollution control. They have the ability to adsorb radionuclides (Krupskaya et al., Reference Krupskaya, Zakusin, Tyupina, Dorzhieva, Zhukhlistov, Belousov and Timofeeva2017), heavy metal ions (Ma et al., Reference Ma, Su, Zhang and Huang2016), dyes (Gök et al., Reference Gök, Özcan and Özcan2010), gases and volatile compounds (Volzone & Ortiga, Reference Volzone and Ortiga2009; Chen & Lu, Reference Chen and Lu2015; Erdoğan Alver & Günal, Reference Erdoğan Alver and Günal2016; Erdoğan Alver et al., Reference Erdoğan Alver, Alver, Günal and Dikmen2016; Erdoğan Alver, Reference Erdoğan Alver2017; Mucha et al., Reference Mucha, Pavlovský and Navrátilová2017). Bentonites are clays consisting essentially of smectites (Fontaine et al., Reference Fontaine, Christidis, Yans, Hollanders, Hoffman and Fagel2020). Smectites consist of repetitions of T–O–T layers, where T corresponds to tetrahedral sheets that consist of one T atom residing at the centre of the tetrahedron (T is usually Si4+) and four O2– atoms are at the corners of the tetrahedron, and O represents octahedral sheets that consist of a metal atom (generally Al3+) and six O2– atoms and OH– (Brigatti et al., Reference Brigatti, Galan, Theng, Bergaya, Theng and Lagaly2006; Do Nascimento, Reference Do Nascimento and Do Nascimento2016). Through isomorphic substitution, the Al atoms in the octahedral sheets may be replaced with divalent cations such as Fe2+ or Mg2+, and in the tetrahedral sheets Al3+ atoms replace Si. These substitutions create a negative net charge, which is balanced with exchangeable interlayer cations (Erdoğan et al., Reference Erdoğan Alver, Alver, Günal and Dikmen2016). Despite their large cation-exchange capacity and basal swelling abilities, the structural properties of smectites need to be improved through the application of various techniques. The most common method is acid activation (Balci, Reference Balci2019). Treatment with inorganic acids changes the chemical composition and structure of smectites, leading to increases in their specific surface areas, Brønsted acidity and gas-adsorption capacity (Didi et al., Reference Didi, Makhoukhi, Azzouz and Villemin2009; Sarıoğlan et al., Reference Sarioğlan, Yuzer and Koral2010; Çağlar et al., Reference Çağlar, Afsin, Koksal, Tabak and Eren2013; Wang et al., Reference Wang, Wang, Song, Wei, Ding and Xiao2013).
Ammonia is a colourless and malodorous gas (Li et al., Reference Li, Lin, Wang, Zhao and Hou2010). It consists of three hydrogen atoms bonded to nitrogen with a H–N–H bond angle of ~107°. It has a molar mass of 17.0312 g mol–1 and a kinetic diameter of 2.6 Å. The ammonia molecule has a dipole moment (1.5 Debye) due to its asymmetrical shape (Appl, Reference Appl2000). Agricultural activities produce various types of ecological pollutants, one of which is ammonia (Ciahotný et al., Reference Ciahotný, Melenová, Jirglová, Boldiš and Kočiřík2002). In particular, ammonia released from livestock and poultry poses a serious environmental risk (Amon et al., Reference Amon, Dobeic, Sneath, Phillips, Misselbrook and Pain1997), and the concentration of ammonia in the air directly affects worker health and production capacity. When the ammonia concentration reaches 50 ppm in poultry, the production capacity decreases and workers display eye irritation (Gülşen & Alagöz, Reference Gülşen and Alagöz2010). In addition, ammonia may cause pharyngitis, laryngitis and pulmonary oedema (Appl, Reference Appl2000). Consequently, the release of ammonia in livestock facilities must be controlled.
Clay minerals may be good candidates for dealing with the problem of controlling the concentration of ammonia in air due to their structural properties and significant gas-adsorbing capacities. Russell (Reference Russel1965) reported the ammonia-adsorption capacity of Wyoming montmorillonite to be 0.58 mmol g–1. Ursu et al. (Reference Ursu, Gros, Nistor and Djelveh2008) used the Al3+-doped K10 commercial clay and natural Romanian Na-bentonite in fixed and fluidized bed gas–solid reactors and reported ammonia-adsorption capacities of 2.09 and 1.07 mmol g–1 for K10 and Na-bentonite, respectively. Panasyugin et al. (Reference Panasyugin, Bondareva and Rat'ko2004) determined the ammonia-adsorption capacity of Fe3+-modified montmorillonite to be ~4 mmol g–1, and Belzunce et al. (Reference Belzunce, Mendioroz and Haber1998) observed that the ammonia-adsorption capacity of sepiolites modified with hydrofluoric acid at various concentrations and at 60°C increased up to 0.320 mmol g–1. Exchangeable cations and moisture affect ammonia adsorption in clay soils (Dontsova et al., Reference Dontsova, Norton and Johnston2005). Seredych et al. (Reference Seredych, Ania and Bandosz2016) reported that the phenolic–formaldehyde resin/bentonite composite material exhibited an ammonia-adsorption capacity of 38 mg g–1 (~2.23 mmol g–1). Liu et al. (Reference Liu, Sarkar, Wang and Naidu2016) observed that the ammonia-adsorption capacity of bentonite treated with 4 M HCl solution for 1 h was 0.828 mmol g–1. Based on previous studies in the literature, it is clear that there is limited work on ammonia adsorption by acid-treated bentonites. In addition, the presence of large bentonite deposits in Turkey would make this material advantageous for use in the removal of harmful gases such as ammonia in the future (İpekoğlu et al., Reference İpekoğlu, Kurşun, Bilge, Barut, Köse and Arslan1997). Therefore, the purpose of the present study is to investigate the effect of HCl treatment on bentonite for adsorbing ammonia at 298 K from environments such as poultry houses.
