With the growing array of medications and their availability on the internet, drug advertising and its regulation is becoming increasingly important to individuals and societies and is widely discussed in the fields of discourse analysis,Footnote 1 policy studiesFootnote 2 and social research.Footnote 3 Drug advertising blends health, scientific and commercial information, and the representation of drug advertising is largely shaped by a wide range of factors from the institutional and social levels. Drug advertising is also a major economic activity. Global pharmaceutical companies spend between 24 per cent and 33 per cent of total sales revenue on marketing, advertising and promotional activities, about twice as much as on research and development.Footnote 4 They are also important political actors on the local, national and international levels.
The Chinese case is particularly interesting and important since the Chinese health system has recently gone through a number of huge transformations since the 1980s. Moreover, the changing and contested role of drugs and drug advertising (particularly in relation to mental health) in China makes it potentially one of the largest and most contested markets for prescription drugs (and antidepressants) in the world. At present, direct-to-consumer advertising for prescription drugs (DTCA-PD) is illegal in China. However, with the growth of globalization and the internet, access to drug advertising is becoming increasingly difficult to control.
In this article, the focus is on the Chinese case. However, in order to understand its relative distinctiveness, we will be comparing it to the UK case. The UK was chosen because it has a key similarity to the Chinese context – in both cases prescription drug advertising is illegal except in recognized medical journals (hence ruling out a China–US comparison). It also has an important difference – the UK has a relatively lengthy history of both antidepressant usage and advertising regulation whereas China has limited experience of both. Using tools from critical discourse analysis to explore the linguistic and discursive patterns of antidepressant advertisements in Chinese and UK medical journals, we will examine how linguistic patterns in anti-depressant drug advertising in China and the UK have evolved in recent times, and explore some of the wider policy contexts that have shaped these developments. The research concludes by exploring what elements are central in the development of the drug advertising policies in China, and what the key implications are, for both academics and policy actors, in understanding governance and policymaking in this area.
Theoretical Framework
Though grounded in complexity theoryFootnote 5 and its overlapping relationship with critical discourse analysis,Footnote 6 the theoretical framework of this article is located in critical discourse analysis (CDA) and its implications for policy learning and transfer. CDA aims “to show how semiotic, including linguistic, properties of the text connect with what is going on socially in the interaction.”Footnote 7 What is essential for CDA, then, is to analyse structures of language use, and to systematically relate them to structures of the sociocultural context of language use. From this sense, CDA goes beyond linguistic analysis, and “seeks to locate discourse within a social world of conflict and inequality,”Footnote 8 looking at common concerns shared by social theory and sociolinguistics, and contributing to explain the interaction between social structures and language use.
CDA has been recently applied to policy studies in the areas of educational policy,Footnote 9 public housing policy,Footnote 10 urban policy research,Footnote 11 adult literacy policyFootnote 12 and a range of other areas. CDA is distinct from other approaches in policy studies in the standing which it gives to language analysis – analysis of ‘texts’ in a comprehensive sense – within discourse analysis. As Fairclough argues, in policymaking and policy debate, there is a focal relationship between problem and solution; CDA, although it does not aim to find such solutions, can contribute to developing models for analysing them that accentuate this relationship.”Footnote 13 Hence, the work of this article is to explore the features of language-use in Chinese antidepressant advertisements and connect them with the multiple levels of discursive events and policy-related socioeconomic contexts that give rise to such language use.
Given the inherent complexity and subtlety of multi-level, political-linguistic policymaking, we intend to question traditional implications of policy learning and transfer. There is a very lengthy history within different fields of policy studies regarding the importance and implications of policy learning and transfer between different countries and policy contexts. For example, post-Second World War modernization theory promoted the simplistic belief that fundamentally all that developing countries had to do in order to develop was to copy, as closely as possible, the policy strategies of developed nations and they would automatically develop. This was followed in the 1970s and 1980s by the emphasis of the World Bank/IMF on a common “structural adjustment framework” for all developing countries.Footnote 14 Again, the implication was that Western policies were more advanced and that imitating their policy structures and strategies was the quickest road to success. More subtle concepts of “policy learning and transfer” and “governance” instead of “government” emerged in the 1990s and 2000s which recognized the highly contextual nature of policymaking and limits of direct policy learning and transfer.Footnote 15 However, they were countered by the narratives of “new public management” and “evidence-based policymaking” which often reasserted the dominance of traditional forms of Western policymaking.Footnote 16
In this article, we do not have the space to explore these debates in detail. Nevertheless, our comparative discourse analysis of the Chinese and UK policies shines a distinctive light on the importance of policy learning and transfer, and at the same time, the inherent limits and difficulties of such learning and the need to continually critically evaluate and assess it.
