Biocatalysis opens a door to green and sustainable approaches in synthetic chemistry owing to its unprecedented chemo-, region and stereo-selectivity under mild reaction conditions. As an important element in bio-catalysts, enzymes have exhibited high levels of efficiency in terms of catalysis and selectivity for a broad range of organic reactions (Santaniello et al., Reference Santaniello, Ferraboschi, Grisenti and Manzocchi1992; García-Urdiales et al., Reference García-Urdiales, Alfonso and Gotor2005). However, because most enzyme molecules show high activity in the aqueous phase only while many substrates are poorly soluble in water, enzyme-catalysed reactions are often performed in biphasic aqueous–organic reaction systems. To enhance the efficiency of two-phase enzymatic reactions, an emulsion is usually formed via the addition of surfactants (Wiese et al., Reference Wiese, Spiess and Richtering2013). The consequent problem is the detrimental effects of surfactants on enzyme activity and the difficulties with separation and product recovery (Wang et al., Reference Wang, van Oers, Rutjes and van Hest2012; Chen et al., Reference Chen, Zhou, Bing, Zhang, Li, Ren and Qu2014).
Pickering emulsions have been the focus of recent, intensive research following their introduction at the beginning of the twentieth century. Being stabilized by solid particles, Pickering emulsions may avoid the disadvantages of traditional surfactant-stabilized emulsions, and therefore hold great potential for enzyme-catalysed reactions in biphasic media (Liu et al., Reference Liu, Lan, Peng, Li, Li and Yang2013; Yang et al., Reference Yang, Zhou and Zhang2013). The first successful application of Pickering emulsions in biocatalysis was conducted with non-porous silica nanoparticles as the stabilizer, whereby the enzyme molecules were encapsulated in the aqueous core of a water/oil (W/O) emulsion (Wang et al., Reference Wang, van Oers, Rutjes and van Hest2012). In addition, confining the enzyme to the nanopores of mesoporous silica particles to form Pickering emulsions might simplify the purification operation (Liu et al., Reference Liu, Lan, Peng, Li, Li and Yang2013; Villalonga et al., Reference Villalonga, Diez, Sanchez, Aznar, Martinez-Manez and Pingarron2013; Jiang et al., Reference Jiang, Liu, Chen, Zhou, He, Ma and Gao2014). Another efficient approach to encapsulating the enzyme is to take the Pickering emulsion droplets as templates for polymerization (Shchukina & Shchukin, Reference Shchukina and Shchukin2012; Wang et al., Reference Wang, van Oers, Rutjes and van Hest2012; Qi et al., Reference Qi, Wu, Molina, Sun, Schulz, Griesinger, Gradzielski, Haag, Ansorge-Schumacher and Schalley2013; Scott et al., Reference Scott, Roy, Abul-Haija, Fleming, Bai and Ulijn2013; Shi et al., Reference Shi, Wang, Zhang, Jiang, Liang, Zhu and Zhang2013; Zhang et al., Reference Zhang, Hu, Zhao, Moller, Yan and Zhu2013). Direct immobilization of enzyme on the surface of Pickering emulsifiers might be achieved via covalent bonds (Motornov et al., Reference Motornov, Zhou, Pita, Tokarev, Gopishetty, Katz and Minko2009; Chen et al., Reference Chen, Zhou, Bing, Zhang, Li, Ren and Qu2014; Gao et al., Reference Gao, Yang, Wu, Wang, Wang, Guo and Yin2014; Zhou et al., Reference Zhou, Fang, Fan, Albela, Bonneviot, Campo, Pera-Titus and Clacens2014). Despite their novelty and advantages, all of these methods involve complicated chemical synthesis, which is generally tedious and time-consuming.
In addition to traditional Pickering emulsifiers, such as silica gels, clay particles and polymer gels, proteins have also been applied for the stabilization of Pickering emulsions (Gao et al., Reference Gao, Li, Jiang, Ma, Zhou and He2014; Liu & Tang, Reference Liu and Tang2014). Because most enzymes are globular proteins, they should be able to stabilize Pickering emulsions. However, perhaps due to their high costs, there have been no reports of such investigations.
The preparation of an oil/water (O/W) Pickering emulsion stabilized by palygorskite particles was reported recently (Lu et al., Reference Lu, Tian, Jin, Chen, Walters and Ding2014, Reference Lu, Zhou, Chen, Jin, Walters and Ding2015). Here the immobilization of a lipase via a palygorskite–enzyme synergistically stabilized Pickering emulsion is reported. To our knowledge, such an investigation has not previously been reported, although there have been reports on the immobilization of lipase on the surface of modified palygorskite particles via adsorption/crosslinking (Huang et al., Reference Huang, Liu and Wang2008, Reference Huang, Liu and Wang2009; Floody et al., Reference Floody, Theng, Reyes and Mora2009; An et al., Reference An, Zhou, Zhuang, Tong and Yu2015; Zhou et al., Reference Zhou, Zhao, Wang, Chen and He2016). Lipases are classified as hydrolases that may catalyse the breakdown of fats and oils with subsequent release of free fatty acids, diacylglycerols, monoglycerols and glycerol at a W/O interface in nature (Huang et al., Reference Huang, Liu and Wang2008). In this work, it is reported that a small amount of lipase may co-stabilize Pickering emulsions with hydrophilic palygorskite particles. Therefore, the lipase may be easily ‘immobilized’ on the surface of O/W Pickering emulsion droplets. Upon the completion of one batch, the Pickering emulsion-immobilized enzyme may be recovered via centrifugation and prepared for the next batch, resulting in an easy separation and reutilization process. The catalytic performance of the Pickering emulsion-immobilized lipase was compared with that of free lipase in terms of thermal stability, storage stability and reusability.
