Layered minerals are omnipresent in nature and are used in a wide variety of applications; for example, as mineral fillers in composite materials such as polymers, paints, etc. (Konta, Reference Konta1995; Zazenski et al., Reference Zazenski, Ashton, Briggs, Chudkowski, Kelse, MacEachern, McCarthy, Nordhauser, Roddy and Teetsel1995; Murray, Reference Murray2000; Ding et al., Reference Ding, Kloprogge, Frost, Lu and Zhu2001; Carretero, Reference Carretero2002). Despite their applications, they have some disadvantages (heterogeneous particle sizes and chemical compositions, presence of impurities) that may restrict their use, especially for fine chemicals or petrochemicals (Bergaya & Lagaly, Reference Bergaya, Lagaly, Bergaya, Theng and Lagaly2006). To overcome these drawbacks, the synthesis of phyllosilicates such as talc (Si4Mg3O10(OH)2) was envisaged by scientists in the last century (Claverie et al., Reference Claverie, Dumas, Careme, Poirier, Le Roux, Micoud, Martin and Aymonier2018).
The synthesis of phyllosilicates plays an important role in the development of new materials. Since 2015, there has been a renewed interest in extending mineral synthesis for industrial purposes (Schaef et al., Reference Schaef, Loring, Glezakou, Miller, Chen, Owen, Lee, Ilton, Felmy, McGrail and Thompson2015; Dumas et al., Reference Dumas, Claverie, Slostowski, Aubert, Careme, Le Roux, Micoud, Martin and Aymonier2016; Diez-Garcia et al., Reference Diez-Garcia, Gaitero, Dolado and Aymonier2017; Cecilia et al., Reference Cecilia, García-Sancho, Vilarrasa-García, Jiménez-Jiménez and Rodriguez-Castellón2018; Cirillo et al., Reference Cirillo, Kozlowski and Spizzirri2018). In those studies, the innovative process highlighted combines a continuous set-up with the use of supercritical water for the synthesis of talc (Si4Mg3O10[OH]2) and tobermorite (Ca5Si6O16(OH)2.nH2O). In a general way, the supercritical solvothermal synthesis underlines the advantage of combining a fast process (a few seconds compared with hours to many days for a batch synthesis) with an opportunity to scale up the process.
This study adopts a new strategy in phyllosilicate supercritical synthesis with the use of a different solvent: a water/ethanol mixture. Interactions between water and ethanol allowed modification of the critical temperature and pressure of the solvent as shown in Fig. 1 (critical coordinates of ethanol: T c = 241°C, p c = 6.27 MPa; critical coordinates of water: T c = 374°C, p c = 22.06 MPa). This phenomenon is due to the change in water molecule organization when ethanol is added (Griswold et al., Reference Griswold, Haney and Klein1943; Laaksonen et al., Reference Laaksonen, Kusalik and Svishchev1997; Bazaev et al., Reference Bazaev, Abdulagatov, Bazaev and Abdurashidova2007).
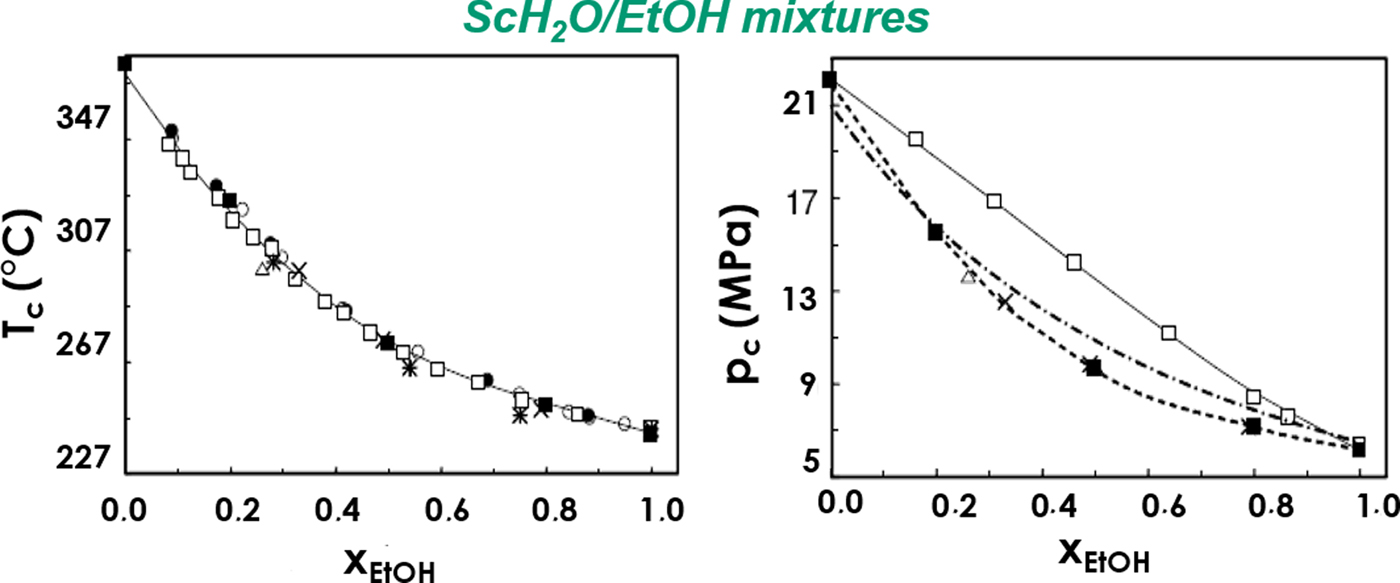
Fig. 1. Evolution of the critical T and p as a function of the water/ethanol mixture composition. ●: data from Marshall & Jones (Reference Marshall and Jones1974); □: data from Griswold et al. (Reference Griswold, Haney and Klein1943); ×: data from Barr-David & Dodge (Reference Barr-David and Dodge1959); ■: data from Bazaev et al. (Reference Bazaev, Abdulagatov, Bazaev and Abdurashidova2007). Reprinted with permission from Elsevier (from Bazaev et al., Reference Bazaev, Abdulagatov, Bazaev and Abdurashidova2007).