Prescription Drug Advertising Regulations in the UK
In the UK, the control of advertising of medicines was originally based on self-regulation.Footnote 17 However, DTCA-PD was very limited and made illegal by the March 1992 EU directive on the advertising of medical products for human useFootnote 18 which prohibited DTCA-PD within the European Union.Footnote 19 As early as 1958, guidance for advertising to medical practitioners was provided by the Association of British Pharmaceutical Industry (ABPI). In 1974, the ABPI began to provide the guidelines that resulted in the current form of prescription drug adverts and the most updated version was published in 2016.Footnote 20 These guidelines largely conform with the requirements of prescription drug adverts to medical practitioners recommended by the Ethical Criteria for Medicinal Drug Promotion and developed by the World Health Organization (WHO);Footnote 21 both organizations require information related to names of medicine (both generic and brand), active ingredients, dosage, side-effects, precautions, major interactions, etc. In addition, a separate body, the Prescription Medicines Code of Practice Authority (PMCPA),Footnote 22 operates a code of practice for the pharmaceutical industry independently of the ABPI.
Hence, the UK has had a highly regulated environment for prescription drug advertising to medical practitioners for over 30 years and conforms with WHO standards. This is confirmed in a study by LexchinFootnote 23 who notes that although medical journals from developed countries contain the information suggested by the ethical criteria developed by WHO,Footnote 24 the safety information is systematically ignored in developing countries. As we will explore, less developed regulatory systems and sociocultural issues surrounding mental health conditions may play an important role in shaping the current forms of antidepressant adverts to medical practitioners.
Sociocultural Context of Mental Depression and the Transformation of Anti-depressant Drug Advertising Policy in China
Social, cultural and historical context of mental depression in China
In China, one central concept of Chinese culture and medicine appears to be the tian ren xiang ying 天人相应 (“correspondence between microcosm and macrocosm”).Footnote 25 This means that health is maintained when there is a balanced and harmonious relationship between the human body and the environment. Mental depression can be traced back to the concept of yu zheng 郁症 (“stagnation syndrome”) in traditional Chinese medicine, which explains it as an imbalance of different elements in the human body.Footnote 26 The treatment of such a condition, therefore, often relies on the improvement of the social environment or the balance of different elements in the human body. KleinmanFootnote 27 pointed out the overwhelmingly social foundations of mental illnesses in Chinese society and, in his later work, argued that “attributions of illness onset to social sources, the symbolic linking of symptoms to life context, and the alleviation of distress with improvement in circumstances point to the sociosomatic mediation of sickness.”Footnote 28
Therefore, the use of antidepressants has been marginalized in Chinese society for much of the 20th century and there is still strong resistance to biomedical treatments for mental and emotional disorders. BaumFootnote 29 examined the tension between legislative change (at the level of the state) and phenomenological consistency (at the level of the society and individual) surrounding mental health problems in China in the early 1920s, and concluded that “to many contemporary Chinese minds, the theoretical underpinnings of biomedicine and scientific psychiatry were not only inscrutably foreign, but improbably useless in their approach to psychobiological disorder.”Footnote 30 A relevant point here is that the side-effects of biomedicines have not been generally acceptable for Chinese people, who had a long-established tradition of using natural herbal medicine. As we will argue later, this may be connected with the omission of information related to adverse side-effects in Chinese antidepressant adverts.
However, a significant change in the Chinese discussion of depression and the use of antidepressants has taken place in the last ten years. WangFootnote 31 conducted a longitudinal study examining the discussions about mental depression in British and Chinese newspapers in the last three decades, finding that Chinese people still frame the discussion of mental depression with traditional Chinese concepts, but the depiction of depression as a chemical condition has started to appear in a significant number of Chinese articles since 2009.Footnote 32 At the same time, rising healthcare needs combined with pharmaceutical industry development have led to a large-scale market expansion of antidepressants. Sales of antidepressants have risen from 25.1 to 56.74 billion yuan in the last five years and are estimated to exceed 60 billion yuan in 2017.Footnote 33 This mixture has led to a re-conceptualization that differs in important ways from the Western concept. Therefore, it is important to look into the ways in which antidepressants were advertised and how the language reflected the sociocultural context.
The next section briefly discusses the policy-related socioeconomic contexts of antidepressants advertising, including the upheaval of the Chinese healthcare system and China's prescription drug advertising regulatory background, its current status and challenges. This will not only contribute to unravelling the interaction between language use in antidepressant adverts and the related policy practices but will also provide more insight into comparative policy learning and transfer.
