Acute rheumatic fever is a well-known non-suppurative complication of pharyngitis resulting from group A β-haemolytic streptococcal infection. Steroids are used in the treatment of cases with moderate-to-severe carditis due to acute rheumatic fever.Reference Gewitz, Baltimore and Tani 1 Corticosteroids are among the main therapeutic options for several autoimmune or rheumatic diseases both in adults and children. Many side effects such as diabetes mellitus, osteoporosis, and fungal infections may develop during steroid treatment.Reference Klein-Gitelman and Pachman 2 Corticosteroids has several cardiovascular side effects that are more common in adults than in children. Corticosteroid-related bradycardia is a rarely seen side effect of steroids and it is mostly related to intravenous steroid treatment.Reference Taylor and Gaco 3 Although adult patients presenting with bradycardia following oral corticosteroid use have been reported, children with bradycardia have rarely been reported previously. In this report, we present a child who developed bradycardia following oral corticosteroid use for the treatment of acute rheumatic fever with concurrent Wolff–Parkinson–White pattern and aimed to draw attention to bradycardia as a rare side effect of oral steroid treatment.
Case
A 12-year-old girl presented with pain in her right knee joint. The patient had experienced fever and abdominal pain 3 weeks ago. Her medical and family history revealed no noteworthy signs. On physical examination, S1 and S2 were normal, cardiac sounds were rhythmical, and no additional sound was heard. Out of six, two to three systolic and diastolic murmurs were heard at the apex. Blood pressure was measured as 100/60 mmHg. Laboratory examination results were as follows: white blood count – 11,000/mm3, C-reactive protein – 14 mg/dl, sedimentation rate – 106 mm/hour, and antistreptolysin O – 865 IU. Serum electrolytes levels were normal. At electrocardiographic examination, heart rate was measured as 95/bpm, QTc as 413 ms, and P as 200 ms (Fig 1). Echocardiographic examination revealed moderate mitral regurgitation with peak velocity of 4 m/second, moderate-to-severe aortic regurgitation with peak velocity of 4.4 m/second, left ventricular end-diastolic diameter at 44 mm (z score: 0.48), ejection fraction at 75%, and shortening fraction at 44%. The patient was consulted to the Department of Rheumatology. Rheumatological parameters and serological tests were found to be negative. On the basis of these findings, the patient was diagnosed with acute rheumatic fever carditis. Prednisolone was started at a dose of 2 mg/kg in four divided doses per day. Follow-up electrocardiographic examination taken during steroid therapy revealed bradycardia attacks at Day 5, with heart rate falling to 30 bpm (Fig 2a). The delta wave was observed in the 24-hour rhythm Holter records (Fig 2b). After cessation of prednisolone treatment, the bradycardia attacks did not recur, and acetylsalicylic acid treatment was commenced for acute rheumatic fever. The patient was electrophysiologically studied. The accessory pathway was seen on anteroseptal localisation at electrophysiologic study by using catheters of radiofrequency ablation, and successful ablation was performed. Any recurrence of pre-excitation was not seen at basal condition and following adenosine injection after the electrophysiologic study. Our patient was asymptomatic during bradycardia and after the accessory pathway became evident. No additional complaint developed during the 8-month follow-up period.

Figure 1 Electrocardiography during admission of the patient.
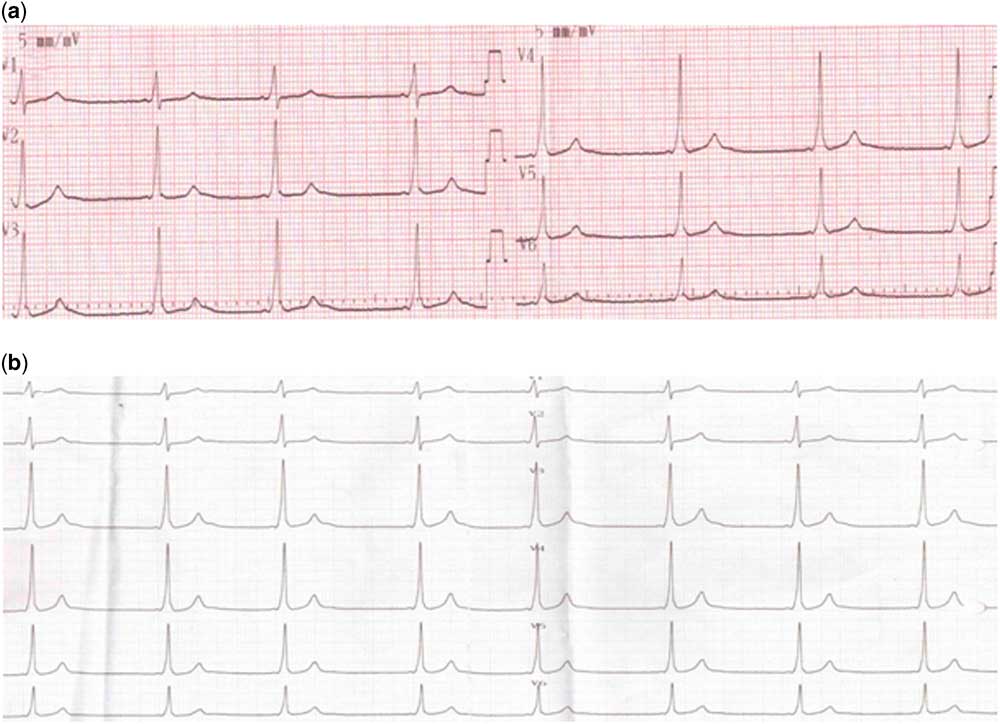
Figure 2 ( a ) Bradycardia and pre-excitation seen during steroid therapy; ( b ) pre-excitation on 24-hour rhythm Holter.