Materials and methods
Materials
Raw palygorskite was obtained from Xuyi Zhongyuan Minerals Co. Ltd (China) with the following chemical composition: SiO2 65.1%, MgO 16.4%, Al2O3 9.35%, Fe2O3 5.33%, CaO 1.23% and trace amounts of K2O, TiO2, P2O5 and MnO. The clay was milled and collected through a 200 mesh (<74 µm) sieve. The as-received palygorskite contained a few mineral impurities, such as quartz, feldspars, smectite and carbonates. A refining process was carried out according to a previously reported work (Xue et al., Reference Xue, Reinholdt and Pinnavaia2006). Water (pH 6.7) was obtained from a Milli-Q Direct 8 reagent water system. Toluene was obtained from Jiuyi Chemical Reagent Co. Ltd (Shanghai). NaCl, NaOH and HCl were purchased from Sinopharm Chemical Reagent Co. Ltd (China). Lipase from Aspergillus oryzae (300,000 U g−1), sodium phosphates (dibasic and monobasic dehydrate, respectively), cupric acetate (anhydrous), pyridine, Brilliant Blue G250, oleic acid, olive oil, phosphoric acid, ethanol (absolute) and bovine serum albumin (BSA) were obtained from Aladdin (China).
Preparation of Pickering emulsion-immobilized lipase
A certain amount of palygorskite particles was put into phosphate-buffered saline (PBS; 0.01 M, pH = 7.4) and sonicated for 5 min. The lipase–PBS solution was then added. The combined volume of PBS solution was 5.0 mL. A total of 5.0 mL of organics was added into the aqueous dispersion and emulsified by a homogenizer at 10,000 rpm for 2 min.
Physical measurements of the Pickering emulsions
The conductivity of the emulsions was determined using a DDS-307 digital conductivity meter with Pt/Pt black electrodes (INESA Scientific Instrument Co. Ltd, China). The emulsion type was determined by observing the dropping of the emulsion into pure water. If the droplet was dissolved, then the emulsion was O/W, otherwise it was W/O. The stability of O/W emulsions to creaming was assessed by monitoring the increase of the height of the clear water (serum)–emulsion interface with time. Optical microscopy of the emulsions was performed by diluting the samples with their continuous phases and viewing them with a PH100-DB500U digital microscope (Phenix Optical Co. Ltd, China). Images were obtained and processed using the NanoMeasure 1.2 software to determine the emulsion droplet size and size distributions. The average droplet size was calculated from at least 100 individual measurements of the drop diameters. Polarizing micrographs of emulsions were obtained using a Leica DM 2500P polarizing optical microscope (POM). Photographs of the palygorskite/lipase-stabilized emulsions were recorded using a digital camera (DMC-LX5GK, Panasonic, Japan). The zeta potentials of the dispersions were measured using a Zetasizer Nano ZS 90 (Malvern Instruments, UK). Each measurement was performed in triplicate to obtain a mean value and standard deviation. The contact angle measurements were performed as follows: the palygorskite particles were pressed to form a disc and immersed in toluene and a droplet of the lipase solution was dropped onto the surface of the palygorskite disc to be measured by the contact angle meter.
Determination of protein content
The protein content in the crude enzyme and immobilized enzyme was determined by the Bradford method (Bradford, Reference Bradford1976), using BSA as the standard. The standard curve was given as y = 0.0017x + 0.0321, R2 = 0.9945. The protein content was 27.9% for the free lipase and 22.3% for the immobilized lipase. The amount of immobilized protein was determined indirectly from the difference between the initial total protein exposed and the amount of protein recovered.
Activity assay of lipase
Standard curve method for determination of fatty acid
A series of solutions with different concentrations of fatty acid was prepared. A certain amount of the solution was mixed with a chromogenic reagent (5% cupric acetate at pH = 6.1 adjusted with pyridine) under stirring to form a green complex, the absorbance of which was determined at 710 nm, with a blank sample as the control. The standard calibration was given as y = 0.11557x + 0.07912, R2 = 0.9937.
The protocol of the lipase activity assay and the recycling of immobilized lipase
The activity of the free and immobilized lipase was analysed using an ultraviolet–visible spectrophotometer. The fatty acid was liberated by hydrolysis of olive oil under the catalysis of enzymes in phosphate buffer at 37°C. The activities of the (immobilized) lipase were examined by adding a certain amount of the (immobilized) lipase to the phosphate buffer, with olive oil as the substrate. After stirring for 10 min at 37°C, the reaction was stopped by adding toluene and stirring was repeated for another 2 min. The reaction mixture was centrifuged at 4000 rpm for 10 min. The 4 mL upper layer was taken, to which 1 mL of the chromogenic reagent was added and stirred for another 3 min. (The remaining contents of the centrifuge tube above were retained for the later recycling cycle to determine the immobilized enzyme.) After being centrifuged at 4000 rpm for 10 min, the supernatant was collected and the absorbance was determined at the wavelength of 710 nm. By comparing the value with that of the control samples, and according to the standard curve obtained above, the concentrations of fatty acid could be obtained. One unit of the activity of (immobilized) lipase was defined as 1 μmol of the fatty acid produced by the catalysis of 1 g (immobilized) of lipase in 1 min under the assay conditions. The results were presented as percentages of the greatest level of activity.