This study reports the influence of the composition of the water/ethanol mixture on the structure of the phyllosilicates formed. Various types of phyllosilicates were synthesized using a continuous-flow reactor described in a recent patent (Aymonier et al., Reference Aymonier, Slostowski, Dumas, Micoud, Le Roux and Martin2015) and developed at the Institut de Chimie de la Matière Condensée de Bordeaux (ICMCB) laboratory for the synthesis of different types of materials, such as oxides, metals, nitrides, etc. (Fig. 2) (Aymonier et al., Reference Aymonier, Philippot, Erriguible and Marre2018).
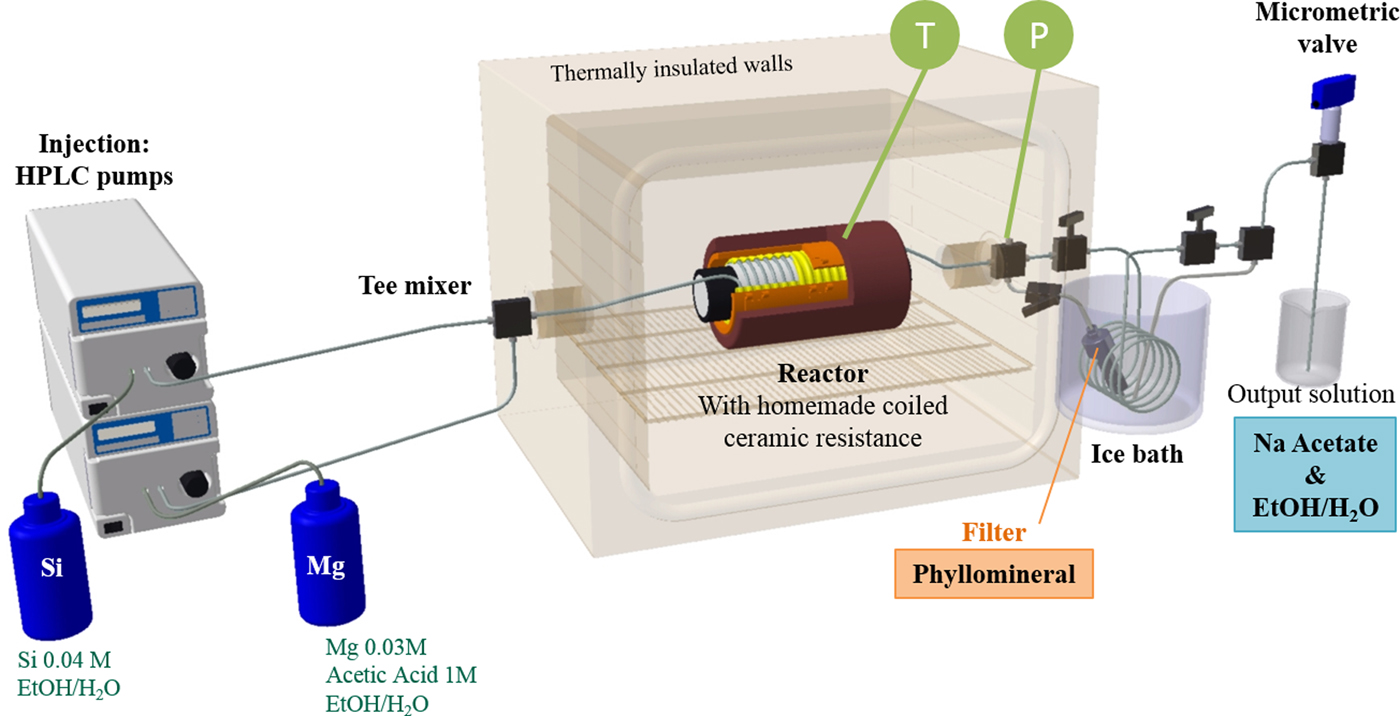
Fig. 2. Custom-built continuous process used for synthesis of layered minerals under supercritical conditions. T = thermocouple; P = manometer; HPLC = high-performance liquid chromatography. Reprinted with permission from Wiley-VCH (from Dumas et al., Reference Dumas, Claverie, Slostowski, Aubert, Careme, Le Roux, Micoud, Martin and Aymonier2016).