Materials
Natural bentonite (B) from the Ünye region was used in this study. The bentonite sample was ground and sieved to obtain the <63 μm fraction. A total of 5 g of each bentonite was activated with 100 mL of 0.5–2.5 M HCl solutions at 75°C for 4 h under reflux conditions. The suspensions were then filtered, and the solid samples were washed several times with deionized water at the approximate boiling point and dried at 100°C overnight. The dried bentonite samples were kept in a desiccator containing silica gel. Acid-treated bentonites were labelled as ‘BH-X’, where X indicates the acid molarity.
Experimental
The mineralogy of the samples was determined using X-ray diffraction (XRD) with a Panalytical Emperian device using Cu-Kα radiation (λ = 1.54 Å) in the range 5–40°2θ with a step size of 0.02° at room temperature using the glass slide method. The chemical composition of the bentonites was determined using X-ray fluorescence (XRF) spectroscopy with a Rigaku ZSX Primus XRF spectrometer. Simultaneous thermogravimetric/differential thermal analysis (TG/DTA) was conducted using Setaram Setsys Evolution equipment in the temperature range 30–1000°C at a heating rate of 10°C min−1. Approximately 35 mg of sample was used for each run. Nitrogen gas adsorption isotherms of the bentonites were obtained at 77 K using 3Flex equipment (Micromeritics, USA). The adsorption isotherms of ammonia were determined volumetrically at 298 K and up to 100 kPa using the same 3Flex instrument. The nitrogen- and ammonia-adsorption measurements were made after degassing in vacuum at 125°C for 12 h. The specific surface areas and the micropore data were calculated using the Brunauer–Emmett–Teller (BET) and t-plot methods, respectively.
Results and discussion
Chemical composition
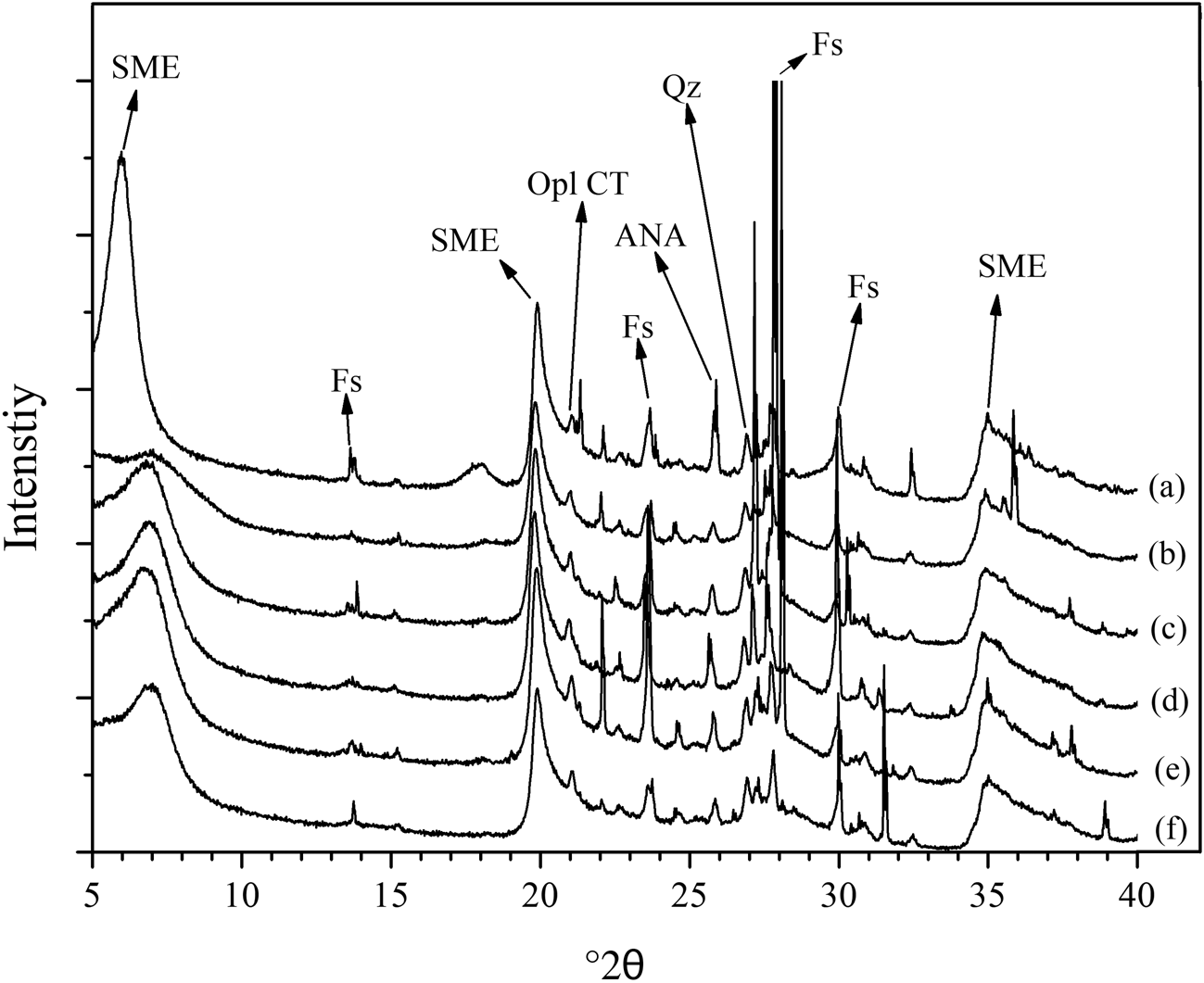
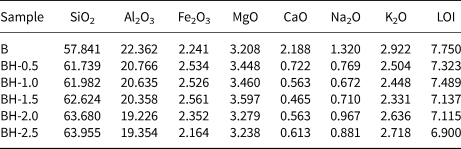
The chemical compositions (mass% of metal oxides) of the raw bentonite and the acid-treated samples (BH-0.5, BH-1.0, BH-1.5, BH-2.0 and BH-2.5) are listed in Table 1. For the BH-0.5 sample, the Ca2+, Na+ and K+ contents of the bentonite decreased rapidly due to the initial attack of the H+ ions of HCl on the exchangeable interlayer cations (Na+, Ca2+ and K+) and the resulting cation exchange (Carrado & Komadel, Reference Carrado and Komadel2009). However, CaO, Na2O and K2O were not totally depleted from the acid-treated samples due to the presence of impurities such as feldspar, which resisted leaching (Bendou & Amrani, Reference Bendou and Amrani2014). These results are consistent with the XRD data (Fig. 1).

Fig. 1. XRD traces of (a) the original bentonite sample, (b) BH-0.5, (c) BH-1.0, (d) BH-1.5, (e) BH-2.0 and (f) BH-2.5. ANA = analcime; Fs = feldspar; Opl CT = opal-CT; Qz = quartz; SME = smectite.
Table 1. Chemical composition (mass%) of the raw and acid-treated bentonite samples.
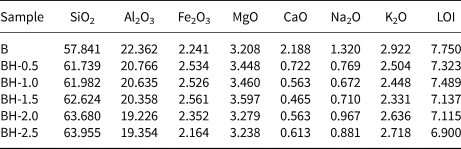
LOI = loss on ignition.