The remaining part of the reaction mixture was removed carefully and the immobilized lipase in the Pickering emulsion added was retained. The same amount of olive oil was added, and the mixture was stirred at 37°C for 10 min. The reaction was stopped by adding toluene and the same analytical protocol was repeated as above. The results were presented as percentages of the initial activity retained.
Results and discussion
Preparation of palygorskite–lipase co-stabilized Pickering emulsions
Because lipase is soluble in PBS, 0.01 M PBS at pH 7.4 was used as the dispersion medium for palygorskite particles/lipase to make the aqueous phase. It was homogenized with the same volume of toluene (the oil phase) to prepare the palygorskite/lipase-stabilized Pickering emulsions.
First, the concentration of palygorskite particles was set constant (~1.5 wt.%) for the preparation of Pickering emulsions while varying the concentrations of lipase. Photographs of the as-prepared Pickering emulsions are shown in Fig. 1. Immediately after the homogenization, the emulsions with different lipase concentrations were all well emulsified. However, after 24 h, the sample containing only palygorskite (Fig. 1, top, a) started creaming, while the remaining samples were stable. After 24 days, the sample without lipase (Fig. 1, lower, a) demulsified completely, while other samples showed comparatively stable emulsions, which indicated a synergistic stabilization by lipase and palygorskite.

Fig. 1. Effect of lipase concentration (a: 0 wt.%, b: 0.005 wt.%, c: 0.010 wt.%, d: 0.015 wt.%, e: 0.020 wt.%, f: 0.025 wt.%, g: 0.030 wt.%) on emulsion stability with constant palygorskite particle concentration at 1.5 wt.% prepared after 24 h (upper) and 24 days (lower).
Figure 2 (top) shows the optical images of emulsion droplets taken 24 h after the emulsions were prepared with different concentrations of lipase. Average emulsion droplet diameters are shown in Fig. 2 (bottom) as a function of lipase concentration. With increased lipase concentration, the emulsion droplet diameters first increased and then decreased until the concentration reached 0.020 wt.%, after which the particle size reached a plateau, with a slight decline in droplet size at 0.025 and 0.030 wt.%. To reduce cost, the lipase concentration of 0.020 wt.% was chosen for the subsequent experiments.
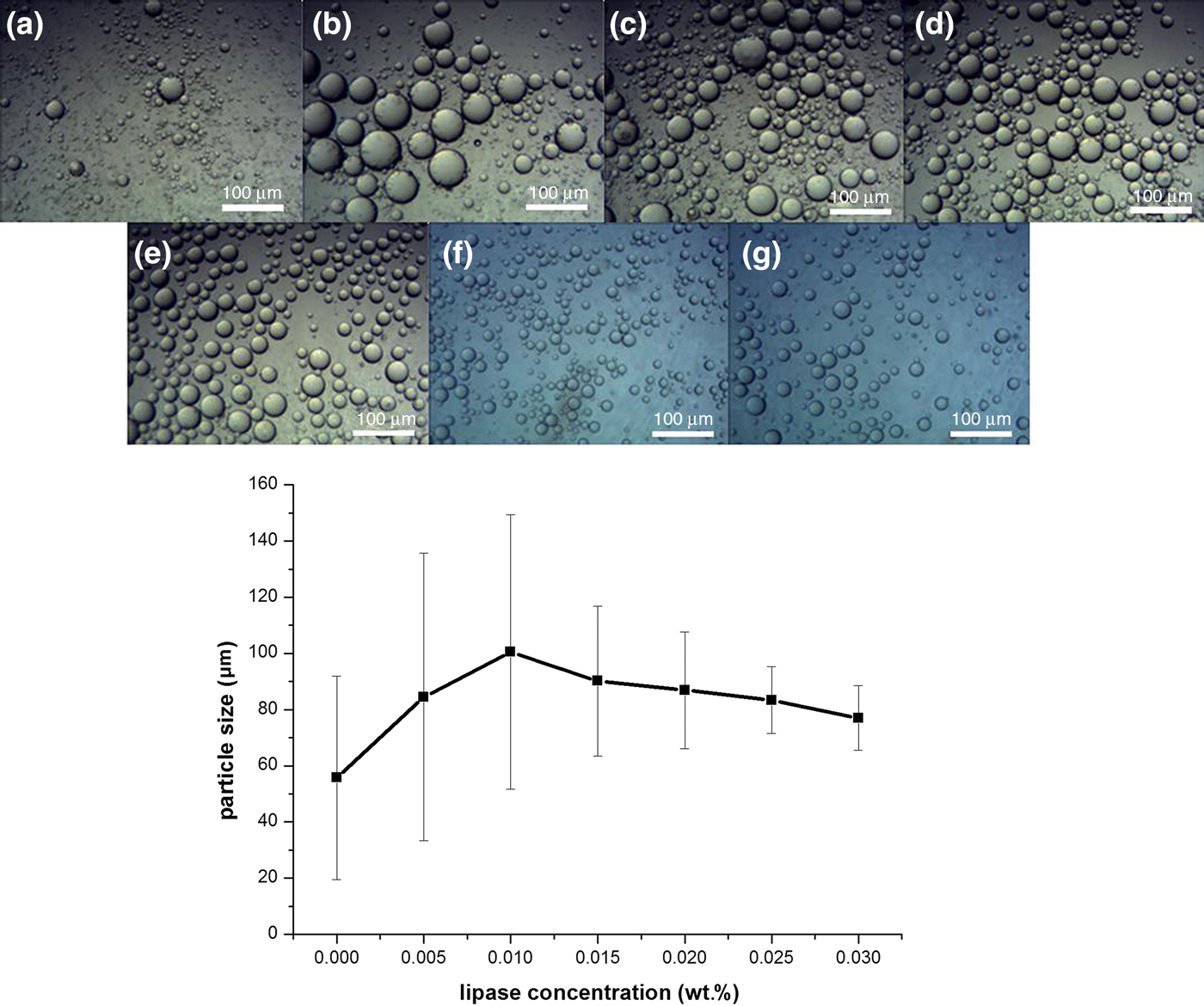
Fig. 2. (Top) Optical images of Pickering emulsions stabilized with 1.5 wt.% palygorskite particles and lipase at varied concentrations (a: 0 wt.%, b: 0.005 wt.%, c: 0.010 wt.%, d: 0.015 wt.%, e: 0.020 wt.%, f: 0.025 wt.%, g: 0.030 wt.%). Scale bar = 100 µm. (Bottom) The size (average diameter) of emulsion droplets and their size distribution vs. lipase concentrations.