To perform the phyllosilicate synthesis, two separate injection lines of solutions were used. One involved 0.03 M magnesium acetate (Mg(CH3COO)2.4H2O) and 1 M acetic acid in the water/ethanol mixture and the second involved 0.04 M potassium silicate (K2SiO3.nH2O) in the water/ethanol mixture. These two solutions were mixed separately at room temperature before being injected into the reactor via a tee mixer point. The reactor, made of 1/8 inch, 316 L stainless steel coiled tubing, has an internal diameter of 1.57 mm for a total volume of 8 cm3. The temperature (up to 500°C) was controlled by a homemade coiled ceramic resistor. The pressure is consistent throughout the whole system, monitored using manometers and controlled with a micrometre needle valve (Autoclave Engineers). In the reactor operating at supercritical conditions, the formation of phyllosilicate particles occurs. An ice bath, placed downstream of the reactor, allows thermal quenching of the reaction. The phyllosilicate product was recovered in a filter, while the solvent solution containing salt was collected for reuse after depressurization through the micrometric valve. The pressure was set to 25 MPa, the temperature to 400°C and the residence time was fixed at 20 s in each experiment. The appropriate flow rate Q (m3 s–1) for a residence time of 20 s at 400°C and 25 MPa has been calculated for each solvent mixture using the following equation:
where τ is the residence time, V reactor is the volume of the reactor and ρi and ρr are the solvent mixture densities at a pressure of 25 MPa and temperatures of 25°C and 400°C, respectively.
One set of samples was obtained to investigate the role of the water/ethanol molar ratio under supercritical conditions at 400°C (nomenclature and description of the samples are given in Table 1). Figure 3 illustrates the X-ray diffraction (XRD) patterns of powdered samples synthesized at 25 MPa for ~20 s by varying the ethanol content in the reaction medium from 0 to 50 mol.%. The ethanol content of the reaction medium drastically affects the nature of the phase synthesized. Indeed, at a lower ethanol content (<20 mol.%), the tetrasilicic magnesium mica (KSi4(Mg2.5□0.5)O10(OH)2; TMM) structure was developed. Mica was synthesized using potassium silicate as the silicon source. The crystal order of these samples increased with the ethanol content (from 0 to 20 mol.%).

Fig. 3. Powder XRD patterns of minerals synthesized at 400°C and 25 MPa in 20 s with various amounts of ethanol.
Table 1. Synthesis conditions of layered mineral samples. The nomenclature of samples in the present study is in the form X-N%, where X represents the first letter of the mineral obtained (M for mica, S for serpentine and B for brucite) and N is the ethanol content.

By contrast, when synthesis was performed in a reaction medium composed of 28 mol.% ethanol, the XRD patterns of the end product indicated a poorly crystallized phyllosilicate structure (reflections at ~7.97 and 1.53 Å) (Grauby et al., Reference Grauby, Baronnet, Devouard, Schoumacker and Demirdjian1998) and the absence of mica. Moreover, 001 reflections shifted towards large angles, indicating a decrease in the interlayer distance (Fig. 3). The S-28% sample is designated as a 1:1 serpentine-like phyllosilicate with an elementary layer of ~8 Å. Lastly, sample B-50%, synthesized in a reaction medium composed of 50 mol.% of ethanol, exhibits all reflection characteristics of a brucite structure (JCPDS no. 44-1482, space group P̅3̅m1 with unit-cell parameters of a = 3.144 Å and c = 4.777 Å). These first XRD results reveal an evolution of the tetrahedral–octahedral–tetrahedral arrangements with the ethanol content in the reaction medium.