Background to drug advertising policies in China
In the past 30 years China has gone through huge changes in its health policy and systems. During the 1960s and 1970s, China's health system was prevention oriented, public health based, and people-centred using local “barefoot doctors.” At that time, it was praised by WHO for its successful vaccination programmes, elimination of malaria, improving mortality rates for the under-fives, elimination of opium usage, and a range of other basic health achievements. Key changes occurred following the transition to a more market-oriented approach to development in the early to mid-1990s. Under the new system, state-funded healthcare was replaced with care funded by a mix of local government, employer and individual contributions, and private provision of healthcare was promoted. At the time of huge economic upheaval, this led to a range of problems including: huge regional variation and growing inequality of access to care, growing business orientation and loss of public health-oriented culture of the profession,Footnote 34 and poor regulation and corruption. By the mid-2000s, WHO, World Bank and even The Economist criticized China for pushing the health market too far and too fast.Footnote 35 In response, in 2007 President Hu Jintao 胡锦涛 announced a major health reform to increase funding, make the system more patient-centred and strengthen its public orientation. However, problems persist in relation to growing costs, huge variation in outcomes and access, and hidden corruption.
In relation to prescription drug advertising, early regulation from 1978 to 1993 made no distinction between prescription and non-prescription drugs. However, from 1994 onwards, the state authorities defined advertising and separated prescription and non-prescription drugs. The main regulations concerning the pharmaceutical advertising in China are the Advertising Law (promulgated in 1995 and amended in 2015); the Law for the Administration of Pharmaceuticals (adopted in 2002); the Standards for the Examination and Publication of Drug Advertisements (effective from 1 May 2007 and now under revision); and the Measures for the Examination of Drug Advertisements (effective from 1 May 2007). The latest Advertisement Law of the People's Republic of China (2015), amended and revised by the Standing Committee of the National People's Congress (NPC) and State Administration for Industry & Commerce (SAIC),Footnote 36 strengthened the inclusion of the critical information about drugs and reaffirmed the ban on prescription drug advertising (except in professional journals):
Article 16: Drug and device advertisements must clearly state critical information about the products, such as contraindication and adverse effects; Drug and device advertisements must not contain absolute assurances or guarantees of the product's safety.
Article 19: Mass media (including broadcasting, television and the internet) cannot feature drug, device, health supplement and medical service advertisements in disguised forms, such as in news reports or health-related public education programs, so as to circumvent being regulated as advertisements.
China's prescription drug advertising regulatory challenges
Ma and LouFootnote 37 pointed out that although the Chinese government oversees prescription drug advertisements and relies on strict pre-approval requirements, compared to the huge sums spent on drug promotion, government resources are limited to regulate and enforce. BaiFootnote 38 notes that approval authorities for prescription drug advertising in China are only regularly trained to understand relevant policies and regulations and to improve their competence in implementing them, without being provided sufficient, efficient and reliable drug information. Moreover, China now operates an Essential Drugs List (EDL), a list of core medicines that are reimbursable under basic medical insurance at the national and provincial level. The importance of the EDL weakens the role of drug advertising strategies for those drugs on the list since manufacturers can be guaranteed a significant market share.Footnote 39 However, it also pushes those producers who are not on the list to use unbalanced and untruthful advertising to increase market share.
A Critical Discourse Analysis of Antidepressant Advertisements in China
Chinese drug advertising polices state that prescription drugs are only allowed to be advertised in officially approved medical journals. This research looks at all the antidepressant advertisements published in China's leading journal in relation to psychiatric illness, the Chinese Journal of Psychiatry (Zhonghua jingshenke zazhi 中华精神科杂志), from 1996, when the journal was founded, to 2015. The journal was a quarterly publication from 1996 to 2011 and became a bi-monthly from 2012. As Figure 1 shows, the number of advertisements in the journal has increased significantly over the last 20 years. From 1996 to 2003, the number rose steadily, despite a slight fall in 2001. In 2004 the total number of advertisements almost doubled from 2003. This is because in 2003, the Chinese government started to increase investment in the public health system due to the SARS crisis. As a result, the prescription drugs market began to be more active. In 2005 there was a slight decrease in the frequency of the advertisements, but subsequently the number of advertisements kept increasing until 2008, when the frequency spiked. The dip between 2012 and 2014 is related primarily to editorial changes in the Chinese Journal of Psychiatry.

Figure 1: Number of Antidepressant Advertisements in Chinese Journal of Psychiatry, 1996–2015
Table 1 shows the top-ten most frequently advertised antidepressants in the Chinese Journal of Psychiatry. It shows four Chinese brands manufactured by Chinese companies (Bo Le Xin, Yan Suan Liu Li Da Qin, Mi Er Ning) and three Western brands manufactured by Chinese-Western joint ventures (Seroxat, Cipramil, Lexapro). The remaining four are Western brands produced by Western companies (Cymbalta, Effexor XR, Remeron, Zoloft). In this article, we will be making a brief comparison with UK antidepressant drug advertising and examine the differences between British and Chinese advertisements in the context of their respective prescription drug advertising policies.
Table 1: Top-Ten Most Frequently Advertised Antidepressants in Chinese Journal of Psychiatry, 1996–2015

Source:
Compiled by the authors.