The effect of the concentration of palygorskite particles at a constant lipase concentration of 0.02 wt.% on the stability of emulsions was then examined. Immediately after the homogenization, water was precipitated out for the sample containing only lipase (Fig. 3a). Over time, the amount of water released was increased to a much greater extent than that of the samples with increased concentrations of palygorskite particles (Fig. 3b,c,d), indicating the effect of the synergistic stabilization of lipase and palygorskite particles. However, from the ninth day after preparation, the samples with increased concentrations of palygorskite particles showed lower stability. The sample with 2.0 wt.% palygorskite (Fig. 3e) showed both water and oil release, while the sample with 3.0 wt.% palygorskite (Fig. 3f) showed phase separation. This might be due to the fact that the relative amount of lipase was decreased in the samples with increased concentrations of palygorskite. Consequently, the effect of lipase was reduced, suggesting that the Pickering emulsions were co-stabilized by lipase and palygorskite particles.

Fig. 3. Effect of palygorskite particle concentration (a: 0 wt.%, b: 0.5 wt.%, c: 1.0 wt.%, d: 1.5 wt.%, e: 2.0 wt.%, f: 3.0 wt.%) with lipase concentration at 0.02 wt.% on emulsion stability prepared after 9 days.
For the samples stabilized with a constant concentration (0.02 wt.%) of lipase and varying concentrations of palygorskite particles, optical images were also obtained and then processed using NanoMeasure 1.2 software to determine the emulsion droplet sizes and size distributions. The average droplet size was calculated from 120 individual measurements of drop diameters. Combining the calculated results (similar to Fig. 2, lower, not shown) with the results of Fig. 3, the concentration of 1.5 wt.% as optimal for palygorskite particles was chosen for as optimal the subsequent experiments.
Characterization of palygorskite–lipase co-stabilized Pickering emulsions
A Pickering emulsion with an optimal composition of 0.02 wt.% lipase and 1.5 wt.% palygorskite particles in PBS as the aqueous phase and the same volume of toluene as the oil phase were prepared. The conductivity value of 80 μs cm–1 suggested the formation of an O/W emulsion. The drop test – placing a drop of the emulsion in water and observing whether the drop disappears – also gave the same result. The prepared emulsion was then observed using a POM. Because palygorskite crystal bundles are birefringent, in polarizing microscopy images, birefringent domains appear bright (Lu et al., Reference Lu, Tian, Jin, Chen, Walters and Ding2014, Reference Lu, Zhou, Chen, Jin, Walters and Ding2015). Light haloes surrounding the emulsion droplets can be seen in Fig. 4a,b, indicating that most palygorskite particles were absorbed at the W/O interface. Because lipase is also highly hydrophilic, the next step should be the exploration of the location of the lipase to determine whether it was in the continuous water phase or on the surface of emulsion droplets.