As the acid concentration increased, the SiO2 content of acid-modified bentonites increased, while the abundances of octahedral cations (Al3+, Fe3+ and Mg2+) decreased. This is due to the resistance of Si tetrahedral sheets in acid solution. Although the acid molarity increased up to 2.5 M (Fig. 1f), the Si–O network was not fully destroyed in accordance with previous studies (Brindley & Youell, Reference Brindley and Youell1951; Erdoğan Alver et al., Reference Erdoğan Alver, Alver, Günal and Dikmen2016). The dissolution behaviour of bentonites at low acid concentrations depends largely on the experimental conditions (e.g. acid concentration and contact time). For example, Timofeeva et al. (Reference Timofeeva, Volcho, Mikhalchenko, Panchenko, Krupskaya and Tsybulya2015) found that the Al contents of 0.25 and 0.50 M HCl acid-activated bentonites from Russia and Kazakhstan increased slightly. On the other hand, Arus et al. (Reference Arus, Nousir, Sennour, Shiao, Nistor, Roy and Azzouz2018) determined that the Fe content of the bentonite first increased and then decreased, while the Al content of a commercial bentonite decreased continuously. In the present study, low acid concentrations generally affected the exchangeable cations more substantially, and the SiO2/Al2O3 ratio increased with increasing acid molarity (Table 1), in accordance with similar previous studies (Venaruzzo et al., Reference Venaruzzo, Volzone, Rueda and Ortiga2002; Amari et al., Reference Amari, Chlendi, Gannouni and Bellagi2010; Bendou & Amrani, Reference Bendou and Amrani2014; Erdoğan Alver et al., Reference Erdoğan Alver, Alver, Günal and Dikmen2016; Komadel, Reference Komadel2016; Alexander et al., Reference Alexander, Ahmad Zaini, Abdulsalam, El-Nafaty and Aroke2018).
X-ray diffraction
The XRD traces of the original and acid-treated samples are shown in Fig. 1. Sample B consists predominantly of smectite, feldspar, opal-CT and quartz. The identification of the clay minerals and non-clay minerals in the raw bentonite followed Moore & Reynolds (Reference Moore and Reynolds1997). The 001 reflection was mainly affected by acid treatment and the intensity of this peak decreased signifiantly after treatment with 0.5 M HCl solution (Fig. 1). As the acid concentration increased to 2.5 M, the intensities of the non-basal reflections of montmorillonite (4.43 and 2.56 Å) also decreased. The presence of feldspar, quartz and analcime in the sample treated with 2.5 M HCl indicates that these phases are more resistant to acid attack than smectite (Venaruzzo et al., Reference Venaruzzo, Volzone, Rueda and Ortiga2002). As the acid concentration increased, the crystal order of smectite decreased. Moreover, the XRD traces of the acid-treated samples show the characteristic hump between 19 and 30°2θ, which becomes clearer due to partial destruction of the smectite structure and precipitation of amorphous SiO2. A similar characteristic hump for acid-treated bentonites has also been reported in previous works (Gonzales-Pradas et al., Reference González-Pradas, Villafranca-Sánchez, Villafranca-Sánchez, del Rey-Bueno, Valverde-García and García-Rodríguez1991; Christidis et al., Reference Christidis, Scott and Dunham1997; Venaruzzo et al., Reference Venaruzzo, Volzone, Rueda and Ortiga2002). Acid treatment caused significant changes in the smectite structure, especially at HCl molarities greater than 1.0 M.