Content analysis of Chinese antidepressant advertisements
Our content analysis is focused on all 67 advertisementsFootnote 40 published in the Chinese Journal of Psychiatry from 1996 to 2015. To highlight the distinctive features of Chinese antidepressant advertisements, the equivalent British version is considered for comparison. A brief review of the British version shows clearly that all the advertisements have a very similar structure, covering almost all the categories shown in medical instructions. Therefore, only five British adverts randomly chosen from the British Journal of Psychiatry from 1996 to 2015 are included for this comparative analysis. Table 2 shows the average percentage figures for coverage of all categories of information in Chinese and British adverts.
Table 2: Percentage of Information Categories Covered by Chinese and British Advertisements

Source:
Compiled by the authors.
It is clear that around 89 per cent of the advertisements in the Chinese Journal of Psychiatry include the use of antidepressants. Around two-thirds cover the dosage and contraindication categories, while precaution and drug interaction are under-represented. Meanwhile, adverse effects of antidepressants are clearly indicated in around 37 per cent of Chinese adverts. British adverts cover all the above-mentioned six categories of information, without exceptions. Presentation is not frequently mentioned in Chinese adverts but is consistently provided in the British ones. The overdosage category is covered by 40 per cent of the British adverts, and NHS basic price information appears in 80 per cent of the British adverts. In Chinese adverts, only around 4 per cent include information related to overdosage and 11 per cent indicate the price information about products.
Based on a manual calculation, the average number of the categories that these 67 Chinese adverts cover is 3.57. This indicates that the information Chinese antidepressants adverts provide is neither complete nor balanced (with the precaution, drug interaction and overdosage categories underrepresented). Furthermore, a brief look into the content of Chinese antidepressant adverts indicates that the medical information is oversimplified relative to the UK. We separated all the adverts based on different categories listed in Table 2 and compared the average word count in each category in the Chinese and British adverts. Figure 2 shows that the content in relation to categories of dosage, precaution, drug interaction, adverse effects and overdosage in British adverts significantly exceeds those in the Chinese. Interestingly, all of these categories focus on the use of antidepressants, and critical information concerning them, indicating the concepts of caution, danger and adverse effects. Chinese adverts clearly underrepresented such categories.

Figure 2: Average Word Count of Each Category of Medical Information in Chinese and British Antidepressant Advertisements
The only category in which Chinese adverts use more words than British ones is contraindication. The average word count of this category is 67 in Chinese adverts, and 15 in British ones. It will be interesting to see a comparison of representation in this category. Here, we extract two typical ones from each side.
British adverts
1) Contraindication: hypersensitivity to Paroxetine. Use with MAOIs (refer to drug interactions).
2) Contraindications: hypersensitivity to any component of the product.
Chinese adverts
1) [Translation]
Contraindication: 1. hyperactivity to Tianeptine or other components). 2. Children aged below 15 years. 3. Concomitant use with MAOIs. 4. Do not use Tianeptine within two weeks after MAOIs treatment. Patients who change treatment from Tianeptine to MAOIs only need to leave one day before starting MAOIs.
2) [Translation]
Contraindication: hyperactivity to Trazodone Hydrochloride. Patients with severe liver function impairment, severe cardiac conditions and mental disabilities should not use Tianeptine treatment.
Clearly, in British adverts, the content in the category of contraindication is predominantly represented as “hypersensitivity to any components” while in Chinese adverts, apart from the issue of hypersensitivity, general descriptions related to drug interaction and precaution are included. The word count information provided in Figure 2 indicates that British adverts describe the information of these two categories in a much more detailed way. Therefore, it is interesting to find out here that Chinese adverts tend to mix the information related to contraindication, drug interaction and precaution together and put them all in one category of contraindication, with oversimplified language. This forms another distinctive feature of Chinese antidepressant advertising.
Corpus-assisted critical discourse analysis of Chinese antidepressant advertisements
To discover the predominant ways of representing antidepressants in Chinese adverts, a special corpus is built for this research including all 424 advertisements that appeared in the Chinese Journal of Psychiatry from 1996 to 2015, and the size of the corpus is 73,719 words. To draw a comparison between Chinese and British texts, five British adverts have been randomly chosen, with a corpus word count of 3,842. Three of the main corpus research methodologies: frequency analysis, collocation analysis and keyword analysis, will be used in this study.
Frequency analysis: information on use and dosage
Using a frequency analysis, from two sets of word lists extracted from the Chinese and British antidepressant advertisements, we can see the top 20 most frequent words in the two corpora (Table 3). The top 20 words in Chinese adverts fall neatly into the following four categories of information:
1) Indication:焦虑 (anxiety), 治疗 (treatment, treat), 抑郁症 (depression), 抑郁 (depressive), 症 (symptoms)
Table 3: Top 20 Most Frequent Words in Chinese and British Antidepressants Advertisements

Source:
Compiled by the authors.