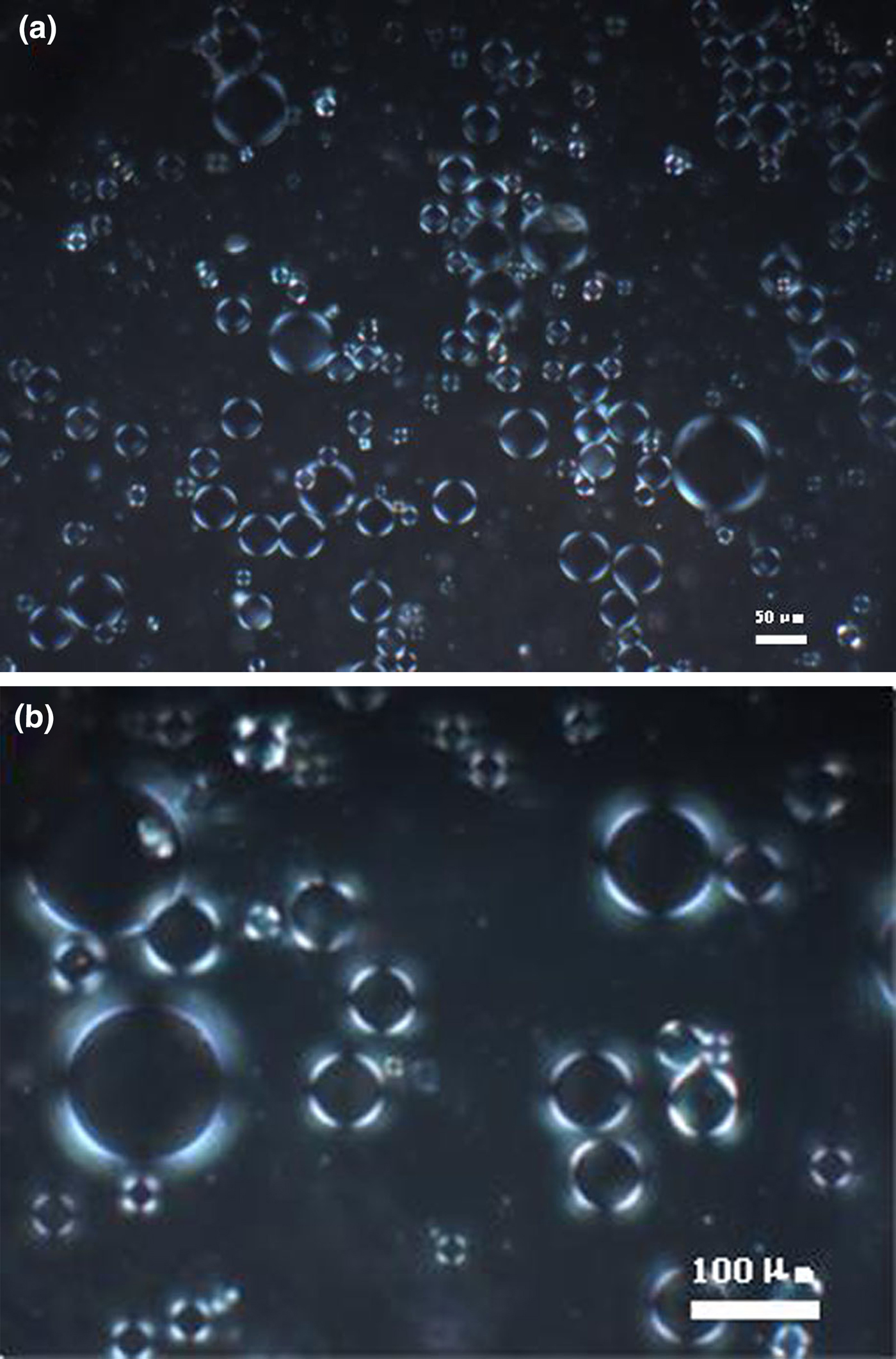
Fig. 4. POM images of the as-prepared Pickering emulsion. The scale bar is 50 µm in (a) and 100 µm in (b).
In order to answer this question, the adsorption rate of lipase onto the palygorskite particles in the aqueous dispersion was determined. The concentration of palygorskite particles was kept at 1.5 wt.% in PBS, while a different amount of lipase was added. The dispersion stood still for 2 h and then was filtered. The amount of lipase in the filtrate was determined by the Bradford method. Thus, the amount of lipase adsorbed onto the palygorskite was determined indirectly from the difference between the initial total lipase exposed and the amount of lipase recovered. The adsorption rate was calculated as the percentage of the amount of lipase absorbed over the amount of the lipase added. The amount of lipase absorbed divided by the amount of palygorskite particles was also calculated and taken as the specific adsorption (Fig. 5).