Discussion
Steroid therapy is routinely used in the treatment of acute rheumatic fever with severe carditis. Arrhythmia has been reported to occur in 1–82% of patients, especially those taking intravenous steroids.Reference Klein-Gitelman and Pachman 2 Sinus bradycardia, atrial fibrillation/flutter, and ventricular tachycardia are the most common rhythm problems that may be encountered.Reference Vasheghani-Farahani, Sahraian, Darabi, Aghsaie and Minagar 4
Corticosteroid-related bradycardia is less common than corticosteroid-related tachycardia, and it usually occurs following high-dose intravenous steroid treatment.Reference Kucukosmanoglu, Karabay, Ozbarlas, Noyan and Anarat 5 , Reference Akikusa, Feldman, Gross, Silverman and Schneider 6 Although oral steroid-related bradycardia has been reported in adults, there are few such reports for children in the literature.Reference Al Shibli, Al Attrach and Hamdan 7
Arrhythmia has been reported to occur after both a single dose and consecutive daily doses. Arrhythmia may develop during or after steroid administration. Akikusa et al.Reference Akikusa, Feldman, Gross, Silverman and Schneider 6 examined five rheumatologic children with steroid-related bradycardia and reported that bradycardia develops within 24–60 hours after first dose of steroid treatment. Our patient developed bradycardia on the 5th day after initiation of oral steroid treatment.
Arrhythmias can be symptomatic or asymptomatic, with the most common symptoms being palpitation, loss of consciousness, and cardiac arrest.Reference Kucukosmanoglu, Karabay, Ozbarlas, Noyan and Anarat 5 , Reference Al Shibli, Al Attrach and Hamdan 7 Treatment may vary according to the needs of the patient, such as only observation and monitoring, administration of chronotropic or anti-arrhythmic agents, and temporary cardiac pacing.Reference Kucukosmanoglu, Karabay, Ozbarlas, Noyan and Anarat 5 The duration of reported arrhythmias varies from hours to days, after steroid dose reduction or cessation.Reference Van der Gugten, Bierings and Frenkel 8 Our patient was asymptomatic and followed up under observation and monitoring. Bradycardia was not observed after 24 hours of oral steroid withdrawal.
The incidence of Wolff–Parkinson–White syndrome varies from 0.1 to 3 per 1000 in healthy patients.Reference Olgin and Zipes 9 This syndrome may be associated with various congenital and acquired defects such as Ebstein anomaly, mitral valve prolapse, and cardiomyopathy.Reference Olgin and Zipes 9 Observation of the Wolff–Parkinson–White syndrome in our case has been evaluated as incidental, asymptomatic, and was not associated with another congenital or acquired defect. Because Wolff–Parkinson–White syndrome is associated with lifetime cardiac arrest or sudden cardiac death risk, catheter ablation in asymptomatic patients is the preventive step to avoid sudden cardiac death. Wolff–Parkinson–White patients with some clinical features such as younger age, male gender, associated structural heart disease, posteroseptal localisation, ability to conduct anterogradely at short intervals of ≤250 ms, and inducibility of sustained atrioventricular re-entrant tachycardia and/or atrial fibrillation have higher risk of sudden cardiac death.Reference Pappone and Santinelli 10 Radiofrequency ablation was performed in our case for Wolff–Parkinson–White syndrome with established high risk on electrophysiological study.
In conclusion, as is the case with acute rheumatic fever, patients receiving steroids should be followed up carefully because of the seriousness and the multiplicity of side effects. Bradycardia can occur as a side effect of steroid treatment in children. It is usually asymptomatic and recovers after cessation of steroid.
Financial Support
This research received no specific grant from any funding agency or from commercial or not-for-profit sectors.
Conflicts of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Acknowledgements
None.