Complementary analyses with transmission electron microscopy (TEM) and electron microprobe analysis confirmed by inductively coupled plasma-atomic emission spectroscopy (ICP-AES) allowed precise identification of the nature of the three main minerals obtained (Fig. 4). For synthesis in a reaction medium containing <20 mol.% ethanol, TEM images present a sample with folded and tortuous very thin sheets up to 200 nm in diameter. The microprobe analysis shows a Si/Mg weight ratio of ~1.78 (equivalent to an atomic ratio Si/Mg of ~1.54) and 8.46 wt.% potassium. The atomic Si/Mg ratio of 1.54 is in agreement with the presence of mica (Figs 3, 4). This ratio differs from the 4:3 injected into the tee mixer, confirming the influence of the solvent composition on the material formed. The Mg deficit created octahedral vacancies and induced a negative charge to the layers. Subsequently, K from the silicon source was introduced in the interlayer space. Mid-infrared (MIR) spectra in the hydroxyl stretching zone of these samples are presented in Fig. 5a. The spectra of the M-N% samples in the OH-stretching region consist of two N-bands at 3725 and 3695 cm–1 corresponding to the OH bonded to 3Mg2+ and one V-type band at 3595 cm–1 corresponding to the OH adjacent to an octahedral vacancy of a TMM (Robert & Kodama, Reference Robert and Kodama1988; Robert et al., Reference Robert, Beny, Ventura and Hardy1993). Moreover, to confirm the non-swelling nature of the particles synthesized in a reaction medium containing <20 mol.% of ethanol, various treatments were performed on the oriented aggregate samples: untreated, ethylene glycol solvated and heated at 550°C (Fig. 5b). The respective 001 reflections obtained in the XRD traces are ~10–11 Å without modification after saturation with ethylene glycol or heating at 550 °C. Therefore, the MIR and XRD results confirm the mica nature of these samples.

Fig. 4. Chemical analyses by electron microprobe (solid bars) and by ICP-AES (hatched bars) and transmission electron microscopy images of (a) M-0%, M-5%, M-10% and M-20%, (b) S-28% and (c) B-50%.

Fig. 5. (a) MIR spectra of mica solid samples in the OH-stretching region. (b) X-ray diffraction patterns of the oriented aggregate sample M-0% untreated, glycolated and heated at 550°C.
For synthesis in a reaction medium containing 28 mol.% of ethanol, the TEM images display a poorly crystallized sample with a Si/Mg weight ratio of ~0.78, similar to lizardite (weight ratio Si/Mg of ~0.78). This ratio and the XRD results suggest the synthesis of a poorly crystallized proto-serpentine (Andreani et al., Reference Andreani, Grauby, Baronnet and Muñoz2008). This 1:1 phyllosilicate is composed of only one tetrahedral sheet of silicon with one octahedral sheet of magnesium (T-O phyllosilicate) and is poorly crystalline.
Finally, the sample obtained in a reaction medium containing at least 50 mol.% ethanol is characterized by small polygonal particles composed mainly of magnesium oxide. These results confirm the synthesis of the brucite phase.
The synthesis of minerals adopting diverse structures according to the water/ethanol ratio of the solvent is demonstrated in Fig. 6. The layered mineral synthesized presents a brucitic structure (O), a brucitic structure associated with a Si-tetrahedral sheet (T-O) or a brucitic sheet inserted between two Si-tetrahedral sheets (T-O-T). Therefore, we demonstrated that the introduction of ethanol in the supercritical reaction medium modifies the metasilicate solubility in relation to the evolution of the dielectric constant of the water/ethanol mixture as a function of its composition, with a direct consequence on the formation of tetrahedral sheets of silicon (Akerlof, Reference Akerlof1932; Akerlof & Short, Reference Akerlof and Short1936; Albright & Gosting, Reference Albright and Gosting1946; Rochester, Reference Rochester1972; Karaskova & Mollin, Reference Karaskova and Mollin1993; Fernández et al., Reference Fernández, Mulev, Goodwin and Sengers1995; Bandura & Lvov, Reference Bandura and Lvov2005).

Fig. 6. Schematic diagram of mineral synthesis in a supercritical water/ethanol mixture.
This continuous method is quick (requiring only a few tens of seconds), sustainable and scalable and gives access to high-quality nanostructured minerals with unique physicochemical properties that cannot be obtained with other synthetic methods. Specifically, this process, using solvothermal treatment, enables us to control the structure of the layered material synthesized. This innovative route provides the first proof of synthesis in a few tens of seconds of various minerals in a continuous process simply by varying the solvent composition in the water/ethanol system. This appears to be a promising approach for preparing new synthetic minerals with tuneable layered structures.
ACKNOWLEDGMENTS
The authors acknowledge sincerely the reviewers for their constructive and helpful comments, E. Lebraud for XRD analyses and M. Lahaye for microprobe analyses. The authors also acknowledge financial support from Imerys Company for the PhD grant of M. Claverie. Lastly, we acknowledge the Conseil Régional Nouvelle-Aquitaine.