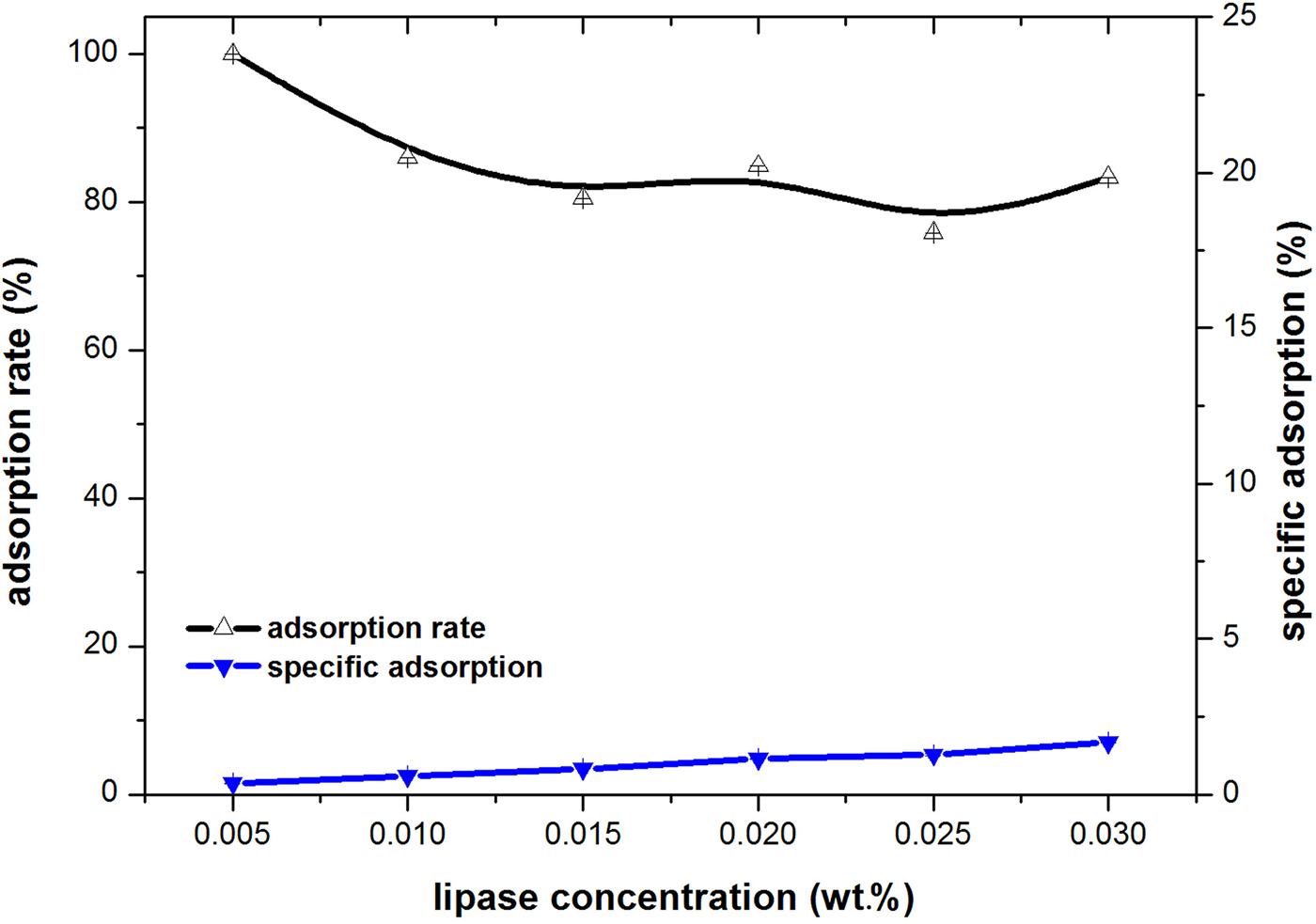
Fig. 5. Adsorption rate and specific adsorption of lipase in the dispersion of palygorskite.
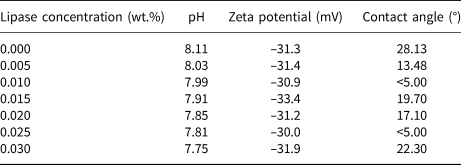
The adsorption rate of lipase did not change with the varying concentration of the lipase, remaining at ~80% of the lipase added (Fig. 5). This suggests that the adsorption of lipase was saturated. While most of the lipase added was adsorbed onto palygorskite particles, the value of specific adsorption of lipase was small, at <2%, which indicates that the amount of lipase adsorbed was quite small compared to the amount of palygorskite. This could be confirmed by the fact that the addition of lipase showed no significant effects on the properties of the aqueous dispersion, such as electric charge and wettability (Table 1). Although the isoelectric points of lipase and palygorskite were both in the range of 4.0–5.5, which suggests that they both bore negative charges in the working environment (pH = 7.75–8.11; Table 1), they did not show repulsion or competitive adsorption onto the surface of emulsion droplets (Binks et al., Reference Binks, Desforges and Duff2007a, Reference Binks, Rodrigues and Frith2007b). By contrast, most of lipase was adsorbed onto the palygorskite particles via electric attraction to the positive charge centre of the palygorskite particles or via hydrogen bonding of amino acids with silanol groups of the palygorskite particles. This might partially reduce the flocculation of palygorskite particles induced by PBS (Lu et al., Reference Lu, Tian, Jin, Chen, Walters and Ding2014). Combined with the results shown in Fig. 4, it could be concluded that the lipase should be on the O/W interface together with palygorskite particles.
Table 1. pH values and zeta potentials of palygorskite dispersions with different concentrations of lipase and the contact angles of lipase solutions on a palygorskite disc immersed in toluene.
Stability and reusability of the Pickering emulsion-immobilized lipase
The preparation of the Pickering emulsion stabilized by palygorskite particles and lipase was indeed a process of the immobilization of lipase. The lipase was immobilized on the surface of emulsion droplets. Because temperature is an important factor regarding the activity of lipase, the thermal stability of lipase was investigated. A certain amount of the (immobilized) lipase was stored at a set temperature for 1 h. Then, 50 μL of emulsion were used to measure the enzyme action. The thermal stability was expressed as the relative activity in relation to the percentage of the greatest level activity, which was 10.30 × 105 U g–1 for the immobilized lipase and 9.49 × 105 U g–1 for the free lipase, both at 45°C. The immobilized lipase had better heat resistance (Fig. 6). At 70°C in particular, its enzymatic activity was four times greater than that of the free lipase, indicating that the thermal stability of the lipase was improved after immobilization. At high temperatures, the free lipase was probably denatured while the immobilized lipase might have been more rigid in terms of its conformation; therefore, it remained stable. Although heat could reduce the conformational flexibility of the free and immobilized lipase, the immobilized lipase was still able to perform its catalytic activity efficiently (Rahman et al., Reference Rahman, Tajudin, Hussein, Rahman, Salleh and Basri2005).