Thermal properties
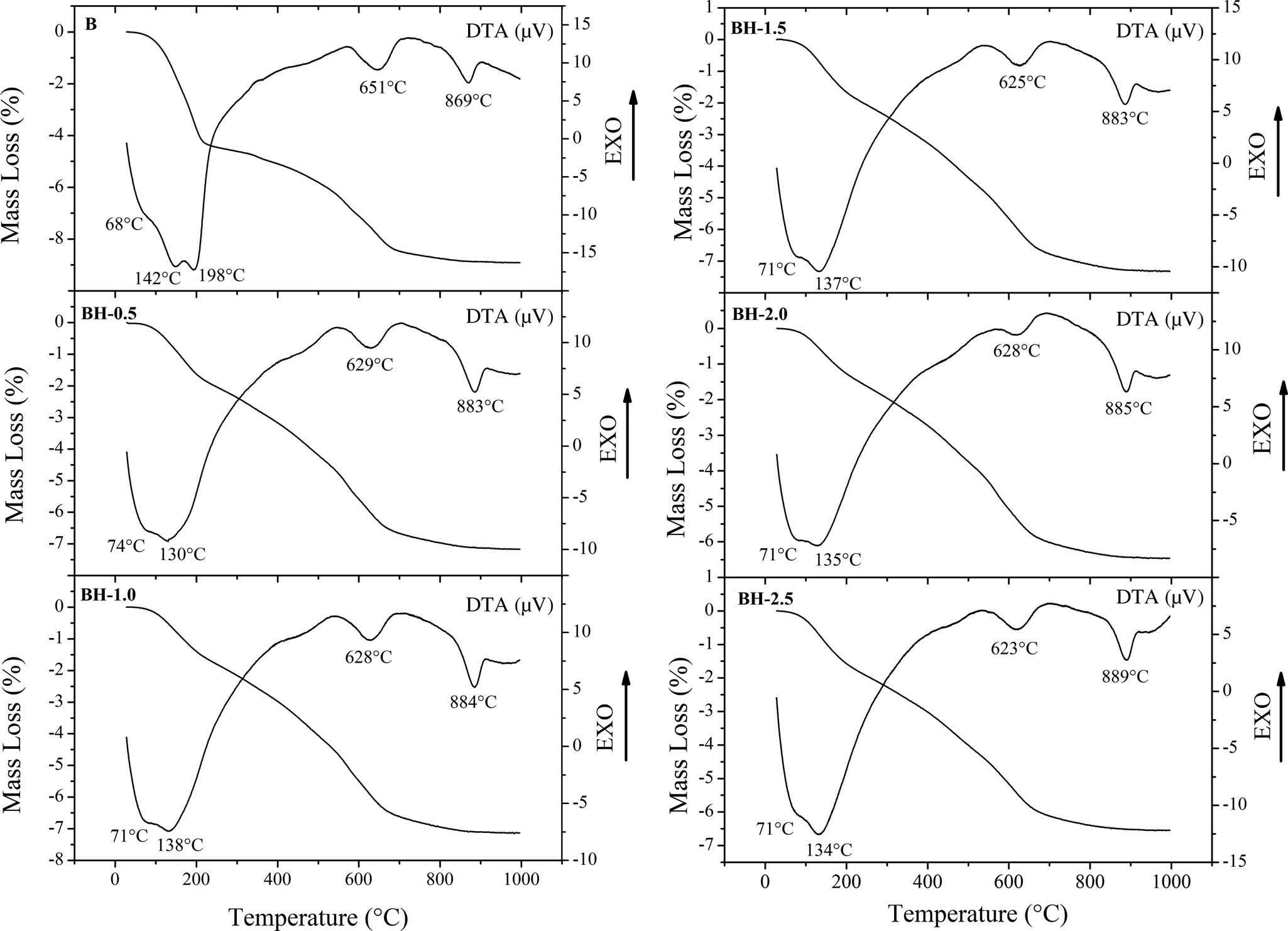
The TG/DTA curves of the original and the acid-treated bentonites are shown in Fig. 2. The shoulder at 68°C and endothermic events at ~140°C and 198°C correspond to the removal of adsorbed water. The double endotherm in the original bentonite at 142°C and 198°C is associated with the presence of exchangeable divalent cations (Fontaine et al., Reference Fontaine, Christidis, Yans, Hollanders, Hoffman and Fagel2020), and its intensity decreased in the acid-activated bentonites. The lower abundances of CaO after acid treatment (Table 1) support the relationship between the presence of divalent cations and double-peak behaviour. The endothermic peak at 651°C is attributed to the dehydroxylation of smectite. Increasing acid concentration affected the thermal behaviour of the bentonite samples. The dehydroxylation peaks of samples BH-0.5, BH-1.0, BH-1.5, BH-2.0 and BH-2.5 became more diffuse and shifted to lower temperatures (623–629°C) compared to the original bentonite sample (651°C). This result may be attributed to the easier loss of hydroxyl units from the broken edges of the acid-treated bentonites (Srasra et al., Reference Srasra, Bergaya, Van Damme and Ariguib1989; Çağlar et al., Reference Çağlar, Afsin, Koksal, Tabak and Eren2013). The endothermic peak at 869°C indicates collapse of the structure and recrystallization.