Notes:
*In presenting the research results generated from language software, only Chinese characters and their English translations are provided. For all Chinese characters incorporated within the text, both English translations and pinyin will be provided.
†In the Chinese language, zhiliao 治疗 can be used as both a noun (treatment) and a verb (to treat).
2) Dosage and administration: 剂量 (dosage), 片 (tablets), 服用 (administration), 法 (method), 用 (using), 一 (one), 天 (day), 次 (time)
3) Adverse effects: 不良 (adverse), 反应 (effects)
4) Contraindication: 对 (for), 本 (this), 品 (product), 过敏 (hypersensitivity), 患者 (patients)
It is interesting to find that the anxiety (jiaolü 焦虑) is the most frequent word in the Chinese corpus, with treatment/treat” (zhiliao 治疗) as the second. Examining the texts in which “anxiety” occurs shows that it is uniformly used in the category of indication with rare exceptions, to describe the symptom that antidepressants can treat.
A more detailed analysis of “treatment/treat” (zhiliao 治疗) in Chinese adverts shows that the following sentence is overwhelmingly used in the category of indication: “[this product is used to] treat all types of depression (including mild, moderate and severe), depressive symptoms, generalized anxiety disorder.” The dominant use of such a structure contributes to the high frequencies of words like “anxiety” (jiaolü 焦虑), “treatment/treat” (zhiliao 治疗), “depression” (yiyuzheng 抑郁症), “depressive” (yiyu 抑郁) and “symptoms” (zheng 症). This indicates that in Chinese adverts, the category of indication has been over-generalized into two words or phrases: “anxiety” and “depressive symptoms,” without pointing out important differences between brands of antidepressants and the different types of depression that they treat. Thus, antidepressants have been represented as a one-for-all medicine that doctors can prescribe for patients who have depressed feeling or a generalized condition of anxiety.”
By contrast, in British adverts, the description for usage of the antidepressant is much clearer and more concise:
Use: treatment of schizophrenia. Treatment of manic or major depressive episodes associated with bipolar disorder. Seroquel XL is effective in preventing relapse in stable schizophrenic patients who have been maintained on Seroquel XL.
Here, the use of a particular brand of antidepressant is described in relation to specific types of depression with distinctive symptomatic features, providing more detailed information for prescribing doctors.
To look into the category of dosage information, we directly searched the texts in which the most frequent words about dosage (see Table 3) occurred: “dosage” (jiliang 剂量), and “method” (fa 法), and “using” (yong 用). Surprisingly, the short sentence “One tablet a day; convenient using method” (mei tian/ri yi pian 每天/日一次, fu fa fang bian 服法方便) stands out as a predominant structure and there is hardly any further information about dosage. By contrast, we find detailed information provided in the British adverts, for example:
Dosage: Adults: Depression: 20 mg a day recommended. If necessary, increase dose in 10 mg increments to a maximum of 50 mg according to response. Obsessive compulsive disorder: 40 mg a day recommended. Starting dose 20 mg a day increased weekly in 10 mg increments. Maximum dose 60 mg a day. Panic disorder: 40 mg a day recommended. Starting dose 10 mg a day; dose increased weekly in 10 mg increments. Maximum dose 50 mg a day. Social anxiety disorder/social phobia: 20 mg a day recommended. Starting dose 20 mg per day. If no improvement after at least two weeks, increase by 10 mg per week up to a maximum of 50 mg/day according to response. Effective in 12-week placebo-controlled trials. Only limited evidence of efficacy after 12 weeks of treatment. Post-traumatic stress disorder: 20 mg a day is recommended starting and maintenance dose. Some patients may benefit from dose increases in 10 mg increments as required up to a maximum of 50 mg/day according to response. Effectiveness has not been evaluated beyond 12 weeks in placebo-controlled trials.
In this typical dosage information extracted from the British corpus, we find six words (emboldened in the above text) which enter the top 20 words list of the British corpus shown in Table 3. It provides detailed information for different types of depression, such as general “depression,” “obsessive compulsive disorder,” “panic disorder,” “social anxiety disorder/social phobia” and “post-traumatic stress disorder.” It also includes the instruction based on patients’ response to their starting dose, such as “if no improvement after two weeks, increase by 10 mg per week up to a maximum of 50 mg/day.” Similarly, readers are reminded of the importance of continuous treatment after recovery, and the danger of abrupt discontinuation. It is therefore fair to conclude that in this comparison of Chinese and UK antidepressant adverts, the Chinese dosage and administration information has been heavily simplified, echoing the average word count analysis shown in Figure 2.