Fig. 6. The activity of the immobilized lipase and free lipase at different temperatures.
The storage life of the immobilized lipase was then investigated and compared with that of the free lipase. A certain amount of the immobilized lipase was prepared and kept at 4°C. A volume of 50 μL of emulsion was taken at a set point in time to examine its activity. The storage stability was presented as the percentage of the initial activity retained, which was 12.66 × 105 U g–1 for the immobilized lipase and 11.64 × 105 U g–1 for the free lipase. The results are shown in Fig. 7. During the first several days (≤8 days), the activities of both lipases remained high, decreasing slightly with extension of the storage time. However, after 10 days, the immobilized lipase showed greater activity. The improved storage stability of the immobilized lipase might be attributed to a decline in the rate of denaturation of the enzyme as a result of fixation in the hydrophilic environment. This helped to preserve the essential monolayer of water surrounding the enzyme molecules and to prevent the disruption of the enzyme-bound water, which was required to maintain the three-dimensional structure of the enzyme for catalysis (Rahman et al., Reference Rahman, Tajudin, Hussein, Rahman, Salleh and Basri2005).
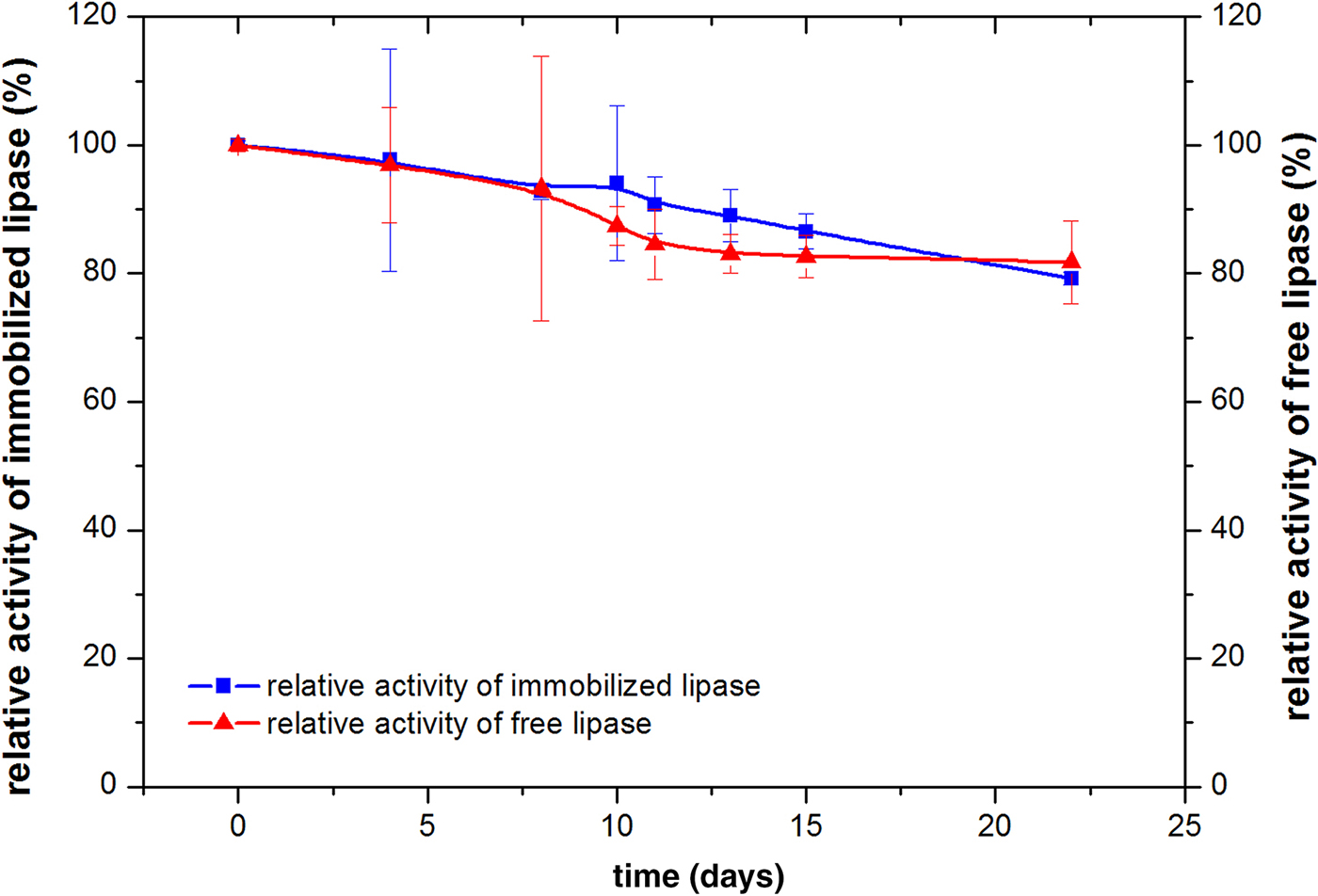
Fig. 7. Storage stability of the lipases.