Fig. 2. TG/DTA curves for the raw and acid-activated bentonite samples.
The total mass-loss values of all bentonite samples were determined using TG analysis (Fig. 2). The first mass-loss event of the bentonite samples due to removal of adsorbed interlayer water occurred between 30 and 200°C. In addition, the total mass losses of the BH-0.50, BH-1.0, BH-1.5, BH-2.0 and BH-2.5 samples (6.463–7.325%) were smaller than that of the original bentonite sample (8.915%). This result is due to the loss of exchangeable cations and disorganization of the tetrahedral and octahedral layers as a result of acid activation (Srasra et al., Reference Srasra, Bergaya, Van Damme and Ariguib1989; Christidis et al., Reference Christidis, Scott and Dunham1997; Önal et al., Reference Önal, Sarıkaya, Alemdaroğlu and Bozdoğan2002; Çağlar et al., Reference Çağlar, Afsin, Koksal, Tabak and Eren2013).
N2 adsorption
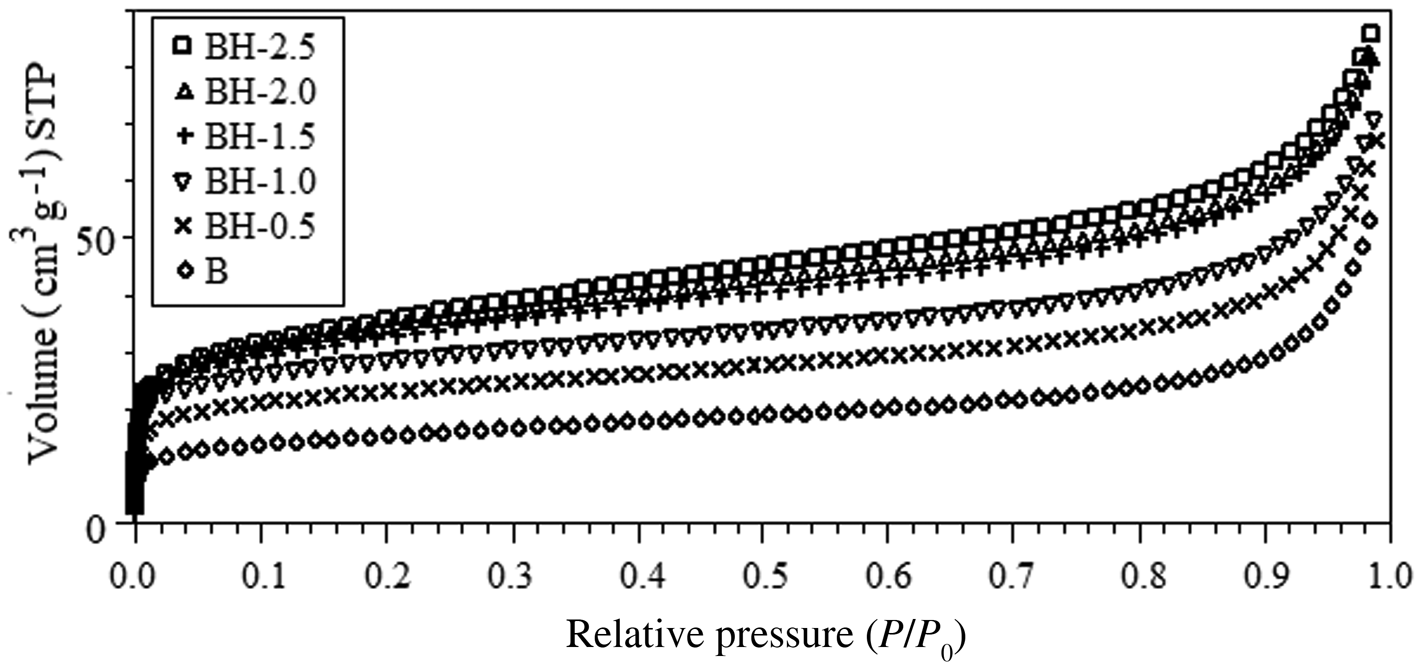
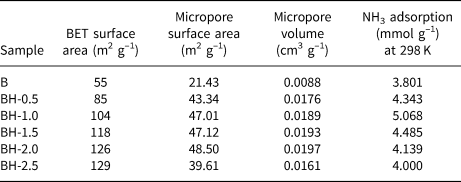
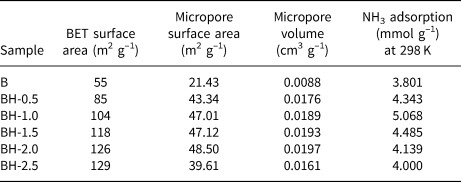
The N2 adsorption isotherms of the original and acid-treated bentonite samples are presented in Fig. 3. The isotherms of bentonites are of type II (Gregg & Sing, Reference Gregg and Sing1982; Lowell et al., Reference Lowell, Shields, Thomas and Thommes2004). Table 2 presents some important parameters obtained from the analysis of the nitrogen-adsorption isotherms. The t-plot method (P/P 0 > 0.1) was used to evaluate the micropore surface areas and micropore volumes of the samples. The specific surface areas were calculated using data in the interval 0.05 < P/P 0 < 0.35 (Table 2). As the acid concentration increased, the N2-adsorption capacities gradually increased. The specific surface area of natural bentonite increased from 55 to 129 m2 g–1 after acid treatment with 2.5 M HCl solution (BH-2.5). The increase in specific surface area is due to the gradual disruption of the smectite structure and the formation of amorphous SiO2, generating greater microporosity (Venaruzzo et al., Reference Venaruzzo, Volzone, Rueda and Ortiga2002; Horri et al., Reference Horri, Sanz-Pérez, Arencibia, Sanz, Srasra-Frini and Srasra2020). However, it was not possible to increase the micropore surface area by increasing the acid concentration beyond a certain value. In addition, the sample modified with 2.5 M HCl had a smaller micropore surface area (39.61 m2 g–1) and micropore volume (0.0161 cm3 g–1) than the BH-2.0 sample (48.50 m2 g–1 and 0.0197 cm3 g–1, respectively). This is attributed to an increase in average pore diameter, condensations of silanol groups and a partial collapse of the smectite crystal structure (Gonzalez-Pradas et al., Reference González-Pradas, Villafranca-Sánchez, Villafranca-Sánchez, del Rey-Bueno, Valverde-García and García-Rodríguez1991; Horri et al., Reference Horri, Sanz-Pérez, Arencibia, Sanz, Srasra-Frini and Srasra2020), in accordance with the XRF data (Table 1) and the XRD data (Fig. 1).