In the top 20 words list, we find buliang 不良 (“adverse”), and fanying 反应 (“effects”), but none of the rest of the words indicate the content of this category. Similarly, in the British word list, we do not find “adverse effects” or words that might be used to describe the actual side-effects of antidepressants. Therefore, the corpus research method of collocate analysis was used to investigate the features of the language describing the side-effects of antidepressants.
Collocate analysis: “adverse effects” and “contraindications”
In this section, we look at all the words that co-occur with “adverse effects” (buliang fanying 不良反应) within five positions to the left and right.Footnote 41 Wordsmith Tools enables the generation of a list of such word collocates based on their raw frequencies, called a collocation profile. Table 4 shows the top ten words in this profile. The lines in which “adverse effects” (buliang fanying 不良反应) and these listed words co-occur represent the side-effects as comparatively rare. We find citations like “the ‘common’ side-effects of antidepressants ‘include’ very light stomach discomfort,” and “for ‘detailed’ information in relation to ‘adverse effects and precautions’ please refer to medical instruction.” The following provides six complete sentences with more contextual information:
[Translations]
Table 4: Top Collocates of 不良反应 (“Adverse Effects”) in the Chinese Corpus of Antidepressant Advertisements

Source:
Generated using Wordsmith 5.0.
1. … has comparatively little influence on cytochrome oxidase, and the side-effects are comparatively rare.
2. … is convenient to take and its dosage is easy to adjust. The toxins brought by the medicine are easy to be excreted. Besides, its side-effects are comparatively rare, and the safety range is relatively wide.
3. Adverse effects include: very light stomach discomfort and nerve disorders.
5. … is effective for long-term treatment with low relapse rate. It is also with light side-effects, high drug tolerance and unique package design.
6. … causes extremely rare drug interaction, and its side-effects are rare and light.
By contrast, British adverts adopt a universal way in displaying very clear and straightforward contents about adverse effects. Instead of looking into the collocation profile of “side-effects,” the following typical example provides a better picture:
SIDE-EFFECTS: nausea, headache, insomnia, somnolence, dry mouth, dizziness, constipation, asthenia, sweating, nervousness, anorexia, dyspepsia, abdominal pain, anxiety, impotence, abnormality of accommodation, vasodilation, vomiting, tremor, paraesthesia, abnormal ejaculation/orgasm, chills, hypertension, palpitation, weight gain, agitation, decreased libido, rise in blood pressure, postural hypotension, reversible increases in liver enzymes, slight increase in serum cholesterol, hyponatraemia.
It is important to point out that the above displayed side-effects are equivalent to the information included in the medical instructions. Therefore, when we revisit the regulation for prescription drug advertising issued by SAIC and SFDA: “Such contents… shall conform to the instructions approved by the food and drug administrative department of the State Council,” and “The publicity may not be conducted by exaggerating or maliciously hiding certain information…,” we can assume that the implementation of such regulation needs to be improved in China.
Lastly, in the category of contraindication, as the analysis in the section “Content analysis of Chinese antidepressant advertisements” shows, one of the distinctive features of the Chinese antidepressant adverts is that they try to mix the information related to contraindication, drug interaction and precaution under one category of contraindication and describe them in simplified terms. Therefore, we do not see any words related to drug interaction or precaution in the Chinese word list, and thus the significant collocates extracted for such low-frequency words are not revealing. However, when we look directly into the texts of adverts that include the content related to contraindication in Chinese adverts, we found that the following sentence is overwhelmingly used: dui ben pin guomin zhe ji zhengzai fuyong dan an yang hua mei yi zhi ji de huanzhe jinyong ben pin 对本品过敏者及正在服用单胺氧化酶抑制剂的患者禁用本品 (“not recommended in patients who have hypersensitivity to this product and patients taking MAOIs”). This explains why the words “for” (dui 对), “this” (ben 本), “product” (pin 品), “hypersensitivity” (guomin 过敏) and “patients” (huanzhe 患者) fall within the top 20 words list, and this over-simplified sentence has actually been used as the only information concerning the risks (negative side) of antidepressants.
By contrast, in the British word list, words like “patients,” “caution,” “severe” and “recommended” are very likely to occur in the categories of drug interaction and precaution. The word “patients” occurs as the most frequent word in the British corpus. Therefore, it is important to find out how British adverts construct patients, which might throw light on the content of the categories of contraindication, drug interaction and precaution. Using a Wordsmith “word cluster” function, an extended application of collocate analysis, the top ten three-word clusters in the British corpus can be identified as seen in Table 5. Of these ten three-word clusters, five contain “patients,” with the first one as “in patients with,” and the second as “caution in patients.” Another two frequent clusters containing “caution” are “with caution in” and “use with caution.” It is fair to assume that the frame “use with caution in patients with/taking” should form a repeated pattern. Therefore, all the lines containing “in patients” were extracted, to ascertain whether this assumption is right – see Table 6.