Reusability is an important characteristic of immobilized enzymes in terms of costs. Therefore, a certain amount of the Pickering emulsion (i.e. the immobilized lipase) was taken for the recovery and repeated use, with olive oil as the substrate. At the end of each cycle, the reaction mixture was centrifuged and collected, while the immobilized lipase part was kept at the bottom of the tube. Thus, the same amount of olive oil/PBS was added and stirred with the solid particles to form a new cycle of Pickering emulsion. Then, a new cycle of reaction was started once again. The reusability of the immobilized lipase was presented as the relative activity in relation to the percentage of the initial activity, which was 13.25 × 105 U g–1. Figure 8 shows that, with increasing cycle numbers, the lipase activity decreased, which might be due to the partial leaching of the lipase. However, after being recycled/reused seven times, the lipase activity was still close to 3.00 × 105 U g–1. This indicates that the immobilized lipase was stable and still remained highly active after being recycled seven times.
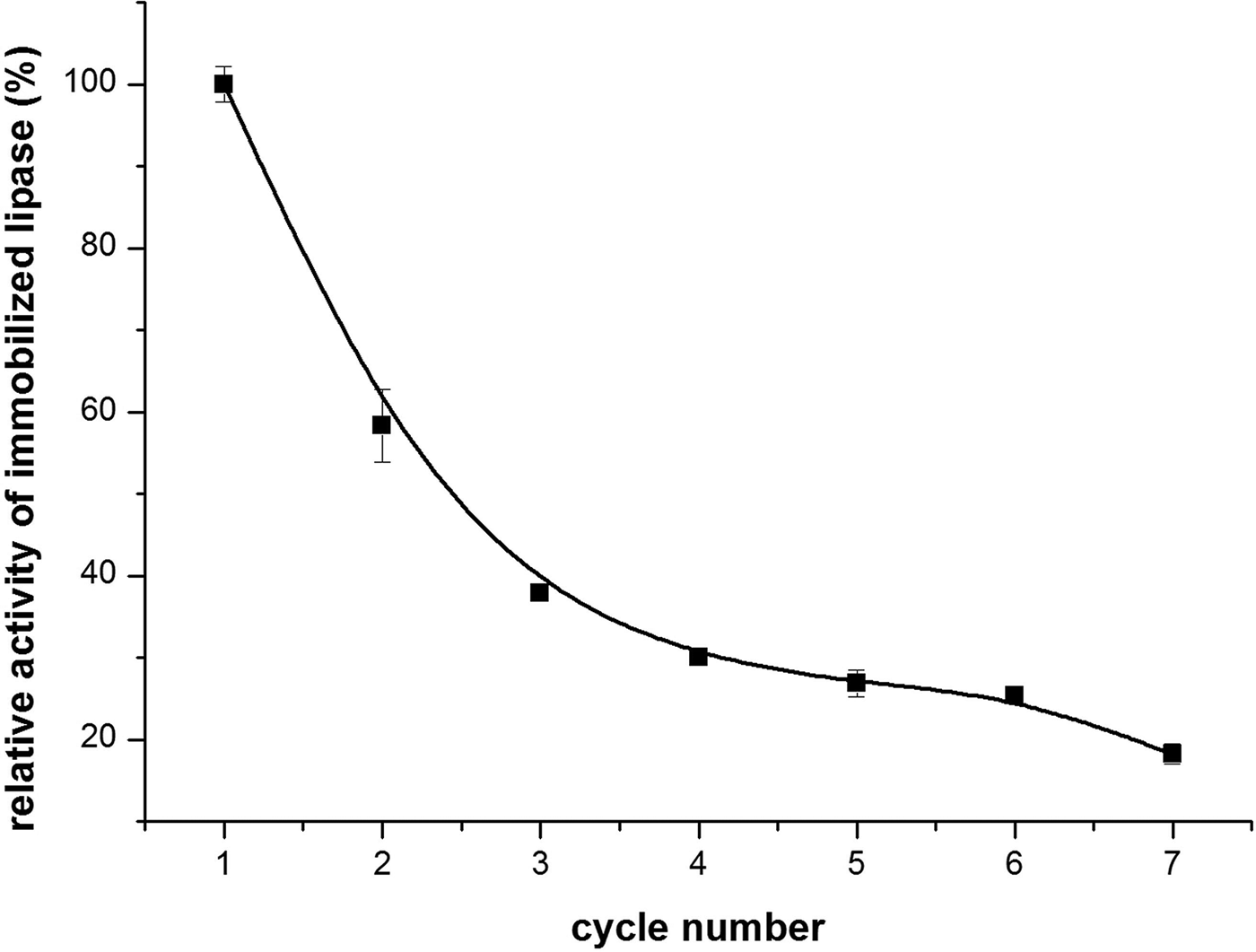
Fig. 8. Change in activity of the immobilized lipase in relation to recycle/reuse number.
Conclusion
In this work, the immobilization of lipase was achieved via the preparation of a Pickering emulsion, which was synergistically stabilized by lipase and palygorskite particles. The immobilized lipase showed greater thermal stability and better storage stability than its free lipase counterpart, and it could be recycled and reused over at least seven cycles. Thus, we have offered a novel and simple route for the immobilization of lipase, which may be extended to other enzymes.
Acknowledgements
The authors are grateful to the National Natural Science Foundation of China (21174046 and 51174096) for financial support of this work.