Fig. 3. N2-adsorption isotherms of the raw and acid-activated bentonite samples. STP = standard temperature and pressure.
Table 2. N2- and NH3-adsorption data of the raw and acid-treated bentonite samples.
NH3 adsorption
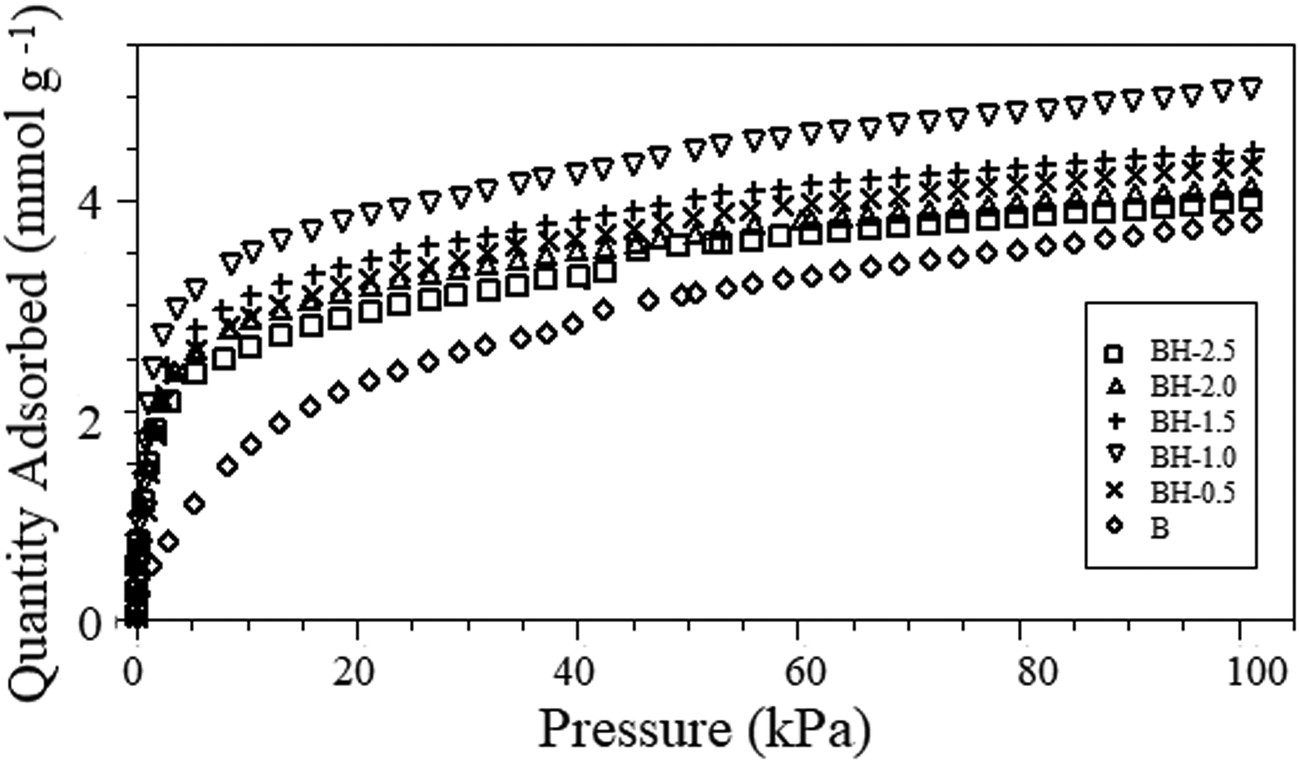
The ammonia-adsorption isotherms for the original and acid-treated bentonites are shown in Fig. 4, and the amounts of adsorbed NH3 per gram of bentonite are listed in Table 2. NH3, an extremely polar molecule, penetrates easily between the smectite layers. In addition, its relatively small kinetic diameter (2.6 Å) should render it available to the whole clay surface (Siegel, Reference Siegel1956). Slightly greater amounts of ammonia were adsorbed on acid-treated bentonites at lower partial pressures. The difference in adsorption of ammonia between different samples decreased with increasing partial pressure. Sorption of ammonia gas on all of the bentonites increased in the following sequence: B < BH-2.5 < BH-2.0 < BH-0.5 < BH-1.5 < BH-1.0 (Table 2). The NH3-adsorption capacities of acid-treated bentonite samples varied from 4.000 to 5.068 mmol g–1. Sample B (3.801 mmol g–1) demonstrated a much lower adsorption capacity compared to the acid-treated forms. The stronger affinity for ammonia of acid-treated bentonites is attributed to the presence of active acidic sites, which attract basic ammonia molecules (Siegel, Reference Siegel1956).