Table 5: Most Frequent Word Clusters of “Patients” in the British Corpus of Antidepressant Advertisements

Source:
Generated using Wordsmith 5.0.
Table 6: Concordance Lines of “In Patients” in the British Corpus of Antidepressant Advertisements

Source:
Generated using Wordsmith 5.0.
As predicted, we find six lines containing “caution in patients with/taking.” It is obvious to see that the majority of these lines are used to refer to the precaution and drug interaction categories (with lines about “in patients with” for precaution, and “in patients taking” for drug interaction), with a small portion of the content being used in the category of dosage information (line 23). This content provides detailed information concerning patients’ medical conditions, such as patients with “congenital long DT syndrome,” “epilepsy,” “myocardial infarction” or “established cardiac disease.” The concept of caution is continuously emphasized, keeping readers alert. This content is equivalent to that provided in the medical instructions accompanying antidepressants. By contrast, Chinese adverts remain silent in such areas. This echoes our analyses shown in Table 2, that only around 12 per cent of adverts include information related to precaution, around 10.45 per cent to drug interaction, and the contents in these two categories are usually over-simplified.
Overall, the differences between Chinese and British adverts are significant. First, most Chinese adverts cover the categories of information related to the use, dosage, side-effects and contraindications of antidepressants. British adverts, on the other hand, not only cover the above-mentioned categories, but use more space for the categories of precaution and drug interaction. Second, the content of every category in Chinese adverts is generally oversimplified, for example, with the use of antidepressants described as to “treat depressive symptoms and anxiety,” with the dosage information as “once a day,” with side-effects as very “rare (light),” and with contraindication as “hypersensitivity to this product.” British adverts, by contrast, provide a significantly more detailed picture for each category, especially in the dosage information. Lastly, British adverts have highlighted the concept of “caution” by providing large-scale detailed information in relation to precaution and drug interaction while Chinese adverts keep silent on this issue.
Keyword analysis: changes over time
Using Wordsmith keyword analysis, it is even more clear which aspects of antidepressants have been focused on, and which ones less talked about over time. As the British adverts have adopted a consistent way (in both structure and content) of displaying antidepressant information, we will mainly look at what categories of information Chinese adverts have emphasized from 1996 to 2015, and whether they have changed over time.
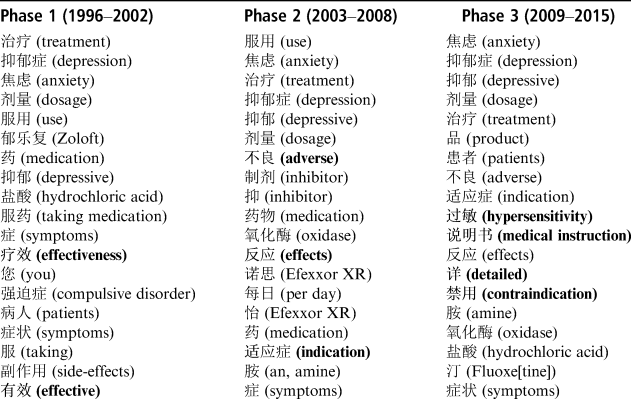
Based on the analysis of the frequency changes of Chinese adverts and their related social contexts shown in Figure 1, we divide the Chinese corpus into three time phases: 1996–2002, 2003–2008 and 2009–2015. Table 7 shows the three sets of keyword lists for the defined phases (the words in bold indicate the distinctiveness of each time phase). These three keyword lists overlap each other to a large extent, including a certain amount of common words such as “treatment/treat,” “depression,” “anxiety,” “dosage,” etc., and words indicating the chemical or brand names of antidepressants, such as “Efexxor XR” (yinuosi 怡诺思) and “Zoloft” (yulefu, 郁乐复). Some distinctive phasal keywords stand out, carrying important connotations relating to the development of Chinese antidepressant advertisements. For example, in the first phase, two phasal key words are “effectiveness” and “effective,” implying that from around the mid-1990s to the beginning of the 2000s, the basic theme of Chinese adverts is that antidepressants are “effective” in treating depressive symptoms, and such “effectiveness” is repeatedly emphasized. From the second phase onwards, more formal and scientific names of the categories of information start to appear, such as “indication” (shiyingzheng 适应症), and “adverse effects,” implying that Chinese adverts began to develop in a more scientific and objective way. In a similar way, in the third phase, “contraindication” (jinyong 禁用) and “hypersensitivity” entered as new key words, indicating that Chinese adverts in the last ten years have begun to include information related to the contraindications of antidepressants, though these are oversimplified as “not recommended for patients who have hypersensitivity to any components of the product.” At the same time, we found the words “detailed” (xiang 详), and “medical instruction” (shuomingshu 说明书) enter this key word list, and an examination of these two words in the context of Chinese adverts indicates that they occur in the typical sentence “For detailed information related to contraindication and adverse effects of this product, please refer to the medical instructions.” This implies that drug advertising regulations have been tightened to require the inclusion of the categories of contraindication and adverse effects, but the pharmaceutical companies have responded to this simply by adding a standard clause referring the reader to the medical instructions.