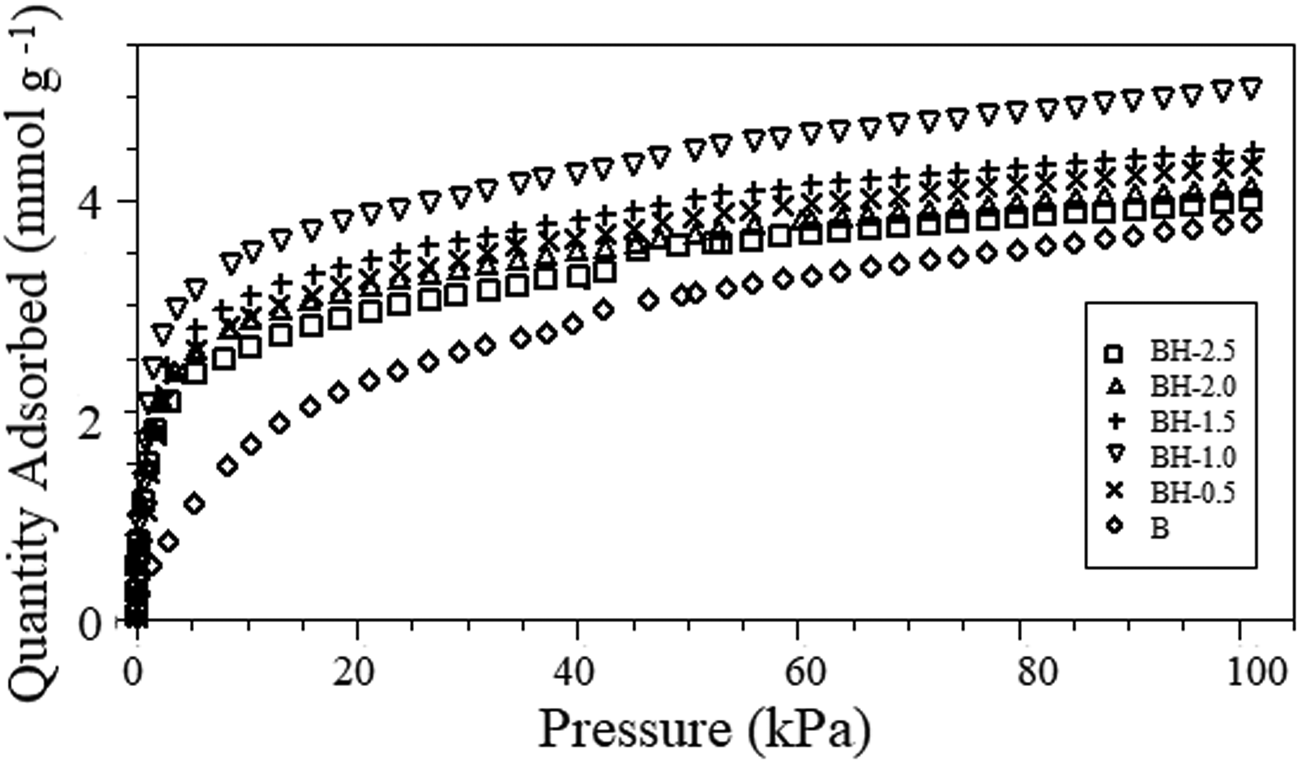
Fig. 4. NH3-adsorption isotherms of the raw and acid-activated bentonite samples.
Increasing the acid concentration to greater than 1.0 M did not further increase the NH3-adsorption capacity (Fig. 4). Sample BH-1.0 showed the highest NH3-adsorption capacity (5.068 mmol g–1) compared to the remaining samples (Table 2). In addition to the removal of exchangeable cations and the loss of octahedral cations, acid treatment increases the number of Brønsted and Lewis acid sites in the clay structure and leads to the formation of Si-OH, Al-OH and (SiO3) Si–OH groups. Thus, interaction of ammonia with silanol groups and surface oxygens is more probable for acid-modified bentonites (Ashworth, Reference Ashworth1978; Christidis et al., Reference Christidis, Scott and Dunham1997; Venaruzzo et al., Reference Venaruzzo, Volzone, Rueda and Ortiga2002). However, although the density of the Brønsted acid sites increased with the acid concentration, the total number of acid sites on the montmorillonite surface decreased due to dealumination (Flessner et al., Reference Flessner, Jones, Rozière, Zajac, Storaro and Lenarda2001).
The ammonia-adsorption capacities of the bentonite samples were not directly proportional to their specific surface areas (Fig. 5). Although the specific surface area values of BH-1.5, BH-2.0 and BH-2.5 (118–129 m2 g–1) were greater than that of BH-1.0 (104 m2 g–1), the ammonia-adsorption capacities were lower (4.000–4.485 mmol g–1) owing to the greater extent of dealumination and partial destruction of the structure (Fig. 1 & Table 1). This is probably because ammonia adsorption is associated with specific sites on the surface. Retention of ammonia by sample BH-1.0 (5.068 mmol g–1) was greater than those of the commercial adsorbent K10-montmorillonite (2.09 mmol g–1) and Na-bentonite (1.07 mmol g–1) (Ursu et al., Reference Ursu, Gros, Nistor and Djelveh2008), carbon/bentonite composites (14.8 mg g–1, 0.869 mmol g–1) (Seredych et al., Reference Seredych, Tamashausky and Bandosz2008), phenolic–formaldehyde resin/bentonite composites (~2.23 mmol g–1) (Seredych et al., Reference Seredych, Ania and Bandosz2016), Fe3+-modified montmorillonite (~4.0 mmol g–1) (Panasyugin et al., Reference Panasyugin, Bondareva and Rat'ko2004) and bentonite treated with 4 M HCl solution for 1 h (0.828 mmol g–1) due to the structural and textural differences in the origin, the mineral and impurity contents and the conditions of acid treatment. The abundance and large sorption capacity of the bentonite-type clay mineral contribute to low-cost and eco-friendly solutions. Therefore, bentonite treated with 1.0 M HCl solution is a promising material for removing ammonia from the air in livestock facilities.
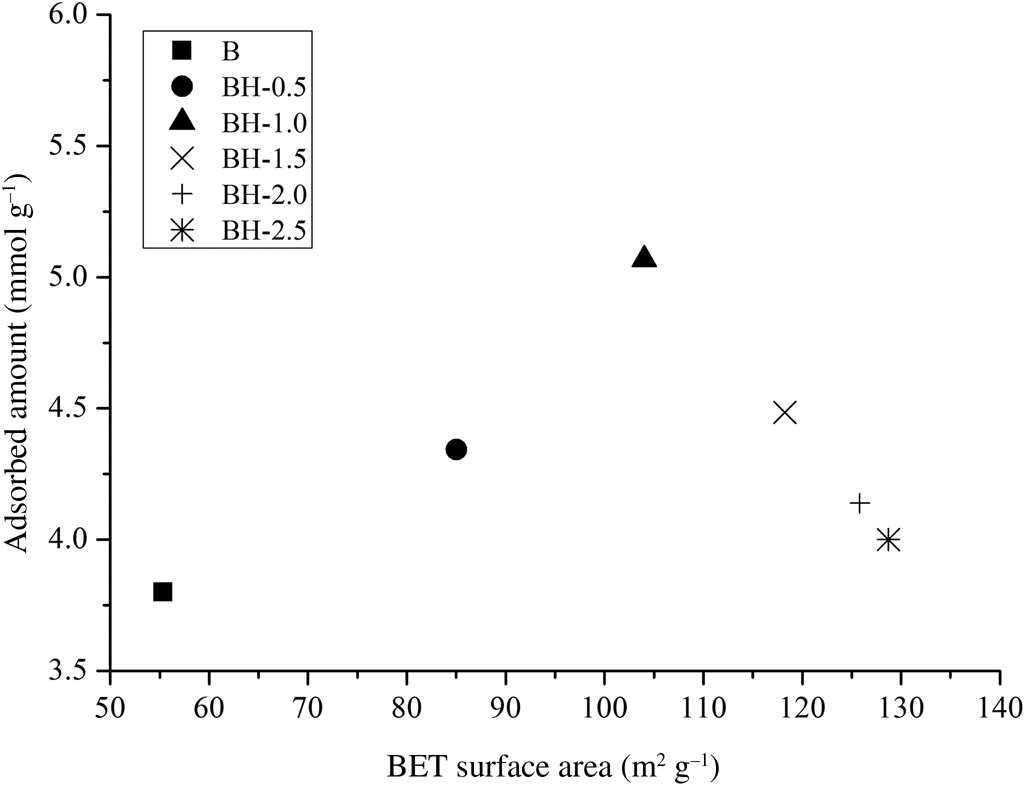
Fig. 5. NH3-adsorption vs BET surface area values of the bentonite samples.
Summary and conclusions
The adsorption characteristics of bentonite and its acid-activated counterparts towards NH3 were investigated. All samples were examined using XRF, XRD, TG/DTA and N2 adsorption techniques to determine their structural, chemical and thermal properties, and the following conclusions were drawn:
(1) Significant structural changes occurred as a result of the acid activation, and the smectite structure dissolved significantly with increasing acid molarity.
(2) All chemical elements except for Si decreased with increasing HCl concentration.
(3) HCl concentrations up to 1.0 M increased the micropore surface area and volume.
(4) Thermal analysis of bentonite samples showed that the total mass losses of the acid-treated samples were lower than that of the natural form of bentonite.
(5) The acid-treated forms of bentonite were more efficient in terms of ammonia adsorption than the raw material. Although the specific surface area of the acid-modified samples increased as the acid concentration increased, treatment with HCl concentrations greater than 1.0 M did not increase the uptake of ammonia gas. Consequently, the BH-1.0 sample with the greatest ammonia-adsorption capacity (5.068 mmol g–1) is recommended as an effective adsorbent of ammonia gas in environments such as poultry houses.
Financial support
Financial support from the Eskisehir Technical University Commission of Scientific Research Project under Grant No. 19ADP054 is gratefully acknowledged.