Table 7: Distribution of Keywords in the Chinese Corpus of Antidepressant Advertisements
Source:
Generated using Wordsmith 5.0.
Conclusion and Discussion
On a linguistic level, Chinese adverts tend to use generic language to represent antidepressants as very effective, with rare side-effects, and as a convenient-to-use medication. In describing the use of antidepressants, they over-emphasize the symptomatic problems that antidepressants can treat, especially “anxiety” and “depressiveness,” catering to the localization and conceptualization of mental illness and antidepressants within Chinese culture. That is, Chinese people tend to somatize depressionFootnote 42 due to the social stigma attached to psychological or psychiatric conditions. The adverse effects of antidepressants have been largely represented in highly generic language such as “very rare” and “extremely light.” This leads to the unbalanced representation of the information in Chinese medical journals. The basic theme of caution, which is continuously reiterated in British adverts, does not exist in Chinese adverts, because the information related to the categories of drug interaction and precaution is largely omitted. Being exposed to such limited, inaccurate and unbalanced sources of information about antidepressants may lead doctors to inaccurately or over-prescribe antidepressants.
The findings of the discourse analysis of Chinese antidepressant advertisements reflect that, from the policy level, more nuanced Chinese regulatory laws related to the contents of prescription drug advertising need to be established. As addressed earlier, the current regulation related to the content of advertisements states that they “shall conform to the instructions approved by the food and drug administrative department of the State Council.” A recent development here is that in 2015, China's Advertising Law saw its first amendment in two decades highlighting that: “Drug and device advertisements must clearly state critical information about the products, such as contraindication and adverse effects; Drug and device advertisements must not contain absolute assurances or guarantees of the product's safety.”Footnote 43 This indicates that China has realized the necessity of including critical information in the advertisements. However, as our diachronic analysis of the keywords shows, pharmaceutical companies tend to use the sentence: “Please refer to the medical instructions for detailed information related to adverse effects and contraindication.” Therefore, to ensure that detailed and balanced information is provided, regulatory laws should further indicate that all the categories of information displayed in the instructions should be covered, and the content in each category should reach a certain specified threshold of detail.
In policy terms, to ensure the accessibility, reliability and balanced supply of information in medical advertisements, advertising law also needs to be effectively implemented and enforced. This clearly depends on a complex mix of coordinated action between many interest and stakeholder groups: government, regulatory authorities, medical organizations, pharmaceutical companies, media, public health service and others. Moreover, due to the large financial stakes involved and the complex financial and political relationships between these groups, enforcing these laws can be very difficult. Complicating this, as our discourse analysis shows, Chinese advertisements describe the use of antidepressants as treating an inappropriately wide range of symptomatic problems, minimizing their side-effects. These problems are closely related to the cultural understanding of mental depression and antidepressants in China. Recognizing this, it is important to draw some of the conclusions in regard to debates around policy learning and transfer. In the area of antidepressant drug advertising, Chinese policymakers must remember:
• they cannot simply copy an existing policy structure from another area/country and expect it to work in the same way. As our analysis demonstrates, there are too many interdependent factors for this to occur.
• policies always have social and cultural meanings. Mental health is one of the most socially and culturally difficult areas for societies to confront. Hence, this will continue to be a highly contested policy area.
• nevertheless, learning from other policy actors and contexts can be very useful. The limited two-country comparison (China–UK) utilized in this article highlighted a number of weaknesses in the Chinese regulatory framework. This does not imply that copying UK regulation would necessarily solve Chinese policy issues. However, as a method of reflecting upon internal structures and strategies, sensitive and contextual international policy comparison is an excellent choice.
• lastly, this is a continual policy process. Medications, information technologies and advertising do not stand still. Although Chinese antidepressant policy could be seen to be “catching up” with British regulation, this does not mean that it ends there.
Biographical notes
Robert GEYER is a professor of politics, complexity and policy at Lancaster University. His main research focus is on complexity theory and public policy. His publications in this field include Handbook on Complexity and Public Policy (Edward Elgar, 2015); Complexity and Public Policy (Routledge, 2010). His other core area of research is in EU social and health policy with a particular emphasis on drug advertising policy. Other areas of interest are in international political economy, Scandinavian social democracy, and European politics in general.
Fang WANG is currently working at the Centre for Translation Studies, University of Surrey. Her research interests are in corpus linguistics, critical discourse analysis, and translation studies. In the field of critical discourse analysis, her areas of interest include social construction of mental depression, discourse analysis of antidepressant advertising, and news representation of global warming in Britain and China.












